Abstract
Objective:
To investigate if MRI-assessed tumour volumetry correlates with histological tumour response to neoadjuvant chemotherapy (NACT) and subsequent chemoradiotherapy (CRT) in locally advanced rectal cancer (LARC).
Methods:
Data from 69 prospectively enrolled patients with LARC receiving NACT followed by CRT and radical surgery were analysed. Whole-tumour volumes were contoured in T2 weighted MR images obtained pre-treatment (VPRE), after NACT (VNACT) and after the full course of NACT followed by CRT (VCRT). VPRE, VNACT and tumour volume changes relative to VPRE, ΔVNACT and ΔVCRT were calculated and correlated to histological tumour regression grade (TRG).
Results:
61% of good histological responders (TRG 1–2) to NACT followed by CRT were correctly predicted by combining VPRE < 10.5 cm3, ΔVNACT > −78.2% and VNACT < 3.3 cm3. The highest accuracy was found for VNACT, with 55.1% sensitivity given 100% specificity. The volume regression after completed NACT and CRT (VCRT) was not significantly different between good and poor responders (TRG 1–2 vs TRG 3–5).
Conclusion:
MRI-assessed small tumour volumes after NACT correlated with good histological tumour response (TRG 1–2) to the completed course of NACT and CRT. Furthermore, by combining tumour volume measurements before, during and after NACT, more good responders were identified.
Advances in knowledge:
MRI volumetry may be a tool for early identification of good and poor responders to NACT followed by CRT and surgery in LARC in order to aid more individualized, multimodal treatment.
Chemoradiotherapy (CRT) followed by complete surgical removal is regarded as a standard of care for patients diagnosed with locally advanced rectal cancer (LARC).1 Therapeutic approaches incorporating additional agents to the standard fluorouracil (FU)-based CRT could potentially improve clinical outcome. Experimental studies have shown that intensified pre-operative treatment approaches with systemic neoadjuvant chemotherapy (NACT) before conventional long-course CRT have promising long-term outcome and acceptable safety profiles.2–4 On the other hand, modern multimodal treatment based on MRI leads to excellent local control, but distant control remains a challenge.5,6 Reliable identification of good and poor responders at an early stage could allow for more individualized, effective and less toxic treatment of these patients.
Traditionally, image-based evaluation of treatment response has been performed by unidimensional measurements of tumour diameters.7 Volumetry may provide more accurate assessment, especially for irregular-shaped tumours, and the volumetric information can readily be obtained from standard high-resolution two-dimensional MR images. MRI volumetry is accepted as a sensitive predictive tool for assessment of treatment outcome after NACT and radiotherapy (RT) of cervical cancer.8,9 In LARC, MRI-based tumour volume changes have been investigated as a parameter of treatment response after the full course of CRT.10–14 Recognizing the dose–volume relationship,15 it is anticipated that small tumour volume at the time of CRT is correlated to good treatment response. To our knowledge, the potential of MRI volumetry after NACT alone has been explored only in two studies; a pilot study of 16 patients16 and a recent study of 40 patients;17 the latter also assessing the performance of fluorine-18 fludeoxyglucose positron emission tomography (18F-FDG-PET). By performing a third, independent and larger study, the reliability of MRI volumetry after NACT as a clinical tool is addressed.
The main objective of the present study was to investigate if MRI-assessed tumour volumetry correlates with histological tumour response to NACT and subsequent CRT in LARC.
METHODS AND MATERIALS
Patients
The prospective Phase II trial LARC-Radiation Response Prediction (LARC-RRP) was approved by the institutional review board at Oslo University Hospital and the Regional Committee for Medical and Health Research Ethics South East. The study was performed in accordance with the Helsinki declaration, and written informed consent was required for participation. Of the total enrolled 113 patients, complete MRI data sets and histopathology from 69 patients were analysed (Figure 1). These patients were enrolled between October 2005 and April 2009.
Figure 1.
Study flow chart showing that from a total of 113 enrolled patients in the prospective trial Locally Advanced Rectal Cancer—Radiation Response Prediction, complete MRI data sets and histopathology from 69 patients were analysed. The patients underwent pre-treatment MRI (MRIPRE) response evaluation after neoadjuvant chemotherapy (NACT) and the first three radiotherapy fractions (MRINACT) as well as after the completion of chemoradiotherapy (CRT) MRICRT. *Not patient specific (breakdown of two out of three scanners).
Eligibility
The study eligibility criteria were MRI-assessed rectal tumour with histologically confirmed adenocarcinoma scheduled to receive pre-operative CRT according to the national guidelines;18 T4 tumour, T3 tumour with predicted circumferential resection margin ≤3 mm or any T stage with lymph node involvement within 3 mm of the predicted circumferential resection margin, age 18 years or more, Eastern Co-operative Oncology Group performance 0–1 and adequate haematologic, hepatic, renal and cardiac function. Diagnostic T and N stages were assessed by MRI according to Brown et al19,20 and the fifth edition TNM staging system. The study protocol allowed inclusion of patients with synchronous, potentially resectable metastases; thus, one patient with lung metastases and five patients with liver metastases were enrolled. Patient and tumour characteristics are provided in Table 1.
Table 1.
Patient characteristics
| Variable | Value |
|---|---|
| Number of patients | 69 |
| Gender | |
| Male | 38 (55.1%) |
| Female | 31 (44.9%) |
| Median age (years)a | |
| Male | 61 (30–73) |
| Female | 59 (30–70) |
| T stage at diagnosisb | |
| mrT2 | 2 (2.9%) |
| mrT3 | 42 (60.9%) |
| mrT4 | 25 (36.2%) |
| Median tumour volume at diagnosisa | 16.1 (1.1–293.4) |
| T stage after chemoradiotherapyc | |
| ypT0 | 14 (20.3%) |
| ypT1 | 5 (7.2%) |
| ypT2 | 17 (24.6%) |
| ypT3 | 22 (31.9%) |
| ypT4 | 11 (15.9%) |
| Tumour regression graded | |
| 1 (good response) | 13 (18.8%) |
| 2 (good response) | 36 (52.2%) |
| 3 (poor response) | 10 (14.5%) |
| 4 (poor response) | 9 (13.0%) |
| 5 (poor response) | 1 (1.4%) |
Except where indicated, data are numbers of patients, with percentages in parentheses.
Numbers in parentheses are ranges.
Assessed with MRI according to the TNM system.
Determined by histopathological evaluation of the surgical specimens.
Tumour regression grade (TRG) is classified into good (TRGs 1–2) and poor histological response (TRGs 3–5).
Pre-operative treatment and surgery
The experimental pre-operative treatment protocol consisted of two cycles of NACT, the Nordic flurouracil-leucovorin-oxaliplatin (FLOX) regimen: oxaliplatin (85 mg m−2, Day 1) and bolus 5-FU (500 mg m−2, Days 1 and 2) and folinic acid (100 mg m−2, Days 1 and 2) every second week followed by CRT. The RT was delivered in daily 2-Gy fractions, 5 days per week; the initial 23 fractions to the macroscopic tumour and areas at risk and the two final fractions adapted to the macroscopic tumour, as planned by CT. During the course of RT, concomitant chemotherapy was given as 50 mg m−2 of oxaliplatin once weekly and 825 mg m−2 of capecitabine twice daily on the days of RT. The median times to completed NACT and CRT, respectively, were 4 weeks (range, 4–4) and 5 weeks (range, 5–7 weeks and 4 days). The patients were scheduled for curatively intended surgery 6–8 weeks after CRT completion. None of the patients received post-operative chemotherapy.
MRI
The patients underwent diagnostic MRI prior to treatment, MRIPRE and radiological response evaluation by MRI 4 weeks after the completion of CRT, MRICRT. In addition, a study-specific MRI was performed after NACT and the first three RT fractions, MRINACT. The median time from MRIPRE to the start of NACT was 5 days (range, 3–19), and the median time from MRICRT to surgery was 20 days (range, 7–98). 41 of the study patients were imaged using a 1.5-T GE Signa® LS scanner (GE Healthcare, Milwaukee, WI) with a phased-array torso coil. Owing to the upgrading of the GE MRI scanner, the last 28 study patients were imaged using a 1.5 T Siemens Espree scanner (Siemens, Erlangen, Germany) equipped with a phased-array body matrix coil. The same scanner was always used for the same patient.
All MR examinations were performed according to the MERCURY study.21 High-resolution fast spin-echo T2 weighted (T2W) images of the pelvic cavity and rectum were obtained in the sagittal and transversal planes as well as perpendicular to the tumour axis. The imaging parameters for the transversal T2W sequence used in tumour volume assessment are summarized in Table 2. All MR images were stored in the institutional picture archiving and communication system.
Table 2.
Imaging parameters for the T2 weighted transversal fast spin-echo MR sequence
| Parameter | GE scannera | Siemens scannera |
|---|---|---|
| Repetition time (ms) | 3000–4000 | 3000 |
| Echo time (ms) | 81–84 | 81 |
| Echo train length | 12–16 | 13 |
| Number of excitations | 1 | 2 |
| Acquisition matrix | 256 × 256 | 320 × 256 |
| Reconstructed matrix | 512 × 512 | 640 × 640 |
| Section thickness (mm) | 4 | 4 |
| Gap (mm) | 1 | 1 |
| In-plane image resolution (mm2) | 0.39 × 0.39 | 0.38 × 0.38 |
41 study patients were imaged using a 1.5-T GE Signa® LS scanner (GE Healthcare, Milwaukee, WI) with a phased-array torso coil. Owing to an upgrade of the GE MRI scanner, the last 28 study patients were imaged using a 1.5-T Siemens Espree scanner (Siemens, Erlangen, Germany) equipped with a phased-array body matrix coil.
MRI volumetry
All examinations were transferred to the Oncentra Masterplan treatment planning system (3.0 SP1; Nucletron, Veenendaal, Netherlands) for tumour volume measurements. The tumour was manually contoured in transversal T2W MRIs by a radiologist with 12 years' experience in pelvic MRI. The radiologist has participated in the MERCURY trial21 and has gained in-depth knowledge of MR-pathological correlation of LARC specimens through a joint collaboration with a dedicated pathologist.22 The study radiologist was unaware of the therapeutic outcome. Solid tumours (medium T2W signal intensity) as well as mucinous tumours (high T2W signal intensity) were included in the delineation of tumour. After neoadjuvant treatment, fibrosis within the pre-treatment tumour area was included in the tumour volume since scattered residual tumour within fibrosis cannot reliably be excluded at MRI.22 Radiation-induced fibrosis in normal tissue was not included in the volume. The time needed to contour the tumour in each MR examination ranged from 5 min for well-defined small tumours up to 20 min for large infiltrating tumours.
Whole-tumour volumes were obtained by multiplying the cross-sectional tumour area in individual slices by the sum of the slice thickness and the slice gap. The percentage change in the MRI-assessed tumour volumes from pre-treatment (VPRE) to completed NACT and CRT (ΔVNACT and ΔVCRT) was calculated as ΔVNACT = [(VNACT − VPRE)/VPRE] × 100 and ΔVCRT = [(VCRT − VPRE)/VPRE] × 100, respectively.
Histopathological assessment
The resected primary tumour specimens were prepared according to validated protocols. Briefly, specimens were opened from the resection edge to 2 cm above and below macroscopically identifiable tumours and fixed in formalin. Slices of 5 mm were cut transversely through the tumour area and large-mount preparations were made to enable the examination of maximum depth of penetration, as well as the oral, anal and circumferential resection margins. To evaluate histopathological response, deep serial sections of the remaining paraffin-embedded tissue blocks from the initial tumour size were investigated for tumour cells.
A rectal cancer pathologist with 10 years' experience reviewed all histological sections. The histological characteristics included tumour and nodal stages (ypTN), in addition to the CRT-induced tumour regression grade (TRG) quantified using the five-point scale proposed by Bouzourene et al:23
TRG 1: the absence of residual tumour (pathological complete response)
TRG 2: rare residual tumour cells scattered throughout fibrosis
TRG 3: increased number of residual tumour cells but with fibrosis dominating
TRG 4: residual tumour cells outgrowing fibrosis
TRG 5: the absence of any tumour regression (no fibrosis).
In this study, TRGs 1–2 were defined as good histological response, and TRGs 3–5 were defined as poor histological response.
Historadiological correlation and statistical analysis
The one-sample Kolmogorov–Smirnov test of normality revealed that pre-treatment tumour volumes were non-gaussian distributed, thus, data were analysed using non-parametric tests. Correspondingly, continuous variables are presented as median and range, and categorical variables as numbers and percentages. The Mann–Whitney U test was used to compare VPRE, VNACT, ΔVNACT and ΔVCRT with TRG (TRGs 1–2 vs TRGs 3–5). The Fisher's exact test was used to assess if the proportion of good and poor responders differed between the two MRI scanners. The diagnostic performance of MRI volumetry for prediction of good histological tumour response was assessed by area under the receiver operating characteristic (ROC) curves and calculation of the sensitivity, specificity, positive-predictive value (PPV) and negative-predictive value (NPV). From the ROC curve, the optimal cut-off was identified by giving equal weights to sensitivity and specificity (overall accuracy). A p-value <0.05 was considered statistically significant. Statistical analysis was performed using GraphPad Prism® v. 6.0c for Mac OS X (GraphPad Software, San Diego, CA).
RESULTS
Pre-treatment tumour volumes vs tumour regression grade
49 (71%) of the 69 patients showed good histological tumour response (TRGs 1–2), whereas 20 (29%) patients were poor responders (TRGs 3–5). There was no significant difference in the proportion of good and poor responders between the two MRI scanners (p > 0.05). The VPRE was significantly smaller for patients achieving good histological response (median, 13.4 cm3; range, 1.1 to 144.1 cm3) than for poor-responding patients (median, 32.8 cm3; range, 11.1 to 293.4 cm3) (p < 0.0001).
Figure 2a shows the sensitivity and specificity for prediction of good histological tumour response using different cut-off values of VPRE. The highest accuracy was obtained for VPRE = 17.1 cm3 [area under the ROC curve (AUC) = 0.80; 95% confidence interval (CI) = 0.70–0.91; p < 0.0001] (Figure 2b), yielding 71.4% of sensitivity, 85.0% of specificity, 86.8% (33 of 38) of PPV and 51.6% (16 of 31) of NPV. 38 (55%) patients had VPRE < 17.1 cm3. The cut-off that exclusively identified good responders was VPRE < 10.5 cm3, yielding 36.7% of sensitivity, 100% of specificity, 100% (18 of 18) PPV and 39.2% (20 of 51) NPV. In our study population, 18 (26%) patients had VPRE < 10.5 cm3, whereas 51 (74%) patients had VPRE > 10.5 cm3.
Figure 2.
The accuracy of pre-treatment tumour volume [VPRE (cm3)] for prediction of good histological tumour response [tumour regression grades (TRGs) 1–2] to neoadjuvant chemotherapy followed by chemoradiotherapy was assessed by receiver operating characteristic (ROC) analysis. The sensitivity and specificity for different cut-off values for VPRE are shown. The dashed line indicates the highest cut-off for VPRE yielding 100% specificity (a). A VPRE of 10.5 cm3 corresponded to an area under the ROC curve of 0.80 (95% confidence interval = 0.70–0.91; p < 0.0001), a sensitivity of 36.7%, a positive-predictive value of 100% (18 of 18), and a negative-predictive value of 39.2% (20 of 51) (b). AUC, area under the curve.
Tumour volume changes following neoadjuvant chemotherapy vs tumour regression grade
The ΔVNACT ranged from a decrease of 96.4% to an increase of 26.2%. Median ΔVNACT was significantly different between tumours with poor (−45.5%; range, −77.1% to 26.2%) and good (−68.4%; range, −96.4% to −0.55%) histological response (p =0.005) (Figure 3).
Figure 3.
Transversal fast spin-echo T2 weighted MRIs of two patients with locally advanced T3 (a–c) and T4 (d–f) rectal tumours, achieving tumour regression grade (TRG) 1 and TRG 4, respectively. The two tumours' central slices are shown. Prior to treatment their whole-tumour volumes were 31.0 cm3 (a) and 36.2 cm3 (d). After neoadjuvant chemotherapy and three 2-Gy fractions of radiotherapy, the TRG 1 tumour showed 96.4% volume regression (b), whereas for the TRG 4 tumour, the volume regression was 16.2% (e). After the completion of chemoradiotherapy, both tumours obtained large volume regressions; 99.9% for the TRG 1 tumour (c) and 84.1% for the TRG 4 tumour (f). Imaging parameters are listed in Table 2.
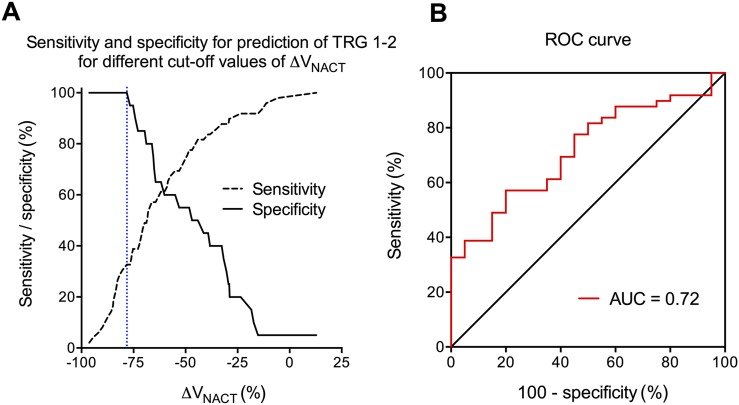
Figure 4a shows the sensitivity and specificity for prediction of good histological tumour response using different cut-off values of ΔVNACT. The highest accuracy was obtained for ΔVNACT > −78.2% (AUC = 0.72; 95% CI = 0.59–0.84; p = 0.005) (Figure 4b), yielding 32.7% sensitivity, 100% specificity, 100% (16 of 16) PPV and 37.7% (20 of 53) NPV. 16 (23%) patients had ΔVNACT > 78.2%. The cut-off that exclusively identified good responders was, therefore, also ΔVNACT > −78.2%.
Figure 4.
The accuracy of tumour volume regression from pre-treatment to after neoadjuvant chemotherapy (NACT) and three 2-Gy fractions of radiotherapy [ΔVNACT (%)] for prediction of good histological tumour response [tumour regression grade (TRG) 1–2] to NACT followed by chemoradiotherapy (CRT) was assessed by receiver operating characteristic (ROC) analysis. The sensitivity and specificity for different cut-off values of ΔVNACT are shown, with the optimal cut-off of ΔVNACT identified as −78.2% (dashed line) (a). A ΔVNACT cut-off of −78.2% corresponded to an area under the ROC curve (AUC) of 0.72 (95% confidence interval = 0.59–0.84; p = 0.005), a sensitivity of 32.7%, a specificity of 100%, a positive-predictive value of 100% (16 of 16), and a negative-predictive value of 37.7% (20 of 53) (b).
Tumour volumes after neoadjuvant chemotherapy vs tumour regression grade
The VNACT was significantly smaller for patients achieving good histological response (median, 2.8 cm3; range, 0.2 to 89.9 cm3) than was for poor-responding patients (median, 17.6 cm3; range, 3.4 to 102.1 cm3) (p < 0.0001). Figure 5a shows the sensitivity and specificity for prediction of good histological tumour response using different cut-off values of VNACT. The highest accuracy was obtained for VNACT < 3.3 cm3 (AUC = 0.84; 95% CI = 0.74–0.93; p < 0.0001) (Figure 5b), yielding 55.1% sensitivity, 100% specificity, 100% (27 of 27) PPV and 47.6% (20 of 42) NPV. The cut-off that exclusively identified good responders was therefore also VNACT < 3.3 cm3. 27 (39%) patients had VNACT < 3.3 cm3.
Figure 5.
The accuracy of tumour volume after neoadjuvant chemotherapy (NACT) and three 2-Gy fractions of radiotherapy [VNACT (cm3)] for prediction of good histological tumour response [tumour regression grade (TRG) 1–2] to NACT followed by chemoradiotherapy (CRT) was assessed by receiver operating characteristic (ROC) analysis. The sensitivity and specificity for different cut-off values of VNACT are shown, with the optimal cut-off of VNACT identified as 3.3 cm3 (dashed line) (a). A VNACT cut-off of 3.3 cm3 corresponded to an area under the ROC curve (AUC) of 0.84 (95% confidence interval = 0.74–0.93; p < 0.0001), a sensitivity of 55.1%, a specificity of 100%, a positive-predictive value of 100% (27 of 27), and a negative-predictive value of 47.6% (20 of 42) (b).
Tumour volume changes following chemoradiotherapy completion vs tumour regression grade
The median ΔVCRT was −86.0% (range, −99.9% to +1.1%), and there was no statistically significant difference between the two histological responder groups (poor responders: median, −88.6%; range, −97.4% to −25.3% and good responders: median, −85.8%; range, −99.9% to +1.1%) (p =0.797).
Combined volumetric measurements vs tumour regression grade
In total, 30 (61%) out of 49 good responders were predicted by either small pre-treatment tumour volumes (<10.5 cm3; n = 18), large volume regressions to NACT (>−78.2%; n = 16) or small volumes after NACT (<3.3 cm3; n = 27). Small pre-treatment volume and large volume regression identified different patients; only six patients had both small treatment volume and large volume regression. Small tumour volume after NACT identified the majority of good responders, but the combined use of all three volumetric measurements identified the most.
DISCUSSION
This study showed that small pre-treatment tumour volume (VPRE), large tumour volume regression to NACT (ΔVNACT) and small tumour volume after NACT (VNACT) correlated with good histological tumour response (TRGs 1–2) to the completed course of NACT followed by CRT in LARC. The combined use of VPRE, ΔVNACT and VNACT identified most good responders.
To our knowledge, two other studies have evaluated the predictive value of MRI volumetry following NACT in patients with LARC (Table 3).16,17 Both studies explored high-resolution T2W-based MRI volumetry and used the same end point: good responders were defined as complete response or scattered microscopic residual foci at histopathology.23,24 Oxaliplatin-based NACT was used, although in different dosage, duration and combination with other agents. The study populations consisted of tumour stages T3–4. Our study included T3 tumours with a larger distance to the mesorectal fascia than the other two studies. The pre-treatment tumour volumes were comparable to Aiba et al,17 but substantially smaller than Nougaret et al.16 Despite the differences between these three studies, the results are encouragingly congruent: the optimal cut-off for tumour volume regression after NACT for differentiating good and poor responders at histopathology was 68–78%. This indicates that MRI volumetry is a robust method for assessment of NACT and CRT response in LARC. Furthermore, the results of the MRI volumetry support that the dose–volume relationship is an essential mechanism.
Table 3.
Overview of the three studies investigating MRI volumetry of neoadjuvant chemotherapy (NACT) response in locally advanced rectal cancer as a predictor of tumour regression grade (TRG) after NACT followed by chemoradiotherapy
| Author | Nougaret et al16 | Aiba et al17 | Present study |
|---|---|---|---|
| Patient characteristics | |||
| Number of patients | 16 | 40 | 69 |
| T stage | Locally advanced | T3 (32%) + T4 (67%) | T2 (3%) + T3 (61%) + T4 (36%) |
| Tumour volume (cm3)a | 132 ± 166 | 29.3 (3.5–262.3) | 16.1 (1.1–293.4) |
| Method | |||
| Good responder | TRGs 3–4 (Dworak) | TRGs 3–4 (Dworak et al24) | TRGs 1–2 (Bouzourene et al23) |
| MRI | T2W ≤1 mm3 | T2W ≤1 mm3 | T2W ≤1 mm3 |
| Reading | Single radiologist | Experienced radiologist and surgeon | Experienced radiologist |
| Treatment | |||
| NACT | FOLFIRINOX | XELOX, FOLFOX | Nordic FLOX |
| Time point (weeks) | 8 | 4–8 | 4 |
| Results | |||
| ∆V (%)a | −68 (±27) | −60 (+23.5 to −93.9) | −65 (+26.2 to −96.4) |
| Accuracy—receiver operating characteristics analyses (%) | 68 | 70 | 78 |
| Sensitivity for 100% specificity (%) | 86 | 55–60 | 38 |
| Cut-off ∆V (%) for 100% specificity | 70 | NA | 78 |
FLOX, fluorouracil + oxaliplatin; FOLFIRINOX, folinic acid + fluorouracil + irinotecan + oxaliplatin; FOLFOX, folinic acid + fluorouracil + oxaliplatin; NA, not applicable; T2W, T2 weighted; XELOX, capecitabine + oxaliplatin.
Mean ± standard deviation or median (range).
Modern multimodal treatment of LARC commonly leads to excellent local control, but distant metastases remains a problem.2,5,6 At present, the challenge is to reduce treatment morbidity and prevent metastatic disease.5 It has recently been advocated that customized and individualized treatment strategies may result in a more optimal balance between over- and undertreatment.9,25 The promising results of our study and the studies of Nougaret et al16 and Aiba et al17 support that MRI volumetry after NACT can be used to stratify good responders into studies exploring individualized, less extensive treatment regimens, such as omission of RT and less extensive surgery, or even deferral of surgery.6,25,26 Another strategy, as suggested by Schrag,9 is to intensify the systemic treatment or to administer the systemic treatment earlier in the treatment course to prevent dissemination of micrometastases. The recent updated results from the German CAO/ARO/AIO-94 trial27 showed a significant association between local treatment efficacy (TRG) and disease-free survival. Thus, it is likely that MRI volumetry of the primary tumour can predict systemic treatment response and therefore can be used to customize subsequent treatment regimens.
Appropriate choice of cut-off value for tumour volume regression is a trade-off between specificity and sensitivity, although optimal accuracy is the best combination of sensitivity and specificity, 100% specificity is required to exclusively identify good responders, notwithstanding that some good responders are overlooked. Although the accuracies in the three aforesaid studies were similar, 100% specificity yielded substantial variation in sensitivity (38–86%).
In our study, the cut-off value with optimal accuracy (78%) was higher than in the two other studies (68% and 70%), and the sensitivity for 100% specificity (38%) was lower (86% and 55%). There may be several possible explanations for these discrepancies: time to assessment, variation in study population and that CRT in our study was initiated shortly before MRI volumetry. Both Nougaret et al16 and Aiba et al17 used more intensive and prolonged NACT, probably leading to larger differences between good and poor responders and consequently higher sensitivity. The population in our study was larger and more heterogeneous with respect to T stage and tumour volume, leading to larger variations in volume changes and thereby also in sensitivity and specificity. Furthermore, we included mucinous tumours with extracellular mucin pools that do not necessarily shrink despite good cellular response. For practical reasons, MRI volumetry was performed after 3 out of 25 RT fractions. Radiation-induced oedema is known to occur quickly during RT and may have caused overall volume increase masking differences between good and poor responders. A highly diffusion-weighted sequence could have reduced the interpretation difficulties caused by oedema and improved the delineation of residual tumour. Radiation-induced fibrosis commonly manifests weeks to months after treatment completion and is unlikely to have occurred.
The tumour volume regression after the completed course of NACT and CRT (∆VCRT) did not correlate with TRG. The experimental study-specific NACT induced extensive tumour volume regression for almost all patients, possibly masking the differences in tumour volume regression to CRT between tumours with good and poor histological response. Other studies have demonstrated a clear correlation between CRT-induced tumour volume regression and TRG, but the median tumour volume regression in these studies28 was substantially less than in our study. Furthermore, none of these studies used NACT prior to CRT. In accordance with our results, Nougaret et al16 found a correlation between tumour volume regression after NACT alone, but not after the full course of NACT and CRT.
In rectal cancer as well as in other solid tumours, functional MRI techniques and PET are increasingly integrated into staging and treatment response evaluation.29–32 However, the acquisition and evaluation of these additional sequences demand more resources, prolonged scan time and additional time for interpretation. Aiba et al17 found that the addition of 18F-FDG PET to MRI volumetry did not improve the performance of NACT response assessment. The real potential of functional imaging techniques is the ability to depict treatment response occurring prior to tumour volume regression, thereby allowing early adjustment of the therapeutic strategy.
A potential weakness of our study is the time point to assess change in volume after only two 2-week cycles of NACT. The findings of Nougaret et al16 and Aiba et al17 indicate that MRI volumetry after four 2-week cycles of NACT identifies good responders with higher sensitivity. Ideally, to establish the optimal time point for assessment, repeat imaging at different time points during NACT and CRT should be considered.
Volumetry is challenging, particularly after NACT, and may lead to considerable intra- and interobserver variability. A limitation in our study was that volumetry was performed by a single study radiologist, hence, interobserver variations could not be assessed. However, the congruent tumour volume changes predicting good response in our study and the studies reported by Nougaret et al and Aiba et al, the latter even without subsequent CRT, support that MRI volumetry is a robust method.
CONCLUSION
For patients with LARC receiving NACT followed by CRT and surgery, MRI-assessed small tumour volumes after NACT correlated with good histological tumour response (TRGs 1–2) to the completed course of NACT and CRT. Furthermore, by combining tumour volume measurements before, during and after NACT, more good responders were identified.
FUNDING
We acknowledge the financial support by the South-Eastern Norway Regional Health Authority (grant 2012002) and the Norwegian Cancer Society (grant D04085/003).
Contributor Information
T Seierstad, Email: therese@student.matnat.uio.no.
K H Hole, Email: khh@ous-hf.no.
K K Grøholt, Email: kkg@ous-hf.no.
S Dueland, Email: svedue@ous-hf.no.
A H Ree, Email: a.h.ree@medisin.uio.no.
K Flatmark, Email: Kjersti.Flatmark@rr-research.no.
K R Redalen, Email: kathrine.roe@medisin.uio.no.
REFERENCES
- 1.Weber GF, Rosenberg R, Murphy JE, Meyer Zum Büschenfelde C, Friess H. Multimodal treatment strategies for locally advanced rectal cancer. Expert Rev Anticancer Ther 2012; 12: 481–94. doi: 10.1586/era.12.3 [DOI] [PubMed] [Google Scholar]
- 2.Chua YJ, Barbachano Y, Cunningham D, Oates JR, Brown G, Wotherspoon A, et al. Neoadjuvant capecitabine and oxaliplatin before chemoradiotherapy and total mesorectal excision in MRI-defined poor-risk rectal cancer: a phase 2 trial. Lancet Oncol 2010; 11: 241–8. doi: 10.1016/s1470-2045(09)70381-x [DOI] [PubMed] [Google Scholar]
- 3.Chau I, Brown G, Cunningham D, Tait D, Wotherspoon A, Norman AR, et al. Neoadjuvant capecitabine and oxaliplatin followed by synchronous chemoradiation and total mesorectal excision in magnetic resonance imaging-defined poor-risk rectal cancer. J Clin Oncol 2006; 24: 668–74. doi: 10.1200/jco.2005.04.4875 [DOI] [PubMed] [Google Scholar]
- 4.Schou JV, Larsen FO, Rasch L, Linnemann D, Langhoff J, Høgdall E, et al. Induction chemotherapy with capecitabine and oxaliplatin followed by chemoradiotherapy before total mesorectal excision in patients with locally advanced rectal cancer. Ann Oncol 2012; 23: 2627–33. doi: 10.1093/annonc/mds056 [DOI] [PubMed] [Google Scholar]
- 5.Engelen SM, Maas M, Lahaye MJ, Leijtens JW, van Berlo CL, Jansen RL, et al. Modern multidisciplinary treatment of rectal cancer based on staging with magnetic resonance imaging leads to excellent local control, but distant control remains a challenge. Eur J Cancer 2013; 49: 2311–20. doi: 10.1016/j.ejca.2013.03.006 [DOI] [PubMed] [Google Scholar]
- 6.Schrag D, Weiser MR, Goodman KA, Gonen M, Hollywood E, Cercek A, et al. Neoadjuvant chemotherapy without routine use of radiation therapy for patients with locally advanced rectal cancer: a pilot trial. J Clin Oncol 2014; 32: 513–18. doi: 10.1200/JCO.2013.51.7904 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer 2009; 45: 228–47. doi: 10.1016/j.ejca.2008.10.026 [DOI] [PubMed] [Google Scholar]
- 8.Wang JZ, Mayr NA, Zhang D, Li K, Grecula JC, Montebello JF, et al. Sequential magnetic resonance imaging of cervical cancer: the predictive value of absolute tumour volume and regression ratio measured before, during, and after radiation therapy. Cancer 2010; 116: 5093–101. doi: 10.1002/cncr.25260 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Schrag D. Evolving role of neoadjuvant therapy in rectal cancer. Curr Treat Options Oncol 2013; 14: 350–64. doi: 10.1007/s11864-013-0242-8 [DOI] [PubMed] [Google Scholar]
- 10.Curvo-Semedo L, Lambregts DM, Maas M, Thywissen T, Mehsen RT, Lammering G, et al. Rectal cancer: assessment of complete response to preoperative combined radiation therapy with chemotherapy—conventional MR volumetry versus diffusion-weighted MR imaging. Radiology 2011; 260: 734–43. doi: 10.1148/radiol.11102467 [DOI] [PubMed] [Google Scholar]
- 11.Barbaro B, Fiorucci C, Tebala C, Valentini V, Gambacorta MA, Vecchio FM, et al. Locally advanced rectal cancer: MR imaging in prediction of response after preoperative chemotherapy and radiation therapy. Radiology 2009; 250: 730–9. doi: 10.1148/radiol.2503080310 [DOI] [PubMed] [Google Scholar]
- 12.Yeo SG, Kim DY, Kim TH, Jung KH, Hong YS, Chang HJ, et al. Tumor volume reduction rate measured by magnetic resonance volumetry correlated with pathologic tumour response of preoperative chemoradiotherapy for rectal cancer. Int J Radiat Oncol Biol Phys 2010; 78: 164–71. doi: 10.1016/j.ijrobp.2009.07.1682 [DOI] [PubMed] [Google Scholar]
- 13.Yeo SG, Kim DY, Kim TH, Chang HJ, Oh JH, Park W, et al. Pathologic complete response of primary tumor following preoperative chemoradiotherapy for locally advanced rectal cancer: long-term outcomes and prognostic significance of pathologic nodal status (KROG 09-01). Ann Surg 2010; 252: 998–1004. doi: 10.1097/SLA.0b013e3181f3f1b1 [DOI] [PubMed] [Google Scholar]
- 14.Nougaret S, Rouanet P, Molinari N, Pierredon MA, Bibeau F, Azria D, et al. MR volumetric measurement of low rectal cancer helps predict tumor response and outcome after combined chemotherapy and radiation therapy. Radiology 2012; 263: 409–18. doi: 10.1148/radiol.12111263 [DOI] [PubMed] [Google Scholar]
- 15.Jakobsen A, Ploen J, Vuong T, Appelt A, Lindebjerg J, Rafaelsen SR. Dose-effect relationship in chemoradiotherapy for locally advanced rectal cancer: a randomized trial comparing two radiation doses. Int J Radiat Oncol Biol Phys 2012; 84: 949–54. doi: 10.1016/j.ijrobp.2012.02.006 [DOI] [PubMed] [Google Scholar]
- 16.Nougaret S, Fujii S, Addley HC, Bibeau F, Pandey H, Mikhael H, et al. Neoadjuvant chemotherapy evaluation by MRI volumetry in rectal cancer followed by chemoradiation and total mesorectal excision: initial experience. J Magn Reson Imaging 2013; 38: 726–32. doi: 10.1002/jmri.23905 [DOI] [PubMed] [Google Scholar]
- 17.Aiba T, Uehara K, Nihashi T, Tsuzuki T, Yatsuya H, Yoshioka Y, et al. MRI and FDG-PET for assessment of response to neoadjuvant chemotherapy in locally advanced rectal cancer. Ann Surg Oncol 2014; 21: 1801–8. doi: 10.1245/s10434-014-3538-4 [DOI] [PubMed] [Google Scholar]
- 18.Bernstein TE, Endreseth BH, Romundstad P, Wibe A; Norwegian Colorectal Cancer Group. Circumferential resection margin as a prognostic factor in rectal cancer. Br J Surg 2009; 96: 1348–57. doi: 10.1002/bjs.6739 [DOI] [PubMed] [Google Scholar]
- 19.Brown G, Richards CJ, Newcombe RG, Dallimore NS, Radcliffe AG, Carey DP, et al. Rectal carcinoma: thin-section MR imaging for staging in 28 patients. Radiology 1999; 211: 215–22. doi: 10.1148/radiology.211.1.r99ap35215 [DOI] [PubMed] [Google Scholar]
- 20.Brown G, Richards CJ, Bourne MW, Newcombe RF, Radcliffe AG, Dallimore NS, et al. Morphologic predictors of lymph node status in rectal cancer with use of high-spatial-resolution MR imaging with histopathologic comparison. Radiology 2003; 227: 371–7. doi: 10.1148/radiol.2272011747 [DOI] [PubMed] [Google Scholar]
- 21.MERCURY Study Group. Diagnostic accuracy of preoperative magnetic resonance imaging in predicting curative resection of rectal cancer: prospective observational study. BMJ 2006; 333: 779. doi: 10.1136/bmj.38937.646400.55 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hole KH, Larsen SG, Grøholt KK, Giercksky KE, Ree AH. Magnetic resonance-guided histopathology for improved accuracy of tumor response evaluation of neoadjuvant treatment in organ-infiltrating rectal cancer. Radiother Oncol 2013; 107: 178–83. doi: 10.1016/j.radonc.2013.03.017 [DOI] [PubMed] [Google Scholar]
- 23.Bouzourene H, Bosman FT, Seelentag W, Matter M, Coucke P. Importance of tumor regression assessment in predicting the outcome in patients with locally advanced rectal carcinoma who are treated with preoperative radiotherapy. Cancer 2012; 94: 1121–30. doi: 10.1002/cncr.10327 [DOI] [PubMed] [Google Scholar]
- 24.Dworak O, Keilholz L, Hoffmann A. Pathological features of rectal cancer after preoperative radiochemotherapy. Int J Colorectal Dis 1997; 12: 19–23. doi: 10.1007/s003840050072 [DOI] [PubMed] [Google Scholar]
- 25.Valentini V, Minsky BD. Tumor regression grading in rectal cancer: is it time to move forward? J Clin Oncol 2014; 32: 1534–6. doi: 10.1200/JCO.2014.55.4766 [DOI] [PubMed] [Google Scholar]
- 26.Habr-Gama A, Sabbaga J, Gama-Rodrigues J, São Julião GP, Proscurshim I, Bailão Aguilar P, et al. Watch and wait approach following extended neoadjuvant chemoradiation for distal rectal cancer: are we getting closer to anal cancer management? Dis Colon Rectum 2013; 56: 1109–17. doi: 10.1097/DCR.0b013e3182a25c4e [DOI] [PubMed] [Google Scholar]
- 27.Fokas E, Liersch T, Fietkau R, Hohenberger W, Beissbarth T, Hess C, et al. Tumor regression grading after preoperative chemoradiotherapy for locally advanced rectal carcinoma revisited: updated results of the CAO/ARO/AIO-94 trial. J Clin Oncol 2014; 32: 1554–62. doi: 10.1200/jco.2013.54.3769 [DOI] [PubMed] [Google Scholar]
- 28.Wu LM, Zhu J, Hu J, Yin Y, Gu HY, Hua J, et al. Is there a benefit in using magnetic resonance imaging in the prediction of preoperative neoadjuvant therapy response in locally advanced rectal cancer? Int J Colorectal Dis 2013; 28: 1225–38. doi: 10.1007/s00384-013-1676-y [DOI] [PubMed] [Google Scholar]
- 29.Beets-Tan RG, Beets GL. MRI for assessing and predicting response to neoadjuvant treatment in rectal cancer. Nat Rev Gastroenterol Hepatol 2014; 11: 480–8. doi: 10.1038/nrgastro.2014.41 [DOI] [PubMed] [Google Scholar]
- 30.Joye I, Deroose CM, Vandecaveye V, Haustermans K. The role of diffusion-weighted MRI and (18)F-FDG PET/CT in the prediction of pathologic complete response after radiochemotherapy for rectal cancer: a systematic review. Radiother Oncol 2014; 113: 158–65. doi: 10.1016/j.radonc.2014.11.026 [DOI] [PubMed] [Google Scholar]
- 31.Petrillo A, Fusco R, Petrillo M, Granata V, Sansone M, Avallone A, et al. Standardized Index of Shape (SIS): a quantitative DCE-MRI parameter to discriminate responders by non-responders after neoadjuvant therapy in LARC. Eur Radiol Jan 2015. Epub ahead of print. doi: 10.1007/s00330-014-3581-3 [DOI] [PubMed] [Google Scholar]
- 32.Janssen MH, Öllers MC, van Stiphout RG, Riedl RG, van den Bogaard J, Buijsen J, et al. PET-based treatment response evaluation in rectal cancer: prediction and validation. Int J Radiat Oncol Biol Phys 2012; 82: 871–6. doi: 10.1016/j.ijrobp.2010.11.038 [DOI] [PubMed] [Google Scholar]