Abstract
Objective:
Intensity-modulated radiotherapy (IMRT) for anal canal carcinoma (ACC) is associated with favourable toxicity outcomes. Side effects include sexual dysfunction, skin desquamation, pain and fibrosis to perineum and genitalia region. The genitalia are situated anterior to the primary ACC between two inguinal regions providing a challenging structure to avoid. Techniques improving outcomes require robust, consistent genitalia contouring to ensure standardization and production of fully optimized IMRT plans. Official recommendations for genitalia contouring are lacking. We describe a potential genitalia contouring atlas for ACC radiotherapy.
Methods:
Following a review of genitalia CT anatomy, a contouring atlas was generated for male and female patients positioned prone and supine. Particular attention was paid to the reproducibility of the genitalia contour in all planes.
Results:
Male and female genitalia positioned prone and supine are described and represented visually through a contouring atlas. Contoured areas in males include penis and scrotum, and in females include clitoris, labia majora and minora. The muscles, bone, prostate, vagina, cervix and uterus should be excluded. The genitalia contour extends laterally to inguinal creases and includes areas of fat and skin anterior to the symphysis pubis for both genders.
Conclusion:
This atlas provides descriptive and visual guidance enabling more consistent genitalia delineation for both genders when prone and supine. The atlas can be used for other sites requiring radiotherapy planning.
Advances in knowledge:
This atlas presents visual contouring guidance for genitalia in ACC radiotherapy for the first time. Contouring methods provide reproducible genitalia contours that allow the provision of accurate dose toxicity data in future studies.
The standard of care for anal cancer treatment is chemoradiation, combining external beam radiotherapy with fluoropyrimidine and mitomycin-C chemotherapy.1 Intensity-modulated radiotherapy (IMRT) in anal cancer is the preferred method of radiotherapy delivery owing to its ability to highly conform to target areas and spare normal tissues compared with conventional radiotherapy delivery of two to four fields.2 During the radiotherapy planning process, a clinician contours the planning target volume (PTV) and organs at risk (OARs) on axial CT slices. PTV dose objectives and OAR dose constraints are assigned in the treatment planning system and prioritized to provide maximum homogeneous dose to the PTV whilst minimizing dose to OARs. The dose constraints assigned to OARs are usually based upon treatment planning studies and toxicity outcome data for the particular OAR. Many OARs were discussed within the Quantitative Analyses of Normal Tissue Effects in the Clinic data,3 however, only the penile bulb is discussed within genitalia structures. To produce a successful IMRT plan that meets predefined acceptance criteria, realistic dose constraints must be applied, and this depends upon robust contouring of the OAR.4
Such radiotherapy plans will also need to consider if regional lymph nodes are involved, which requires an additional boost dose as part of the radiotherapy regime. The lymphatic drainage of the anal canal mirrors the vascular supply and is related to the embryological origin of the canal from both ectoderm (lower one-third of the canal) and endoderm (upper two-third). The dentate line demarcates the division between these regions. The lower part of the anal canal is drained by the superficial inguinal nodes, whereas the upper part drains via the lymphatics accompanying the middle and inferior rectal vessels that either drain directly into the internal iliac vessels or via the internal pudendal vessels. The lymphatics drain into the internal iliac nodes.
Major radiotherapy acute side effects for anal cancer include skin toxicity to the perineum and genitalia. Skin reactions vary from minor (erythema) to major (moist desquamation), and may require hospital admission and require a “break” in treatment, potentially affecting the probability of local control of the tumour.1,5 Long-term reactions include local fibrosis with pain, loss of mobility and impaired sexual function.6,7 The genitalia are situated anterior to the primary anal carcinoma and between two inguinal nodal regions meaning this is a difficult structure to completely avoid. Planning studies comparing conventional radiotherapy vs IMRT report a dose reduction to the genitalia when using IMRT,8,9 with outcome data from the use of IMRT reporting favourable skin toxicity outcomes.10–12 Following the results of these studies, a planning study conducted by our institution,2 along with our IMRT experience of other sites, we implemented anal cancer IMRT in 2011. However, we found the contouring of genitalia to be inconsistent, influencing our ability to meet normal tissue constraints and thus affecting the quality of the radiotherapy plan. Ideally, implementation and evaluation of new techniques should be set within clinical trials, facilitating robust and consistent contouring of the OAR. However, anal cancer trials to date have not provided detailed guidance on outlining genitalia, so there is no standardization of approach, with a wide variation in the plans produced. We have therefore proposed a contouring atlas for the delineation of male and female genitalia for use in anal cancer radiotherapy.
METHODS AND MATERIALS
Four patients with primary anal cancer, no regional nodal involvement, were identified who had previously received IMRT. Two patients were male and two were female, one treated prone and one supine in each gender group. Informed consent was obtained for all patients regarding the use of their radiotherapy images for study and publication. This atlas provides representative examples for the four main clinical situations encountered in radiotherapy voluming for anal cancer. These were derived from our series of >70 patients treated with IMRT as being representative examples of the series, and the chosen examples have been reviewed and approved by a gastrointestinal specialist radiologist. A multidisciplinary team consisting of a gastrointestinal radiologist, two clinical oncologists, a medical physicist and a therapy radiographer reviewed the genitalia anatomy and the surrounding tissues as seen on CT. An external genitalia contouring atlas was generated for male and female patients in the prone and supine positions. The specific structures outlined included the scrotum, perineal body, corpus cavernosum penis for males and clitoris, labia majora and minora for females. For both genders, the surrounding fat and tissue anterior to the symphysis pubis excluding the muscle and bone was also delineated. Particular attention was paid to the likelihood of reproducibility and consistency of the genitalia contour in the superior/inferior, anterior/posterior and lateral directions. All patients also had a diagnostic MRI scan of the pelvis, which was available to refine the location and extent of the primary tumour on planning CT. The superior soft-tissue contrast afforded by MRI added confidence to contouring of the OARs included on the diagnostic MR scan.
RESULTS
The male and female genitalia in the prone and supine positions are described and represented visually through a high-resolution CT contouring atlas as seen in Figures 1–4. Two different windowing options are presented that demonstrate the internal anatomy and external contour. The window width and level to show the external contour were set to 1601 and −1300 HU, respectively. The window width and level to show the internal anatomy were set to 501 and −250 HU, respectively. The genitalia volume size for the supine and prone male was 412.9 and 267.1 cm3 on axial CT slices, respectively. The genitalia volume size for the supine and prone females was 110.6 and 180.6 cm3, respectively.
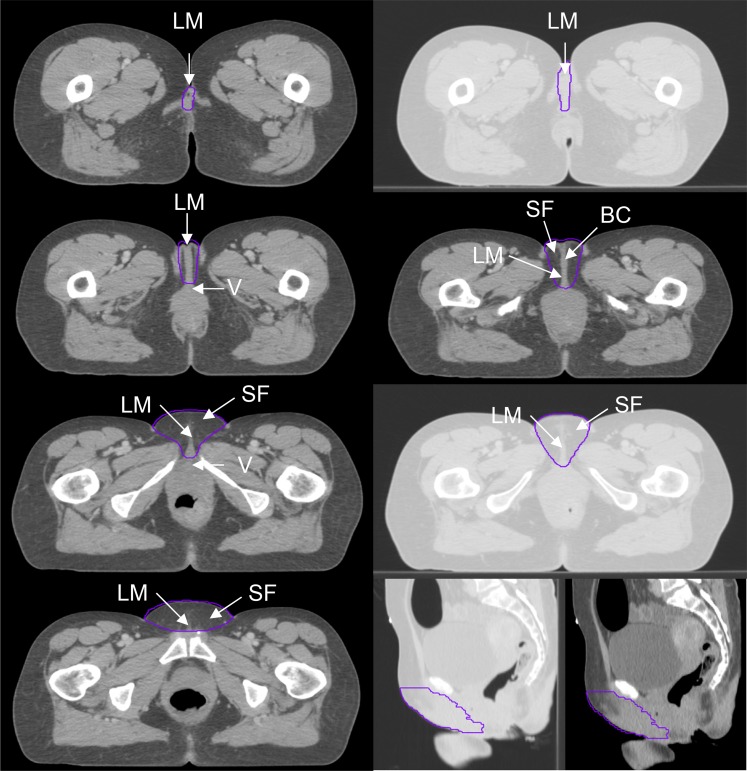
Figure 1.
Genitalia outlined for a female in the supine position. BC, body of clitoris; LM, labium majus; SF, surrounding fat; V, vagina.
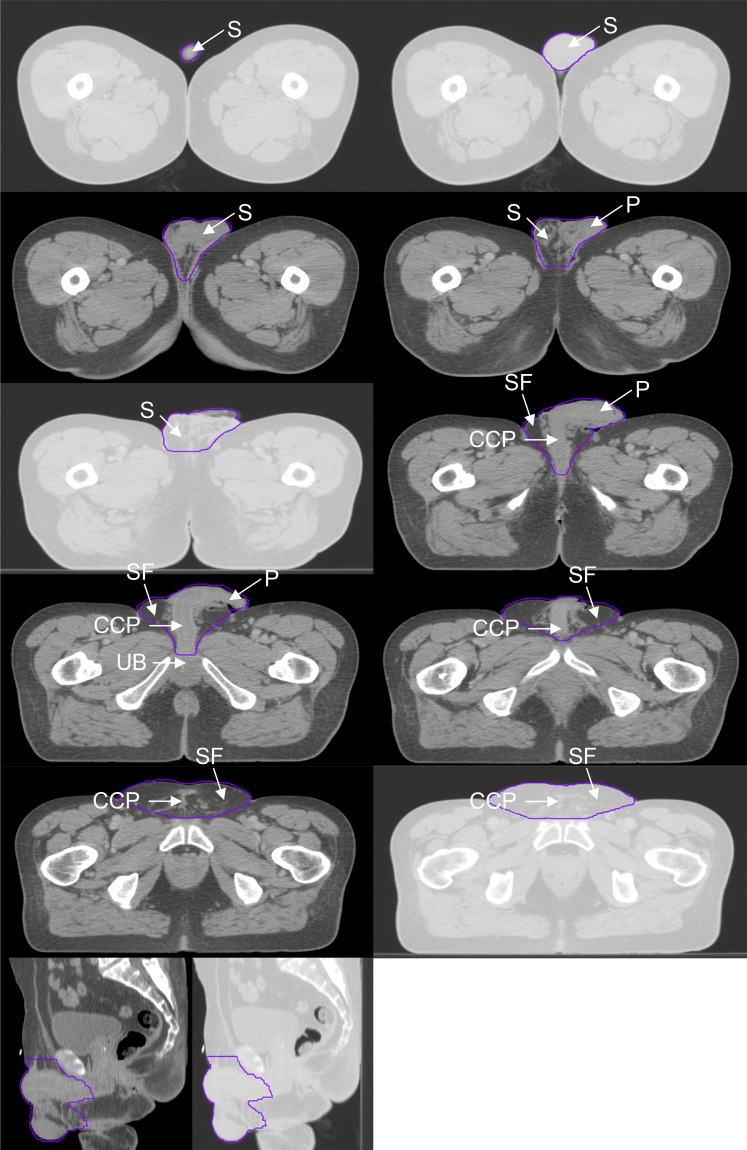
Figure 4.
Genitalia outlined for a male in the supine position. CCP, corpus cavernosum penis; P, penis; S, scrotum; SF, surrounding fat; UB, urethral bulb.
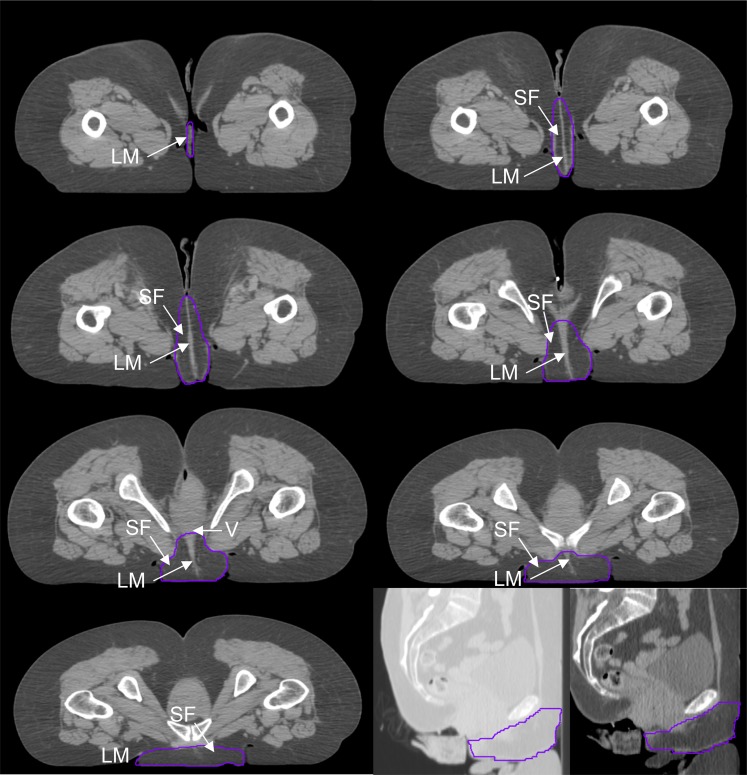
Figure 2.
Genitalia outlined for a female in the prone position. LM, labium majus; SF, surrounding fat; V, vagina.
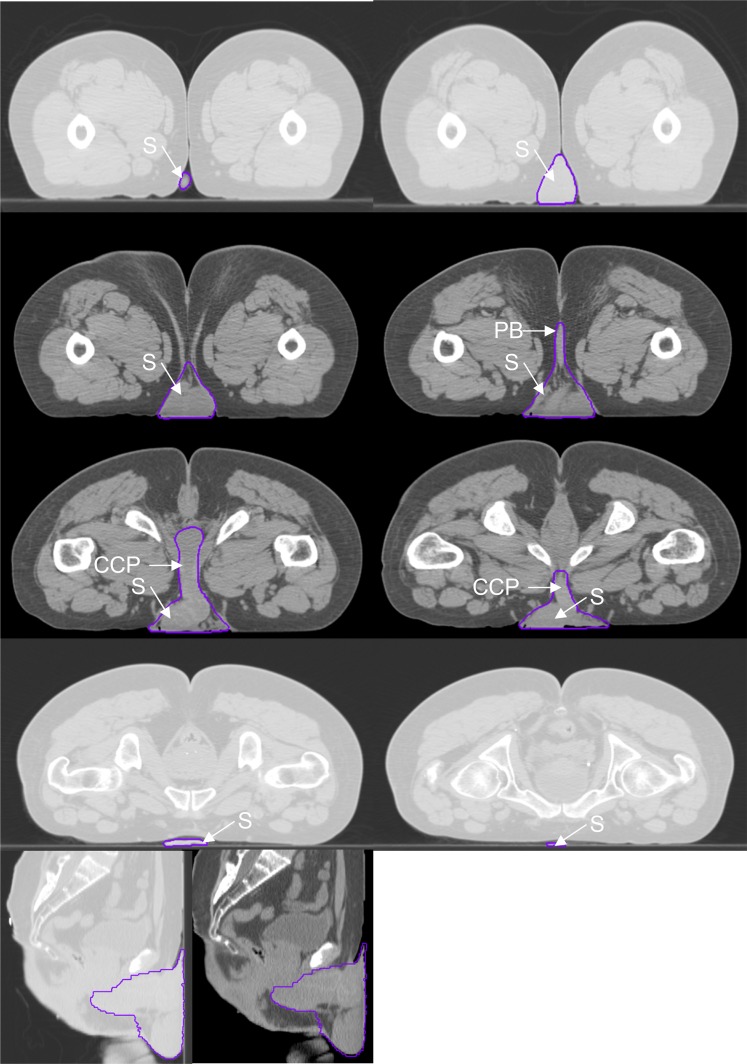
Figure 3.
Genitalia outlined for a male in the prone position. CCP, corpus cavernosum penis; PB, perineal body; S, scrotum.
In males, the scrotum, perineal body, corpus cavernosum penis and surrounding pre-pubic fat were contoured. The posterior border of the contour was defined by the exclusion of the rectum, prostate, urethral bulb and symphysis pubis. The lateral border extended from the inguinal crease at the anterior skin surface and joined the posterior border, excluding muscle and bone. The surrounding fat and tissue anterior to the symphysis pubis was included within the lateral borders. The inferior/superior extent of the contour was defined as the first and last slices seen containing scrotal and penile tissue.
In females, the area contoured consisted of the clitoris, labia majora and minora, and surrounding fat. The posterior border of the contour was defined by the exclusion of the vagina, cervix, uterus and symphysis pubis. The lateral border extended from the inguinal crease at the anterior skin surface and joined the posterior border, excluding muscle and bone. The mons pubis, surrounding fat and tissue anterior to the symphysis pubis was included within the lateral borders. The inferior/superior extent of the contour was defined as the first and last slices seen containing labial tissue.
DISCUSSION
To our knowledge this work presents for the first time concise, informed guidance for contouring the genitalia through a contouring atlas. This atlas was designed for radiotherapy purposes only and not as an anatomical and/or diagnostic tool. The aim of this atlas was to define the genitalia region to be considered in radiotherapy with a high emphasis on reproducibility and consistency. As with any normal tissue structure in radiotherapy volume localization, tissue exclusion depends on the clinical setting with the priority being appropriate tumour volume coverage. Therefore, the atlas acts as a tool to aid identification of genital structures in a consistent fashion, but their implementation in clinical practice should incorporate the radiotherapy planning principals common to other tumour treatment sites.
Unlike other studies often describing the contouring of the penis and scrotum in males and the clitoris, labia majora and minora in females, we included the surrounding fat and used the inguinal crease as an anatomical reference for the lateral border. Whilst it is important to spare the penis and scrotum in males and the clitoris, labia majora and minora in females, it is also important to include tissues surrounding the structures such as the skin and fat in the inguinal crease. These areas can develop moist desquamation and if not contoured as a sparing structure may receive high doses. The atlas has been produced as an aid to help generic planning of treatment of the primary tumour. Should regional lymph nodes be considered to be involved, these would require a boost dose that may alter the target volumes delineated in the atlas. Therefore, we consider that the atlas will be used as the foundation for treatment planning and that target volumes will be modified to adequately treat individual patients dependent on factors such as regional nodal involvement.
Ng et al13 acknowledges the lack of no established genitalia guidelines and describes the area to be contoured; the penis and scrotum (males), and the clitoris, labia majora and minora (females); the skin and fat anterior to the symphysis pubis for both genders should also be included. Myerson et al14 present a contouring atlas for clinical target volumes in anal cancer and recommend contouring the femoral heads, bladder and bowel but not the genitalia. Gay et al15 also present guidance for contouring normal tissues in pelvic radiotherapy but do not include the genitalia. Within the literature regarding IMRT for anal cancer, there is variation regarding the contouring method and dose constraints applied to the genitalia (Table 1) and genitalia dose reported (Table 2).
Table 1.
Common genitalia dose constraints applied in anal intensity-modulated radiotherapy plans
| Study | Prescription (Gy) | V20Gy (%) | V30Gy (%) | V40Gy (%) | V50Gy (%) | Max (Gy) | Mean (Gy) |
|---|---|---|---|---|---|---|---|
| James et al1 | 45 | <50–60 | 50 | ||||
| Menkarios et al9 | Unclear | 50 | <30 | ||||
| Brooks et al2 | 50.4 | <50 | <35 | <5 | |||
| Salama et al12 | 63 | 35–45 | 48 | ||||
| Gay et al15 | 50.4–54 | <50 | <35 | <5 | |||
| Lin and Ben-Josef8 | 59.4 | 35–45 | <5–10 | 48 | |||
| Das et al7 | Unclear | 36 | |||||
| Kachnic et al16 | 54 | <50 | <35 | <5 | |||
| Modal constraints | Median (range) = 54 (45–63) | <50 | <35 | <5 | 48–50 |
VXGy = percentage of genitalia volume receiving X Gy.
Table 2.
The median (range) genitalia dose reported from a review of the literature
| Study | Prescription (Gy) | n | V20Gy (%) | V30Gy (%) | V40Gy (%) | V50Gy (%) | Mean (Gy) |
|---|---|---|---|---|---|---|---|
| James et al1 | 45 | 7 | 5 | 29.1 | |||
| Brooks et al2 | 50.4 | 10 | 57.7 | 52.4 | 2.6 | 22.9 | |
| Myerson et al14 | 63 | 39 | 15.3 | 1.6 | |||
| Joseph et al17 | 59.4 | 10 | 62.1 | 45 | 24.5 | ||
| Vieillot et al18 | 50–54 | 10 | 21.7 | <5 | |||
| Das et al7 | Unclear | 9 | 22.1 | ||||
| Kachnic et al16 | 54 | 8 (male) | 74.8 | 35.2 | 3.2 | ||
| Kachnic et al16 | 54 | 8 (female) | 79.9 | 44.1 | 16 | ||
| Median (range) | 54 (45–63) | 9.5 (7–39) | 59.9 | 48.7 (15.3–79.9) | 5 (1.6–44.1) | 9.6 | 23.7 (22.1–29.1) |
The data used in this atlas were limited to an example of one male and one female patient, in each of the clinical treatment positions. However, these are examples, and the authors have outlined more patients. This permitted demonstration of the genital structures in atlas format for clinicians to refer to and base their outlining on. As with all radiotherapy planning atlases, it did not aim to cover all anatomical variations. The difference in genitalia volume size between and within the genders indicates this potential variation and further studies at our institution are looking at a wider application of the genitalia atlas both to a retrospective series and prospective group of patients treated with IMRT for anal cancer. From this, an assessment will be made of whether or not the current genitalia dose constraints need to be revised and incorporate some gender- and tumour stage-specific recommendations.
Whilst structures such as the penis, scrotum and labia may be easier to identify in practice, it is very difficult to identify the full anatomical extent of the genitalia in all dimensions, and consequently, there has until now been a lack of consistency in outlining. Our method, using standardized lateral borders should reduce this inconsistency, and it is ultimately hoped that dose constraint data will be more meaningful and achievable as a result.
There is an ever increasing role of MRI for radiotherapy planning, given the advantages of the higher contrast resolution of soft tissues and the multiplanar capability. In the future, it may be possible to refine the contours of the external genitalia using MRI planning scans or when fused with the CT planning data set. However, this would require a change in practice, as patients would need to be scanned using MRI in their treatment position, and scan protocols would need to be modified, as currently much of the external genitalia is excluded from diagnostic MRI.
CONCLUSION
Optimization of IMRT plans depends on accurate delineation of OARs as the planning algorithm will attempt to minimize dose to these structures, possibly at the expense of other normal structures not outlined or compromise the PTV coverage. There has been little work on genitalia dose constraints, partly because of the paucity of detailed data on outlining this OAR, and this atlas provides an opportunity to establish standardized outlining of these structures in males and females. This will subsequently allow the collection of toxicity data based on accurate and consistent OAR outlining and will help to optimize IMRT plan generation for this group of patients.
Furthermore, the atlas can be applied to other tumour sites receiving pelvic radiotherapy such as rectal, gynaecological and prostate cancers. Pelvic radiotherapy dose to the genitalia is responsible for both acute and late toxicities that have important long-term consequences in terms of sexual dysfunction and other quality of life issues. This is therefore an important area on which to focus and to ensure that there is the same level of accuracy and reproducibility as with other OARs.
Contributor Information
C Brooks, Email: corrie11@hotmail.com.
V N Hansen, Email: vibeke.hansen@icr.ac.uk.
A Riddell, Email: angela.riddell@rmh.nhs.uk.
V A Harris, Email: victoria.harris@rmh.nhs.uk.
D M Tait, Email: diana.tait@rmh.nhs.uk.
REFERENCES
- 1.James RD, Glynne-Jones R, Meadows HM, Cunningham D, Myint AS, Saunders MP, et al. Mitomycin or cisplatin chemoradiation with or without maintenance chemotherapy for treatment of squamous-cell carcinoma of the anus (ACT II): a randomised, phase 3, open-label, 2 × 2 factorial trial. Lancet Oncol 2013; 14: 516–24. doi: 10.1016/S1470-2045(13)70086-X [DOI] [PubMed] [Google Scholar]
- 2.Brooks CJ, Lee YK, Aitken K, Hansen VN, Tait DM, Hawkins MA. Organ-sparing intensity-modulated radiotherapy for anal cancer using the ACTII schedule: a comparison of conventional and intensity-modulated radiotherapy plans. Clin Oncol (R Coll Radiol) 2013; 25: 155–61. doi: 10.1016/j.clon.2012.08.008 [DOI] [PubMed] [Google Scholar]
- 3.Roach M, 3rd, Nam J, Gagliardi G, El Naqa I, Deasy JO, Marks LB. Radiation dose-volume effects and the penile bulb. Int J Radiat Oncol Biol Phys 2010; 76: S130–4. doi: 10.1016/j.ijrobp.2009.04.094 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Czito BG, Pepek JM, Meyer JJ, Yoo S, Willett CG. Intensity-modulated radiation therapy for anal cancer. Oncology (Williston Park) 2009; 23: 1082–9. [PubMed] [Google Scholar]
- 5.Glynne-Jones R, Sebag-Montefiore D, Adams R, McDonald A, Gollins S, James R, et al. ; UKCCCR Anal Cancer Trial Working Party. “Mind the gap”—the impact of variations in the duration of the treatment gap and overall treatment time in the first UK Anal Cancer Trial (ACT I). Int J Radiat Oncol Biol Phys 2011; 81: 1488–94. doi: 10.1016/j.ijrobp.2010.07.1995 [DOI] [PubMed] [Google Scholar]
- 6.Milano MT, Jani AB, Farrey KJ, Rash C, Heimann R, Chmura SJ. Intensity-modulated radiation therapy (IMRT) in the treatment of anal cancer: toxicity and clinical outcome. Int J Radiat Oncol Biol Phys 2005; 63: 354–61. doi: 10.1016/j.ijrobp.2005.02.030 [DOI] [PubMed] [Google Scholar]
- 7.Das P, Cantor SB, Parker CL, Zampieri JB, Baschnagel A, Eng C, et al. Long-term quality of life after radiotherapy for the treatment of anal cancer. Cancer 2010; 116: 822–9. doi: 10.1002/cncr.24906 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lin A, Ben-Josef E. Intensity-modulated radiation therapy for the treatment of anal cancer. Clin Colorectal Cancer 2007; 6: 716–19. doi: 10.3816/CCC.2007.n.041 [DOI] [PubMed] [Google Scholar]
- 9.Menkarios C, Azria D, Laliberté B, Moscardo CL, Gourgou S, Lemanski C, et al. Optimal organ-sparing intensity-modulated radiation therapy (IMRT) regimen for the treatment of locally advanced anal canal carcinoma: a comparison of conventional and IMRT plans. Radiat Oncol 2007; 2: 41. doi: 10.1186/1748-717X-2-41 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chuong MD, Freilich JM, Hoffe SE, Fulp W, Weber JM, Almhanna K, et al. Intensity-modulated radiation therapy vs. 3D conformal radiation therapy for squamous cell carcinoma of the anal canal. Gastrointest Cancer Res 2013; 6: 39–45. [PMC free article] [PubMed] [Google Scholar]
- 11.Bazan JG, Hara W, Hsu A, Kunz PA, Ford J, Fisher GA, et al. Intensity-modulated radiation therapy versus conventional radiation therapy for squamous cell carcinoma of the anal canal. Cancer 2011; 117: 3342–51. doi: 10.1002/cncr.25901 [DOI] [PubMed] [Google Scholar]
- 12.Salama JK, Mell LK, Schomas DA, Miller RC, Devisetty K, Jani AB, et al. Concurrent chemotherapy and intensity-modulated radiation therapy for anal canal cancer patients: a multicenter experience. J Clin Oncol 2007; 25: 4581–6. doi: 10.1200/JCO.2007.12.0170 [DOI] [PubMed] [Google Scholar]
- 13.Ng M, Leong T, Chander S, Chu J, Kneebone A, Carroll S, et al. Australasian Gastrointestinal Trials Group (AGITG) contouring atlas and planning guidelines for intensity-modulated radiotherapy in anal cancer. Int J Radiat Oncol Biol Phys 2012; 83: 1455–62. doi: 10.1016/j.ijrobp.2011.12.058 [DOI] [PubMed] [Google Scholar]
- 14.Myerson RJ, Garofalo MC, El Naqa I, Abrams RA, Apte A, Bosch WR, et al. Elective clinical target volumes for conformal therapy in anorectal cancer: a radiation therapy oncology group consensus panel contouring atlas. Int J Radiat Oncol Biol Phys 2009; 74: 824–30. doi: 10.1016/j.ijrobp.2008.08.070 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gay HA, Barthold HJ, O'Meara E, Bosch WR, El Naqa I, Al-Lozi R, et al. Pelvic normal tissue contouring guidelines for radiation therapy: a Radiation Therapy Oncology Group consensus panel atlas. Int J Radiat Oncol Biol Phys 2012; 83: e353–62. doi: 10.1016/j.ijrobp.2012.01.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kachnic LA, Tsai HK, Coen JJ, Blaszkowsky LS, Hartshorn K, Kwak EL, et al. Dose-painted intensity-modulated radiation therapy for anal cancer: a multi-institutional report of acute toxicity and response to therapy. Int J Radiat Oncol Biol Phys 2012; 82: 153–8. doi: 10.1016/j.ijrobp.2010.09.030 [DOI] [PubMed] [Google Scholar]
- 17.Joseph KJ, Syme A, Small C, Warkentin H, Quon H, Ghosh S, et al. A treatment planning study comparing helical tomotherapy with intensity-modulated radiotherapy for the treatment of anal cancer. Radiother Oncol 2010; 94: 60–6. doi: 10.1016/j.radonc.2009.10.003 [DOI] [PubMed] [Google Scholar]
- 18.Vieillot S, Fenoglietto P, Lemanski C, Moscardo CL, Gourgou S, Dubois JB, et al. IMRT for locally advanced anal cancer: clinical experience of the Montpellier Cancer Center. Radiat Oncol 2012; 7: 45. doi: 10.1186/1748-717X-7-45 [DOI] [PMC free article] [PubMed] [Google Scholar]