Abstract
In the past decade, electronic brachytherapy (EB) has emerged as an attractive modality for the treatment of skin lesions and intraoperative partial breast irradiation, as well as finding wider applications in intracavitary and interstitial sites. These miniature X-ray sources, which operate at low kilovoltage energies (<100 kV), have reduced shielding requirements and inherent portability, therefore can be used outside the traditional realms of the radiotherapy department. However, steep dose gradients and increased sensitivity to inhomogeneities challenge accurate dosimetry. Secondly, ease of use does not mitigate the need for close involvement by medical physics experts and consultant oncologists. Finally, further studies are needed to relate the more heterogeneous dose distributions to clinical outcomes. With these provisos, the practical convenience of EB strongly suggests that it will become an established option for selected patients, not only in radiotherapy departments but also in a range of operating theatres and clinics around the world.
WHAT IS ELECTRONIC BRACHYTHERAPY?
Brachytherapy literally means “close” or “near” treatment (as opposed to tele- or “far” -therapy). Historically, it refers to the placement of radioactive sources on or within the treatment site, to deliver a very high dose of radiation with rapid fall off of dose to surrounding normal tissues. Radium needles, iridium wire and compact sources made from caesium or iridium have been used, placed in moulds on the skin surface (superficial), within natural body cavities and lumens (intracavitary, intraluminal, intravascular) or inserted into the tissue itself (interstitial). Manual insertion has largely been replaced by automated afterloading to reduce operator doses, and the majority of current treatments are delivered using high dose rate (HDR) units or low dose rate (LDR) seeds.1,2 A recent special issue of the British Journal of Radiology (BJR) reviewed the latest advances in HDR brachytherapy physics.3–8
The past decade has seen the development of a new form of treatment, termed electronic brachytherapy (EB),9 and there are now >400 systems worldwide. These miniature X-ray sources, operating at low kilovoltage energies, have many of the advantages of established radiotherapy approaches, but with further benefits of their own (Table 1). Use of lower energies reduces the requirements for shielding of the surrounding environment and increases the radiobiological effectiveness.10 In addition, the inherent portability means that treatments can be offered beyond the traditional realms of the radiotherapy department. Skin lesions can be treated in the dermatology clinic, and single high doses can be delivered in the operating theatre immediately after surgical excision of a tumour. However, with increased freedom comes increased risk of misuse by those unfamiliar with radiation. It is vital that oncologists and medical physicists are well informed and remain closely involved in these novel approaches. Therefore, the aim of this review is to provide an overview of the range of current clinical systems and applications; to address current issues in dosimetry calibration, safety regulations and staffing; and to consider future directions of this emerging field.
Table 1.
Advantages and disadvantages of electronic brachytherapy and comparable treatments
| Therapy type | Energya | Steep dose gradient | Little shielding | Compact source | Little fading with time | Portable | Switch on/off | No contamination risk |
|---|---|---|---|---|---|---|---|---|
| Electronic brachytherapy | Approximately 50 kV | ✓ | ✓ | ✓ | – | ✓ | ✓ | ✓ |
| Iridium high dose rate | 380 keV | – | × | ✓ | × | × | × | × |
| Iodine seeds | 28 keV | ✓ | ✓ | ✓ | × | ✓ | × | × |
| Superficial X-ray units | 50–150 kV | ✓ | – | – | ✓ | – | ✓ | ✓ |
| Electron linear accelerator intraoperative radiotherapy | 4–12 MeV | – | × | × | ✓ | – | ✓ | ✓ |
Values given are the average energy of radionuclides or the nominal energies of X-ray and electron sources. X-ray systems are often described in terms of their peak accelerating voltage (e.g. 50 kV), although only a few photons will possess the corresponding maximum energy (50 keV), and the effective energy of the beam spectrum will be lower (e.g. 20–30 keV). For electron beams produced by linear accelerators, it is conventional to use units of mega electron volts, since the energy spread will be lower, and to distinguish them from photon beams of the same nominal energy.
CLINICAL SYSTEMS
Operating parameters for the range of currently available clinical systems are given in Table 2, and images of each system are shown in Figure 1. They may be broadly divided into three classes: steered, stepped and collimated, as described below. There is some overlap between collimated systems advertised as EB devices and conventional superficial units for treatment of surface lesions. Typically, the former are mobile and at the lower end of the conventional range for beam energy, field size and focus-to-surface distance (FSD). However, there is currently no clear division between the two.
Table 2.
Operating parameters and usage of current clinical systems
| Machine name | Manufacturer | Approximate number of units worldwide (UK and Europe/USA)a | Clinical applications | Approximate treatment time | Accelerating potential (kV), tube current (mA) | Half-value layer (mmAl) | Geometry | Applicator or cone size | Focus-to-surface distance | Study |
|---|---|---|---|---|---|---|---|---|---|---|
| INTRABEAM® | Carl Zeiss Surgical (Oberkochen, Germany) | 250 (160/60) | Skin, breast,b intracranial, kyphoplasty, other | 25–40 min (spheres) 5–30 min (surface applicator) |
50, 0.04 | 0.1 (bare source) 0.8–1.3 (spherical applicators) 1.6–2.1 (sphere and 1–2 cm water) |
Point source (probe tip, steered) | 15- to 50-mm spheres, 10- to 60-mm surface applicators, 20- to 35-mm cylinders | 10–26 mm (surface) | Eaton11 and Schneider et al12 |
| Xoft® | iCAD Inc. (Nashua, NH) | >150 (10/140) | Skin,b breast,b vaginal | 10–25 min (balloon) 5–10 min (surface) 10–15 min (endocavitary) |
50, 0.3 | 0.5 (bare source), 1.6 (endocavitary) | Point source (catheter, stepped) | 30- to 60-mm balloons, 10- to 50-mm cones, 20- to 35-mm cylinders | 20–30 mm (cones) | Liu et al,13 Richardson et al,14 Bhatnagar15 and Dickler et al16 |
| Papillon | Ariane Medical Systems Ltd (Derby, UK) | 11 (11/0) | Rectum,b skin, breast | 2 min | 50, 2.7 | 0.6 (cone) | Collimated source | 22- to 30-mm cones | 29–38 mm | Gerard et al17 |
| Esteya® | Elekta AB-Nucletron (Stockholm, Sweden) | 10 (2/8) | Skinb | 2 min | 70, 0.5–1.6 | 1.9 (surface applicator) | Collimated source | 10- to 30-mm surface applicators | 60 mm | Garcia-Martinez et al18 |
| Photoelectric therapy | Xstrahl Ltd (Camberley, UK) | 1 (1/0) | Skinb | 1–2 min | 80, 1.3 | 2.9 (surface applicator) | Collimated source | 10- to 50-mm surface applicators | 50 mm | M Robinson, 2014, personal communication |
| SRT-100™ | Sensus Healthcare (Boca Raton, FL) | 150 (7/130) | Skinb | 1–2 min | 50–100, 8–10 | 0.5–2.1 (cone) | Collimated source | 10- to 50-mm (100 mm) cones | 150 mm (250 mm) | User manual |
Data provided by manufacturers, as of February 2015.
Primary application.
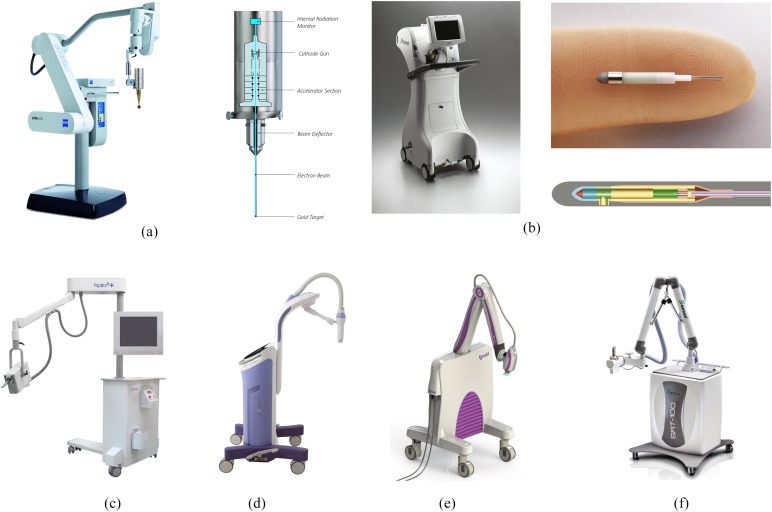
Figure 1.
Images of (a) INTRABEAM® source (Carl Zeiss Surgical, Oberkochen, Germany) on gantry arm with spherical applicator attached, and internal schematic (image provided by Carl Zeiss Surgical); (b) Xoft® Axxent® system (iCAD Inc., Nashua, NH) external trolley, and close up of source (image provided by Xoft/iCad); (c) Papillon (Ariane Medical Systems Ltd, Derby, UK) source on gantry arm (image provided by Ariane Medical Systems), (d) Esteya® EB system (Elekta AB-Nucletron, Stockholm, Sweden) (image provided by Elekta), (e) Photoelectric therapy system (Xstrahl Ltd, Camberley, UK) (image provided by Xstrahl), (f) SRT-100™ (Sensus Healthcare, Boca Raton, FL) unit (image provided by Sensus Healthcare).
The INTRABEAM® PRS500 system (Carl Zeiss Surgical, Oberkochen, Germany) is a compact mobile X-ray source originally developed for intracranial stereotaxy in the early 1990s (Figure 1a). Electrons are accelerated and steered down a drift tube, striking a gold target on the inside surface of the probe tip. Photons are then produced in an approximately isotropic distribution. Accessories are provided to measure output in fixed geometry and isotropy before each treatment. Dosimetry and quality assurance (QA) for this device have been reviewed previously.11 Solid spherical applicators were developed to deliver intraoperative radiotherapy (IORT) to the tumour bed for breast cancer, as described below. Further applicators have been designed for gynaecological, skin and kyphoplasty treatments,12,19,20 but only a few clinical results have been reported.21,22
The Xoft® Axxent® system (iCAD Inc., Nashua, NH) is a miniature X-ray tube integrated with a cooling sheath into a multilumen catheter, first released in 2006 (Figure 1b). The position of the source may be stepped along the length of the catheter, similar to an HDR source. Measured and simulated dosimetry for this device has been described by Rivard et al23 and Liu et al.13 Unlike the INTRABEAM system, Xoft sources have a limited lifetime of about 3 h or 10 treatments. However, the dose rate is higher and the depth dose falls off less steeply. Source strength is verified using an internal well chamber before each treatment. Balloon catheters have been used to treat early stage breast cancer, initially with multiple fractions24 and then with single fraction IORT, as described below. Dose distributions are similar to the MammoSite® balloon catheter (Cytec Industries Inc., Mountain View, CA) used with iridium-192 (192Ir) HDR sources, but with higher doses close to the applicator surface, which may increase the risk of fat necrosis. Conversely, doses to surrounding organs at risk are lower, because of the relatively rapid dose fall off.25,26 Dosimetric characteristics have also been described for endometrial,27 surface28 and endocavitary applicators.14 Output variation between sources, flatness and symmetry for surface applicators were all within 5%.
The Papillon 50 contact radiotherapy system (Ariane Medical Systems Ltd, Derby, UK) is a short FSD collimated X-ray source, developed to replace the Phillips RT50 (Philips Healthcare, Amsterdam, Netherlands) and released in 2008 (Figure 1c). Electrons are accelerated into an evacuated copper tube to hit a rhenium transmission target. Photons are produced in an approximately isotropic distribution but collimated by cones of increasing diameter and FSD to give a fixed opening angle of 45°. A plastic chamber holder is provided for output constancy checks. Measured and simulated dosimetry for this device has been described by Croce et al.29 The primary use of the system is as a boost for external beam treatment (EBRT) of rectal cancers, providing a convenient and efficient alternative to surgery. A dose of 90 Gy to the tissue surface in three fractions is delivered by an “internal superficial” method where the applicator end is inserted into the rectum and placed against the lesion.17 Applicators for skin are also available, and breast IORT is under development.
The Esteya® EB system (Elekta AB-Nucletron, Stockholm, Sweden) is a mobile collimated miniature X-ray source released in late 2013 and designed specifically for treatment of skin lesions. A QA device is provided to check consistency of output, flatness and depth doses. Surface applicators with a flattening filter are used to give a dose distribution similar to the Valencia 192Ir HDR applicator, produced by the same manufacturer. Tube current is varied to give an approximately constant treatment time. Dosimetry of the unit has been described by Garcia-Martinez et al.18 They found the system had flatness and symmetry within 5%, along with a sharper penumbra and shallower depth dose than the Valencia or Leipzig HDR applicators (Elekta AB-Nucletron).
Photoelectric therapy (Xstrahl Ltd, Camberley, UK) is a new product launched in late 2014, also aimed at treating skin lesions. The system is a compact ultralight mobile unit with built-in cooling, easy to shape collimation and flattening filters to give a uniform dose profile. Simple QA tools allow precise placement of ion chambers for dose output checks [M Robinson, 2014, personal communication].
Finally, the SRT-100™ (Sensus Healthcare, Boca Raton, FL) is another mobile collimated low kilovoltage unit specifically aimed at treating skin lesions, but with FSD and field sizes comparable to a standard kilovoltage therapy unit, such as the Xstrahl 100 or 150 series. It is included in this review to show the overlap between conventional superficial units and EB devices. The system features a built-in QA check of output, which is performed before each treatment.
RADIATION DOSIMETRY
Basic dosimetry
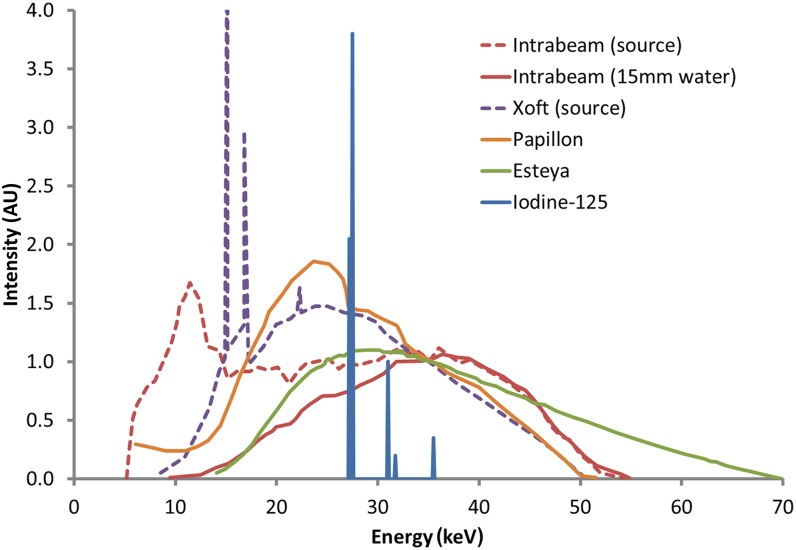
Energy spectra for the INTRABEAM, Xoft, Papillon and Esteya devices are shown in Figure 2. Differences are apparent at lower energies, where the spectrum is dependent on unit-specific filtration and characteristic emission, but the curves are similar in range from 35 to 50 keV, where bremsstrahlung interactions dominate. When point-like sources such as INTRABEAM are filtered by small depths of water, or alternatively the spherical applicators, the majority of low energy photons are absorbed and the resulting spectrum is similar to other devices, except the Esteya that has higher total accelerating potential of 70 kV rather than 50 kV. The same effect is expected for the Xoft system with balloon applicators attached. Overall the mean energies of approximately 25–35 keV are similar to or slightly higher than iodine-125 (125I, 28 keV) but much lower than 192Ir (380 keV).
Figure 2.
Energy spectra of electronic brachytherapy devices: INTRABEAM® (Carl Zeiss Surgical, Oberkochen, Germany) (source only, and 15-mm depth in water),30 Xoft® (iCAD Inc., Nashua, NH) (source only),23 Papillon29 (Ariane Medical Systems Ltd, Derby, UK) and Esteya® EB system (Elekta AB-Nucletron, Stockholm, Sweden).18 All of these data are normalized to the value at 35 keV. Emission lines for iodine-125 are also shown for comparison, with data from Task Group-43U1.31 AU, arbitary units.
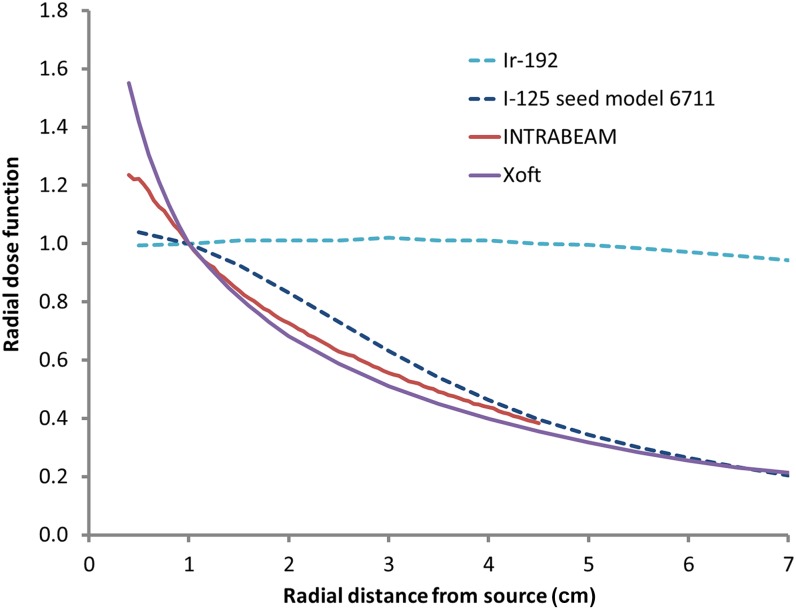
Radial dose functions for the INTRABEAM and Xoft sources are shown in Figure 3, alongside data for 125I (LDR seeds) and 192Ir (HDR source). This function, defined in the Task Group (TG)-43 formalism,31,32 shows the fall off of dose independent of inverse square dependence on distance. Both these sources have a steeper dose fall off than do125I seeds close to the source but are similar at larger distances. However, these effects will be mitigated by the addition of spherical applicators as described above.
Figure 3.
Radial dose functions for iridium-192 (Ir-192) and iodine-125 (I-125) seed model 6711,32 INTRABEAM® (data courtesy of the Royal Free Hospital, London, UK) and Xoft® (iCAD Inc., Nashua, NH) devices.23 All these data are normalized to the value at 1-cm distance.
Depth dose data for devices used with surface applicators and cones are shown in Figure 4. Again, there is some variation, for example, the Esteya device has a higher accelerating potential of 70 kV and longer FSD of 6 cm, which will contribute to the shallower depth dose curve. However, the SRT-100 curve is similar to those for the Papillon and Xoft devices, in spite of the larger field size of 5 cm and much longer FSD of 15 cm. Residual differences may depend on the specific filtration used in each system, which could explain why the INTRABEAM curve is much steeper, in spite of the similar energy, field size and FSD. Centres should verify depth dose data for their own system as part of the commissioning process, although published values may be useful for comparison.
Figure 4.
Percentage depth dose curves for electronic brachytherapy devices with surface or cone applicators: Esteya® EB system (Elekta AB-Nucletron, Stockholm, Sweden) (30-mm surface applicator),18 Papillon (Ariane Medical Systems Ltd, Derby, UK) (30-mm cone),29 SRT-100 (50-mm cone; data courtesy of Sensus Healthcare, Boca Raton, FL), Xoft® (iCAD Inc., Nashua, NH) (35-mm cone)28 and INTRABEAM® (Carl Zeiss Surgical, Oberkochen, Germany) (30-mm flat applicator, extrapolated to zero depth as surface data not reported).12
Output calibration
Radioactive source strength is commonly measured in a well-type ionization chamber with 4π geometry. The well chamber is typically calibrated using a free air chamber with a common source inserted into both systems. Absorbed dose to water is then calculated using the TG-43 formalism.31,32 (These articles are among the most highly cited radiotherapy physics papers of all time,33 suggesting they have become standard methodology, and hence appropriate for extension to novel brachytherapy applications.) The TG-43 formalism specifies the absorbed dose to a reference point 1 cm from the source, in a direction perpendicular to the long axis, and relates this dose to the air kerma strength at 1 m from the source. The Xoft device was originally characterized using this formalism,21 because of the similar source geometry and catheter delivery as other LDR/HDR sources. The formalism also allows standard geometry parameters to be determined independent of intersource variation. The formalism has also been extended to generalized tungsten target EB sources.34
For other EB systems, the output has been verified using a soft X-ray parallel plate ion chamber, in a liquid water tank or solid water phantom. This is the approach taken when INTRABEAM sources are calibrated in the factory, and also matches the usual approach for collimated kilovoltage units, where the dose is prescribed to the applicator end surface. In this case, the reference point is in line with either the electron or collimated photon beam, at the end of the applicator cone or 1 cm from the surface of spherical applicators. For the INTRABEAM and Papillon units, the 0.02-cm3 PTW23342 chamber (PTW Freiburg, Freiburg, Germany) has been used following the Institute of Physics and Engineering in Medicine very low energy code of practice.35,36 Air kerma calibration was derived from the UK National Physical Laboratory free air chamber, and the chamber correction factor for the difference between measurement in air and in phantom, kch, was found to be 1.06–1.07.37,38 The main uncertainties in this approach are positioning accuracy and the energy dependence of any solid water materials used.
When measurements are made using solid water, it is important to verify the phantom composition and that the water equivalence extends to the low kilovoltage energy range of EB sources. The following materials have been recommended in two review articles for energies down to 50 kV:A150, Gammex Solid Water™/RMI-457/WT1, MS11, PAGAT, Plastic Water® DT, Plastic Water LR, PTW RW1 and Virtual Water. Unsuitable materials include: Perspex/polymethyl methacrylate (PMMA), Plastic Water, Polystyrene, PRESAGE, RW3 11,39 (the reader is advised to refer to the original sources for details of composition and manufacturer). However, some materials such as PMMA may be suitable for backscatter but not depth dose measurement. Local verification of water equivalence is advised for specific EB units.
A recent review by Sander5 describes reference dosimetry for brachytherapy in more detail. Direct primary standards are being developed for EB sources by National Institute of Standards and Technology/University of Wisconsin Accredited Dosimetry Calibration Laboratory (NIST/UWADCL) in the USA40 and a German/Czech collaboration in Europe. There is also a desire to derive new codes of practice based on direct dose-to-water calibration, with consistent quantities and a clear transition from current methods. The overall aim remains to have a total uncertainty in delivered dose of <10% (k = 2), although this may be difficult to achieve.5
Dosimetry audit
Interdepartmental audit has been a foundation of UK radiotherapy for over 20 years.41 Several errors in calibration have been discovered through this process, but recent regional audit rounds have shown increasingly close agreement for standard treatments.42 This has led to greater confidence in the safe, consistent and accurate delivery of standard radiotherapy regardless of geographical location or level of experience. The focus of national audits has now shifted to ensuring the safe introduction of complex or unusual techniques, such as volumetric modulated arc therapy43 and IORT,44 or end-to-end system audits such as for cervix HDR treatment.45 In the IORT audit, a single centre visited all clinical INTRABEAM units in the UK in 2013. Measured output compared with the prescribed system dose was −3.2% ± 2.7%, which was considered acceptable compared with a tolerance level of 5% and action level of 10%. Tolerances for agreement are higher than conventional superficial units given the steep dose gradients involved and sensitivity to positional uncertainty.11 The systematic offset of mean agreement may reflect the lack of direct UK primary standard calibration for this unit, as described above. The audit also found that dose anisotropy around the source and spherical applicator was 11% ± 4%, which had not been previously reported for this unit. Finally, depth dose curves were verified using radiochromic film. An average three dimensional gamma index pass rate of 97% (range, 88–100%) was found using a gradient-appropriate threshold of 7%/0.5 mm and hybrid local/global normalization.
Routine quality assurance
Many EB systems are provided with internal tests to verify key parameters such as output and isotropy or uniformity before each treatment. Some also use calibration data provided by the manufacturer. However, independent and traceable verification of dosimetry is a core philosophy of clinical medical physics.46 This includes both commissioning a new system or source, and routine QA checks on existing equipment. UK guidance on radiotherapy QA is currently being updated, but the previous report47 included the following recommendations for kilovoltage units:
• daily checks on output constancy (tolerance 5%), interlocks and mechanical fixtures
• monthly checks on output (3%), timer or monitor chamber accuracy (2%) and half-value layer (HVL) constancy (10%)
• annual checks on field size (2 mm), uniformity (5%), HVL (10%) and applicator factors (3%).
The American Association of Physicists in Medicine (AAPM) recommendations for EB regulation48 include spot checks before each treatment session of output (3%), dose distribution (3% from baseline), position (1 mm) and inspection of all components. They also require each EB source calibration to be validated in terms of output (2% of expected), timer accuracy, relative dose distribution (5%) and positional accuracy (1 mm). However, dose gradients can be up to 10% per millimetre, so positional uncertainties often dominate measurement accuracy. Therefore, tolerances that are appropriate for fixed geometry collimated units may be too low for point source-like units. Output constancy may also have a lower tolerance than absolute output measurement, representing what is achievable in a fixed geometry compared with baselines, rather than the overall accuracy of dose calibration.
Modern approaches to QA should include prospective risk-based evaluation, taking into account the clinical use of a particular device in a particular centre.49 Suggested QA programmes have been published for the INTRABEAM11,50 and Xoft devices.51 These have been incorporated into Table 3 to give a suggested program for all EB devices. It should be emphasized that this includes the minimum suggested frequencies and tolerances. If a centre feels that a tighter tolerance would be achievable and useful to highlight drifts in performance, then local values should be determined and used to implement preventative maintenance. Checks on interlocks and safety features should reflect the areas of greatest risk for the local implementation. For example, in breast IORT the greatest risks may be the surgical set-up and manual data entry, so resources could be better directed to staff training and double checks of dose calculation, rather than exhaustive repeats of other tests.
Table 3.
Quality assurance tests, with suggested minimum frequencies and tolerancesa
| Frequency | Test | Source typeb | Tolerance | Suitable detectors |
|---|---|---|---|---|
| Before each treatment session | Visual checks, interlocks and connectivity | All | Functional | N/A |
| Output constancy (fixed geometry) | All | 3% from baseline | Ion chamber, well chamber, manufacturer-provided or other constancy device | |
| Isotropy constancy | Steered | 5% | Manufacturer jig (diodes) | |
| Source positioning | Stepped | 1 mm | Manufacturer jig (film) | |
| Monthly | Other internal tests | All | Functional | As provided by manufacturer or equivalent |
| Annual (or following service or repair) | Output (absolute) | All | 10% | Ion chamber |
| Isotropy | Steered or stepped | 10% from baseline | Thermoluminescent dosemeter, film, ion chamber | |
| Field size | Collimated | 1 mm | Film | |
| Uniformity | Collimated | 10% | Film, diode array | |
| Applicator factors | Collimated | 3% from baseline | Ion chamber | |
| Beam quality or depth dose | All | 10% or 1 mm | Film, ion chamber, solid state detector |
N/A, not applicable.
These are based on Eaton,11 the Institute of Physics and Engineering in Medicine,47 the American Association of Physicists in Medicine,48 Schneider et al50 and Hiatt et al51 along with the author's own experience. Local frequencies and tolerances may be adjusted to reflect the clinical use and achievable accuracy of the specific system.
Steered units include INTRABEAM® (Carl Zeiss Surgical, Oberkochen, Germany); stepped units include Xoft® (iCAD Inc., Nashua, NH); and collimated units are those with cone-type applicators to restrict the beam angle.
Treatment planning
Most EB systems calculate the treatment time based on standard data tables for fixed geometries. However, using the TG-43 formalism allows calculation of Xoft doses for variable dwell positions and with commercial treatment planning systems. For example, outlines can be drawn on CT scans of patients with the balloon catheter inserted to retrospectively calculate doses to different organs in the breast.52 It should be noted, however, that the TG-43 formalism assumes a full scatter volume of water, so material inhomogeneities are not taken into account in this type of analysis. Monte Carlo modelling of breast IORT for both INTRABEAM and Xoft devices showed a reduction in dose to the skin of 20–60%, but increase in dose to the bone of two to five times, although this was not always clinically significant.53–55 Surface doses are reduced by lack of backscatter from underlying tissue, which is not always accounted for in manual calculations.56 This effect can also be caused by barium impregnation in balloon catheters.57 White et al58 compared TG-43-based calculation of the Xoft source in the breast to the model-based dosimetry recommended recently by TG-186.59 They too found reduction in dose to the skin, but only of 10–15%, mainly because of lack of backscatter, and increased dose to ribs of up to three times, especially when reported as dose to medium.
For IORT, it has been argued that patient-specific treatment planning is not useful, because acquiring a CT scan in theatre is impractical, variations between patients' anatomy close to the source are likely to be minimal and the target volume is localized by direct visualization.11 For kyphoplasty, however, Monte Carlo simulation was used to characterize dose to the bone when developing the technique,60 and the close proximity of critical organs makes individual calculation potentially useful. In addition, modulation of energy and dwell times to give intensity-modulated brachytherapy is only practical with rapid and accurate dose calculation for individual patients. Likewise, CT simulation is not required in situations where the target and normal tissues may be directly visualized but is useful when the exact proximity of underlying structures is needed. Further research would be useful into how best to quantify tissue composition, and how much to account for inhomogeneities close to EB sources.
RADIATION PROTECTION AND STAFFING REQUIREMENTS
Regulations and safety
Radiation safety in the UK is governed by two main pieces of legislation: “The Ionising Radiations Regulations 1999”61 for protection of staff and members of the public, and “Ionising Radiation Medical Exposure Regulations 2000” (IRMER2000)62 for protection of the patient. These are derived from the European Union directives 96/29 and 97/43, so similar principles apply across Europe. Every employer using ionizing radiation must appoint a radiation protection adviser (referred to in directive 96/29 as the qualified radiation expert). They should be consulted regarding controlled area definition and working instructions; prior risk assessment; critical examination and acceptance of new equipment; regular checking and calibration; dose monitoring and training. Local rules for safe use of the radiation technique must be drawn up and compliance supervised by the radiation protection supervisor, usually a local staff member. Prior authorization (from the Health and Safety Executive in the UK) is required for sites not already using radiation. Under IRMER2000, every exposure must be justified by a defined practitioner and delivered by defined operators. A medical physics expert (MPE) must also be closely involved in all radiotherapeutic practices. In the USA, EB devices are not currently covered by Nuclear Regulatory Commission regulations, and requirements may vary from state to state. However, AAPM task group 152 has provided guidelines, which require the services of an authorized medical physicist in similar areas to those described above.48
These points may be well known to established radiotherapy users, but EB devices have the potential to be used in settings where staff and/or management are unfamiliar with the hazards of therapy-level sources. For example, theatre staff may use mobile image intensifiers, but both the dose and dose rate of EB devices are much greater, up to 1 Gy per minute for some unshielded sources. If an external supplier provides the service, then responsibilities must be clearly spelt out for each employer, and the co-operation between them should be fully documented.
Radiation protection for the INTRABEAM device has been recently reviewed,63 following an earlier investigation of breast IORT.64 Prospective dose surveys should be performed in every operating theatre to be used for IORT, although reference data for diagnostic kilovoltage units may be used to estimate shielding by common materials. Environmental surveys should be repeated at regular intervals to confirm the sufficiency of protection measures for clinical patients. It may be prudent to designate the whole theatre as a controlled area, including any side rooms if they are difficult to restrict access to separately. Doors should be clearly signed and locked to prevent unauthorized access. During irradiation, all non-essential staff should leave, with just the two operators and an anaesthetist remaining to monitor the patient. These staff can be given personal monitors, although negligible doses were recorded in clinical practice when using a lead screen and patient surface shielding.64 At a typical operator position of 2 m from the source, dose rates of 12–15 µSv per hour have been reported,9 but <5 µSv per hour should be achievable.64 Shielding sheets placed on the patient surface over the treatment area will reduce external dose rates by 95–99% and may be useful to keep doses as low as reasonably practicable. Lead screens >0.3 mm in thickness will reduce transmitted radiation to effectively zero, however, scattered dose around the screen can still give small measured dose rates. Lead aprons could also be used but are cumbersome to wear and do not provide whole-body protection. Either way, no permanent modification of the theatre should be necessary.
Schneider et al65 compared environmental dose rates with the INTRABEAM kyphoplasty technique and found dose rates were similar to fluoroscopy also used during this procedure. Therefore, the same operating theatres can be used, so long as specific risk assessments are still performed, since the operators stand much closer during fluoroscopy than during IORT irradiation. Mobit et al66 recently investigated radiation protection for the Xoft device, reporting similar conclusions to those of the above.
Staffing models
Under IRMER2000, an MPE must be closely involved in every radiotherapeutic practice.55 They must be contracted full time to the radiation employer and should be contactable throughout EB treatments. Two trained operators are required to operate the equipment and to provide independent verification of treatment parameters. These may be clinical scientists, therapeutic radiographers or clinical technologists. For IORT, the surgeon should be defined as an additional operator. Where the treatment is delivered on the same site as an established radiotherapy department, a clinical scientist need only be available in case of problems. They should also perform regular QA of the system. In one large acute hospital, a team of radiographers have delivered many treatments using INTRABEAM following this model.67 They also established the role of “pathway radiographer” to co-ordinate the patient pathway and liaise between the different staff groups. This role has been performed in another centre by a nurse specialist.68
Alternatively, where the treatment is delivered on a different site, such as a district hospital supported by a local cancer centre, it is more practical for the clinical scientist to act as one of the operators. The resource implications of having two members of staff out of the main department for up to 2–3 h should be considered in any business model. However, for breast IORT with INTRABEAM, as part of the ongoing National Institute of Clinical Excellence evaluation, the total provider cost per treatment was found to be very similar to 15 fractions of EBRT.69 Further cost-effectiveness studies are needed to compare other EB treatments with conventional approaches. A third model of service provision is to procure the complete service from a private practitioner. This reduces the capital outlay risk and potentially allows greater flexibility in patient numbers. Breast IORT with the Xoft device is currently delivered to a number of private hospitals in the UK following this model.
In the USA, AAPM recommend that the qualified medical physicist be physically present for every EB treatment. They should also be responsible for evaluation of output, establishing and reviewing QA checks.48 The UK model allows greater resource flexibility but relies on other staff groups who are confident working in remote multidisciplinary environments, for example, those who also deliver prostate brachytherapy in an operating theatre. Further publications describing implementation in other countries would be useful.
CLINICAL APPLICATIONS
Skin
Radiotherapy is commonly used to treat both malignant and benign skin conditions, but a wide variety of doses, fractionations and techniques abound.70,71 In a recent survey of non-melanoma skin cancer, 81% of respondents used kilovoltage therapy.70 A retrospective analysis of 1715 lesions treated over 10 years (including the past 2 years with the SRT-100 unit) found 5-year recurrence rates of 4.2% for basal cell carcinoma (BCC) and 5.8% for squamous cell carcinoma (SCC) tumours.72 Initial experience of using the Xoft device for these patients has also been reported.73 37 patients were treated using a dose of 40 Gy in 8 fractions, prescribed to a depth of 3–7 mm. After a median follow-up of 4 months, no severe toxicities were reported along with good-to-excellent cosmesis for all patients. Updated results for 122 patients with 171 lesions were similarly positive, although the length of follow-up was still short compared with other studies.15 After a mean follow-up of 10 months, no recurrences or severe toxicities were reported, and 98% good-to-excellent cosmesis. At 1 year or more of follow-up, late adverse events such as hypopigmentation were reported in 10/46 (22%) lesions.
In spite of these initial findings, in 2013, the American Academy of Dermatology issued a position statement on EB.74 They stated that surgery remains the primary treatment intervention for BCC and SCC, but that EB may be considered as an option where surgery is contraindicated or refused. They suggested that further research and data on long-term outcomes of EB is needed and that devices should not be operated by dermatologists. Finally, they expressed concern that aggressive marketing of these devices in the USA has led to inaccurate reimbursement coding on the basis of financial gain alone. Further investigation is clearly needed into the efficacy of EB for skin lesions, ideally high-level evidence in the form of randomized clinical trials.
Breast
Breast cancer is the most common cancer in females worldwide. Extensive screening and self-awareness campaigns have led to most tumours being diagnosed at an early stage, when they can be treated by surgical excision, sentinel node biopsy and EBRT. This combination is well established and highly successful. However, there has been much recent research into the potential for reduction of the irradiated area, to the partial breast or even just to the tumour bed.75,76 IORT, once confined to advanced or recurrent disease, has been investigated as a minimally invasive method for delivering a single high dose to the tumour bed at the time of surgery.
A recent review in BJR describes the use of IORT for breast cancer.77 This is a form of conformal radiotherapy, only in this case the patient is conformed to the dose, rather than the dose to the patient. Both INTRABEAM and Xoft devices have been used to deliver breast IORT with spherical applicators and a similar geometry.16,78 The main differences are in the design of applicator: either solid plastic (INTRABEAM) or water-filled balloon catheter (Xoft); and the higher dose rate and average energy of the Xoft device. However, the uncertainties in this technique are likely to be more dependent on the device placement than on equipment type. IORT relies heavily on the skill of the surgeon in positioning the applicator in the tumour cavity, and the role of the oncologist could be seen as merely authorization of the prescription. However, a multidisciplinary approach is essential when combining these two disciplines in one treatment session.
In addition, the clinical evidence for EB in the breast is limited to various single centre reports, multicentre observational studies79 and one randomized controlled trial.80 This trial has itself come under heavy criticism for the lack of reported follow-up data, confounding factors for differences in mortality, and statistical interpretation.81–83 One conclusion that has been reached at this stage is that IORT should only take place immediately after surgical excision, rather than as a second procedure a few weeks later, to avoid the risk of geographical miss. However, doubt remains on the true difference in local recurrence risk at 5 years, and whether absolute recurrence rates are low enough for IORT to be offered to selected patients as an alternative to EBRT, if these uncertainties are clearly explained.84
When the lesion is close to the chest wall, internal shielding may be used to reduce dose to the ribs, lung and heart.16 These shields are typically made from the same tungsten–rubber material as the external shielding sheets for the patient surface, but in small circular shapes that can be affixed to the applicator surface. For INTRABEAM, shields with effective lead thickness of 0.1 mm reduced the dose rate by >95%.64,85 However, internal shields should be used with caution, as parts of the target volume may also be occluded, and they are rarely used in the UK.
In many centres, a dose of 6 Gy is prescribed at 1 cm from the applicator surface, since the dose at this point may be more easily verified by measurement, and this distance is consistent with other brachytherapy devices used for partial breast irradiation such as MammoSite. All UK centres have followed this approach for consistency nationwide. In some centres in other countries, 20 Gy is prescribed to the applicator surface, but this is not equivalent. The different sizes of applicator will harden the beam by different amounts, therefore, the dose fall off will be less steep with larger applicators. Prescription at depth also reduces the range of doses across different applicator sizes, and therefore is recommended to give the most accurate and consistent dose reporting.11,53
Other treatment sites
Current and future applications of IORT have been reviewed by Debenham et al,86 including the INTRABEAM and Xoft devices. They include head and neck, breast, sarcoma, colorectal, gastric, pancreas, anal, gynaecological, genitourinary, thoracic and palliative applications, such as kyphoplasty. Outcomes are improved when complete resection is possible, and typical maximum doses of 10–20 Gy seem to avoid major side effects. Further clinical trials are recommended, however, and proper training and expertise are essential. Rectal cancer treatment with the Papillon unit is being investigated by the CONTEM observational studies,17 and the proposed OPERA Phase III randomized trial.
FUTURE DIRECTIONS
A recent review of kilovoltage dosimetry stated that this modality continues to provide a useful treatment option in many departments, but that further research is needed into treatment planning systems, three-dimensional dosemeters, Monte Carlo simulation and direct dose-to-water calibration.39 Radiobiology of kilovoltage X-rays is also an area of significant uncertainty.
In the past decade, EB devices have joined conventional superficial units as an attractive option for the treatment of skin lesions and have also found wider applications in intracavitary and interstitial sites.
5 years ago, it was argued that EB would ultimately replace 192Ir HDR treatment, primarily because of the simplified operating conditions, and the ability to produce more heterogeneous dose distributions.87,88 However, many physicists and oncologists remain sceptical as to the applications of this new approach, and regulators are wary of its use by unqualified personnel. Highly localized dose deposition will need to be further correlated with clinical outcomes through appropriate trials with adequate follow up. MPEs and consultant radiation oncologists will need to be closely involved in all therapeutic applications. Given these safeguards, however, the practical convenience of EB strongly suggests that it will become an established option for selected patients, not only in radiotherapy departments but also in a wide range of operating theatres and clinics around the world.
REFERENCES
- 1.Keevil SF. Physics and medicine: a historical perspective. Lancet 2012; 379: 1517–24. doi: 10.1016/S0140-6736(11)60282-1 [DOI] [PubMed] [Google Scholar]
- 2.Williams JR, Thwaites DI, eds. Radiotherapy physics in practice. Oxford, UK: Oxford University Press; 2000. [Google Scholar]
- 3.Palmer AL. BJR brachytherapy dosimetry special feature. Br J Radiol 2014; 87: 20140506. doi: 10.1259/bjr.20140506 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kertzscher G, Rosenfeld A, Beddar S, Tanderup K, Cygler JE. In vivo dosimetry: trends and prospects for brachytherapy. Br J Radiol 2014; 87: 20140206. doi: 10.1259/bjr.20140206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sander T. Air kerma and absorbed dose standards for reference dosimetry in brachytherapy. Br J Radiol 2014; 87: 20140176. doi: 10.1259/bjr.20140176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Papagiannis P, Pantelis E, Karaiskos P. Current state of the art brachytherapy treatment planning dosimetry algorithms. Br J Radiol 2014; 87: 20140163. doi: 10.1259/bjr.20140163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lee C. Recent developments and best practice in brachytherapy treatment planning. Br J Radiol 2014; 87: 20140146. doi: 10.1259/bjr.20140146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Palmer AL, Bradley DA, Nisbet A. Dosimetric audit in brachytherapy. Br J Radiol 2014; 87: 20140105. doi: 10.1259/bjr.20140105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Park CC, Yom SS, Podgorsak MB, Harris E, Price RA, Jr, Bevan A, et al. ; Electronic Brachytherapy Working Group. American Society for Therapeutic Radiology and Oncology (ASTRO) emerging technology committee report on electronic brachytherapy. Int J Radiat Oncol Biol Phys 2010; 76: 963–72. doi: 10.1016/j.ijrobp.2009.10.068 [DOI] [PubMed] [Google Scholar]
- 10.Brenner DJ, Leu CS, Beatty JF, Shefer RE. Clinical relative biological effectiveness of low-energy x-rays emitted by miniature x-ray devices. Phys Med Biol 1999; 44: 323–34. doi: 10.1088/0031-9155/44/2/002 [DOI] [PubMed] [Google Scholar]
- 11.Eaton DJ. Quality assurance and independent dosimetry for an intraoperative x-ray device. Med Phys 2012; 39: 6908–20. doi: 10.1118/1.4761865 [DOI] [PubMed] [Google Scholar]
- 12.Schneider F, Clausen S, Thölking J, Wenz F, Abo-Madyan Y. A novel approach for superficial intraoperative radiotherapy (IORT) using a 50 kV X-ray source: a technical and case report. J Appl Clin Med Phys 2014; 15: 4502. doi: 10.1120/jacmp.v15i1.4502 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Liu D, Poon E, Bazalova M, Reniers B, Evans M, Rusch T, et al. Spectroscopic characterization of a novel electronic brachytherapy system. Phys Med Biol 2008; 53: 61–75. doi: 10.1088/0031-9155/53/1/004 [DOI] [PubMed] [Google Scholar]
- 14.Richardson S, Garcia-Ramirez J, Lu W, Myerson RJ, Parikh P. Design and dosimetric characteristics of a new endocavitary contact radiotherapy system using an electronic brachytherapy source. Med Phys 2012; 39: 6838–46. doi: 10.1118/1.4757915 [DOI] [PubMed] [Google Scholar]
- 15.Bhatnagar A. Nonmelanoma skin cancer treated with electronic brachytherapy: results at 1 year. Brachytherapy 2013; 12: 134–40. doi: 10.1016/j.brachy.2012.08.003 [DOI] [PubMed] [Google Scholar]
- 16.Dickler A, Ivanov O, Francescatti D. Intraoperative radiation therapy in the treatment of early-stage breast cancer utilizing xoft axxent electronic brachytherapy. World J Surg Oncol 2009; 7: 24. doi: 10.1186/1477-7819-7-24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gerard J-P, Myint AS, Croce O, Lindegaard J, Jensen A, Myerson R, et al. Renaissance of contact x-ray therapy for treating rectal cancer. Expert Rev Med Devices 2011; 8: 483–92. doi: 10.1586/erd.11.28 [DOI] [PubMed] [Google Scholar]
- 18.Garcia-Martinez T, Chan J-P, Perez-Calatayud J, Ballester F. Dosimetric characteristics of a new unit for electronic skin brachytherapy. J Contemp Brachytherapy 2014; 6: 45–53. doi: 10.5114/jcb.2014.40770 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Schneider F, Fuchs H, Lorenz F, Steil V, Ziglio F, Kraus-Tiefenbacher U, et al. A novel device for intravaginal electronic brachytherapy. Int J Radiat Oncol Biol Phys 2009; 74: 1298–305. doi: 10.1016/j.ijrobp.2009.01.082 [DOI] [PubMed] [Google Scholar]
- 20.Wenz F, Schneider F, Neumaier C, Kraus-Tiefenbacher U, Reis T, Schmidt R, et al. Kypho-IORT—a novel approach of intraoperative radiotherapy during kyphoplasty for vertebral metastases. Radiat Oncol 2010; 5: 11. doi: 10.1186/1748-717X-5-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Reis T, Schneider F, Welzel G, Schmidt R, Bludau F, Obertacke U, et al. Intraoperative radiotherapy during kyphoplasty for vertebral metastases (Kypho-IORT): first clinical results. Tumori 2012; 98: 434–40. doi: 10.1700/1146.12636 [DOI] [PubMed] [Google Scholar]
- 22.Reis T, Sperk E, Abo-Madyan Y, Ehmann M, Bludau F, Wenz F. Other applications of INTRABEAM. In: Keshtgar M, Pigott K, Wenz F, eds. Targeted intraoperative radiotherapy in oncology. Berlin, Germany: Springer-Verlag; 2014. [Google Scholar]
- 23.Rivard MJ, Davis SD, DeWerd LA, Rusch TW, Axelrod S. Calculated and measured brachytherapy dosimetry parameters in water for the Xoft Axxent X-Ray Source: an electronic brachytherapy source. Med Phys 2006; 33: 4020–32. doi: 10.1118/1.2357021 [DOI] [PubMed] [Google Scholar]
- 24.Mehta VK, Algan O, Griem KL, Dickler A, Haile K, Wazer DE, et al. Experience with an electronic brachytherapy technique for intracavitary accelerated partial breast irradiation. Am J Clin Oncol 2010; 33: 327–35. doi: 10.1097/COC.0b013e3181d79d9e [DOI] [PubMed] [Google Scholar]
- 25.Dickler A, Kirk MC, Seif N, Griem K, Dowlatshahi K, Fancescatti D, et al. A dosimetric comparison of MammoSite high-dose-rate brachytherapy and Xoft Axxent electronic brachytherapy. Brachytherapy 2007; 6: 164–8. doi: 10.1016/j.brachy.2007.01.005 [DOI] [PubMed] [Google Scholar]
- 26.Smitt MC, Kirby R. Dose-volume characteristics of a 50-kV electronic brachytherapy source for intracavitary accelerated partial breast irradiation. Brachytherapy 2007; 6: 207–11. doi: 10.1016/j.brachy.2007.03.002 [DOI] [PubMed] [Google Scholar]
- 27.Dickler A, Kirk MC, Coon A, Bernard D, Zusag T, Rotmensch J, et al. A dosimetric comparison of Xoft Axxent Electronic Brachytherapy and iridium-192 high-dose-rate brachytherapy in the treatment of endometrial cancer. Brachytherapy 2008; 7: 351–4. doi: 10.1016/j.brachy.2008.05.003 [DOI] [PubMed] [Google Scholar]
- 28.Rong Y, Welsh JS. Surface applicator calibration and commissioning of an electronic brachytherapy system for nonmelanoma skin cancer treatment. Med Phys 2010; 37: 5509–17. doi: 10.1118/1.3489379 [DOI] [PubMed] [Google Scholar]
- 29.Croce O, Hachem S, Franchisseur E, Marcie S, Gerard J-P, Bordy J-M. Contact radiotherapy using a 50kV X-ray system: evaluation of relative dose distribution with the Monte Carlo code PENELOPE and comparison with measurements. Radiat Phys Chem 2012; 81: 609–17. doi: 10.1016/j.radphyschem.2012.01.033 [DOI] [Google Scholar]
- 30.Ebert MA, Carruthers B, Lanzon PJ, Haworth A, Clarke J, Caswell NM, et al. Dosimetry of a low-kV intra-operative X-ray source using basic analytical beam models. Australas Phys Eng Sci Med 2002; 25: 119–23. doi: 10.1007/BF03178772 [DOI] [PubMed] [Google Scholar]
- 31.Rivard MJ, Coursey BM, DeWerd LA, Hanson WF, Huq MS, Ibbott GS, et al. Update of AAPM Task Group No. 43 Report: a revised AAPM protocol for brachytherapy dose calculations. Med Phys 2004; 31: 633–74. doi: 10.1118/1.1646040 [DOI] [PubMed] [Google Scholar]
- 32.Nath R, Anderson LL, Luxton G, Weaver KA, Williamson JF, Meigooni AS. Dosimetry of interstitial brachytherapy sources: recommendations of the AAPM radiation-therapy committee Task Group No. 43. American Association of Physicist in Medicine. Med Phys 1995; 22: 209–34. doi: 10.1118/1.597458 [DOI] [PubMed] [Google Scholar]
- 33.Eaton DJ. Highly cited papers in Medical Physics. Med Phys 2014; 41: 080401. doi: 10.1118/1.4887822 [DOI] [Google Scholar]
- 34.Safigholi H, Faghihi R, Jashni SK, Meigooni AS. Characteristics of miniature electronic brachytherapy x-ray sources based on TG-43U1 formalism using Monte Carlo simulation techniques. Med Phys 2012; 39: 1971–9. doi: 10.1118/1.3693046 [DOI] [PubMed] [Google Scholar]
- 35.Klevenhagen SC, Auckett RJ, Harrison RM, Moretti C, Nahum AE, Rosser KE. The IPEMB code for practice for the determination of absorbed dose for x-rays below 300 kV generating potential (0.035 mm Al–4 mm Cu HVL; 10–300 kV generating potential). Institution of Physics and Engineering in Medicine and Biology. Phys Med Biol 1996; 41: 2605–25. doi: 10.1088/0031-9155/41/12/002 [DOI] [PubMed] [Google Scholar]
- 36.Aukett RJ, Burns JE, Greener AG, Harrison RM, Moretti C, Nahum AE, et al. ; IPEM Working Party. Addendum to the IPEMB code for practice for the determination of absorbed dose for x-rays below 300 kV generating potential (0.035 mm Al-4 mm Cu HVL). Phys Med Biol 2005; 50: 2739–48. doi: 10.1088/0031-9155/50/12/001 [DOI] [PubMed] [Google Scholar]
- 37.Eaton DJ, Duck S. Dosimetry measurements with an intra-operative x-ray device. Phys Med Biol 2010; 55: N359–69. doi: 10.1088/0031-9155/55/12/N02 [DOI] [PubMed] [Google Scholar]
- 38.Carver A, Gately A, Clements R, Nahum A. Monte Carlo dosimetry for the Papillon P50 contact radiotherapy and IORT device. Radiother Oncol 2013; 109: 367–9. doi: 10.1016/j.radonc.2013.10.032 [DOI] [PubMed] [Google Scholar]
- 39.Hill R, Healy B, Holloway L, Kuncic Z, Thwaites D, Baldock C. Advances in kilovoltage x-ray beam dosimetry. Phys Med Biol 2014; 59: R183–231. doi: 10.1088/0031-9155/59/6/R183 [DOI] [PubMed] [Google Scholar]
- 40.Seltzer SM, Brien MO, Mitch MG. New national air-kerma standard for low-energy electronic brachytherapy sources. J Res Nat Inst Stand Tech 2014; 119: 554–74. doi: 10.6028/jres.119.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Eaton DJ. Inter-departmental dosimetry audits—the UK experience. J Med Phys 2014; 39: 287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Palmer A, Mzenda B, Kearton J, Wills R. Analysis of regional radiotherapy dosimetry audit data and recommendations for future audits. Br J Radiol 2011; 84: 733–42. doi: 10.1259/bjr/18691638 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Clark CH, Hussein M, Tsang Y, Thomas R, Wilkinson D, Bass G, et al. A multi-institutional dosimetry audit of rotational intensity-modulated radiotherapy. Radiother Oncol 2014; 113: 272–8. doi: 10.1016/j.radonc.2014.11.015 [DOI] [PubMed] [Google Scholar]
- 44.Eaton DJ, Earner B, Faulkner P, Dancer N. A national dosimetry audit of intraoperative radiotherapy. Br J Radiol 2013; 86: 20130447. doi: 10.1259/bjr.20130447 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Palmer AL, Diez P, Gandon L, Wynn-Jones A, Bownes P, Lee C, et al. A multicentre “end to end” dosimetry audit for cervix HDR brachytherapy treatment. Radiother Oncol 2015; 114: 264–71. doi: 10.1016/j.radonc.2014.12.006 [DOI] [PubMed] [Google Scholar]
- 46.Institute of Physics and Engineering in Medicine. Medical and dental guidance notes. York, UK: IPEM; 2002. [Google Scholar]
- 47.Institute of Physics and Engineering in Medicine. Report 81: physics aspects of quality control in radiotherapy. York, UK: IPEM; 1999. [Google Scholar]
- 48.American Association of Physicists in Medicine. AAPM report 152: the 2007 AAPM response to the CRCPD request for recommendations for the CRCPD’s model regulations for electronic brachytherapy. College Park, MD: AAPM; 2009. [Google Scholar]
- 49.Huq MS, Fraass BA, Dunscombe PB, Gibbons JP, Jr, Ibbott GS, Medin PM, et al. A method for evaluating quality assurance needs in radiation therapy. Int J Radiat Oncol Biol Phys 2008; 71: S170–3. doi: 10.1016/j.ijrobp.2007.06.081 [DOI] [PubMed] [Google Scholar]
- 50.Schneider F, Clausen S, Eaton DJ. Quality assurance and commissioning In: Keshtgar M, Pigott K, Wenz F, eds. Targeted intraoperative radiotherapy in oncology. Berlin, Germany: Springer-Verlag; 2014. [Google Scholar]
- 51.Hiatt J, Cardarelli G, Hepel J, Wazer D, Sternick E. A commissioning procedure for breast intracavitary electronic brachytherapy systems. J Appl Clin Med Phys 2008; 9: 2775. doi: 10.1120/jacmp.v9i3.2775 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Dooley WC, Wurzer JC, Megahy M, Schreiber G, Roy T, Proulx G, et al. Electronic brachytherapy as adjuvant therapy for early stage breast cancer: a retrospective analysis. Onco Targets Ther 2011; 4: 13–20. doi: 10.2147/OTT.S15297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ebert MA, Carruthers B. Dosimetric characteristics of a low-kV intra-operative x-ray source: implications for use in a clinical trial for treatment of low-risk breast cancer. Med Phys 2003; 30: 2424–31. doi: 10.1118/1.1595611 [DOI] [PubMed] [Google Scholar]
- 54.Shi C, Guo B, Cheng CY, Eng T, Papanikolaou N. Applications of tissue heterogeneity corrections and biologically effective dose volume histograms in assessing the doses for accelerated partial breast irradiation using an electronic brachytherapy source. Phys Med Biol 2010; 55: 5283–97. doi: 10.1088/0031-9155/55/18/003 [DOI] [PubMed] [Google Scholar]
- 55.Mille MM, Xu G, Rivard MJ. Comparison of organ doses for patients undergoing balloon brachytherapy of the breast with HDR 192Ir or electronic sources using Monte Carlo simulations in a heterogeneous human phantom. Med Phys 2010; 37: 662–71. doi: 10.1118/1.3292292 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Eaton DJ, Doolan P. Review of backscatter measurement in kilovoltage radiotherapy using novel detectors and reduction from lack of underlying scattering material. J Appl Clin Med Phys 2013; 14: 4358. doi: 10.1120/jacmp.v14i6.4358 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Segala JJ, Cardarelli GA, Jiatt JR, Curran BH, Sternick ES. Interface dosimetry for electronic brachytherapy breast balloon applicators. J Appl Clin Med Phys 2011; 12: 3221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.White SA, Landry G, Fonseca GP, Holt R, Rusch T, Beaulieu L, et al. Comparison of TG-43 and TG-186 in breast irradiation using a low energy electronic brachytherapy source. Med Phys 2014; 41: 061701. doi: 10.1118/1.4873319 [DOI] [PubMed] [Google Scholar]
- 59.Beaulieu L, Carlsson Tedgren A, Carrier JF, Davis SD, Mourtada F, Rivard MJ, et al. Report of the Task Group 186 on model-based dose calculation methods in brachytherapy beyond the TG-43 formalism: current status and recommendations for clinical implementation. Med Phys 2012; 39: 6208–36. doi: 10.1118/1.4747264 [DOI] [PubMed] [Google Scholar]
- 60.Schneider F, Greineck F, Clausen S, Mai S, Obertacke U, Reis T, et al. Development of a novel method for intraoperative radiotherapy during kyphoplasty for spinal metasatases (Kypho-IORT). Int J Radiat Oncol Biol Phys 2011; 81: 1114–19. doi: 10.1016/j.ijrobp.2010.07.1985 [DOI] [PubMed] [Google Scholar]
- 61.Her Majesty's Stationary Office. The Ionising Radiations Regulations 1999. Statutory Instrument 3232. London, UK: HMSO; 1999. [Google Scholar]
- 62.Her Majesty's Stationary Office. The Ionising Radiation Medical Exposure Regulations 2000. Statutory Instrument 1059. London, UK: HMSO; 2000. [Google Scholar]
- 63.Eaton DJ, Schneider F. Radiation protection. In: Keshtgar M, Pigott K, Wenz F, eds. Targeted intraoperative radiotherapy in oncology. Berlin, Germany: Springer-Verlag; 2014. [Google Scholar]
- 64.Eaton DJ, Gonzalez R, Duck S, Keshtgar M. Radiation protection for an intraoperative X-ray device. Br J Radiol 2011; 84: 1034–39. doi: 10.1259/bjr/29466902 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Schneider F, Clausen S, Jahnke A, Steil V, Bludau F, Sütterlin M, et al. Radiation protection for an intraoperative X-ray source compared to C-arm fluoroscopy. Z Med Phys 2014; 24: 243–51. doi: 10.1016/j.zemedi.2013.10.006 [DOI] [PubMed] [Google Scholar]
- 66.Mobit PN, Rajaguru P, Brewer M, Baird M, Packinathan S, Yang CC. Radiation safety considerations during intraoperative radiation therapy. Rad Prot Dosimetry Sep 2014. Epub ahead of print. doi: 10.1093/rpd/ncu292 [DOI] [PubMed] [Google Scholar]
- 67.Eaton DJ, Ghaus T, Gonzalez R, Best B, Hourihane C, Mastroianni B, et al. Multidisciplinary intra-operative breast radiotherapy. Clin Oncol (R Coll Radiol) 2009; 21: 255. [Google Scholar]
- 68.Armoogum K, Ackland C, Gardner J. Implementation and experiences of an intraoperative radiotherapy service. J Radiother Pract 2006; 5: 203–10. doi: 10.1017/S146039690600029X [DOI] [Google Scholar]
- 69.Picot J, Copley V, Colquitt JL, Kalita N, Hartwell D, Bryant J. The clinical and cost effectiveness of the INTRABEAM photon radiotherapy system for the adjuvant treatment of early breast cancer. Health Technology Assessment. Available from: https://www.org.uk/guidance/gid-tag353/documents/breast-cancer-intrabeam-radiosurgery-system-assessment-report-consultation2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.McPartlin AJ, Slevin NJ, Sykes AJ, Rembielak A. Radiotherapy treatment of non-melanoma skin cancer: a survey of current UK practice and commentary. Br J Radiol 2014; 87: 20140501. doi: 10.1259/bjr.20140501 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Eaton DJ, Barber E, Ferguson L, Simpson GM, Collis CH. Radiotherapy treatment of keloid scars with a kilovoltage x-ray parallel pair. Radiother Oncol 2012; 102: 421–3. doi: 10.1016/j.radonc.2011.08.002 [DOI] [PubMed] [Google Scholar]
- 72.Cognetta AB, Howard BM, Heaton HP, Stoddard EA, Hong HG, Green WH. Superficial x-ray in the treatment of basal and squamous cell carcinomas: a viable option in select patients. J Am Acad Dermatol 2012; 67: 1235–41. doi: 10.1016/j.jaad.2012.06.001 [DOI] [PubMed] [Google Scholar]
- 73.Bhatnagar A, Loper A. The initial experience of electronic brachytherapy for the treatment of non-melanoma skin cancer. Radiat Oncol 2010; 5: 87. doi: 10.1186/1748-717X-5-87 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.American Academy of Dermatology. Position statement on electronic surface brachytherapy for basal cell carcinoma (BCC) and squamous cell carcinomas (SCC). 2013. [Cited December 2014.] Available from: www.aad.org/Forms/Policies/
- 75.Offerson BV, Overgaard M, Kroman N, Overgaard J. Accelerated partial breast irradiation as part of breast conserving therapy of early breast carcinoma: a systematic review. Radiother Oncol 2009; 90: 1–13. doi: 10.1016/j.radonc.2008.08.005 [DOI] [PubMed] [Google Scholar]
- 76.Stewart AJ, Khan AJ, Devlin PM. Partial breast irradiation: a review of techniques and indications. Br J Radiol 2010; 83: 369–78. doi: 10.1259/bjr/11505970 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Hanna GG, Kirby AM. Intraoperative radiotherapy in early stage breast cancer: potential indications and evidence to date. Br J Radiol 2015; 88: 20140686. doi: 10.1259/bjr.20140686 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Vaidya JS, Baum M, Tobias JS, D’Souza DP, Naidu SV, Morgan S, et al. Targeted intra-operative radiotherapy (Targit): an innovative method of treatment for early breast cancer. Ann Surg Oncol 2001; 12: 1075–80. [DOI] [PubMed] [Google Scholar]
- 79.Beitsch PD, Patel RR, Lorenzetti JD, Wurzer JC, Tucker JC, Laduzinsky SJ, et al. Post-surgical treatment of early-stage breast cancer with electronic brachytherapy: an intersociety, multicenter brachytherapy trial. Onco Targets Ther 2010; 3: 211–18. doi: 10.2147/OTT.S14514 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Vaidya JS, Wenz F, Bulsara M, Tobias JS, Joseph DJ, Keshtgar M, et al. ; TARGIT trialists' group. Risk-adapted targeted intraoperative radiotherapy versus whole-breast radiotherapy for breast cancer: 5-year results for local control and overall survival from the TARGIT-A randomised trial. Lancet 2014; 383: 603–13. doi: 10.1016/S0140-6736(13)61950-9 [DOI] [PubMed] [Google Scholar]
- 81.Haviland JS, A’Hern R, Bentzen SM, Whelan T, Bliss JM. Radiotherapy for breast cancer, the TARGET-A trial. Lancet 2014; 383: 1716–17. doi: 10.1016/S0140-6736(14)60826-6 [DOI] [PubMed] [Google Scholar]
- 82.Yarnold J, Offerson BV, Olivotto I, Poortmans P, Sarin R. Radiotherapy for breast cancer, the TARGET-A trial. Lancet 2014; 383: 1717–18. doi: 10.1016/S0140-6736(14)60828-X [DOI] [PubMed] [Google Scholar]
- 83.Moran MS, Truong PT. Intraoperative accelerated partial breast irradiation: caution still warranted. Int J Radiat Oncol Biol Phys 2014; 89: 496–8. doi: 10.1016/j.ijrobp.2014.01.034 [DOI] [PubMed] [Google Scholar]
- 84.National Institute for Clinical Excellence. Breast cancer (early)–intrabeam radiotherapy system: appraisal consultation document. 2014. [Cited December 2014.] Available from: www.nice.org.uk
- 85.Avanzo M, Rink A, Dassie A, Massarut S, Roncardin M, Borsatti E, et al. In vivo dosimetry with radiochromic films in low-voltage intraoperative radiotherapy of the breast. Med Phys 2012; 39: 2359–68. doi: 10.1118/1.3700175 [DOI] [PubMed] [Google Scholar]
- 86.Debenham BJ, Hu KS, Harrison LB. Present status and future directions of intraoperative radiotherapy. Lancet Oncol 2013; 14: e457–64. doi: 10.1016/S1470-2045(13)70270-5 [DOI] [PubMed] [Google Scholar]
- 87.Holt RW, Thomadsen BR, Orton CG. Point/Counterpoint. Miniature x-ray tubes will ultimately displace Ir-192 as the radiation sources of choice for high dose rate brachytherapy. Med Phys 2008; 35: 815–17. doi: 10.1118/1.2836415 [DOI] [PubMed] [Google Scholar]
- 88.Sternick ES, Todor DA, Orton CG. Point/Counterpoint. Intensity modulated electronic brachytherapy will soon become the brachytherapy treatment of choice for irregularly shaped tumor cavities or those closely bounded by critical structures. Med Phys 2009; 36: 681–3. doi: 10.1118/1.3075768 [DOI] [PubMed] [Google Scholar]