Abstract
Osseous metastases are a source of significant morbidity for patients with a variety of cancers. Radiotherapy is well established as an effective means of palliating symptoms associated with such metastases. The role of external beam radiotherapy is limited where sites of metastases are numerous and widespread. Low linear energy transfer (LET) radionuclides have been utilized to allow targeted delivery of radiotherapy to disparate sites of disease, with evidence of palliative benefit. More recently, the bone targeting, high LET radionuclide 223Ra has been shown to not only have a palliative effect but also a survival prolonging effect in metastatic, castration-resistant prostate cancer with bone metastases. This article reviews the different radionuclide-based approaches for targeting bone metastases, with an emphasis on 223Ra, and key elements of the underlying radiobiology of these that will impact their clinical effectiveness. Consideration is given to the remaining unknowns of both the basic radiobiological and applied clinical effects of 223Ra as targets for future research.
A significant burden is imposed on patients with cancer by osseous metastases; they are common in many forms of malignancy and are often highly symptomatic. In an early autopsy series of 1000 patients dying from disseminated malignancy of various primary sites, Abrams et al1 found bone metastases present in 27.2% of their series. Metastases have biologically defined tropisms related to their site of origin. Although the biochemical determinants of these tropisms remain poorly understood, their effects are clear in the clinic and mean that certain sites of cancers have a particular preponderance for forming bone metastases. Autopsy data adapted by Coleman2 show that 73% of patients dying of breast cancer, 68% of patients dying of prostate cancer and 36% of patients dying of lung cancer have bone metastases present at post-mortem. Of those patients with osseous metastases, a significant proportion goes on to develop skeletal-related events (SREs) in relation to their metastases. The precise definition of SREs differs between trials, but they generally refer to any of the following four clinical outcomes: the need for external beam radiotherapy (EBRT) for bone pain, development of malignant spinal cord compression, pathological fracture (symptomatic or asymptomatic) or the need for orthopaedic intervention to bone metastasis. In the context of a life-limiting illness, any of the above is obviously of huge detriment to a patient's well-being on a number of fronts; pain by its very nature, time spent attending hospital, immobility associated with fracture/surgery etc. In their review of Phase 3 randomized trials of bone-modifying agents, Poon et al3 show that in the placebo arms of 11 such trials, the percentage of patients with bone metastases experiencing SREs ranged from 23.47% to 67.2%. Thus bone metastases are a common finding in advanced malignancy and within the cohort of patients experiencing them; SREs are a common outcome, with obvious detriment to the quality of life of the patients affected.
EBRT is a proven and well-established means of managing pain associated with bone metastases. In their systematic review of studies examining the palliative benefit of EBRT for bone metastases, Chow et al4 found overall response rates, with regard to pain, of 58% for single fraction and 59% for multiple fraction treatments. The same authors found complete pain response rates of 23% in single fraction and of 24% in multiple fraction treatments. A large determinant of the degree of toxicity associated with EBRT treatment is the volume of normal tissue that is irradiated in pursuit of optimally irradiating disease. Thus, with increasing number of metastases requiring treatment by EBRT, there is increasing likelihood of significant toxicity. For this reason, when targeting multiple disparate sites of bone metastases within a single field (wide-field radiotherapy) dose deliverable is severely constrained by normal tissue toxicity; the alternative approach of targeting individual metastases as its own clinical target volume rapidly becomes impractical as the number of metastases increases, from the point of view of planning and set-up time. Thus, noting both the beneficial effect of radiotherapy and the limitations inherent to EBRT, an alternative strategy of delivering radiotherapy is needed for those patients with very widespread disease. This cohort is not small; in a recent Phase 3, randomized trial, patients with prostate cancer metastatic to bone at a minimum of two sites, but not metastatic to the viscera were recruited. Within the placebo group, 12% of patients had <6 metastases, 48% had 6–20 metastases, 30% had >20 metastases and 10% had a so-called “superscan”, which is a scan with diffuse uptake throughout the entire visualized skeleton, corresponding to very widespread and advanced metastatic infiltration.5 It is in this cohort of patients that the systemic delivery of radiotherapy by bone-seeking radionuclide has long been utilized to circumvent the limitations of EBRT. Any proposed radionuclide needs to exhibit certain characteristics as outlined in Table 1—a hypothetical “ideal” radioisotope.
Table 1.
Characteristics of hypothetical “ideal radionuclide”
| Characteristic | Ideal requirement |
|---|---|
| Availability | Relative ease of manufacture making it economically viable across a range of healthcare systems |
| Mechanism of uptake | Innate affinity for areas of bone metastases or easily chelated with other species conferring such affinity |
| Efficacy | Response rates, with regard to pain, similar to or better than those seen with external beam radiotherapy |
| Safety—patient | Acceptable toxicity profile |
| Safety—public | Minimal radiation protection issues—avoiding isolation/inpatient treatment if possible |
| Safety—environment | Physical half-life allowing practical time from manufacture for delivery and administration but ensuring safe and timely decay within subject or shortly after excretion |
In the remainder of this article, first the radiobiological principles underpinning the competing strengths and weakness of different types of radionuclide therapies are examined, namely linear energy transfer (LET) and DNA damage, Bystander effect and influence of LET thereon, and oxygen enhancement ratio (OER). Second, the clinical uses of radionuclides are reviewed with particular emphasis on 223Ra.
Quality of radiation influences DNA damage and thus biological effects
Radionuclides may obviously emit alpha, beta and gamma radiation or a combination thereof. A full comparison of the physics of decay resulting in these different forms of radiation is beyond the scope of this review and is covered elsewhere in the literature.6,7 However, as might be expected, the significant differences in decay properties of various radionuclides lead to significant differences in their mechanisms of action. Alpha particles, having a significantly higher LET than either beta particles or photons, deposit more energy (cause more ionizations) along a shorter track and are thus significantly more densely ionizing than either secondary electrons from photons or beta particles. To apply this to a cellular model of cell kill resulting from ionizing radiation, one must factor in the means by which charged particles and their resultant ionization events result in cell death. As summarized well by Joiner and van der Kogel,8 there is significant evidence that cell death is related to DNA lesions, with the most lethal lesion being clustered, double-stranded DNA breaks. Being more densely ionizing, alpha particles are associated with a high probability of inducing densely clustered lesions and are thus associated with significantly higher cell kill per unit dose than either beta or gamma radiation. Relative biological effectiveness (RBE),8 being an expression of the dose of radiation of different types required to generate the same fraction cell kill, is calculated as:
The classic work by Barensden9 showed that in human tumour cell lines, RBE rose with increasing LET up to an optimum LET around 100 keV μm−1 after which RBE fell; the fall off is taken to be as a result of the phenomenon of “overkill” whereby with very high LET radiation, more DNA double-strand breaks (DSBs) occur than are actually required to kill the cell. Thus, alpha particles, by virtue of this LET/RBE relationship, are likely to result in higher cell kill per unit dose than either secondary electrons from a photon beam or beta particles.
DNA damage markers
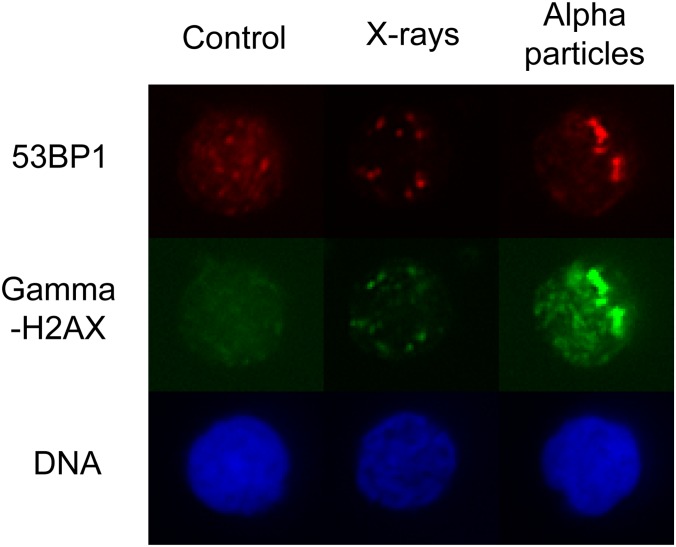
That survival is heavily dependent on the LET of incident radiation, supporting the idea that high LET radiation is having a more damaging effect on DNA than low LET radiation. In order to further verify this idea, investigators have sought evidence of this DNA damage itself in addition to work above using cell death as a surrogate for such damage. Previously, the formation of foci of γ-phosphorylated histone protein H2AX (to form γH2AX) has been shown to be a sensitive marker of DNA DSBs that occur following irradiation of cells.10 Different patterns of γH2AX foci are seen after low LET vs high LET radiation; this was demonstrated recently by Dokic et al11 in glioblastoma cell lines. They found that an increased proportion of cells develop γH2AX foci following irradiation with high LET (carbon ion) compared with low LET (320 kV X-ray) radiation. Furthermore, foci were larger following irradiation with the high LET radiation.11 High LET radiation has also been shown to cause not only foci of γH2AX but also a lower intensity, pan-nuclear background phosphorylation of H2AX in chromatin that has not suffered a DSB after some part of the DNA within the same nucleus has suffered such a break.12 A similar finding has been observed in human lymphocytes; namely, high LET radiation induces a pan-nuclear signal in addition to discrete foci of γH2AX with only discrete foci being seen following low LET radiation as shown in Figure 1 (S Horn, Queen's University Belfast, 2014, personal communication).
Figure 1.
DNA changes following irradiation with low linear energy transfer (LET) (X-rays) vs high LET (alpha particle) radiation. Note foci of H2AX phosphorylation at sites of DNA strand breaks present in both X-ray and alpha-irradiated cells but background pan-nuclear phosphorylation in alpha irradiated only Figure provided by S Horn, Queen's University Belfast, 2014.
Bystander effect
In addition to the direct tumouricidal effect of ionized particles causing double-stranded DNA breaks as described above, a further effect giving rise to cell death is the bystander effect, whereby cells that have themselves not been traversed by ionized particles but that are in close proximity to traversed cells experience cell damage and death via the bystander effect. The bystander effect with regard to radionuclides specifically has been comprehensively reviewed by Brady et al13 who cite evidence of the phenomenon both in vitro and in vivo. In experiments by Bishayee et al,14 Chinese hamster cells were labelled with tritiated thymidine and mixed with unlabelled cells to form clusters. Tritiated thymidine was selected owing to short range of the beta particles resulting from its decay, allowing selective self-irradiation of those cells labelled without irradiation of adjacent cells. They found that in the case of 100% labelling of cells, the survival of cells was dependent exponentially on the activity of the cluster (in kilobecquerel). By contrast, labelling 50% of cells yielded a two-component dose response curve indicating that labelled cells were certainly being killed but that also, unlabelled cells continue to be killed as the activity in the labelled cells was increased. Furthermore, the addition of gap-junction inhibitor lindane had no effect on the survival for 100% labelled clusters, however, in 50% labelled clusters, survival increased with the addition of lindane in a dose-dependent manner up to a plateau at a concentration of 100 μm.14 Thus there is evidence, in vitro, for a bystander effect that is abrogated by the addition of a gap-junction inhibitor. Xue et al15 investigated the same phenomenon in vivo. They used a human colonic adenocarcinoma cell line (LS174T) to grow subcutaneous tumours in nude mice, with various combinations of radiolabelled, unlabelled and dead cells used as initial inoculate. Radiolabelled cells had thymidine analogue 5-[(125)I]iodo-2′-deoxyuridine (125IUdR) incorporated into their DNA to a lethal dose. When only 125IUdR-labelled cells were inoculated, as expected, no tumour growth resulted. However, when a combination of 125IUdR and live LS174T were coadministered, significant tumour growth retardation was seen relative to controls that had the same proportions of unlabelled live and dead LS174T inoculated.15
Influence of linear energy transfer on bystander effect
There is evidence of an LET effect on the bystander phenomenon with regard to radionuclides. Boyd et al16 investigated this using noradrenaline transporter (NAT)-labelled human cell lines. NAT specifically accumulates meta-iodobenzyl-guanidine (MIBG), thus radiolabelling MIBG allows specific uptake of radioactive species into NAT-expressing cells. They then irradiated donor cells by external beam, low LET 131I-MIBG or high LET 123I-MIBG and At211-MABG. When media from irradiated donor cells was transferred into non-irradiated recipient cells, decrease in clonogenic survival was evident. For external beam, this effect plateaued with 30–40% cell kill after 2 Gy and was maintained but did not increase with further dose increase. By contrast, low LET radionuclide exposure showed no plateau in bystander cell kill with a range of concentrations of 131I-MIBG. High LET radionuclides showed U-shaped survival curves for recipient cells, with decreasing survival over the lower dose range but relatively higher survival as dose increased above a maximally lethal level.16 In a further series of elegant bystander experiments, the same group compared the high LET source 123I and the low LET source 131I as radiolabels to the compounds IUdR (localizing to DNA) and MIBG (localizing to extranuclear sites in NAT-expressing cells). This allowed them to test both the effect of radiation quality and the subcellular site of radiolabel incorporation on the bystander effect. They found that for low LET radiation (131I), when a culture of cells was exposed and then the media from these irradiated donor cells was transferred to unirradiated recipient cells, a dose-dependent decrease in survival of recipient cells was seen. This was independent of whether the carrier molecule was IUdR or MIBG, suggesting independence on the subcellular location of the radioactive species within the donor cells. For high LET radiation using 123I, which again was used to irradiate a donor population whose media was then transferred to a recipient population, at low doses, a dose-dependent decrease in survival of donor cells was seen. However, as dose increased further, this effect diminished resulting in relative increase in survival and a U-shaped survival curve. Again, there was no significant difference between those cells targeted with IUdR and those targeted with MIBG. This led the authors to conclude that at high doses, bystander effect of high LET radiations may be in opposition to the effect on irradiated cells; furthermore, the effect did not appear to be dependent on subcellular location of radionuclide accumulation.
Oxygen enhancement ratio
The tumouricidal effect of radiation on cells depends on factors inherent to the radiation as seen above. However, the relative radiosensitivity of tumour cells can depend on their local environment as well as on the quality, fractionation etc. of the radiation to which they are exposed. This is particularly true of the state of oxygenation of the tumour cells. Early work, subsequently repeated many times, showed that the tumouricidal effect of radiation was significantly greater in oxic as opposed to hypoxic or anoxic conditions.17 This has important clinical consequences, as it is also well established that oxygenation status within solid tumours is heterogeneous and significant areas of hypoxia exist owing to oxygen requirements not being met by the disordered tumour microcirculation.18 Indeed, a body of evidence exists for tumour hypoxia being associated with a poor clinical outcome, reviewed in detail by Vaupel and Mayer.19 This may be particularly true in bone metastases since, physiologically, bone is a relatively hypoxic tissue.20 In clonogenic experiments, this effect can be expressed as the OER, that is, the ratio of dose required to kill a given fraction of cells in hypoxic vs oxic conditions. Various investigators have demonstrated that OER decreases with increasing LET, with Barensden9 finding that OER reached 1 with LET of 165 keV μm−1.21 Thus, cells within relatively hypoxic and therefore radioresistant areas of tumour are likely to be more effectively killed by radiation of LET >100 keV μm−1.
Clinical use of low linear energy transfer radionuclides
Sources of low LET radiation used in the clinic tend to be beta emitters with the two most extensively studied being 89Sr and 153Sm. 89Sr decays with a half-life of 50.5 days releasing beta particles with mean energy 1.46 MeV and maximum range of 7 mm.22 153Sm has a half-life of 1.9 days and releases beta particles with mean energy of 0.81 MeV and maximum range of 2.5 mm.22 89Sr has the advantage of sharing Group 2 of the periodic table with calcium, thus its metabolism follows similar pathways to that of calcium, and it has natural affinity for areas of high bone turnover. This is not the case for 153Sm; in order to impart a tropism for areas of high bone turnover, it must be chelated with a phosphate group, in the form 153Sm-ethylenediamine tetra(methylene phosphonic acid) (153Sm-EDTMP). Finlay et al22 have reviewed the literature with regard to 89Sr and identified 16 observational studies and 11 randomized controlled trials, where it was utilized as a palliative agent. Systems used to monitor pain are, by their nature, subjective, and thus difficulties arise with intertrial variability in reporting systems; however, Finlay et al22 concluded that complete response of pain to treatment with 89Sr occurred in between 8% and 77% of patients (mean = 32%), whilst no response occurred in between 14% and 52% (mean of 25%). A randomized, double-blind, placebo-controlled trial from the 1990s showed 153Sm-EDTMP to significantly reduce pain from bony metastases associated with a range of cancers.23 Sartor et al24 more recently conducted a randomized, controlled, double-blinded trial in which patients were randomized to receive either radioactive 153Sm-EDTMP or non-radioactive 152Sm-EDTMP. Both, the subjective end point of patient-reported pain and the objective end point of analgesia consumption were reduced by the active agent, however, there was no improvement in survival. Although these low LET emitters have continued to be used, given that no survival advantage was seen and problems with their common side effect of bone marrow toxicity were relatively common, alternatives have been sought (Table 2).
Table 2.
Summary of commonly used therapeutic bone-targeting radionuclides
| Treatment | Targeting | Radiation form | Physical T1/2 (days) | Maximum particle energy (MeV) | Maximum particle range in tissue | Efficacy |
|---|---|---|---|---|---|---|
| 89Sr | Calcium mimetic | Beta particle | 50.5 | 1.46 | Approximately 7 mm | RCT evidence of symptomatic benefit from bone pain in metastatic cancer22 |
| 153Sm | Chelated to phosphate moiety [ethylenediaminetetra (methylene phosphonic acid)] | Beta particle | 1.9 | 0.81 | Approximately 2.5 mm | Placebo controlled RCT evidence of symptomatic benefit from bone pain in castration resistant prostate cancer24 |
| 223Ra | Calcium mimetic | Alpha particle | 11.4 | 27.78 | <100 μm | Double-blind, placebo controlled RCT evidence of survival benefit and symptomatic benefit in castration resistant prostate cancer5 |
RCT, randomized controlled trial; T1/2, physical half-life.
Clinical use of high linear energy transfer radionuclides
Given the radiobiological differences between high and low LET radiation as discussed above, it was postulated that high LET radionuclides (and in particular alpha particles) might offer a therapeutic advantage over those with low LET in a number of realms:
(1) higher RBE
(2) greater bystander effect at low-to-moderate dose
(3) reduced OER
(4) shorter range of alpha particles making crossfire into highly radiosensitive bone marrow compartment less likely.
With these potential advantages in mind, 223Ra has been developed as an alpha particle-emitting, bone-seeking radionuclide. Like 89Sr, it is a group 2 element and therefore has natural bone-seeking affinity and accumulates at areas of high bone turnover. 223Ra decays via a six-stage process to 207Pb; the fraction of energy released by alpha, beta and gamma radiation is 95.3%, 3.6% and 1.1%, respectively; the alpha particles released have mean energy in the range 5.0–7.5 MeV.25
Pre-clinical/Phase 1 data
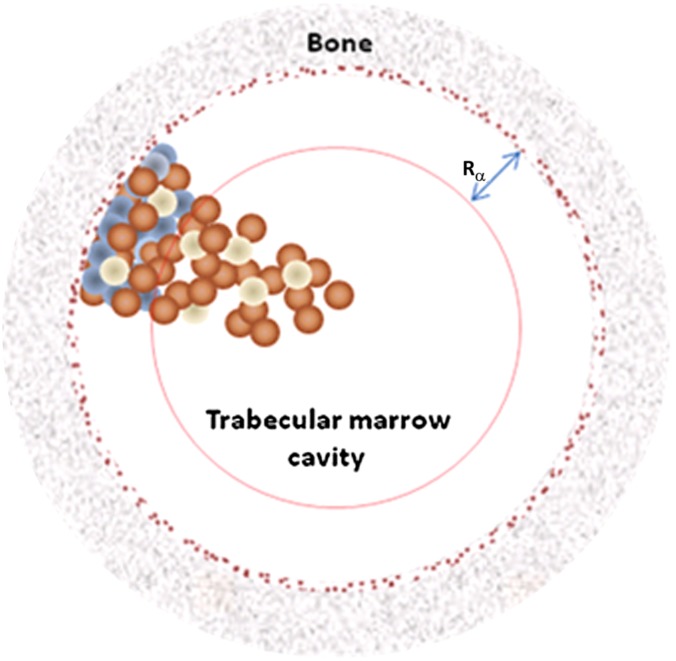
Initial pre-clinical work with mice confirmed that 223Ra preferentially accumulated in the bone with only small amounts of daughter radionuclides migrating from skeletal site of 223Ra decay.26 Furthermore, a dosimetry estimate was made and found that, as expected, high LET, short-range alpha radiation from 223Ra showed substantial sparing of the bone marrow—the tissue associated with dose-limiting toxicity, compared with beta-emitting 89Sr.26 This is shown schematically in Figure 2, demonstrating the sparing of haematopoietic marrow cavity by short-range alpha particles emitted from endosteal layer at sites of bone metastasis.
Figure 2.
Representation of the marrow cavity. Small spheres represent mix of various marrow cell types including osteoprogenitor (blue), haematopoietic (brown) and adipose (white). Dark speckled ring represents 10 µm endosteal layer. Rα is the range of the alpha particles from 223Ra decay, thus showing significant sparing of deep marrow haematopoietic stem cells. Reproduced from Hobbs et al27 with permission from IOP Publishing. For colour images please see online.
In a Phase 1 clinical trial, increasing doses of 223Ra (from 46 up to 250 kBq kg−1) were administered to 25 patients with either prostate or breast cancer, metastatic to the bone. The investigators utilized the small amount of penetrating gamma radiation released by decaying 223Ra to image the pattern of uptake of the compound. Comparing these treatment images to pre-treatment, 99mTc scans showed high concordance in terms of sites of uptake, demonstrating that 223Ra preferentially targets bone metastases rather than diffusely targeting healthy bone.28 In the same trial, blood clearance experiments showed that 12% of post-injection activity remained in blood 10 min after infusion, falling to 6% at 1 h and <1% after 24 h. The drug was well tolerated with some reversible myelosuppression of generally grades 1 and 2; two patients experienced grade 3 leucopenia and both these patients along with one further experienced grade 3 neutropenia. Although a small study, patients were asked about pain scores and benefit to most individuals was seen at 4 weeks post infusion at which time 60% of patients reported some improvement, 20% reported no change and 20% reported worse pain.28
Phase 2/3 data
These pre-clinical and Phase 1 trials were followed by three Phase 2 trials in metastatic castration-resistant prostate cancer (mCRPC). One of these was a single-dose dose–response trial involving 100 patients; each given a single infusion of 223Ra at one of four dose levels (5, 25, 50 or 100 kBq kg−1). This found a dose-dependent improvement in pain and further found 223Ra to be well tolerated at all dose levels up to the maximum of 100 kBq kg−1. The commonest toxicities were haematological and gastrointestinal with 43%, 24% and 22% experiencing nausea, vomiting and diarrhoea, respectively, whilst grade 3–4 anaemia, leucopenia, neutropenia and thrombocytopaenia were seen in 8%, 1%, 3% and 6% of patients treated, respectively.29 A further Phase 2 trial administered each subject three injections at a dose level of 25, 50 or 80 kBq kg−1 with doses given at 6-week intervals. A dose-dependent fall in alklaline phosphatase (ALP) and prostate-specific antigen (PSA) were seen, and again 223Ra was well tolerated. Gastrointestinal toxicity was again relatively common with 21% of participants experiencing diarrhoea and 16% nausea. Grades 3 or 4 haematological side effects were seen in 2 of 41 patients in 25 kBq kg−1 group, 6 of 39 in the 50 kBq kg−1 group and 7 of 42 in the 80 kBq kg−1 group.30 In a randomized, multicentre, placebo-controlled, Phase 2 trial, patients due to receive EBRT for pain were additionally assigned to receive either four 223Ra injections at a dose of 50 kBq kg−1 at 4-week intervals or placebo on the same schedule. The group receiving 223Ra showed a significant fall in ALP and delay in time to PSA progression, with a tendency towards reduced rate of SRE and improved overall survival (OS) also being seen. The safety profile was acceptable, with the only statistically significant difference between treatment and placebo groups being increased constipation in the treatment group that was mild to moderate in all but one patient (Table 3).31
Table 3.
Summary of 223Ra Phase 2/3 efficacy and safety data
| Name | Phase | Method | Number | Outcomes |
|---|---|---|---|---|
| BC-10231 | 2 | –Four injections 223Ra of 50 kBq kg−1 (or placebo) at 4-week intervals –vs placebo |
N = 33 223Ra N = 31 placebo |
–Significant delay in PSA progression and fall in ALP in the 223Ra group –Tendency towards reduced rate of skeletal-related event and improved survival in 223Ra group –Well tolerated |
| BC-10329 | 2 | –Single injection 223Ra 5, 25, 50 or 100 kBq kg−1 |
N = 26 at 5 kBq kg−1 N = 25 at 25 kBq kg−1 N = 25 at 50 kBq kg−1 N = 24 at 100 kBq kg−1 |
–Dose-dependent improvement in pain –Well tolerated all dose levels |
| BC-10430 | 2 | –Three injections 223Ra per subject at 6-week intervals –Either 25, 50 or 80 kBq kg−1 (no dose escalation within groups) |
N = 37 at 25 kBq kg−1 N = 36 at 50 kBq kg−1 N = 39 at 80 kBq kg−1 (These N are those treated per protocol and analysed in efficacy calculations. In each group, respectively, 4, 3 and 3 additional patients received 1 or 2 injections and are analysed as part of the safety population.) |
–Dose-dependent fall in PSA and ALP –Well tolerated all dose levels |
| ALSYMPCA5 | 3 | –Six injections of 223Ra of 50 kBq kg−1 (or placebo) at 4-week intervals –vs placebo –Plus best standard of care |
N = 614 223Ra N = 307 placebo |
–223Ra associated with significant improvement in overall survival (14.9 vs 11.3 months p < 0.001) –223Ra associated with significant delay to first symptomatic skeletal event (15.6 vs 9.8 months p < 0.001) –Number of patients experiencing adverse events lower in 223Ra group (all grades) –Signal to increased (low-grade) diarrhoea in 223Ra group –Signal to increased (low-grade) myelosuppression in 223Ra group |
ALP, alkaline phosphatase; PSA, prostate-specific antigen.
These positive Phase 2 data led to the large, multicentre, randomized, placebo-controlled trial of 223Ra in mCRPC—ALSYMPCA.5 This trial randomized males with mCRPC in a 2 : 1 fashion to receive either six cycles of 223Ra given 4 weeks apart or placebo given along the same schedule. There was significant improvement in survival among the patients treated with 223Ra [14.9 vs 11.3 months; hazard ratio (HR), 0.7; p < 0.001]. This result is ground breaking in so far as it was the first time any form of palliative radiotherapy had been shown to improve survival in any form of metastatic cancer. Secondary end points involving biochemical markers of disease also showed improvement in 223Ra group, with prolongation in time to increase in PSA (HR, 0.64; p < 0.001) and in time to increase in ALP (HR, 0.17; p < 0.001). 223Ra also showed benefit from a quality of life perspective with significant improvement in time to first symptomatic SREs of 5.8 months (p < 0.001) and a significantly higher proportion of patients reporting an improvement in quality of life (as measured by the Functional Assessment of Cancer Therapy–Prostate questionnaire32). 223Ra was well tolerated. The investigators provide a detailed breakdown of toxicities by type and grade. The total number of patients experiencing adverse events (AEs) was consistently lower in the treatment group than in placebo across all grades of AE, grades 3 and 4 AE, serious AE and study drug discontinuation owing to AE.5 The authors report that “no clinically meaningful differences” in the frequency of grades 3 or 4 AEs were seen between groups. There is a signal pointing towards increased, low-grade diarrhoea in 223Ra-treated individuals with 25% of 223Ra vs 15% placebo experiencing diarrhoea in all grades, 2% in each group experiencing grade 3 and none in either group experiencing grades 4 or 5.
What we do not yet know—biological
From the above discussion, it should be clear that the radiobiology of high LET radiation suggests a therapeutic advantage over low LET radiation in the context of radionuclides. This has been born out in the results from early trials and, most impressively, from the first large Phase 3 trial of 223Ra. These results are encouraging, however, there is much still to understand with regard to the biological action of high LET radionuclides. Much of the above basic science research is comparative in nature, comparing a given outcome following low LET vs high LET irradiation. There is still little known regarding the different biological processes that underpin these differences in behaviour. In particular, future work will be interesting in so far as it clarifies how much the contribution of the increase in lethality with high LET radiation is owing to a direct effect and what component of it is owing to bystander effects. Furthermore, what is the underlying biological system responsible for the bystander effect in general and what allows high LET radiation to accentuate its effect? The work quoted above by (separately) Dokic, Mayer and Horn into γH2AX signalling following radiation exposure is exciting in so far as it is beginning to show subcellular structural changes correlating with quality of incident radiation.
What we do not yet know—clinical
The proven efficacy and safety of 223Ra make it a drug that rightly inspires hope among mCRPC sufferers and those treating them. With the ALSYMPCA data, 223Ra has joined a small number of treatments proven to extend life in mCRPC namely docetaxel,33 cabazitaxel,34 abiraterone,35 enzalutamide36 and sipuleucel-T.37 With regard to 223Ra alone, uncertainty exists regarding dosing. Doses higher than the 50 kBq kg−1 used in ALSYMPCA were well tolerated in Phase 2 studies,29,30 and it is unknown if dose escalation could provide extra benefit. Furthermore, in those patients who achieve a good result with initial six cycles (as trialled in ALSYMPCA), it is as yet unknown if re-challenge with the same or dose-escalated regime on progression would result in disease response. Then with regard to the position of 223Ra in the overall treatment landscape of mCRPC, uncertainty exists as to the sequence in which the above life-prolonging treatments should be used. It is also unknown if 223Ra can safely and efficaciously be given in combination with one or more of these other agents. Trials examining the above questions are under way. An exciting possibility is that combination treatments may show a synergistic rather than simply additive effect. Recently, it has been shown that an isoform of the androgen receptor encoded by the AR-V7 splice variant is associated with resistance to both abiraterone and enzalutamide. In groups of AR-V7-positive patients treated with abiraterone acetate or enzalutmide, 0% of patients in either group showed a PSA response to respective treatment; this resistance was also manifest as shorter OS in AR-V7 groups compared with patients with wild-type receptor.38 It is possible that owing to its unique mechanism of action, 223Ra may be less susceptible to acquired or innate mechanisms of resistance; certainly less susceptible than those relying on drug–receptor interactions as in the case of AR-V7 resistance above. A final area of combination therapy that engenders much hope but also much uncertainty is the use of 223Ra in combination with EBRT. It is known from renal cancer that aggressive cytoreduction (by surgery) of the site of a primary lesion can provide an improvement in OS even in the metastatic setting.39 The use of advanced EBRT techniques in combination with 223Ra offers for the first time the option of using highly conformal and targeted radiotherapy to provide cytoreduction at distinct harbours of disease, that is, intensity-modulated radiotherapy to prostate primary and pelvic nodes and targeted 223Ra to bone metastases. This approach is to be trialled in the ADRRAD (neo-adjuvant androgen deprivation therapy, pelvic radiotherapy and radium-223 for new presentation T1-4 N0/1 M1B adenocarcinoma of prostate) clinical trial due to open shortly in the Belfast—Prostate Cancer UK Centre of Excellence. Finally, it was noted in the opening paragraph of this review that there are other cancers with a predilection for bone metastases, including breast and lung cancer. Early trials of 223Ra in the breast have already begun. Pre-clinical experiments in nude mice inoculated with breast cancer cell lines show that treatment with 223Ra inhibits tumour growth and osteolysis, and increases survival both when mice are treated prior to inoculation of tumour cells, at the stage of micrometastases or with established metastases.40 A Phase 2a non-randomized study of 223Ra in advanced breast cancer treated patients failing endocrine therapy with four cycles of 223Ra at 50 kBq kg−1. This found treatment with 223Ra to be associated with a reduction in markers of bone turnover along with a reduction in metabolic activity within one-third of the total number of bone metastases visualized across 23 patients; 223Ra was well tolerated.41 Phase 3 trials in breast cancer are set to open shortly.
CONCLUSION
There are fundamental differences in the radiobiology of low vs high LET sources of radiation. The underlying biological processes responsible for this are as yet poorly understood and represent an exciting opportunity in primary radiation research. Low LET radionuclides have proven efficacy in symptom control in mCRPC, however, they are associated with significant side effects relating to marrow suppression and have no proven survival advantage. Basic radiobiology suggests a higher LET source radionuclide with shorter range could have a therapeutic advantage, and indeed, this has been borne out in the ALSYMPCA data that showed 223Ra to be a well-tolerated, alpha-emitting, bone-seeking radionuclide with both symptom and survival improvement. The best time to use 223Ra in sequence or combination with other survival-prolonging agents is as yet uncertain, as is its utility in cancers other than that of the prostate. These, along with the possibility of combined EBRT/223Ra, are exciting avenues of clinical research.
CONFLICTS OF INTEREST
Dr PG Turner has attended Bayer and Janssen funded educational/trial events. Professor JM O'Sullivan has received payment for participating in lectures and advisory boards from Bayer, Janssen, Astellas, Lilly and Sanofi.
Contributor Information
P G Turner, Email: pturner03@qub.ac.uk.
J M O'Sullivan, Email: joe.osullivan@qub.ac.uk.
REFERENCES
- 1.Abrams HL, Spiro R, Goldstein N. Metastases in carcinoma; analysis of 1000 autopsied cases. Cancer 1950; 3: 74–85. doi: 10.1002/1097-0142(1950)3:13.0.CO;2-7 [DOI] [PubMed] [Google Scholar]
- 2.Coleman RE. Clinical features of metastatic bone disease and risk of skeletal morbidity. Clin Cancer Res 2006; 12: 6243s–9s. doi: 10.1158/1078-0432.CCR-06-0931 [DOI] [PubMed] [Google Scholar]
- 3.Poon M, Zeng L, Zhang L, Lam H, Emmenegger U, Wong E, et al. Incidence of skeletal-related events over time from solid tumour bone metastases reported in randomised trials using bone-modifying agents. Clin Oncol (R Coll Radiol) 2013; 25: 435–44. doi: 10.1016/j.clon.2013.03.003 [DOI] [PubMed] [Google Scholar]
- 4.Chow E, Harris K, Fan G, Tsao M, Sze WM. Palliative radiotherapy trials for bone metastases: a systematic review. J Clin Oncol 2007; 25: 1423–36. doi: 10.1200/JCO.2006.09.5281 [DOI] [PubMed] [Google Scholar]
- 5.Parker C, Nilsson S, Heinrich D, Helle SI, O'Sullivan JM, Fosså SD, et al. ; ALSYMPCA Investigators. Alpha emitter radium-223 and survival in metastatic prostate cancer. N Engl J Med 2013; 369: 213–23. doi: 10.1056/NEJMoa1213755 [DOI] [PubMed] [Google Scholar]
- 6.Sofou S. Radionuclide carriers for targeting of cancer. Int J Nanomedicine 2008; 3: 181–99. doi: 10.2147/ijn.s2736 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kassis AI, Adelstein SJ. Radiobiologic principles in radionuclide therapy. J Nucl Med 2005; 46(Suppl. 1): 4S–12S. [PubMed] [Google Scholar]
- 8.Joiner MC, van der Kogel A, eds. Basic clinical radiobiology. 4th edn. London, UK: Hodder Arnold; 2009. [Google Scholar]
- 9.Barensden GW. Responses of cultured cells, tumours and normal tissues to radiations of different linear energy transfer. In: Ebert M, Howard A, eds. Current Topics in Radiation Research. Volume IV. Amsterdam, Netherlands: North-Holland; 1968. pp. 293–356. [Google Scholar]
- 10.Rogakou EP, Pilch DR, Orr AH, Ivanova VS, Bonner WM. DNA double-stranded breaks induce histone H2AX phosphorylation on serine 139. J Biol Chem 1998; 273: 5858–68. doi: 10.1074/jbc.273.10.5858 [DOI] [PubMed] [Google Scholar]
- 11.Dokic I, Mairani A, Brons S, Schoell B, Jauch A, Krunic D, et al. High resistance to X-rays and therapeutic carbon ions in glioblastoma cells bearing dysfunctional ATM associates with intrinsic chromosomal instability. Int J Radiat Biol 2015; 91: 157–65. doi: 10.3109/09553002.2014.937511 [DOI] [PubMed] [Google Scholar]
- 12.Meyer B, Voss KO, Tobias F, Jakob B, Durante M, Taucher-Scholz G. Clustered DNA damage induces pan-nuclear H2AX phosphorylation mediated by ATM and DNA-PK. Nucleic Acids Res 2013; 41: 6109–18. doi: 10.1093/nar/gkt304 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Brady D, O'Sullivan JM, Prise KM. What is the role of the Bystander response in radionuclide therapies? Front Oncol 2013; 3: 215. doi: 10.3389/fonc.2013.00215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bishayee A, Rao DV, Howell RW. Evidence for pronounced bystander effects caused by nonuniform distributions of radioactivity using a novel three-dimensional tissue culture model. Radiat Res 1999; 152: 88–97. doi: 10.2307/3580054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Xue LY, Butler NJ, Makrigiorgos GM, Adelstein SJ, Kassis AI. Bystander effect produced by radiolabeled tumor cells in vivo. Proc Natl Acad Sci U S A 2002; 99: 13765–70. doi: 10.1073/pnas.182209699 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Boyd M, Ross SC, Dorrens J, Fullerton NE, Tan KW, Zalutsky MR, et al. Radiation-induced biologic bystander effect elicited in vitro by targeted radiopharmaceuticals labeled with alpha-, beta-, and auger electron-emitting radionuclides. J Nucl Med 2006; 47: 1007–15. [PubMed] [Google Scholar]
- 17.Gray LH, Conger AD, Ebert M, Hornsey S, Scott OC. The concentration of oxygen dissolved in tissues at the time of irradiation as a factor in radiotherapy. Br J Radiol 1953; 26: 638–48. doi: 10.1259/0007-1285-26-312-638 [DOI] [PubMed] [Google Scholar]
- 18.Vaupel P, Höckel M, Mayer A. Detection and characterization of tumor hypoxia using pO2 histography. Antioxid Redox Signal 2007; 9: 1221–35. doi: 10.1089/ars.2007.1628 [DOI] [PubMed] [Google Scholar]
- 19.Vaupel P, Mayer A. Hypoxia in cancer: significance and impact on clinical outcome. Cancer Metastasis Rev 2007; 26: 225–39. doi: 10.1007/s10555-007-9055-1 [DOI] [PubMed] [Google Scholar]
- 20.Hirao M, Hashimoto J, Yamasaki N, Ando W, Tsuboi H, Myoui A, et al. Oxygen tension is an important mediator of the transformation of osteoblasts to osteocytes. J Bone Mineral Metab 2007; 25: 266–76. doi: 10.1007/s00774-007-0765-9 [DOI] [PubMed] [Google Scholar]
- 21.Tinganelli W, Ma NY, Von Neubeck C, Maier A, Schicker C, Kraft-Weyrather W, et al. Influence of acute hypoxia and radiation quality on cell survival. J Radiat Res 2013; 54(Suppl. 1: i23–30. doi: 10.1093/jrr/rrt065 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Finlay IG, Mason MD, Shelley M. Radioisotopes for the palliation of metastatic bone cancer: a systematic review. Lancet Oncol 2005; 6: 392–400. doi: 10.1016/S1470-2045(05)70206-0 [DOI] [PubMed] [Google Scholar]
- 23.Serafini AN, Houston SJ, Resche I, Quick DP, Grund FM, Ell PJ, et al. Palliation of pain associated with metastatic bone cancer using samarium-153 lexidronam: a double-blind placebo-controlled clinical trial. J Clin Oncol 1998; 16: 1574–81. [DOI] [PubMed] [Google Scholar]
- 24.Sartor O, Reid RH, Hoskin PJ, Quick DP, Ell PJ, Coleman RE, et al. ; Quadramet 424Sm10/11 Study Group. Samarium-153-lexidronam complex for treatment of painful bone metastases in hormone-refractory prostate cancer. Urology 2004; 63: 940–5. doi: 10.1016/j.urology.2004.01.034 [DOI] [PubMed] [Google Scholar]
- 25.European Medicines Agency. Radium 223 Xofigo (223 Ra dichloride) summary of product characteristics. [Updated 10 February 2015; accessed 9 April 2015.] Available from: http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Product_Information/human/002653/WC500156172.pdf
- 26.Henriksen G, Fisher DR, Roeske JC, Bruland ØS, Larsen RH. Targeting of osseous sites with alpha-emitting 223Ra: comparison with the beta-emitter 89Sr in mice. J Nucl Med 2003; 44: 252–9. [PubMed] [Google Scholar]
- 27.Hobbs RF, Song H, Watchman CJ, Bolch WE, Aksnes AK, Ramdahl T, et al. A bone marrow toxicity model for 223Ra alpha-emitter radiopharmaceutical therapy. Phys Med Biol 2012; 57: 3207–22. doi: 10.1088/0031-9155/57/10/3207 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nilsson S, Larsen RH, Fosså SD, Balteskard L, Borch KW, Westlin JE, et al. First clinical experience with alpha-emitting radium-223 in the treatment of skeletal metastases. Clin Cancer Res 2005; 11: 4451–9. doi: 10.1158/1078-0432.CCR-04-2244 [DOI] [PubMed] [Google Scholar]
- 29.Nilsson S, Strang P, Aksnes AK, Franzèn L, Olivier P, Pecking A, et al. A randomized, dose-response, multicenter phase II study of radium-223 chloride for the palliation of painful bone metastases in patients with castration-resistant prostate cancer. Eur J Cancer 2012; 48: 678–86. doi: 10.1016/j.ejca.2011.12.023 [DOI] [PubMed] [Google Scholar]
- 30.Parker CC, Pascoe S, Chodacki A, O'Sullivan JM, Germá JR, O'Bryan-Tear CG, et al. A randomized, double-blind, dose-finding, multicenter, phase 2 study of radium chloride (Ra 223) in patients with bone metastases and castration-resistant prostate cancer. Eur Urol 2013; 63: 189–97. doi: 10.1016/j.eururo.2012.09.008 [DOI] [PubMed] [Google Scholar]
- 31.Nilsson S, Franzén L, Parker C, Tyrrell C, Blom R, Tennvall J, et al. Bone-targeted radium-223 in symptomatic, hormone-refractory prostate cancer: a randomised, multicentre, placebo-controlled phase II study. Lancet Oncol 2007; 8: 587–94. [DOI] [PubMed] [Google Scholar]
- 32.Cella D, Nichol MB, Eton D, Nelson JB, Mulani P. Estimating clinically meaningful changes for the Functional Assessment of Cancer Therapy–Prostate: results from a clinical trial of patients with metastatic hormone-refractory prostate cancer. Value Health 2009; 12: 124–9. doi: 10.1111/j.1524-4733.2008.00409.x [DOI] [PubMed] [Google Scholar]
- 33.Tannock IF, de Wit R, Berry WR, Horti J, Pluzanska A, Chi KN, et al. ; TAX 327 Investigators. Docetaxel plus prednisone or mitoxantrone plus prednisone for advanced prostate cancer. N Engl J Med 2004; 351: 1502–12. doi: 10.1056/NEJMoa040720 [DOI] [PubMed] [Google Scholar]
- 34.de Bono JS, Oudard S, Ozguroglu M, Hansen S, Machiels JP, Kocak I, et al. ; TROPIC Investigators. Prednisone plus cabazitaxel or mitoxantrone for metastatic castration-resistant prostate cancer progressing after docetaxel treatment: a randomised open-label trial. Lancet 2010; 376: 1147–54. doi: 10.1016/S0140-6736(10)61389-X [DOI] [PubMed] [Google Scholar]
- 35.de Bono JS, Logothetis CJ, Molina A, Fizazi K, North S, Chu L, et al. ; COU-AA-301 Investigators. Abiraterone and increased survival in metastatic prostate cancer. N Engl J Med 2011; 364: 1995–2005. doi: 10.1056/NEJMoa1014618 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Scher HI, Fizazi K, Saad F, Taplin ME, Sternberg CN, Miller K, et al. ; AFFIRM Investigators. Increased survival with enzalutamide in prostate cancer after chemotherapy. N Engl J Med 2012; 367: 1187–97. doi: 10.1056/NEJMoa1207506 [DOI] [PubMed] [Google Scholar]
- 37.Kantoff PW, Higano CS, Shore ND, Berger ER, Small EJ, Penson DF, et al. ; IMPACT Study Investigators. Sipuleucel-T immunotherapy for castration-resistant prostate cancer. N Engl J Med 2010; 363: 411–22. doi: 10.1056/NEJMoa1001294 [DOI] [PubMed] [Google Scholar]
- 38.Antonarakis ES, Lu C, Wang H, Luber B, Nakazawa M, Roeser JC, et al. AR-V7 and resistance to enzalutamide and abiraterone in prostate cancer. N Engl J Med 2014; 371: 1028–38. doi: 10.1056/NEJMoa1315815 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Flanigan RC, Mickisch G, Sylvester R, Tangen C, Van Poppel H, Crawford ED. Cytoreductive nephrectomy in patients with metastatic renal cancer: a combined analysis. J Urol 2004; 171: 1071–6. doi: 10.1097/01.ju.0000110610.61545.ae [DOI] [PubMed] [Google Scholar]
- 40.Suominen MI, Rissanen JP, Käkönen R, Fagerlund KM, Alhoniemi E, Mumberg D, et al. Survival benefit with radium-223 dichloride in a mouse model of breast cancer bone metastasis. J Natl Cancer Inst 2013; 105: 908–16. doi: 10.1093/jnci/djt116 [DOI] [PubMed] [Google Scholar]
- 41.Coleman R, Aksnes AK, Naume B, Garcia C, Jerusalem G, Piccart M, et al. A phase IIa, nonrandomized study of radium-223 dichloride in advanced breast cancer patients with bone-dominant disease. Breast Cancer Res Treat 2014; 145: 411–18. doi: 10.1007/s10549-014-2939-1 [DOI] [PMC free article] [PubMed] [Google Scholar]