Abstract
Objective:
To assess the diagnostic quality of low dose (100 kV) CT angiography (CTA), by using ultra-low contrast medium volume (30 ml), for thoracic and abdominal aorta evaluation.
Methods:
67 patients with thoracic or abdominal vascular disease underwent multidetector CT study using a 256 slice scanner, with low dose radiation protocol (automated tube current modulation, 100 kV) and low contrast medium volume (30 ml; 4 ml s−1). Density measurements were performed on ascending, arch, descending thoracic aorta, anonymous branch, abdominal aorta, and renal and common iliac arteries. Radiation dose exposure [dose–length product (DLP)] was calculated. A control group of 35 patients with thoracic or abdominal vascular disease were evaluated with standard CTA protocol (automated tube current modulation, 120 kV; contrast medium, 80 ml).
Results:
In all patients, we correctly visualized and evaluated main branches of the thoracic and abdominal aorta. No difference in density measurements was achieved between low tube voltage protocol (mean attenuation value of thoracic aorta, 304 HU; abdominal, 343 HU; renal arteries, 331 HU) and control group (mean attenuation value of thoracic aorta, 320 HU; abdominal, 339; renal arteries, 303 HU). Radiation dose exposure in low tube voltage protocol was significantly different between thoracic and abdominal low tube voltage studies (490 and 324 DLP, respectively) and the control group (thoracic DLP, 1032; abdomen, DLP 1078).
Conclusion:
Low-tube-voltage protocol may provide a diagnostic performance comparable with that of the standard protocol, decreasing radiation dose exposure and contrast material volume amount.
Advances in knowledge:
Low-tube-voltage-setting protocol combined with ultra-low contrast agent volume (30 ml), by using new multidetector-row CT scanners, represents a feasible diagnostic tool to significantly reduce the radiation dose delivered to patients and to preserve renal function, while also maintaining adequate diagnostic quality images in assessment of aorta.
Since the introduction of multidetector CT (MDCT), CT angiography (CTA) has become a standard imaging tool for the evaluation of diseases affecting the aorta and its major branches.1 CTA has been advocated for pre-operative evaluation of thoracic and abdominal aortic aneurysms and their relationship with the main branches. Moreover, it is crucial to detect other vascular morbidities, such as dissections and arterial occlusive diseases.2 CTA allows the proper visualization of main vascular structures and has several advantages: minimal invasiveness, with a lower complication rate than that of angiography; generation of high spatial resolution images of both the arterial wall and the lumen; availability of multiplanar reconstructions (MPR) and three-dimensional (3D) reconstructions; and short examination times, allowing extended scan ranges.1,3
The extended use of MDCT in the clinical practice, however, may result in an increase of both the frequency of CTA studies and patient's radiation exposure compared with single-slice CT.4 Therefore, CT protocols should be properly designed and carefully applied in order to obtain the highest amount of information by using the lowest radiation dose achievable,5–10 since the theoretical risk of radiation-induced cancer from CT examinations has been reported as not negligible.1 As the radiation exposure is linearly dependent on the tube current, a helpful technique for reducing radiation dose involves the modulation of tube current itself, according to real-time local attenuation (i.e. Siemens Medical Solution, Forchheim, Germany and Philips Medical Systems, Best, Netherlands)5,11 or predictive calculation or sinusoidal interpolation between anteroposterior and lateral views, depending on the different manufacturers.
Moreover, the reduction of the X-ray tube voltage, keeping a constant current, can theoretically reduce the radiation exposure exponentially. However, it has to be considered that a lower radiation dose is associated with higher image mottle, and may therefore degrade image quality.4
The use of large amounts of contrast media (CM) is another concern for CTA, because patients with aortic aneurysms generally tend to be aged and suffer from other comorbidities, such as atherosclerotic renal vascular disease and diabetes. Some studies showed that contrast-induced impairment of renal function directly depends on the amount of CM.2,12–15
Lowering the tube voltage represents the most widely reported technique for reducing the radiation burden in body CTA.16 This approach allows a significant radiation dose reduction because the dose changes with the square of the tube voltage.17 Moreover, higher attenuation levels for iodine-based CM are achieved at lower X-ray tube voltages owing to greater photoelectric effect and decreased Compton scattering.18 The closer the tube voltage approaches the K-edge of iodine (33.2 keV), the greater the inherent attenuation of the iodinated CM is.19 Hence, a lower CM volume can be injected while obtaining the same vessel attenuation. If current values are not increased correspondingly, the low tube voltage scanning can lead to an increased image noise,18 but this does not necessarily result in reduced subjective image quality. The increased attenuation of the iodine-containing arterial vessels and the high attenuation differences between the arterial system and poorly enhanced surrounding tissues can partially offset the higher image noise.16,19
The aim of our study was to assess, by using a 256 MDCT scanner, the feasibility of reducing both the radiation dose exposure and the CM volume, in the assessment of thoracic or abdominal aorta and their major branches.
METHODS AND MATERIALS
Study population
This study was performed in a single university centre and was approved by our institutional review board; written informed consent was obtained from all patients.
From July 2010 to March 2012, 67 consecutive patients, with known or clinical suspicion of aortic (and/or its major branches) disease, were prospectively enrolled in our study (Table 1). We performed, independently, a total of 41 studies for thoracic aorta and 26 for abdominal aorta. A control group of 35 consecutive patients, with clinical suspicion of aortic disease, was prospectively evaluated using a standard CTA protocol and compared with the study group (Table 1). We performed a total of 22 studies of thoracic aorta and 13 of abdominal aorta.
Table 1.
Summarizing table of study and control group patient characteristics
| Patient characteristics | Study group | Control group |
|---|---|---|
| Number of patients | 67 | 35 |
| Sex (male/female) | 45/12 | 30/5 |
| Age (years), mean (range) | 65.4 (35–83) | 66 (39–83) |
| Body mass index (kg m−2), mean (range) | 24.6 (17.4–29.3) | 25.1 (16.5–29.7) |
In both groups, exclusion criteria for contrast-enhanced CT (CE-CT) examination were: (a) patients showing renal failure [serum creatine level >2 mg dl−1 (152 mmol l−1)] or other contraindications for iodinated contrast material injections, such as previous allergic reaction; (b) patients referred for follow-up after aneurysm repair or stent-graft placement, since the low dose contrast volume cannot provide the proper vessel opacification required to evaluate endoleaks, in the venous phase; (c) heart rate >100 beats per minute (bpm) in thoracic aorta studies; (d) body mass index (BMI) ≥30 kg m−2 at the time of the examination. In order to obtain a feasible CTA protocol, useful to evaluate all non-obese patients in the clinical practice, the included patients were not distinguished in different categories according to BMI values.
CT angiography protocol
All the patients, including those of the control group, underwent a 256 MDCT scan examination of thoracic or abdominal aorta (Brilliance iCT; Philips Medical Systems, Best, Netherlands) with the following scan parameters: thickness, 1 mm; increment, 1 mm; collimation, 128.000 × 0.625; pitch, 0.915 (0.17 for the chest); rotation time, 0.4 s (0.27 s for the chest); field of view, 350 mm; matrix, 512 × 512.
In each patient, an 18-gauge intravenous catheter was placed in an antecubital vein in the upper limb, and CM was injected using a double-syringe injector (Medrad Stellant®; Medrad Inc., Pittsburgh, PA). In order to obtain an optimal intraluminal contrast enhancement, the start of scanning in both groups was individually obtained for each patient by using bolus-tracking (B-T) technique, with a trigger level of 120 HU and a delay time between 5 and 7 s. The triggering area was manually placed at the top of the ascendant aorta in the thoracic study and at the aortic hiatus in the abdominal study, in order to reduce the time motion of the CT bed from the trigger area to the start level of acquisition. All scans were obtained in a single breath-hold, acquired from the upper level of the clavicles to the aortic hiatus in the chest studies (mean total scan duration of 6.2 s; range, 5.4–7.1 s) and from the aortic hiatus to proximal femoral bifurcation in the abdominal examinations (mean total scan duration of 2.9 s; range, 2.1–3.8 s), respectively. No B-blockers or heart-rate-lowering agents were administered before scanning.
The study group was examined using a low-dose-radiation protocol, with 100 kV and automated tube current modulation. In all 67 patients of this group, low CM volume (30 ml, Xenetix® 350, 350 mgI ml−1; Guerbet, Aulnay-sous-Bois France) was administered, with a flow rate of 4 ml s−1 followed by saline flushing (50 ml; flow rate, 4 ml s−1). Moreover, in the evaluation of thoracic aorta, in order to minimize motion artefacts owing to cardiac pulsation, we used a retrospective electrocardiogram (ECG)-gated protocol with EGC-based tube modulation. Using this protocol, the current is dropped to 20% of the reference current when the cardiac phase is outside 35–70% of the R-R interval. The scanner software automatically reconstructed data acquired at 60% of the R-R interval from the scans obtained with retrospective gating.
In the control group, we used a standard CTA protocol both for the thoracic and abdominal aorta, with 120 kV tube voltage and automated tube current modulation. In the CTA study of the chest, the same ECG-gating protocol was used. In this group, a total of 80 ml of CM (Xenetix 350, 350 mgI ml−1) was administered with a flow rate of 4 ml s−1, followed by saline flushing (50 ml; flow rate, 4 ml s−1). The coverage area for both thoracic and abdominal CTA studies was the same as in the study group.
Image analysis
Images were processed on a dedicated workstation (Brilliance Workspace; Philips) in order to produce MPR (thickness, 2 mm; increment, 1 mm; standardized smooth reconstruction kernel), maximum intensity projections (MIPs) and volume-rendering (VR) images.
Vessel contrast enhancement (mean attenuation value, Hounsfield units) was measured in the source axial images by manually placing a circular region of interest (ROI) in the centre of the vascular lumen at four different levels along the z-axis in the thoracic study, as follows: (1) ascending aorta; (2) aortic arch; (3) anonymous trunk; (4) descending aorta. In the abdominal study, ROIs were placed at three different levels as follows: (1) aorta (above renal arteries origin); (2) both renal arteries; (3) both common iliac arteries. The ROI's size was as large as possible but varied depending on the target artery and avoiding wall calcifications. We also evaluated the vessel opacification after CM volume injection, considering adequate for uniform CTA a difference <50 HU in attenuation values among the different levels along z-axis.2 These methods of analysis were derived from previous studies.1–4,20
Two radiologists with 7 and 3 years' experience, respectively, in CTA and 3D vascular images interpretation, who were blinded to the two different scanning parameters, performed qualitative evaluations of the randomized 3D aortograms on VR and MIP-reconstructed images, in a blinded manner. For qualitative evaluation, the diagnostic quality of images and the visualization of major aortic branches were evaluated using a three-point subjective scale, derived from ones previously proposed in literature:1–3 (1) as “inadequate” (major artefacts, unsatisfactory delineation of the boundary or poor visualization of major branches); (2) as “diagnostic” (delineation of the boundary equivocal but within an acceptable range, minor artefacts and sufficient visualization of major branches); (3) as “good” (no artefacts and excellent visualization of boundary and major branches).
To analyse the radiation dose exposure, the CT dose–length product (DLP; mGy cm) and the CT dose index (CTDIvol; mGy) were recorded for all scans. We also compared the image noise in the two groups by calculating the signal-to-noise ratio (SNR) using the following formula: mean attenuation values of the aorta (Hounsfield units)/standard deviation (SD).
Statistical analysis
All statistical analysis was performed using commercially available software (MedCalc v. 11.0.0; MedCalc Software, Mariakerke, Belgium). The Mann–Whitney U test was used to evaluate differences between the study group (100 kV; 30 ml) and the control group (120 kV; 80 ml) in terms of age, weight, mean attenuation values of the aorta (Hounsfield units) and SNR. Using the same test, we also compared the radiation dose exposure (DLP and CTDIvol) and image quality scores between the two groups. A p < 0.05 was considered statistically significant.
The interobserver agreement of mean attenuation values, image noise and SNR were calculated using intraclass correlation coefficient (ICC). Additionally, the interobserver agreement of image quality scores was determined by reliability statistics using ICC based on the Cronbach’s alpha model. Three levels of ICC were defined as follows: moderate agreement (<0.61), good agreement (between 0.61 and 0.81) and very good agreement (>0.81).20
RESULTS
All CTA examinations were completed in a single breath-hold, without any complications and without any adverse effect after contrast material injection.
There were no statistically significant differences in terms of age and body weight between the study and the control groups, both in the thoracic and in the abdominal evaluation (Table 1). The concomitant pathological vascular findings (e.g. aneurism, stenosis and atherosclerosis) obtained in all the patients are summarized in Table 2.
Table 2.
Summarizing table of CT vascular findings in the study and control groups
| Diagnosis | Study group | Control group |
|---|---|---|
| Normal finding | 13/67 | 5/35 |
| Atherosclerosis | 32/67 | 22/35 |
| Aortic aneurysm | 21/67 | 16/35 |
| Aortic dissection | 3/67 | 3/35 |
| Penetrating aortic ulcer | 1/67 | 0/35 |
| Branch aneurysm | 4/67 | 5/35 |
| Branch stenosis (>70%) | 5/67 | 2/35 |
In the study group, images of the aorta and its major branches were evaluated, in terms of quality, as good in 52/67 patients (78%) and diagnostic in 15/67 patients (22%). No inadequate findings were obtained. Contrast enhancement >200 HU (reported as necessary value for diagnostic efficacy of CTA1) was obtained in 282/294 of measurements (96%). In 12/294 of measurements (4%), we observed contrast enhancement <200 HU and >153 HU. The main branches of thoracic and abdominal aorta (e.g. renal arteries, mesenteric arteries) were always properly visualized up to almost their second order of ramifications; no diagnostic differences between the study and the control groups were found.
Thoracic aorta
In the thoracic aorta examination, the two readers obtained similar density measurements in terms of Hounsfield units at the four different levels evaluated, both in the low tube voltage protocol and in the control group, without any statistical difference: the mean attenuation values obtained in the ascending aorta, in the arch, in the anonymous trunk and in the descending aorta were summarized in Table 3. No statistical differences (p > 0.05) were also found in the control group compared with the study group in terms of enhancement values.
Table 3.
Summarizing table of calculated values for enhancement in the thoracic study and control group
| Readers | Aortic levels | Parameters |
||
|---|---|---|---|---|
| Study group (100 kV; 30 ml) enhancement (mean HU ± SD) | Control group (120 kV; 80 ml) enhancement (mean HU ± SD) | p-value | ||
| First reader | Ascending aorta | 301 ± 82.64 | 284 ± 66.67 | 0.5573 |
| Arch | 314 ± 83.88 | 279 ± 63.73 | 0.1522 | |
| Descending aorta | 323 ± 90.15 | 272 ± 68.11 | 0.0528 | |
| Anonymous artery | 327 ± 92.93 | 282 ± 66.18 | 0.0910 | |
| Second reader | Ascending aorta | 295 ± 85.65 | 289 ± 70.2 | 0.6619 |
| Arch | 314 ± 86.86 | 277 ± 63.03 | 0.1736 | |
| Descending aorta | 321 ± 92.6 | 277 ± 74.21 | 0.1351 | |
| Anonymous artery | 326 ± 91.13 | 285 ± 69.35 | 0.1895 | |
HU, Hounsfield unit; SD, standard deviation.
p < 0.05 indicates the difference between the study and control groups.
Concerning radiation dose exposure (Table 4), significant statistical differences (p = 0.0005) were found in the DLP displayed in the 100 kV ECG-gated group (490 mGy cm) compared with the control group (1032 mGy cm), with an average DLP reduction of 61%.
Table 4.
Summarizing table of mean radiation dose exposure in the thoracic and abdominal studies
| Parameters | Study group (100 kV; 30 ml) (mean value ± SD) | Control group (120 kV; 80 ml) (mean value ± SD) | p-value |
|---|---|---|---|
| DLP chest | 490 ± 100.60 | 1032 ± 89.21 | <0.0005 |
| DLP abdomen | 334 ± 126.23 | 1078 ± 59.60 | <0.0001 |
| CTDIvol chest | 13.85 ± 6.02 | 27.78 ± 3.13 | <0.0005 |
| CTDIvol abdomen | 5.83 ± 1.9 | 22.51 ± 1.94 | <0.0001 |
CTDIvol, CT dose index volume; DLP, dose–length product; SD, standard deviation.
p < 0.05 indicates the difference between study and control group.
The image noise (evaluated by SD) measured by the first reader was higher in the study group (SD = 24.5) than in the control group (SD = 14.1). The SNR of the study group (11.6) significantly differed (p = 0.0067) from the SNR of control group (18.32) (Table 5). Same results about SD and SNR were obtained for the second reader, with mean SD of 26.66 and SNR of 11.3 for the study group and mean SD of 12.61 and SNR of 21.53 for the control group, respectively (Table 5).
Table 5.
Summarizing table of calculated values for noise measurements in the study and control groups
| Readers | SNR | Parameters |
||
|---|---|---|---|---|
| Study group (100 kV; 30 ml) (mean value ± SD) | Control group (120 kV; 80 ml) (mean value ± SD) | p-value | ||
| First reader | Chest | 11.6 ± 7.91 | 18.32 ± 7 | 0.0067 |
| Abdomen | 10.8 ± 2.93 | 14.55 ± 4.21 | 0.0365 | |
| Second reader | Chest | 12.6 ± 6.82 | 21.53 ± 6.42 | 0.0026 |
| Abdomen | 11.2 ± 3.63 | 14.8 ± 5.31 | 0.0412 | |
SD, standard deviation; SNR, signal-to-noise ratio.
p < 0.05 indicates the difference between the study and control groups.
Abdominal aorta
The abdominal low-dose CTA studies have shown homogeneous and diagnostic enhancement at all the different levels of the aorta.
The two readers have found no statistical differences between the mean attenuation values in suprarenal aorta and common iliac arteries in the study group in comparison with the control group, as reported in Table 6. However, in the study group, we found higher values of vessel density in the renal arteries in comparison with control group (Table 6), maybe in relation to the high flow rate coupled with low resistance that characterizes these branches. No significant differences of density measurement or image degradation between proximal and distal aortic levels were observed in the study group (i.e. mean attenuation value of renal arteries 335 HU vs common iliac arteries 323 HU) (Table 6).
Table 6.
Summarizing table of calculated values for enhancement in the abdominal study and control group
| Readers | Aortic levels | Parameters |
||
|---|---|---|---|---|
| Study group (100 kV; 30 ml) enhancement (mean HU ± SD) | Control group (120 kV; 80 ml) enhancement (mean HU ± SD) | p-value | ||
| First reader | Abdominal aorta | 350 ± 80.39 | 312 ± 37.5 | 0.1248 |
| Renal arteries | 335 ± 70.85 | 282 ± 37.45 | 0.0008 | |
| Common iliac arteries | 323 ± 81.84 | 323 ± 42.76 | 0.2425 | |
| Second reader | Abdominal aorta | 350 ± 81.55 | 314 ± 43.33 | 0.1233 |
| Renal arteries | 339 ± 79.99 | 290 ± 41.73 | 0.0025 | |
| Common iliac arteries | 315 ± 82.24 | 329 ± 49.95 | 0.0767 | |
HU, Hounsfield unit; SD, standard deviation.
p < 0.05 indicate the difference between the study and control groups.
The radiation dose exposure (DLP) in the abdominal aorta studies with low-tube-voltage setting was significant lower (p < 0.0001) than the mean value measured in the control group (324 vs 1078 mGy cm). This corresponds to an average reduction in DLP of 69.9% in the study group (Table 4).
The image noise, obtained by the first reader, differed significantly between the study group (SD, 31.2) and the control group (SD, 20.63), with a corresponding reduction of SNR from 14.55 in the control group to 10.8 in the study group (p = 0.0365) (Table 5). The second reader's evaluation was similar, with an increase in image noise (SD, 22.1 in the study group vs SD, 15.8 in the control group) and a significant reduction (p = 0.0412) of SNR from the control group (SNR, 14.8) to the study group (SNR, 11.2) (Table 5).
A very good interobserver agreement level was found in the evaluation of density measurements such as in the qualitative analysis, both in thoracic (ICC, 0.99) and in abdominal (ICC, 0.96) CTA studies. The interobserver agreement of SNR was very good in thoracic examinations and good in the abdominal scans (Table 7). No moderate agreement was found in all the evaluations.
Table 7.
Summarizing table of interobserver agreement between the first and the second reader
| Interobserver agreement | ICC study group | ICC control group |
|---|---|---|
| Thoracic density measurements | 0.99 | 0.97 |
| Abdominal density measurements | 0.96 | 0.98 |
| Thoracic SD | 0.94 | 0.92 |
| Abdominal SD | 0.69 | 0.74 |
| Thoracic SNR | 0.94 | 0.93 |
| Abdominal SNR | 0.79 | 0.77 |
| Thoracic quality | 0.98 | 0.99 |
| Abdominal quality | 0.94 | 0.93 |
ICC, interclass correlation coefficient; SD, standard deviation; SNR, signal-to-noise ratio.
Agreement <0.61 was considered moderate, between 0.61 and 0.81 was considered good and >0.81 was considered very good.
DISCUSSION
In the past years, several strategies have been considered for dose reduction in body CTA studies, such as the use of automated or patient size-adjusted tube current (mA) modulation, virtual non-CE imaging from dual-energy CT and iterative reconstruction algorithms.
In accordance with the as low as reasonably practicable principle to maintain radiation exposure as far below the dose limits as practical, the aim of our study was to reduce the radiation dose exposure in CTA studies of thoracic or abdominal aorta, lowering the tube voltage at 100 kV and also employing automatically adapted tube current modulation along with ECG-controlled tube current system. Exploiting the higher X-ray attenuation at low voltage of iodine-based CM, we also reduced the CM volume administered during CTA (30 ml) in order to maintain an adequate and diagnostic opacification of arterial vessels.
In spite of an increased noise, the images obtained in our study were adequate for diagnosis, with an overall good visualization and evaluation of the aorta and its major branches in all the patients included (Figures 1 and 2). Contrast enhancement achieved was >200 HU in 92% of patients (96% of measurements) and lower (up to 153 HU) in only 4% of cases. These patients were characterized by heart failure that reduced vascular flow rate with the decrease of CM concentration in the vascular lumen. However, even in these cases, image quality was adequate.
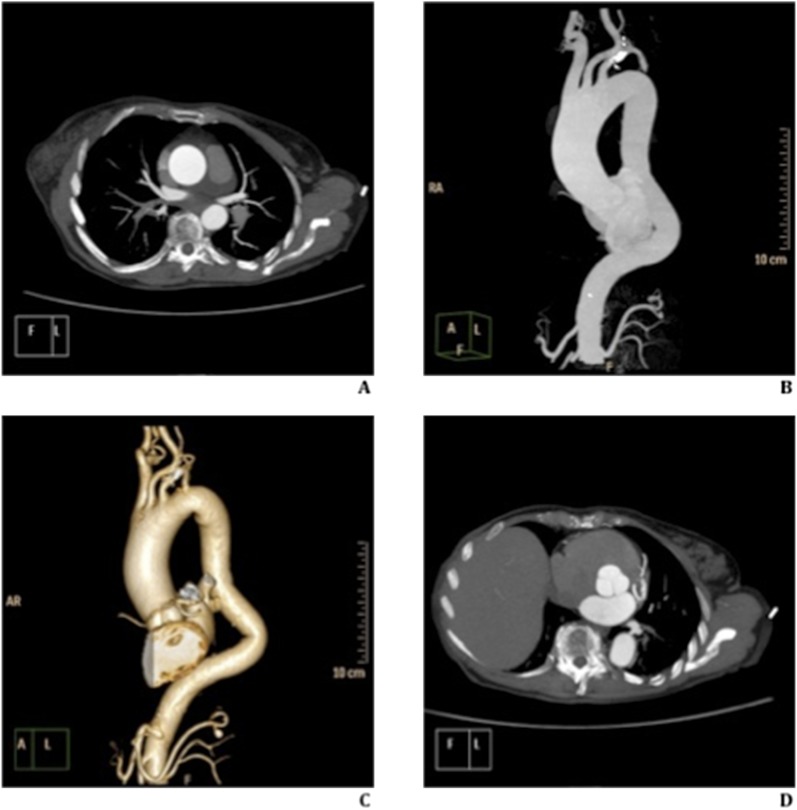
Figure 1.
73 year old-female with ascending aorta aneurysm. Thoracic aorta ECG-gated study, with low tube voltage (100 kV) and ultra low contrast material (40 ml). (a–d) Axial (a and d) and parasagittal (b) maximum intensity projections and volume-rendering reconstruction (c) show homogeneous and uniform contrast enhancement of vascular structures analysed. High definition of ECG-gated study, without cardiac motion artefacts, highlights the aortic valve (d).
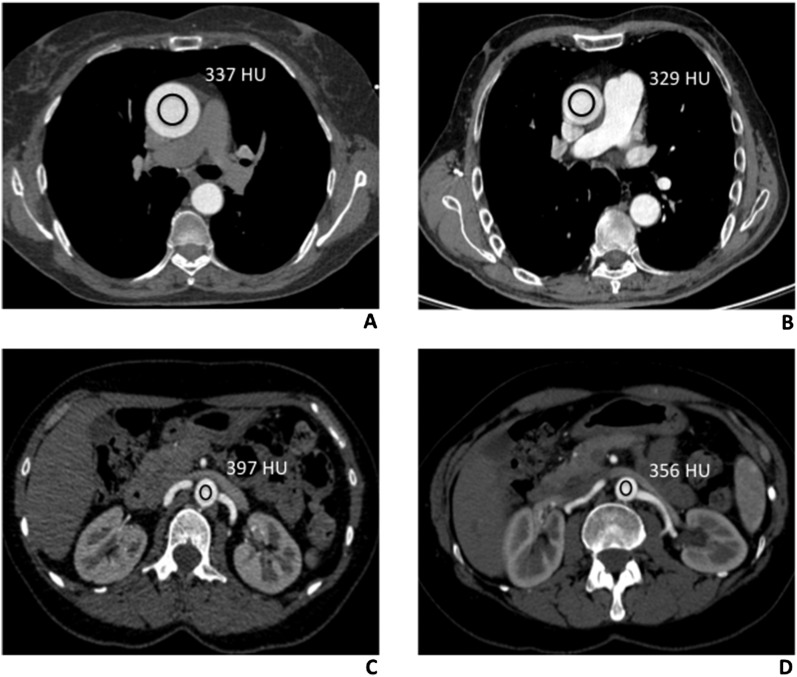
Figure 2.
Density measurements (Hounsfield units) of thoracic and abdominal aorta in low and standard tube voltage CT angiography (CTA) studies. (a) 100 kV protocol CT scan of a 57-year-old male, with no aortic disease. Axial CTA thoracic image showing a region of interest (ROI) in the lumen of the ascending aorta, at the level of the common pulmonary artery (337 HU). (b) Comparative image of a 62-year-old male, with no aortic disease, evaluated with the 120 kV protocol. Axial CTA thoracic image, acquired at the same level, showing the ROI in the ascending aortic lumen, with the corresponding value of 329 HU. (c) A 68-year-old male, who had undergone CTA study with the 100 kV protocol. Axial CT abdominal scan, with the rounded ROI drawn into the abdominal aortic lumen (397 HU), at the origin of renal arteries. (d) Comparative image of a 65-year-old male, evaluated with the 120 kV protocol. Axial CTA scan, with the ROI drawn into the lumen of the abdominal aorta (356 HU), at the same level.
In the study group, we obtained a good and homogeneous magnitude of vascular enhancement throughout the whole scanning coverage, both in thoracic (i.e. mean attenuation of ascending aorta, 301 HU vs descending aorta, 323 HU, Reader 1) and abdominal studies (i.e. mean attenuation of renal arteries, 339 HU vs common iliac arteries, 315 HU, Reader 2). No statistical differences in opacification of proximal and distal vascular structures analysed were found (Figure 2). These results were significant for diagnostic quality of images and also allowed high-quality 3D reconstructions to be obtained (Figure 1), proving that low-KV protocol represents a feasible tool to establish a correct evaluation of vascular lumen.
Our results are in line with those previously published by Nakayama et al.1 Using low-tube-voltage protocol (90 kV) with low CM (40 ml), they reported a mean attenuation value >200 HU in 89.5% and did not find any significant difference in average aortic CT attenuation compared with standard protocol (120 kV, 100 ml of CM). However, they reported that the intravessel attenuation at the level of the distal iliac branches was lower using low tube voltage and low CM protocol. Such decay was previously described also by Kubo et al,2 which compared three different doses of CM (100, 75 and 50 ml) in the CT study of thoracic and abdominal aortic aneurysms, performed with 120 kV and automatic tube current modulation technique (100–220 mAs). The authors found that by injecting 50 ml of CM with 20 ml of saline flush, the mean attenuation value was significantly lower (p < 0.05) at Level 3 (distal) than those calculated at Levels 1 and 2 of the aortic axis. This decay of uniformity and homogeneity in the low CM group was related to the delayed blood flow in patients with aortic aneurysms. We did not find the same deterioration as described by Nakayama et al1 and Kubo et al2 using low CM volume. This occurrence may be explained by several factors: the different CT scanners (16- and 8-row vs 256-row); the different range of scan, as we studied separately the thoracic and the abdominal aorta; and the reduction of tube voltage in our protocol (not performed by Kubo et al) that could have increased the enhancement.
Using the 100 kV study protocol, we were also able to reduce significantly the radiation dose exposure. Particularly, in the study of abdominal aorta, we reduced the DLP approximately 70%, compared with the 120 kV protocol. Also, in the thoracic study, using retrospectively ECG-gated protocol with two systems of tube current modulation dose (automated tube current modulation dose along z-axis and EGC-based tube modulation), we obtained a decreased radiation exposure (about 60%) in the low tube voltage group. In our study protocol, the image noise increased and the vessel enhancement remained constant; consequently, a decrease in SNR value both of the chest (from 18.32 ± 7 to 11.6 ± 7.91) and the abdomen (from 14.55 ± 4.21 to 10.8 ± 2.93) between the two protocols was achieved.
Our results were in accordance with those of Manousaki et al21 who have shown the possibility of obtaining diagnostic angiographic images of the aorta and renal arteries at tube voltages as low as 100 kVp, using 60 mAs and 100 ml of iodinated contrast material (370 mg ml−1). The authors estimated that the reduction of the tube voltage to 100 kVp resulted in 44.5% dose reduction compared with the 120 kV CTA protocol. Image quality in the 100 kV study was moderately downgraded compared with 120 kV images but remained satisfactory and adequate for diagnosis without any statistical difference in image quality.
The DLP reduction in our study was higher than that reported by Wintersperger et al.4 In their abdominal aortic studies, they have shown a DLP lowering within the 100 kV group of about 37% compared with the 120 kV group. The differences in terms of DLP reduction between our study and the one by Wintersperger et al (60% vs 37%) could be related to the different acquisition protocols (i.e. pitch) and the different CT scanner employed (256-row vs 16-row, respectively). Our results disagree with those of d'Agostino et al22 that, using two dose modulation systems and 120 kV for chest examinations, obtained high-quality ECG-gated examinations of the entire thorax with DLP values (260.57 mGy cm−1) lower than that obtained during standard non-ECG-gated examinations of the chest (650 mGy cm−1). They achieved greater DLP reduction than that of our study because they used similar tube current modulation but higher pitch parameter (0.33 vs 0.17).
Some limitations in our study have to be acknowledged. Firstly, we did not include patients after endovascular stent placement, because they require the acquisition of venous phase to evaluate late endoleaks, and this may not be properly obtained with 30 ml of CM. Furthermore, based on ethical concerns, we did not compare the same group of patients but two different cohorts; so, the haemodynamic differences and body habitus may have partially influenced our results. The potential advantage of our CTA protocol for scanning patients with renal failure is highly predictable owing to the low amount of CM administered but a direct correlation with renal function was not performed in our study. The sample size evaluated was small, and therefore theoretically, the absence of statistically significance obtained in terms of image quality in our series should be related to the low power of the study that was not calculated before the enrollment of patients. For these reasons, our preliminary results need to be confirmed by further investigations in a larger series of patients, in order to validate the proposed CTA protocol.
Lastly, a large number of tests with different outcomes were performed, using two different blinded readers, and we did not employ any correction's test for multiple tests, so an increase of unreal significant differences may be found. However, the purpose of our study was to demonstrate that low tube voltage with low CM protocol does not differ significantly to standard CT-angiographic protocol. The absence of statistically significant differences between the two protocols may be even strengthened by the lack of correction's test.
In conclusion, our results suggest that the low tube voltage (100 kV) CTA protocol with low contrast material (30 ml) represents a feasible, reproducible and useful technique that allows, in routine clinical practice, the thoracic or abdominal aorta to be properly evaluated, with a significant reduction of radiation dose exposure and contrast material volume injected.
Contributor Information
D Ippolito, Email: davide.atena@tiscalinet.it.
C Talei Franzesi, Email: ctfdoc@hotmail.com.
D Fior, Email: davidefior85@gmail.com.
P A Bonaffini, Email: pa.bonaffini@gmail.com.
O Minutolo, Email: orazio.minutolo@hotmail.it.
S Sironi, Email: sandrosironi@libero.it.
REFERENCES
- 1.Nakayama Y, Awai K, Funama Y, Liu D, Nakaura T, Tamura Y, et al. Lower tube voltage reduces contrast material and radiation doses on 16 MDCT aortography. AJR Am J Roentgenol 2006; 187: W490–7. [DOI] [PubMed] [Google Scholar]
- 2.Kubo S, Tadamura E, Yamamuro M, Kanao S, Kataoka ML, Takahashi M, et al. Multidetector-row computed tomographic angiography of thoracic and abdominal aortic aneurysms: comparison of arterial enhancement with 3 different doses of contrast material. J Comput Assist Tomogr 2007; 31: 422–9. [DOI] [PubMed] [Google Scholar]
- 3.Fujioka C, Horiguchi J, Kiguchi M, Yamamoto H, Kitagawa T, Ito K. Survey of aorta and coronary arteries with prospective ECG-triggered 100-kV 64-MDCT angiography. AJR Am J Roentgenol 2009; 193: 227–33. doi: 10.2214/AJR.08.1722 [DOI] [PubMed] [Google Scholar]
- 4.Wintersperger B, Jakobs T, Herzog P, Schaller S, Nikolaou K, Suess C, et al. Aorto-iliac multidetector-row CT angiography with low kV settings: improved vessel enhancement and simultaneous reduction of radiation dose. Eur Radiol 2005; 15: 334–41. [DOI] [PubMed] [Google Scholar]
- 5.Mastora I, Remy-Jardin M, Delannoy V, Duhamel A, Scherf C, Suess C, et al. Multi-detector row spiral CT angiography of the thoracic outlet: dose reduction with anatomically adapted online tube current modulation and preset dose savings. Radiology 2004; 230: 116–24. [DOI] [PubMed] [Google Scholar]
- 6.Naidich DP, Marshall CH, Gribbin C, Arams RS, McCauley DI. Low-dose CT of the lungs: preliminary observations. Radiology 1990; 175: 729–31. [DOI] [PubMed] [Google Scholar]
- 7.Zwirewich CV, Mayo JR, Müller NL. Low-dose high-resolution CT of lung parenchyma. Radiology 1991; 180: 413–17. [DOI] [PubMed] [Google Scholar]
- 8.Mayo JR, Jackson SA, Müller NL. High-resolution CT of the chest: radiation dose. AJR Am J Roentgenol 1993; 160: 479–81. [DOI] [PubMed] [Google Scholar]
- 9.Lee KS, Primack SL, Staples CA, Mayo JR, Aldrich JE, Müller NL. Chronic infiltrative lung disease: comparison of diagnostic accuracies of radiography and low- and conventional-dose thin-section CT. Radiology 1994; 191: 669–73. [DOI] [PubMed] [Google Scholar]
- 10.Lucaya J, Piqueras J, García-Peña P, Enríquez G, García-Macías M, Sotil J. Low-dose high-resolution CT of the chest in children and young adults: dose, cooperation, artifact incidence and image quality. AJR Am J Roentgenol 2000; 175: 985–92. [DOI] [PubMed] [Google Scholar]
- 11.Kalender WA, Wolf H, Suess C, Gies M, Greess H, Bautz WA. Dose reduction in CT by on-line tube current control: principles and validation on phantoms and cadavers. Eur Radiol 1999; 9: 323–8. [DOI] [PubMed] [Google Scholar]
- 12.Joshi SB, Mendoza DD, Steinberg DH, Goldstein MA, Lopez CF, Raizon A, et al. Ultra-low-dose intra-arterial contrast injection for iliofemoral computed tomographic angiography. JACC Cardiovasc Imaging 2009; 2: 1404–11. doi: 10.1016/j.jcmg.2009.08.010 [DOI] [PubMed] [Google Scholar]
- 13.Kubo S, Tadamura E, Yamamuro M, Hosokawa R, Kimura T, Kita T, et al. Thoracoabdominal-aortoiliac MDCT angiography using reduced dose of contrast material. AJR Am J Roentgenol 2006; 187: 548–54. [DOI] [PubMed] [Google Scholar]
- 14.Martin ML, Tay KH, Flak B, Fry PD, Doyle DL, Taylor DC, et al. Multidetector CT angiography of the aortoiliac system and lower extremities: a prospective comparison with digital subtraction angiography. AJR Am J Roentgenol 2003; 180: 1085–91. [DOI] [PubMed] [Google Scholar]
- 15.Tepel M, Aspelin P, Lameire N. Contrast-induced nephropathy: a clinical and evidence-based approach. Circulation 2006; 113: 1799–806. [DOI] [PubMed] [Google Scholar]
- 16.Alkadhi H, Schindera ST. State of the art low-dose CT angiography of the body. Eur J Radiol 2011; 80: 36–40. doi: 10.1016/j.ejrad.2010.12.099 [DOI] [PubMed] [Google Scholar]
- 17.Huda W, Scalzetti EM, Levin G. Technique factors and image quality as functions of patient weight at abdominal CT. Radiology 2000; 217: 430–5. [DOI] [PubMed] [Google Scholar]
- 18.Leschka S, Stolzmann P, Schmid FT, Scheffel H, Stinn B, Marincek B, et al. Low kilovoltage cardiac dual-source CT: attenuation, noise, and radiation dose. Eur Radiol 2008; 18: 1809–17. doi: 10.1007/s00330-008-0966-1 [DOI] [PubMed] [Google Scholar]
- 19.Schindera ST, Graca P, Patak MA, Abderhalden S, von Allmen G, Vock P, et al. Thoracoabdominal-aortoiliac multidetector-row CT angiography at 80 and 100 kVp: assessment of image quality and radiation dose. Invest Radiol 2009; 44: 650–5. doi: 10.1097/RLI.0b013e3181acaf8a [DOI] [PubMed] [Google Scholar]
- 20.Farrelly C, Davarpanah A, Keeling AN, Sheehan J, Ragin A, Yaghmai V, et al. Low dose dual-source CT angiography of the thoracic aorta. Int J Cardiovasc Imaging 2011; 27: 1025–34. doi: 10.1007/s10554-010-9742-9 [DOI] [PubMed] [Google Scholar]
- 21.Manousaki E, Perisinakis K, Karantanas A, Tsetis D. MDCT angiography assessment of renal artery in-stent restenosis: can we reduce the radiation exposure burden? A feasibility study. Eur J Radiol 2011; 79: 224–31. doi: 10.1016/j.ejrad.2010.03.017 [DOI] [PubMed] [Google Scholar]
- 22.d'Agostino AG, Remy-Jardin M, Khalil C, Delannoy-Deken V, Flohr T, Duhamel A, et al. Low-dose ECG-gated 64-slices helical CT angiography of the chest: evaluation of image quality in 105 patients. Eur Radiol 2006; 16: 2137–46. [DOI] [PubMed] [Google Scholar]