Abstract
Objective
To examine agreement between patient and proxy responses on the Quality of Life in Neurological Disorders (Neuro-QoL) instruments after stroke.
Design
Cross-sectional observational sub-study of the longitudinal, multi-site, multi-condition Neuro-QoL validation study.
Setting
In-person interview-guided patient-reported outcomes.
Participants
Convenience sample of 86 dyads of community-dwelling persons with stroke and their proxy respondents.
Interventions
Not applicable.
Main Outcome Measures
Dyads concurrently completed short-forms of 8 or 9 items for the 13 Neuro-QoL adult domains using the patient-proxy perspective. Agreement was examined at the scale-level with difference scores, intraclass correlation coefficients (ICCs), effect size statistics, and Bland-Altman plots, and at the item-level with kappa coefficients.
Results
We found no mean differences between patients and proxies on the Applied Cognition-General Concerns, Depression, Satisfaction with Social Roles and Activities, Stigma, and Upper Extremity Function (Fine Motor) short forms. Patients rated themselves more favorably on the Applied Cognition-Executive Function, Ability to Participate in Social Roles and Activities, Lower Extremity Function (Mobility), Positive Affect and Well-Being, Anxiety, Fatigue, Emotional and Behavioral Dyscontrol, and Fatigue short-forms. The largest mean patient-proxy difference observed was 3 T-score points on the Lower Extremity Function (Mobility). ICCs ranged from 0.34 to 0.59. However, limits of agreement showed dyad differences exceeding ±20 T-score points, and item-level agreement ranged from not significant to κw=0.34.
Conclusions
Proxy responses on Neuro-QoL short forms can complement responses of moderate to high-functioning community-dwelling persons with stroke and augment group-level analyses, but do not substitute for individual patient ratings. Validation is needed for other stroke populations.
Keywords: Stroke, Proxy, Quality of Life
Individuals who sustain a stroke may experience negative health-related quality of life (HRQOL) effects including physical activity limitations, cognitive impairments, speech and language problems, anxiety, decreased work and leisure activity, social isolation, and depressive symptoms.1 HRQOL is typically measured as a patient-reported outcome (PRO); however, patients with stroke may have difficulty responding to PRO items due to cognitive, linguistic, and motor deficits.2 Further, persons who are unable to respond to PROs may be systematically excluded from HRQOL stroke research studies, consequently limiting generalizability of findings to this population.
When patients are unable to respond, a proxy respondent, such as spouse, relative, friend, other caregiver, or a health care professional may complete the assessment on the patient’s behalf. Of nine studies included in a systematic review of proxy response on measures of HRQOL for patients with stroke, five excluded persons with aphasia and cognitive impairments and two excluded persons with severe cognitive or communicative impairments, while only two specifically included persons with aphasia due to stoke.3 Exclusion of patients who have aphasia or cognitive deficits limits our ability to fully assess the impact of clinical treatments, may impede alleviation of suffering, and limits the generalizability of studies attempting to detect changes in HRQOL.3,4 Proxy respondents tend to overestimate the HRQOL impairments for persons with stroke,3 although caregivers may overestimate deficits related to hearing and self-care and underestimate deficits to speech and ambulation;5 the magnitude of disagreement tends to increase with impairment severity.2,6–8
Validity is inferred from evidence and theory that support interpretation of test scores for the proposed use of the test,9 thus substitution of a proxy’s response for a patient’s requires agreement for the context in which scores will be interpreted. Oczkowski and O’Donnell reported intraclass correlation coefficients (ICCs) and kappa statistics ranging from 0.32 to 0.77 for generic HRQOL instruments and 0.41 to 0.83 for stroke-specific instruments.3 As HRQOL instruments tend to be multi-dimensional, one single overall correlation may not adequately reflect differences in patient-proxy agreement across sub-scale scores. For example, patient and proxy responses on Stroke-Specific Quality of Life (SSQOL) summary scores were correlated at ICC=0.41, but subscale score ICCs ranged from 0.30 for the Role Function to 0.59 for the Physical Function subscales.10 Agreement was higher for studies conducted farther from the stroke event and higher for observable domains such as physical functioning than for unobservable domains such as social and emotional functioning.3
The Quality of Life in Neurological Disorders (Neuro-QoL) measurement system was developed to standardize PROs across a range of neurological conditions using contemporary test development methods. Neuro-QoL cover 13 adult domains of physical, mental, and social functioning calibrated using Item-Response Theory-based methods, and are available as computer-adaptive tests or short forms.11–13 The short forms (online supplement table S1) have been validated for use with people with major neurological disorders11, 12 including stroke.14
The objective of this study was to examine agreement between stroke patients and their proxy respondents using the patient-proxy perspective with difference scores, ICCs, effect size, Bland-Altman plots, and item-level kappa for the Neuro-QoL short form instruments.
Methods
We recruited patient-proxy dyads at five clinical sites from across the United States, including: Dartmouth-Hitchcock Medical Center, NorthShore University HealthSystem, Rehabilitation Institute of Chicago, University of Chicago, and University of Texas Health Science Center. Institutional Review Boards at each site approved this study.
Eligible patients were community-dwelling persons who sustained a stroke and completed inpatient rehabilitation at least 6 months prior to recruitment, were 18 years of age or older, English speaking, did not have cognitive or communicative impairments that prohibited providing informed consent, and had an available proxy. Participants were recruited by physician referral, mailings to persons on a stroke registry, and through fliers posted publicly. Participants received an honorarium of $100 upon completing the study (i.e., baseline and 6-month follow-up) or $50 upon withdrawal at follow-up.
Stroke patients selected proxies as someone who knew them well and could respond on their behalf; no limits were set on the nature of the relationship between the patient and proxy. Stroke patients and their proxies completed the Neuro-QoL short forms independently on the same day, using a touchscreen tablet computer guided by an interviewer; data were stored using the Assessment Center web-based survey administration platform.15 Persons with expressive aphasia were excluded because of the requirement to communicate verbally with the interviewer.
The Neuro-QoL instruments were developed for research and clinical measurement of persons with neurological conditions including stroke, multiple sclerosis, and Parkinson’s disease.13 Initial validation for these populations was reported previously.14 The 13 adult domains are each comprised of an item bank from which fixed-length short forms of 8–9 items were derived and validated.11 Scores are reported as T-scores which are standardized to a mean of 50 and a standard deviation (SD) of 10. Ability to Participate in Social Roles and Activities, Applied Cognition-Executive Function, Applied Cognition-General Concerns, Lower Extremity Function (Mobility), Positive Affect and Well-Being, Satisfaction with Social Roles and Activities, and Upper Extremity Function (Fine Motor) are scored positively such that higher scores represent better functioning. Anxiety, Depression, Fatigue, Emotional and Behavioral Dyscontrol, Sleep Disturbance, and Stigma are scored negatively, such that a higher score represents poorer functioning.11,12
Proxies were instructed to answer questions utilizing the “patient-proxy” perspective, in which they respond from the patient’s perspective rather than their own.4 This approach is based on previous perspective-taking research16 that posits caregivers respond with greater correspondence to patients’ reports when they imagine themselves as the patient, compared to providing their personal evaluation of the patients’ condition.
We calculated descriptive statistics for demographic and stroke characteristics to describe our sample, and ICCs to examine the associations between patient and proxy ratings on the Neuro-QoL short forms. We considered agreement to be poor for ICCs <0.40, good for 0.40 to 0.70, and excellent for >0.70.17 We calculated difference scores as the proxy’s score minus the patient’s score to facilitate interpretation of the proxy’s response as an over- or underestimate of the patient’s score. We computed the mean, SD, minimum, and maximum values for the patient, proxy, difference scores, the 95% confidence intervals (CIs) for the difference scores, and examined Bland-Altman plots18 for each Neuro-QoL short form. We examined the distributions of the difference scores for normality, and calculated paired-sample t-tests to identify differences between the patient and proxy responses. Item-level kappa coefficients provided insight to agreement differences within each short form. Neuro-QoL short forms produce summary scores so we examined the relative magnitude of the coefficients and noted the number of items with non-significant p-values, rather than interpreting the absolute magnitudes of the coefficients. To facilitate comparisons of results with other studies, we standardized differences by computing pooled effect sizes. We considered d=0.2 to indicate a small effect, d=0.5 a moderate effect, and d=0.8 a large effect.2,19 We used IBM SPSSa Version 20 statistical software for all analyses.
Results
Patient and Proxy Characteristics
Eighty-six stroke patients and their proxies completed the Neuro-QoL short forms and HRQOL-related measures; however personal characteristics are missing for 29 proxies. Patients (n=86) and proxies (n=57) were roughly the same age (mid to late fifties), with most being either Caucasian (63% and 57% respectively) or African American (29% and 30%, respectively). Most patients and proxies sought education beyond high school (40% and 80%, respectively). More than half of the proxy respondents were spouses or romantic partners; one fifth were other relatives. Most patients (58%) were married, whereas most proxies (60%) were divorced (table 1). Most patients presented with mild to moderate stroke-related impairments but were functioning independently (table 2).
Table 1.
Socio-Demographic Characteristics of Patients and Proxies
| Patients (All) n=86 | Patients (With Proxy Characteristics) n=57 | Proxies* n=57 | |
|---|---|---|---|
| Age | |||
| Mean (SD) | 58 (14) | 60 (14) | 58 (15) |
| Min-Max | 21–87 | 31–85 | 23–89 |
| n=57 | n=57 | ||
| Sex (% male) | 52% | 46% | 35% |
| Race | n=57 | n=56 | |
| Caucasian | 63% | 61% | 57% |
| African American | 29% | 30% | 30% |
| Other ethnicity | 8% | 9% | 13% |
| Education | n=55 | n=56 | |
| Less than high school | 26% | 4% | 4% |
| Only high school | 31% | 18% | 16% |
| Beyond high school | 41% | 78% | 80% |
| Marital status | n=57 | n=57 | |
| Married | 58% | 54% | 60% |
| Divorced | 13% | 14% | 12% |
| Never married | 12% | 9% | 18% |
| Widowed | 7% | 9% | 5% |
| Separated | 5% | 7% | 2% |
| Living with partner | 6% | 7% | 3% |
| Income | n=51 | n=47 | |
| Less than $20,000 | 26% | 25% | 21% |
| Between $20,000 to $49,000 | 34% | 33% | 26% |
| Between $50,000 to $99,999 | 19% | 24% | 34% |
| More than $100,000 | 15% | 18% | 19% |
|
| |||
| Proxy Relationship to Patient | n=86 | n=57 | |
| Spouse | 48% | 47% | |
| Romantic partner | 5% | 7% | |
| Parent | 6% | 9% | |
| Other relative | 19% | 21% | |
| Caregiver | 3% | 4% | |
| Other | 13% | 12% | |
| Missing | 7% | 0% | |
|
| |||
| Patient-Proxy living together | n=57 | ||
| Yes | 68% | ||
| No | 32% | ||
Data on personal characteristics are missing for 29 proxies.
Table 2.
Patient Stroke Characteristics (n=86)
| American Heart Association Stroke Outcome Classification | |
|---|---|
| Impairment Severity (neurological deficit) | |
| None or Minimal | 19.8% |
| Mild or Moderate | 54.7% |
| Severe | 18.6% |
| Function | |
| Independent | 57.0% |
| Partially dependent | 16.3% |
| Completely dependent | 3.5% |
|
| |
| Stroke Type | |
|
| |
| Non-Hemorrhagic | 70.8% |
| Hemorrhagic | 29.2% |
|
| |
| Time Since Stroke (years) | |
|
| |
| Median (interquartile range) | 2.9 (5.9) |
| Minimum | 0.1 |
| Maximum | 39.3 |
Patient and Proxy Responses on Neuro-QoL Short Forms
Good, positive ICCs were observed across nine short forms with the highest coefficient of 0.59 for Lower Extremity Function (mobility) (Table 3). ICCs were poor for the Anxiety, Stigma, Emotional and Behavioral Dyscontrol, and Sleep Disturbance short forms. Positively scored Neuro-QoL short forms generally had higher correlations than the negatively scored domains. ICCs higher than 0.50 were observed in physical function and mental-cognitive (both Executive Function and General Concerns) domains, however, no clear pattern of was apparent in the social, physical symptom, or mental emotional domains.
Table 3.
Patient and proxy ratings and difference scores for Neuro-QOL short forms
| Neuro-QOL domain | Scale Range | Patient Rating | Proxy Rating | t-test value | ICC** | 95% CI for ICC | Patient-Proxy Difference* | 95% CI for Difference | Effect Size |
|---|---|---|---|---|---|---|---|---|---|
| T-Score Min–Max |
Mean (SD) Min–Max |
Mean (SD) Min–Max |
Mean (SD) Min–Max |
||||||
| Social Domains | |||||||||
| Ability to Participate in Social Roles and Activities | 45.9 (7.1) | 44.3 (6.6) | 2.12† | 0.50 | 0.31 to 0.64 | −1.6 (6.9) | −3.1 to −0.1 | −0.23 | |
| 24–60 | 29–60 | 31–60 | −22.3 to 15.3 | ||||||
| Satisfaction with Social Roles and Activities | 44.9 (5.0) | 44.0 (5.2) | 1.64 | 0.41 | 0.21 to 0.57 | −0.9 (5.5) | −2.2 to 0.2 | −0.18 | |
| 28–60 | 29–61 | 28–61 | −16.3 to 13.7 | ||||||
| Mental Cognitive Domains | |||||||||
| Applied Cognition - Executive Function | 44.5 (10.4) | 41.8 (10.8) | 2.57† | 0.56 | 0.40 to 0.69 | −2.8(10.0) | −4.9 to −0.6 | −0.25 | |
| 13–58 | 16–58 | 13–58 | −38.0 to 17.9 | ||||||
| Applied Cognition - General Concerns | 43.6 (8.7) | 43.1 (7.9) | 0.62 | 0.53 | 0.40 to 0.70 | −0.5 (8.0) | −2.3 to 1.2 | −0.06 | |
| 20–59 | 20–59 | 23–59 | −21.2 to 17.8 | ||||||
| Mental Emotional Domains | |||||||||
| Anxiety | 51.0 (6.9) | 53.0 (5.5) | −2.68‡ | 0.38 | 0.18 to 0.55 | 2.0 (7.0) | 0.5 to 3.6 | 0.32 | |
| 36–77 | 36–67 | 36–65 | −14.1 to 23.2 | ||||||
| Depression | 47.5 (7.8) | 48.6 (6.7) | −1.26 | 0.42 | 0.23 to 0.58 | 1.1 (7.8) | −0.6to 2.8 | 0.15 | |
| 37–75 | 37–69 | 37–61 | −17.0 to 19.0 | ||||||
| Stigma | 52.2 (6.4) | 52.5 (5.1) | −0.37 | 0.34 | 0.14 to 0.52 | 0.3 (6.6) | −1.2 to 1.7 | 0.05 | |
| 39–81 | 44–74 | 44–65 | −24.3 to 16.0 | ||||||
| Emotional and Behavioral Dyscontrol | 45.4 (8.0) | 48.4 (8.2) | −2.95‡ | 0.32 | 0.12 to 0.50 | 3.0 (9.4) | 1.0 to 5.0 | 0.37 | |
| 32–83 | 32–67 | 32–70 | −19.2 to 23.7 | ||||||
| Positive Affect and Well-Being | 54.5 (8.2) | 52.6 (7.1) | 2.22† | 0.48 | 0.30 to 0.63 | −1.9 (7.8) | −3.6 to −0.2 | −0.25 | |
| Physical Function Domains | |||||||||
| Upper Extremity Function (Fine Motor) | 37.9 (9.1) | 38.1 (8.4) | −0.17 | 0.52 | 0.34 to 0.66 | 0.2 (8.6) | −1.7 to 2.0 | 0.02 | |
| 13–54 | 16–54 | 16–54 | −24.8 to 24.2 | ||||||
| Lower Extremity Function (Mobility) | 42.7 (8.1) | 39.8 (7. 6) | 3.49‡ | 0.59 | 0.42 to 0.72 | −3.0 (7.1) | −4.6 to −1.3 | −0.36 | |
| 16–57 | 27–59 | 26–59 | −15.0 to 19.9 | ||||||
| 26–68 | 31–68 | 37–68 | −21.2 to 18.0 | ||||||
| Physical Symptom Domains | |||||||||
| Sleep Disturbance | 46.3 (8.1) | 49.0 (6.7) | −2.94‡ | 0.34 | 0.13 to 0.51 | 2.8 (8.6) | 0.9 to 4.6 | 0.36 | |
| 32–84 | 32–66 | 37–68 | −17.4 to 23.8 | ||||||
| Fatigue | 45.6 (8.9) | 47.7 (7.3) | −2.24† | 0.45 | 0.26 to 0.61 | 2.1 (8.5) | 0.2 to 3.9 | 0.26 | |
| 30–74 | 30–7 | 30–66 | −21.2 to 18.7 | ||||||
Difference scores are calculated as proxy score minus patient score, and include rounding error
Significant at p<0.05
Significant at p<0.01
Significant at p<0.001
Intraclass correlation coefficients (ICCs) are reported as Shrout and Fleiss Type (2, 1). Due to missing data, n varies from 84–86 for all Neuro-QoL short forms except Lower Extremity (Mobility) for which n=71
Paired-sample t-tests revealed no differences between patient and proxy responses on the Applied Cognition-General Concerns, Depression, Satisfaction with Social Roles and Activities, Stigma, and Upper Extremity Function (Fine Motor) short forms (table 3). Mean differences on these short forms ranged from −0.9 to 1.1. The 95% CIs ranged from ±1.3 to ±1.8 points (i.e., less than 0.2 SD units). The extreme difference scores (+24 and −24.8 points,) were observed on the Upper Extremity Function (Fine Motor) short form. Proxies systematically rated patients worse on the remaining short forms (i.e., lower on positively-scaled and higher on negatively-scaled domains).
Mean differences on the positively-scored Ability to Participate in Social Roles and Activities, Applied Cognition-Executive Function, Lower Extremity Function (Mobility), and Positive Affect and Well-Being short forms ranged from −1.6 to −3.0 T-score units, and the 95% CIs ranged from ±1.5 to ±2.3 points. The extreme difference scores observed were +20 points on the Lower Extremity Function (Mobility) short form and −38 points on the Applied Cognition-Executive Function short form. Mean differences on the negatively-scored Anxiety, Fatigue, Emotional and Behavioral Dyscontrol, and Sleep Disturbance short forms ranged from 2.0 to 3.0 points, and the 95% CIs ranged from ±1.5 to ±1.9 points. The extreme difference scores observed were +20 points on the Emotional and Behavioral Dyscontrol short forms and −21 points on the Fatigue short form.
Effect sizes ranged from 0.02 for Upper Extremity Function (Fine Motor) to 0.37 for Emotional and Behavioral Control. The smallest effect sizes were observed for the five Neuro-QoL short forms that had no mean patient-proxy differences. With one exception, effect sizes were largest for the negatively scored domains that exhibited differences, ranging from d=0.26 to d=0.37. Of the positively scored domains, only Lower Extremity Function (Mobility) had a higher effect size (d=0.36), while the remaining three domains ranged from d=0.23 to d=0.25.
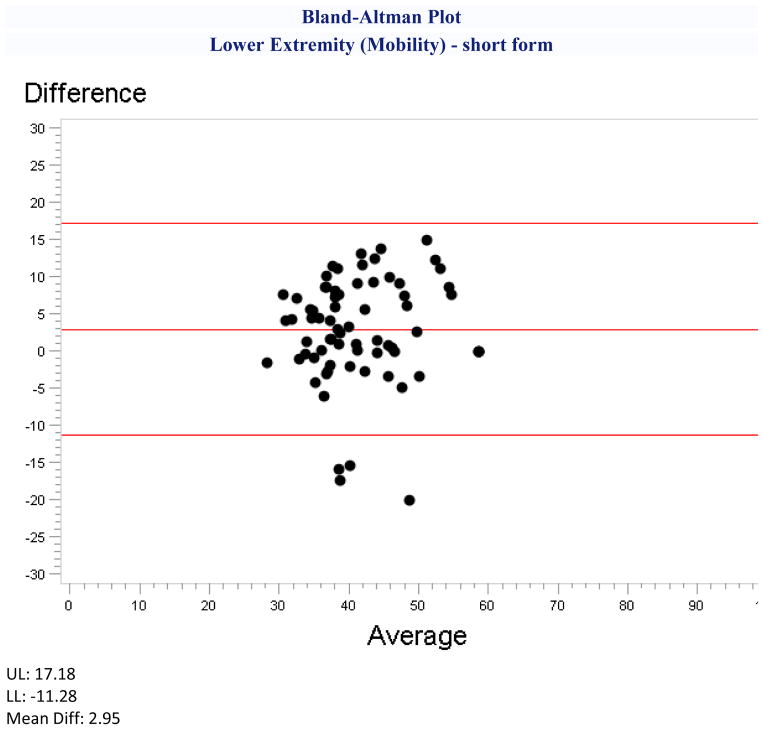
The Bland-Altman plots (online supplement figure S1) corresponded to the magnitude and direction of the difference scores, but none demonstrated interactions between score level and direction of the difference. Three plots presented with outliers clustered at one end of the scale suggesting that proxies tend to rate patients worse for lower extremity function (figure 1), stigma, and fatigue.
Figure 1.
Item-level weighted kappa coefficients ranged from not significant to κw=0.34 across the short forms (online supplement table S2). The Lower Extremity Function (Mobility) and Applied Cognition - General Concerns each had agreement on seven of 8 items, whereas the Anxiety and Emotional and Behavioral Dyscontrol short forms each had agreement on one of eight items. Internalized constructs generally had more items with non-significant coefficients; however there was no consistent pattern, as Depression had fewer non-significant items but lower coefficients than Upper Extremity Function (Fine Motor, ADL).
Discussion
The purpose of this study was to examine agreement between community-dwelling persons with stroke and their proxies on the Neuro-QoL short forms using the patient-proxy perspective. Our findings will facilitate interpretation of proxy responses as complementary reports or substitutes for patient responses in research and clinical measurement applications.
We found consistently moderate ICCs between patient and proxy responses for all Neuro-QoL short forms, with relatively stronger associations on more “observable” domains such as physical function and cognitive function, and weaker associations on some emotional and symptom domains. The lower and upper extremity functioning domains had two of the highest correlations; whereas latent states of Emotional and Behavioral Dyscontrol and Anxiety, which can be more variable and internalized, produced weaker correlations. These results are consistent with previous studies where agreement is stronger for observable HRQOL domains and weaker for internally experienced psychological or evaluative domains.2,3,6,17,20,21 However, ICCs are not, on their own, suitable indices of agreement,9 given that two physical functioning domains had higher correlations yet Lower Extremity Function (Mobility) demonstrated patient-proxy differences whereas Upper Extremity Function (Fine Motor) did not.
However, the results deviate from previous studies on difference scores. We found no significant differences on two mental emotional health domains (Depression and Stigma) and two social domains (Applied Cognition-General Concerns and Satisfaction with Social Roles and Activities).2,6,17 Also, we found proxies rated patients worse on the Lower Extremity Motor (Mobility) domain, which is an observable physical function. Results of some previous studies were consistent with ours;2,10 however others found no difference.17 Proxies also rated patients worse on the remaining positively scored domains, which include one social, one mental-cognitive, and mental-emotional. Proxies also rated patients worse on two mental-emotional health domains and the two physical-symptom domains, all of which are negatively scored. Differences in our findings may relate to differences in organization of the Neuro-QoL domain map compared to other instruments, the item content of the short forms relative to other instruments, or to factors associated with the study participants and their proxies. For example, the SSQOL mobility subscale22 and the Neuro-QoL Lower Extremity Function (mobility) short form23 differ structurally as the former has six items rated on a 3-point scale and the latter has eight items rated on a 5-point scale. The scales also differ in content as the former includes balance and stair climbing items which the latter does not, while the latter include mobility items for curbs, toileting, car entry/exit, and running errands where the former does not. It is possible that the two scales cover different ranges of the mobility continuum, or even differ in terms of the dimensionality of construct of mobility that they measure.
Use of the patient-proxy perspective assumes that proxies adopt the perspective of the patient and thus transcend expectations and personal projections, yielding greater accuracy.4 Rather than assuming their own viewpoint, we might expect to see higher levels of agreement than for studies that use the proxy-proxy perspective. Researchers studying agreement between patient and proxy responses on the Stroke Impact Scale employed the proxy-proxy perspective on the assumption that this perspective was more reliable than the patient-proxy perspective.17 They reported effect sizes ranging from d=0.01 to 0.21.17 In contrast, we found larger effect sizes on eight Neuro-QoL domains, perhaps reflecting our instructions to proxy to attempt to answer as the patient would.
The effect size standardizes the differences in score variability. Standardization facilitates comparisons of scores from different instruments collected concurrently on the same sample, and perhaps from different samples that present with similar variability in scores, but may not do so where the sample variances differ substantially. Consequently, effect size statistics should be examined more closely for the relative contributions of sample variances when making comparisons across studies. Others posit that the two perspectives measure different constructs that are comparable only under limited circumstances, and that the patient-proxy perspective is suitable for examining agreement between the patient and the proxy.4
Overall, the Neuro-QoL instruments were comparable in their performance to the other instruments applied in this study. However, the characteristics of the Neuro-QoL instruments provide advantages that are not available in typical legacy questionnaires. In addition to interval level scaling, normative scoring and item-level reliability, the Neuro-QoL short forms have score-level reliability statistics and large scale ranges on all domains. In the context of normative scoring, the largest observed mean patient-proxy difference was 3 points on the Lower Extremity Function (Mobility) short form, which represents 0.3 SD on the T-score. With interval-level scoring, measured proxy scores could be adjusted by the mean patient-proxy differences for the domains on differences were apparent.
The item-level analysis produced kappa coefficients similar to proxy responses of parents and their children on pediatric PROMIS instruments.24 Comparably, externalized constructs like Lower Extremity Function (Mobility) showed better agreement on items than internalized constructs such as Anxiety (online supplement table S2). Varni and colleagues noted that item-level disagreement may represent the influence of the parent’s context in addition to measurement error24 thus context may warrant further exploration in proxy response for adults.
Researchers and clinicians can be confident in using proxy responses in lieu of patient responses for the Neuro-QoL short forms when measuring group-level aspects of HRQOL for community dwelling persons with stroke with the patient-proxy perspective. We suggest the patient-proxy perspective, using the same item wording as presented to the patient, for estimates that contextually approach those provided by patients themselves. Mean proxy responses adequately represented patient scores for observable physical function and on mental cognitive domains, and could be adjusted for the domains where differences were apparent. However, adjusted scores should be interpreted along with the original proxy scores, and may be less suitable for interpreting individual scores as differences could be ±20 points or more. Since patient-proxy differences may diminish with time following the stroke,2,4,6 these findings may not hold for proxy response of persons in acute care, inpatient rehabilitation, skilled or long-term nursing facilities, or recently discharged to the community. Also, our findings may not reflect patient-proxy differences for persons with communicative or cognitive impairments, particularly since communicative impairment precludes comparison of patient and proxy ratings.2 However, until evidence becomes available for the validity of proxy responses in these settings, our findings provide the best available evidence for the use and interpretation of proxy responses of Neuro-QoL scores for any stroke population.
Future research should focus on the experience and characteristics of the proxies themselves, including the influence of relationship to the patient, frequency of contact, level of caregiver burden, gender, and other demographic, social support and relationship information. For example, when a person develops a disability or chronic illness, the impact of the individual’s diagnosis extends to family, friends, and other caregivers. Family caregivers often experience distress, burden, impaired self-care, and increased psychological and physical morbidity.25 Thus, proxies must be cognizant of and distinguish their own functioning from that of the patient for HRQOL reports to be accurate. If not, proxies may unintentionally allow the distressing nature of the patient’s disability or illness to affect their perceptions of the patient. Additionally, the impairment severity should also be considered as previous research has shown that level of proxy and patient agreement on HRQOL measures decreases with increasing impairment severity.2,7,10,21 We did not ask proxy respondents to provide a proxy-proxy perspective. Perhaps if proxies are first allowed to respond based on how they feel the patient is functioning, their subsequent patient-proxy judgment may be more aligned with patients. Finally, it may be important to debrief proxies in future studies where they are asked to describe their experience rating the patient-proxy perspective, how they derived their judgments, and how they would rate their level of agreement. Again, there are multiple external factors not considered in this study which could provide researchers with future directions when further investigating the patient-proxy perspective in stroke patients.
Limitations
We had incomplete descriptive data for proxies. Our sample was limited to moderate to high functioning community-dwelling persons with stroke, and may not represent persons with more severe impairments and limitations; persons in acute care, inpatient rehabilitation, or other non-community settings; or persons who sustained a stroke more recently than our sample. We excluded persons with cognitive and communicative impairments that precluded administering informed consent, and may not represent this population; however this represents a methodological problem inherent in all proxy studies.
Conclusions
HRQOL is a multifaceted outcome inherently difficult to assess due to the complex interactions of physical, psychological, and social constructs. Proxy ratings using a patient-proxy perspective can complement patient responses, but do not replace them at the individual level. While further validation of the Neuro-QoL instruments is necessary, this study provides an important step toward understanding and measuring agreement in HRQOL between community-dwelling persons with stroke and their proxy respondents.
Supplementary Material
Acknowledgments
Financial Support
The study was supported from grants from the National Institute of Neurological Disorders and Stroke (NINDS) contract number HHSN265200423601C (PI: David Cella) and the National Institute on Disability and Rehabilitation Research (NIDRR), grant number H133B090024 (PI: Allen Heinemann). The contents represent original work and have not been published elsewhere. No commercial party having a direct financial interest in the results of the research supporting this article has or will confer a benefit upon the author(s) or upon any organization with which the author(s) is/are associated.
List of Abbreviations
- CTT
Classical Test Theory
- HRQOL
Health-related quality of life
- ICC
Intraclass correlation coefficient
- IRT
Item-Response Theory
- Neuro-QoL
Neurological Quality of Life
- PRO
Patient-reported outcome
- SSQOL
Stroke-Specific Quality of Life
Footnotes
Supplier’s List
IBM Corporation, 1 New Orchard Road, Armonk, New York 10504-1722, United States
Reprints are not available.
Disclosures:
We certify that no party having a direct interest in the results of the research supporting this article has or will confer a benefit on us or on any organization with which we are associated AND, if applicable, we certify that all financial and material support for this research (eg, NIH or NHS grants) and work are clearly identified in the title page of the manuscript. (Allan J. Kozlowski, Ritika Singh, David Victorson, Ana Miskovic, Jin-Shei Lai, Richard L. Harvey, David Cella, Allen W. Heinemann)
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Pan JH, Song XY, Lee SY, Kwok T. Longitudinal analysis of quality of life for stroke survivors using latent curve models. Stroke. 2008;39:2795–802. doi: 10.1161/STROKEAHA.108.515460. [DOI] [PubMed] [Google Scholar]
- 2.Sneeuw KC, Aaronson NK, de Haan RJ, Limburg M. Assessing quality of life after stroke. The value and limitations of proxy ratings. Stroke. 1997;28:1541–9. doi: 10.1161/01.str.28.8.1541. [DOI] [PubMed] [Google Scholar]
- 3.Oczkowski C, O’Donnell M. Reliability of proxy respondents for patients with stroke: a systematic review. J Stroke Cerebrovasc Dis. 2010;19:410–6. doi: 10.1016/j.jstrokecerebrovasdis.2009.08.002. [DOI] [PubMed] [Google Scholar]
- 4.Pickard AS, Knight SJ. Proxy evaluation of health-related quality of life: a conceptual framework for understanding multiple proxy perspectives. Med Care. 2005;43:493–9. doi: 10.1097/01.mlr.0000160419.27642.a8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mathias SD, Bates MM, Pasta DJ, Cisternas MG, Feeny D, Patrick DL. Use of the Health Utilities Index with stroke patients and their caregivers. Stroke. 1997;28:1888–94. doi: 10.1161/01.str.28.10.1888. [DOI] [PubMed] [Google Scholar]
- 6.Dorman PJ, Waddell F, Slattery J, Dennis M, Sandercock P. Are proxy assessments of health status after stroke with the EuroQol questionnaire feasible, accurate, and unbiased? Stroke. 1997;28:1883–7. doi: 10.1161/01.str.28.10.1883. [DOI] [PubMed] [Google Scholar]
- 7.Duncan PW, Lai SM, Tyler D, Perera S, Reker DM, Studenski S. Evaluation of proxy responses to the Stroke Impact Scale. Stroke. 2002;33:2593–9. doi: 10.1161/01.str.0000034395.06874.3e. [DOI] [PubMed] [Google Scholar]
- 8.Poulin V, Desrosiers J. Participation after stroke: comparing proxies’ and patients’ perceptions. J Rehabil Med. 2008;40:28–35. doi: 10.2340/16501977-0115. [DOI] [PubMed] [Google Scholar]
- 9.American Educational Research Association, American Psychological Association, National Council on Measurement in Education. Standards for educational and psychological testing. Washington, DC: American Educational Research Association; 2014. [Google Scholar]
- 10.Williams LS, Bakas T, Brizendine E, Plue L, Tu W, Hendrie H, et al. How valid are family proxy assessments of stroke patients’ health-related quality of life? Stroke. 2006;37:2081–5. doi: 10.1161/01.STR.0000230583.10311.9f. [DOI] [PubMed] [Google Scholar]
- 11.Cella D, Nowinski CJ, Peterman A, Victorson D, Miller D, Bethoux F, et al. Neuro-QOL: Brief measures of health-related quality of life for clinical research neurology. Neurology. 2012;78:1860–7. doi: 10.1212/WNL.0b013e318258f744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gershon RC, Lai JS, Bode R, Choi S, Moy C, Bleck T, Miller D, Peterman A, Cella D. Neuro-QOL: quality of life item banks for adults with neurological disorders: item development and calibrations based upon clinical and general population testing. Qual Life Res. 2012;21:475–86. doi: 10.1007/s11136-011-9958-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.National Institute of Neurological Disorders and Stroke (NINDS) NINDS User Manual: Quality of Life in Neurological Disorders (Neuro-QOL) Measures. 2012 Available from: http://www.neuroqol.org/Resources/Resources%20documents/Neuro-QOL_UserManualv2Dec2014.pdf.
- 14.National Institute on Neurological Disorders and Stroke (NINDS) Measuring quality of life in neurological disorders: Final report of the Neuro-QOL Study. 2013 Available from: http://www.neuroqol.org/Resources/Resources%20documents/NeuroQOL-Final%20report-2013.pdf.
- 15.National Institutes of Health. Assessment Center web page. 2013 [cited 2014 March 14]. Available from: http://www.assessmentcenter.net/
- 16.Lobchuk MM, Vorauer JD. Family caregiver perspective-taking and accuracy in estimating cancer patient symptom experiences. Soc Sci Med. 2003;57:2379–84. doi: 10.1016/s0277-9536(03)00132-1. [DOI] [PubMed] [Google Scholar]
- 17.Carod-Artal FJ, Ferreira Coral L, Stieven Trizotto D, Menezes Moreira C. Self- and proxy-report agreement on the Stroke Impact Scale. Stroke. 2009;40:3308–14. doi: 10.1161/STROKEAHA.109.558031. [DOI] [PubMed] [Google Scholar]
- 18.Bland JM, Altman DG. Statistical methods for assessing agreement between two methods of clinical measurement. Lancet. 1986;1(8476):307–10. [PubMed] [Google Scholar]
- 19.Cohen J. Statistical Power Analysis for the Behavioral Sciences. 2. Hillsdale, NJ: Lawrence Erlbaum Associates; 1988. [Google Scholar]
- 20.Lobchuck MM, Degner LF. Symptom esperiences: perceptual accuracy between advanced-stage cancer patients and familiy caregivers in the home care setting. J Clin Oncol. 2002;20:3495–507. doi: 10.1200/JCO.2002.01.153. [DOI] [PubMed] [Google Scholar]
- 21.Weinfurt KP, Trucco SM, Willke RJ, Schulman KA. Measuring agreement between patient and proxy responses to multidimensional health-related quality-of-life measures in clinical trials. An application of psychometric profile analysis. J Clin Epidemiol. 2002;55:608–18. doi: 10.1016/s0895-4356(02)00392-x. [DOI] [PubMed] [Google Scholar]
- 22.Williams LS, Weinberger M, Harris LE, Clark DO, Biller J. Development of a stroke-specific quality of life scale. Stroke. 1999;30:1362–9. doi: 10.1161/01.str.30.7.1362. [DOI] [PubMed] [Google Scholar]
- 23.National Institute of Neurological Disorders and Stroke. Neuro-QOL: Quality of Life in Neurological Disorders website. [cited 2014 March 14]. Available from: http://www.neuroqol.org/Pages/default.aspx.
- 24.Varni JW, Thissen D, Stucky BD, Liu Y, Magnus B, He J, et al. Item-level informant discrepancies between children and their parents on the PROMIS pediatric scales. Qual Life Res. 2015 doi: 10.1007/s11136-014-0914-2. Epub 2015/01/07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Burton LC, Zdaniuk B, Schulz R, Jackson S, Hirsch C. Transitions in spousal caregiving. Gerontologist. 2003;43:230–41. doi: 10.1093/geront/43.2.230. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.