Abstract
Background
Bone marrow (BM) dysfunction following experimental lung contusion (LC) resolves in 7 days, however, if followed by chronic stress (CS) following, BM dysfunction is persistent. Mesenchymal stem cells (MSC) have protective immunomodulatory effects. We hypothesize that MSC can protect the BM against the deleterious effect of CS following LC.
Methods
Male Sprague-Dawley rats (n=6–7/group) underwent LC or LC/CS ± MSC injection. CS consisted of a daily 2-hour period of restraint with repositioning and alarming every 30 minutes to prevent habituation. A single intravenous dose of 5 × 106 MSC was given within ten minutes following LC. Animals were sacrificed at day seven and peripheral blood (PB) and BM were collected. Flow cytometry was used to assess hematopoietic progenitor cells (HPCs) mobilized to PB. Plasma G-CSF levels were measured by ELISA. BM cellularity and growth of BM HPC colonies (CFU-E, BFU-E, CFU-GEMM) were also evaluated.
Results
As previously reported, the addition of CS to LC resulted in a 32% decrease in BM cellularity, significant decreases in CFU-GEMM, BFU-E, and CFU-E and marked increase in HPC in the PB as compared naïve animals. The addition of MSC to LC/CS resulted in a 22% increase in BM cellularity and significant increases in CFU-GEMM, BFU-E, and CFU-E cultured from the BM. MSCs additionally reduced plasma G-CSF, prevented prolonged mobilization of HPC to PB, and restored colony growth to naïve levels.
Conclusion
Chronic stress following LC results in persistent BM dysfunction manifested by a significant decrease in cellularity, HPC colony growth, and increased G-CSF levels and HPC mobilization to the PB at seven days following injury. The addition of a single dose of MSCs following acute traumatic injury reverses the deleterious effects of CS on BM function. Further study is warranted to better understand the mechanisms behind MSC-mediated protection of BM function in the setting of CS.
Keywords: Cell-based therapy, MSCs, Chronic Stress, BM dysfunction, CFU-E
BACKGROUND
Trauma-associated BM dysfunction manifests as increased susceptibility to infection and persistent anemia despite transfusion1. In our rodent lung contusion (LC) model, BM dysfunction is observed soon following injury and is characterized by depression of BM cellularity, decreased growth of hematopoietic progenitor cell (HPC) colonies, and mobilization of HPCs to the peripheral blood2,3. Despite this early dysfunction, rats demonstrate recovery to normal usually by seven days following injury. This pattern of recovery following injury is not typically seen in severely injured trauma patients who frequently have prolonged bone marrow (BM) dysfunction, lasting more than 14 days following ICU admission4,5. One critical difference between our patients and our rodent model is that severely injured patients are subjected to repeated insults, which result in a prolonged elevation of catecholamine levels that persists for several weeks6. The addition of chronic restraint stress to our lung contusion model prolongs BM dysfunction following injury7.
Mesenchymal stem cells (MSCs) have generated much interest as potential cell based therapy for various clinical applications including wound healing, acute lung injury, sepsis, myocardial infarction, and many inflammatory/autoimmune disorders8–12. These multipotent cells are normally found within the bone marrow (BM) as well as many other tissues. They can be allogeneically transplanted without evidence of rejection or need for immunosuppression. While one of the defining characteristics of MSCs is plasticity, it appears that their paracrine and immunomodulatory effects may have more of an impact in their potential as a cell-based therapy. We have previously investigated the effect of MSCs given at the time of lung injury on the healing of the injured lung and found them to hasten wound healing13. We now hypothesize that MSCs can protect the BM against the deleterious effects of CS following LC.
METHODS
Experimental Groups
Male Rats (n=6–7/group) were assigned to experimental groups as follows: lung contusion (LC) alone, lung contusion plus mesenchymal stem cells (LC+MSC), lung contusion followed by six days of chronic restraint stress (LC/CS), and lung contusion plus mesenchymal stem cells followed by six days of chronic restraint stress (LC/CS+MSC). Additional rats were assigned to a control group consisting of uninjured animals that only underwent daily handling. Rats were sacrificed on day seven and bone marrow and plasma were harvested.
Animals
Male Sprague-Dawley rats weighing between 250 and 350g (Charles River, Wilmington, MA) were housed in a barrier-sustained animal facility with free access to water and chow (Teklad 22/5 Rodent Diet W-8640; Harlen, Madison WI) at 25° C with 12-hour light/dark cycle. All rats were maintained in accordance with the recommendations of the Guide for the Care and Use of Laboratory Animals and experiments were approved by the Rutgers New Jersey Medical School Animal Care and Use Committee.
Lung Contusion Model
Following administration of intraperitoneal sodium pentobarbital (50mg/kg), unilateral lung contusion (LC) was induced using a blast wave of a percussive nail gun (Craftsman 968514 Stapler, Sears Brands, Chicago IL) applied to a 12mm metal plate secured to the right axilla of the rat.
Chronic Stress Model
Daily restraint stress was performed for two hours between 8am and 12pm daily. Animals were placed in restraint containers measuring 16.5cm in length and 7.5cm in diameter. In order to prevent acclimation to the containers, rats underwent stimulation consisting of two minutes of continuous alarming (80–85 decibels) as well as repositioning at 30, 60, and 90 minutes. At the completion of two hours of restraint stress, animals were removed from their restraint containers and returned to normal housing. Animals in the chronic stress groups underwent two hours of restraint stress daily for six days following LC prior to sacrifice on experimental day seven. As the animals undergoing CS were not permitted feed or water during the stress period, those animals in other experimental groups were also not permitted feed or water during the stress period.
Mesenchymal Stem Cell Culture
Sprague-Dawley rat mesenchymal stem cells (MSCs) were purchased from Cyagen Biosciences (Santa Clara, CA) and established and expanded. Briefly, cells were thawed and transferred into 15mL OriCell MSC Growth Medium. This suspension was then centrifuged at 250 × g for 5 minutes and supernatant aspirated. Cells were then suspended 2–3 mL fresh growth medium and seeded into a T25 flask where additional growth medium was added prior to incubation in a 37°C humidified 5% CO2 incubator. Medium was changed on the first day after initial incubation as well as every three days thereafter. When the cells were 80–90% confluent, they were dissociated with Trypsin-EDTA and re-seeded at 3 × 103/cm2. Cell cultures were similarly expanded until adequate numbers of cells were available for harvest. On the day of injection, MSCs from the third passage were quantified and aliquoted into 5 × 106 cells/vial. The cells were then washed with IMDM twice and a volume of 5 × 106 cells in one mL IMDM was incubated at 37°C until time of injection.
Mesenchymal Stem Cell Injection
Animals undergoing MSC injection were anesthetized with intraperitoneal sodium pentobarbital (50mg/kg). Prior to undergoing LC, animals underwent cannulation of the right internal jugular vein with polyethylene tubing ((PE-50; Becton Dickinson and Co., Sparks, MD). In accordance with our previous protocol13,, a dose of 5 × 106 MSC in one mL IMDM was injected within ten minutes of injury and was given over five minutes.
Bone Marrow Cellularity
The right femur was used to obtain BM cells for determination of cellularity and to establish cell culture. Following removal of the femoral epiphysis, BM was aspirated with an 18-gauge needle on a 5cc syringe filled with 1mL IMDM. Cells were then made into a suspension, stained with 0.4% Tryptan blue, and total viable cell count was determined by hemocytometer.
Bone Marrow Progenitor Cell Cultures
Growth of early BM progenitor cells was assessed by culturing colony-forming unit-granulocyte- erythrocyte-, monocyte-, megakaryocyte (CFU-GEMM) while burst-forming unit-erythroid (BFU-E) and colony-forming unit-erythroid (CFU-E) were used to specifically assess the effect on erythroid cell lines. Based on the cellularity, a stock solution of BM cells was prepared at a concentration of 1×106 cells/mL of IMDM. 1.5×105 cells were taken from this stock solution and plated in duplicate in IMDM supplemented with 30% FBS, 2%BSA, rat growth factor, 1% methylcellulose, 2×10−4 mol/L 2-mercaptoethanol, penicillin/streptomycin, and glutamine. Those plates examining BFU-E/CFU-E were further supplemented with 1.3 U/mL rhEpo and 6 U/mL rhIL-3. Plates assessing CFU-GEMM were supplemented with 3 U/mL rhGM-CSF. Cultures were incubated incubated at 37°C in 5% CO2 until the time of counting by an observer blinded to sample origin. CFU-E colonies were counted on day 7, BFU-E on day 14, and CFU-GEMM on day 17.
Cultures with Experimental Plasma
Bone marrow was collected from naïve rats and cellularity and stock solutions prepared as described above. Cells were plated in duplicate with media as described above, however, with the addition of 5% volume per volume of plasma from rats in one of the following four experimental groups: naïve, LC, LC/CS, and LC/CS+MSC. Plasma obtained following sacrifice on day 7 after injury. CFU-E colonies were counted on day 7, BFU-E on day 14, and CFU-GEMM on day 17.
Flow Cytometry
Peripheral blood was obtained by via cardiac puncture using a 10-mL heparinized syringe. The frequency of CD 71+ and CD117+ cells was quantified in unfractionated peripheral blood samples using a well established, single-platform enumeration method. Briefly, 100μl of peripheral blood was labeled with 10μL of BD Pharmingen™ mouse anti-rat CD71 antibody conjugated with fluorescein isothiocyanate (FITC) and 10μL of BD Pharmingen™ rat anti-mouse CD117 (c-Kit) antibody conjugated to phycoerythrin (PE) (BD Biosciences, Franklin Lakes, NJ) for 30 minutes. Following erythrocyte lysis by ammonium chloride in a 37°C in 5% CO2 incubator, cells were centrifuged at 300 × g for five minutes and supernatant was discarded. Cells were washed 3 times and fixed with BD Cytofix™ solution (BD). Cells were analyzed using BD FACSCalibur flow cytometer (BD) equipped with CellQuest software (BD). Samples from each group were stained and run in duplicate and an event count of 50,000 was obtained for each run. A representative gating scheme is presented in Figure 1.
Figure 1. Flow Cytometry Gating Scheme for Identification of Viable Hematopoietic Progenitor Cells in Peripheral Blood.
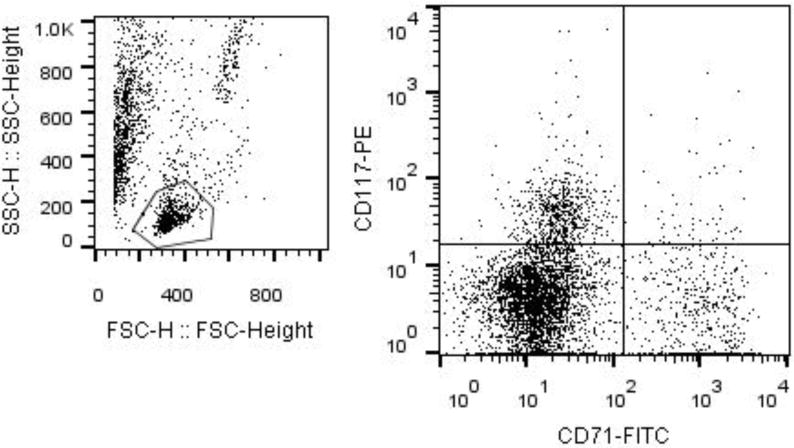
Gate 1 was made to isolate the lymphocyte population. The plot depicts the events gated in G1 made on CD71-FITC vs CD117-PE to identify the cells stained with both FITC and PE.
Plasma G-CSF Levels
Plasma G-CSF Levels were determined by a commercial ELISA kit (R&D Systems, Minneapolis, MN) run in duplicate. Briefly, 50μL of assay dilutent RD1–54 was added to each well followed by 50μL of standard, control, or sample. Wells were was precoated with polyclonal anti-mouse G-CSF. Following gentle mixing, plate was covered and incubated at room temperature for two hours. Wells were then aspirated and washed with wash buffer a total of five times. Following the last wash and sufficient blotting, 100μL Mouse G-CSF conjugate was added to each well and plate was covered and incubated at room temperature for two hours. At the end of incubation, the plate was again washed a total of five times. 100μL substrate solution was then added to each well and plate incubated in the dark for 30 minutes. Following incubation, 100μL stop solution was added to each well and the plate was read using 450–540nm wavelength correction.
Reagents
Bovine serum albumin (BSA), and 2-mercaptoethanol were purchased from Sigma-Aldrich (St. Louis, MO). Methylcellulose was purchased from Stemcell Technologies (Vancouver, Canada). Fetal bovine serum (FBS), Iscove’s Modified Dulbecco’s Medium (IMDM), glutamine, penicillin/streptomycin, and trypan blue were obtained from Invitrogen (Carlsbad, CA). All cytokines rhEpo, rhIL-3, rhGM-CSF were purchased from R&D Systems (Minneapolis, MN). Sodium pentobarbital was purchased from B&B Pharmacy (Bellflower, CA) and heparin was obtained from Hospira Inc. (Lakefront, IL).
Statistical Analysis
All data are expressed as mean ± SD. Statistical analyses were performed using one-way analysis of variance (ANOVA) followed by Tukey-Kramer’s multiple comparison post-test or Student’s t-test with GraphPad Prism (Version 4.0, San Diego, CA). Results were considered significant if *p <0.05 vs. LC or **p <0.05 vs. LC/CS.
RESULTS
Effects of Mesenchymal Stem Cells on Bone Marrow Cellularity
There was no significant difference in BM cellularity seven days following LC alone or LC+MSC as compared to controls (213±12, 214±3 vs. 225±6). As previously reported7, adding CS to LC resulted in a significant reduction in BM cellularity (163±8* vs. 225±6). The addition of MSCs to animals undergoing LC/CS resulted in a 22% increase in BM cellularity as compared to LC/CS alone, returning cellularity to naïve levels (204±2** vs. 225±6) (Figure 2A).
Figure 2. A–D: Effect of MSCs on BM seven days following injury and stress.
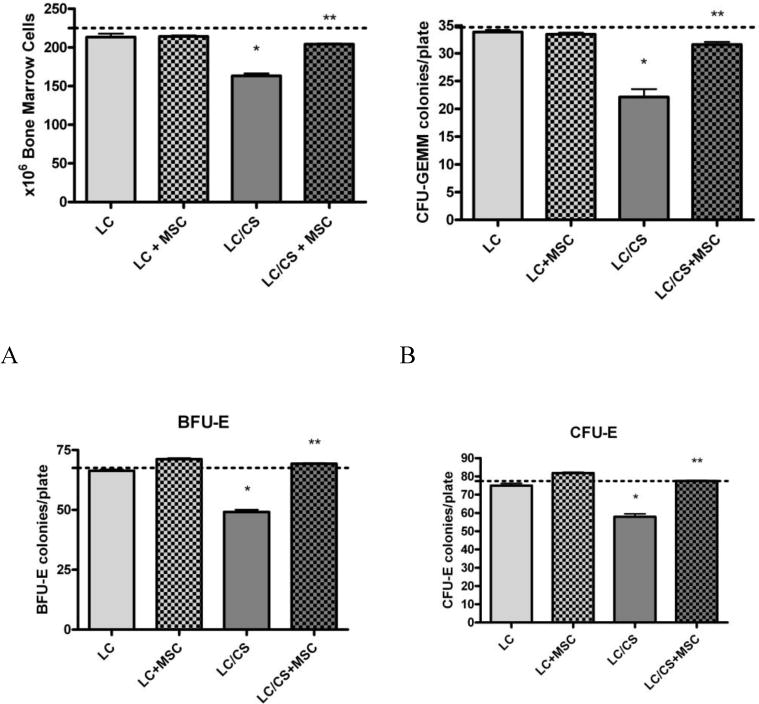
A. The addition of MSCs to LC/CS causes a significant increase in BM cellularity, returning cellularity to naïve levels. B. The addition of MSCs to LC/CS causes a significant increase in BM CFU-GEMM colony growth, returning it to naïve levels. C. The addition of MSCs to LC/CS causes a significant increase in BM BFU-E colony growth, returning growth to naïve levels. D. The addition of MSCs to LC/CS causes a significant increase in BM CFU-E colony growth, returning growth to naïve levels. N=6–7/group (dotted line represents naïve; BM= Bone Marrow (dotted line represents naïve; BM= Bone Marrow; LC = lung contusion; CS = chronic stress; MSC = mesenchymal stem cell). *p<0.05 vs naïve, **p<0.05 vs LC/CS
Effects of Mesenchymal Stem Cells on Bone Marrow Hematopoietic Progenitor Cell Cultures
Seven days following injury, there was no significant difference in CFU-GEMM growth as compared to naïve in either LC or LC+MSC groups. As previously shown, the addition of CS to LC resulted in a significant decrease in BM CFU-GEMM colony growth as compared to LC alone (22±4* vs. 34±1). The addition of MSCs at the time of injury to LC/CS resulted in a 35% increase in BM CFU-GEMM cultures as compared to LC/CS alone, returning colony counts to naïve levels (32±1** vs 35±1) (Figure 2B).
BM BFU-E colony growth was also significantly reduced following LC/CS as compared to LC alone (49±3* vs. 66±2). The addition of MSCs to LC/CS returned BFU-E colony growth to naïve levels (69±1** vs. 68±3), a 34% increase as compared to LC/CS alone (Figure 2C).
Both the effects of CS and MSC persisted when looking at CFU-E, the late erythroid progenitor cells. Again, the addition of CS following LC resulted in significantly depressed colony growth. MSCs conferred a 29% increase as compared to LC/CS (78±1** vs. 58±4), again returning colony growth to naïve levels (Figure 2D).
Effect of Mesenchymal Stem Cells on Hematopoietic Progenitor Cell Mobilization and G-CSF
Seven days following LC alone or LC+MSC, the percentage of HPCs found in the peripheral blood has returned to naïve levels (0.85±0.1, 0.93±0.6 vs. 0.48±0.5 respectively). As previously found, addition of chronic stress to LC results in prolonged mobilization of HPCs to the periphery (5.6±0.8* vs. 0.48±0.5). When MSCs are given at the time of injury, animals undergoing LC/CS no longer displayed prolonged mobilization of HPCs to the periphery (0.56±0.2** vs. 5.6±0.8) (Figure 3A).
Figure 3. A–B: Effect of MSCs on HPC mobilization and G-CSF seven days following injury and stress.
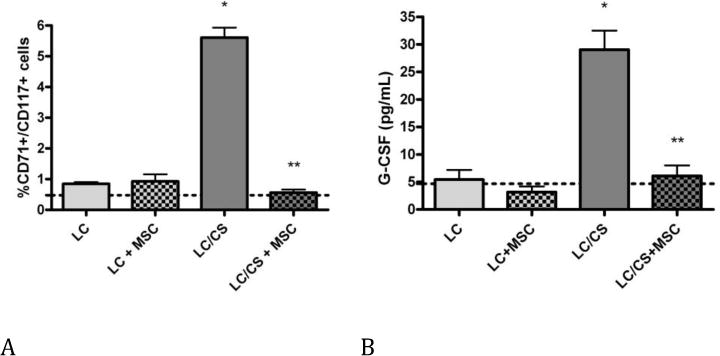
A. The addition of MSCs to LC/CS causes a significant decrease in the percentage of HPCs found in the peripheral blood, returning cell count to naïve levels. B. The addition of MSCs to LC/CS causes a significant decrease in the plasma G-CSF level, returning plasma G-CSF to naïve levels. N=6–7/group (dotted line represents naïve; HPC= hematopoietic stem cell; LC = lung contusion; CS = chronic stress; MSC = mesenchymal stem cell). *p<0.05 vs naïve, **p<0.05 vs LC/CS
Plasma G-CSF levels in naïve rats average 4.69±2 pg/mL. Seven days following injury, there was no significant difference in G-CSF levels in LC alone or LC+MSC groups as compared to naïve. Rats undergoing combined LC/CS had significantly elevated plasma G-CSF levels following injury (29.0±8* vs. 4.69±2). MSCs given at the time of injury returned plasma G-CSF levels to naïve levels, a 130% decrease as compared to LC/CS (6.12±5** vs. 29.0±8) (Figure 3B).
Effect of Exposure to Plasma from Injured Animals on Naïve Bone Marrow Cells
There was no statistically significant difference between colony counts in naive BM incubated with media alone and naïve BM incubated with naïve plasma. BM incubated with 5% plasma from 7 day LC/CS animals demonstrated 22.9, 28.6, and 17.6% decreases in CFU-GEMM, BFU-E, and CFU-E growth respectively (39.8±4* vs. 50.1±4, 50.4±6* vs. 67.2±9, and 62.0±4 vs. 74.0±4). BM colony growth of CFU-GEMM, BFU-E and CFU-E returned to naïve levels when incubated with plasma from LC+MSC rodents (51.6±2, 71. 5±5, and 75.0±6). (4A–C).
DISCUSSION
BM dysfunction following acute traumatic injury has been long recognized4,5. This is characterized by both depression of BM and cellularity and HPC growth within the BM itself as well as mobilization of HPCs to the periphery with an associated elevation in plasma G-CSF2,3. With exposure to CS, this response is further potentiated, resulting in persistent BM dysfunction lasting more than seven days after injury7. In the current study, we demonstrate that the addition of a single IV dose of MSCs given shortly following injury can reverse all of the manifestations of stress-induced persistent BM dysfunction, effectively returning the BM to normal function.
MSCs are multipotent cells that ordinarily comprise an essential part of the BM niche and contribute to hematopoietic homeostasis by production of cytokines and adhesion molecules involved in HPC homing14–16. Their role in the setting of post-traumatic BM dysfunction has not previously been examined. Our data demonstrates that a single IV dose of MSCs shortly following injury reversed the BM dysfunction seen following LC/CS and resulted in an increase in both BM cellularity and HPC growth within the BM to levels observed in naïve animals. The role of MSCs on the BM microenvironment has been studied in the setting of co-transplantation with HPCs and in autoimmune diseases marked by chronic inflammation17–19. When MSCs are co-transplanted with HPCs at the time of BM transplantation, they enhance the speed of recovery and reduce the incidence of graft-versus host disease (GVHD)17–18. MSCs have also been shown to improve blood cell counts in the treatment of refractory cytopenia in patients with systemic lupus erythemaosus19. While MSCs have shown dramatic benefit in these populations, their mechanism of action within the BM and in the setting of stress remains unknown.
Animals undergoing chronic stress exhibit prolonged mobilization of HPCs to the periphery7. This prolonged egress is associated with a persistent elevation of plasma G-CSF, which is not seen following LC without CS. Petit et al. investigated the mechanism by which this BM mobilization occurs and determined that the interaction of G-CSF with SDF-1 in the BM releases HPCs from their stromal attachments, allowing migration20. Katayama et al. implicated the sympathetic nervous system, norepinephrine (NE) in particular, in regulating this cellular egress21. Repeated exposure to supraphysiologic levels of NE caused by CS may be responsible for prolonged HPC mobilization leading to BM dysfunction in this model. Kawada et al. determined that stress positively influences migration of BM-resident MSCs into the PB or to sites of injury16, however, no prior studies have examined the effects of MSCs on HPC mobilization in the setting of stress. In the current study, we demonstrate that treatment with MSCs reduces plasma G-CSF to naïve levels and causes a return of HPCs from the periphery, effectively negating the effect of CS. While G-CSF has long been administered to mobilize both HPCs and MSCs to the periphery in preparation for BM transplantation, there have been no studies examining the effects of MSC injection on plasma G-CSF levels. This decrease in plasma G-CSF and homing of HPC back to the BM may represent one of the mechanisms by which MSCs mediate protection of the BM in the setting of trauma and CS.
The protective effect of MSCs is secondary to both local changes within the bone marrow, as is evidenced by increased HPC colony growth, as well as systemic changes at the level of the plasma. Plasma from trauma patients inhibits HPC colony growth for up to two weeks following injury22. In this work, we show that while naïve BM HPCs cultured in vitro with plasma from animals undergoing LC/CS have significantly depressed growth, those cultured with plasma from LC/CS animals receiving MSCs had no suppression of erythroid progenitor growth. We have previously shown changes in the plasma cytokine profile following injection of MSC, marked by a decrease in levels of the proinflammatory cytokines IL-2, IL-4, and IFN- γ and a concomitant increase in plasma anti-inflammatory cytokine IL-1023. Others have also shown a systemic change in the plasma following MSC injection24–25 (Pedrazza, Weil). Pedrazza et al. demonstrated a decrease in plasma levels of pro-inflammatory cytokines TNF-α and IL-6 with a concomitant increase in anti-inflammatory IL-10 when MSCs were injected in a mouse model of sepsis using cecal ligation and puncture24. Similarly, Weil et al. showed a decrease in serum levels of TNF-α, IL-1β, and IL-6 when MSCs were given following exposure to LPS25. MSCs likely function both at the local level as well as systemically to decrease inflammatory signals and reestablish homeostasis following injury and stress.
Although MSCs have been shown to be of benefit in myriad disease states, their mechanism of action has not been fully elucidated. Initially, it was believed that the therapeutic effect of MSCs relied on their ability to structurally engraft into damaged tissues26,27. More recently, however, research in MSCs favors both the paracrine and immunomodulatory roles of MSCs in reducing inflammation. In multiple non-traumatic models of injury, MSCs have been shown to have anti-inflammatory effects, reducing the levels of proinflammatory cytokines including TNF-a, IL-1, and IL-6, and increasing levels of anti-inflammatory cytokines such as IL-1025, 28–30. Further evidence for a paracrine effect lies in reports by Curley et al that treatment with MSC-conditioned media alone retains the anti-inflammatory properties seen when MSCs themselves were injected28. In the current study we provide evidence supporting the paracrine function of MSCs and presence of soluble anti-inflammatory mediators in that exposure of naïve BM cells to plasma from animals receiving MSCs were not suppressed.
Furthermore, MSCs have been shown to have immunomodulatory function via both paracrine and cell-contact mediated mechanisms. Ortiz et al, demonstrated secretion of IL-1ra by MSCs, thereby inhibiting stimulation of helper T lymphocytes and suppressing TNF-a production by macrophages28. Spaggiari et al reported further immunomodulation by MSCs via a PGE2-dependent inhibition of dendritic cell maturation31. Our lab has recently reported an expansion of the peripheral population of T regulatory cells (Treg) following MSC injection using the same LC/CS model that is associated with improved lung healing32. The function of Tregs is to maintain immune homeostasis, and this observed expansion may be integral to the ability of MSCs to exert their anti-inflammatory effects.
In summary, MSCs given at the time of injury reverse the persistent BM dysfunction seen seven days following LC/CS. This protection is associated with an increase in BM cellularity and HPC growth within the BM itself as well as a reduction in the number of HPCs mobilized to the PB and decrease in plasma G-CSF levels. Furthermore, MSCs have a protective effect at the systemic level as plasma from animals receiving MSCs is no longer suppressive to BM colony growth in vitro. Cellular therapy with MSCs given shortly after severe injury may provide a robust clinical benefit in preventing BM dysfunction and its resultant morbidities. Further study is warranted to better understand the mechanisms behind MSC-mediated protection of BM function both at the local and systemic level in the setting of CS.
Figure 4. A–C: Effect of experimental plasma on CFU-GEMM Colony Growth.
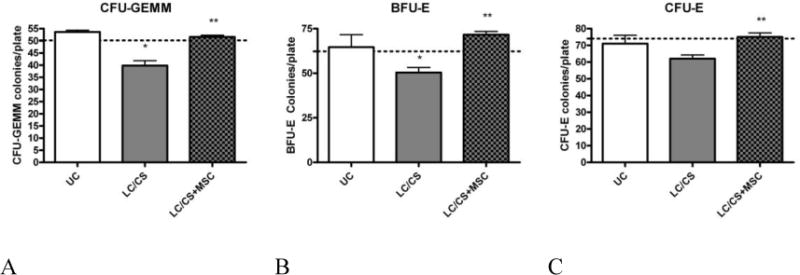
A. Naïve BM cells cultured with media plus the addition of 5% plasma from 7-day animals undergoing LC/CS suppresses CFU-GEMM colony growth. When incubated with plasma from animals receiving MSCs suppression is no longer seen. B. Naïve BM cells cultured with media plus the addition of 5% plasma from 7-day animals undergoing LC/CS suppresses BFU-E colony growth. When incubated with plasma from animals receiving MSCs suppression is no longer seen. C. Naïve BM cells cultured with media plus the addition of 5% plasma from 7-day animals undergoing LC/CS results in a trend toward suppression of CFU-E colony growth. When incubated with plasma from animals receiving this trend is no longer seen. N=3–6/group (dotted line represents naïve BM incubated with media alone; UC= naïve BM incubated with naïve plasma; LC = lung contusion; CS = chronic stress; MSC = mesenchymal stem cell). *p<0.05 vs naïve, **p<0.05 vs LC/CS
Acknowledgments
This research was supported by the National Institutes of Health grants R01 GM105893–01A1 and T32 GM069330. No conflicts of interest to report.
Footnotes
This work was presented as a Podium Presentation at the 34th Annual Meeting of the Surgical Infection Society, May 2014.
Level of Evidence: III
Study Type: Therapeutic
Authorship
The role of each author is as described below. Amy Gore was involved in experimental design, data acquisition, analysis and interpretation of data, and manuscript preparation. Letitia Bible in data acquisition and analysis. David Livingston, Alicia Mohr, and Ziad Sifri in design, data analysis and interpretation, and critical revision.
Contributor Information
Amy V. Gore, Email: goreav@njms.rutgers.edu.
Letitia E. Bible, Email: biblele@njms.rutgers.edu.
David H. Livingston, Email: livingst@njms.rutgers.edu.
Alicia M. Mohr, Email: Alicia.mohr@surgery.ufl.edu.
Ziad C. Sifri, Email: sifrizi@njms.rutgers.edu.
References
- 1.Corwin HL, Surgenor SD, Gettinger A. Transfusion practice in the critically ill. Crit Care Med. 2003;31:S668–S671. doi: 10.1097/01.CCM.0000099348.99451.84. [DOI] [PubMed] [Google Scholar]
- 2.Baranski G, Offin M, Sifri Z, Elhassan IO, Hannoush EJ, Alzate WD, Rameshwar P, Livingston DH, Mohr AM. Beta-blockade protection of bone marrow following trauma: the role of G-CSF. J Surg Res. 2011;170:325–331. doi: 10.1016/j.jss.2011.03.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Elhassan IO, Hannoush EJ, Sifri ZC, Jones E, Alzate WD, Rameshwar P, Livingston DH, Mohr AM. Beta-Blockade prevents hematopoietic progenitor cell suppression after hemorrhagic shock. Sur Infect. 2011;12:273–278. doi: 10.1089/sur.2010.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Livingston DH, Gentile PS, Malangoni MA. Bone marrow failure after hemorrhagic shock. Circ Shock. 1990;30:255–263. [PubMed] [Google Scholar]
- 5.Livingston DH, Anjaria D, Wu J, Hauser CJ, Chang V, Deitch EA, Rameshwar P. Bone marrow failure following severe injury in humans. Ann Surg. 2003;238:748–753. doi: 10.1097/01.sla.0000094441.38807.09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fonseca RB, Mohr AM, Wang L, Clinton E, Sifri ZC, Rameshwar P, Livingston DH. Adrenergic modulation of erythropoiesis following severe injury is mediated through bone marrow stroma. Surg Infect (Larchmt) 2004 Winter;5(4):385–393. doi: 10.1089/sur.2004.5.385. [DOI] [PubMed] [Google Scholar]
- 7.Bible L, Pasupuleti L, Sifri Z, Alzate W, Livingston D, Mohr A. Chronic stress impairs bone marrow function. Shock. 2013;39(S2):176. [Google Scholar]
- 8.Ren G, Chen X, Dong F, Li W, Ren X, Zhang Y, Shi Y. Concise Review: Mesenchymal Stem Cells and Translational Medicine: Emerging Issues. Stem Cells Transl Med. 2012;1(1):51–58. doi: 10.5966/sctm.2011-0019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yi TG, Song SU. Immunomodulatory Properties of Mesenchymal Stem Cells and Their Therapeutic Applications. Arch Pharm Res. 2012;35(2):213–221. doi: 10.1007/s12272-012-0202-z. [DOI] [PubMed] [Google Scholar]
- 10.Jackson WM, Nesti LJ, Tuan RS. Concise Review: Clinical Translation of Wound Healing Therapies Based on Mesenchymal Stem Cells. Stem Cells Transl Med. 2012;1(1):44–50. doi: 10.5966/sctm.2011-0024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ghannam S, Bouffi C, Djouad F, Jorgensen C, Noel D. Immunosuppression by Mesenchymal Stem Cells: Mechanisms and Clinical Applications. Stem Cell Res Ther. 2010;1(1):2. doi: 10.1186/scrt2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chen AK, Reuveny S, Oh SK. Application of Human Mesenchymal and Pluripotent Stem Cell Microcarrier Cultures in Cellular Therapy: Achievements and Future Direction. Biotechnol Adv. 2013;31(7):1032–1046. doi: 10.1016/j.biotechadv.2013.03.006. [DOI] [PubMed] [Google Scholar]
- 13.Hannoush EJ, Elhassan I, Sifri ZC, Mohr AM, Alzate WD, Livingston DH. Role of bone marrow and mesenchymal stem cells in healing after traumatic injury. Surgery. 2013;153(1):44–51. doi: 10.1016/j.surg.2012.06.020. [DOI] [PubMed] [Google Scholar]
- 14.Majumdar MK, Thiede MA, Mosca JD, Moorman M, Gerson SL. Phenotypic and functional comparison of cultures of marrow-derived mesenchymal stem cells(MSCs) and stromal cells. J Cell Physiol. 1998;176:57–66. doi: 10.1002/(SICI)1097-4652(199807)176:1<57::AID-JCP7>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- 15.Haynesworth SE, Baber MA, Caplan AI. Cell surface antigens on human marrow-derived mesenchymal cells are detected by monoclonal antibodies. Bone. 1992;13:69–80. doi: 10.1016/8756-3282(92)90363-2. [DOI] [PubMed] [Google Scholar]
- 16.Kawada H, Fujita J, Kinjo K, Matsuzaki Y, Tsuma M, Miyatake H, Mugu ruma Y, Tsuboi K, Itabashi Y, Ikeda Y. Nonhematopoietic mesenchymal stem cells can be mobilized and differentiate into cardiomyocytes after myocardial infarction. Blood. 2004;104:3581–3587. doi: 10.1182/blood-2004-04-1488. [DOI] [PubMed] [Google Scholar]
- 17.Jin J, Wang X, Wang Q, Guo X, Cao J, Zhang X, Zhu T, Zhang D, Wang W, Wang J, Shen B, Gao X, Shi Y, Zhang J. Chronic Psychological Stress induces the accumulation of myeloid-derived suppressor cells in mice. PLoS One. 2013;8:e74497. doi: 10.1371/journal.pone.0074497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Romana-Souza B, Porto LC, Monte-Alto-Costa A. Cutaneous wound healing of chronically stressed mice is improved through catecholamine blockade. Exp Derm. 2010;19:821–829. doi: 10.1111/j.1600-0625.2010.01113.x. [DOI] [PubMed] [Google Scholar]
- 19.Wu Y, Cao Y, Li X, Xu L, Wang Z, Liu P, Yan P, Liu Z, Wang J, Jiang S, Wu X, Gao C, Da W, Han Z. Cotransplantation of haploidentical hematopoietic and umbilical cord mesenchymal stem cells for severe aplastic anemia: successful engraftment and mild GVHD. Stem Cell Res. 2014;12(1):132–8. doi: 10.1016/j.scr.2013.10.001. [DOI] [PubMed] [Google Scholar]
- 20.Petit I, Szyper-Kravitz M, Nagler A, Lahav M, Peled A, Habler L, Ponomaryov T, Taichman RS, Arenzana-Seisdedos F, Fugii N, Sandbank J, Zipori D, Lapidot T. G-CSF induces stem cell mobilization by decreasing bone marrow SDF-1 and up-regulating CXCR4. Nat Immunol. 2002;3(7):687–787. doi: 10.1038/ni813. [DOI] [PubMed] [Google Scholar]
- 21.Katayama Y, Battista M, Kao WM, Hidalgo A, Peired AJ, Thomas SA, Frenette PS. Signals from the sympathetic nervous system regulate hematopoietic stem cell egress from bone marrow. Cell. 2006;124(2):407–21. doi: 10.1016/j.cell.2005.10.041. [DOI] [PubMed] [Google Scholar]
- 22.Wu JC, Livingston DH, Hauser CJ, Deitch EA, Rameshwar P. Trauma inhibits erythroid burst-forming unit and granulocyte-monocyte colony-forming unit growth through the production of TGF-beta1 by bone marrow stroma. Ann Surg. 2001;234(2):224–232. doi: 10.1097/00000658-200108000-00013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cook K, Sifri Z, Baranski G, Rameshwar P, Mohr A, Livingston D. The Role of T Regulatory Cells in Mesenchymal Stromal Cell (MSC)-Medicated Wound Healing. Surgical Infections. 2012;13(1):S15. [Google Scholar]
- 24.Pedrazza L, Lundardelli A, Luft C, Cruz CU, de Mesquita FC, Bitencourt S, Nunes FB, de Oliveira JR. Mesenchymal stem cells decrease splenocytes apoptosis in a sepsis experimental model. Inflamm Res. 2014;63:719–728. doi: 10.1007/s00011-014-0745-1. [DOI] [PubMed] [Google Scholar]
- 25.Weil Brent R, Manukyan MC, Herrmann JL, et al. Mesenchymal stem cells attenuate myocardial functional depression and reduce systemic and myocardial inflammation during endotoxemia. Surgery. 2010;148(2):444–452. doi: 10.1016/j.surg.2010.03.010. [DOI] [PubMed] [Google Scholar]
- 26.Liechty KW, MacKenzie TC, Shaaban AF, Radu A, Moseley AM, Deans R, Marshak DR, Flake AW. Human mesenchymal stem cells engraft and demonstrate site-specific differentiation after in utero transplantation in sheep. Nat Med. 2000;6:1282–86. doi: 10.1038/81395. [DOI] [PubMed] [Google Scholar]
- 27.Prockop DJ. Marrow stromal cells as stem cells for nonhematopoietic tissues. Science. 1997;276:71–74. doi: 10.1126/science.276.5309.71. [DOI] [PubMed] [Google Scholar]
- 28.Curley GF, Hayes M, Ansari B, Shaw G, Ryan A, Barry F, O’Brien T, O’Toole D, Laffey JG. Mesenchymal stem cells enhance recovery and repair following ventilator-induced lung injury in the rat. Thorax. 2012;67:496–501. doi: 10.1136/thoraxjnl-2011-201059. [DOI] [PubMed] [Google Scholar]
- 29.Ortiz LA, Dutreil M, Fattman C, Pandey AC, Torres G, GO K, Phinney DG. Interleukin 1 receptor antagonist mediates the anti-inflammatory and antifibrotic effect of mesenchymal stem cells during lung injury. Proc Natl Acad Sci USA. 2007;104(26):11002–7. doi: 10.1073/pnas.0704421104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mei SH, Haitsma JJ, Dos Santos CC, Deng Y, Lai PF, Slutsky AS, Liles WC, Stewart DJ. Mesenchymal stem cells reduce inflammation while enhancing bacterial clearance and improving survival in sepsis. Am J Respir Crit Care Med. 2010;182(8):1047–57. doi: 10.1164/rccm.201001-0010OC. [DOI] [PubMed] [Google Scholar]
- 31.Spaggiaria GM, Abdelrazik H, Becchetti F, Moretta L. MSCs inhibit monocyte-derived DC maturation and function by selectively interfering with the generation of immature DCs: central role of MSC-derived prostaglandin E2. Blood. 2009;113(26):6576–83. doi: 10.1182/blood-2009-02-203943. [DOI] [PubMed] [Google Scholar]
- 32.Gore AG, Bible LE, Livingston DH, Mohr AM, Sifri ZC. Can mesenchymal stem cells reverse chronic stress-induced impairment of lung healing following trauma? J Trauma. 2015;78(4):76–72. doi: 10.1097/TA.0000000000000592. [DOI] [PMC free article] [PubMed] [Google Scholar]


