Abstract
Objective:
To compare dynamic contrast-enhanced (DCE) MRI parameters from scans of breast lesions at 1.5 and 3.0 T.
Methods:
11 patients underwent paired MRI examinations in both Philips 1.5 and 3.0 T systems (Best, Netherlands) using a standard clinical fat-suppressed, T1 weighted DCE-MRI protocol, with 70–76 s temporal resolution. Signal intensity vs time curves were fit with an empirical mathematical model to obtain semi-quantitative measures of uptake and washout rates as well as time-to-peak enhancement (TTP). Maximum percent enhancement and signal enhancement ratio (SER) were also measured for each lesion. Percent differences between parameters measured at the two field strengths were compared.
Results:
TTP and SER parameters measured at 1.5 and 3.0 T were similar; with mean absolute differences of 19% and 22%, respectively. Maximum percent signal enhancement was significantly higher at 3 T than at 1.5 T (p = 0.006). Qualitative assessment showed that image quality was significantly higher at 3 T (p = 0.005).
Conclusion:
Our results suggest that TTP and SER are more robust to field strength change than other measured kinetic parameters, and therefore measurements of these parameters can be more easily standardized than measurements of other parameters derived from DCE-MRI. Semi-quantitative measures of overall kinetic curve shape showed higher reproducibility than do discrete classification of kinetic curve early and delayed phases in a majority of the cases studied.
Advances in knowledge:
Qualitative measures of curve shape are not consistent across field strength even when acquisition parameters are standardized. Quantitative measures of overall kinetic curve shape, by contrast, have higher reproducibility.
Dynamic contrast-enhanced MRI (DCE-MRI) of the breast is a valuable tool for the detection, diagnosis and staging of breast cancer as well as for evaluation of treatment response.1–3 The use of contrast agents increases the visibility of lesions in T1 weighted images, and the kinetics of the uptake and washout of contrast media in lesions reflect underlying physiology and provide radiologists with an indicator of the likelihood of malignancy.4 Lesions exhibiting a rapid increase in signal intensity followed by washout resulting in decreased signal intensity are more likely to be malignant. Lesion kinetics are routinely evaluated qualitatively utilizing the classification developed by Kuhl et al,4 and quantitative measures of the signal curves are also diagnostically useful.5
However, signal intensity is highly dependent on scanner properties and acquisition parameters. Relaxation times of tissue, for example, are dependent on field strength.6 This means that tissues at higher field strengths will show greater enhancement as their native T1 will be greater.
Comparison of DCE-MRI parameters measured at 1.5 and 3.0 T are of interest since both field strengths are commonly used for screening and diagnosis of breast cancer. It is important to identify standardized parameters that are independent of field strength so that the same diagnostic criteria can be used regardless of field strength.
Previous studies found significant differences in lesion kinetic parameters across different scanners, acquisition protocols and field strengths.7,8 These previous results suggest that lesion kinetics may be more dependent on acquisition and scanner parameters than on the intrinsic physiology of the lesions. Thus, it would be beneficial to compare the signal enhancement curve intensity and shape between 1.5 and 3.0 T, when all acquisition parameters, including temporal resolution, are kept comparable.
Quantitative analysis—i.e. conversion of signal intensity to concentration and estimation of parameters descriptive of physiology—can potentially allow standardization between different field strengths. However, such calculations require additional calibration scans, can be very sensitive to errors in the estimation of native T1 and B1 maps9 and are not currently part of the clinical standard of care. Therefore in this report, we focus on semi-quantitative parameters associated with signal intensity and its changes during uptake and washout of contrast media.
The purpose of this prospective pilot study was to measure the differences in the semi-quantitative parameters describing the kinetic curves of lesions of patients scanned at both 1.5 and 3.0 T using similar acquisition parameters, with the goal of identifying the diagnostic variables that are reproducible across repeated scans at different field strengths.
METHODS AND MATERIALS
Patient recruitment
11 patients were scanned after informed consent was obtained under an institutional review board-approved Health Insurance Portability and Accountability Act-compliant protocol, over a 16-month recruitment period. In total, 11 biopsy-proven lesions were imaged, 7 benign and 4 malignant. Patients' ages ranged from 34 to 61 years, with a median age of 49 years. Inclusion criteria were: knowledge of a biopsy-proven lesion or an enhancing lesion identified in prior imaging studies, and for volunteers to be able to return for a second scan in a timely fashion. Two volunteers were excluded from the study, one owing to the inability to reidentify any abnormal enhancement in the images, and another because post-biopsy changes affected the images in the initial scan. Volunteers were scanned on both 1.5 T Philips Achieva and 3.0 T-TX Philips Achieva scanners (Philips Healthcare, Best, Netherlands) using 16-channel bilateral breast coils (MammoTrak; Philips Healthcare). The median time between scans was 7 days, and eight patients were scanned within 7 days of the initial scan; with a range of 1–22 days between scans. Pre-menopausal patients were scanned during the second week of the menstrual cycle. When patient or scanner scheduling did not allow a second scan within a few days, these patients were asked to return during the second week of their following cycle (two patients). This was carried out to minimize differences in parenchymal enhancement owing to the menstrual cycle.10 Patients presenting with malignant lesions underwent both MRI examinations before the beginning of any treatment.
MRI acquisition
The DCE portion of the MRI was based on the standard clinical protocol used at the University of Chicago and consisted of a series of T1 weighted, fat-suppressed, three-dimensional gradient echo acquisitions in the axial plane with the following parameters: repetition time, 4.9–5.4 ms; echo time, 2.5–2.7 ms; 10° flip angle at 1.5 T and 12° at 3 T; 0.8 × 0.8 × 1.6 mm spatial resolution (reconstructed to 0.8 mm isotropic); and temporal resolution of 70–76 s. Multiple element radiofrequency transmission and patient-adaptive radiofrequency shimming were utilized on the 3.0-T system.11 Two images were acquired before, and five images after the injection of contrast media, 0.1 mm kg−1 gadodiamide (OmniScan®; GE Healthcare, Waukesha, WI) at an injection rate of 2 ml s−1 followed by a 20-ml saline solution flush at a rate of 2 ml s−1.
Data analysis
Image analysis was performed with in-house software written in MATLAB® (MathWorks®, Natick, MA). Regions of interest (ROIs) were drawn, under radiologists' guidance, around the entire visible volume of the lesion, in multiple slices, on the dynamic series. Voxel values were averaged across the entire ROI at each time point to produce a signal vs time series [S(t)] for each lesion. Then, percent signal enhancement (PSE) was calculated as follows:
| (1) |
where S0 is the baseline signal intensity.
PSE(t) was fit to a three-parameter empirical mathematical model (EMM) that has been shown in previous studies to accurately describe the uptake and washout of contrast media in breast lesions and provide diagnostically useful information.12–14
| (2) |
with the three parameters being upper enhancement limit “A”, uptake rate “α” (min−1) and contrast media washout rate “β” (per minute). The EMM provides a method for quantifying differences in uptake and washout of contrast media by comparing values measured at 1.5 T to those measured at 3 T. The time-to-peak (TTP) enhancement can be derived directly from the EMM parameters:
| (3) |
Signal enhancement ratio (SER) is defined as the ratio of the change in signal intensity soon after contrast injection to the change at a later time:15
| (4) |
where Searly and Sdelayed were measured at t = 1.2 min and at t = 6 min, respectively.
For all the semi-quantitative parameters described above, percent difference between the values of each parameter at 1.5 and 3.0 T was calculated as the difference between the two values divided by the average of the two. Absolute percent difference was also calculated.
In addition, lesion kinetics were classified using a three time-point method,16 according to standard clinical protocol. The PSE at the second post-contrast acquisition was used to evaluate the initial rise and at 6 min to classify the delayed phase. Lesions were then assigned to one of the three categories for each of the two phases (for a total of six categories): slow (<50% PSE), medium (50% < PSE < 100%) or rapid (>100% PSE) rise; and persistent (>10% increase between early and delayed phases), plateau (<10% change between phases) or washout (decrease >10%) for the delayed phase.
Qualitative image assessment
Two radiologists, experienced in reading breast MRI (Observer A, 20 years' experience in MRI mammography and Observer B, 9 years' experience) were asked to review the images for all scans. They rated several aspects of the images on discrete scales. Parenchymal enhancement was rated as none, minimal, mild, moderate or marked. Breast volume was also rated on a qualitative basis and assigned a score based on quartiles: <25%, 25–50%, 51–75% and >75%. Lesion conspicuity, margins and internal lesion sharpness, lymph node conspicuity, as well as technical aspects of the images such as quality of fat saturation and the amount of noise and artefacts present in the images were rated on a five-point scale. Conspicuity of other findings, such as oedema, cysts or clips was also rated. A total image quality score was determined for each scan by aggregating the scores for the parameters listed above, excluding breast volume and parenchymal enhancement.
Statistical analysis
All statistical tests were performed in MATLAB with built-in functions. Statistical significance of differences in parameters measured at the two field strengths was determined with a paired, two-tailed Wilcoxon signed rank test. Levels of statistical significance were adjusted for multiple comparisons using Bonferroni's method; results were significant if the p-value found was lower than α/n = 0.008 (α = 0.05 and n = 6).17 To determine if the differences in the parameters were dependent on the mean value of that parameter, Kendall's tau rank correlation coefficient was calculated between the absolute difference and the mean measured value of each parameter.18 A one-way analysis of variance (ANOVA) was performed on both the signed and absolute percent differences measured with post hoc multiple comparisons. A two-tailed Wilcoxon signed rank test was performed on the total image quality score from the radiologists' evaluation to determine statistical significance of the differences between 1.5 and 3.0 T.
RESULTS
Semi-quantitative image assessment
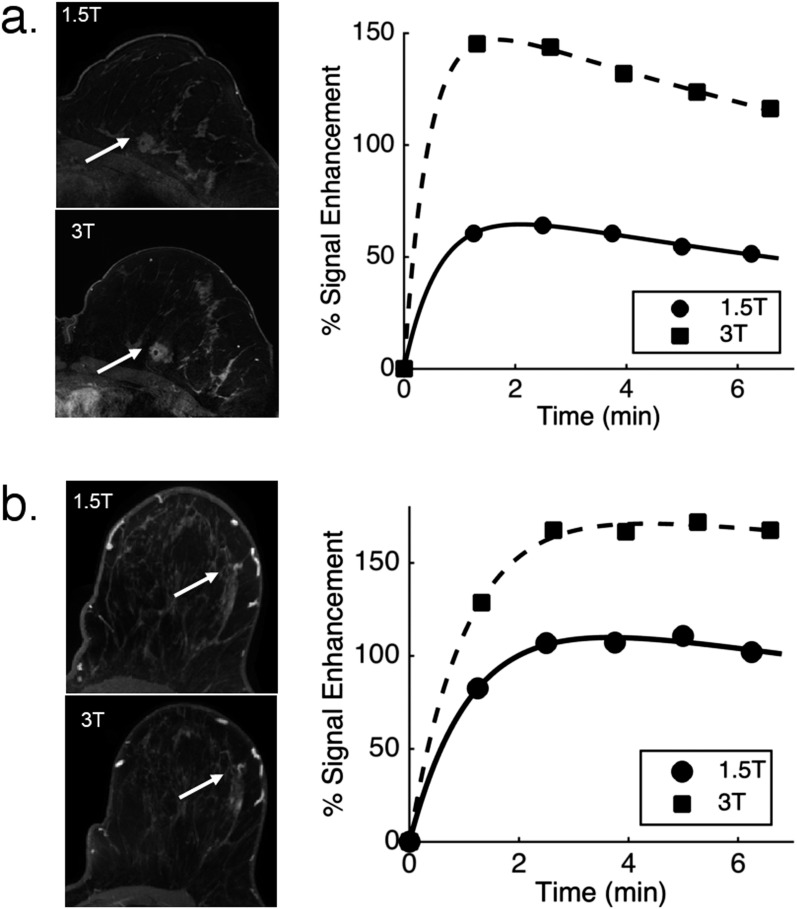
Figure 1 shows the measured PSE in two lesions, one malignant (invasive ductal carcinoma) and one benign (fibroadenoma), at both field strengths as well as the EMM fits to the data. DCE-MRI images from a representative slice (first post-contrast) are also shown. PSE curves at 3.0 T were higher than those at 1.5 T for all lesions.
Figure 1.
Dynamic contrast-enhanced MRI images and kinetic curves at 1.5 and 3.0 T for: (a) an invasive ductal carcinoma; (b) a fibroadenoma. The images of the affected breast at both fields are given on the left. Lesions are indicated by arrows.
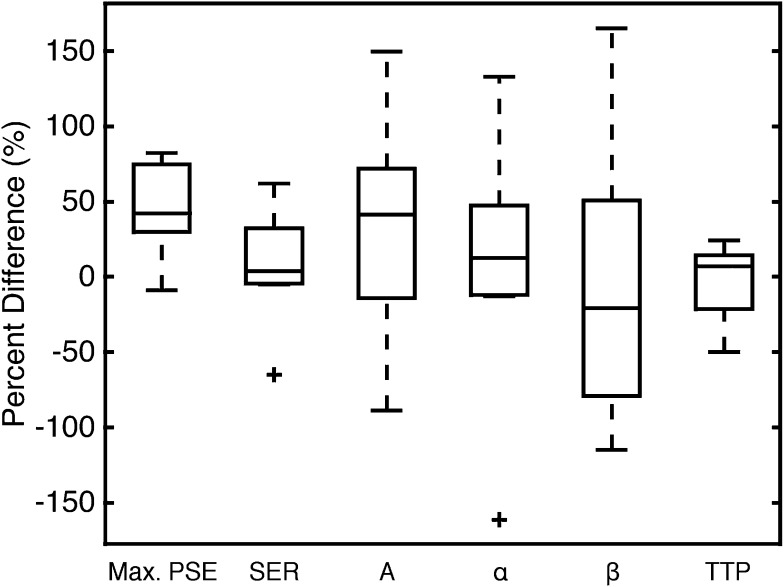
Mean values of maximum PSE, SER, three EMM fitting parameters and TTP, averaged over all lesions, are reported in Table 1. Percent differences and absolute percent differences for all parameters are also given in Table 1. On average, maximum PSE for each lesion measured at 3.0 T was greater than that measured at 1.5 T (p = 0.006). Paired tests showed that there was no significant difference in the mean value of any other parameter at 1.5 vs 3.0 T. The average value of TTP was greater than 5 min owing to the persistent kinetics of benign lesions imaged. Figure 2 shows box plots of the (signed, rather than absolute) percent difference for all the parameters. Of the three primary EMM parameters, the uptake rate (α) had the lowest variability, and washout rate (β) had the largest absolute percent difference between 1.5 and 3.0 T. TTP had an average signed and absolute percent difference lower than any of the individual EMM parameters. SER had the second lowest variability of all the semi-quantitative parameters measured. Post hoc multiple comparisons on the ANOVA table determined that there was no significant difference between the percent differences (signed and absolute) of any of the parameters measured. Kendall's tau rank correlation coefficient showed no significant (p > 0.05) correlation between the absolute difference and mean measured value for all the parameters measured. This demonstrates that the magnitude of the difference between parameters measured at the two fields was not significantly dependent on the parameter value in the sample measured.
Table 1.
Measured values of semi-quantitative parameters describing lesion kinetics across all lesions and percent differences between 1.5 and 3.0 T
| Kinetic parameter | Mean value ± standard deviation |
Percent difference (%) | Absolute percent difference (%) | |
|---|---|---|---|---|
| 1.5 T | 3.0 T | |||
| Max signal enhancementa (%) | 95 ± 32 | 148 ± 43 | 43 ± 30 | 45 ± 27 |
| Signal enhancement ratio | 0.66 ± 0.28 | 0.68 ± 0.27 | 7 ± 34 | 22 ± 25 |
| Enhancement upper limit (A) | 219 ± 145 | 308 ± 208 | 32 ± 67 | 59 ± 43 |
| Uptake rate (α) (min−1) | 0.54 ± 0.45 | 0.62 ± 0.64 | 14 ± 76 | 51 ± 55 |
| Washout rate (β) (min−1) | 0.06 ± 0.03 | 0.03 ± 0.03 | −11 ± 84 | 67 ± 46 |
| Time to peak enhancement (min) | 5.9 ± 2.7 | 5.7 ± 2.3 | −2 ± 24 | 19 ± 13 |
p = 0.006 for comparison between 1.5 and 3.0 T.
Figure 2.
Box plots of signed percent difference between the 1.5 and 3.0 T for all lesions (3.0 T minus 1.5 T divided by their average); A, α, β and time-to-peak enhancement (TTP) are determined from empirical mathematical model fits. The crosses denote outliers. Max., maximum; SER, signal enhancement ratio.
According to the breast imaging-reporting and data system (BIRADS) descriptors, the initial phase classification at the two field strengths differed in 6 of the 11 lesions; in these 6 cases, the uptake kinetics at 3.0 T were rated as greater than that at 1.5 T. In the delayed phase ten of the lesions had the same classification between both field strengths, one of the lesions was rated as having plateau kinetics at 1.5 T and persistent at 3.0 T.
Qualitative image assessment
In the evaluations performed by two readers, breast volume was rated exactly the same for both field strengths in all cases. One of the observers reported no differences in parenchymal enhancement between the two field strengths for all cases. The other observer reported differences in five of the cases, with no systematic difference in the ratings between 1.5 and 3.0 T. A summary of the rest of the evaluation criteria can be seen in Table 2. Images at 3.0 T consistently rated higher than those at 1.5 T for lesion conspicuity, delineation of margins, internal enhancement and technical aspects such as quality of fat suppression and noise/artefact level. The total image quality score was significantly higher at 3.0 T, with an average value of 27.7 (out of a possible 30 points), while the average image quality score at 1.5 T was 23.4 (p = 0.005).
Table 2.
Radiologists' evaluation of image quality
| Criterion | Scalea |
Average rating |
|||
|---|---|---|---|---|---|
| 1 | 5 | 1.5 T | 3.0 T | ||
| Total image quality scoreb | Low | High |
23.4 | 27.7 | |
| Margin sharpness | Low | High |
4 | 4.8 | |
| Internal lesion sharpness | Low | High |
4 | 4.9 | |
| Lesion conspicuity | Low | High |
4.1 | 5 | |
| Fat suppression quality | Poor | Very good |
3.9 | 4.6 | |
| Artefact/noise level | High noise | Low noise |
3.7 | 4.2 | |
| Lymph node conspicuity | Low | High | 3.7 | 4.2 | |
Total quality score scale ranged from 6 to 30.
p = 0.005.
DISCUSSION
This pilot study is the first study to compare DCE-MRI scans of the same breast lesions performed an average of 1 week apart using very similar protocols at 1.5 and 3.0 T. Despite the difficulty of recruiting patients to volunteer for successive MRI scans, we were able to scan 11 patients following this protocol, and this allowed for a comparison of semi-quantitative kinetic parameters, as well as image quality.
Our study showed that images at 3.0 T consistently received higher ratings for conspicuity of lesions, margins and lymph nodes as well as quality of fat suppression and lower noise level. These results were expected owing to increased signal-to-noise ratio at higher field strengths and the increased separation of fat and water resonances at 3.0 T. The evaluations reported here are consistent with those of previous studies demonstrating that scanning at 3.0 T is desirable, owing to increased image quality and sensitivity.8,19–22 However, imaging at 3.0 T is not always a possibility, thus identification of parameters that can be standardized between different field strengths remains a clinically relevant challenge.
Maximum PSE in lesions at 3 T was significantly higher than that at 1.5 T, consistent with the increased native T1 of tissues at higher field strength. This result is contrary to what was found in a previous prospective study, where females were imaged at both 1.5 and 3.0 T.8 In that study, Kuhl et al determined that signal enhancement was lower at 3.0 T than at 1.5 T. However, acquisition parameters varied at both fields, thus the results did not reflect the increased T1 of tissues at 3.0 T, and further highlight the need for identification of parameters that can be standardized across systems and acquisition parameters.
The estimated TTP, derived from the EMM parameters, performed best in terms of having the lowest percent difference between 1.5 and 3.0 T. SER had the second lowest average difference. Greater differences in washout rate could be attributed to the fact that the washout phase was not sampled for long enough, especially in lesions exhibiting persistent uptake kinetics, limiting the accuracy of the estimated washout rate. Uncertainty in fitting the washout rate (β) affects, in turn, fitting for the uptake rate (α) and can cause its wide range of percent differences. Higher temporal resolution would allow more accurate estimation of parameters descriptive of the kinetics of lesions, as the transition between uptake and washout would be better sampled. The fact that TTP exhibited a lower variability than did both uptake and washout rates is an indication that TTP is relatively insensitive to temporal sampling that is sparse and/or of insufficient duration. SER, on the other hand, is dependent on the times at which the early and delayed signals are measured, and thus on the temporal resolution of the acquisition.
TTP and SER are associated with the overall shape of the kinetic curve. The results demonstrate that the overall shape of the lesion contrast media kinetic curve, an important clinical variable, is consistent between the two fields when measured semi-quantitatively and not classified discretely (i.e. with set thresholds for classification).
A previous retrospective study showed that the BIRADS descriptors of curve shape can vary for malignant lesions across different systems and acquisition protocols (at the same field strength).7 Our results showed differences in a majority of the cases in the descriptors for the initial rise. The delayed phase, however, had the same classification in all but one case. Differences in the initial rise can be explained by greater enhancement at 3.0 T and the fact that the same thresholds were used for the classification at both field strengths, as is the case in standard clinical evaluation of lesion kinetics. Therefore, the thresholds for the classification of slow, medium or rapid rise should be adjusted for field strength and acquisition protocol; or alternatively, measure curve shape semi-quantitatively.
TTP has shown promise as a diagnostic parameter in a previous study of DCE-MRI of the breast, with a sensitivity of 92% and specificity of 83%.13 SER has been shown to have diagnostic utility with a previous study reporting a sensitivity of 95%; specificity, however, was 47%.23 SER has also been reported to be useful for evaluating tumour response to therapy and has been shown to correlate with a parameter associated with tumour physiology (redistribution rate constant kep).24–26 These results, along with the lower absolute variability of TTP and SER, suggest that they should be further investigated as primary diagnostic variables.
Although TTP and SER exhibited the lowest variability, these differences were not significantly less than those in other parameters. A repeatability study, with the same patients imaged at the same field strength, would establish baseline values for the variability of each parameter owing to physiological changes and measurement errors. The variability in the parameters between two fields seen here could then be compared with the baseline variations to determine whether imaging at a different field strength significantly changes the resulting estimate for each parameter. However, this would require scanning a large number of patients repeatedly within a short timeframe.
The results suggest that radiologists should exercise caution when classifying lesion kinetics by BIRADS descriptors when a practice uses scanners of different field strengths. This includes the use of computer-aided visualization stations that may use fixed thresholds for classification and colour coding of lesion kinetics. Curve shape has been shown to be valuable in the classification of lesions, and the results presented here indicate that semi-quantitative descriptors of curve shape have the potential to standardize DCE-MRI across different scanners and field strengths and increase diagnostic accuracy.
CONCLUSION
The reproducibility of TTP and SER indicate consistency in the measurement of the overall shape of contrast media kinetic curves for each lesion at different field strengths, when acquisition parameters are standardized. In contrast to TTP and SER, assessments of the washout and uptake rates according to the BIRADS descriptors had higher variability. Conversion of signal intensity to concentration and calculation of pharmacokinetic parameters (e.g. Ktrans and ve) could, in principle, reduce variability and provide more quantitative information, but this requires additional calibration scans that are not part of routine clinical DCE-MRI acquisitions. Further work is underway to determine whether quantitative analysis increases the reproducibility of kinetic parameters derived from DCE-MRI.
Acknowledgments
ACKNOWLEDGMENTS
The authors would like to thank Sharon Harris for her support with patient recruitment.
Contributor Information
F D Pineda, Email: fdp@uchicago.edu.
M Medved, Email: mmedved@uchicago.edu.
X Fan, Email: xfan@uchicago.edu.
M K Ivancevic, Email: marko.ivancevic@philips.com.
H Abe, Email: habe@uchicago.edu.
A Shimauchi, Email: akikoshima@gmail.com.
G M Newstead, Email: gnewstead@radiology.bsd.uchicago.edu.
G S Karczmar, Email: gskarczm@uchicago.edu.
CONFLICT OF INTEREST
Dr Karczmar received research funding from Philips Healthcare.
FUNDING
This work was supported by Army Breast Cancer Research Program; grant number: BC101131, Philips Healthcare. Supported, in part, by the National Cancer Institute of the National Institutes of Health under grant number R01 CA167785. Supported, in part, by the National Institute of Biomedical Imaging and Bioengineering of the National Institutes of Health under grant number T32 EB002103.
REFERENCES
- 1.Kuhl CK, Schrading S, Leutner CC, Morakkabati-Spitz N, Wardelmann E, Fimmers R, et al. Mammography, breast ultrasound, and magnetic resonance imaging for surveillance of women at high familial risk for breast cancer. J Clin Oncol 2005; 23: 8469–76. [DOI] [PubMed] [Google Scholar]
- 2.Yankeelov TE, Lepage M, Chakravarthy A, Broome EE, Niermann KJ, Kelley MC, et al. Integration of quantitative DCE-MRI and ADC mapping to monitor treatment response in human breast cancer: initial results. Magn Reson Imaging 2007; 25: 1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mahoney MC, Newell MS, Bailey L, Barke LD, D'Orsi CJ, Haffty BG, et al. ACR practice guideline for the performance of contrast-enhanced magnetic resonance imaging (MRI) of the breast. MRI Breast 2013; 12. [Google Scholar]
- 4.Kuhl CK, Mielcareck P, Klaschik S, Leutner C, Wardelmann E, Gieseke J, et al. Dynamic breast MR imaging: are signal intensity time course data useful for differential diagnosis of enhancing lesions? Radiology 1999; 211: 101–10. [DOI] [PubMed] [Google Scholar]
- 5.El Khouli RH, Macura KJ, Jacobs MA, Khalil TH, Kamel IR, Dwyer A, et al. Dynamic contrast-enhanced MRI of the breast: quantitative method for kinetic curve type assessment. AJR Am J Roentgenol 2009; 193: W295–300. doi: 10.2214/AJR.09.2483 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rakow-Penner R, Daniel B, Yu H, Sawyer-Glover A, Glover GH. Relaxation times of breast tissue at 1.5T and 3T measured using IDEAL. J Magn Reson Imaging 2006; 23: 87–91. [DOI] [PubMed] [Google Scholar]
- 7.Jansen SA, Shimauchi A, Zak L, Fan X, Wood AM, Karczmar GS, et al. Kinetic curves of malignant lesions are not consistent across MRI systems: need for improved standardization of breast dynamic contrast-enhanced MRI acquisition. AJR Am J Roentgenol 2009; 193: 832–9. doi: 10.2214/AJR.08.2025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kuhl CK, Jost P, Morakkabati N, Zivanovic O, Schild HH, Gieseke J. Contrast-enhanced MR imaging of the breast at 3.0 and 1.5 T in the same patients: initial experience. Radiology 2006; 239: 666–76. [DOI] [PubMed] [Google Scholar]
- 9.Schabel MC, Parker DL. Uncertainty and bias in contrast concentration measurements using spoiled gradient echo pulse sequences. Phys Med Biol 2008; 53: 2345–73. doi: 10.1088/0031-9155/53/9/010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Delille JP, Slanetz PJ, Yeh ED, Kopans DB, Garrido L. Physiologic changes in breast magnetic resonance imaging during the menstrual cycle: perfusion imaging, signal enhancement, and influence of the T1 relaxation time of breast tissue. Breast J 2005; 11: 236–41. [DOI] [PubMed] [Google Scholar]
- 11.Rahbar H, Partridge SC, Demartini WB, Gutierrez RL, Parsian S, Lehman CD. Improved B1 homogeneity of 3 Tesla breast MRI using dual-source parallel radiofrequency excitation. J Magn Reson Imaging 2012; 35: 1222–6. doi: 10.1002/jmri.23571 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fan X, Medved M, River JN, Zamora M, Corot C, Robert P, et al. New model for analysis of dynamic contrast-enhanced MRI data distinguishes metastatic from nonmetastatic transplanted rodent prostate tumors. Magn Reson Med 2004; 51: 487–94. [DOI] [PubMed] [Google Scholar]
- 13.Fan X, Medved M, Karczmar GS, Yang C, Foxley S, Arkani S, et al. Diagnosis of suspicious breast lesions using an empirical mathematical model for dynamic contrast-enhanced MRI. Magn Reson Imaging 2007; 25: 593–603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jansen SA, Fan X, Karczmar GS, Abe H, Schmidt RA, Newstead GM. Differentiation between benign and malignant breast lesions detected by bilateral dynamic contrast‐enhanced MRI: a sensitivity and specificity study. Magn Reson Med 2008; 59: 747–54. doi: 10.1002/mrm.21530 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Esserman L, Hylton N, George T, Weidner N. Contrast-enhanced magnetic resonance imaging to assess tumor histopathology and angiogenesis in breast carcinoma. Breast J 1999; 5: 13–21. [DOI] [PubMed] [Google Scholar]
- 16.Hauth EA, Stockamp C, Maderwald S, Mühler A, Kimmig R, Jaeger H, et al. Evaluation of the three-time-point method for diagnosis of breast lesions in contrast-enhanced MR mammography. Clin Imaging 2006; 30: 160–5. [DOI] [PubMed] [Google Scholar]
- 17.Bland JM, Altman DG. Multiple significance tests: the Bonferroni method. BMJ 1995; 310: 170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Padhani AR, Hayes C, Landau S, Leach MO. Reproducibility of quantitative dynamic MRI of normal human tissues. NMR Biomed 2002; 15: 143–53. [DOI] [PubMed] [Google Scholar]
- 19.Elsamaloty H, Elzawawi MS, Mohammad S, Herial N. Increasing accuracy of detection of breast cancer with 3-T MRI. AJR Am J Roentgenol 2009; 192: 1142–8. doi: 10.2214/AJR.08.1226 [DOI] [PubMed] [Google Scholar]
- 20.Lourenco AP, Donegan L, Khalil H, Mainiero MB. Improving outcomes of screening breast MRI with practice evolution: initial clinical experience with 3T compared to 1.5T. J Magn Reson Imaging 2014; 39: 535–9. doi: 10.1002/jmri.24198 [DOI] [PubMed] [Google Scholar]
- 21.Pinker K, Grabner G, Bogner W, Gruber S, Szomolanyi P, Trattnig S, et al. A combined high temporal and high spatial resolution 3 Tesla MR imaging protocol for the assessment of breast lesions: initial results. Invest Radiol 2009; 44: 553–8. doi: 10.1097/RLI.0b013e3181b4c127 [DOI] [PubMed] [Google Scholar]
- 22.Lee JH, Kim SH, Kang BJ, Choi JJ, Lee AW. Clinical experience of 3T breast MRI in detecting the additional lesions in breast cancer patients. J Korean Soc Magn Reson Med 2010; 14: 121–5. [Google Scholar]
- 23.Lee SH, Kim JH, Cho N, Park JS, Yang Z, Jung YS, et al. Multilevel analysis of spatiotemporal association features for differentiation of tumor enhancement patterns in breast DCE-MRI. Med Phys 2010; 37: 3940–56. [DOI] [PubMed] [Google Scholar]
- 24.Li KL, Henry RG, Wilmes LJ, Gibbs J, Zhu X, Lu Y, et al. Kinetic assessment of breast tumors using high spatial resolution signal enhancement ratio (SER) imaging. Magn Reson Med 2007; 58: 572–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hylton N. Dynamic contrast-enhanced magnetic resonance imaging as an imaging biomarker. J Clin Oncol 2006; 24: 3293–8. [DOI] [PubMed] [Google Scholar]
- 26.Hylton NM, Blume JD, Bernreuter WK, Pisano ED, Rosen MA, Morris EA, et al. ; ACRIN 6657 Trial Team and I-SPY 1 TRIAL Investigators. Locally advanced breast cancer: MR imaging for prediction of response to neoadjuvant chemotherapy—results from ACRIN 6657/I-SPY TRIAL. Radiology 2012; 263: 663–72. doi: 10.1148/radiol.12110748 [DOI] [PMC free article] [PubMed] [Google Scholar]