Abstract
Objective:
This article describes the external audit measurements conducted in two UK centres implementing total skin electron beam therapy (TSEBT) and the results obtained.
Methods:
Measurements of output, energy, beam flatness and symmetry at a standard distance (95 or 100 cm SSD) were performed using a parallel plate chamber in solid water. Similarly, output and energy measurements were also performed at the treatment plane for single and dual fields. Clinical simulations were carried out using thermoluminescent dosemeters (TLDs) and Gafchromic® film (International Specialty Products, Wayne, NJ) on an anthropomorphic phantom.
Results:
Extended distance measurements confirmed that local values for the beam dosimetry at Centres A and B were within 2% for outputs and 1-mm agreement of the expected depth at which the dose is 50% of the maximum for the depth–dose curve in water (R50,D) value. Clinical simulation using TLDs) showed an agreement of −1.6% and −6.7% compared with the expected mean trunk dose for each centre, respectively, and a variation within 10% (±1 standard deviation) across the trunk. The film results confirmed that the delivery of the treatment technique at each audited centre complies with the European Organisation for Research and Treatment of Cancer recommendations.
Conclusion:
This audit methodology has proven to be a successful way to confirm the agreement of dosimetric parameters for TSEBT treatments at both audited centres and could serve as the basis for an audit template to be used by other audit groups.
Advances in knowledge:
TSEBT audits are not established in the UK owing to a limited number of centres carrying out the treatment technique. This article describes the audits performed at two UK centres prior to their clinical implementation.
External audits are a vital component in the implementation of any radiotherapy treatment programme. The aim is primarily to ensure dosimetric acceptability, as well as to maintain and improve consistency while transferring clinical experience between centres,1 particularly when implementing less established treatment techniques.2
The UK has a well-organized network of regional audit groups. External audit is now a requirement of the National Cancer Service Standards.3
Megavoltage and kilovoltage audits are well established within the radiotherapy community, although these are being redesigned to encompass newer treatment techniques such as intensity-modulated radiotherapy and volumetric arc therapy. Electron audits for total skin electron beam therapy (TSEBT) are not so established in the UK owing to a limited number of centres carrying out the treatment technique. This article describes two external dosimetry audits performed by the Guy's and St Thomas' NHS Foundation Trust (London, UK), hereon referred to as “the auditing centre”, at two centres (A and B) in the UK in April 2012 and August 2013, respectively, prior to their clinical implementation.
At the auditing centre, TSEBT is performed using an adaptation of the Stanford technique4 on an Elekta Precise digital linear accelerator (Elekta AB, Stockholm, Sweden) to mainly treat mycosis fungoides (MF). Since the first treatment in January 2006, well over 100 patients have been treated.
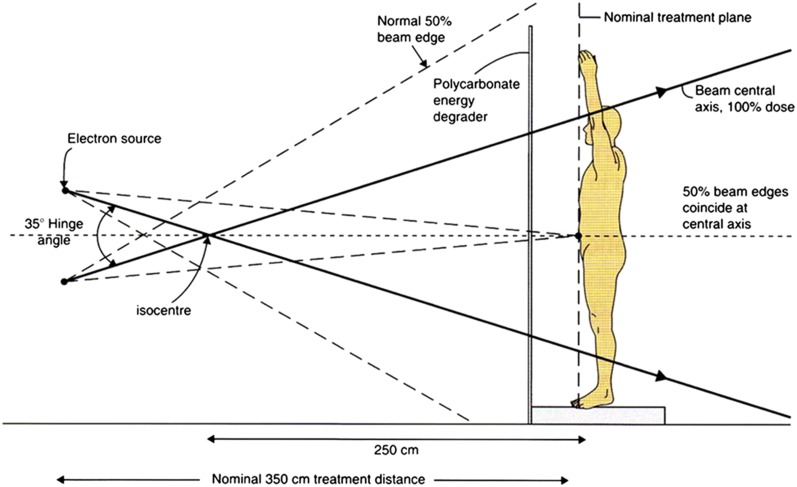
In the two audited centres, treatment is delivered using an Elekta Precise linear accelerator with a nominal beam energy of 6 MeV in high-dose rate electron mode also using a modified Stanford technique. This consists of the patient standing and adopting six different positions to maximize unfolding of the skin: three anteriorly then three posteriorly (Figure 1). Dual angled beams are then delivered—with a specified hinge angle (Figure 2)—at each position to achieve total skin coverage leading to a total of 12 beams at each treatment session.
Figure 1.
Representation of the six positions to be adopted during total skin electron beam therapy treatment. From top left to bottom right, these are: right anterior oblique, anterior–posterior, left anterior oblique, posterior–anterior, right posterior oblique 5Consent was received for use of this figure.
Figure 2.
Auditing centre total skin electron beam therapy-modified Stanford technique geometrical arrangement of the dual-field treatment technique, given equal exposures per beam. Calibration point is centred in the patient's truncal skin at the nominal treatment plane.5
A recent review of the clinical implementation of TSEBT reported that the modified Stanford technique is employed at >80% of centres worldwide6 owing to its ease of implementation on standard linear accelerators (linacs) with sufficiently sized treatment rooms. The hinge angles used range from 20° to 50° (average of 35°) depending on the treatment distance and beam energy employed and the optimal angle required for beam flatness.6
Modified Stanford technique
The typical prescription and fractionation used at the auditing centre for the treatment of MF is 1.5 Gy per fraction in 8, 16 or 20 fractions delivered 4 days per week prescribed to the skin surface. All 12 fields at the 6 adopted positions are treated during approximately 45-min morning sessions.
To achieve the depth dose distribution recommended4,7 for TSEBT treatments, the mean energy, E0, at the skin surface, of a single horizontal electron beam should be set close to 4.2 MeV at the treatment plane, which is achieved using an energy degrader with a standard 6-MeV electron beam. The beam degrader is typically a large Perspex® screen set in front of the treatment plane.
Routine shielding requires wearing leaded goggles to protect the eyes and finger nail shields. Further shielding may be required for the dosimetric results obtained on the first fraction from thermoluminescent dosemeter (TLD) placed on various parts of the body.
Techniques at Centres A and B
Summarized in Table 1 are the key differences between the set-ups at Centre A, Centre B and the auditing centre, which, namely, are the dose per fraction, location of the degrader, degrader thickness and treatment distance. At Centre A, the Perspex degrader is placed immediately before the patient and at Centre B, the degrader is at the treatment head, i.e. incorporated within the TSEBT applicator.
Table 1.
Total skin electron beam therapy set-up at Guy's and St Thomas' NHS Foundation Trust, Centres A and B
| Centre | Nominal beam energy (MeV) | Perspex® degrader location and thickness | Treatment distance (cm) | Dual-beam treatment hinge angles (degrees) | Dose per fraction (cGy) | Monitor unit delivered per field |
|---|---|---|---|---|---|---|
| Auditing centre | 6 | At patient; 4 mm | 350 | 35 | 150 | 93 |
| Centre A | 6 | At patient; 6 mm | 400 | 35 | 200 | 157 |
| Centre B | 6 | In treatment head; 3 mm | 400 | 40 | 150 | 120 |
METHODS AND MATERIALS
There is minimal guidance on how to conduct external audits for TSEBT treatments. Technique and dosimetry recommendations are given in two major reports, the American Association of Physicists in Medicine Task Group 30 report 23 published in 19884 and, more recently, the 2002 European Organisation for Research and Treatment of Cancer (EORTC) guidelines.7
The measurements performed at these two centres were based around the quality control (QC) performed at the auditing centre and split into:
– standard electron treatment measurements—beam output, energy, flatness and symmetry measurements at 95 or 100 cm source to surface distance (SSD)
– extended treatment distance (400 cm SSD)— for both single- and dual-beam arrangements
– clinical simulation tests—using an anthropomorphic phantom with skin doses assessed utilizing TLDs (lithium fluoride-100) placed on the head and trunk, and Gafchromic® film (International Specialty Products, Wayne, NJ) placed in the pelvic region to measure the transverse dose distribution. These simulations were used to verify the delivered dose based on the monitor units (MU) set per field and to assess the dose coverage.
Other than the clinical simulation tests, all measurements were performed using an NACP-02 parallel plate chamber (IBA-Dosimetry, Schwarzenbruck, Germany) paired with a 2620 electrometer (Elektron Technology, Berkshire, UK) calibrated against an (NACP-02) electron secondary standard and electrometer using the Institute of Physics and Engineering in Medicine (IPEM) electron code of practice 20038 with calibration factors derived from the host centre's depth at which the dose is 50% of the maximum for the depth–dose curve in water (R50,D) values.
Standard treatment distance
Measurements at the standard treatment distance (95 or 100 cm SSD) with gantry at 0° were carried out to obtain the beam output, energy, flatness and symmetry parameters. For Centre B, the output measurement was performed with the degrader in place as this is the method used to calibrate the output locally.
Beams of 100 MU were delivered using electron solid water (WTe; St Bartholomew's Hospital, London, UK) with the required build-up to the depth of maximum dose (dmax) added on top, where 1 MU was calibrated to deliver 10 cGy at the standard SSD. Readings were taken in a high-dose-rate mode. TSEBT treatments require high dose rates (3000 cGy min−1 at the standard treatment distance of 95 cm at the auditing centre) to reduce the treatment time as the patient is located at an extended distance, hence the difference in calibration with respect to standard electron beams (typically 1 cGy MU−1).
The beam energy was measured as the ratio of the dose delivered at two different depths matching those used at the host centre.
Flatness was measured by moving the couch 12 cm from the beam central axis in the conventional G (gun), T (target), A (left as facing the linac) and B (right as facing the linac) positions. Results from the four couch positions were normalized to the central axis. TG and AB symmetry ratios were then calculated.
Flatness and symmetry measurements at extended SSD were not performed owing to the complexity involved measuring at the patient's treatment distance. However, these were implicitly measured during clinical simulation.
Extended treatment distance
Single-field measurements at 400 cm SSD were performed with the chamber aligned on the beam central axis for a 90° gantry angle.
The beam output was measured at dmax, and energy measurements were carried out at the host centre's depths. Readings were taken for 100 MU delivered in high-dose-rate mode.
Dual-field measurements were performed under the same set-up as per single fields at the site-specific hinge angles.
For these measurements, the chamber cables were kept out of the extensive main electron fields or shielded with lead to minimize cable effects.
Clinical simulations
Clinical simulations were performed using the centre's RANDO® (The Phantom Laboratory, Greenwich, NY) anthropomorphic phantom (RANDO male for Centre A and RANDO female for Centre B) to measure the skin dose and the isodose distribution in the pelvic region. The RANDO phantom was placed on a stable custom support stool that enabled 60° rotations in between each set of dual fields to recreate the various treatment positions. The phantom was placed at the correct treatment distance, centred on the beam centre line with its axis of revolution at the nominal treatment distance. The MU set for each field in the simulation was supplied by both centres (157 MU for Centre A and 120 MU for Centre B) and were delivered in normal clinical mode via the record and verify system MOSAIQ® (Elekta AB).
Skin dose
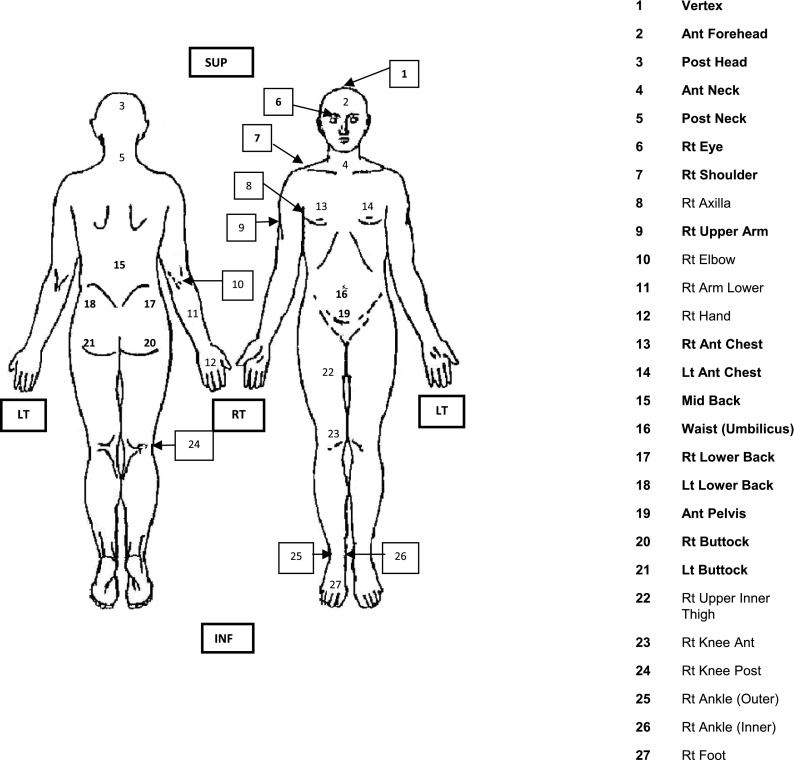
A series of TLD sachets (two chips per sachet) were attached to the surface of the anthropomorphic phantom at positions corresponding to those used clinically at the auditing centre for the head and trunk regions as highlighted in Figure 3. The TLD were positioned at known stable anatomical positions from the vertex to the right (RT) buttock, Positions 1–21 in Figure 3, excluding Position 8, RT axilla, and Position 12, RT hand.
Figure 3.
Typical clinical thermoluminescent dosemeter positions at auditing centre, those used during the simulation tests are shown in bold. Ant, anterior; inf, inferior; Lt and LT, left; mid, middle; post, posterior; Rt and RT, right; sup, superior.
A batch calibration factor (BCF) for the TLD was derived by irradiating five chips to the prescription dose at each centre in a conventional 6-MeV electron beam at 95-cm SSD at Centre A and 100-cm SSD at Centre B using a 10 × 10-cm applicator. Read out was performed at the auditing centre using a Thermo Scientific™ Harshaw TLD™ Model 5500 automatic reader (Thermo Scientific, Waltham, MA). The average trunk dose (Positions 13–21 in Figure 3) was then determined from the dose measured for TLD placed on the main trunk of the anthropomorphic phantom. The dose from each individual TLD was obtained as a product of their individual charge (nC), individual calibration factor and the BCF. A supralinearity correction factor was also applied to account for the non-linear response of lithium fluoride-100 at doses different to the calibration dose.
Isodose distribution in the patient
Sheets of Gafchromic EBT2 film were cut to shape and placed in the pelvic region of the anthropomorphic phantom.
The film calibration curve was established in a 6-MeV (non-TSEBT) beam at the auditing centre, at dmax.
All films taken at the auditing and host centres were scanned in the same orientation and position on an Epson A3 Model 1900 flatbed scanner (48 channels) (Epson, Suwa, Japan). Images were saved as TIFF files, with the open source program ImageJ (National Institutes of Health, Bethesda, MD) used to split the RGB channels with the red channel file (16-bit) saved separately. The latter file was then analysed using VeriSoft® v. 3.1 software (PTW Freiburg). A calibration correction was then applied to the films. This correction was established from the calibration film using PTW Film Scan software (PTW Freiburg). This enabled the red channel optical density to be mapped to dose (cGy). The subsequent images were then renormalized to the appropriate dose per fraction, i.e. 200 cGy for Centre A and 150 cGy for Centre B and auditing centre.
RESULTS
The beam dosimetry and TLD results are summarized in Table 2. The standard SSD measurements were compared with local values at Centre B where QC measurements are performed at 100-cm SSD using tolerances from the IPEM National Interdepartmental Dosimetry Audit of the electron code of practice 2003:1 2% for outputs and 1 mm for R50,D determination. For Centre A, measurements are not routinely made at a standard SSD, so the results are compared with the expected values from the auditing centre. However, in both cases, the final test of dosimetric acceptability was based on the clinical simulation where a tolerance of 5% of the prescribed mean trunk dose was considered acceptable.
Table 2.
Summary of the dosimetric results obtained at the audited Centres A and B
| Beam dosimetry | Measured values at standard distance |
Single/dual field at treatment distance |
Clinical simulation thermoluminescent dosemeter results | |||||
|---|---|---|---|---|---|---|---|---|
| Centre | Output (cGy MU−1) | Energy ratio | Flatness (% average TGAB) | Symmetry (%) |
Output (cGy MU−1) | Energy ratio | Mean trunk dose (percentage of prescribed dose ±1 standard deviation) | |
| TG | AB | |||||||
| Centre A | 10.98 | 0.650 | 98.1 | 99.7 | 100.8 | 0.500 (S); 0.465 (D) | 0.495 (S); 0.490 (D) | 98.4 ± 6.9 |
| (95-cm SSD) | ||||||||
| Centre B | 12.69 | 0.529 | 85.0 | 101 | 99.5 | 0.406 (S); 0.446 (D) | 0.511 (S); (D) not measured | 93.3 ± 3.6 |
| (100-cm SSD) | ||||||||
| Auditing centre baseline values | 10.0 | 0.405 | 98.5 | 100 | 100 | 0.600 (S and D) | 0.465 | 99.3 ± 8.1 (for all patients to date) |
| (95-cm SSD) | ||||||||
A, left as facing linac; B, right as facing linac; D, dual; G, gun; MU, monitor unit; S, single; SSD, source to surface distance; T, target.
Standard source to surface distance results
The beam output varied between centres depending on their calibration method owing to the differing distances (95 and 100 cm SSD) and positions of the Perspex degrader. Thus, for Centre B, as the degrader is fitted with the TSEBT applicator in the head, the standard output is affected compared with the centres using the degrader only at the treatment distance.
The nominal 6-MeV energy for Centre A was found to be higher than the corresponding beam at the auditing centre. For the clinical set-up, this is compensated by the increased treatment distance and screen thickness employed. For Centre B, a very good agreement was achieved with locally expected values (within 0.2% and 0.2 mm). Note that the expected energy was lower for Centre B owing to the degrader location being at the head; therefore, the energy ratio was measured at depths of 18 and 10 mm as opposed to 24 and 12 mm at the auditing centre and at Centre A.
The overall flatness (85%) at Centre B was lower than at Centre A. This is again owing to the Perspex degrader being located at the head of the machine as opposed to the extended treatment distance. With the degrader removed, the overall flatness increased to 97%, which is comparable to measurements at 95-cm SSD at the auditing centre.
Beam symmetry at both centres was within ±1.0% at the 12-cm points.
Extended source to surface distance results
The single- and dual-field output measurements agreed within 1.0% at Centre A and 1.5% at Centre B compared with the local values.
The dose delivered in Centre A by each of the dual beams was measured as 46.5% of the direct central axis dose. The energy measured at the treatment distance agrees closely (within 1 mm at R50,D) with the auditing centre's value, indicating a slightly higher energy.
The dose delivered in Centre B by each of the dual beams was measured as 54.9% of the direct central axis dose.
This indicates that the beams overlap slightly for Centre B as opposed to underlapping for Centre A. The implication is that the superior–inferior coverage may be affected, and the dual-beam flatness profile will look different, being higher on the central axis for Centre B and lower for Centre A, as indicated in Table 2.
However, the overall flatness and clinically useful extent of the dual beams will have been determined at each centre during commissioning and will be a function of the location of the degrader, hinge angle, beam profile and treatment distance. This would be an interesting area to investigate further.
Thermoluminescent dosemeter simulation
The TLD results showed that the phantom had received an average trunk dose of 196.8 cGy [standard deviation (SD) = 13.6 cGy (6.9%)] at Centre A and 139.9 cGy [SD = 5.1 cGy (3.6%)] at Centre B. These results agree within −1.6% and −6.7% of the expected dose per fraction of 200 and 150 cGy, respectively. The result at Centre B was obtained with an initial commissioning MU setting, which has since been adjusted as a consequence of the audit and further local measurements.
For Centre A, there was some variation seen between the different positions, with the TLD placed on the left side of the body receiving slightly more dose than on the right side. This is in all likelihood down to variations in the positioning of the anthropomorphic phantom in the six different treatment positions, as small variations in positioning of both the TLD and the phantom can lead to different doses received by a single point. Overall, the TLD results showed a 6.9% variation for the trunk dose and 7.1% for all measurements excluding the vertex and shielded eye readings. This agrees well with the level of variation seen at the auditing centre of 8.1% for all patients to date. Nevertheless, it was recommended to repeat these measurements on a different occasion so as to appreciate the potential variations under “optimal set-up conditions” and to ascertain what would be acceptable in clinical practice. This way, measurements for the first few patients could be performed and analysed to check that the correct trunk dose had been delivered and that the variation across the trunk was acceptable. For the audits, a dose variation of 10% (±1 SD) across the trunk was deemed acceptable.
For Centre B, the TLD results showed only a small variation between measurements (3.6% for trunk dose, 4.1% for all measurements excluding vertex and shielded eye readings). The average of the TLD results (139.9 cGy for trunk dose, 141.8 cGy overall) indicate that a slightly higher MU is needed to deliver the prescription dose of 150 cGy.
Film analysis
The Gafchromic film results from the pelvis region of the anthropomorphic phantoms used at Centre A, Centre B and the auditing centre can be seen in Figure 4a–c, respectively.
Figure 4.
Gafchromic® film (International Specialty Products, Wayne, NJ) results from the pelvic region of the anthropomorphic phantom at (a) Centre A, (b) Centre B and (c) auditing centre. Note that in (b), the phantom orientation is inverted in comparison with (a) and (c).
The general isodose shape from the Gafchromic film results showed overall agreement between Centre A, Centre B and the auditing centre for the clinical simulations. All the isodoses exhibited a lower dose region laterally where the 80% isodose comes closer to the surface. This is to be expected when there are no lateral fields involved in the delivery technique. In general, the EORTC recommendations7 of at least 80% at a depth of 4 mm and <20% at a depth >2 cm were complied with for a large part of these cross sections.
CONCLUSION
Two new centres implementing TSEBT treatments in the UK were audited successfully. The results confirm that the beam dosimetry at the extended treatment distances at Centres A and B were within the audit tolerances of 2% for outputs and 1 mm of the expected R50,D values.
The TLD results showed that the mean trunk dose was within −1.6% of that expected at Centre A. At Centre B, the dose was −6.7% lower than that expected. This initial commissioning MU was adjusted prior to clinical introduction at Centre B.
The Gafchromic film results showed that the auditing centre and Centres A and B comply with the EORTC recommendations.7
This audit methodology has proven to be a successful way to confirm agreement of dosimetric parameters for TSEBT treatments in both audited centres and could serve as the basis for an audit template to be used by other audit groups.
Acknowledgments
ACKNOWLEDGMENTS
The authors would like to thank all the centres that participated in the audit and the physicists involved (Steve Bolton, Hugh Brown and Andrew Przeslak), with special thanks to Peter Rudd, the lead physicist, who originally commissioned the technique at Guy's and St Thomas' NHS Foundation Trust.
Contributor Information
S Misson-Yates, Email: Sarah.Misson-Yates@gstt.nhs.uk.
R Gonzalez, Email: regina.gonzalez@gstt.nhs.uk.
M McGovern, Email: mark.mcgovern@gstt.nhs.uk.
A Greener, Email: tony.greener@gstt.nhs.uk.
REFERENCES
- 1.Palmer A, Mzenda B, Kearton J, Wills R. Analysis of regional radiotherapy dosimetry audit data and recommendations for future audits. Br J Radiol 2011; 84: 733–42. doi: 10.1259/bjr/18691638 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Eaton DJ, Earner B, Faulkner P, Dancer N. A national dosimetry audit of intraoperative radiotherapy. Br J Radiol 2013; 86: 20130447. doi: 10.1259/bjr.20130447 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.National Cancer Peer Review Team. Manual for cancer services: radiotherapy measures. London, UK: National Cancer Action Team; 2011. [Google Scholar]
- 4.AAPM Task Group. Total skin electron therapy: technique and dosimetry. AAPM report no 23. New York, NY: American Institute of Physics; 1988. [Google Scholar]
- 5.Morris SL. Skin lymphoma. Clin Oncol (R Coll Radiol) 2012; 24: 371–85. doi: 10.1016/j.clon.2012.02.007 [DOI] [PubMed] [Google Scholar]
- 6.Diamantopoulos S, Platoni K, Dilvoi M, Nazos I, Geropantas K, Maravelis G, et al. Clinical implementation of total skin electron beam (TSEB) therapy: a review of the relevant literature. Phys Med 2011; 27: 62–8. doi: 10.1016/j.ejmp.2010.09.001 [DOI] [PubMed] [Google Scholar]
- 7.Jones GW, Kacinski BM, Wilson LD, Willemze R, Spittle M, Hohenberg G, et al. Total skin electron radiation in the management of mycosis fungoides: consensus of the European Organisation for Research and Treatment of Cancer (EORTC). Cutaneous lymphoma project group. J Am Acad Dermatol 2002; 47: 364–70. [DOI] [PubMed] [Google Scholar]
- 8.Thwaites DI, DuSautoy AR, Jordan T, McEwen MR, Nisbet A, Nahum AE, et al. The IPEM code of practice for electron dosimetry for radiotherapy beams of initial energy from 4 to 25MeV based on the absorbed dose to water calibration. Phys Med Biol 2003; 48: 2929–70. [DOI] [PubMed] [Google Scholar]