Abstract
Following early results of recent studies of intraoperative radiotherapy (IORT) in the adjuvant treatment of patients with early breast cancer, the clinical utility of IORT is a subject of much recent debate within the breast oncology community. This review describes the intraoperative techniques available, the potential indications and the evidence to date pertaining to local control and toxicity. We also discuss any implications for current practice and future research.
Adjuvant radiotherapy (RT) following surgery in the treatment of early stage breast cancer delivered with external beam RT (EBRT) permits breast conservation with low rates of in-breast tumour recurrence (IBTR).1 When IBTR occurs, however, the recurrence is most commonly located in the same quadrant as the index tumour.2 Furthermore, pathological examination of mastectomy specimens demonstrates that malignant and/or pre-malignant cells are rarely found >4 cm from the index lesion.3 Such data have given rise to the hypothesis that irradiating only the part of the breast tissue in proximity to the index tumour will be associated with local control rates comparable with those seen after adjuvant whole-breast EBRT. Such partial breast irradiation would also be expected to be associated with less toxicity given the reduced volume of non-target tissue irradiation.
Partial breast RT may be delivered by a number of techniques, including EBRT, interstitial brachytherapy and intraoperative RT (IORT).4,5 The use of IORT has been reported in a range of tumour sites, including the breast, head and neck, lung, limbs (sarcoma), gastrointestinal and genitourinary tracts, and lung.6–12 For most tumour sites, the premise of IORT is to deliver RT directly and therefore potentially more accurately to the tumour itself or to the tumour bed whilst delivering minimal dose to the surrounding normal tissues. Although not in routine clinical practice, in previous studies of IORT, the IORT has been delivered in combination with EBRT as a “boost” or as the sole RT modality.6,13
In the context of adjuvant treatment for early breast cancer, IORT has most commonly been delivered to the tumour bed after surgical excision of the tumour, and a number of technical approaches have been described. IORT to the breast has been used both to deliver a tumour-bed boost in conjunction with EBRT and as definitive adjuvant RT treatment instead of whole-breast EBRT.13,14 Until recently, there has been a lack of randomized Phase III trial evidence comparing IORT with EBRT in either setting. This review describes the different IORT techniques available, the potential clinical utility of IORT, the evidence to date and the implications for standard practice and future research.
SEARCH STRATEGY
The terms “intraoperative”, “radiotherapy” and “breast” along with their derivatives were used to search PubMed. All studies relating to IORT for breast cancer were included in the preparation of the review. No limitations were placed on language or year of publication. This search returned 480 citations, of which 34 studies of relevance were included in this review.5,6,13–44
INTRAOPERATIVE RADIOTHERAPY DELIVERY TECHNIQUES
IORT may be delivered using linear accelerator-based EBRT, electrons, low-energy photons or by high-dose-rate (HDR) brachytherapy. HDR brachytherapy as a truly intraoperative technique has been reported in head and neck cancer.7 Although insertion of brachytherapy after-loading catheters can occur intraoperatively, HDR brachytherapy is usually delivered in several fractions in the days following surgery and hence is not truly intraoperative. Brachytherapy has an advantage permitting customization of dose distribution to better fit the tumour bed or the shape of the chest wall.
Electron IORT may be delivered by a standard linear accelerator in close approximation to the operating theatre or by a mobile linear accelerator within the operating theatre.15,16 The use of a standard linear accelerator poses both infection control and logistical challenges, as the patient under general anaesthetic must be transferred from the operating theatre to the linear accelerator and back again. By virtue of being within the operating theatre, mobile linear accelerators reduce infection control requirements and avoid moving the anaesthetized patient. Examples of mobile linear accelerators include the Novac7® (Hitesys Srl, Latina, Italy) the Liac (Info&Tech, Roma, Italy) and the Mobetron device (IntraOp Medical Corporation, Sunnyvale, CA). These deliver electrons at energies of between 3 and 12 MeV using a small linear accelerator mounted on a robotic arm. Typically, the beam is collimated using perspex applicators, and aluminium and lead discs may be inserted at the base of the tumour bed cavity to reduce the dose to the chest wall.14 Doses of approximately 21 Gy prescribed to the 90% isodose line are delivered, and treatment times are of the order of less than 15 min.
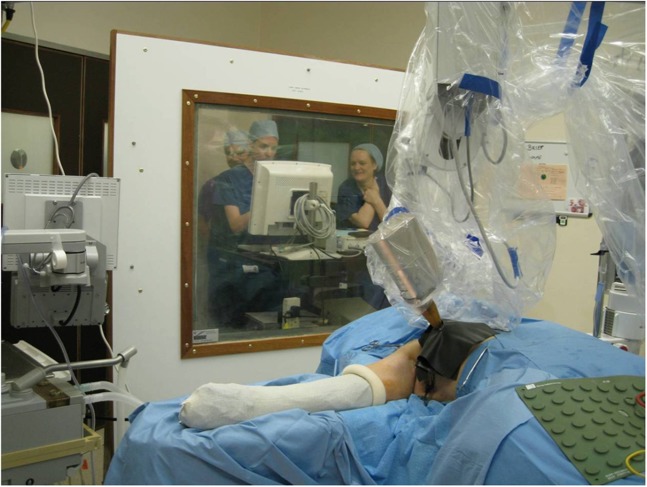
IORT to the tumour bed may also be delivered using low-energy photons. For example, the INTRABEAM® system (Carl Zeiss Surgical, Oberkochen, Germany) uses a miniature X-ray generator mounted on a robotic arm.17,18 This device accelerates electrons that strike a gold target at the tip of a 10-cm long drift tube leading to the emission of low-energy X-rays typically around 50 kV. A spherical applicator keeps the irradiated tissue at a fixed distance and ensures uniformity in dose distribution. The applicator is available in a range of sizes with the larger applicators having lower dose rates. Typically, doses of 20 Gy are delivered in treatment times of between 20 and 45 min depending on the size of the applicator used. As this device uses low-energy X-rays, no modification of the operating theatre is required, but lead shielding between the device and theatre staff is recommended (Figure 1).19
Figure 1.
Intraoperative radiotherapy delivery using the INTRABEAM® (Carl Zeiss Surgical, Oberkochen, Germany) device. Reproduced from Eaton et al19 with permission from the British Institute of Radiology.
POTENTIAL PHYSICAL AND RADIOBIOLOGICAL ADVANTAGES OF INTRAOPERATIVE RADIOTHERAPY
The IORT techniques that deliver RT using brachytherapy, electrons or low-energy X-rays deliver effective radiation dose to between 10- and 30-mm depth dependent on the precise technique and energy used;20 therefore, dose to surrounding normal tissues is significantly reduced.21 With low-energy X-ray systems, there is a possibility of higher dosage to the chest wall owing to the higher electron density (Z) of the bone. However, given the short effective range, this risk is minimized.18 It should be noted that, as no cross-sectional imaging is normally undertaken during the delivery of IORT, it is not possible to report the dose delivered to classical International Commission on Radiation Units and Measurements constructs such as the clinical target volume (CTV) or planning target volume.
Delivering dose in a single large fraction has a number of radiobiological advantages. Firstly, it avoids the problems of repopulation and repair. In general, a single IORT has a presumed biological equivalence of 1.5–2.5 times the EBRT dose.22 When large fractions of RT (e.g. 21 Gy) are delivered, there is concern that this may lead to increased normal tissue effects in late reacting tissues. However, normal tissues repair DNA damage more efficiently and promptly than do malignant tissues, often in minutes. Hence, it has been postulated that with the longer treatment delivery times with IORT techniques compared with EBRT techniques, such as with the INTRABEAM device (25–35 min), repair of normal tissues may occur during treatment delivery, hence, reducing the apparent effect of large fraction sizes on normal tissues.23 Uncertainty exists as to the robustness of radiobiological models such as the linear quadratic model at predicting early and late tissue effects with large single doses.45 However, at the low dose rates used in IORT, it is likely that the linear–quadratic model may provide a reasonable estimate of late effects for single high doses.46
POTENTIAL CLINICAL UTILITY OF IORT IN THE ADJUVANT TREATMENT OF BREAST CANCER
The use of IORT has been reported in a number of settings in the adjuvant treatment of breast cancer. These include IORT prior to excision, IORT delivered as a boost in conjunction with whole-breast EBRT after surgery, IORT as the sole RT adjunct and IORT for reirradiation after prior EBRT to the breast. IORT has also been used when EBRT is not possible owing to other medical conditions contraindicating EBRT. Evidence relating to these different indications is now discussed in turn, but it should be noted that the use of IORT in the various indications discussed below does not yet represent routine clinical practice, and none of these indications is endorsed by any current clinical guideline.
Intraoperative radiotherapy delivery to in situ tumour prior to excision
Potential uncertainties around the delivery of IORT include the definition of the CTV, the radiation dose distribution and confirmation of dose delivery to the CTV. It has been suggested that the delivery of IORT to the intact tumour and surrounding breast tissue prior to surgical excision of the tumour may reduce these uncertainties.24 Kimple et al25 reported outcomes following a single fraction of IORT prior to surgical excision of tumours for patients with early stage breast cancer. In this Phase II study, 71 patients who were node negative and had tumours of <3 cm, as measured on pre-operative imaging, were treated with IORT to a dose of 15 Gy to the tumour prior to excision. Of the 71 patients treated with IORT, 11 received subsequent EBRT owing to adverse features on final histopathological examination, such as lobular histology, nodal positivity and tumour size. At a median follow-up of 3.5 years, the 3-year actuarial local control rate of 92% was lower than expected, with a 6-year follow-up reporting a concerning IBTR rate of 15%.25,26 This high local failure rate is at a level to suggest that this is unlikely to become a standard indication for IORT. The role of pre-operative partial breast irradiation using EBRT, however, remains under investigation in the Netherlands Cancer Institute PAPBI trial (Image Guided Preoperative Accelerated Partial Breast Irradiation).47
Intraoperative radiotherapy as a boost in conjunction with external beam radiotherapy
A boost following EBRT to the whole breast has been shown to nearly halve local recurrence rates in comparison with those reported following EBRT alone.48 Before three-dimensional conformal RT techniques were used to delineate the tumour bed, identification of the lumpectomy cavity was largely based on the surgical scar, and it is estimated that the tumour bed received inadequate radiation dose in up to 50% of patients.49 With modern RT techniques and the use of titanium clips inserted perioperatively into the tumour bed, it is anticipated that the risk of geographic miss has been reduced.50 However, it is suggested that IORT, by physically delivering radiation dose directly to the lumpectomy cavity, may reduce the risk of geographic miss depending on what one believes to be the depth of the CTV beyond the tumour bed.27
Most of the studies identified in the PubMed search used either IORT with intraoperative electrons, INTRABEAM or intraoperative EBRT. One group of researchers used an interesting technique of injecting a solution of avidin into and around the tumour bed.28 On the day following surgery, 3.7 GBq of 90Y-biotin spiked with 185 MBq of 111In-biotin was administered intravenously. Avidin has a very high affinity for biotin and hence caused the uptake of radiolabelled biotin in the tumour bed. Although avidination is not strictly by definition an IORT technique, the reported outcomes of this procedure demonstrate an acceptable toxicity profile. 3 (8.6%) of the 35 patients included had RTOG grade 3 skin toxicity at the completion of EBRT. At a follow-up of 6 months, the highest skin toxicity was grade 1 using the Radiation Therapy Oncology Group (RTOG) scoring system, which was observed in five (14.3%) of the patients.
Six studies report outcomes of IORT used in place of an EBRT tumour-bed boost.13,29–33 The main characteristics are summarized in Table 1. There is potential overlap in reporting of outcomes of the patients included in the studies by Reitsamer et al.31,32 The length of follow-up is variable ranging from 8.9 to 79 months, and the local control rates published are excellent ranging from 96% to 100%. Although not tested in a randomized Phase III study, the disease control outcomes of using IORT in place of an EBRT boost are favourable. But concern exists regarding the late toxicity of using IORT as boost in combination with EBRT. In a subanalysis of the targeted intraoperative radiotherapy A (TARGIT-A) study, the combination of IORT and whole breast radiotherapy (WBRT) had a late normal tissue fibrosis rate at 3 years of 37.5 vs 18.4% for WBRT alone and 5.9% for IORT alone.34 This level of late fibrosis exceeds that reported in the large EBRT boost study (4.4% severe fibrosis rate at 10 years).48
Table 1.
Studies reporting outcomes of the use of intraoperative radiotherapy (IORT) in place of an external beam radiotherapy (EBRT) tumour-bed boost
| Study | Study type | Sample size | IORT technique | IORT dose (Gy) | EBRT boost dose | EBRT whole-breast dose | Follow-up time (months) | IORT group LCR (%) | EBRT boost LCR (%) |
|---|---|---|---|---|---|---|---|---|---|
| Fastner et al29 | Cohort | 1109 | Intraoperative electrons | 10 | N/A | 50–54 Gy in 25–27 fractions | 72.4 | 99.2 | N/A |
| Joseph et al30 | Phase II | 35 | INTRABEAM® | 5 | N/A | 45 Gy in 25 fractions | 8.9 | N/A | N/A |
| Reitsamer et al31 | Prospective cohort | 156 | Intraoperative electrons | 9 | N/A | 51–56 Gy in 30 fractions | 18 | 100 | N/A |
| Reitsamer et al32 | Sequential intervention study | 378 | Intraoperative electrons | 9 | 12 Gy in six fractions | 51–56 Gy in 30 fractions | 25.8 | 100 | 95.70 |
| Vaidya et al13 | Prospective cohort | 299 | INTRABEAM | 20 | N/A | 45–50 Gy in 25 fractions | 60.5 | 98.30 | N/A |
| Wong et al33 | Prospective cohort | 52 | Intraoperative electrons | 10 | N/A | 48 Gy in 24 fractions | 79 | 96 | N/A |
LCR, local control rate; N/A, not available.
INTRABEAM obtained from Carl Zeiss Surgical, Oberkochen, Germany.
One difficulty with the use of IORT to deliver a boost is that the tumour pathological characteristics are not available at the time of surgery. This causes problems in two different directions. Firstly, in patients with positive margins and who require re-excision, an IORT boost may be ineffective; it may make re-excision more prone to complications and may make interpretation of the pathological examination of the re-excision margins more difficult. Secondly, there is a subset of patients with low risk of local recurrence such as patients aged 50 years and who at surgery are found to have low-grade disease, small tumours, clear excision margins, the absence of lymphovascular invasion and favourable biology. In these patients, a boost does not add a sizeable benefit in local control and hence may be avoided. Identifying these patients prior to surgery may be difficult but is essential to achieve the best outcomes when using IORT in place of an EBRT tumour boost. However, given the excellent outcomes with this strategy, the use of IORT for a tumour-bed boost warrants further investigation and the results of currently recruiting studies such as the non-randomized Intra-Operative Electron Boost, the Hypofractionated Whole-Breast Irradiation During Breast-conserving Treatment study (HIOB study) and the NIHR-HTA funded TARGIT-B randomized study are awaited.51 Also awaited are data regarding the cost-effectiveness of using two different RT delivery techniques for the same patient.
Intraoperative radiotherapy as the sole radiotherapy treatment vs whole-breast external beam radiotherapy
As described earlier, it has been suggested that, as most local recurrences occur in the region of tumour bed, for females of low risk of recurrence, it may be possible to avoid whole-breast EBRT and treat the tumour bed only. A range of partial breast techniques have been described including IORT.4 Numerous cohort studies have reported outcomes of IORT delivered as the definitive RT treatment, but in the last year, two randomized studies [electron intraoperative radiotherapy (ELIOT) and TARGIT-A] have published their findings comparing IORT with EBRT as definitive adjuvant RT treatment, and their main findings are summarized in Table 2.14,35 The ELIOT study was conducted in a single Italian institution. In the ELIOT study, IORT was delivered using intraoperative electrons and the dose fractionation regimen for EBRT was standardized for all patients. Patients randomized to receive EBRT received a dose of 50 Gy in 25 fractions to the whole breast followed by a 10-Gy dose in 5 fractions tumour-bed boost. Patient demographics and tumour characteristics were largely balanced between both groups. In the ELIOT study, although IBTR with IORT at 4.4% was within a pre-specified equivalence limit, it was significantly higher that with EBRT (0.4%) and the authors report that IORT was not equivalent to EBRT.14 However, no significant differences in overall survival were noted between the groups, and skin toxicity in the 67% of randomized patients for whom data were available was significantly reduced in patients receiving IORT (p = 0·0002).
Table 2.
Phase III randomized studies comparing intraoperative radiotherapy (IORT) with external beam radiotherapy (EBRT) as the definitive adjuvant radiotherapy (RT) treatment
| Study | Sample size | IORT technique | IORT dose (Gy) | EBRT whole-breast dose | Median follow-up (years) | IBTR for IORT group (%) | IBTR for EBRT group (%) | IBTR hazard ratio in favour of IORT |
|---|---|---|---|---|---|---|---|---|
| ELIOT14 | 1305 | Intraoperative electrons | 21 | 50 Gy/25 fractions to the whole breast, followed by 10 Gy/5 fractions tumour-bed boost | 5.8 | 4.4 | 0.4 | 9.3 (95% confidence interval, 3.3–26.3) |
| TARGIT-A35 | 3451 | INTRABEAM® | 20 | 40–56 Gy with or without a boost of 10–16 Gy | 2.4 | 3.3 | 1.3 | Not reported |
IBTR, in-breast tumour recurrence.
INTRABEAM obtained from Carl Zeiss Surgical, Oberkochen, Germany.
The TARGIT-A trial was a multicentre study involving 33 centres in 11 countries with a 1 : 1 randomization between EBRT and partial breast RT using the INTRABEAM device.35 Of the 3451 patients included in the study, 1153 were randomized post-operatively to tumour-bed boost using IORT vs whole-breast RT such that this subset did not truly test IORT. The remaining 2298 patients were randomized before surgery to IORT vs whole-breast EBRT. Taking the trial population as a whole (n = 3451), local recurrence following EBRT was lower than for those receiving partial breast RT (1.3 vs 3.3%; p = 0.042). However, for the subset randomized pre-surgically to EBRT vs IORT, the difference in local recurrence was smaller and non-significant (EBRT = 1.1; IORT = 2.1%; p = 0.31). Neither was breast cancer mortality nor overall survival significantly different between the two groups. As with the ELIOT study, skin toxicity was significantly better in those who received partial breast RT (4 vs 13 patients with grades 3–4 skin toxicity for IORT vs EBRT, respectively; p = 0.029). The authors reported reduced non-breast cancer deaths in the partial breast group compared with the whole-breast EBRT group [17 (1.4%) vs 35 (3.5%); p = 0.006] with two vs eight deaths being cardiovascular in origin. The reasons for these differences are not clear and, interestingly, from expected death rates in females of the same median age as those treated in TARGIT-A, it appears that the number of deaths in the EBRT group was as expected, but in the IORT group was lower than expected. In any event, it is highly unlikely that the excess of deaths in the WBRT arm has been caused by WBRT itself.36 Based on epidemiological data relating mean heart dose to incidence of major coronary events, the mean heart doses of 1–5 Gy delivered by whole-breast WBRT within the trial would not be expected to result in anywhere near the number of cardiovascular deaths described.52 It would have been informative to see the number of non-breast cancer-related deaths presented by treated side, but the laterality of EBRT was not provided in the most recent publication. If excess non-breast cancer-related deaths are as a result of EBRT, one would expect excess cardiac deaths to be confined to those patients receiving left-sided breast cancer. Neither can the increase in non-cardiovascular deaths be explained by the use of EBRT. It is more likely that the difference in non-breast cancer deaths between groups is owing to the under reporting of deaths in the IORT arm or to imbalances in risk factors.
Local control rates in the post-operative group were lower for IORT than for whole-breast EBRT but were not statistically significant (5.4 vs 1.7%; p = 0·069). Nonetheless, the apparently poorer local control in the post-operative group is concerning. If this difference is real, it may be owing to tumour repopulation or to less accurate geographical localization than in those patients treated intraoperatively. As demonstrated by the Early Breast Cancer Trialists' Collaborative Group analyses, differences in local control and breast cancer survival may become apparent only after 10–15 years of follow-up.53 With a relatively short follow-up of 2.4 years at this time point, this study has, as yet, been unable to provide conclusive evidence of the non-inferiority of IORT over EBRT.
Intraoperative radiotherapy for adjuvant treatment of local recurrence in a previously irradiated breast
When local recurrence occurs after breast-conserving surgery (BCS) and whole-breast RT, mastectomy is the current standard of care. A single institution study reports outcomes of IORT and BCS following local recurrence. In total, 15 patients had IBTR following prior EBRT.37 They were treated with further breast-conserving surgery and IORT using the INTRABEAM device. With a median follow-up of 26 months, no further local recurrences have been reported in this small follow-up. This represents a novel utility for IORT, but the use of IORT in this setting should remain within the setting of clinical trial.
Intraoperative radiotherapy for patients at higher risk of late radiation-induced side effects
A number of comorbidities including connective tissue disease are associated with an increased risk of late RT side effects. Given that with IORT, the skin dose is lower and the volume of irradiated breast tissue is reduced, IORT has been used to treat patients who would have difficulty in receiving EBRT or in whom EBRT is contraindicated.38 Keshtgar et al38 report a 3-centre study of 80 patients who were treated with TARGIT because of either prior EBRT (n = 21), contraindications to EBRT (n = 31) or compelling personal reasons (n = 28). Contraindications to EBRT listed were systemic lupus erythematosus, motor neuron disease, Parkinson's disease, ankylosing spondylitis, morbid obesity and cardiovascular or severe respiratory disease. Two local recurrences (2.5%) have been observed after a median follow-up of 38 months.
Intraoperative radiotherapy in the post-mastectomy setting
For patients with large or multifocal tumours, mastectomy still remains the standard of care. To reduce the effect of mutilation from mastectomy, nipple-sparing mastectomy has been shown to lead to acceptable local control rates when the nipple–areolar complex is retained and transplanted within the reconstructed breast.54 But, concern about recurrence in the nipple–areolar complex remains.39 Post-mastectomy RT is routinely offered to patients with a high risk of recurrence, such as patients with lymph node-positive disease, larger tumours (>5 cm) or those with involvement of the skin or chest wall.55 The use of IORT to “sterilize” the nipple–areolar complex in patients who have had a nipple-sparing mastectomy but who do not fulfil standard criteria for RT has been reported.39,40 In a study by Petit et al,40 800 patients received IORT to the nipple–areolar complex using the ELIOT device. With a reported median follow-up of 20 months, there were 13 (1.6%) local recurrences, but none of these was in the preserved nipple–areolar complex. However, this technique did lead to increased toxicity, and of the 800 patients who received IORT, 3.5% had complete necrosis of the nipple–areolar complex and 5.6% had partial necrosis. Overall, 4.8% of patients needed removal of the nipple–areolar complex after IORT in this setting.
IMPACT OF INTRAOPERATIVE RADIOTHERAPY ON RADIOLOGICAL FOLLOW-UP
Interpretation of mammographic findings after EBRT is often complicated by fat necrosis producing microcalcifications, which may prompt investigations such as ultrasound and/or biopsy.55 But what are the mammographic changes following IORT? In a subgroup analysis study of patients treated in the TARGIT-A study, mammographic findings in 27 patients treated using IORT were compared with those of 21 patients treated using EBRT.41 It was reported that fat necrosis was more common in those treated with IORT (56 vs 24%; p = 0.03) as well as being larger (8.7 vs 1.6 cm2). The same study noted that calcifications were also more frequent after IORT (63 vs 19%; p = 0.003). In a different subset analysis of a subset from TARGIT-A, which examined mammographic findings on 141 patients treated within the TARGIT-A study, no difference in the presence of calcifications between the IORT group (n = 80) and the EBRT group (n = 61) was noted.42 This study did note that those treated with EBRT did have an increase in breast density scores as assessed by the breast imaging-reporting and data system (25 vs 6 patients; p = 0.002) and an increase in generalized skin thickening as documented by the reporting radiologist (25 vs 10 patients; p = 0.03).
In a study of 38 patients reporting ultrasound features of the breast following IORT, all had some degree of seroma at 6 and 12 months following IORT.43 Similarly, a study of MR mammography (MRM) after IORT in 36 patients with a median follow up of 2.8 years, reported wound cavities containing fat necrosis in 81% of patients.44 Hence, the evidence available at this point appears to suggest that IORT has a higher rate of seroma and wound cavity formation with fat necrosis than does EBRT. By comparison, the rates of tumour-bed rim enhancement in keeping with fat necrosis following EBRT is 37% of patients at 1 year following RT.57 The significance of this is uncertain, but at present it does not appear to affect the diagnostic value of mammography, ultrasound or MRM.
IS INTRAOPERATIVE RADIOTHERAPY APPROPRIATE IN ROUTINE PRACTICE?
Use of IORT in the management of patients with breast cancer in the UK has to date been mainly in the context of clinical trials. However, the use of the INTRABEAM device in the adjuvant treatment of females with early breast cancer outside clinical trials has been the subject of a multiple technology appraisal by the National Institute for Health and Care Excellence (NICE). The draft guidance relating to this appraisal provoked a strong reaction from the multidisciplinary team across the UK (and beyond), and indeed, the latest document issued by NICE requests longer term follow-up data from the TARGIT-A trialists before final guidance can be issued. In particular, NICE acknowledged the response from professional organizations, such as the Royal College of Radiologists and the UK Radiotherapy Board that median overall follow-up was not long enough and that at least 5 years of follow up for the entire cohort would be needed before any results would be considered by the oncology community.58 This most recent NICE document also requested more data regarding the 95% confidence intervals around the absolute difference in the Kaplan–Meier estimates of 5-year risk of local recurrence comparing the two treatment groups. This had not been included in the published results of the TARGIT-A study.35 Given the low rate of local recurrence in the EBRT arm of 1.3%, NICE also raised concerns about the non-inferiority criterion of the TARGIT-A trial design, which were based on a 5-year absolute difference in local recurrence between treatment groups of 2.5%. This, in turn, was based on an estimated rate of 5-year local recurrence of 6% in the EBRT group.35 In conclusion, given the lack of equivalence to EBRT, IORT cannot currently be recommended as a standard treatment modality in the adjuvant treatment of breast cancer.
Patients who have long distances to travel for their RT treatment or who have contraindications or relative contraindications to whole-breast EBRT could be considered for IORT as a better alternative to no RT at all. It should be borne in mind that the above issues can, in many cases, be overcome by either hypofractionating whole-breast RT (as per the FAST-Forward trial) or by reducing the irradiated volume (as per the IMPORT-Low trial).59,60
Centres can of course use IORT as an alternative to the EBRT boost and, depending upon local fractionation schedules, this will save patients some hospital visits. However, there are some concerns around the toxicity of the IORT boost in combination with hypofractionated RT, which should be discussed with the patient prior to surgery. Meanwhile, the IMPORT High trial is comparing a sequential vs a simultaneous integrated boost such that, in future, it may be that the boost can be incorporated within the whole-breast plan reducing the number of visits for patients undergoing boost treatment. The use of IORT in reirradiation is of great interest and worthy of further investigation in a trial context. Again, however, partial breast EBRT is an alternative technological approach that would be more immediately widely available.
POTENTIAL FUTURE RESEARCH DIRECTIONS
Given the good outcomes with EBRT following breast-conserving surgery, a number of studies have compared RT with no RT in patients with early breast cancer with a low risk of local recurrence.61–63 Thus far, a population of females at low enough risk of local recurrence to safely avoid RT has not yet been identified, but local recurrence rates continue to fall, and it is likely that a subset of older females with low-stage, low-grade, oestrogen-receptor-positive disease will be able to safely avoid adjuvant RT. Future trials of IORT might therefore include a no RT arm, although the low event rate will necessitate inclusion of many thousands of patients.
Beyond conventional tumour staging factors, predictive assays, which utilize technologies such as gene expression profiling, have been shown to provide additional prognostic information and predictive information on the benefit of adjuvant chemotherapy for individual patients and are now in routine clinical use.64,65 Such predictive assays have not yet had the same impact on decision-making with regard to adjuvant RT, but there is evidence to suggest that these be helpful in this regard.66 If IORT is shown to have inferior local control rates compared with EBRT for the adjuvant treatment of breast cancer, then perhaps IORT may have a role to play in treatment de-escalation. In an era of ever-increasing personalization of oncology treatment, the use of a combination of patient factors, tumour stage and tumour biology may better stratify those patients who require whole-breast EBRT, who may be adequately served by IORT and in whom no adjuvant RT is required.
CONCLUSIONS
The use of IORT in breast cancer has a number of potential applications but most lack long-term randomized comparisons to whole-breast EBRT. The two randomized studies of IORT vs EBRT at present do not show non-inferiority of IORT, and more mature follow-up of the TARGIT-A trial is awaited before NICE can issue guidance regarding the use of IORT as a standard treatment option for females with early breast cancer.
Acknowledgments
ACKNOWLEDGMENTS
We thank Dr David Eaton for providing the image in Figure 1 for reproduction.
Contributor Information
G G Hanna, Email: Gerry.Hanna@gmail.com, g.hanna@qub.ac.uk.
A M Kirby, Email: anna.kirby@rmh.nhs.uk.
REFERENCES
- 1.Clarke M, Collins R, Darby S, Davies C, Elphinstone P, Evans E, et al. ; Early Breast Cancer Trialists' Collaborative Group (EBCTCG). Effects of radiotherapy and of differences in the extent of surgery for early breast cancer on local recurrence and 15-year survival: an overview of the randomised trials. Lancet 2005; 366: 2087–106. [DOI] [PubMed] [Google Scholar]
- 2.Offersen BV, Overgaard M, Kroman N, Overgaard J. Accelerated partial breast irradiation as part of breast conserving therapy of early breast carcinoma: a systematic review. Radiother Oncol 2009; 90: 1–13. doi: 10.1016/j.radonc.2008.08.005 [DOI] [PubMed] [Google Scholar]
- 3.Holland R, Veling SH, Mravunac M, Hendriks JH. Histologic multifocality of Tis, T1-2 breast carcinomas. Implications for clinical trials of breast-conserving surgery. Cancer 1985; 56: 979–90. [DOI] [PubMed] [Google Scholar]
- 4.Smith BD, Arthur DW, Buchholz TA, Haffty BG, Hahn CA, Hardenbergh PH, et al. Accelerated partial breast irradiation consensus statement from the American Society for Radiation Oncology (ASTRO). Int J Radiat Oncol Biol Phys 2009; 74: 987–1001. doi: 10.1016/j.ijrobp.2009.02.031 [DOI] [PubMed] [Google Scholar]
- 5.Veronesi U, Gatti G, Luini A, Intra M, Orecchia R, Borgen P, et al. Intraoperative radiation therapy for breast cancer: technical notes. Breast J 2003; 9: 106–12. [DOI] [PubMed] [Google Scholar]
- 6.Vaidya JS, Joseph DJ, Tobias JS, Bulsara M, Wenz F, Saunders C, et al. Targeted intraoperative radiotherapy versus whole breast radiotherapy for breast cancer (TARGIT-A trial): an international, prospective, randomised, non-inferiority phase 3 trial. Lancet 2010; 376: 91–102. doi: 10.1016/S0140-6736(10)60837-9 [DOI] [PubMed] [Google Scholar]
- 7.Scala LM, Hu K, Urken ML, Jacobson AS, Persky MS, Tran TN, et al. Intraoperative high-dose-rate radiotherapy in the management of locoregionally recurrent head and neck cancer. Head Neck 2013; 35: 485–92. doi: 10.1002/hed.23007 [DOI] [PubMed] [Google Scholar]
- 8.Krempien R, Roeder F, Oertel S, Weitz J, Hensley FW, Timke C, et al. Intraoperative electron-beam therapy for primary and recurrent retroperitoneal soft-tissue sarcoma. Int J Radiat Oncol Biol Phys 2006; 65: 773–9. [DOI] [PubMed] [Google Scholar]
- 9.Guo S, Reddy CA, Kolar M, Woody N, Mahadevan A, Deibel FC, et al. Intraoperative radiation therapy with the photon radiosurgery system in locally advanced and recurrent rectal cancer: retrospective review of the Cleveland clinic experience. Radiat Oncol 2012; 7: 110. doi: 10.1186/1748-717X-7-110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hallemeier CL, Choo R, Davis BJ, Pisansky TM, Gunderson LL, Leibovich BC, et al. Long-term outcomes after maximal surgical resection and intraoperative electron radiotherapy for locoregionally recurrent or locoregionally advanced primary renal cell carcinoma. Int J Radiat Oncol Biol Phys 2012; 82: 1938–43. doi: 10.1016/j.ijrobp.2011.02.026 [DOI] [PubMed] [Google Scholar]
- 11.Jakse G, Kapp KS, Geyer E, Oechs A, Maier A, Gabor S, et al. IORT and external beam irradiation (EBI) in clinical stage I-II NSCLC patients with severely compromised pulmonary function: an 52-patient single-institutional experience. Strahlenther Onkol 2007; 183 Spec No 2: 24–5. doi: 10.1007/s00066-007-2010-9 [DOI] [PubMed] [Google Scholar]
- 12.del Carmen MG, McIntyre JF, Goodman A. The role of intraoperative radiation therapy (IORT) in the treatment of locally advanced gynecologic malignancies. Oncologist 2000; 5: 18–25. [DOI] [PubMed] [Google Scholar]
- 13.Vaidya JS, Baum M, Tobias JS, Wenz F, Massarut S, Keshtgar M, et al. Long-term results of targeted intraoperative radiotherapy (Targit) boost during breast-conserving surgery. Int J Radiat Oncol Biol Phys 2011; 81: 1091–7. doi: 10.1016/j.ijrobp.2010.07.1996 [DOI] [PubMed] [Google Scholar]
- 14.Veronesi U, Orecchia R, Maisonneuve P, Viale G, Rotmensz N, Sangalli C, et al. Intraoperative radiotherapy versus external radiotherapy for early breast cancer (ELIOT): a randomised controlled equivalence trial. Lancet Oncol 2013; 14: 1269–77. doi: 10.1016/S1470-2045(13)70497-2 [DOI] [PubMed] [Google Scholar]
- 15.Hanna SA, de Barros AC, de Andrade FE, Bevilacqua JL, Piato JR, Pelosi EL, et al. Intraoperative radiation therapy in early breast cancer using a linear accelerator outside of the operative suite: an “image-guided” approach. Int J Radiat Oncol Biol Phys 2014; 89: 1015–23. doi: 10.1016/j.ijrobp.2014.04.038 [DOI] [PubMed] [Google Scholar]
- 16.Veronesi U, Gatti G, Luini A, Intra M, Ciocca M, Sanchez D, et al. Full-dose intraoperative radiotherapy with electrons during breast-conserving surgery. Arch Surg 2003; 138: 1253–6. [DOI] [PubMed] [Google Scholar]
- 17.Vaidya JS, Baum M, Tobias JS, D'Souza DP, Naidu SV, Morgan S, et al. Targeted intra-operative radiotherapy (Targit): an innovative method of treatment for early breast cancer. Ann Oncol 2001; 12: 1075–80. [DOI] [PubMed] [Google Scholar]
- 18.Ebert MA, Carruthers B. Dosimetric characteristics of a low-kV intra-operative x-ray source: implications for use in a clinical trial for treatment of low-risk breast cancer. Med Phys 2003; 30: 2424–31. [DOI] [PubMed] [Google Scholar]
- 19.Eaton DJ, Gonzalez R, Duck S, Keshtgar M. Radiation protection for an intra-operative X-ray device. Br J Radiol 2011; 84: 1034–9. doi: 10.1259/bjr/29466902 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Herskind C, Steil V, Kraus-Tiefenbacher U, Wenz F. Radiobiological aspects of intraoperative radiotherapy (IORT) with isotropic low-energy X rays for early-stage breast cancer. Radiat Res 2005; 163: 208–15. [DOI] [PubMed] [Google Scholar]
- 21.Tobias JS, Vaidya JS, Keshtgar M, D'Souza DP, Baum M. Reducing radiotherapy dose in early breast cancer: the concept of conformal intraoperative brachytherapy. Br J Radiol 2004; 77: 279–84. [DOI] [PubMed] [Google Scholar]
- 22.Orecchia R, Ciocca M, Lazzari R, Garibaldi C, Leonardi MC, Luini A, et al. Intraoperative radiation therapy with electrons (ELIOT) in early-stage breast cancer. Breast 2003; 12: 483–90. [DOI] [PubMed] [Google Scholar]
- 23.Vaidya JS, Tobias JS, Baum M, Keshtgar M, Joseph D, Wenz F, et al. Intraoperative radiotherapy for breast cancer. Lancet Oncol. 2004; 5: 165–73. [DOI] [PubMed] [Google Scholar]
- 24.Kimple RJ, Klauber-DeMore N, Kuzmiak CM, Pavic D, Lian J, Livasy CA, et al. Cosmetic outcomes for accelerated partial breast irradiation before surgical excision of early-stage breast cancer using single-dose intraoperative radiotherapy. Int J Radiat Oncol Biol Phys 2011; 79: 400–7. doi: 10.1016/j.ijrobp.2009.10.032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kimple RJ, Klauber-DeMore N, Kuzmiak CM, Pavic D, Lian J, Livasy CA, et al. Local control following single-dose intraoperative radiotherapy prior to surgical excision of early-stage breast cancer. Ann Surg Oncol 2011; 18: 939–45. doi: 10.1245/s10434-010-1392-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Vanderwalde NA, Jones EL, Kimple RJ, Moore DT, Klauber-Demore N, Sartor CI, et al. Phase 2 study of pre-excision single-dose intraoperative radiation therapy for early-stage breast cancers: six-year update with application of the ASTRO accelerated partial breast irradiation consensus statement criteria. Cancer 2013; 119: 1736–43. doi: 10.1002/cncr.27915 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Vaidya JS, Baum M, Tobias JS, Massarut S, Wenz F, Murphy O, et al. Targeted intraoperative radiotherapy (TARGIT) yields very low recurrence rates when given as a boost. Int J Radiat Oncol Biol Phys 2006; 66: 1335–8. [DOI] [PubMed] [Google Scholar]
- 28.Paganelli G, De Cicco C, Ferrari ME, McVie G, Pagani G, Leonardi MC, et al. IART (Intra-Operative Avidination for Radionuclide Therapy) for accelerated radiotherapy in breast cancer patients. Technical aspects and preliminary results of a phase II study with 90Y-labelled biotin. Ecancermedicalscience 2010; 4: 166. doi: 10.3332/ecancer.2010.166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fastner G, Sedlmayer F, Merz F, Deutschmann H, Reitsamer R, Menzel C, et al. IORT with electrons as boost strategy during breast conserving therapy in limited stage breast cancer: long term results of an ISIORT pooled analysis. Radiother Oncol 2013; 108: 279–86. doi: 10.1016/j.radonc.2013.05.031 [DOI] [PubMed] [Google Scholar]
- 30.Joseph DJ, Bydder S, Jackson LR, Corica T, Hastrich DJ, Oliver DJ, et al. Prospective trial of intraoperative radiation treatment for breast cancer. ANZ J Surg 2004; 74: 1043–8. [DOI] [PubMed] [Google Scholar]
- 31.Reitsamer R, Peintinger F, Sedlmayer F, Kopp M, Menzel C, Cimpoca W, et al. Intraoperative radiotherapy given as a boost after breast-conserving surgery in breast cancer patients. Eur J Cancer 2002; 38: 1607–10. [DOI] [PubMed] [Google Scholar]
- 32.Reitsamer R, Peintinger F, Kopp M, Menzel C, Kogelnik HD, Sedlmayer F. Local recurrence rates in breast cancer patients treated with intraoperative electron-boost radiotherapy versus postoperative external-beam electron-boost irradiation. A sequential intervention study. Strahlenther Onkol 2004; 180: 38–44. [DOI] [PubMed] [Google Scholar]
- 33.Wong WW, Pockaj BA, Vora SA, Halyard MY, Gray RJ, Schild SE. Six-year outcome of a prospective study evaluating tumor bed boost with intra-operative electron irradiation followed by whole-breast irradiation for early-stage breast cancer. Breast J 2014; 20: 125–30. doi: 10.1111/tbj.12235 [DOI] [PubMed] [Google Scholar]
- 34.Sperk E, Welzel G, Keller A, Kraus-Tiefenbacher U, Gerhardt A, Sütterlin M, et al. Late radiation toxicity after intraoperative radiotherapy (IORT) for breast cancer: results from the randomized phase III trial TARGIT A. Breast Cancer Res Treat 2012; 135: 253–60. doi: 10.1007/s10549-012-2168-4 [DOI] [PubMed] [Google Scholar]
- 35.Vaidya JS, Wenz F, Bulsara M, Tobias JS, Joseph DJ, Keshtgar M, et al. ; TARGIT Trialists' Group. Risk-adapted targeted intraoperative radiotherapy versus whole-breast radiotherapy for breast cancer: 5-year results for local control and overall survival from the TARGIT-A randomised trial. Lancet 2014; 383: 603–13. doi: 10.1016/S0140-6736(13)61950-9 [DOI] [PubMed] [Google Scholar]
- 36.Yarnold J, Offersen BV, Olivotto I, Poortmans P, Sarin R. Radiotherapy for breast cancer, the TARGIT-A trial. Lancet 2014; 383: 1717–18. doi: 10.1016/S0140-6736(14)60828-X [DOI] [PubMed] [Google Scholar]
- 37.Kraus-Tiefenbacher U, Bauer L, Scheda A, Schoeber C, Schaefer J, Steil V, et al. Intraoperative radiotherapy (IORT) is an option for patients with localized breast recurrences after previous external-beam radiotherapy. BMC Cancer 2007; 7: 178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Keshtgar MR, Vaidya JS, Tobias JS, Wenz F, Joseph D, Stacey C, et al. Targeted intraoperative radiotherapy for breast cancer in patients in whom external beam radiation is not possible. Int J Radiat Oncol Biol Phys 2011; 80: 31–8. doi: 10.1016/j.ijrobp.2010.01.045 [DOI] [PubMed] [Google Scholar]
- 39.Petit JY, Veronesi U, Orecchia R, Luini A, Rey P, Intra M, et al. Nipple-sparing mastectomy in association with intra operative radiotherapy (ELIOT): a new type of mastectomy for breast cancer treatment. Breast Cancer Res Treat 2006; 96: 47–51. [DOI] [PubMed] [Google Scholar]
- 40.Petit JY, Veronesi U, Orecchia R, Rey P, Martella S, Didier F, et al. Nipple sparing mastectomy with nipple areola intraoperative radiotherapy: one thousand and one cases of a five years experience at the European institute of oncology of Milan (EIO). Breast Cancer Res Treat 2009; 117: 333–8. doi: 10.1007/s10549-008-0304-y [DOI] [PubMed] [Google Scholar]
- 41.Engel D, Schnitzer A, Brade J, Blank E, Wenz F, Suetterlin M, et al. Are mammographic changes in the tumor bed more pronounced after intraoperative radiotherapy for breast cancer? Subgroup analysis from a randomized trial (TARGIT-A). Breast J 2013; 19: 92–5. doi: 10.1111/tbj.12049 [DOI] [PubMed] [Google Scholar]
- 42.Elsberger B, Romsauerova A, Vinnicombe S, Whelehan P, Brown DC, Dewar JA, et al. Comparison of mammographic findings after intraoperative radiotherapy or external beam whole breast radiotherapy. Eur J Surg Oncol 2014; 40: 163–7. doi: 10.1016/j.ejso.2013.11.011 [DOI] [PubMed] [Google Scholar]
- 43.Goble RN, Drukteinis JS, Lee MC, Khakpour N, Kiluk JV, Laronga C. Early experience with ultrasound features after intrabeam intraoperative radiation for early stage breast cancer. J Surg Oncol 2014; 109: 751–5. doi: 10.1002/jso.23581 [DOI] [PubMed] [Google Scholar]
- 44.Wasser K, Schnitzer A, Engel D, Krammer J, Wenz F, Kraus-Tiefenbacher U, et al. First description of MR mammographic findings in the tumor bed after intraoperative radiotherapy (IORT) of breast cancer. Clin Imaging 2012; 36: 176–84. doi: 10.1016/j.clinimag.2011.08.024 [DOI] [PubMed] [Google Scholar]
- 45.Brown JM, Carlson DJ, Brenner DJ. The tumor radiobiology of SRS and SBRT: are more than the 5 Rs involved? Int J Radiat Oncol Biol Phys 2014; 88: 254–62. doi: 10.1016/j.ijrobp.2013.07.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Brenner DJ. The linear-quadratic model is an appropriate methodology for determining isoeffective doses at large doses per fraction. Semin Radiat Oncol 2008; 18: 234–9. doi: 10.1016/j.semradonc.2008.04.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Clinical Trials. gov. National Library of Medicine at the National Institutes of Health. Radiation therapy followed by surgery in treating patients with early-stage breast cancer. [Updated 9 August 2013; cited 16 October 2014.] Available from: clinicaltrials.gov/show/NCT01024582
- 48.Bartelink H, Horiot JC, Poortmans PM, Struikmans H, Van den Bogaert W, Fourquet A, et al. Impact of a higher radiation dose on local control and survival in breast-conserving therapy of early breast cancer: 10-year results of the randomized boost versus no boost EORTC 22881-10882 trial. J Clin Oncol 2007; 25: 3259–65. [DOI] [PubMed] [Google Scholar]
- 49.Benda RK, Yasuda G, Sethi A, Gabram SG, Hinerman RW, Mendenhall NP. Breast boost: are we missing the target? Cancer 2003; 97: 905–9. [DOI] [PubMed] [Google Scholar]
- 50.Kirby AN, Jena R, Harris EJ, Evans PM, Crowley C, Gregory DL, et al. Tumour bed delineation for partial breast/breast boost radiotherapy: what is the optimal number of implanted markers? Radiother Oncol 2013; 106: 231–5. doi: 10.1016/j.radonc.2013.02.003 [DOI] [PubMed] [Google Scholar]
- 51.Clinical Trials. gov. National Library of Medicine at the National Institutes of Health. Intra-operative electron boost and hypofractionated whole-breast irradiation during breast-conserving treatment (BCT) (HIOB). [Updated 9 August 2013; cited 16 October 2014.] Available from: http://clinicaltrials.gov/show/NCT01343459Lastaccessed12/10/2014
- 52.Darby SC, Ewertz M, McGale P, Bennet AM, Blom-Goldman U, Brønnum D, et al. Risk of ischemic heart disease in women after radiotherapy for breast cancer. N Engl J Med 2013; 368: 987–98. doi: 10.1056/NEJMoa1209825 [DOI] [PubMed] [Google Scholar]
- 53.Early Breast Cancer Trialists' Collaborative Group (EBCTCG); Darby S, McGale P, Correa C, Taylor C, Arriagada R, Clarke M, et al. Effect of radiotherapy after breast-conserving surgery on 10-year recurrence and 15-year breast cancer death: meta-analysis of individual patient data for 10,801 women in 17 randomised trials. Lancet 2011; 378: 1707–16. doi: 10.1016/S0140-6736(11)61629-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Gerber B, Krause A, Reimer T, Müller H, Küchenmeister I, Makovitzky J, et al. Skin-sparing mastectomy with conservation of the nipple-areola complex and autologous reconstruction is an oncologically safe procedure. Ann Surg 2003; 238: 120–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Eifel P, Axelson JA, Costa J, Crowley J, Curran WJ, Jr, Deshler A, et al. National Institutes of Health Consensus Development Conference Statement: adjuvant therapy for breast cancer, November 1-3, 2000. J Natl Cancer Inst 2001; 93: 979–89. [DOI] [PubMed] [Google Scholar]
- 56.Trombetta M, Valakh V, Julian TB, Werts ED, Parda D. Mammary fat necrosis following radiotherapy in the conservative management of localized breast cancer: does it matter? Radiother Oncol 2010; 97: 92–4. doi: 10.1016/j.radonc.2010.02.021 [DOI] [PubMed] [Google Scholar]
- 57.Li J, Dershaw DD, Lee CH, Joo S, Morris EA. Breast MRI after conservation therapy: usual findings in routine follow-up examinations. AJR Am J Roentgenol 2010; 195: 799–807. doi: 10.2214/AJR.10.4305 [DOI] [PubMed] [Google Scholar]
- 58.National Institute for Health and Care Excellence. Breast cancer (early)–intrabeam radiotherapy system [ID618]. [Updated 1 October 2014; cited 16 October 2014.] Available from: https://www.nice.org.uk/guidance/indevelopment/gid-tag353/documents
- 59.Coles CE, Brunt AM, Wheatley D, Mukesh MB, Yarnold JR. Breast radiotherapy: less is more? Clin Oncol (R Coll Radiol) 2013; 25: 127–34. doi: 10.1016/j.clon.2012.10.013 [DOI] [PubMed] [Google Scholar]
- 60.Coles C, Yarnold J; IMPORT Trials Management Group. The IMPORT trials are launched (September 2006). Clin Oncol (R Coll Radiol) 2006; 18: 587–90. [DOI] [PubMed] [Google Scholar]
- 61.Fyles AW, McCready DR, Manchul LA, Trudeau ME, Merante P, Pintilie M, et al. Tamoxifen with or without breast irradiation in women 50 years of age or older with early breast cancer. N Engl J Med 2004; 351: 963–70. [DOI] [PubMed] [Google Scholar]
- 62.Hughes KS, Schnaper LA, Berry D, Cirrincione C, McCormick B, Shank B, et al. ; Cancer and Leukemia Group B; Radiation Therapy Oncology Group; Eastern Cooperative Oncology Group. Lumpectomy plus tamoxifen with or without irradiation in women 70 years of age or older with early breast cancer. N Engl J Med 2004; 351: 971–7. [DOI] [PubMed] [Google Scholar]
- 63.Kunkler IH, Williams LJ, Jack WJ, Cameron DA, Dixon JM; PRIME II investigators. Breast-conserving surgery with or without irradiation in women aged 65 years or older with early breast cancer (PRIME II): a randomised controlled trial. Lancet Oncol 2015; 16: 266–73. [DOI] [PubMed] [Google Scholar]
- 64.Mulligan JM, Hill LA, Deharo S, Irwin G, Boyle D, Keating KE, et al. Identification and validation of an anthracycline/cyclophosphamide-based chemotherapy response assay in breast cancer. J Natl Cancer Inst 2014; 106: djt335. doi: 10.1093/jnci/djt335 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.McVeigh TP, Hughes LM, Miller N, Sheehan M, Keane M, Sweeney KJ, et al. The impact of Oncotype DX testing on breast cancer management and chemotherapy prescribing patterns in a tertiary referral centre. Eur J Cancer 2014; 50: 2763–70. doi: 10.1016/j.ejca.2014.08.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Mamounas EP, Anderson SJ, Dignam JJ, Bear HD, Julian TB, Geyer CE, Jr, et al. Predictors of locoregional recurrence after neoadjuvant chemotherapy: results from combined analysis of National Surgical Adjuvant Breast and Bowel Project B-18 and B-27. J Clin Oncol 2012; 30: 3960–6. doi: 10.1200/JCO.2011.40.8369 [DOI] [PMC free article] [PubMed] [Google Scholar]