Abstract
Objectives:
The aim of the present study was to analyse the mineralization pattern of enamel and dentin in patients affected by X-linked hypophosphatemic rickets (XLHR) using micro-CT (µCT), and to associate enamel and dentin mineralization in primary and permanent teeth with tooth position, gender and the presence/absence of this disease.
Methods:
19 teeth were collected from 5 individuals from the same family, 1 non-affected by XLHR and 4 affected by XLHR. Gender, age, tooth position (anterior/posterior) and tooth type (deciduous/permanent) were recorded for each patient. Following collection, teeth were placed in 0.1% thymol solution until µCT scan. Projection images were reconstructed and analysed. A plot profile describing the greyscale distance relationship in µCT images was achieved through a line bisecting each tooth in a region with the presence of enamel and dentin. The enamel and dentin mineralization densities were measured and compared. Univariate ANOVA and post hoc Tukey tests were used for all comparisons.
Results:
Teeth of all affected patients presented dentin with a different mineralization pattern compared with the teeth of healthy patients with dentin defects observed next to the pulp chambers. Highly significant differences were found for gray values between anterior and posterior teeth (p < 0.05), affected and non-affected (p < 0.05), as well as when position and disease status were considered (p < 0.05).
Conclusions:
In conclusion, the mineralization patterns of dentin differed when comparing teeth from patients with and without FHR, mainly next to pulp chambers where areas with porosity and consequently lower mineral density and dentin defects were found.
Keywords: familial hypophosphatemic rickets, X-ray microtomography, tooth, tooth calcification, radiology
Introduction
Familial hypophosphatemic rickets (FHR) is a rare condition with an incidence of 1 in 20,000 births.1 Several forms of FHR have been described, distinguished by their pattern of inheritance and genetic cause. The most common form of FHR is known as X-linked hypophosphatemic rickets (XLHR), which has an X-linked dominant pattern of inheritance.2 Mutations in the phosphate-regulating gene with homologies to endopeptidases on the X-chromosome are responsible for the disease in most familial cases.3 The other forms of FHR are rare, and include X-linked recessive, autosomal dominant, autosomal recessive and hereditary hypophosphatemic rickets with hypercalciuria.2
Clinically, XLHR is characterized by short stature, deformities predominantly in the lower limbs, metaphyseal widening, rachitic rosary and frontal bossing.4 At birth, children present normal height, but growth rate reduction is present during the first year of life, resulting in a final deficit in height, which is a consequence of the slow speed of growth that precedes the diagnosis and initiation of therapy.5–7 The differential diagnosis from “vitamin D deficiency rickets” is based on the non-compliance with hypotonia, myopathy, muscle weakness or tetany.8
The recurrence of spontaneous abscesses affecting multiple non-carious primary and permanent teeth is the main oral clinical feature associated with FHR.9,10 Teeth are characterized by deficient dentin mineralization with large pulp chambers11 and the presence of large pulp horns and structural defects in the dentin.12 McWhorter and Seale13 suggested that abscess formation occurs secondary to bacterial invasion into the pulp through microcracks present in the enamel and dentin. Histological sections of the affected teeth show dentin disorganization.14 Radiographically, dental characteristics are generally evaluated through periapical14 or panoramic radiographs.9,12,15 Scanning electron microscopy and immunohistochemistry have also been used to assess the primary and permanent dentitions of patients with FHR.16,17
X-ray micro-CT (µCT) is a high-resolution, non-destructive radiographic method, which has been increasingly used in studies of dental anatomy.18,19 Described by Elliot and Dover,20 µCT is a microscopic system based on the principles of CT, which interprets X-ray attenuation variation within a planar section of a solid object, without the need to make cuts. This technique allows microstructural analysis of small bodies, enabling the study of parameters, such as mineral density,18 volume21 and mineralization pattern.19,22 Although µCT is an important technology in microstructure studies, no previous work has reported its use in teeth of patients diagnosed with XLHR. The aim of the present study was to analyse the mineralization pattern of enamel and dentin in a family of patients affected by XLHR using µCT, and to verify variations in enamel and dentin mineralization in primary and permanent teeth, as a function of tooth position, gender and the presence/absence of this disease.
Methods and materials
Samples
Five individuals from the same family (one non-affected and four affected by XLHR) donated 19 teeth to be used in the present study. These teeth were previously extracted for reasons other than the present study. Hence, the primary cause for extractions was the presence of either periodontal disease or dental caries. The following characteristics were recorded for each patient: gender, age, tooth position (anterior—incisors, canines; posterior—premolars, molars) and tooth type (deciduous/permanent) (Table 1). Following collection, teeth were placed in 0.1% thymol solution until µCT scan. This study was approved by the Ethics Committee of the Federal University of Ceará Medical School (Brazil) (Protocol 004/11). Consent and tooth donation forms were signed by volunteers or legal guardians, prior to patient enrolment in the study.
Table 1.
Teeth distributed by gender, age and the presence/absence of X-linked hypophosphatemic rickets (XLHR) of study volunteers
| Individuals | XLHR | Gender | Age (years) | Tooth | Tooth position | Tooth type | Greyscale value (mean ± standard deviation) |
|
|---|---|---|---|---|---|---|---|---|
| Enamel | Dentin | |||||||
| 1 | Absent | Female | 13 | 1 | Posterior | Deciduous | 151.26 ± 5.01 | 77.91 ± 0.31 |
| 2 | Anterior | Deciduous | 152.22 ± 20.82 | 93.59 ± 1.61 | ||||
| 3 | Posterior | Deciduous | 151.64 ± 3.83 | 79.99 ± 1.79 | ||||
| 4 | Anterior | Deciduous | 176.34 ± 4.79 | 94.95 ± 1.59 | ||||
| 5 | Posterior | Deciduous | 151.64 ± 12.51 | 84.48 ± 0.87 | ||||
| 6 | Posterior | Deciduous | 165.27 ± 9.82 | 84.65 ± 0.55 | ||||
| 2 | Present | Male | 6 | 1 | Anterior | Deciduous | 158.83 ± 30.44 | 73.06 ± 5.95 |
| 2 | Posterior | Deciduous | 148.30 ± 21.41 | 61.64 ± 7.02 | ||||
| 3 | Anterior | Deciduous | 168.64 ± 11.77 | 69.09 ± 4.86 | ||||
| 4 | Anterior | Deciduous | 173.17 ± 8.08 | 76.40 ± 3.88 | ||||
| 5 | Anterior | Deciduous | 161.50 ± 15.82 | 78.40 ± 3.47 | ||||
| 6 | Posterior | Deciduous | 167.88 ± 3.89 | 83.63 ± 2.42 | ||||
| 7 | Anterior | Deciduous | 157.16 ± 23.47 | 66.56 ± 2.79 | ||||
| 8 | Anterior | Deciduous | 178.60 ± 11.89 | 76.54 ± 7.47 | ||||
| 9 | Anterior | Deciduous | 161.19 ± 9.25 | 86.77 ± 2.24 | ||||
| 3 | Present | Female | 10 | 1 | Posterior | Deciduous | 159.42 ± 5.39 | 83.05 ± 1.50 |
| 4 | Present | Female | 31 | 1 | Posterior | Permanent | 140.79 ± 10.69 | 73.90 ± 1.26 |
| 5 | Present | Male | 27 | 1 | Posterior | Permanent | 163.44 ± 5.55 | 71.58 ± 2.03 |
| 2 | Anterior | Permanent | 149.72 ± 22.52 | 72.64 ± 2.82 | ||||
micro-CT analysis
High-resolution µCT (SkyScan 1172; SkyScan, Antwerp, Belgium) was used to scan all teeth. Samples were wrapped in Parafilm® (Bemis NA, Neenah, WI) as a way to prevent drying and affixed to the scanning stage by the use of this same plastic paraffin film. Projection images were obtained using the following scanner settings: voltage, 70 kV; resolution, 8.9 μm; aluminium filter, 0.5 mm; stage rotation, 0.4° per second; and frame averaging, two. Flat-field corrections were used to minimize background noise. Projection images were reconstructed through manufacturer-provided software (NRecon; SkyScan) with the following settings: beam hardening, 33%; ring reduction, 5% and threshold, 0.00–0.13. In addition, post-alignment was optimized separately for each specimen.
Reconstructed images were analysed with the ImageJ 1.43u software (Wayne Rasband, National Institutes of Health, Bethesda, MD). A plot profile describing the greyscale distance relationship in µCT images was achieved through a line bisecting each tooth at a standardized cross-sectional occlusal level with the presence of enamel and dentin. In each tooth, five lines were drawn and the average of greyscale values for enamel and dentin was calculated. The enamel and dentin mineralization densities were measured and compared.
Statistics
All statistical tests were performed with SPSS® 9.0 software (SPSS Inc., Chicago, IL). Univariate ANOVA was used for all comparisons, with either enamel or dentin greyscale as the dependent variable, and variables such as gender, age, tooth position, tooth type and the presence/absence of XLHR as the independent variables. Post hoc Tukey tests were only used when more than two groups were being compared. In these cases the post hoc Tukey test was applied. Values were considered significant when p < 0.05.
Results
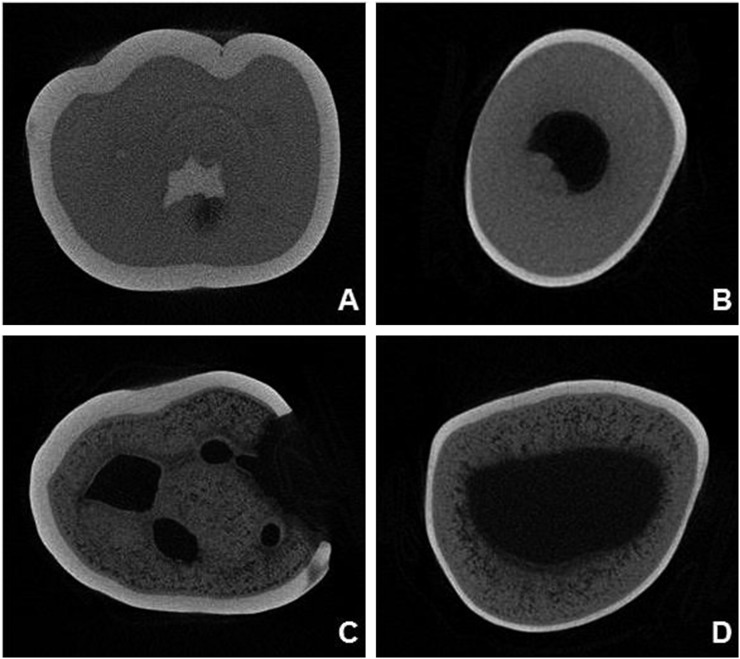
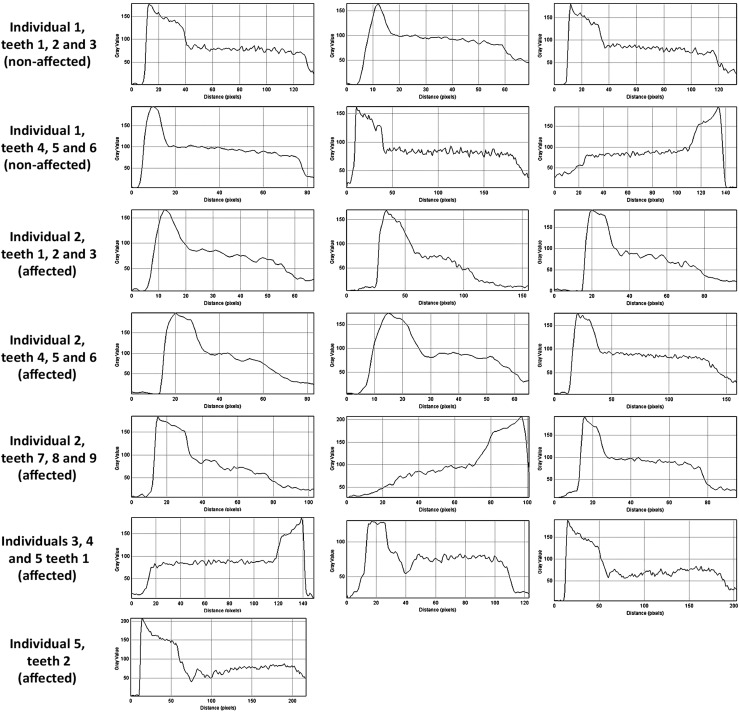
The dentin mineralization pattern differed between teeth of the unaffected and affected individuals (Figure 1). Dentin defects were observed next to the pulp chambers as areas with porosities and consequently lower mineral density. No visual differences were detected in pulp space and enamel. Based on a cross-section of each tooth, a bisecting line was established from enamel towards the pulp. Mineralization patterns were expressed as greyscale–distance plot profiles along this line and are represented by histograms as shown in Figure 2. In Figure 2, higher peaks represent higher greyscale values, so that enamel shows the highest values, followed by dentin and pulp. In addition, parallel to the x-axis in each histogram, a more stable line can be observed in the dentin of the non-affected patient. This finding was not observed among affected individuals.
Figure 1.
Two-dimensional micro-CT reconstructed images (NRecon; SkyScan, Antwerp, Belgium). Cross-section at a more occlusal level showing normal pattern of mineralization in non-affected individuals [(a) molar, (b) incisor]. Cross-section at a more occlusal level showing hypomineralization defects to be more widespread in dentin of affected individual [(c) molar, (d) incisor].
Figure 2.
Histograms expressing mineralization patterns as greyscale–distance plot profiles along the line bisecting teeth of affected and non-affected individuals. Each histogram expresses mineralization patterns from enamel to pulp for: individual 1 (teeth 1–5), individual 2 (teeth 1–7 and tooth 9), individual 4 (tooth 1) and individual 5 (teeth 1–2). Mineralization patterns are expressed from pulp to enamel for: individual 1 (tooth 6), individual 2 (tooth 8) and individual 3 (tooth 1).
When considering tooth position and the presence/absence of XLHR, comparisons of enamel greyscale values (Table 1) differed only between anterior and posterior teeth (p = 0.027), but no difference was found between the affected and non-affected individuals (p = 0.965), nor when all these variables were considered (p = 0.823). However, when these comparisons were carried out with dentin greyscale value as the dependent variable, highly significant differences were found for grey values between anterior and posterior teeth (p < 0.05), affected and non-affected (p < 0.05), as well as when position and disease status were considered (p < 0.05).
Evaluation of dentin greyscale values (Table 1) and gender alone showed a significant difference between males and females (p < 0.05), whereas the female gender expressed the highest values of greyscale. However, enamel greyscale values did not differ between genders (p = 0.057). Dentin grey values differed between dentitions (p < 0.05), hence deciduous teeth presented higher greyscale values than did permanent teeth.
Discussion
µCT is a miniaturized version of medical CT and has been used extensively for in vitro dental research.23–26 The technique allows three-dimensional analyses of both structure and density (or concentration), the latter requiring prior knowledge of composition.27 Although µCT was not previously used to assess the mineral structure of teeth from patients with rickets, other studies have focused on the tooth mineral structure from these individuals by using scanning electron microscopy,17,28 transmission electron microscopy,29 immunohistochemical examination16,17 and radiographic examination.14
Teeth of all affected patients presented dentin with a different mineralization pattern compared with teeth of the unaffected individual. Dentin defects were noted next to pulp chambers as areas with porosities and consequently lower mineral density. This fact was confirmed by dentin greyscale values, which differed between affected and non-affected patients. Thus, a higher number of porosities renders a lower mineral density, numerically observed as reduced greyscale values. Porosities observed in the present study reflect the presence of dentinal canals, which are usually uniform and highly aligned, expressing a regular distribution in healthy teeth. Since dentin is a very anisotropic tissue, it tends to deform differently when loaded along vs across tubule orientation.30 In the present study, teeth from affected individuals expressed lower greyscale values in dentin when compared with the non-affected patient. This finding may result from a greater number and a more irregular distribution of these dentinal tubules in the teeth of affected subjects. Although odontoblast function is normal in patients with XLHR, dental mineralization is inadequate. Hence, hypophosphatemia, which occurs as a consequence of XLHR, generates a dysplastic and poorly mineralized dentinal tissue with areas of interglobular dentin.12 Interglobular dentin and irregular dentinal tubules were histologically observed by Pereira et al.14
In the present study, no visual differences were detected in enamel mineralization and pulp chamber. Enamel greyscale did not differ between affected and non-affected individuals. Harris and Sullivan,31 and Archard and Witkop32 also described the enamel of individuals with FHR as normal but thin. However, enamel hypoplasia has been reported in several studies.12,33,34 Large canals and root canal space were also previously described.14 These abnormalities may explain the common outbreak of periradicular abscesses in the absence of caries or history of trauma, typically found in FHR, caused by bacterial contamination via enamel microfractures, which extend to the pulp and often lead to tissue necrosis.
Enamel and dentin greyscales differed between anterior and posterior teeth in the presence and absence of XLHR. Sánchez-Quevedo et al35 evaluated human teeth with amelogenesis imperfecta through scanning electron microscopy and X-ray microprobe analysis. The authors analysed dental fragments from members of a family clinically and genetically diagnosed as having amelogenesis imperfecta to establish the morphological patterns and the quantitative concentration of calcium in the enamel of anterior and posterior teeth. Calcium levels in the enamel of teeth with and without amelogenesis imperfecta differed significantly between anterior and posterior teeth, indicating that the factors that influence normal mineralization in different regions of the dental arch are not altered in the process of amelogenesis imperfecta.35 However, in the present study, the mineralization level of dental tissues was assessed by quantifying greyscale in both enamel and dentin, and the results showed significantly higher mineralization levels in the analysed posterior dentition.
In this study, female patients affected by XLHR presented a higher mineral density in dentin than did males. Winters et al36 studied hypophosphatemia in a large North Carolina family of English–Scottish descent. They observed that the degree of serum phosphate reduction was the same in males and females, although females expressed less severe bone disease. Dentin defects reflect genetic disorders affecting bone mineralization, such as hypophosphatemic rickets,37 and our results agreed with Winters et al.36 In a healthy population, although males tend to present a 10–12% peak bone mass and greater bone size, density is similar in males and females.38
Deciduous teeth expressed the most elevated greyscale values, suggesting a higher mineralization pattern in the dentin of primary than in permanent dentition. This finding is consistent with the study of Hirayama et al39 that reported that peritubular dentin thickness, which is the dentin with higher mineral content, was two to five times greater in deciduous teeth, with values ranging from 3 to 20 μm, than in permanent teeth. Also supporting our finding, Sumikawa et al40 reported that tubule diameters increased with distance from the dentin–enamel junction, whereas a decrease in peritubular width was observed.
Enamel greyscale values did not differ between deciduous and permanent dentitions. Wilson and Beynon41 measured, by quantitative microradiography, mineralization and compared differences in mineralization between human deciduous and permanent enamel. The authors found an overall lower mineralization pattern in the deciduous dentition in addition to lower mineral levels in the deciduous enamel.41
In the present sample, patients with XLHR showed lower dentinal and enamel mineralization than did unaffected individuals from the same family. Mineralization pattern varied among these patients as a function of gender, tooth position and type of dentition (deciduous or permanent). The utilization of µCT provided a detailed visualization of enamel and dentinal micro-defects, commonly observed among patients with XLHR.
References
- 1.Yong SM, Aik S. X-linked hypophosphatemic rickets—a report of 2 cases and review of literature. Med J Malaysia 2000; 55: 101–4. [PubMed] [Google Scholar]
- 2.Genetics home reference—hereditary hypophosphatemic rickets. [Cited 10 February 2015.] Available from: http://ghr.nlm.nih.gov/condition/hereditary-hypophosphatemic-rickets
- 3.Gaucher C, Walrant-Debray O, Nguyen TM, Esterle L, Garabédian M, Jehan F. PHEX analysis in 118 pedigrees reveals new genetic clues in hypophosphatemic rickets. Hum Genet 2009; 125: 401–11. doi: 10.1007/s00439-009-0631-z [DOI] [PubMed] [Google Scholar]
- 4.Root AW, Diamond FB, Jr. Disorders of calcium metabolism in the child and adolescent. In: Sperling MA, ed. Pediatric endocrinology. 2nd edn. Philadelphia, PA: Saunders; 2002. pp. 650–1. [Google Scholar]
- 5.Ariceta G, Langman CB. Growth in X-linked hypophosphatemic rickets. Eur J Pediatr 2007; 166: 303–9. [DOI] [PubMed] [Google Scholar]
- 6.Haffner D, Nissel R, Wühl E, Mehls O. Effects of growth hormone treatment on body proportions and final height among small children with X-linked hypophosphatemic rickets. Pediatrics 2004; 113: e593–6. [DOI] [PubMed] [Google Scholar]
- 7.Mäkitie O, Doria A, Kooh SW, Cole WG, Daneman A, Sochett E. Early treatment improves growth and biochemical and radiographic outcome in X-linked hypophosphatemic rickets. J Clin Endocrinol Metab 2003; 88: 3591–7. [DOI] [PubMed] [Google Scholar]
- 8.Mughal Z. Rickets in childhood. Semin Musculoskelet Radiol 2002; 6: 183–90. [DOI] [PubMed] [Google Scholar]
- 9.Batra P, Tejani Z, Mars M. X-linked hypophosphatemia: dental and histologic findings. J Can Dent Assoc 2006; 72: 69–72. [PubMed] [Google Scholar]
- 10.Soares EC, Costa FW, Ribeiro TR, Alves AP, Fonteles CS. Clinical approach in familial hypophosphatemic rickets: report of three generations. Spec Care Dentist 2013; 33: 304–7. doi: 10.1111/j.1754-4505.2012.00310.x [DOI] [PubMed] [Google Scholar]
- 11.Seow WK. Diagnosis and management of unusual dental abscesses in children. Aust Dent J 2003; 48: 156–68. [DOI] [PubMed] [Google Scholar]
- 12.Murayama T, Iwatsubo R, Akiyama S, Amano A, Morisaki I. Familial hypophosphatemic vitamin D-resistant rickets: dental findings and histologic study of teeth. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 2000; 90: 310–16. [DOI] [PubMed] [Google Scholar]
- 13.McWhorter AG, Seale NS. Prevalence of dental abscesses in a population of children with vitamin D-resistant rickets. Pediatr Dent 1991; 13: 91–6. [PubMed] [Google Scholar]
- 14.Pereira CM, de Andrade CR, Vargas PA, Coletta RD, de Almeida OP, Lopes MA. Dental alterations associated with X-linked hypophosphatemic rickets. J Endod 2004; 30: 241–5. [DOI] [PubMed] [Google Scholar]
- 15.Baroncelli GI, Angiolini M, Ninni E, Galli V, Saggese R, Giuca MR. Prevalence and pathogenesis of dental and periodontal lesions in children with X-linked hypophosphatemic rickets. Eur J Paediatr Dent 2006; 7: 61–6. [PubMed] [Google Scholar]
- 16.Chaussain-Miller C, Sinding C, Septier D, Wolikow M, Goldberg M, Garabedian M. Dentin structure in familial hypophosphatemic rickets: benefits of vitamin D and phosphate treatment. Oral Dis 2007; 13: 482–9. [DOI] [PubMed] [Google Scholar]
- 17.Boukpessi T, Septier D, Bagga S, Garabedian M, Goldberg M, Chaussain-Miller C. Dentin alteration of deciduous teeth in human hypophosphatemic rickets. Calcif Tissue Int 2006; 79: 294–300. [DOI] [PubMed] [Google Scholar]
- 18.Farah RA, Swain MV, Drummond BK, Cook R, Atieh M. Mineral density of hypomineralised enamel. J Dent 2010; 38: 50–8. doi: 10.1016/j.jdent.2009.09.002 [DOI] [PubMed] [Google Scholar]
- 19.Zhang X, Rahemtulla F, Zhang P, Li X, Beck P, Thomas HF. Normalisation of calcium status reverses the phenotype in dentin, but not in enamel of VDR-deficient mice. Arch Oral Biol 2009; 54: 1105–10. doi: 10.1016/j.archoralbio.2009.09.004 [DOI] [PubMed] [Google Scholar]
- 20.Elliott JC, Dover SD. X-ray microtomography. J Microsc 1982; 126: 211–13. [DOI] [PubMed] [Google Scholar]
- 21.Ikram OH, Patel S, Sauro S, Mannocci F. Micro-computed tomography of tooth tissue volume changes following endodontic procedures and post space preparation. Int Endod J 2009; 42: 1071–6. doi: 10.1111/j.1365-2591.2009.01632.x [DOI] [PubMed] [Google Scholar]
- 22.McKee MD, Yadav MC, Foster BL, Somerman MJ, Farquharson C, Millán JL. Compounded PHOSPHO1/ALPL deficiencies reduce dentin mineralization. J Dent Res 2013; 92: 721–7. doi: 10.1177/0022034513490958 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kühnisch J, Galler M, Seitz M, Stich H, Lussi A, Hickel R, et al. Irregularities below the enamel-dentin junction may predispose for fissure caries. J Dent Res 2012; 91: 1066–70. doi: 10.1177/0022034512458688 [DOI] [PubMed] [Google Scholar]
- 24.Nakata K, Nikaido T, Nakashima S, Nango N, Tagami J. An approach to normalizing micro-CT depth profiles of mineral density for monitoring enamel remineralization progress. Dent Mater J 2012; 31: 533–40. [DOI] [PubMed] [Google Scholar]
- 25.Cochrane NJ, Anderson P, Davis GR, Adams GG, Stacey MA, Reynolds EC. An X-ray microtomographic study of natural white-spot enamel lesions. J Dent Res 2012; 91: 185–91. doi: 10.1177/0022034511429570 [DOI] [PubMed] [Google Scholar]
- 26.Qi YP, Li N, Niu LN, Primus CM, Ling JQ, Pashley DH, et al. Remineralization of artificial dentinal caries lesions by biomimetically modified mineral trioxide aggregate. Acta Biomater 2012; 8: 836–42. doi: 10.1016/j.actbio.2011.10.033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Davis GR, Evershed AN, Mills D. Quantitative high contrast X-ray microtomography for dental research. J Dent 2013; 41: 475–82. doi: 10.1016/j.jdent.2013.01.010 [DOI] [PubMed] [Google Scholar]
- 28.Seow WK, Perham S. Enamel hypoplasia in prematurely-born children: a scanning electron microscopic study. J Pedod 1990; 14: 235–9. [PubMed] [Google Scholar]
- 29.McKee MD, Nakano Y, Masica DL, Gray JJ, Lemire I, Heft R, et al. Enzyme replacement therapy prevents dental defects in a model of hypophosphatasia. J Dent Res 2011; 90: 470–6. doi: 10.1177/0022034510393517 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zaslansky P, Zabler S, Fratzl P. 3D variations in human crown dentin tubule orientation: a phase-contrast microtomography study. Dent Mater 2010; 26: e1–10. doi: 10.1016/j.dental.2009.09.007 [DOI] [PubMed] [Google Scholar]
- 31.Harris R, Sullivan HR. Dental sequelae in deciduous dentition in vitamin D resistant rickets: case report. Aust Dent J 1960; 5: 200–3. [Google Scholar]
- 32.Archard HO, Witkop CJ, Jr. Hereditary hypophosphatemia (vitamin D-resistant rickets) presenting primary dental manifestations. Oral Surg Oral Med Oral Pathol 1966; 22: 184–93. [Google Scholar]
- 33.Herbert FL. Hereditary hypophosphatemia rickets: an important awareness for dentists. ASDC J Dent Child 1986; 53: 223–6. [PubMed] [Google Scholar]
- 34.Ozkan S, Ucok Z, Alagöl F. Dental manifestations of familial hypophosphatemic vitamin-D-resistant rickets: report of case. ASDC J Dent Child 1984; 51: 448–50. [PubMed] [Google Scholar]
- 35.Sánchez-Quevedo MC, Ceballos G, García JM, Rodríguez IA, Gómez de Ferraris ME, et al. Scanning electron microscopy and calcification in amelogenesis imperfecta in anterior and posterior human teeth. Histol Histopathol 2001; 16: 827–32. [DOI] [PubMed] [Google Scholar]
- 36.Winters RW, Graham JB, Williams TF, McFalls VW, Burnett CH. A genetic study of familial hypophosphatemia and vitamin D resistant rickets with a review of the literature. Medicine (Baltimore) 1958; 37: 97–142. [DOI] [PubMed] [Google Scholar]
- 37.Opsahl Vital S, Gaucher C, Bardet C, Rowe PS, George A, Linglart A, et al. Tooth dentin defects reflect genetic disorders affecting bone mineralization. Bone 2012; 50: 989–97. doi: 10.1016/j.bone.2012.01.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bonnick SL. Osteoporosis in men and women. Clin Cornerstone 2006; 8: 28–39. [DOI] [PubMed] [Google Scholar]
- 39.Hirayama A, Yamada M, Miake K. An electron microscopy study on dentinal tubules of human deciduous teeth. [In Japanese.] Dent Sci Rep 1986; 86: 1021–31. [PubMed] [Google Scholar]
- 40.Sumikawa DA, Marshall GW, Gee L, Marshall SJ. Microstructure of primary tooth dentin. Pediatr Dent 1999; 21: 439–44. [PubMed] [Google Scholar]
- 41.Wilson PR, Beynon AD. Mineralization differences between human deciduous and permanent enamel measured by quantitative microradiography. Arch Oral Biol 1989; 34: 85–8. [DOI] [PubMed] [Google Scholar]