Abstract
Major advances in radiotherapy techniques, increasing knowledge of tumour biology and the ability to translate these advances into new therapeutic approaches are important goals towards more individualized cancer treatment. With the development of non-invasive functional and molecular imaging techniques such as positron emission tomography (PET)-CT scanning and MRI, there is now a need to evaluate potential new biomarkers for tumour response prediction, for treatment individualization is not only based on morphological criteria but also on biological tumour characteristics. The goal of individualization of radiotherapy is to improve treatment outcome and potentially reduce chronic treatment toxicity. This review gives an overview of the molecular and functional imaging modalities of tumour hypoxia and tumour cell metabolism, proliferation and perfusion as predictive biomarkers for radiation treatment response in head and neck tumours and in lung tumours. The current status of knowledge on integration of PET/CT/MRI into treatment management and bioimage-guided adaptive radiotherapy are discussed.
Advances in understanding the molecular biology of cancer and the ability to translate these advances into therapeutic approaches are important achievements towards individualized cancer treatment. With the development of non-invasive functional and molecular imaging modalities such as positron emission tomography (PET)-CT scanning and MRI, there is now a need to evaluate potential new biomarkers for tumour response prediction. It is noteworthy that treatment individualization is not only based on morphological criteria but also on biological tumour characteristics such as metabolic and proliferative activity, and hypoxic tumour status before and during treatment.1 The validation and integration of imaging biomarkers before and early during therapy are important tasks for further clinical research and may help to individually select, adapt and optimize treatment schedules for patients in order to improve treatment outcomes, that is, to increase tumour control probability and/or to reduce chronic treatment-related toxicity.2
The primary aim of a predictive biomarker is to accurately determine the outcome of a given treatment. Therefore, the accurate prediction may help facilitate potential interventions early during the course of treatment. By contrast, prognostic markers show an association with patient outcome independent of a given treatment. The increasing use and availability of PET/CT as well as of MRI in radiotherapy will make it feasible to incorporate imaging predictive tests into clinical practice if validation studies confirm the utility of specific PET tracers or functional MRI or CT parameters. In this review, the capacity to use these functional imaging biomarkers is focused on PET, MRI and CT for radiotherapy response detection in head and neck tumours and in lung tumours.
PRE-CLINICAL EVALUATION OF FUNCTIONAL PET IMAGING FOR TREATMENT RESPONSE
Multi-imaging (CT, micro-MRI, micro-PET and autoradiography) registration of tumour xenografts in mice as well as different PET tracers before or during fractionated radiotherapy has been investigated in several pre-clinical studies.3,4 On the microregional level, experiments investigated the correlation between autoradiography of different PET tracers with immunohistochemical stainings.5 [18F]2-fluoro-2-deoxy-D-glucose (18F-FDG) is the most widely used PET tracer for diagnosis, staging and response monitoring in oncology (Figure 1). The results of a proof-of-principle experimental study performed by the author of this review and his coworkers (within the seventh framework of the BioCare project) showed that a higher single radiation dose had a greater effect on local control in tumours with high pre-therapeutic 18F-FDG-uptake than in tumours with low 18F-FDG uptake in a human head and neck squamous cell carcinoma xenograft model.6 The biological mechanisms underlying the observed differences in radiation response between tumours with high and low 18F-FDG uptake are so far not understood. High 18F-FDG uptake reflects tumour subvolumes with high cell density and may identify radioresistant tumour cells owing to hypoxia or other mechanisms of radioresistance, although pre-clinical data comparing 18F-FDG PET, autoradiography and functional histology are partly contradictory.7
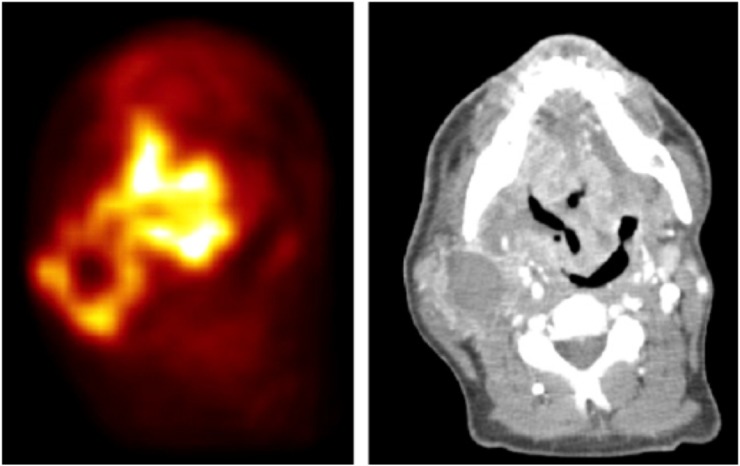
Figure 1.
[18F]2-fluoro-2-deoxy-D-glucose (18F-FDG) positron emission tomography (left) and CT (right) of a patient with tonsil carcinoma and bicervical lymph node metastasis before the start of irradiation therapy showing intratumoral heterogeneity of 18F-FDG uptake. Reprinted from Schütze et al6 with permission from Elsevier Science Publishers.
Therefore, 18F-FDG was compared with one of the hypoxia PET tracers, 2-nitroimidazole [18F]-EF3, on the histological level by Christian et al.8 They found only a small overlap between both tracers, rebutting the assumption that 18F-FDG may reflect mainly tumour hypoxia. For another PET tracer, [18F]fluoromisonidazole (FMISO), an overall good correlation between the FMISO autoradiography signal and pimonidazole immunohistochemistry was found, and the FMISO signal varied with the level of hypoxia, that is, under ambient conditions, carbogen breathing or clamping.9 Investigating the effect of dose escalation in tumours with high 18F-FDG-uptake, an experiment at our institution (Clinical and Experimental Radiotherapy, OncoRay) addressed the question whether dose escalation to high 18F-FDG subvolumes before fractionated radiotherapy or to the residual high 18F-FDG uptake subvolumes after 18 Gy would be more effective [(Clinical and Experimental Radiotheraphy, OncoRay) C Jentsch, R Bergmann, K Brüchner, B Mosch, A Yaromina, M Krause, D Zips, H Thames, J Kotzerke, J Steinbach, M Baumann, B Beuthien-Baumann, manuscript in preparation, 2015]. Only 18F-FDG uptake prior to fractionated irradiation, in contrast to 18F-FDG uptake early during fractionated irradiation at 18 Gy, was found to be predictive for local control.
A proof-of-principle study performed by the author and coworkers investigated the effect of radiation dose escalation on local control in hypoxic vs non-hypoxic tumours defined using FMISO in FaDu human squamous cell carcinoma xenografts growing subcutaneously in nude mice.10 Single irradiation dose escalation resulted in an incremental increase in local tumour control from low-dose hypoxic, over low-dose non-hypoxic and high-dose hypoxic to high-dose non-hypoxic, tumours. The negative effect of FMISO hypoxic volume on permanent local tumour control supports its prognostic value. This result needs to be confirmed using different tumour entities and fractionated radiotherapy schedules.
A novel developed hypoxia marker for PET imaging, 3-[18F]fluoro-2-(4-((2-nitro-1Himidazol-1-yl)methyl)-1H-1,2,3,-triazol-1-yl)-propan-1-ol (HX4), has been evaluated and validated pre-clinically by Dubois et al.11 Recently, a comparative study of the hypoxia PET tracers HX4, [18F]-fluoroazomycinarabinoside (FAZA) and FMISO has been performed in a pre-clinical tumour model by Peeters et al.12 Because of the short half-life and clearance of HX4, a higher tumour-to-background ratio than found for current hypoxia markers such as FMISO is expected. Currently, HX4 is being studied in clinical trials in non-small-cell lung cancer (NSCLC).13,14 Furthermore, an ongoing clinical study investigates HX4-PET in patients with head and neck squamous cell carcinoma (HNSCC) and compares HX4-PET uptake with 18F-FDG PET uptake before and during (chemo)radiotherapy (ClinicalTrials.gov identifier: NCT01347281).
CLINICAL EVALUATION OF FUNCTIONAL PET IMAGING FOR RADIATION TREATMENT RESPONSE IN HEAD AND NECK AND NON-SMALL CELL LUNG CANCER
The non-invasive or minimally invasive nature of molecular imaging techniques allows serial evaluation of changes in tumour biological characteristics. Molecular imaging is therefore suitable to provide not only pre-treatment parameters but also allows for early assessment of treatment effects. In most studies performed to date, however, treatment response using imaging has been evaluated after the end of therapy. Several studies have addressed the potential clinical utility of 18F-FDG, FMISO or [18F]fluorothymidine (FLT) PET for early response assessment during therapy.15–17
In recent years radiotracer-based molecular imaging with PET, mainly using the glucose analogue 18F-FDG, has been shown to improve accuracy in the diagnosis and staging of various tumour types.18–25 Furthermore, it has been proven to be beneficial for both therapy monitoring and differentiating between residual or recurrent tumour and non-specific post-therapeutic changes.26–28 High 18F-FDG uptake reflects mainly tumour subvolumes with high cell density and metabolic activity and may identify areas of radioresistant tumour cells owing to hypoxia or other mechanisms of radioresistance.24,29,30 The hypothesis of a more aggressive tumour phenotype within highly 18F-FDG-avid tumour regions is also supported by the observation that local recurrences after radiotherapy often occur within the irradiated target volume with the highest 18F-FDG uptake.31–35
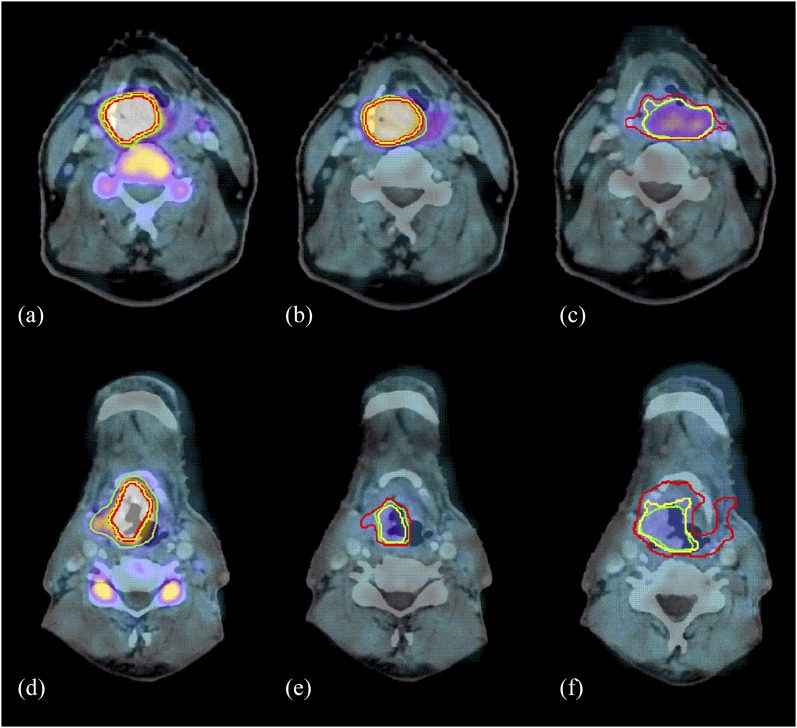
It is well recognized that in different patients, tumours of the same histology may show a varying avidity for 18F-FDG. This intertumoral heterogeneity in 18F-FDG uptake has been demonstrated in a number of clinical studies correlating imaging features with outcome after (chemo)radiotherapy in various tumour entities.36–38 Currently available data concerning an intensive monitoring of HNSCC early in the course of (chemo)radiotherapy are limited.39–44 48 patients with head and neck cancer underwent repeat FLT PET/CT before and during the second and fourth weeks of (chemo)radiotherapy. It was found that a change in FLT uptake early during radiotherapy or chemoradiotherapy was a strong indicator for long-term outcome (Figure 2). FLT PET may thus aid in personalized patient management by steering treatment modifications during an early phase of therapy.
Figure 2.
[18F]fluorothymidine (FLT) positron emission tomography/CT before therapy (a, d), in the second week of therapy (b, e) and in the fourth week of therapy (c, f). The first example (a–c) shows slow decrease in FLT uptake (cT4N2bM0 supraglottic laryngeal carcinoma treated with chemoradiotherapy; local recurrence after 7 months; later distant metastases) and the second one (d–f) fast decrease (cT3N1M0 supraglottic laryngeal carcinoma treated with radiotherapy only; no tumour-related event after 32 months of follow-up). For gross tumour volume (GTV)VIS (green), three dimensional volume change was +8% between A and B, –35% between A and C, –60% between D and E, and –66% between D and F. For GTVSBR (yellow), three-dimensional volume change was +7% between A and B, +4% between A and C, –44% between D and E, and +22% between D and F. For GTV50% (red), three-dimensional volume change was +11% between A and B, +102% between A and C; +30% between D and E, and +247% between D and F. The maximum standardized uptake value changed by –35% between A and B, –70% between A and C, –58% between D and E, and –69% between D and F. Reprinted from Hoeben et al17 with permission from the Society of Nuclear Medicine and Molecular Imaging Inc.
In addition, in an individual patient's tumour, subvolumes with lower and higher 18F-FDG uptake, that is, intratumoral heterogeneity, may be present.20 It is currently unknown whether these subvolumes in individual tumours differ in their radiation response. In HNSCC, Minn et al37 and Allal et al45 demonstrated that pre-therapeutic 18F-FDG uptake above the median value was associated with poor local tumour control. Both authors concluded that patients with high pre-therapeutic 18F-FDG uptake should be considered at increased risk of failure and might benefit from more aggressive multimodality treatment options early during the course of disease. 18F-FDG uptake or decrease in uptake during the first 2 weeks of chemoradiation showed a significant association with treatment outcome in patients with head and neck cancer.20,46 By contrast, Castaldi et al47 could not confirm a predictive role of “early” metabolic response in HNSCC. They found a statistically significant correlation only with the 18F-FDG PET after the end of treatment. The small sample size and heterogeneity of the population may limit interpretation of their data. Similarly, inconsistent results have been reported on the role of baseline and longitudinal 18F-FDG-PET in predicting treatment outcomes in patients with NSCLC.48,49 Considering the possibility of modifying the initially chosen therapeutic approach, however, PET-tracer-guiding treatment alterations early in treatment is mandatory, increasing tumour control probability and preventing patients suffering from potentially unnecessary side effects.
Much effort has been undertaken to develop and improve non-invasive methods for detecting hypoxia by different imaging modalities including PET. FMISO was the first radiotracer proposed for detecting hypoxia with PET50 and has been evaluated clinically.13,14,33 Intertumoral pre-treatment uptake of hypoxia PET tracers has been found in several studies to be prognostic for response to radiotherapy in patients with head and neck cancer.12,13,21,23,29,32,34,40,51,52 It is also well recognized that uptake of hypoxia PET tracers in individual patients shows pronounced heterogeneity, even though it is yet unclear whether this observation has prognostic value for achieving local control in specific tumour subvolumes.21 Far more difficult than to study the impact of hypoxia on outcome in different patients is to investigate also whether information on intratumoral heterogeneity within an individual tumour can be used for the selection of optimal treatment such as combination of radiotherapy with hypoxia modifiers or biologically individualized radiation treatment such as “dose painting”.53,54 However, it is yet unknown whether the biologically individualized treatment based on hypoxia tracer uptake would result in improved local tumour control. During the past decades, controlled clinical trials demonstrated that some of these strategies to overcome tumour hypoxia were able to improve the effect of radiotherapy.55 Other methods are currently being assessed in large international multicentre studies.56,57
FUNCTIONAL BIOIMAGE-TAILORED RADIOTHERAPY FOR HEAD AND NECK CANCER AND NON-SMALL-CELL LUNG CANCER
The integration of 18F-FDG PET/CT fusion imaging into radiation treatment planning by taking into account the metabolic and biological characteristics of tumours has been demonstrated to have significant impact on the selection and delineation of irradiation treatment volumes in HNSCC and NSCLC.46,58–64 However, integration of PET images into the radiotherapy planning process in routine practice should be carried out with caution because of uncertainties about tumour segmentation.65,66 Considering radiation field adaption based on serial 18F-FDG-PET during radiation, data interpretation can be hampered by the inflammatory response of normal tissue, especially in HNSCC.38
Since the introduction of the concept of dose painting of biological image-defined regions within a target, two methods have been proposed: dose painting by contours and dose painting by numbers.54,67,68 The former prescribes dose within biological image-defined contours of the target (subvolume boosting). The latter prescribes dose to voxels throughout the target as a function of signal intensity of the corresponding voxel in a biological image69. Bentzen54 described the potential of molecular and functional imaging for prescription of the distribution of radiation dose in three dimensions and time “theragnostic” (therapeutic and diagnostic) imaging in radiation oncology. Dose painting by numbers relies directly on theragnostic imaging to produce a prescribed dose map. Adaptive dose painting aims to use imaging as a biomarker of (local) response, typically derived from repeat imaging during radiotherapy compared with a scan at baseline (before start of treatment). This response map is then used as the input map to adapt the delineated target volume or, in case of dose painting by numbers, the dose distribution so that relatively more dose is applied in regions showing poor response.
The idea of adaptation of the target volume during the course of the radiation treatment, which would allow for a reduction of the treated volume as radiotherapy progresses and help to spare healthy tissue has been shown in proof-of-principle studies.70,71 With conventional dose prescriptions, Geets et al70 noted no reduction in radiation dose to the unintentionally irradiated organs at risk, but this approach could be especially helpful in combination with dose escalation strategies. Nevertheless, adaptive PET-guided radiotherapy remains an attractive approach, but the safety and clinical effect remain to be shown in clinical studies. The multicentre randomized Phase II PET Boost trial in NSCLC (PET Boost, NCT01024829) thus far has reported no increase in toxicity.72 Moreover, a Phase III randomized study in HNSCC is investigating the redistribution of the radiation dose to the metabolically most 18F-FDG-PET-avid part of the tumour (ARTFORCE, ClinicalTrials.gov identifier: NCT0150481573).
Clinically so far, only the feasibility of inhomogeneous hypoxia dose-painting strategies in tumour subvolumes has been investigated in planning studies. In a Phase I clinical trial, the dose could be homogeneously escalated inside 18F-FDG-PET-defined contour, and treatment was well tolerated in 41 patients with locally advanced HNSCC.32 Following Phase I, studies demonstrated the feasibility of adaptive 18F-FDG-PET-voxel intensity-based intensity modulated radiotherapy (IMRT) using dose painting by numbers in patients with head and neck cancer as well the feasibility of using deformable image co-registration of a three-phase adaptive 18F-FDG-PET-guided dose painting by numbers tool.74,75
In a recent Phase I clinical trial, a dose escalation in 18F-FDG regions and dose painting by numbers approach (i.e. dose prescription to voxels as a function of signal intensity of the corresponding voxel in a biological image) was used for adaptation of radiation treatment volumes based on 18F-FDG-PET/CT-detected biological and anatomical changes at the end of the second week of treatment. With acceptable acute toxicity, the approach resulted in a significant reduction of target volumes in patients with head and neck cancer.74 Besides, a prospective randomized Phase II trial is currently investigating adaptive dose painting by numbers based on 18F-FDG-PET compared with standard IMRT in patients with HNSCC (NCT01341535).
Additionally, it has been shown in 13 patients with head and neck cancer that it was possible to deliver spatially variant doses according to the dynamic FMISO-PET data while respecting the dose constraints for normal tissues.39 Therefore, an ongoing randomized clinical Phase II study in Tübingen, Germany, tests the concept of individual hypoxia dose painting based on FMISO-PET in patients with HNSCC (ClinicalTrials.gov identifier: NCT02352792).
These adaptive PET-guided radiotherapy strategies are expected to result in an increase of local tumour control, but side effects need to be monitored rigorously.
A few radiotherapy-planning studies have been conducted so far on 18F-FDG-PET-based adaptive treatment in NSCLC. The feasibility of boosting areas with residual 18F-FDG uptake at mid-treatment or later during radiotherapy in NSCLC is, however, controversial.76,77 Dose painting planning studies in patients with NSCLC have recently been suggested to improve tumour control probability, and clinical studies are being conducted accordingly.78–80 However, so far no reports are available that demonstrate that an increase in dose to hypoxic subvolumes in individual tumours may improve radiotherapy outcome. The only result reported so far is that no increase in toxicity was observed in the aforementioned PET Boost trial.72
Overall, these studies confirm the value of 18F-FDG-PET for monitoring early treatment response and show the feasibility of integrating PET for radiotherapy optimization before initiation of treatment and/or early during therapy. The optimal time points of imaging, that is, before or during treatment, for accurate response prediction and subsequent modifications or adaptation of treatment protocols need to be defined. In a number of clinical studies, it has been shown that changes in 18F-FDG uptake during (chemo)radiotherapy may better correlate with treatment outcome than baseline measurements.
One concern in the development of hypoxia-directed dose painting is the spatiotemporal stability of the PET hypoxia map. Already the pioneering FMISO-PET study by Koh et al81 showed PET-detected reoxygenation in some but not all tumours investigated. Spatial variability has also been shown in a subset of HNSCC tumours in scan–rescan studies conducted before the onset of radiation therapy.82,83 Conversely, Lee et al84 found that boosting the dose to the hypoxic subvolume on one scan would still lead to a substantial additional dose to the subvolume that was found to be hypoxic on the repeat FMISO-PET scan. For the novel hypoxia PET tracer, HX4, repeat scans were found to be reproducible and spatially stable in HNSCC.85
The magnitude of the required dose to control disease in PET hypoxic regions is not clear. Simplistic estimates based on in vitro oxygen enhancement ratios are likely to be gross overestimates of the dose required in human tumours. Interestingly, in this context, Lee et al86 found excellent locoregional tumour control in a series of 20 patients after standard chemoradiation therapy despite the presence of PET-detected hypoxia in the primary tumour or in positive nodes at baseline or during fractionated therapy in 18 of these cases.
A recent development in the evaluation of bioimaging is the extraction of radiomics features from CT and PET scans and their correlation with clinical outcome. Elegantly, Aerts et al87 performed a radiogenomics analysis and found a prognostic radiomic signature, capturing intratumour heterogeneity, to be associated with underlying gene-expression patterns in both HNSCC and NSCLC. Furthermore, the same group found CT-based radiomics features to predict distant metastasis in adenocarcinoma of the lung.88 Numerous hypotheses concerning radiomics will most certainly be tested in the nearby future, further tailoring the individual patient's treatment.
ONGOING CLINICAL STUDIES EVALUATING BIOIMAGE-GUIDED ADAPTIVE RADIOTHERAPY IN HEAD AND NECK AND LUNG CANCER
Clearly, prospective clinical trials in well-defined populations of patients are needed to test whether redistribution or boosting of radiation dose to metabolically active (i.e. 18F-FDG) or hypoxic (i.e. FMISO) tumour subvolumes results in improved outcome after (chemo)radiotherapy.
A currently recruiting Phase II clinical trial (NCT01341535) illustrates the narrow therapeutic window for radiation dose escalation in HNSCC. Standard IMRT is compared with adaptive 18F-FDG-PET voxel intensity-based IMRT or volumetric-modulated arc therapy using repetitive per-treatment planning 18F-FDG-PET/CT scans for HNSCC. The investigators hypothesize that treatment adaptation to biological and anatomical changes, occurring during treatment, can increase the chance of cure at minimized or equal radiation-induced toxicity in patients with head and neck cancer.
A Phase III clinical study on 18F-FDG-PET in patients with HNSCC randomizes between conventional or adaptive high-dose radiotherapy in an international multicentre setting (NCT01504815). Primary end points include locoregional recurrence-free survival and number of patients experiencing grade 3 or more toxicity.
An ongoing randomized clinical Phase II study in Tübingen, Germany, tests the concept of individual hypoxia dose painting in patients with HNSCC (ClinicalTrials.gov identifier: NCT02352792). This clinical study hypothesizes that patients can be stratified based on pre-treatment dynamic FMISO-PET/CT into three different risk groups (low, intermediate and high) and that dose escalation by 10% improves local control in the group with intermediate risk. Alternatives to this approach include the application of hypoxic cell sensitizers or dose escalation to the full macroscopic tumour in patients stratified by hypoxia PET as having a low chance of local tumour control after standard treatment.
In NSCLC, an ongoing international multicentre randomized Phase II clinical trial investigates whether boosting of radiation dose to the subvolume of the primary tumour with high pre-treatment 18F-FDG uptake [50% maximum standardized uptake value (SUVmax)] results in a better local progression-free survival than escalating the dose to the primary tumour as a whole in patients with lung cancer (NCT01024829).
ROLE OF FUNCTIONAL MRI AND CT IMAGING IN TREATMENT RESPONSE PREDICTION
MRI is a powerful modality for analysing morphological and functional properties of the tumour and normal tissue. MRI has received wide acceptance in diagnostics owing to its superior contrast in soft tissues compared with CT. For this amongst other reasons, for example, real-time in vivo tracking of the target volume, MRI is currently gaining popularity as a modality of choice for delineation of tumours for radiation therapy or even as the only modality for treatment planning.89 In this review, we will focus on the capabilities of MRI to characterize functional properties of the tumour for prognosis, treatment planning and response assessment.
MR spectroscopy
MR spectroscopy (MRS), that is, 1H-MRS and 31P-MRS, can measure levels of cholin, creatine, lipids and lactate, which are known to be prognostic markers, but the method is technically challenging and has not been a widely utilized in the past 15 years.90
Diffusion weighted MRI
Diffusion-weighted MRI (DW-MRI) can estimate diffusion levels of water molecules in tissue, which can be used to detect and characterize malignancies. It was found that a high degree of restriction of water diffusion correlates with increased cellular density and extracellular space, a typical feature of cancerous tissues.91,92 For head and neck cancer, Hauser et al93 demonstrated that parameters derived from diffusion weighting are prognostic (e.g. high value of perfusion-related parameter is associated with poor prognosis) and are changing during therapy. Overall, an increase in diffusion-related coefficient, perfusion-related parameter and apparent-diffusion coefficient (ADC) correlated with good outcome. Srinivasan et al94 demonstrated significant differences in mean ADC between patients showing positive and negative outcomes. Kim et al95 showed in 33 patients that a pre-treatment ADC value of complete responders was significantly lower than that from partial responders or non-responders. Stratification of responders and partial or non-responders based on ADC value was possible with a sensitivity of 65% and a specificity of 86%. The same study showed that an increase in ADC within 1 week of therapy is associated with complete response (86% sensitivity and 83% specificity). At the same time, changes in normalized tumour volumes of responders were not significantly different from those of partial responders indicating higher relevance of changes of diffusion restriction within the tumour under therapy as compared with morphological volumetric changes.
Several technical challenges exist for DW-MRI in the head and neck region: (i) susceptibility, chemical shift and other artefacts—owing to steep density profiles on tissue–air borders; (ii) motion artefacts—owing to head motion and swallowing. There are several workarounds to decrease these artefacts including the use of special sequences, reduction of the imaging time and fixation devices.96 Absolute thresholds of ADC values were used in the studies mentioned above in order to stratify responding and non-responding patients. However, these thresholds vary from study to study making it difficult if possible to derive a generic imaging biomarker. Additionally, most of the studies were based on mean ADC value within the tumour, thus making the result dependent on the segmentation quality. In summary, DW-MRI is a promising though challenging technique for prognosis and early response assessment to chemoradiotherapy in patients with HNSCC.
Dynamic contrast-enhanced MRI
Dynamic contrast-enhanced MRI (DCE-MRI) is a technique allowing the dynamic monitoring of the distribution of the injected contrast agent within the region of interest. For this technique, sequential MR scans are acquired before, during and after injection of contrast agent. Modelling and analysis of contrast agent concentration as a function of time allows for assessment of tissue perfusion and permeability—highly relevant characteristics describing tumour malignancy. There are several approaches for analysis of DCE-MRI: a quantitative approach using pharmacokinetic modelling, for example Toffs model,97,98 and a semi-quantitative one using longitudinal analysis of DCE-MRI signal intensity function, for example by means of DCE time–intensity curve shape analysis.99 Two reviews on utilization of DCE-MRI in HNSCC have recently been published.97,100 In summary, these reviews show that DCE-MRI is a feasible modality for diagnosis and as a source of prognostic markers in HNSCC. It is noteworthy that the reviews also highlighted challenges of high interstudy heterogeneity in DCE-MRI acquisition parameters, lack of standardization, pharmacokinetic modelling issues and the relatively small number of patients in the studies included in the reviews, making DCE-MRI attractive but still a research modality outside standard care.
Currently, several clinical trials are ongoing in order to assess the value of DW-MRI and DCE-MRI in treatment of HNSCC, for example NCT00581906 (320 patients; risk stratification prognosis before therapy based on MRI), NCT01829646 (120 patients; Prediction of outcome after chemoradiotherapy for head and neck cancer and tumour biology), NCT02273778 (MRI and PET-CT for radiotherapy planning for head and neck cancer) and NCT02031250 (Randomized Phase II study of DCE-MRI-based dose escalation for poor-prognosis and neck cancer).
Becker and Zaidi101 have recently addressed the role of the hybrid PET/MRI in diagnosis and follow-up of patients with HNSCC in a review article. The main identified challenges are cross-modality registration, MRI artefacts, number and range of additionally required MR sequences. Despite a promising combination of morphological, functional and molecular information, hybrid PET/MRI still remains within the field of challenging academic research.
CT perfusion
CT perfusion (CTp) is an imaging modality for quantitative assessment of tissue perfusion based on a technique similar to DCE-MRI; multiple CT scans are performed before, during and after injection of an iodinated contrast medium. Advantage of CTp over DCE-MRI is a linear correlation of signal intensity with contrast agent concentration. The main disadvantage is ionizing radiation during multiple CT scans. Preda et al102 reviewed studies of CTp for chemoradiation response monitoring and prediction for HNSCC, concluding that blood volume and blood flow may help in predicting response to and monitoring of radiation and chemotherapy.
Only a few publications on prediction of NSCLC treatment response based on DW-MRI, DCE-MRI or CTp were found. This may be explained by the very challenging acquisition owing to motion and highly variable tissue density in the lung region. Regier et al103 studied the correlation between standard uptake volume of 18F-FDG-PET and ADC of DW-MRI showing significant inverse correlation between SUVmax and ADCmin. CTp was shown to be effective for response assessment of antiangiogenic chemotherapy of NSCLC.104 Moreover, Wang et al105 showed that CTp can predict early tumour response and overall survival, mentioning, however, that image quality was good or moderate in only 68.2% of the cases.
Cone beam CT
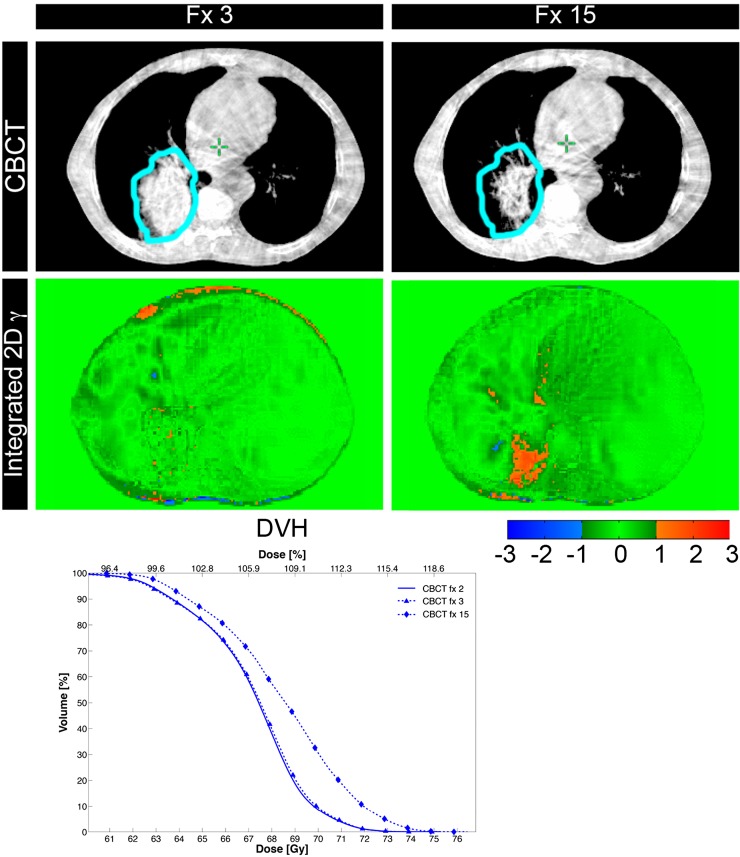
Modern linear accelerators are frequently equipped with an onboard cone beam CT. Apart from position verification prior to and during radiotherapy, the images obtained can be used for dose-guided radiotherapy. Based on this technique, treatment for patients with HNSCC and NSCLC can be adapted, sustaining/increasing the dose to the target volume, while keeping within the normal tissue dose constraints (Figure 3).106,107 Apart from this, attempts have been made to gather predictive information during the course of therapy, ultimately tailoring the chosen therapeutic approach.108
Figure 3.
Patient with a cT3N2M0 non-small-cell lung cancer of the right lower lobe undergoing chemoradiotherapy (23 × 2.75 Gy). During the treatment course, a tumour regression (clinical target volume delineated in sky blue on the kV-cone beam CT images projected in transverse direction, upper row) occurred when comparing kV-cone beam CT imaging at fractions 3 and 15. This resulted in geometric and dosimetric discrepancies as detected by integrated three dimensional postal dosimetry (middle row). From the dose–volume-histogram an increasing dose (blue) to the clinical target volume can be gathered. Image courtesy of Lucas CGG Persoon, MAASTRO Clinic.
CONCLUSIONS AND FUTURE DIRECTIONS
Monitoring of early treatment response by non-invasive molecular imaging methods is a new and challenging research area. PET tracers and functional MRI- and CT imaging-specific biological tumour characteristics offer potential for individualized radiation therapy. As several of these tumour characteristics represent crucial resistance mechanisms for radiotherapy and chemotherapy, as well as for newer biologically modifying molecules, the idea of adaptation of the target volume during the course of the radiation treatment, aiming to increase long-term tumour cure and/or to reduce chronic treatment toxicity, has been evolved. The optimal time points of imaging, that is, before or during treatment, for accurate response prediction and thereby for modifications or adaptation of treatment protocols, need to be defined. Based on current research, modulation of the radiation dose distribution according to specific biological tumour characteristics in an individual tumour is likely to be technically feasible at a level of spatial resolution comparable to the voxel size in clinical PET images. Technical restrictions of the resolution of clinical PET scanners and questions related to segmentation of tumour and non-tumour signals are still challenging. Despite a promising combination of morphological, functional and molecular information, hybrid PET/MRI still remains within the field of challenging academic research. However, none of these strategies has been developed to a stage at which it can be safely introduced into routine clinical practice yet. There is an emerging need for standardization of imaging protocols, image post-processing techniques and methods of extraction of imaging biomarkers, especially for MRI. Robust, repeatable and reproducible imaging protocols are crucial for validation of promising imaging biomarkers and bringing them from research into routine clinical practice. Important steps in this direction are taken within the Quantitative Imaging Network co-ordinated via the Cancer Imaging Program of National Cancer Institute.
Importantly, several clinical correlative studies and early clinical trials addressing the complexity of dose-painting strategies are currently ongoing. Strong data from the early clinical trials will be required to motivate a Phase III trial with an adequate sample size and complexity of the intervention in order to validate the early clinical results whether redistribution or boosting of radiation dose to tumour (sub)volumes results in improved radiotherapy outcome. Therefore, a multicentre or co-operative group format with standardized imaging protocols is mandatory to completed accrual of the calculated patient size within a reasonable time.
The development of new therapy strategies has only been possible owing to the close interaction between nuclear medicine, radiology and radiation oncology. It is essential to co-operate closely, and research areas need to be addressed jointly by nuclear medicine, radiology and radiation oncology. In the meantime, imaging databases correlating features with outcomes on an anonymous basis should be created, facilitating sharing of data, exchanging of analysis methods and ultimately faster gathering of knowledge.
Contributor Information
C Jentsch, Email: christina.jentsch@uniklinikum-dresden.de.
B Beuthien-Baumann, Email: b.beuthien@hzdr.de.
E G C Troost, Email: esther.troost@uniklinikum-dresden.de.
G Shakirin, Email: Georgy.Shakirin@philips.com.
REFERENCES
- 1.Bussink J, Kaanders JH, van der Graaf WT, Oyen WJ. PET-CT for radiotherapy treatment planning and response monitoring in solid tumors. Nat Rev Clin Oncol 2011; 8: 233–42. doi: 10.1038/nrclinonc.2010.218 [DOI] [PubMed] [Google Scholar]
- 2.Grégoire V, Jeraj R, Lee JA, O'Sullivan B. Radiotherapy for head and neck tumours in 2012 and beyond: conformal, tailored, and adaptive? Lancet Oncol 2012; 13: e292–300. doi: 10.1016/S1470-2045(12)70237-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Christian N, Lee JA, Bol A, De Bast M, Gallez B, Grégoire V. Immobilization device for in vivo and in vitro multimodality image registration of rodent tumors. Radiother Oncol 2008; 87: 147–51. doi: 10.1016/j.radonc.2008.02.016 [DOI] [PubMed] [Google Scholar]
- 4.Busk M, Mortensen LS, Nordsmark M, Overgaard J, Jakobsen S, Hansen KV, et al. PET hypoxia imaging with FAZA: reproducibility at baseline and during fractionated radiotherapy in tumour-bearing mice. Eur J Nucl Med Mol Imaging 2013; 40: 186–97. doi: 10.1007/s00259-012-2258-x [DOI] [PubMed] [Google Scholar]
- 5.Troost EG, Laverman P, Philippens ME, Lok J, van der Kogel AJ, Oyen WJ, et al. Correlation of [18F]FMISO autoradiography and pimonodazole immunohistochemistry in human head and neck carcinoma xenografts. Eur J Nucl Med Mol Imaging 2008; 35: 1803–11. (Erratum in: Eur J Nucl Med Mol Imaging 2009; 1836: 1331). doi: 10.1007/s00259-008-0772-7 [DOI] [PubMed] [Google Scholar]
- 6.Schütze C, Bergmann R, Yaromina A, Hessel F, Kotzerke J, Steinbach J, et al. Effect of increase of radiation dose on local control relates to pre-treatment FDG uptake in FaDu tumours in nude mice. Radiother Oncol 2007; 83: 311–15. [DOI] [PubMed] [Google Scholar]
- 7.Bruechner K, Bergmann R, Santiago A, Mosch B, Yaromina A, Hessel F, et al. Comparison of [18F]FDG uptake and distribution with hypoxia and proliferation in FaDu human squamous cell carcinoma (hSCC) xenografts after single dose irradiation. Int J Radiat Biol 2009; 85: 772–80. doi: 10.1080/09553000903043067 [DOI] [PubMed] [Google Scholar]
- 8.Christian N, Deheneffe S, Bol A, De Bast M, Labar D, Lee JA, et al. Is (18)F-FDG a surrogate tracer to measure tumor hypoxia? Comparison with the hypoxic tracer (14)C-EF3 in animal tumor models. Radiother Oncol 2010; 97: 183–8. doi: 10.1016/j.radonc.2010.02.020 [DOI] [PubMed] [Google Scholar]
- 9.Troost EG, Laverman P, Kaanders JH, Philippens M, Lok J, Oyen WJ, et al. Imaging hypoxia after oxygenation-modification: comparing [18F]FMISO autoradiography with pimonidazole immunohistochemistry in human xenograft tumors. Radiother Oncol 2006; 80: 157–64. doi: 10.1016/j.radonc.2006.07.023 [DOI] [PubMed] [Google Scholar]
- 10.Schütze C, Bergmann R, Brüchner K, Mosch B, Yaromina A, Zips D, et al. Effect of [(18)F]FMISO stratified dose-escalation on local control in FaDu hSCC in nude mice. Radiother Oncol 2014; 111: 81–8. doi: 10.1016/j.radonc.2014.02.005 [DOI] [PubMed] [Google Scholar]
- 11.Dubois LJ, Lieuwes NG, Janssen MH, Peeters WJ, Windhorst AD, Walsh JC, et al. Preclinical evaluation and validation of [18F]HX4, a promising hypoxia marker for PET imaging. Proc Natl Acad Sci U S A 2011; 108: 14620–5. doi: 10.1073/pnas.1102526108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Peeters SG, Zegers CM, Lieuwes NG, van Elmpt W, Eriksson J, van Dongen GA, et al. A comparative study of the hypoxia PET tracers [18F]HX4, [18F]FAZA, and [18F]FMISO in a preclinical tumor model. Int J Radiat Oncol Biol Phys 2015; 91: 351–9. doi: 10.1016/j.ijrobp.2014.09.045 [DOI] [PubMed] [Google Scholar]
- 13.Zegers CM, van Elmpt W, Reymen B, Even AJ, Troost EG, Ollers MC, et al. In vivo quantification of hypoxic and metabolic status of NSCLC tumors using [18F]HX4 and [18F]FDG-PET/CT imaging. Clin Cancer Res 2014; 20: 6389–97. doi: 10.1158/1078-0432.CCR-14-1524 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zegers CM, van Elmpt W, Wierts R, Reymen B, Sharifi H, Öllers MC, et al. Hypoxia imaging with [18F]HX4 PET in NSCLC patients: defining optimal imaging parameters. Radiother Oncol 2013; 109: 58–64. doi: 10.1016/j.radonc.2013.08.031 [DOI] [PubMed] [Google Scholar]
- 15.Schöder H, Fury M, Lee N, Kraus D. PET monitoring of therapy response in head and neck squamous cell carcinoma. J Nucl Med 2009; 50(Suppl. 1: 74–88. doi: 10.2967/jnumed.108.057208 [DOI] [PubMed] [Google Scholar]
- 16.van Elmpt W, Ollers M, Dingemans AM, Lambin P, De Ruysscher D. Response assessment using 18F-FDG PET early in the course of radiotherapy correlates with survival in advanced-stage non-small cell lung cancer. J Nucl Med 2012; 53: 1514–20. doi: 10.2967/jnumed.111.102566 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hoeben BA, Troost EG, Span PN, van Herpen CM, Bussink J, Oyen WJ, et al. 18F-FLT PET during radiotherapy or chemoradiotherapy in head and neck squamous cell carcinoma is an early predictor of outcome. J Nucl Med 2013; 54: 532–40. doi: 10.2967/jnumed.112.105999 [DOI] [PubMed] [Google Scholar]
- 18.Ahuja V, Coleman RE, Herndon J, Patz EF, Jr. The prognostic significance of fluorodeoxyglucose positron emission tomography imaging for patients with nonsmall cell lung carcinoma. Cancer 1998; 83: 918–24. doi: [DOI] [PubMed] [Google Scholar]
- 19.Bomanji JB, Costa DC, Ell PJ. Clinical role of positron emission tomography in oncology. Lancet Oncol 2001; 2: 157–64. [DOI] [PubMed] [Google Scholar]
- 20.Brun E, Kjellén E, Tennvall J, Ohlsson T, Sandell A, Perfekt R, et al. FDG PET studies during treatment: prediction of therapy outcome in head and neck squamous cell carcinoma. Head Neck 2002; 24: 127–35. doi: 10.1002/hed.10037 [DOI] [PubMed] [Google Scholar]
- 21.Goerres GW, Schmid DT, Grätz KW, von Schulthess GK, Eyrich GK. Impact of whole body positron emission tomography on initial staging and therapy in patients with squamous cell carcinoma of the oral cavity. Oral Oncol 2003; 39: 547–51. doi: 10.1016/S1368-8375(03)00016-2 [DOI] [PubMed] [Google Scholar]
- 22.Higashi K, Matsunari I, Ueda Y, Ikeda R, Guo J, Oguchi M, et al. Value of whole-body FDG PET in management of lung cancer. Ann Nucl Med 2003; 17: 1–14. doi: 10.1007/BF02988253 [DOI] [PubMed] [Google Scholar]
- 23.Kostakoglu L, Goldsmith SJ. PET in the assessment of therapy response in patients with carcinoma of the head and neck and of the esophagus. J Nucl Med 2004; 45: 56–68. [PubMed] [Google Scholar]
- 24.Rajendran JG, Mankoff DA, O'Sullivan F, Peterson LM, Schwartz DL, Conrad EU, et al. Hypoxia and glucose metabolism in malignant tumors: evaluation by [18F]fluoromisonidazole and [18F]fluorodeoxyglucose positron emission tomography imaging. Clin Cancer Res 2004; 10: 2245–52. [DOI] [PubMed] [Google Scholar]
- 25.Van de Wiele C, Lahorte C, Oyen W, Boerman O, Goethals I, Slegers G, et al. Nuclear medicine imaging to predict response to radiotherapy: a review. Int J Radiat Oncol Biol Phys 2003; 55: 5–15. doi: 10.1016/S0360-3016(02)04122-6 [DOI] [PubMed] [Google Scholar]
- 26.Lonneux M, Lawson G, Ide C, Bausart R, Remacle M, Pauwels S. Positron emission tomography with fluorodeoxyglucose for suspected head and neck tumor recurrence in the symptomatic patient. Laryngoscope 2000; 110: 1493–7. doi: 10.1097/00005537-200009000-00016 [DOI] [PubMed] [Google Scholar]
- 27.Lowe VJ, Boyd JH, Dunphy FR, Kim H, Dunleavy T, Collins BT, et al. Surveillance for recurrent head and neck cancer using positron emission tomography. J Clin Oncol 2000; 18: 651–8. [DOI] [PubMed] [Google Scholar]
- 28.Menda Y, Graham MM. Update on 18F-fluorodeoxyglucose/positron emission tomography and positron emission tomography/computed tomography imaging of squamous head and neck cancers. Semin Nucl Med 2005; 35: 214–19. doi: 10.1053/j.semnuclmed.2005.05.001 [DOI] [PubMed] [Google Scholar]
- 29.Pugachev A, Ruan S, Carlin S, Larson SM, Campa J, Ling CC, et al. Dependence of FDG uptake on tumor microenvironment. Int J Radiat Oncol Biol Phys 2005; 62: 545–53. doi: 10.1016/j.ijrobp.2005.02.009 [DOI] [PubMed] [Google Scholar]
- 30.Busk M, Horsman MR, Kristjansen PE, van der Kogel AJ, Bussink J, Overgaard J. Aerobic glycolysis in cancers: implications for the usability of oxygen-responsive genes and fluorodeoxyglucose-PET as markers of tissue hypoxia. Int J Cancer 2008; 122: 2726–34. doi: 10.1002/ijc.23449 [DOI] [PubMed] [Google Scholar]
- 31.Aerts HJ, van Baardwijk AA, Petit SF, Offermann C, Loon Jv, Houben R, et al. Identification of residual metabolic-active areas within individual NSCLC tumours using a pre-radiotherapy (18)fluorodeoxyglucose-PET-CT scan. Radiother Oncol 2009; 91: 386–92. doi: 10.1016/j.radonc.2009.03.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Madani I, Duthoy W, Derie C, De Gersem W, Boterberg T, Saerens M, et al. Positron emission tomography-guided, focal-dose escalation using intensity-modulated radiotherapy for head and neck cancer. Int J Radiat Oncol Biol Phys 2007; 68: 126–35. doi: 10.1016/j.ijrobp.2006.12.070 [DOI] [PubMed] [Google Scholar]
- 33.Abramyuk A, Tokalov S, Zöphel K, Koch A, Szluha Lazanyi K, Gillham C, et al. Is pre-therapeutical FDG-PET/CT capable to detect high risk tumor subvolumes responsible for local failure in non-small cell lung cancer? Radiother Oncol 2009; 91: 399–404. doi: 10.1016/j.radonc.2009.01.003 [DOI] [PubMed] [Google Scholar]
- 34.van den Bogaard J, Janssen MH, Janssens G, Buijsen J, Reniers B, Lambin P, et al. Residual metabolic tumor activity after chemo-radiotherapy is mainly located in initially high FDG uptake areas in rectal cancer. Radiother Oncol 2011; 99: 137–41. doi: 10.1016/j.radonc.2011.04.004 [DOI] [PubMed] [Google Scholar]
- 35.Bittner MI, Wiedenmann N, Bucher S, Hentschel M, Mix M, Weber WA, et al. Exploratory geographical analysis of hypoxic subvolumes using 18F-MISO-PET imaging in patients with head and neck cancer in the course of primary chemoradiotherapy. Radiother Oncol 2013; 108: 511–16. doi: 10.1016/j.radonc.2013.06.012 [DOI] [PubMed] [Google Scholar]
- 36.Levine EA, Farmer MR, Clark P, Mishra G, Ho C, Geisinger KR, et al. Predictive value of 18-fluoro-deoxy-glucose-positron emission tomography (18F-FDG-PET) in the identification of responders to chemoradiation therapy for the treatment of locally advanced esophageal cancer. Ann Surg 2006; 243: 472–8. doi: 10.1097/01.sla.0000208430.07050.61 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Minn H, Lapela M, Klemi PJ, Grénman R, Leskinen S, Lindholm P, et al. Prediction of survival with fluorine-18-fluoro-deoxyglucose and PET in head and neck cancer. J Nucl Med 1997; 38: 1907–11. [PubMed] [Google Scholar]
- 38.Thorwarth D, Eschmann SM, Holzner F, Paulsen F, Alber M. Combined uptake of [18F]FDG and [18F]FMISO correlates with radiation therapy outcome in head-and-neck cancer patients. Radiother Oncol 2006; 80: 151–6. doi: 10.1016/j.radonc.2006.07.033 [DOI] [PubMed] [Google Scholar]
- 39.Yao M, Smith RB, Hoffman HT, Funk GF, Lu M, Menda Y, et al. Clinical significance of postradiotherapy [18F]-fluorodeoxyglucose positron emission tomography imaging in management of head-and-neck cancer-a long-term outcome report. Int J Radiat Oncol Biol Phys 2009; 74: 9–14. doi: 10.1016/j.ijrobp.2008.07.019 [DOI] [PubMed] [Google Scholar]
- 40.Moeller BJ, Rana V, Cannon BA, Williams MD, Sturgis EM, Ginsberg LE, et al. Prospective risk-adjusted [18F]Fluorodeoxyglucose positron emission tomography and computed tomography assessment of radiation response in head and neck cancer. J Clin Oncol 2009; 27: 2509–15. doi: 10.1200/JCO.2008.19.3300 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lee SW, Nam SY, Im KC, Kim JS, Choi EK, Ahn SD, et al. Prediction of prognosis using standardized uptake value of 2-[(18)F] fluoro-2-deoxy-D-glucose positron emission tomography for nasopharyngeal carcinomas. Radiother Oncol 2008; 87: 211–16. doi: 10.1016/j.radonc.2008.01.009 [DOI] [PubMed] [Google Scholar]
- 42.Kao J, Vu HL, Genden EM, Mocherla B, Park EE, Packer S, et al. The diagnostic and prognostic utility of positron emission tomography/computed tomography-based follow-up after radiotherapy for head and neck cancer. Cancer 2009; 115: 4586–94. doi: 10.1002/cncr.24493 [DOI] [PubMed] [Google Scholar]
- 43.Seol YM, Kwon BR, Song MK, Choi YJ, Shin HJ, Chung JS, et al. Measurement of tumor volume by PET to evaluate prognosis in patients with head and neck cancer treated by chemo-radiation therapy. Acta Oncol 2010; 49: 201–8. doi: 10.3109/02841860903440270 [DOI] [PubMed] [Google Scholar]
- 44.Malone JP, Gerberi MA, Vasireddy S, Hughes LF, Rao K, Shevlin B, et al. Early prediction of response to chemoradiotherapy for head and neck cancer: reliability of restaging with combined positron emission tomography and computed tomography. Arch Otolaryngol Head Neck Surg 2009; 135: 1119–25. doi: 10.1001/archoto.2009.152 [DOI] [PubMed] [Google Scholar]
- 45.Allal AS, Slosman DO, Kebdani T, Allaoua M, Lehmann W, Dulguerov P. Prediction of outcome in head-and-neck cancer patients using the standardized uptake value of 2-[18F]fluoro-2-deoxy-D-glucose. Int J Radiat Oncol Biol Phys 2004; 59: 1295–300. doi: 10.1016/j.ijrobp.2003.12.039 [DOI] [PubMed] [Google Scholar]
- 46.Hentschel M, Appold S, Schreiber A, Abramyuk A, Abolmaali N, Kotzerke J, et al. Serial FDG-PET on patients with head and neck cancer: implications for radiation therapy. Int J Radiat Biol 2009; 85: 796–804. doi: 10.1080/09553000903039180 [DOI] [PubMed] [Google Scholar]
- 47.Castaldi P, Rufini V, Bussu F, Miccichè F, Dinapoli N, Autorino R, et al. : Can “early” and “late”18F-FDG PET-CT be used as prognostic factors for the clinical outcome of patients with locally advanced head and neck cancer treated with radio-chemotherapy? Radiother Oncol 2012; 103: 63–8. doi: 10.1016/j.radonc.2012.03.001 [DOI] [PubMed] [Google Scholar]
- 48.Sasaki R, Komaki R, Macapinlac H, Erasmus J, Allen P, Forster K, et al. [18F]fluorodeoxyglucose uptake by positron emission tomography predicts outcome of non-small-cell lung cancer. J Clin Oncol 2005; 23: 1136–43. [DOI] [PubMed] [Google Scholar]
- 49.Huang W, Zhou T, Ma L, Sun H, Gong H, Wang J, et al. Standard uptake value and metabolic tumor volume of 18F-FDG PET/CT predict short-term outcome early in the course of chemoradiotherapy in advanced non-small cell lung cancer. Eur J Nucl Med Mol Imaging 2011; 38: 1628–35. doi: 10.1007/s00259-011-1838-5 [DOI] [PubMed] [Google Scholar]
- 50.Rasey JS, Grunbaum Z, Magee S, Nelson NJ, Olive PL, Durand RE, et al. Characterization of radiolabeled fluoromisonidazole as a probe for hypoxic cells. Radiat Res 1987; 111: 292–304. doi: 10.2307/3576986 [DOI] [PubMed] [Google Scholar]
- 51.Horsman MR, Mortensen LS, Petersen JB, Busk M, Overgaard J. Imaging hypoxia to improve radiotherapy outcome. Nat Rev Clin Oncol 2012; 9: 674–87. doi: 10.1038/nrclinonc.2012.171 [DOI] [PubMed] [Google Scholar]
- 52.Rischin D, Hicks RJ, Fisher R, Binns D, Corry J, Porceddu S, et al. ; Trans-Tasman Radiation Oncology Group Study 98.02. Prognostic significance of [18F]-misonidazole positron emission tomography-detected tumor hypoxia in patients with advanced head and neck cancer randomly assigned to chemoradiation with or without tirapazamine: a substudy of Trans-Tasman Radiation Oncology Group Study 98.02. J Clin Oncol 2006; 24: 2098–104. doi: 10.1200/JCO.2005.05.2878 [DOI] [PubMed] [Google Scholar]
- 53.Begg AC, Stewart FA, Vens C. Strategies to improve radiotherapy with targeted drugs. Nat Rev Cancer 2011; 11: 239–53. doi: 10.1038/nrc3007 [DOI] [PubMed] [Google Scholar]
- 54.Bentzen SM. Theragnostic imaging for radiation oncology: dose-painting by numbers. Lancet Oncol 2005; 6: 112–17. doi: 10.1016/S1470-2045(05)01737-7 [DOI] [PubMed] [Google Scholar]
- 55.Overgaard J. Hypoxic radiosensitization: adored and ignored. J Clin Oncol 2007; 25: 4066–74. doi: 10.1200/JCO.2007.12.7878 [DOI] [PubMed] [Google Scholar]
- 56.Overgaard J. Hypoxic modification of radiotherapy in squamous cell carcinoma of the head and neck—a systematic review and meta-analysis. Radiother Oncol 2011; 100: 22–32. doi: 10.1016/j.radonc.2011.03.004 [DOI] [PubMed] [Google Scholar]
- 57.Kaanders JH, Pop LA, Marres HA, Bruaset I, van den Hoogen FJ, Merkx MA, et al. ARCON: experience in 215 patients with advanced head-and-neck cancer. Int J Radiat Oncol Biol Phys 2002; 52: 769–78. [DOI] [PubMed] [Google Scholar]
- 58.Daisne JF, Duprez T, Weynand B, Lonneux M, Hamoir M, Reychler H, et al. Tumor volume in pharyngolaryngeal squamous cell carcinoma: comparison at CT, MR imaging, and FDG PET and validation with surgical specimen. Radiology 2004; 233: 93–100. doi: 10.1148/radiol.2331030660 [DOI] [PubMed] [Google Scholar]
- 59.Geets X, Daisne JF, Tomsej M, Duprez T, Lonneux M, Grégoire V. Impact of the type of imaging modality on target volumes delineation and dose distribution in pharyngo-laryngeal squamous cell carcinoma: comparison between pre- and per-treatment studies. Radiother Oncol 2006; 78: 291–7. doi: 10.1016/j.radonc.2006.01.006 [DOI] [PubMed] [Google Scholar]
- 60.Koshy M, Paulino AC, Howell R, Schuster D, Halkar R, Davis LW. F-18 FDG PET-CT fusion in radiotherapy treatment planning for head and neck cancer. Head Neck 2005; 27: 494–502. doi: 10.1002/hed.20179 [DOI] [PubMed] [Google Scholar]
- 61.Nestle U, Kremp S, Grosu AL. Practical integration of [18F]-FDG-PET and PET-CT in the planning of radiotherapy for non-small cell lung cancer (NSCLC): the technical basis, ICRU-target volumes, problems, perspectives. Radiother Oncol 2006; 81: 209–25. doi: 10.1016/j.radonc.2006.09.011 [DOI] [PubMed] [Google Scholar]
- 62.van Baardwijk A, Baumert BG, Bosmans G, van Kroonenburgh M, Stroobants S, Gregoire V, et al. The current status of FDG-PET in tumour volume definition in radiotherapy treatment planning. Cancer Treat Rev 2006; 32: 245–60. doi: 10.1016/j.ctrv.2006.02.002 [DOI] [PubMed] [Google Scholar]
- 63.Bussink J, van Herpen CM, Kaanders JH, Oyen WJ. PET-CT for response assessment and treatment adaptation in head and neck cancer. Lancet Oncol 2010; 11: 661–9. doi: 10.1016/S1470-2045(09)70353-5 [DOI] [PubMed] [Google Scholar]
- 64.MacManus M, Nestle U, Rosenzweig KE, Carrio I, Messa C, Belohlavek O, et al. Use of PET and PET/CT for radiation therapy planning: IAEA expert report 2006–2007. Radiother Oncol 2009; 91: 85–94. doi: 10.1016/j.radonc.2008.11.008 [DOI] [PubMed] [Google Scholar]
- 65.Grégoire V, Bol A, Geets X, Lee J. Is PET-based treatment planning the new standard in modern radiotherapy? The head and neck paradigm. Semin Radiat Oncol 2006; 16: 232–8. [DOI] [PubMed] [Google Scholar]
- 66.Schinagl DA, Vogel WV, Hoffmann AL, van Dalen JA, Oyen WJ, Kaanders JH. Comparison of five segmentation tools for 18F-fluoro-deoxy-glucose-positron emission tomography-based target volume definition in head and neck cancer. Int J Radiat Oncol Biol Phys 2007; 69: 1282–9. doi: 10.1016/j.ijrobp.2007.07.2333 [DOI] [PubMed] [Google Scholar]
- 67.Ling CC, Humm J, Larson S, Amols H, Fuks Z, Leibel S, et al. Towards multidimensional radiotherapy (MD-CRT): biological imaging and biological conformality. Int J Radiat Oncol Biol Phys 2000; 47: 551–60. doi: 10.1016/S0360-3016(00)00467-3 [DOI] [PubMed] [Google Scholar]
- 68.Galvin JM, De Neve W. Intensity modulating and other radiation therapy devices for dose painting. J Clin Oncol 2007; 25: 924–30. doi: 10.1200/JCO.2007.10.6716 [DOI] [PubMed] [Google Scholar]
- 69.Vanderstraeten B, De Gersem W, Duthoy W, De Neve W, Thierens H. Implementation of biologically conformal radiation therapy (BCRT) in an algorithmic segmentation-based inverse planning approach. Phys Med Biol 2006; 51: N277–286. doi: 10.1088/0031-9155/51/16/N02 [DOI] [PubMed] [Google Scholar]
- 70.Geets X, Tomsej M, Lee JA, Duprez T, Coche E, Cosnard G, et al. Adaptive biological image-guided IMRT with anatomic and functional imaging in pharyngo-laryngeal tumors: impact on target volume delineation and dose distribution using helical tomotherapy. Radiother Oncol 2007; 85: 105–15. doi: 10.1016/j.radonc.2007.05.010 [DOI] [PubMed] [Google Scholar]
- 71.Henriques de Figueiredo B, Barret O, Demeaux H, Lagarde P, De-Mones-Del-Pujol E, Kantor G, et al. Comparison between CT- and FDG-PET-defined target volumes for radiotherapy planning in head-and-neck cancers. Radiother Oncol 2009; 93: 479–82. doi: 10.1016/j.radonc.2009.09.010 [DOI] [PubMed] [Google Scholar]
- 72.van Elmpt W, De Ruysscher D, van der Salm A, Lakeman A, van der Stoep J, Emans D, et al. The PET-boost randomised phase II dose-escalation trial in non-small cell lung cancer. Radiother Oncol 2012; 104: 67–71. doi: 10.1016/j.radonc.2012.03.005 [DOI] [PubMed] [Google Scholar]
- 73.Heukelom J, Hamming O, Bartelink H, Hoebers F, Giralt J, Herlestam T, et al. Adaptive and innovative radiation treatment for improving cancer treatment outcome (ARTFORCE); a randomized controlled phase II trial for individualized treatment of head and neck cancer. BMC Cancer 2013; 13: 84. doi: 10.1186/1471-2407-13-84 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Duprez F, De Neve W, De Gersem W, Coghe M, Madani I. Adaptive dose painting by numbers for head-and-neck cancer. Int J Radiat Oncol Biol Phys 2011; 80: 1045–55. doi: 10.1016/j.ijrobp.2010.03.028 [DOI] [PubMed] [Google Scholar]
- 75.Berwouts D, Olteanu LA, Duprez F, Vercauteren T, De Gersem W, De Neve W, et al. Three-phase adaptive dose-painting-by-numbers for head-and-neck cancer: initial results of the phase I clinical trial. Radiother Oncol 2013; 107: 310–16. doi: 10.1016/j.radonc.2013.04.002 [DOI] [PubMed] [Google Scholar]
- 76.Gillham C, Zips D, Pönisch F, Evers C, Enghardt W, Abolmaali N, et al. Additional PET/CT in week 5-6 of radiotherapy for patients with stage III non-small cell lung cancer as a means of dose escalation planning? Radiother Oncol 2008; 88: 335–41. doi: 10.1016/j.radonc.2008.05.004 [DOI] [PubMed] [Google Scholar]
- 77.Feng M, Kong FM, Gross M, Fernando S, Hayman JA, Ten Haken RK. Using fluorodeoxyglucose positron emission tomography to assess tumor volume during radiotherapy for non-small-cell lung cancer and its potential impact on adaptive dose escalation and normal tissue sparing. Int J Radiat Oncol Biol Phys 2009; 73: 1228–34. doi: 10.1016/j.ijrobp.2008.10.054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.De Ruysscher D, Wanders S, Minken A, Lumens A, Schiffelers J, Stultiens C, et al. Effects of radiotherapy planning with a dedicated combined PET-CT-simulator of patients with non-small cell lung cancer on dose limiting normal tissues and radiation dose-escalation: a planning study. Radiother Oncol 2005; 77: 5–10. doi: 10.1016/j.radonc.2005.06.014 [DOI] [PubMed] [Google Scholar]
- 79.van Baardwijk A, Bosmans G, Boersma L, Buijsen J, Wanders S, Hochstenbag M, et al. PET-CT-based auto-contouring in non-small-cell lung cancer correlates with pathology and reduces interobserver variability in the delineation of the primary tumor and involved nodal volumes. Int J Radiat Oncol Biol Phys 2007; 68: 771–8. doi: 10.1016/j.ijrobp.2006.12.067 [DOI] [PubMed] [Google Scholar]
- 80.van Der Wel A, Nijsten S, Hochstenbag M, Lamers R, Boersma L, Wanders R, et al. Increased therapeutic ratio by 18FDG-PET CT planning in patients with clinical CT stage N2-N3M0 non-small-cell lung cancer: a modeling study. Int J Radiat Oncol Biol Phys 2005; 61: 649–55. doi: 10.1016/j.ijrobp.2004.06.205 [DOI] [PubMed] [Google Scholar]
- 81.Koh WJ, Rasey JS, Evans ML, Grierson JR, Lewellen TK, Graham MM, et al. Imaging of hypoxia in human tumors with [F-18]fluoromisonidazole. Int J Radiat Oncol Biol Phys 1992; 22: 199–212. doi: 10.1016/0360-3016(92)91001-4 [DOI] [PubMed] [Google Scholar]
- 82.Nehmeh SA, Lee NY, Schröder H, Squire O, Zanzonico PB, Erdi YE, et al. Reproducibility of intratumor distribution of (18)F-fluoromisonidazole in head and neck cancer. Int J Radiat Oncol Biol Phys 2008; 70: 235–42. doi: 10.1016/j.ijrobp.2007.08.036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Okamoto S, Shiga T, Yasuda K, Ito YM, Magota K, Kasai K, et al. High reproducibility of tumor hypoxia evaluated by 18F-fluoromisonidazole PET for head and neck cancer. J Nucl Med 2013; 54: 201–7. doi: 10.2967/jnumed.112.109330 [DOI] [PubMed] [Google Scholar]
- 84.Lee NY, Mechalakos JG, Nehmeh S, Lin Z, Squire OD, Cai S, et al. Fluorine-18-labeled fluoromisonidazole positron emission and computed tomography-guided intensity-modulated radiotherapy for head and neck cancer: a feasibility study. Int J Radiat Oncol Biol Phys 2008; 70: 2–13. doi: 10.1016/j.ijrobp.2007.06.039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Zegers CML, van Elmpt W, Szardenings K, Kolb H, Waxman A, Subramaniam RM, et al. Repeatability of hypoxia PET imaging using [18F]HX4 in lung and head and neck cancer patients: a prospective multicenter trial. Eur J Nucl Med Mol Imag 2015; in press. [DOI] [PMC free article] [PubMed]
- 86.Lee N, Nehmeh S, Schöder H, Fury M, Chan K, Ling CC, et al. Prospective trial incorporating pre-/mid-treatment [18F]-misonidazole positron emission tomography for head-and-neck cancer patients undergoing concurrent chemoradiotherapy. Int J Radiat Oncol Biol Phys 2009; 75: 101–8. doi: 10.1016/j.ijrobp.2008.10.049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Aerts HJ, Velazquez ER, Leijenaar RT, Parmar C, Grossmann P, Cavalho S, et al. Decoding tumour phenotype by noninvasive imaging using a quantitative radiomics approach. Nat Commun 2014; 5: 4006. doi: 10.1038/ncomms5006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Coroller TP, Grossmann P, Hou Y, Rios Velazquez E, Leijenaar RT, Hermann G, et al. CT-based radiomic signature predicts distant metastasis in lung adenocarcinoma. Radiother Oncol 2015; 114: 345–50. doi: 10.1016/j.radonc.2015.02.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Nyholm T, Jonsson J. Counterpoint: opportunities and challenges of a magnetic resonance imaging-only radiotherapy work flow. Semin Radiat Oncol 2014; 24: 175–80. doi: 10.1016/j.semradonc.2014.02.005 [DOI] [PubMed] [Google Scholar]
- 90.Abdel Razek AA, Poptani H. MR spectroscopy of head and neck cancer. Eur J Radiol 2013; 82: 982–9. doi: 10.1016/j.ejrad.2013.01.025 [DOI] [PubMed] [Google Scholar]
- 91.Malayeri AA, El Khouli RH, Zaheer A, Jacobs MA, Corona-Villalobos CP, Kamel IR, et al. Principles and applications of diffusion-weighted imaging in cancer detection, staging, and treatment follow-up. Radiographics 2011; 31: 1773–91. doi: 10.1148/rg.316115515 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Padhani AR, Liu G, Koh DM, Chenevert TL, Thoeny HC, Takahara T, et al. Diffusion-weighted magnetic resonance imaging as a cancer biomarker: consensus and recommendations. Neoplasia 2009; 11: 102–25. doi: 10.1593/neo.81328 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Hauser T, Essig M, Jensen A, Gerigk L, Laun FB, Münter M, et al. Characterization and therapy monitoring of head and neck carcinomas using diffusion-imaging-based intravoxel incoherent motion parameters-preliminary results. Neuroradiology 2013; 55: 527–36. doi: 10.1007/s00234-013-1154-9 [DOI] [PubMed] [Google Scholar]
- 94.Srinivasan A, Chenevert TL, Dwamena BA, Eisbruch A, Watcharotone K, Myles JD, et al. Utility of pretreatment mean apparent diffusion coefficient and apparent diffusion coefficient histograms in prediction of outcome to chemoradiation in head and neck squamous cell carcinoma. J Comput Assist Tomogr 2012; 36: 131–7. doi: 10.1097/RCT.0b013e3182405435 [DOI] [PubMed] [Google Scholar]
- 95.Kim S, Loevner L, Quon H, Sherman E, Weinstein G, Kilger A, et al. Diffusion-weighted magnetic resonance imaging for predicting and detecting early response to chemoradiation therapy of squamous cell carcinomas of the head and neck. Clin Cancer Res 2009; 15: 986–94. doi: 10.1158/1078-0432.CCR-08-1287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Thoeny HC, De Keyzer F, King AD. Diffusion-weighted MR imaging in the head and neck. Radiology 2012; 263: 19–32. doi: 10.1148/radiol.11101821 [DOI] [PubMed] [Google Scholar]
- 97.Noij DP, de Jong MC, Mulders LG, Marcus JT, de Bree R, Lavini C, et al. Contrast-enhanced perfusion magnetic resonance imaging for head and neck squamous cell carcinoma: a systematic review. Oral Oncol 2014; 51: 124–38. doi: 10.1016/j.oraloncology.2014.10.016 [DOI] [PubMed] [Google Scholar]
- 98.Tofts PS, Brix G, Buckley DL, Evelhoch JL, Henderson E, Knopp MV, et al. Estimating kinetic parameters from dynamic contrast-enhanced T(1)-weighted MRI of a diffusable tracer: standardized quantities and symbols. J Magn Reson Imaging 1999; 10: 223–32. [DOI] [PubMed] [Google Scholar]
- 99.Lavini C, Buiter M, Maas M. Use of dynamic contrast enhanced time intensity curve shape analysis in MRI: theory and practice. Rep Med Imaging 2013; 2013: 6 71–82. doi: 10.2147/RMI.S35088 [DOI] [Google Scholar]
- 100.Bernstein JM, Homer JJ, West CM. Dynamic contrast-enhanced magnetic resonance imaging biomarkers in head and neck cancer: potential to guide treatment? A systematic review. Oral Oncol 2014; 50: 963–70. doi: 10.1016/j.oraloncology.2014.07.011 [DOI] [PubMed] [Google Scholar]
- 101.Becker M, Zaidi H. Imaging in head and neck squamous cell carcinoma: the potential role of PET/MRI. Br J Radiol 2014; 87: 20130677. doi: 10.1259/bjr.20130677 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Preda L, Calloni SF, Moscatelli ME, Cossu Rocca M, Bellomi M. Role of CT perfusion in monitoring and prediction of response to therapy of head and neck squamous cell carcinoma. Biomed Res Int 2014; 2014: 917150. doi: 10.1155/2014/917150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Regier M, Derlin T, Schwarz D, Laqmani A, Henes FO, Groth M, et al. Diffusion weighted MRI and 18F-FDG-PET/CT in non-small cell lung cancer (NSCLC): does the apparent diffusion coefficient (ADC) correlate with tracer uptake (SUV)? Eur J Radiol 2012; 81: 2913–18. doi: 10.1016/j.ejrad.2011.11.050 [DOI] [PubMed] [Google Scholar]
- 104.Tacelli N, Santangelo T, Scherpereel A, Duhamel A, Deken V, Klotz E, et al. Perfusion CT allows prediction of therapy response in non-small cell lung cancer treated with conventional and anti-angiogenic chemotherapy. Eur Radiol 2013; 23: 2127–36. doi: 10.1007/s00330-013-2821-2 [DOI] [PubMed] [Google Scholar]
- 105.Wang J, Wu N, Cham MD, Song Y. Tumor response in patients with advanced non-small cell lung cancer: perfusion CT evaluation of chemotherapy and radiation therapy. AJR Am J Roentgenol 2009; 193: 1090–6. doi: 10.2214/AJR.08.1367 [DOI] [PubMed] [Google Scholar]
- 106.Persoon LC, Egelmeer AG, Ollers MC, Nijsten SM, Troost EG, Verhaegen F. First clinical results of adaptive radiotherapy based on 3D portal dosimetry for lung cancer patients with atelectasis treated with volumetric-modulated arc therapy (VMAT). Acta Oncol 2013; 52: 1484–9. doi: 10.3109/0284186X.2013.813642 [DOI] [PubMed] [Google Scholar]
- 107.Hermans BCM, Persoon LCGG, Hoebers FJP, Verhaegen F, Troost EGC. Weekly kilovoltage cone beam computed tomography for detection of dose discrepancies in head and neck cancer patients. Provisionally accepted 2015. [DOI] [PubMed] [Google Scholar]
- 108.Elstrøm UV, Wysocka BA, Muren LP, Petersen JB, Grau C. Daily kV cone-beam CT and deformable image registration as a method for studying dosimetric consequences of anatomic changes in adaptive IMRT of head and neck cancer. Acta Oncol 2010; 49: 1101–8. doi: 10.3109/0284186X.2010.500304 [DOI] [PubMed] [Google Scholar]