Abstract
Objective:
This study investigated the effectiveness of stereotactic body radiotherapy with helical TomoTherapy (T-SBRT) for treating medically inoperable primary and second-primary early stage non-small-cell lung neoplasm (SPLN) and evaluated whether the movement of organizing pneumonia (OP) within the irradiation field (IF) can be detected via analysis of radiological changes.
Methods:
Patients (n = 16) treated for 1 year (2011–12) at our hospital by T-SBRT at a total dose of 60 Gy in five fractions were examined retrospectively. Outcome and toxicity were recorded and were separately described for SPLN. CT scans were reviewed by a single radiologist.
Results:
Of the 16 patients, 5 (31.3%) had primary lung malignancies, 10 (62.5%) had SPLN, and 1 case (6.3%) had isolated mediastinal metastasis of lung neoplasm. Pathological evidence was obtained for 72.2% of all lesions. The median radiological follow-up was 11 months (10.5 months for SPLN). For all cases, the 6- and 12-month survival rates were 100% and 77.7% (100% and 71.4%, respectively, for SPLN), and the 6- and 12-month locoregional control rates were 100% in all cases. 2 (12.5%) of 16 patients developed grade 3 late transient radiation pneumonitis following steroid therapy and 1 (6.3%) presented asymptomatic infiltrates comparable to OP opacities.
Conclusion:
T-SBRT seems to be safe and effective.
Advances in knowledge:
Mild OP is likely associated with radiation-induced anomalies in the IF, identification of migrating opacities can help discern relapse of radiation-induced opacities.
Treating early stage primary non-small-cell lung neoplasm (NSCLN) still requires surgery. Given that comorbidities associated with tobacco consumption frequently limit patients' eligibility for surgery,1 stereotactic body radiotherapy (SBRT) has become an alternative treatment in primary—and more recently also in second-primary—early stage (Ia–Ib) medically inoperable lung neoplasm (SPLN). Several prospective studies using SBRT to treat patients with primary Stage I NSCLN unfit for surgical resection found good local control rates (LCRs) at 1 year (92%2) and 2 years (70%3), with overall survival (OS) of 84%2 and 65%,3 respectively. On the other hand, reports on the use of SBRT in operable cases are limited. A prospective database of patients with operable Stage I disease treated with SBRT showed a 30-day mortality rate of 0%;4 the 30-day lobectomy mortality in these patients according to the thoracoscore predictive model was 2.6%.5 However, randomized controlled trials comparing surgery and SBRT in patients at operable stage have not yet been completed and propensity-matched analyses have not found significant differences in either the OS6 or early morbidity.7 Only one study has reported oncological outcomes of patients affected with primary early stage NSCLN irradiated with SBRT delivered by helical TomoTherapy (T-SBRT).3
SPLNs are arbitrarily classified as synchronous or metachronous.8 Only a few retrospective studies have reported outcomes of SPLN following SBRT,9,10 but none has employed helical TomoTherapy.
Concerning the radiological evolution of lung toxicity from a helical radiation delivery, only limited data are published. Radiation pneumonitis (RP) is a well-known complication of NSCLN treated with SBRT that develops in the irradiation field (IF). Organizing pneumonia (OP) was only recently identified as a symptomatic and transient complication developing outside the IF.11 Classical OP developing after breast irradiation has been proven to originate in and successively move outside the IF; we therefore hypothesize that mild, asymptomatic OP may be present in the IF, and may be identified by its dynamic advancement.
METHODS AND MATERIALS
We retrospectively analysed the 1-year (04/2011–11/2012) experience of patients with medically inoperable early stage primary and second-primary NSCLN treated by T-SBRT at our hospital (Centre Hospitalier Universitaire Vaudois, Lausanne, Switzerland). The TNM classification was made according to the criteria established by the International Association for the Study of Lung Cancer. Patients presenting a distant disease were excluded except in the case of a second ipsilateral nodule in a different lobe. We defined early stage tumour lesions as classified as between Ia and IIIa. Patients were staged using contrast-enhanced thoracic CT and positron emission tomography (PET)-CT scans. The NSCLN diagnosis was based on pathology according to the World Health Organisation classification. Peripheral pulmonary lesions were identified by bronchoscopy with radial probe endobronchial ultrasound (B-EBUS). If the histocytological analysis was inconclusive or medically contraindicated, irradiation treatment was not initiated unless all experts on the tumour board agreed that the pre-test probability of cancer was high enough to decline an invasive procedure. Inoperable patients were determined using both the American College of Chest Physicians guidelines and the Eastern Cooperative Oncology Group performance status. All other concomitant extrapulmonary malignancies were recorded. The severity of post-SBRT toxicity was scored according to common terminology criteria for adverse events. For asymptomatic radiological changes, an infectious disease was excluded by examining the patient's history. Paraclinical examinations were performed for symptomatic patients.
SPLN was defined using established criteria and patients had to have been previously treated with curative intent for primary NSCLN.8 All malignancies that could not be differentiated from a unique lung metastasis were classified as SPLN.
Patients were treated according to the same dose prescription. The total dose was 60 Gy in five fractions administered twice a week over 10–27 days. Patients were treated with multiple chest CT simulation scans, and daily image guidance was performed by slow TomoTherapy megavoltage CT. The planning target volume (PTV) included a margin of 0.5 cm around the internal target volume (ITV). The ITV was defined based on the volumetric sum of the clinical target volumes of an inspiratory, an expiratory and a free breathing planning CT. All fractionation schemes used were prescribed to the encompassing 95% isodose. Treatment plans were optimized to limit high-dose radiation to regions adjacent to organs at risk according to the Radiation Therapy Oncology Group guidelines. The IF was defined as 1% isodose. Dose–volume T-SBRT metrics are reported with descriptive statistics.
Patients with radiological follow-up during a period less than 4 months were excluded. Patients were initially followed up between 1 and 4 months after the end of SBRT and then every 3–5 months. Patients underwent a clinical follow-up at 1, 3 and 6 months, and 1 year. The radiological follow-up included a review of both non-enhanced and enhanced CT scans by a single radiologist experienced in chest imaging and blinded to the clinical course. Consecutive CT scans during a minimum period of 6 months showing loco-regional findings that suggested progressive disease were confirmed by a PET-CT scan. All radiological changes were interpreted in three-dimensional mode after deformable fusion of the IF(s) on the diagnostic CT scan using Velocity software (Varian Medical Systems, Palo Alto, CA).
OP was defined as follows: peribronchial-vascular opacities dynamically migrating inside and/or outside the IF in symptomatic or asymptomatic patients with no evidence of other specific aetiologies. A distinction was made between dynamic migration and advancing: for the former, it was supposed that if the new area adjacent to the lung was affected by infiltrates, previous adjacent abnormal areas of the lung had healed; for the latter, new pulmonary areas were presumed to be affected by infiltrate and previous adjacent abnormal areas were abnormal until maximal extension and then regression occurred.
The Kaplan–Meier method was used to calculate cumulative outcomes [locoregional control rate (LRCR), distant metastasis-free and survival rates] at 6 and 12 months after the end of T-SBRT; results for SPLN were described separately.
RESULTS
A total of 17 early stage NSCLN cases were treated by T-SBRT and 16 were included (1 patient had not reached the minimum radiological follow-up). Patient characteristics are summarized in Table 1. 5 (31.3%) were treated for a primary malignancy, 10 (62.5%) for SPLN and 1 (6.3%) for an isolated mediastinal metastasis of the primary lung neoplasm. A total of 18 pulmonary lesions were detected; detailed characteristics are summarized in Table 2. Pathological confirmation could not be obtained for five of the lesions (three had inconclusive results in histocytological analyses, and B-EBUS was not performed for two of the lesions). Stage I was observed in 81.2% of the total cases. There were no Stage II patients, and three were staged as IIIa.
Table 1.
Patient characteristics
| Variables | Consecutive cases (16) | Second-primary lung neoplasm (10) |
|---|---|---|
| Gender | ||
| Male | 12 (75.0%) | 9 (90%) |
| Female | 4 (25.0%) | 1 (10%) |
| Mean age (years) | 70 | 70.5 |
| Previous surgery | 11 (68.8%) | 10 (100%) |
| Lobectomy | 9 (56.3%) | 8 (80%) |
| Pneumonectomy | 2 (12.5%) | 2 (20%) |
| Histology | 18 | 12 |
| Adenocarcinoma | 7 (38.9%) | 5 (41.7%) |
| Squamous cell | 6 (33.3%) | 2 (16.7%) |
| Not conclusive | 3 (16.7%) | 3 (25%) |
| Not performed | 2 (11.1%) | 2 (16.7%) |
| Stage | ||
| Ia | 10 (62.5%) | 7 (70%) |
| Ib | 3 (18.8%) | 1 (10%) |
| IIa,b | 0 | 0 |
| IIIa | 3 (18.8%) | 2 (20%) |
| Localization | 18 | 12 |
| Central | 2 (11.1%) | 1 (8.3%) |
| Peripheral | 16 (88.9%) | 11 (91.7%) |
| Multiple ipsilateral neoplasm | 2 (12.5%) | 2 (10%) |
| Malignancy presentation | ||
| Metachronous | 5 (31.3%) | 5 (50%) |
| Synchronous | 2 (12.5%) | 2 (20%) |
| Synchronous or metastasis of primary lung cancer | 3 (18.8%) | 3 (30%) |
| Primary | 5 (31.3%) | 0 |
| Metastasis of primary lung cancer | 1 (6.3%) | 0 |
| Oncological comorbidities | 4 (25%) | 2 (20%) |
| Previous radiation therapy and chemotherapy for primary lung neoplasm | 3 (18.8%) | 2 (20%) |
Table 2.
Lesion characteristics
| No. | T | N | M | Stage | Localization | Histology | Classification | Previous neoplasm histology | Previous neoplasm localization | Lagtime between the two neoplasms (years)a |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 1 | 0 | 0 | Ia | R2 | ADC | Metachronous | ADC | L | >2 |
| 2 | 1 | 0 | 0 | Ia | L2 | Not performed | Synchronous or metastasis of primary cancer | Useless | R | ≤2 |
| 3 | 1 | 0 | 0 | Ia | L2 | Not conclusive | Synchronous or metastasis of primary cancer | Useless | R | ≤2 |
| 4 | × | 0 | 1b | IIIa | R2 (medium) R2 (inferior) |
Not performed ADC | Metachronous with intrapulmonary metastasis | SQC | L | >2 |
| 5 | 2 | 0 | 0 | Ib | L2 | SQC | Primary | – | – | – |
| 6 | 1 | 0 | 0 | Ia | L2 | Not conclusive | Synchronous or metastasis of primary cancer | Useless | R | ≤2 |
| 7 | 1 | 0 | 0 | Ia | L2 | ADC | Synchronous | SQC | R | ≤2 |
| 8 | × | 2 | 0 | IIIa | Mediastinal | SQC | Metastasis of primary cancer | SQC | L | ≤2 |
| 9 | × | 0 | 1b | IIIa | R1 (medium) R2 (inferior) |
ADC ADC |
Metachronous with intrapulmonary metastasis | ADC | R | >2 |
| 10 | 1 | 0 | 0 | Ia | L2 | SQC | Metachronous | ADC | R | >2 |
| 11 | 1 | 0 | 0 | Ia | L2 | ADC | Primary | – | – | – |
| 12 | 1 | 0 | 0 | Ib | L2 | SQC | Synchronous | Neuro-endocrine | R | ≤2 |
| 13 | 1 | 0 | 0 | Ia | R1 | SQC | Primary | – | – | – |
| 14 | 1 | 0 | 0 | Ia | L2 | ADC | Primary | – | – | – |
| 15 | 2 | 0 | 0 | Ib | L2 | SQC | Primary | – | – | – |
| 16 | 1 | 0 | 0 | Ia | L2 | Not conclusive | Metachronous | Useless | R | >2 |
1, central; 2, peripheral; ADC, adenocarcinoma; L, left lung; No., consequent cases; R, right lung; SQC, squamous cell cancer.
Lagtime was calculated between the start of the two treatments (surgery for primary and stereotactic body radiotherapy delivered by helical TomoTherapy for the second-primary lung neoplasm).
M1 was limited to the presence of an ipsilateral second neoplasm.
At 6 and 12 months, survival rates were 100% and 77.7%, respectively; LRCRs were both 100%; and distant metastasis-free rates were 93.4% and 82.9%, respectively, for all cases. For SPLN, survival rates were 100% and 71.4%, respectively; LRCRs were both 100%; and distant metastasis-free rates were 100% and 80%, respectively, at 6 and 12 months. Four patients presented a recurrent disease. Two patients developed an isolated local failure and two a distant failure (pleural carcinomatosis and brain metastasis). The pleural carcinomatosis was classified as a distant failure because the volume of the irradiated mass decreased. Two patients—both treated for SPLN—died. No deaths occurred in the first 6 months after the end of T-SBRT. One patient died owing to a recurrent distant disease and one developed fatal urosepsis. Mean dosimetric values are summarized in Table 3.
Table 3.
Stereotactic body radiotherapy (SBRT) delivered by helical TomoTherapy characteristics
| Variables | Consecutive case (16) |
|---|---|
| Total dose (Gy) | 60 |
| Dose/fraction (Gy) | 12 |
| Number of fractions | 5 |
| Mean lung dose (Gy) | 5.3 (range, 0.3–20.0) |
| Biologically effective dose with α/β of 10 Gy (Gy) | 132 |
| Mean planning target volume (cm3) | 53.2 (range, 12.8–203.8) |
| Mean V5 controlateral (%) | 21.0 (range, 3.0–44.5) |
| Mean V15 ipsilateral (%) | 15.5 (range, 0.1–40.7) |
| Mean V30 ipsilateral (%) | 7.7 (range, 0.0–30.5) |
| Mean duration of stereotactic body radiotherapy (days) | 17.2 (range, 10–27) |
VX, proportion of lung receiving at least XGy.
Toxicities and radiological findings following T-SBRT are presented in Table 4. Two patients (Table 2, Cases 5 and 7) developed grade 3 late RP (5 months after the T-SBRT ended in both cases) and were treated with corticosteroids (0.5–1.0 mg kg−1 for 5–6 months) with subsequent resolution of radiological changes. No grade 2 RP was recorded. Five patients developed asymptomatic infiltrates (Figure 1) compatible with grade 1 RP. No patient presented infiltrates outside the IF. Only one asymptomatic patient (Table 2, Case 9) presented infiltrates (Figure 2) that dynamically migrated (while remaining in the IF). No major toxicity was reported.
Table 4.
Toxicities and radiological findings
| Variables | All consecutive cases (16) | SPLN (10) |
|---|---|---|
| Patients with acute dyspnoea interfering with DLA | 4 (25%) | 3 (30%) |
| Patients with acute dyspnoea interfering with DLA resulting from RP | 2 (12.5%) | 1 (10%) |
| Acute dyspnoea aetiology | 6 | 4 |
| Infectious | 3 | 3 |
| RP (grade 3) | 2 | 1 |
| Others | 1 | 0 |
| Radiological findings | ||
| RP (grade 1) | 5 (31%) | 3 (30%) |
| Organizing pneumonia | 1 (6.3%) | 1 (10%) |
| Radiological follow-up | ||
| Median (months) | 11.0 | 10.5 |
| Mean (months) | 10.8 | 11.6 |
| Range (months) | 4–19 | 6–19 |
DLA, daily living activities; RP, radiation pneumonitis; SPLN, second-primary lung neoplasm.
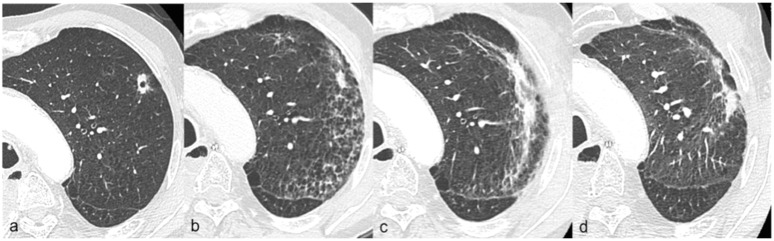
Figure 1.
Radiation pneumonitis (grade 1) radiological evolution Case 6, Table 2; (a) CT scan at 1 month after the end of stereotactic body radiotherapy delivered by helical TomoTherapy showing the pulmonary lesion; (b) CT scan at 5 months with the development of ground-glass opacities; (c) CT scan at 9 months showing the maximal extension of infiltrates and appearance of solid opacities; (d) CT scan at 13 months showing the opacity regression.
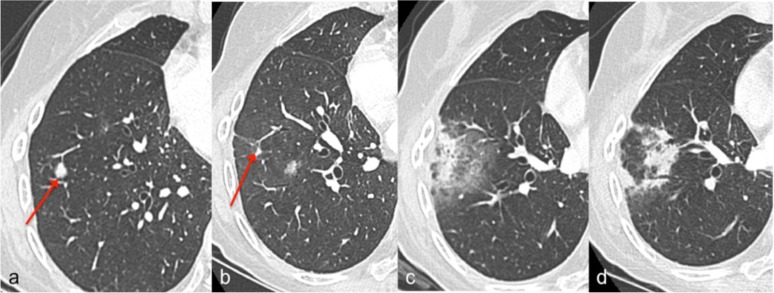
Figure 2.
Organizing pneumonia radiological evolution Case 9, Table 2; (a) CT scan at 2 months before the start of stereotactic body radiotherapy delivered by helical TomoTherapy (T-SBRT), the arrow indicates one of the two irradiated lesions; (b) CT scan at 4 months after the T-SBRT, the arrow shows a decrease in the volume of the lesion; (c) CT scan at 6 months (maximal extension of infiltrates) showing solid and ground-glass peripheral opacities that prevented the recognition of the malignant lesion; (d) CT at 9 months showing the substantial resolution of previous solid infiltrates and the development of other solid infiltrates in place of previous ground-glass opacities.
DISCUSSION
In one retrospective analysis, poor SBRT outcomes for inoperable, early stage NSCLN were usually (66%) associated with a distant recurrence, with disease recurrence accounting for 18% of cases.12 Another study of early stage primary NSCLN found a 1-year LRCR after SBRT of 96.8% and a 20% failure rate (62% of cases being distant relapses).6 Prospective studies of medically inoperable Stage I patients found a 1-year LCR and OS of 92% and 84%, respectively.2 The present analysis found comparable failure rate (25%), 1-year distant metastasis-free rate (82.9%), and 1-year LRCR (100%), while loco-regional and distant failures were observed at equal frequencies. Many factors seem to have not played a role on the observed outcomes; as in our study, PET-CT scans have typically been used to investigate persistent suspicious infiltrates on routine CT scans.2,6,12 With respect to differences in SBRT delivery schema, a systematic review of primary NSCLN found an LCR ≥85% when the biologically equivalent dose (BED)10 was ≥100 Gy and the BED3 was ≤210 Gy.13 In this study, BED10 and BED3 were 132 and 180 Gy, respectively, for all patients, and three patients staged as IIIa—which are typically not classified as early stage—were also included.
Only one study has reported oncological outcome for patients with primary early stage (Ia–Ib) NSCLN treated by T-SBRT.3 The total LRCR of 100% and total recurrence rate of 25% observed at the 12-month follow-up were in accordance with values reported by these authors (>95% and 33%, respectively). The minimum and median duration of the radiological follow-up (6 and 12 vs ours 4 and 11 months), local failure diagnostic method, and BED10 value (100–120 vs our 132 Gy) were also comparable. Unlike these investigators, however, we recorded two cases (12.5%) of acute or late grade ≥2 RP. The frequency after SBRT for early and/or late grade NSCLN with ≥2 RP varies (0% and 21%, respectively3,14–16), and few dosimetric risk factors such as a high PTV (≥37.7 cm3 15) have been identified when using the linear accelerator as a delivery system. The mean PTV of 53 cm3 obtained here for patients with grade ≥2 RP is within the range obtained in other studies.
Few studies have analysed the outcome of SPLN after SBRT, and none has employed TomoTherapy.9,10 The OS of 71.4%, metastasis-free rate of 80.0%, and LCR of 100% at 1 year in this study were similar to the values (91%, 92.3% and 100%, respectively) in another report in which patients were treated by SBRT after pneumonectomy.9 These investigators made a diagnosis based on pathology in only a minority of patients (20%), whereas we achieved pathological proof for 60% of SPLN cases. However, in agreement with their study in which one patient (6.6%) developed grade >2 RP, we also found one patient treated for SPLN who developed RP of grade >2 (10%). Given these results, T-SBRT seems to be a low-risk and effective treatment.
Only limited data are published concerning the radiological evolution of lung toxicity from a helical radiation delivery, the association between OP and SBRT was recently demonstrated. In one study, nine patients (5%) developed OP that migrated outside the IF after SBRT.11 The authors defined this as a mixture of patchy and ground-glass opacities developing in the lung volume after receiving <0.5 Gy, in the presence of general or respiratory symptoms and with no evidence of specific cause. Based on the previous definition, we did not detect changes outside of the IF but found one patient (6% of the total) whose infiltrates moved within the IF. In one report that examined serial changes on a CT scan after SBRT, a local consolidation was detected in 73% of irradiated lesions, and for six of the cases (38%), movement of the opacity was observed: consolidations disappeared in previously affected lung areas and appeared in new areas.17 The authors interpreted these findings as fibrosis. Radiological findings within the IF are difficult to interpret and the priority is to differentiate radiation-induced opacities from a recurrent disease. However, it can be conjectured that mild OP is associated with radiation-induced anomalies in the IF and its recognition by the dynamic migration in an asymptomatic patient can reasonably, considering as unlikely a local relapse, postpone the PET-CT scan execution.
CONCLUSIONS
T-SBRT for early stage NSCLN seems to be safe and effective. Mild OP was likely associated with radiation-induced anomalies in the IF.
FUNDING
Our institution Centre Hospitalier Universitaire Vaudois, Lausanne, Switzerland, funded the study.
Contributor Information
A Casutt, Email: alessio.casutt@unil.ch.
H Bouchaab, Email: Hasna.bouchaab@chuv.ch.
C Beigelman-Aubry, Email: catherine.beigelman-aubry@chuv.ch.
J Bourhis, Email: jean.bourhis@chuv.ch.
A Lovis, Email: alban.lovis@chuv.ch.
O Matzinger, Email: oscar.matzinger@hopitalrivierachablais.ch.
REFERENCES
- 1.Myrdal G, Gustafsson G, Lambe M, Hörte LG, Ståhle E. Outcome after lung cancer surgery. Factors predicting early mortality and major morbidity. Eur J Cardiothorac Surg 2001; 20: 694–9. [DOI] [PubMed] [Google Scholar]
- 2.Taremi M, Hope A, Dahele M, Pearson S, Fung S, Purdie T, et al. Stereotactic body radiotherapy for medically inoperable lung cancer: prospective, single-center study of 108 consecutive patients. Int J Radiat Oncol Biol Phys 2012; 82: 967–73. doi: 10.1016/j.ijrobp.2010.12.039 [DOI] [PubMed] [Google Scholar]
- 3.Marcenaro M, Vagge S, Belgioia L, Agnese D, Lamanna G, Mantero E, et al. Ablative or palliative stereotactic body radiotherapy with helical TomoTherapy for primary or metastatic lung tumor. Anticancer Res 2013; 33: 655–60. [PubMed] [Google Scholar]
- 4.Lagerwaard FJ, Verstegen NE, Haasbeek CJ, Slotman BJ, Paul MA, Smit EF, et al. Outcomes of stereotactic ablative radiotherapy in patients with potentially operable stage I non-small cell lung cancer. Int J Radiat Oncol Biol Phys 2012; 83: 348–53. doi: 10.1016/j.ijrobp.2011.06.2003 [DOI] [PubMed] [Google Scholar]
- 5.Falcoz PE, Conti M, Brouchet L, Chocron S, Puyraveau M, Mercier M, et al. The Thoracic Surgery Scoring System (Thoracoscore): risk model for in-hospital death in 15,183 patients requiring thoracic surgery. J Thorac Cardiovasc Surg 2007; 133: 325–32. [DOI] [PubMed] [Google Scholar]
- 6.Verstegen NE, Oosterhuis JW, Palma DA, Rodrigues G, Lagerwaard FJ, van der Elst A, et al. Stage I-II non-small-cell lung cancer treated using either stereotactic ablative radiotherapy (SABR) or lobectomy by video-assisted thoracoscopic surgery (VATS): outcomes of a propensity score-matched analysis. Ann Oncol 2013; 24: 1543–8. doi: 10.1093/annonc/mdt026 [DOI] [PubMed] [Google Scholar]
- 7.Crabtree T, Puri V, Timmerman R, Fernando H, Bradley J, Decker PA, et al. Treatment of stage I lung cancer in high-risk and inoperable patients: comparison of prospective clinical trials using stereotactic body radiotherapy (RTGO 0236), sublobar resection (ACOSOG Z4032), and radiofrequency ablation (ACOSOG Z4033). J Thorac Cardiovasc Surg 2013; 145: 692–9. doi: 10.1016/j.jtcvs.2012.10.038 [DOI] [PubMed] [Google Scholar]
- 8.Antakli T, Schaefer RF, Rutherford JE, Read RC. Second primary lung cancer. Ann Thorac Surg 1995; 59: 863–6. [DOI] [PubMed] [Google Scholar]
- 9.Haasbeek CJ, Lagerwaard FJ, de Jaeger K, Slotman BJ, Senan S. Outcomes of stereotactic radiotherapy for a new clinical stage I lung cancer arising postpneumonectomy. Cancer 2009; 115: 587–94. doi: 10.1002/cncr.24068 [DOI] [PubMed] [Google Scholar]
- 10.Senthi S, Haasbeek CJ, Lagerwaard FJ, Verbakel WF, de Haan PF, Slotman BJ, et al. Radiotherapy for second primary lung cancer arising post-pneumonectomy: planning considerations and clinical outcomes. J Thorac Dis 2013; 5: 116–22. doi: 10.3978/j.issn.2072-1439.2013.02.07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Murai T, Shibamoto Y, Nishiyama T, Baba F, Miyakawa A, Ayakawa S, et al. Organizing pneumonia after stereotactic ablative radiotherapy of the lung. Radiat Oncol 2012; 7: 123. doi: 10.1186/1748-717X-7-123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Senthi S, Lagerwaard FJ, Haasbeek CJ, Slotman BJ, Senan S. Patterns of disease recurrence after stereotactic ablative radiotherapy for early stages non-small-cell lung cancer: a retrospective analysis. Lancet Oncol 2012; 13: 802–9. doi: 10.1016/S1470-2045(12)70242-5 [DOI] [PubMed] [Google Scholar]
- 13.Senthi S, Haasbeek CJ, Slotman BJ, Senan S. Outcomes of stereotactic ablative radiotherapy for central lung tumours: a systematic review. Radiother Oncol 2013; 106: 276–82. doi: 10.1016/j.radonc.2013.01.004 [DOI] [PubMed] [Google Scholar]
- 14.Takeda A, Ohashi T, Kunieda E, Sanuki N, Enomoto T, Takeda T, et al. Comparison of clinical, tumour-related and dosimetric factors in grade 0-1, grade 2 and grade 3 radiation pneumonitis after stereotactic body radiotherapy for lung tumours. Br J Radiol 2012; 85: 636–42. doi: 10.1259/bjr/71635286 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Matsuo Y, Shibuya K, Nakamura M, Narabayashi M, Sakanaka K, Ueki N, et al. Dose–volume metrics associated with radiation pneumonitis after stereotactic body radiation therapy for lung cancer. Int J Radiat Oncol Biol Phys 2012; 83: e545–9. doi: 10.1016/j.ijrobp.2012.01.018 [DOI] [PubMed] [Google Scholar]
- 16.Shibamoto Y, Hashizume C, Baba F, Ayakawa S, Manabe Y, Nagai A, et al. Stereotactic body radiotherapy using a radiobiology-based regimen for stage I nonsmall cell lung cancer: a multicenter study. Cancer 2012; 118: 2078–84. doi: 10.1002/cncr.26470 [DOI] [PubMed] [Google Scholar]
- 17.Takeda T, Takeda A, Kunieda E, Ishizaka A, Takemasa K, Shimada K, et al. Radiation injury after hypofractionated stereotactic radiotherapy for peripheral small lung tumors: serial changes on CT. AJR Am J Roentgenol 2004; 182: 1123–8. [DOI] [PubMed] [Google Scholar]