Abstract
We have infected primary embryonic mouse limb chondrocytes with a retrovirus carrying simian virus 40 early regions and have obtained a monoclonal mouse chondrocyte line, MC615, that was able to grow on culture dishes for at least 7 months and 20 passages. MC615 cells show expression of simian virus 40 large T (tumor) antigen and express markers characteristic of cartilage in vivo, such as types II, IX, and XI collagen, as well as cartilage aggrecan and link protein. These data show that cell growth induced by large T oncogene expression does not prevent the maintenance of the chondrocytic phenotype.
Full text
PDF
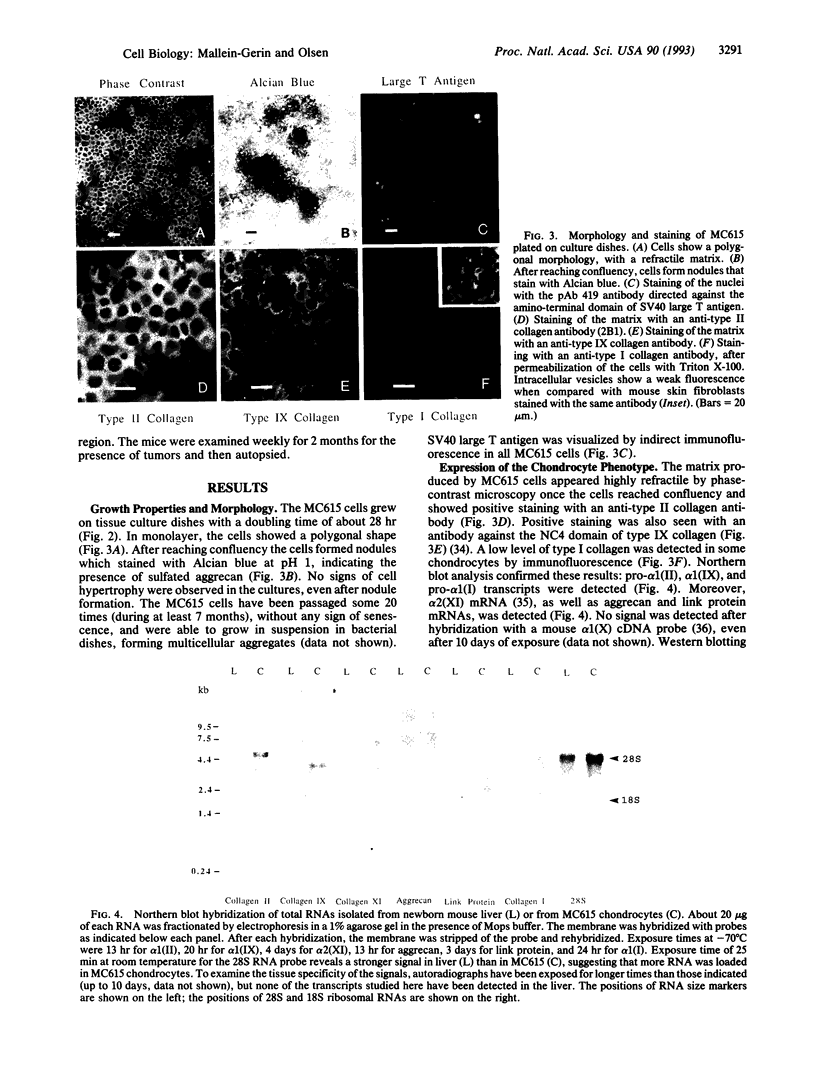
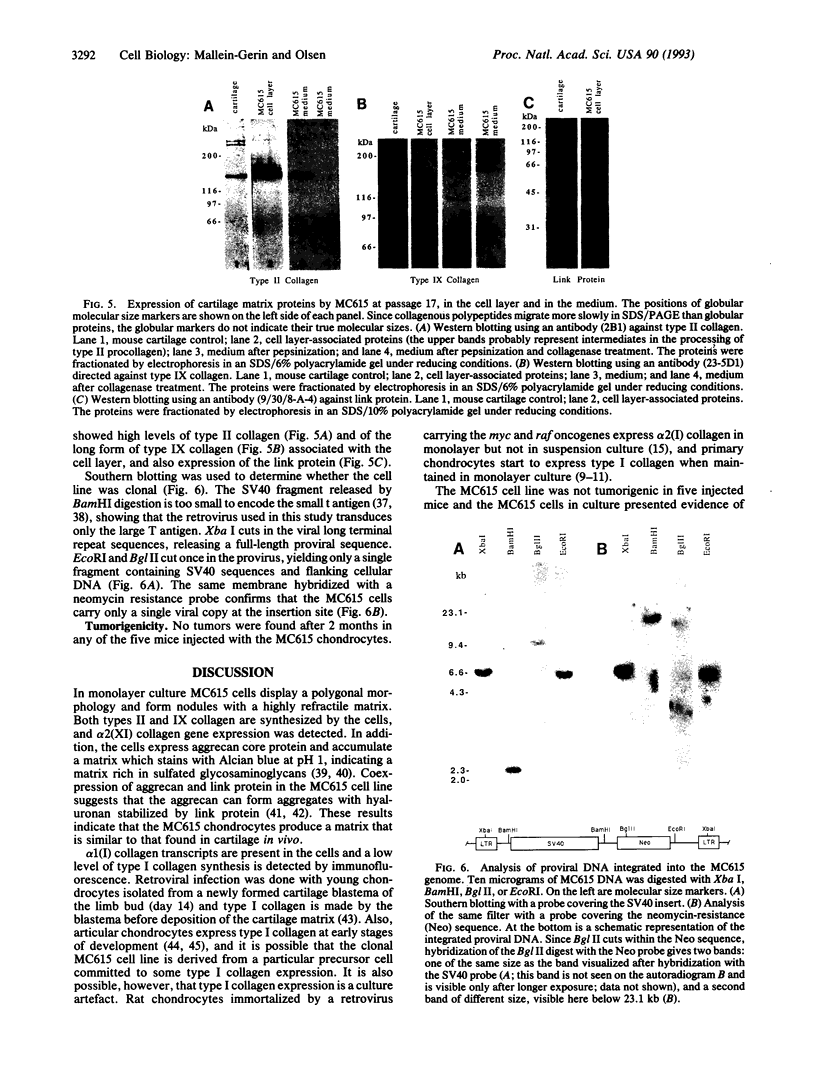

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ahrens P. B., Solursh M., Reiter R. S. Stage-related capacity for limb chondrogenesis in cell culture. Dev Biol. 1977 Oct 1;60(1):69–82. doi: 10.1016/0012-1606(77)90110-5. [DOI] [PubMed] [Google Scholar]
- Alema S., Tato F., Boettiger D. myc and src oncogenes have complementary effects on cell proliferation and expression of specific extracellular matrix components in definitive chondroblasts. Mol Cell Biol. 1985 Mar;5(3):538–544. doi: 10.1128/mcb.5.3.538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Apte S. S., Seldin M. F., Hayashi M., Olsen B. R. Cloning of the human and mouse type X collagen genes and mapping of the mouse type X collagen gene to chromosome 10. Eur J Biochem. 1992 May 15;206(1):217–224. doi: 10.1111/j.1432-1033.1992.tb16919.x. [DOI] [PubMed] [Google Scholar]
- Arnheim N. Characterization of mouse ribosomal gene fragments purified by molecular cloning. Gene. 1979 Oct;7(2):83–96. doi: 10.1016/0378-1119(79)90025-8. [DOI] [PubMed] [Google Scholar]
- Benya P. D., Padilla S. R., Nimni M. E. Independent regulation of collagen types by chondrocytes during the loss of differentiated function in culture. Cell. 1978 Dec;15(4):1313–1321. doi: 10.1016/0092-8674(78)90056-9. [DOI] [PubMed] [Google Scholar]
- Berk A. J., Sharp P. A. Spliced early mRNAs of simian virus 40. Proc Natl Acad Sci U S A. 1978 Mar;75(3):1274–1278. doi: 10.1073/pnas.75.3.1274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruckner P., Hörler I., Mendler M., Houze Y., Winterhalter K. H., Eich-Bender S. G., Spycher M. A. Induction and prevention of chondrocyte hypertrophy in culture. J Cell Biol. 1989 Nov;109(5):2537–2545. doi: 10.1083/jcb.109.5.2537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Capasso O., Gionti E., Pontarelli G., Ambesi-Impiombato F. S., Nitsch L., Tajana G., Cancedda R. The culture of chick embryo chondrocytes and the control of their differentiated functions in vitro. I. Characterization of the chondrocyte-specific phenotypes. Exp Cell Res. 1982 Nov;142(1):197–206. doi: 10.1016/0014-4827(82)90423-2. [DOI] [PubMed] [Google Scholar]
- Caterson B., Baker J. R., Christner J. E., Lee Y., Lentz M. Monoclonal antibodies as probes for determining the microheterogeneity of the link proteins of cartilage proteoglycan. J Biol Chem. 1985 Sep 15;260(20):11348–11356. [PubMed] [Google Scholar]
- Cepko C. L., Roberts B. E., Mulligan R. C. Construction and applications of a highly transmissible murine retrovirus shuttle vector. Cell. 1984 Jul;37(3):1053–1062. doi: 10.1016/0092-8674(84)90440-9. [DOI] [PubMed] [Google Scholar]
- Chirgwin J. M., Przybyla A. E., MacDonald R. J., Rutter W. J. Isolation of biologically active ribonucleic acid from sources enriched in ribonuclease. Biochemistry. 1979 Nov 27;18(24):5294–5299. doi: 10.1021/bi00591a005. [DOI] [PubMed] [Google Scholar]
- DeCaprio J. A., Ludlow J. W., Figge J., Shew J. Y., Huang C. M., Lee W. H., Marsilio E., Paucha E., Livingston D. M. SV40 large tumor antigen forms a specific complex with the product of the retinoblastoma susceptibility gene. Cell. 1988 Jul 15;54(2):275–283. doi: 10.1016/0092-8674(88)90559-4. [DOI] [PubMed] [Google Scholar]
- Doege K., Fernandez P., Hassell J. R., Sasaki M., Yamada Y. Partial cDNA sequence encoding a globular domain at the C terminus of the rat cartilage proteoglycan. J Biol Chem. 1986 Jun 25;261(18):8108–8111. [PubMed] [Google Scholar]
- Doege K., Hassell J. R., Caterson B., Yamada Y. Link protein cDNA sequence reveals a tandemly repeated protein structure. Proc Natl Acad Sci U S A. 1986 Jun;83(11):3761–3765. doi: 10.1073/pnas.83.11.3761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faltz L. L., Caputo C. B., Kimura J. H., Schrode J., Hascall V. C. Structure of the complex between hyaluronic acid, the hyaluronic acid-binding region, and the link protein of proteoglycan aggregates from the swarm rat chondrosarcoma. J Biol Chem. 1979 Feb 25;254(4):1381–1387. [PubMed] [Google Scholar]
- Feinberg A. P., Vogelstein B. "A technique for radiolabeling DNA restriction endonuclease fragments to high specific activity". Addendum. Anal Biochem. 1984 Feb;137(1):266–267. doi: 10.1016/0003-2697(84)90381-6. [DOI] [PubMed] [Google Scholar]
- Fitch J. M., Mentzer A., Mayne R., Linsenmayer T. F. Acquisition of type IX collagen by the developing avian primary corneal stroma and vitreous. Dev Biol. 1988 Aug;128(2):396–405. doi: 10.1016/0012-1606(88)90301-6. [DOI] [PubMed] [Google Scholar]
- Gerstenfeld L. C., Landis W. J. Gene expression and extracellular matrix ultrastructure of a mineralizing chondrocyte cell culture system. J Cell Biol. 1991 Feb;112(3):501–513. doi: 10.1083/jcb.112.3.501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gionti E., Pontarelli G., Cancedda R. Avian myelocytomatosis virus immortalizes differentiated quail chondrocytes. Proc Natl Acad Sci U S A. 1985 May;82(9):2756–2760. doi: 10.1073/pnas.82.9.2756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harlow E., Crawford L. V., Pim D. C., Williamson N. M. Monoclonal antibodies specific for simian virus 40 tumor antigens. J Virol. 1981 Sep;39(3):861–869. doi: 10.1128/jvi.39.3.861-869.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hassell J. R., Horigan E. A. Chondrogenesis: a model developmental system for measuring teratogenic potential of compounds. Teratog Carcinog Mutagen. 1982;2(3-4):325–331. doi: 10.1002/1520-6866(1990)2:3/4<325::aid-tcm1770020314>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- Heinegård D., Hascall V. C. Aggregation of cartilage proteoglycans. 3. Characteristics of the proteins isolated from trypsin digests of aggregates. J Biol Chem. 1974 Jul 10;249(13):4250–4256. [PubMed] [Google Scholar]
- Heinegård D., Oldberg A. Structure and biology of cartilage and bone matrix noncollagenous macromolecules. FASEB J. 1989 Jul;3(9):2042–2051. doi: 10.1096/fasebj.3.9.2663581. [DOI] [PubMed] [Google Scholar]
- Horton W. E., Jr, Cleveland J., Rapp U., Nemuth G., Bolander M., Doege K., Yamada Y., Hassell J. R. An established rat cell line expressing chondrocyte properties. Exp Cell Res. 1988 Oct;178(2):457–468. doi: 10.1016/0014-4827(88)90414-4. [DOI] [PubMed] [Google Scholar]
- Jacenko O., Olsen B. R., LuValle P. Organization and regulation of collagen genes. Crit Rev Eukaryot Gene Expr. 1991;1(4):327–353. [PubMed] [Google Scholar]
- Jat P. S., Cepko C. L., Mulligan R. C., Sharp P. A. Recombinant retroviruses encoding simian virus 40 large T antigen and polyomavirus large and middle T antigens. Mol Cell Biol. 1986 Apr;6(4):1204–1217. doi: 10.1128/mcb.6.4.1204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jat P. S., Sharp P. A. Large T antigens of simian virus 40 and polyomavirus efficiently establish primary fibroblasts. J Virol. 1986 Sep;59(3):746–750. doi: 10.1128/jvi.59.3.746-750.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson P., Gray D., Mowat M., Benchimol S. Expression of wild-type p53 is not compatible with continued growth of p53-negative tumor cells. Mol Cell Biol. 1991 Jan;11(1):1–11. doi: 10.1128/mcb.11.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaelin W. G., Jr, Ewen M. E., Livingston D. M. Definition of the minimal simian virus 40 large T antigen- and adenovirus E1A-binding domain in the retinoblastoma gene product. Mol Cell Biol. 1990 Jul;10(7):3761–3769. doi: 10.1128/mcb.10.7.3761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimura T., Cheah K. S., Chan S. D., Lui V. C., Mattei M. G., van der Rest M., Ono K., Solomon E., Ninomiya Y., Olsen B. R. The human alpha 2(XI) collagen (COL11A2) chain. Molecular cloning of cDNA and genomic DNA reveals characteristics of a fibrillar collagen with differences in genomic organization. J Biol Chem. 1989 Aug 15;264(23):13910–13916. [PubMed] [Google Scholar]
- LEV R., SPICER S. S. SPECIFIC STAINING OF SULPHATE GROUPS WITH ALCIAN BLUE AT LOW PH. J Histochem Cytochem. 1964 Apr;12:309–309. doi: 10.1177/12.4.309. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Laird P. W., Zijderveld A., Linders K., Rudnicki M. A., Jaenisch R., Berns A. Simplified mammalian DNA isolation procedure. Nucleic Acids Res. 1991 Aug 11;19(15):4293–4293. doi: 10.1093/nar/19.15.4293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lane D. P., Crawford L. V. T antigen is bound to a host protein in SV40-transformed cells. Nature. 1979 Mar 15;278(5701):261–263. doi: 10.1038/278261a0. [DOI] [PubMed] [Google Scholar]
- Linzer D. I., Levine A. J. Characterization of a 54K dalton cellular SV40 tumor antigen present in SV40-transformed cells and uninfected embryonal carcinoma cells. Cell. 1979 May;17(1):43–52. doi: 10.1016/0092-8674(79)90293-9. [DOI] [PubMed] [Google Scholar]
- Ludlow J. W., DeCaprio J. A., Huang C. M., Lee W. H., Paucha E., Livingston D. M. SV40 large T antigen binds preferentially to an underphosphorylated member of the retinoblastoma susceptibility gene product family. Cell. 1989 Jan 13;56(1):57–65. doi: 10.1016/0092-8674(89)90983-5. [DOI] [PubMed] [Google Scholar]
- Ludlow J. W., Shon J., Pipas J. M., Livingston D. M., DeCaprio J. A. The retinoblastoma susceptibility gene product undergoes cell cycle-dependent dephosphorylation and binding to and release from SV40 large T. Cell. 1990 Feb 9;60(3):387–396. doi: 10.1016/0092-8674(90)90590-b. [DOI] [PubMed] [Google Scholar]
- Mayne R., Vail M. S., Mayne P. M., Miller E. J. Changes in type of collagen synthesized as clones of chick chondrocytes grow and eventually lose division capacity. Proc Natl Acad Sci U S A. 1976 May;73(5):1674–1678. doi: 10.1073/pnas.73.5.1674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendler M., Eich-Bender S. G., Vaughan L., Winterhalter K. H., Bruckner P. Cartilage contains mixed fibrils of collagen types II, IX, and XI. J Cell Biol. 1989 Jan;108(1):191–197. doi: 10.1083/jcb.108.1.191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Metsäranta M., Toman D., De Crombrugghe B., Vuorio E. Specific hybridization probes for mouse type I, II, III and IX collagen mRNAs. Biochim Biophys Acta. 1991 Jun 13;1089(2):241–243. doi: 10.1016/0167-4781(91)90014-d. [DOI] [PubMed] [Google Scholar]
- Quarto R., Dozin B., Tacchetti C., Robino G., Zenke M., Campanile G., Cancedda R. Constitutive myc expression impairs hypertrophy and calcification in cartilage. Dev Biol. 1992 Jan;149(1):168–176. doi: 10.1016/0012-1606(92)90273-j. [DOI] [PubMed] [Google Scholar]
- Rhodes C., Doege K., Sasaki M., Yamada Y. Alternative splicing generates two different mRNA species for rat link protein. J Biol Chem. 1988 May 5;263(13):6063–6067. [PubMed] [Google Scholar]
- Sandberg M., Mäkelä J. K., Multimäki P., Vuorio T., Vuorio E. Construction of a human pro alpha 1(III) collagen cDNA clone and localization of type III collagen expression in human fetal tissues. Matrix. 1989 Mar;9(2):82–91. doi: 10.1016/s0934-8832(89)80025-3. [DOI] [PubMed] [Google Scholar]
- Takigawa M., Pan H. O., Kinoshita A., Tajima K., Takano Y. Establishment from a human chondrosarcoma of a new immortal cell line with high tumorigenicity in vivo, which is able to form proteoglycan-rich cartilage-like nodules and to respond to insulin in vitro. Int J Cancer. 1991 Jul 9;48(5):717–725. doi: 10.1002/ijc.2910480515. [DOI] [PubMed] [Google Scholar]
- Thenet S., Benya P. D., Demignot S., Feunteun J., Adolphe M. SV40-immortalization of rabbit articular chondrocytes: alteration of differentiated functions. J Cell Physiol. 1992 Jan;150(1):158–167. doi: 10.1002/jcp.1041500121. [DOI] [PubMed] [Google Scholar]
- Treilleux I., Mallein-Gerin F., le Guellec D., Herbage D. Localization of the expression of type I, II, III collagen, and aggrecan core protein genes in developing human articular cartilage. Matrix. 1992 Jun;12(3):221–232. doi: 10.1016/s0934-8832(11)80065-x. [DOI] [PubMed] [Google Scholar]
- Ulrich E., Boehmelt G., Bird A., Beug H. Immortalization of conditionally transformed chicken cells: loss of normal p53 expression is an early step that is independent of cell transformation. Genes Dev. 1992 May;6(5):876–887. doi: 10.1101/gad.6.5.876. [DOI] [PubMed] [Google Scholar]
- Vasios G., Nishimura I., Konomi H., van der Rest M., Ninomiya Y., Olsen B. R. Cartilage type IX collagen-proteoglycan contains a large amino-terminal globular domain encoded by multiple exons. J Biol Chem. 1988 Feb 15;263(5):2324–2329. [PubMed] [Google Scholar]
- Vaughan L., Mendler M., Huber S., Bruckner P., Winterhalter K. H., Irwin M. I., Mayne R. D-periodic distribution of collagen type IX along cartilage fibrils. J Cell Biol. 1988 Mar;106(3):991–997. doi: 10.1083/jcb.106.3.991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von der Mark K., Gauss V., von der Mark H., Müller P. Relationship between cell shape and type of collagen synthesised as chondrocytes lose their cartilage phenotype in culture. Nature. 1977 Jun 9;267(5611):531–532. doi: 10.1038/267531a0. [DOI] [PubMed] [Google Scholar]
- von der Mark K. Immunological studies on collagen type transition in chondrogenesis. Curr Top Dev Biol. 1980;14(Pt 2):199–225. doi: 10.1016/s0070-2153(08)60195-7. [DOI] [PubMed] [Google Scholar]