Abstract
Purpose
Clonal loss of PTEN expression occurs frequently in endometrial carcinoma and endometrial hyperplasia. Limited data from immunohistochemical studies suggest that PTEN-null appearing endometrial glands are detectable in women without pathologic abnormalities, but the relationship of PTEN expression to endometrial cancer risk factors has not been extensively explored. We evaluated relationships between endometrial cancer risk factors and loss of PTEN expression in a set of benign endometrial samples prospectively collected from women undergoing hysterectomy and in endometrial cancer tissues from a population-based case-control study.
Methods
We used a validated PTEN immunohistochemical assay to assess expression in epidemiological studies designed to assess benign endometrium (Benign Reproductive Tissue Evaluation Study (n=73); Einstein Endometrium Study (n=19)), and endometrial cancer (Polish Endometrial Cancer Study (n=148)) tissues. Associations between endometrial cancer risk factors (collected via study-specific risk factor questionnaires) and PTEN expression in endometrial tissues were determined using Fisher's exact tests.
Results
PTEN loss was detected in 19% of benign endometrial tissues versus 55% in endometrial cancers. NSAID use was statistically significantly associated with PTEN loss in the benign endometrium (p=0.02).
Conclusion
Our data demonstrate that PTEN loss is detectable in endometrial tissues that are benign and malignant, with substantially more frequent loss in endometrial cancer compared with benign endometrium. However, alterations in expression were unrelated to most risk factors in this analysis, except for the association with NSAID use, which may represent a chance finding or reverse causality among patients with endometriosis who may have PTEN pathway abnormalities in eutopic endometrium. Further evaluation of factors associated with PTEN loss and long-term follow-up of women with PTEN-null endometrial glands may be useful in understanding early events in endometrial carcinogenesis.
Keywords: PTEN, endometrial cancer, risk factors
Introduction
Endometrial cancer is the most common and second most lethal gynecological cancer among women in the United States, with 54,780 new cases and 10,170 deaths expected in 2015[1]. Endometrial cancer risk factors include menstrual, reproductive and lifestyle factors that are proposed to result in excess exposure to estrogens relative to progesterone or growth factor exposures favoring endometrial proliferation over differentiation and apoptosis [2]. Increased proliferation may lead to more frequent development of mutations in tumor suppressor genes through random errors in DNA replication or to expansion of cells bearing such mutations, resulting in endometrial cancer.
The PTEN tumor suppressor gene is a dual specificity phosphatase located on chromosome 10 (10q23) that acts through an Akt-dependent pathway to suppress cell division and enable apoptosis [3,4]. In endometrial cancer, loss of heterozygosity at the PTEN region has been reported in approximately 40% of cases and somatic PTEN mutations have been identified in 37% to 83% of tumors [5,6]. In animal models PTEN knockout mice develop endometrial cancer precursors and cancer and women with Cowden's disease, who carry germline PTEN mutations, are at elevated risk of endometrial cancer [7-9]. Accordingly, it is proposed that loss of PTEN function represents an important early event in endometrial carcinogenesis.
Loss of PTEN protein expression (“PTEN-null glands”) in microscopically normal appearing endometrial glands have been identified by immunohistochemistry in 43% of samples from healthy premenopausal women and data suggest that PTEN-null glands continued to be present in some women (83% or 10 of 12 women) on follow-up approximately a year later [10]. Under the influence of growth promoting stimuli, PTEN-null glands may undergo clonal expansion to form histopathologically recognizable cancer precursors and cancer. Consistent with this view, several studies show that PTEN loss is more frequent in endometrial hyperplasia and carcinoma as compared with normal endometrium, although comparisons between hyperplastic lesions of varying severity and cancer are less consistent [11,12]. In addition, Lin et al. reported that PTEN loss is less frequent in normal endometrium of women who have used oral contraceptives or intrauterine devices, two factors that are associated with reduced endometrial cancer risk [13]. Relationships with other established endometrial cancer risk factors, such as obesity, were not assessed.
Accordingly, we describe the prevalence of PTEN loss and evaluate the relationships between endometrial cancer risk factors and PTEN expression in benign endometrium prospectively collected from women undergoing a hysterectomy and in endometrial cancers from a population-based case-control study.
Material and methods
Study populations
We evaluated PTEN expression in three epidemiological studies: Benign Reproductive Tissue Evaluation (BRTE) Study, Einstein Normal Endometrium Study (Einstein), and Polish Endometrial Cancer Study (PECS). Briefly, BRTE enrolled 150 consecutive eligible (18-54 years of age; no use of exogenous hormones within 3 months of enrollment; and surgical indication other than cancer) consenting women undergoing hysterectomy for benign indications (such as adenomyosis, leiomyomata, uterine prolapse, endometriosis, abnormal uterine bleeding, and pelvic pain) at Magee Women's Hospital from 2006-2011, of which 73 were included in the current analysis [14]. To augment samples from older and postmenopausal women, we added samples from postmenopausal women undergoing hysterectomy for uterine prolapse at Einstein and Montefiore Medical Center. In brief, subjects were patients from January 2010 onwards, who consented to having endometrial tissue used for research purposes, and who completed an epidemiologic questionnaire. Following exclusion of women using exogenous hormone therapy, 19 subjects from this study, resulting in a total of 92 women with benign endometrial samples for inclusion in the current analysis. PECS is a population-based endometrial cancer case-control study conducted in Poland (Warsaw, Lodz) from 2001 to 2003 that included 551 histologically confirmed incident endometrial cancer cases, of which 148 represented in a tissue microarray were included in this analysis [15]. Written informed consent was obtained from all women enrolled in these studies and Institutional Review Board approval was provided by the US National Cancer Institute and the respective institutions.
Endometrial cancer risk factors and other exposures
Subjects completed a self-administered study-specific questionnaire at time of study enrollment. All three questionnaires assessed basic risk factors for endometrial cancer including demographic factors, anthropometry, reproductive factors, lifestyle factors, and medical history in slightly different formats. Medication use and certain medical history data were not collected in PECS. The data was harmonized to enable pooling. For the BRTE samples, the study pathologist (MES) additionally reviewed the PTEN stained slides for menstrual cycle at time of surgery (menstruation, proliferative, or secretory phase).
Endometrial tissue, immunohistochemistry (IHC) and interpretation
Full tissue sections of formalin-fixed paraffin-embedded normal endometrial tissues were prepared as 5-micron sections that were stained with hematoxylin and eosin for histologic assessment and for PTEN immunohistochemistry. For PECS, routinely prepared formalin-fixed paraffin-embedded blocks of invasive endometrial cancers were used to construct tissue microarrays blocks with 2-fold representation as 0.6 mm diameter cores per tumor. Tissue microarrays were prepared as 5-μm thick sections mounted on glass slides and stored at room temperature under nitrogen to prevent oxidation-related loss of immunoreactivity prior to staining. Majority (86%) of the endometrial cancer cases in PECS were of type I histological type (endometrioid, mucinous).
Paraffin sections from tissue slides for all three studies were immunostained for PTEN expression using a monoclonoal antibody that has been validated to sensitively detect PTEN loss as previously described [16] at Johns Hopkins University (AM) in batches. In brief, antigen unmasking was performed by steaming in EDTA buffer (pH 8.0) for 45 min. Nonspecific binding was blocked and slides were incubated with an anti-PTEN (rabbit monoclonal; clone D4.3, #9188, 1:100; Cell Signaling Technologies, Beverly, MA, USA). A horseradish peroxidase-labeled anti-rabbit polymer (PowerVision Poly-HRP Anti-Rabbit IgG; Leica Microsystems, Bannockburn, IL, USA) was then applied for 30 min at room temperature. Signal detection for PTEN was then performed using 3,3′-diaminobenzidine tetrahydrochloride (DAB) as the chromagen. Slides were counterstained with hematoxylin, dehydrated, mounted and cover-slipped.
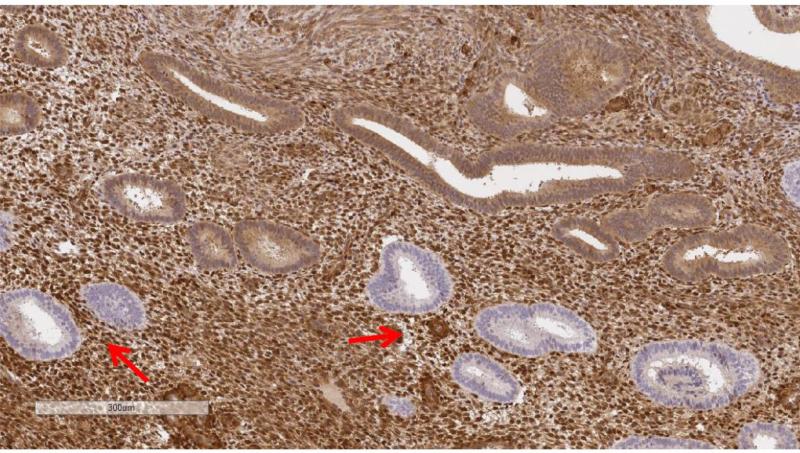
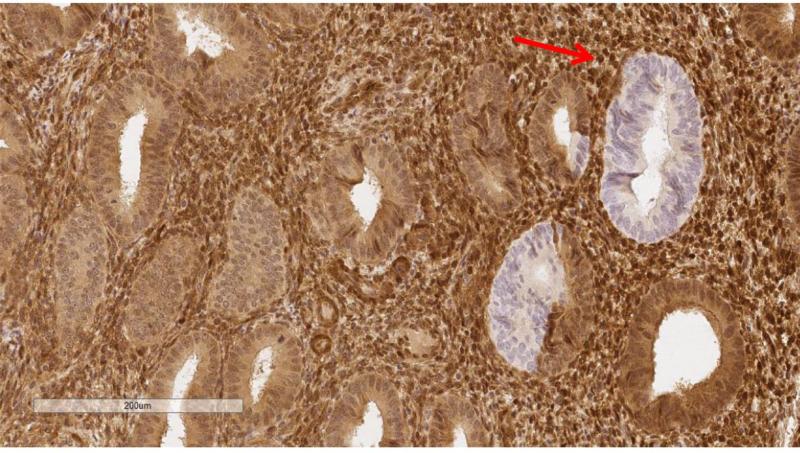
PTEN protein expression was dichotomized as normal vs. null and representative immunohistochemical stains of PTEN null are indicated with arrows in Figure 1 (Figure 1B at higher magnification; ruler included for size comparison). Benign PTEN-null samples were defined as tissues containing PTEN-null glands associated with normal appearing PTEN expressing glands and/or stroma. Benign samples in which staining was not identified in most of the tissue were considered unsatisfactory. Scoring was performed masked to risk factor annotation by the study pathologist (MES). BRTE and PECS slides were independently assessed twice in blinded fashion by the same pathologist. In addition, for 15 BRTE subjects, two sections of normal endometrium were available and independently evaluated. Duplicate cores of endometrial cancers in TMAs were also scored separately.
Figure 1A and 1B.
Representative PTEN protein expression immunohistochemical stains among endometrial tissue samples. Figure 1B is at higher magnification. Note ruler in lower left of images for size comparison..
Statistical analysis
The frequency of PTEN-null glands in two benign studies was tabulated, and then combined, based on similar percentages. BRTE and PECS samples were read twice; overall, independently masked scoring agreed in both studies (overall percent agreement: 85% (95% CI: 76-93%) for BRTE; 85% (95% CI: 78-91%) for PECS) as were estimates of numbers of PTEN null glands for concordant readings. Therefore, to simplify our presentation, we present results henceforth for the PTEN expression results from the first reading for BRTE and PECS.
Associations between endometrial cancer risk factors and PTEN expression were determined using Fisher's exact tests. Statistical analyses were done in Stata13 (Statacorp, College Station, TX).
Results
Patient and tissue sample characteristics
Key characteristics of the women included in our analyses are shown in Table 1 by study. The majority of women were White and overweight. Among the women with benign endometrial tissue, as expected the BRTE participants were younger (median=44 years old) compared with the Einstein participants (median=61.5 years old). We detected PTEN-null glands in 19% of the benign endometrial samples as compared with 55% of endometrial cancers (Table 2; Pearson χ2 p = 2.61e-07; Supplementary Table 1 presented separately for BRTE and Einstein women).
Table 1.
Selected characteristics of the three studies evaluated for PTEN protein expression in endometrial tissue samples
| BRTE (n=73) | Einstein (n=19) | PECS (n=148) | |
|---|---|---|---|
| White, % of total | 85% | 67% | 100% |
| Age, median (range) | 44 years (28-53) | 61.5 years (49-72) | 62.5 (37-75) |
| Body mass index, median (range) | 28.3 (18.1-50.1) | 27.4 (18.5-43.0) | 28.2 (18.2-45.2) |
Table 2.
PTEN protein expression in endometrial tissue samples and its association with endometrial cancer risk factors
| BENIGN (n=92) | MALIGNANT (n=148) | |||
|---|---|---|---|---|
| N | % | N | % | |
| Normal | 70 | 81% | 57 | 45% |
| Null | 16 | 19% | 71 | 55% |
| Equivocal/unsatisfactory | 6 | 20 | ||
| Null Glands (n=16) | Normal (n=70) | Null Glands (n=71) | Normal (n=57) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| N | % | N | % | P-value* | N | % | N | P-value* | |||
| Age (years) | <35 | 0 | 0% | 7 | 10% | 0.45 | 0.89 | ||||
| 35-45 | 9 | 56% | 27 | 40% | 0 | 0% | 1 | 2% | |||
| 46-55 | 4 | 25% | 25 | 37% | 13 | 18% | 10 | 18% | |||
| 56-65 | 2 | 13% | 7 | 10% | 30 | 42% | 24 | 42% | |||
| >65 | 1 | 6% | 2 | 3% | 28 | 39% | 22 | 39% | |||
| Race | White | 14 | 93% | 55 | 80% | 0.55 | 71 | 100% | 57 | 100% | |
| Black | 1 | 7% | 13 | 19% | |||||||
| Asian | 0 | 0% | 1 | 1% | |||||||
| Body mass index** | <25 | 4 | 25% | 19 | 28% | 0.69 | 1 | 1% | 1 | 2% | 0.51 |
| 25-<30 | 6 | 38% | 18 | 26% | 20 | 29% | 11 | 20% | |||
| 30+ | 6 | 38% | 32 | 46% | 49 | 70% | 44 | 79% | |||
| Age at menarche (years) | <11 | 3 | 19% | 10 | 14% | 0.79 | 2 | 3% | 3 | 5% | 0.55 |
| 11-13 | 10 | 63% | 42 | 60% | 37 | 53% | 25 | 44% | |||
| 13+ | 3 | 19% | 18 | 26% | 31 | 44% | 29 | 51% | |||
| Ever pregnant | No | 4 | 25% | 9 | 13% | 0.25 | 13 | 3% | 7 | 12% | 0.46 |
| Yes | 12 | 75% | 61 | 87% | 58 | % | 50 | 88% | |||
| Ever OC use | No | 2 | 13% | 12 | 17% | 1.00 | 68 | 96% | 55 | 96% | 1.00 |
| Yes | 14 | 88% | 58 | 83% | 3 | 4% | 2 | 4% | |||
| Menopausal status | Pre | 13 | 81% | 56 | 80% | 1.00 | 0 | 0% | 1 | 2% | 0.45 |
| Post | 3 | 19% | 14 | 20% | 71 | 100% | 56 | 98% | |||
| Any NSAID use*** | No | 1 | 7% | 29 | 42% | 0.02 | Not available | ||||
| Yes | 14 | 93% | 40 | 58% | |||||||
| NSAID type*** | Aspirin | 9 | 64% | 25 | 63% | 1.00 | Not available | ||||
| Non-aspirin | 5 | 36% | 15 | 38% | |||||||
| Ever smoke | No | 11 | 69% | 34 | 49% | 0.18 | 49 | 69% | 10 | 37% | 1.00 |
| Yes | 5 | 31% | 35 | 51% | 22 | 31% | 17 | 63% | |||
| Diabetes | No | 15 | 100% | 63 | 90% | 0.34 | 56 | 81% | 46 | 84% | 0.82 |
| Yes | 0 | 0% | 7 | 10% | 13 | 19% | 9 | 16% | |||
| Hypertension | No | 12 | 75% | 55 | 79% | 0.75 | Not available | ||||
| Yes | 4 | 25% | 15 | 21% | |||||||
| Endometriosis | No | 9 | 56% | 55 | 80% | 0.06 | Not available | ||||
| Yes | 7 | 44% | 14 | 20% | |||||||
| Fibroid tumors | No | 8 | 50% | 22 | 32% | 0.25 | Not available | ||||
| Yes | 8 | 50% | 46 | 68% | |||||||
| Ovarian cysts | No | 10 | 67% | 40 | 59% | 0.77 | Not available | ||||
| Yes | 5 | 33% | 28 | 41% | |||||||
| Menstrual cycle | Menstruation | 2 | 15% | 3 | 5% | 0.36 | Not available | ||||
| Proliferative phase | 5 | 38% | 30 | 55% | |||||||
| Secretory phase | 6 | 46% | 22 | 40% | |||||||
Numbers do not add up to total due to missingness.
Fisher's exact test
For BRTE and PECS: current BMI. For Einstein: based on weight 12 months ago.
Among those who had used NSAID. For BRTE: based on use in past 12 months for aspirin and ibuprofen. For Einstein: based on regular use of aspirin, acetaminophen, and other anti-inflammation drugs.
Association of endometrial cancer risk factors and loss of PTEN expression
Most endometrial cancer risk factors were not significantly associated with detection of PTEN loss in either benign or malignant endometrial samples (Table 2). We observed similar results when limiting the benign cases to White women and the cancer cases to type I histological type. Use of non-steroidal anti-inflammatory drugs (NSAIDs) was statistically significantly associated with more frequent PTEN loss in benign endometrium (among non-users 3% vs. among users 26%; p=0.02), but we did not find significant differences by NSAID type, aspirin versus non-aspirin (p=1.00). PTEN loss in benign tissues was marginally associated with self-reported endometriosis (p=0.06). Neither NSAID use nor endometriosis was significantly associated with PTEN loss after adjusting for the other factor (data not shown). We did not observe differences in frequency of PTEN loss by menstrual cycle phase (p=0.36).
Discussion
We detected PTEN-null glands in 19% of normal endometrial samples among 86 women undergoing hysterectomy for benign indications, which is similar to results from some previous studies (11% and 20%), but slightly lower than another (43%) [10,17,18]. We also observed that PTEN loss was substantially more frequent in endometrial cancer (55%) compared with benign endometrium, which is consistent with existing literature [2,10] and the proposed mechanistic role of this tumor suppressor gene in carcinogenesis.
Using a different immunohistochemical assay, Lacey et al reported that PTEN loss was identified in 47% of biopsies reported as endometrial hyperplasia or disordered proliferative endometrium; however, PTEN status was unrelated to risk of progression of hyperplasia to endometrial carcinoma or to other major risk factors [12]. Of interest, this previous study reported that three of four women with PTEN-null glands and mutations in their endometrial biopsies and subsequent carcinomas demonstrated identical point mutations in both lesions, suggesting clonal progression over time. Thus, PTEN mutation may represent an early event in endometrial carcinogenesis, which is nonetheless common in normal tissues and unlikely to have clinical utility as a biomarker for predicting progression risk. Although increased risk of progression of endometrial hyperplasia to carcinoma has been associated with obesity and diabetes and reduced risk for use of oral contraceptives [19], these factors were unrelated to PTEN status in benign endometrium and cancer in our study. Furthermore, the factors that influence progression of PTEN abnormalities remain ill-defined.
In our study, we found that endometrial cancer risk factors were generally unrelated to detection of PTEN-null glands in normal endometrium, apart from two possible associations with NSAID use and endometriosis.. Finally, we did not confirm previously reported results linking PTEN-null glands to oral contraceptive use [13].
Our identification of a borderline association of PTEN-null glands with endometriosis is compatible with evidence that the molecular profile of eutopic endometrium of women with endometriosis differs from that of women without endometriosis [20], including some studies that that show altered expression in the AKT-PTEN pathway [21,22]. Further, endometriosis increases risk of ovarian endometrioid and clear cell carcinomas [23], which may also harbor PTEN mutations. Given that endometriosis may form through retrograde menstruation with implantation of exfoliated endometrium, these data may indicate that PTEN-null status may be an indicator of increased risk of developing endometriosis, and indirectly, possibly ovarian clear and endometrioid carcinomas. Many women with endometriosis and other sources of pelvic pain use NSAIDs, hence the association between PTEN null-glands and NSAID use may represent reverse causality. However, given the limited numbers in our analysis, the marginal level of statistical significance of this association, and that the endometriosis is based on self-report rather than more valid laparoscopically-confirmed diagnosis, this association remains speculative, unless confirmed in future studies. We do not have information on indication for NSAID use to further explore this possibility.
A recent meta-analysis found that aspirin use was associated with a slight but significant risk reduction for endometrial cancer, but that use of NSAIDs was not significantly related [24]. The overall pooled estimate for any use versus no use of aspirin yielded an odds ratio of 0.87 (95% confidence intervals: 0.70-0.96), with stronger reduced risk among obese women. However, more recent reports are conflicting, both supporting [25] and not supporting [26] these findings. The proposed mechanisms to account for the potential protective effects of aspirin remain undefined, and both inflammatory and non-inflammatory processes should be considered.
Limitations of our study include a relative small number of samples with limited statistical power from different studies with different age distributions and tissue fixation and storage methods. We also did not examine the influences of indications of hysterectomy, given the small sample size. In addition, our analysis of PTEN expression is based on a single section of the endometrium and uncertainties about whether PTEN-null glands are randomly distributed.. In our analysis of 15 BRTE subjects with multiple samples, we had approximately equal number of anterior/anterior, posterior/posterior, and anterior/posterior combination of samples, with concordance rates varying, perhaps giving no indication that PTEN-null glands located preferentially within certain anatomical aspects of the endometrium. Furthermore, based on these small numbers, we ostulate that different sections from a single uterus may represent independent measures of PTEN status. In using a core of a tissue section for the PECS cases, we might be missing PTEN loss as a result of small sampling on the TMA core. Larger studies focused on extensive sampling methods would inform these sampling method questions. Another limitation of our study is our inability to identify the temporality of the significant relationship between PTEN status and NSAIDs that were observed. It is possible that either PTEN expression influences NSAID use or NSAID use influences PTEN expression. Furthermore, we cannot determine from our data whether NSAIDs alters PTEN status, the fate of PTEN mutant clones (transform to cancer precursors and cancer, regress, remain dormant, or undergo apoptosis), or both. Finally, a more exact analysis would incorporate metrics about the number of endometrial glands assessed. For example, with increasing age, the endometrium undergoes atrophy, suggesting the possibility that when older women harbor PTEN- null glands, these may comprise a greater percentage of their total endometrium. Similarly, oral contraceptive use typically leads to a paucity of benign glands, which are small and inactive appearing.
A major strength of this analysis was the use of a recently validated monoclonal PTEN assay [16] and the assessment of positive staining in each sample as a positive internal control. PTEN IHC has repeatedly been documented to be inconsistent due to variability in antibody characteristics and laboratory processes [27]. Lotan et al optimized the staining protocol with the PTEN IHC showing 100% sensitivity and 97.8% specific for detection of genomic alteration in over 50 cell lines [16]. PTEN loss can result from somatic mutations, abnormalities in PTEN transcriptional and post-transcriptional regulation, and other epigenetic mechanisms that influence PTEN protein stability and degradation [28,27]. The other major strengths of this include prospective rather than retrospective systematic data collection, assessment of epidemiologic risk factors, and standardized preparation of normal hysterectomy tissues samples.
In conclusion, our data demonstrates that PTEN loss occurs in a substantial percentage of women having hysterectomies for benign indications, albeit as a focal finding consisting of few glands detectable using immunohistochemistry. Using the same PTEN assay, PTEN loss was detected nearly three times as frequently in carcinoma. However, apart from possible associations between a history of endometriosis or NSAID use and PTEN-null glands in normal endometrium, other risk factor associations examined were not significant. Given that PTEN loss is a frequent finding in both benign endometrium and endometrial cancer, future studies to assess the extent of PTEN loss per case and to identify factors that may affect persistence of PTEN-null glands and their possible evolution into neoplastic lesions may provide insights into endometrial carcinogenesis.
Supplementary Material
Acknowledgment
The study was supported by the Intramural Research Program of the National Cancer Institute.
References
- 1.American Cancer Society . Cancer Facts & Figures 2015. American Cancer Society; Atlanta: 2015. [Google Scholar]
- 2.Cramer DW. The epidemiology of endometrial and ovarian cancer. Hematology/oncology clinics of North America. 2012;26(1):1–12. doi: 10.1016/j.hoc.2011.10.009. doi:10.1016/j.hoc.2011.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sansal I, Sellers WR. The biology and clinical relevance of the PTEN tumor suppressor pathway. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2004;22(14):2954–2963. doi: 10.1200/JCO.2004.02.141. doi:10.1200/JCO.2004.02.141. [DOI] [PubMed] [Google Scholar]
- 4.Hollander MC, Blumenthal GM, Dennis PA. PTEN loss in the continuum of common cancers, rare syndromes and mouse models. Nature reviews Cancer. 2011;11(4):289–301. doi: 10.1038/nrc3037. doi:10.1038/nrc3037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Matias-Guiu X, Prat J. Molecular pathology of endometrial carcinoma. Histopathology. 2013;62(1):111–123. doi: 10.1111/his.12053. doi:10.1111/his.12053. [DOI] [PubMed] [Google Scholar]
- 6.Mutter GL, Lin MC, Fitzgerald JT, Kum JB, Baak JP, Lees JA, Weng LP, Eng C. Altered PTEN expression as a diagnostic marker for the earliest endometrial precancers. Journal of the National Cancer Institute. 2000;92(11):924–930. doi: 10.1093/jnci/92.11.924. [DOI] [PubMed] [Google Scholar]
- 7.Wang H, Douglas W, Lia M, Edelmann W, Kucherlapati R, Podsypanina K, Parsons R, Ellenson LH. DNA mismatch repair deficiency accelerates endometrial tumorigenesis in Pten heterozygous mice. The American journal of pathology. 2002;160(4):1481–1486. doi: 10.1016/S0002-9440(10)62573-4. doi:10.1016/S0002-9440(10)62573-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Stambolic V, Tsao MS, Macpherson D, Suzuki A, Chapman WB, Mak TW. High incidence of breast and endometrial neoplasia resembling human Cowden syndrome in pten+/− mice. Cancer research. 2000;60(13):3605–3611. [PubMed] [Google Scholar]
- 9.Podsypanina K, Ellenson LH, Nemes A, Gu J, Tamura M, Yamada KM, Cordon-Cardo C, Catoretti G, Fisher PE, Parsons R. Mutation of Pten/Mmac1 in mice causes neoplasia in multiple organ systems. Proceedings of the National Academy of Sciences of the United States of America. 1999;96(4):1563–1568. doi: 10.1073/pnas.96.4.1563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mutter GL, Ince TA, Baak JP, Kust GA, Zhou XP, Eng C. Molecular identification of latent precancers in histologically normal endometrium. Cancer research. 2001;61(11):4311–4314. [PubMed] [Google Scholar]
- 11.Allison KH, Tenpenny E, Reed SD, Swisher EM, Garica RL. Immunohistochemical markers in endometrial hyperplasia: is there a panel with promise? A review. Applied immunohistochemistry & molecular morphology : AIMM / official publication of the Society for Applied Immunohistochemistry. 2008;16(4):329–343. doi: 10.1097/PAI.0b013e318159b88e. doi:10.1097/PAI.0b013e318159b88e. [DOI] [PubMed] [Google Scholar]
- 12.Lacey JV, Jr., Mutter GL, Ronnett BM, Ioffe OB, Duggan MA, Rush BB, Glass AG, Richesson DA, Chatterjee N, Langholz B, Sherman ME. PTEN expression in endometrial biopsies as a marker of progression to endometrial carcinoma. Cancer research. 2008;68(14):6014–6020. doi: 10.1158/0008-5472.CAN-08-1154. doi:10.1158/0008-5472.CAN-08-1154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lin MC, Burkholder KA, Viswanathan AN, Neuberg D, Mutter GL. Involution of latent endometrial precancers by hormonal and nonhormonal mechanisms. Cancer. 2009;115(10):2111–2118. doi: 10.1002/cncr.24218. doi:10.1002/cncr.24218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wentzensen N, Bakkum-Gamez JN, Killian JK, Sampson J, Guido R, Glass A, Adams L, Luhn P, Brinton LA, Rush B, d'Ambrosio L, Gunja M, Yang HP, Garcia-Closas M, Lacey JV, Jr., Lissowska J, Podratz K, Meltzer P, Shridhar V, Sherman ME. Discovery and validation of methylation markers for endometrial cancer. International journal of cancer Journal international du cancer. 2014;135(8):1860–1868. doi: 10.1002/ijc.28843. doi:10.1002/ijc.28843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Brinton LA, Sakoda LC, Lissowska J, Sherman ME, Chatterjee N, Peplonska B, Szeszenia-Dabrowska N, Zatonski W, Garcia-Closas M. Reproductive risk factors for endometrial cancer among Polish women. British journal of cancer. 2007;96(9):1450–1456. doi: 10.1038/sj.bjc.6603731. doi:10.1038/sj.bjc.6603731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lotan TL, Gurel B, Sutcliffe S, Esopi D, Liu W, Xu J, Hicks JL, Park BH, Humphreys E, Partin AW, Han M, Netto GJ, Isaacs WB, De Marzo AM. PTEN protein loss by immunostaining: analytic validation and prognostic indicator for a high risk surgical cohort of prostate cancer patients. Clinical cancer research : an official journal of the American Association for Cancer Research. 2011;17(20):6563–6573. doi: 10.1158/1078-0432.CCR-11-1244. doi:10.1158/1078-0432.CCR-11-1244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Feng ZZ, Chen JW, Yang ZR, Lu GZ, Cai ZG. Expression of PTTG1 and PTEN in endometrial carcinoma: correlation with tumorigenesis and progression. Medical oncology. 2012;29(1):304–310. doi: 10.1007/s12032-010-9775-x. doi:10.1007/s12032-010-9775-x. [DOI] [PubMed] [Google Scholar]
- 18.Lee H, Choi HJ, Kang CS, Lee HJ, Lee WS, Park CS. Expression of miRNAs and PTEN in endometrial specimens ranging from histologically normal to hyperplasia and endometrial adenocarcinoma. Modern pathology : an official journal of the United States and Canadian Academy of Pathology, Inc. 2012;25(11):1508–1515. doi: 10.1038/modpathol.2012.111. doi:10.1038/modpathol.2012.111. [DOI] [PubMed] [Google Scholar]
- 19.Lacey JV, Jr., Chia VM. Endometrial hyperplasia and the risk of progression to carcinoma. Maturitas. 2009;63(1):39–44. doi: 10.1016/j.maturitas.2009.02.005. doi:10.1016/j.maturitas.2009.02.005. [DOI] [PubMed] [Google Scholar]
- 20.Bulun SE. Endometriosis. The New England journal of medicine. 2009;360(3):268–279. doi: 10.1056/NEJMra0804690. doi:10.1056/NEJMra0804690. [DOI] [PubMed] [Google Scholar]
- 21.Govatati S, Kodati VL, Deenadayal M, Chakravarty B, Shivaji S, Bhanoori M. Mutations in the PTEN tumor gene and risk of endometriosis: a case-control study. Human reproduction. 2014;29(2):324–336. doi: 10.1093/humrep/det387. doi:10.1093/humrep/det387. [DOI] [PubMed] [Google Scholar]
- 22.Laudanski P, Szamatowicz J, Kowalczuk O, Kuzmicki M, Grabowicz M, Chyczewski L. Expression of selected tumor suppressor and oncogenes in endometrium of women with endometriosis. Human reproduction. 2009;24(8):1880–1890. doi: 10.1093/humrep/dep175. doi:10.1093/humrep/dep175. [DOI] [PubMed] [Google Scholar]
- 23.Pearce CL, Templeman C, Rossing MA, Lee A, Near AM, Webb PM, Nagle CM, Doherty JA, Cushing-Haugen KL, Wicklund KG, Chang-Claude J, Hein R, Lurie G, Wilkens LR, Carney ME, Goodman MT, Moysich K, Kjaer SK, Hogdall E, Jensen A, Goode EL, Fridley BL, Larson MC, Schildkraut JM, Palmieri RT, Cramer DW, Terry KL, Vitonis AF, Titus LJ, Ziogas A, Brewster W, Anton-Culver H, Gentry-Maharaj A, Ramus SJ, Anderson AR, Brueggmann D, Fasching PA, Gayther SA, Huntsman DG, Menon U, Ness RB, Pike MC, Risch H, Wu AH, Berchuck A, Ovarian Cancer Association C. Association between endometriosis and risk of histological subtypes of ovarian cancer: a pooled analysis of case-control studies. The lancet oncology. 2012;13(4):385–394. doi: 10.1016/S1470-2045(11)70404-1. doi:10.1016/S1470-2045(11)70404-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Neill AS, Nagle CM, Protani MM, Obermair A, Spurdle AB, Webb PM, Australian National Endometrial Cancer Study G Aspirin, nonsteroidal anti-inflammatory drugs, paracetamol and risk of endometrial cancer: a case-control study, systematic review and meta-analysis. International journal of cancer Journal international du cancer. 2013;132(5):1146–1155. doi: 10.1002/ijc.27717. doi:10.1002/ijc.27717. [DOI] [PubMed] [Google Scholar]
- 25.Brasky TM, Moysich KB, Cohn DE, White E. Non-steroidal anti-inflammatory drugs and endometrial cancer risk in the VITamins And Lifestyle (VITAL) cohort. Gynecologic oncology. 2013;128(1):113–119. doi: 10.1016/j.ygyno.2012.10.005. doi:10.1016/j.ygyno.2012.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Setiawan VW, Matsuno RK, Lurie G, Wilkens LR, Carney ME, Henderson BE, Kolonel LN, Goodman MT. Use of nonsteroidal anti-inflammatory drugs and risk of ovarian and endometrial cancer: the Multiethnic Cohort. Cancer epidemiology, biomarkers & prevention : a publication of the American Association for Cancer Research, cosponsored by the American Society of Preventive Oncology. 2012;21(9):1441–1449. doi: 10.1158/1055-9965.EPI-12-0390-T. doi:10.1158/1055-9965.EPI-12-0390-T. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Djordjevic B, Hennessy BT, Li J, Barkoh BA, Luthra R, Mills GB, Broaddus RR. Clinical assessment of PTEN loss in endometrial carcinoma: immunohistochemistry outperforms gene sequencing. Modern pathology : an official journal of the United States and Canadian Academy of Pathology, Inc. 2012;25(5):699–708. doi: 10.1038/modpathol.2011.208. doi:10.1038/modpathol.2011.208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhang S, Yu D. PI(3)king apart PTEN's role in cancer. Clinical cancer research : an official journal of the American Association for Cancer Research. 2010;16(17):4325–4330. doi: 10.1158/1078-0432.CCR-09-2990. doi:10.1158/1078-0432.CCR-09-2990. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.