Abstract
Interleukin-27 (IL-27) is a new member of the IL-12 family. It is produced by activated antigen-presenting cells and plays an important role in the regulation of CD4+ T cell differentiation and immune response. IL-27 activates multiple signaling cascades, including the JAK-STAT and p38 MAPK pathways. Several studies have revealed that IL-27 promotes the differentiation of Th1 and Tr1, but inhibits Th2, Th17, and Treg cells. However, a few studies have shown an opposite effect on certain T cell subsets, such as Treg. IL-27 displays both pro- and anti- inflammatory activities in different autoimmune diseases. Here, we have discussed the role of IL-27 in rheumatoid arthritis, multiple sclerosis, colitis, lupus, psoriasis, type 1 diabetes, and uveitis. Most of this information is derived from experimental models of these autoimmune diseases. The mechanistic basis of the dual role of IL-27 in inflammation and autoimmunity is still not fully defined. In general, the pro-/ anti- inflammatory activity of IL-27 is influenced by the underlying immune effector pathways, the phase of the disease, the presence or absence of counter-regulatory cytokines/T cell subsets, and the tissue/cell type under study. Despite a spectrum of outcomes in various autoimmune diseases, mostly anti-inflammatory and immunomodulatory effects of IL-27 have been observed in this category of diseases. Accordingly, IL-27 represents a novel, promising target/agent for the treatment of autoimmune diseases.
Keywords: Interleukin-27, CD4+ T cell differentiation, Autoimmunity, Arthritis, Multiple sclerosis, Colitis, Lupus, Psoriasis, Diabetes, Uveitis
1. Introduction
Cytokines play a pivotal role in the pathogenesis of various autoimmune and other immune-mediated diseases. Cytokines serve as mediators of cellular and humoral immune responses. Interleukin-27 (IL-27) is a relatively new cytokine, which is a member of the IL-12 family (Figure 1) [1]. IL-27 was first identified in the year 2002 by Pflanz and colleagues [1]. IL-27 is mainly produced by antigen presenting cells (APCs), including dendritic cells (DCs), monocytes, and macrophages following stimulation by microbial products or other immune stimuli. This cytokine binds to, and signals through, the IL-27 receptor (IL-27R) (Figure 1) expressed on dendritic cells, monocytes, macrophages, T and B lymphocytes, natural killer cells, mast cells, and endothelial cells. IL-27 regulates both adaptive and innate immune responses. In regard to the T cells, IL-27 modulates their differentiation and response. The CD4+ T cells carry out multiple functions, including the activation of cells of the innate immune system (e.g., macrophages), B-lymphocytes, cytotoxic T lymphocytes (CTL), and certain non-immune cells, as well as regulation of immune responses. Under specific activation conditions, including the cytokine milieu, CD4+ T cells can differentiate into different T helper (Th) or T regulatory cell subsets. The Th cell subsets include Th1, Th2, Th17 and T follicular helper (Tfh) cells, whereas the regulatory T cell subsets include type 1 regulatory (Tr1) cells and forkhead box P3 (Foxp3)-expressing CD4+CD25+ T regulatory cells (Treg) [2-4] (Figure 2). Many of the functional attributes of IL-27 were initially unraveled through studies based on infectious pathogens, including parasitic protozoa, bacteria, and nematodes [5-13]. In this article, we elaborate on the structure and signaling aspects of IL-27 (Figure 1, 3), the role of this cytokine on T cell subset differentiation and T/B cell response, the pro- versus anti-inflammatory activity of IL-27 in different autoimmune diseases, and its therapeutic potential in controlling these diseases.
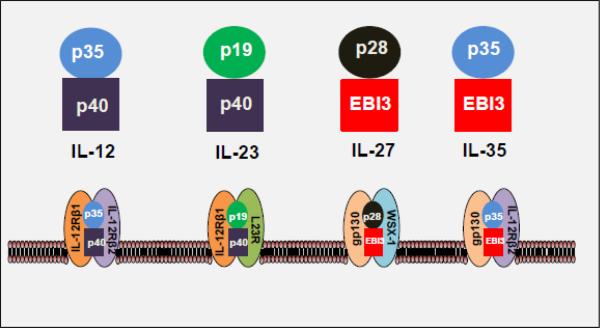
Figure 1. A schematic representation of IL-12 cytokine family members and their receptors.
These cytokines are heterodimeric complexes, and they, as well as their respective receptors, share subunits among them. IL-27 consists of p28 and Epstein-Barr virus-induced gene 3 (EBI3), and its receptor is composed of gp130 and WSX1. In comparison, IL-12 consists of p35 and p40, and it binds to the IL-12 receptor comprised of IL-12Rβ1 and IL-12Rβ2. IL-23 is composed of p19 and p40, and its receptor is formed by the association of IL-23R and IL-12Rβ1. IL-35 contains p35 and EBI3 subunits, and its receptor consists of gp130 and IL-12Rβ2.
Figure 2. Differential effect of IL-27 on T cell subsets and antibody response.
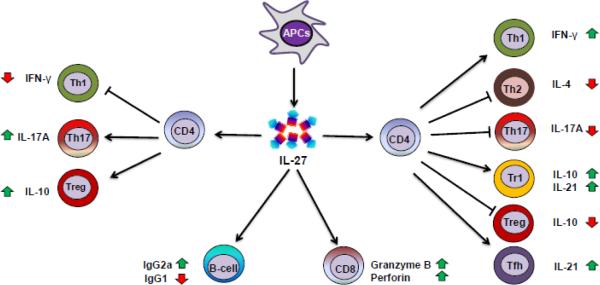
Antigen-presenting cells (APCs) including dendritic cells (DCs) and macrophages produce IL-27 on exposure to microbial products or other immune stimuli. IL-27 generally promotes the differentiation of Th1 and IL-10-producing type 1 regulatory (Tr1) cells, but inhibits Th2, Th17 cells, and Treg cell differentiation. However, contrary effects of IL-27 on Th1 (decrease), Treg (increase), and Th17 (increase) cells have also been reported. Furthermore, IL-27 promotes cytotoxic T lymphocyte (CTL) response involving CD8+ T cells, and induces IgG1 but suppresses IgG2a production (in mice).
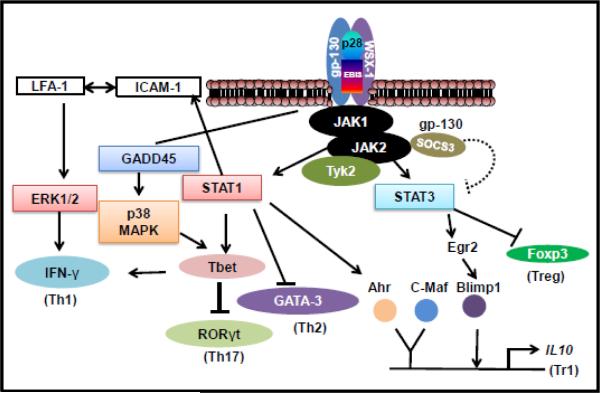
Figure 3. IL-27 signaling pathways.
The schematic pathways shown in the figure are derived from the findings of multiple studies described in the main text. The binding of IL-27 to its receptor leads to activation of JAK/STAT and P38/MAPK pathways. IL-27 can induce Th1 and Tr1 differentiation through multiple signaling pathways. Activation of STAT1 promotes Th1 differentiation either through activating T-bet or via upregulating membrane ICAM-1, which interacts with LFA-1 and signals via ERK. Another pathway of T-bet activation is through GADD45, leading to p38/MAPK activation. All these pathways of T-bet activation lead to IFN-γ secretion. SOCS3 represents one of the negative inhibitory loops for regulation of Th1 response. IL-27 signaling induces Tr1 differentiation by activating IL-10-related transcription factors Ahr and c-Maf. An additional pathway operates via Egr2 and Blimp-1. In addition, IL-27 can inhibit Foxp3 and suppress Treg generation.
2. Structure of IL-27 and its receptor
IL-27 shares structural similarities with IL-12, IL-23 and IL-35 (Figure 1). IL-27 consists of two subunits, an IL-12 p40-related protein, EBI3 (Epstein-Barr virus induced gene 3) (also known as IL-27β or IL-27B) and an IL-12 p35 (p35)-related polypeptide, p28 (also known as IL-27α, IL-27A, IL-27p28 or IL-30) [1]. EBI3 is secreted as a 34 kDa glycoprotein, whereas the p28 subunit is a 24.5 kDa polypeptide [1, 14]. The two subunits bind non-covalently to form IL-27. EBI3 also associates with IL-12p35 to form IL-35, whereas IL-12p35 and IL-12p40 form IL-12. Similarly, IL-12p40 and IL-12p35-related protein p19 constitute IL-23 (Figure 1).
IL-27R is a heterodimer composed of the orphan cytokine receptor WSX-1 (also known as T-cell cytokine receptor (TCCR) or IL-27Rα) and a signal-transducing chain, glycoprotein 130 (gp130) (also known as interleukin 6 signal transducer (IL6ST), IL-6 receptor beta, or CD130) [15]. IL-27Rα is unique to IL-27R, whereas the gp130 subunit of IL-27R is shared with receptors for IL-6 and IL-35, among other cytokines. Both the receptor subunits are required for IL-27 signaling.
3. IL-27 Signaling
IL-27 signals through the Janus kinase (JAK)/ signal transducer and activator of transcription (STAT) and p38 mitogen-activated protein kinase (MAPK) pathways (Figure 3). The binding of IL-27 to its receptor, IL-27Rα, activates cytoplasmic domain Box 1 motif-binding sites for JAK1/2, which subsequently activates STAT1 and STAT3 pathways [16, 17]. Primarily STAT1/STAT3 pathway is believed to be involved in Th1/Tr1 differentiation, respectively, although both STATs might participate in certain events in these pathways [16-18]. The gp130 subunit of the IL-27 receptor is utilized in other receptors as well, and therefore, it has been difficult to comprehend how different cytokines such as IL-6 and IL-27 can signal via a shared receptor but yield different outcomes. In this context, it has recently been shown that asymmetric action of STAT1/STAT3 can influence the repertoire of transcriptional products as well as determine cytokine specificity [19].
For Th1 differentiation, STAT1 signaling leads to interferon-γ (IFN-γ) production by activating T-box expressed in T cells (T-bet), also known as T-box transcription factor (TBX21) (Figure 3). Alternatively, STAT1 signaling increases the surface expression and interaction of intercellular adhesion molecule 1 (ICAM-1)/ lymphocyte function-associated antigen 1 (LFA-1) [20]. This interaction induces extracellular signal-regulated kinase (ERK) activation. However, JAK/STAT-independent, p38/MAPK signaling by IL-27 has also been reported, which ultimately leads to IFN-γ secretion by activating T-bet [17, 20]. In this case, IL-27 signaling induces GADD45γ (growth arrest and DNA damage-inducible 45 gamma), which then activates p38 MAPK, leading to T-bet activation and IFN-γ production [17, 20].
Tr1 differentiation involves cooperation between aryl hydrocarbon receptor (Ahr) and STAT3-induced c-musculoaponeurotic fibrosarcoma oncogene (c-Maf), which leads to IL-10 induction in Tr1 cells (Figure 3) [21]. In an additional pathway, STAT3 upregulates early growth response gene-2 (Egr-2), which is a transcriptional regulator for B lymphocyte-induced maturation protein-1 (Blimp-1), and it leads to IL-10 induction in these cells [18, 22]. IL-27 can induce Tr1, but inhibit the generation of another type of regulatory T cell, the Foxp3-expressing Treg cell [23]. This effect of IL-27 involves STAT3-mediated inhibition of Foxp3, the key transcription factor for Treg (Figure 3).
Besides Th1 differentiation, IL-27-induced STAT1 signaling has been shown to play a role in inhibition of Th17 and activation of natural killer (NK) cells. In the case of Th17, STAT1 is involved in IL-27-induced upregulation of programmed cell death ligand 1 (PD-L1), which in turn inhibits Th17 in-trans via PD-1/PD-L1 interaction [24]. In the case of NK cells, IL-27-induced STAT1 signaling leads to increased IFN-γ secretion and thereby, enhanced effector function of these cells [25]. Furthermore, IL-27 acts on osteoclast precursors and suppresses receptor activator of nuclear factor kappa B ligand (RANKL)-mediated osteoclastogenesis through STAT1-dependent inhibition of c-Fos and nuclear factor of activated T cells c1 (NFATc1) [26].
4. Influence of IL-27 on T cells and B cells
Taking together the results of several studies described below, a general pattern of IL-27-induced modulation of CD4+ T differentiation has emerged. IL-27 promotes the differentiation of Th1 and Tr1 cells, but inhibits the differentiation of Th2, Th17, and Treg cells (Figure 2). However, as the local milieu of cytokines and other mediators affecting T cell activation and differentiation can differ in different pathophysiological conditions, including inflammation and autoimmunity, it is conceivable that the above pattern of IL-27 action can deviate for one or more T cell subset(s). For example, under certain conditions, IL-27 can inhibit Th1, but promote Treg generation.
4.1. IL-27 promotes Th1 differentiation
Th1 cells produce IFN-γ and play a pathogenic role in many autoimmune and inflammatory diseases. Naïve Th cells, in the presence of IFN-γ and IL-12, express transcription factor T-bet and differentiate into Th1 cells [2, 27]. IL-27 induces STAT-1 phosphorylation and increases T-bet expression [28]. Furthermore, IL-27 also increases both the surface expression of IL-12Rβ2 and IL-12-dependent IFN-γ production [28, 29]. IL-27 also facilitates Th1 differentiation by inhibiting basal levels of the transcription factor GATA-binding protein-3 (GATA-3), which otherwise serves to inhibit Th1 development by suppressing STAT4 [29]. A T-bet-independent pathway has also been described, which involves IL-27-mediated enhanced surface expression and interaction of ICAM/LFA-1; downstream of this interaction lies ERK 1/2, which leads to the differentiation of T cells into Th1 cells [20]. Furthermore, as discussed below, IL-27 and transforming growth factor β (TGF-β) have some cross-regulatory effects such that TGF-β can inhibit the effect of IL-27 on Th1 differentiation, whereas IL-27 can inhibit Treg generation [23].
IL-27 also has the ability to regulate excessive Th1 response via increased production of IL-10 by Th1 cells and inhibition of IL-2 production. IL-27 increases IL-10 production by IFN-γ-producing CD4 T cells [30]. This effect is STAT1-dependent, but IL-12-independent, and is not because of differentiation of T cells into Th2 or Treg cells. Similarly, IL-2 plays pivotal roles in proliferation and survival of Th1 cells, and IL-27 suppresses CD28-mediated IL-2 production [31]. This effect is STAT1-dependent and mediated via increased expression of suppressor of cytokine signaling 3 (SOCS3), forming an inhibitory loop to regulate excessive Th1 response [31].
4.2. IL-27 suppresses Th2 response
Th2 cells produce IL-4 and play a role in immune response to large extracellular pathogens. In the presence of IL-2 and IL-4, naïve Th cells express the transcription factor GATA-3 and differentiate into Th2 cells [32, 33]. IL-27 suppresses Th2 differentiation by inhibiting GATA-3, and this effect is STAT1 dependent [29]. It also inhibits T cell proliferation and production of Th2 cytokines [34]. Furthermore, mast cells have been shown to express IL-27 receptor [15], and IL-27 can regulate mast cell functions and thereby influence Th2-type immune response [34].
4.3. IL-27-induced inhibition of Th17
Th17 cells produce pro-inflammatory cytokines IL-17, IL-22, and granulocyte macrophage colony-stimulating factor (GM-CSF), which play a role in the pathogenesis of several autoimmune disorders [35, 36]. Naïve Th cells (murine) in the presence of TGF-β and IL-6 (or IL-21/IL-1β) express retinoic acid-related orphan receptor gamma t (RORγt) and differentiate into Th17 cells. IL-27 suppresses the expression of RORα and RORγ and inhibits the differentiation of Th17 cells [37-40]. Thus, in regard to Th17 differentiation, IL-27 acts in a manner antagonistic to IL-6. IL-27 also inhibits the production of IL-17 from Th17 cells, and this effect is STAT1- but not T-bet-dependent and is independent of SOCS3, which antagonizes gp130 signaling [41, 42]. In addition, IL-27 suppresses the production of IL-22 and GM-CSF, which mediate some of the effectors functions of Th17 cells [40, 43]. While multiple reports support the inhibitory effect of IL-27 on Th17 differentiation, there is disparity in regard to the effect of IL-27 on established or committed Th17 [22, 38, 41].
4.4. T follicular helper (Tfh) cell activation by IL-27
Tfh cells play a role in the development of antigen-specific B-cell immunity [44]. Tfh cells help B cells and generate antibody-producing plasma cells and memory B cells. In the presence of IL-6 and IL-21, activated CD4+ cells express the transcription factor B-cell lymphoma 6 protein (Bcl-6) and differentiation into Tfh cells [45]. Inducible T-cell co-stimulator (ICOS)-induced c-Maf also is involved in Tfh generation [46]. IL-27 acts directly on Tfh cells and results in the production of IL-21, which mediates some of Tfh functions, but it may not influence Tfh differentiation per se [47]. However, in another study, signaling via DC-SIGN (dendritic cell-specific intracellular adhesion molecule-3-grabbing non-integrin) has been implicated in Tfh differentiation [48]. Thus, the precise role of IL-27 in the differentiation of Tfh is not yet fully clear [47, 48].
4.5. IL-27 induces Type 1 regulatory cell (Tr1) differentiation
Tr1 cells are an important subset of CD4+T cells, and they control excessive inflammatory responses [49-51]. A typical phenotype of this T cell subset is Foxp3− IFN-γ+ IL-10+ T cell. In the presence of IL-10, naïve Th cells differentiate into Tr1 cells. IL-27 induces the expression of IL-21, which acts as an autocrine growth factor for Tr1 cells [49]. IL-27 also induces the transcription factor c-Maf, which is vital for Tr1 differentiation. Under the influence of IL-27, AhR and c-Maf activate the transcription of IL-10 and IL-21 [21, 49, 52]. As a parallel pathway independent of Ahr and c-Maf, IL-27 induces Egr-2 and Blimp-1, which play an important role in IL-10 production [18, 21]. Thus, both STAT1 and STAT3 are involved in IL-10 production (Figure 3). In contrast to the positive regulators, metallothioneins can inhibit Tr1 generation by inhibiting phosphorylation of STAT1 and STAT3 [53]. Besides increasing the production of IL-10 by Tr1, IL-27 can induce IL-10 production in other activated T cell subsets, including Th1, Th2, Th17, and Treg [13, 50, 54].
4.6. Effect of IL-27 on regulatory T cells (Treg)
Naïve T cells in the presence of TGF-β express transcription factor Foxp3 and differentiate into T regulatory (Treg) cells [55]. It has been reported that IL-27 suppresses the development of Treg [23, 56, 57]. It was shown that this effect is STAT1-independent [56], and suggested to be mediated in part via STAT3 [23]. The outcome of IL-27 action is the reduced expression of Foxp3, CD25, and cytotoxic T-lymphocyte-associated protein 4 (CTLA-4) [23]. Although IL-2 is required for Treg development and IL-27 inhibits IL-2 production, the inhibitory effect of IL-27 on Treg generation has been shown to be IL-2-independent [56]. However, there is contrary evidence from another study showing that IL-27 does not inhibit Treg generation, but rather endows them with IL-10 production and more effective suppression besides facilitating their survival and proliferation [58]. These effects are attributable to IL-27-induced T-bet and CXCR3 coupled with increased IL-10 production in Treg, imparting these cells with the ability to control pathogenic Th1 effector responses at local sites [13, 58]. These effects of IL-27 are STAT1-mediated. In addition, IL-27 promotes the survival of T cells, including Treg, and this in turn enhances the inhibitory effect of Treg on pathogenic T cells [59]. IL-27 can also enhance the suppressive effect of Treg by increasing the expression of CTLA-4 and PD-1 [60], or lymphocyte-activation gene 3 (Lag3) (on human Treg) [61] on their cell surface. Thus, IL-27 displays a dual effect on Treg.
4.7. Impact of IL-27 on CD8+ T cells
IL-27 induces the expression of IL-12Rβ2 and secretion of IFN-γ in CD8+ T cells, and it promotes CTL responses by inducing the expression of granzyme B and perforin in CD8+ T cells [62, 63]. The generation of CTL response by IL-27 is dependent on STAT1 activation, and both T-bet-dependent and T-bet-independent pathways have been reported [63]. The findings of IL-27-induced CTL activation is relevant in view of the observed effects of IL-27 on the induction of antigen-specific CTL response as well as the enhancement of anti-tumor immunity via CD8 T cells [63]. This effect on CD8 is also of interest in regard to vaccinations [13].
4.8. Influence of IL-27 on B cell response
IL-27 can induce T-bet expression and regulate immunoglobulin (Ig) class switching in B cells. IL-27 induces IgG2a production, but inhibits IgG1 production by mouse B cells [64]. However, IL-27 enhances IgG1 production by human B cells [65]. In the case of mouse antibodies, IgG2a but not IgG1 class switching is STAT-1-dependent, but IFN-γ-independent [64]. IL-27 also promotes B-cell expansion indirectly by inducing CD4 + T cells to produce IL-21. IL-21 then induces Blimp-1 and Bcl-6, which are critical for plasma cell differentiation [44]. Thus, IL-27 influences class switching and plasma cell differentiation. These findings are relevant for immune deviation for the control of allergic diseases as well as for antibody-mediated autoimmune diseases. However, at present, it is not fully clear if IL-27 has any direct effect on B cells per se [13].
5. Involvement of IL-27 in the pathogenesis of autoimmune diseases
IL-27 has been shown to play an important role in a variety of autoimmune diseases. General mechanisms of T cell differentiation and modulation of immune response by IL-27 have been discussed above. IL-27 activates multiple signaling cascades and possesses both pro- and anti- inflammatory activities. In general, the pro- or anti- inflammatory roles of IL-27 depend on the type of the disease in humans, the animal model studied, and the tissue/cell type under study. However, because of obvious limitations in determining the precise role of IL-27 from the serum or urine levels of this cytokine in patients with autoimmunity, we have mostly relied on results from animal models of autoimmunity in elaborating its likely role in the disease processes. These results in turn would help plan and guide additional studies to investigate the role of IL-27 in patients with autoimmunity.
5.1. Rheumatoid arthritis (RA)
RA is a systemic autoimmune disease characterized by synovial inflammation that is initiated by the infiltration of pathogenic cells into the joints, leading eventually to cartilage and bone damage. The pro-inflammatory cytokines such as tumor necrosis factor α (TNFα), IL-1, IL-6 and IL-17 promote the migration of mononuclear cells into the joints, the differentiation of pathogenic Th17 cells, the activation of osteoclasts, the formation of new blood vessels (angiogenesis), and the production of additional mediators of bone/cartilage damage (Figure 4) [66, 67]. Various animal models have been used to study the pathogenesis of RA as well as to test new therapeutic interventions [68]. Two of the commonly used models are collagen-induced arthritis (CIA) in mice and adjuvant-induced arthritis (AA) in rats. IL-27 has been shown to be expressed in the synovial tissue of RA joints and it is present in the RA synovial fluid [69-71]. Studies in RA and animal models of this disease have revealed both anti- and pro-inflammatory activities of IL-27.
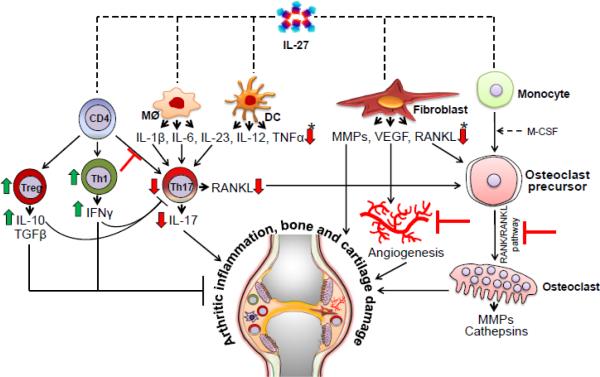
Figure 4. Modulation of autoimmune arthritis by IL-27.
The major mechanisms of IL-27-induced suppression of arthritic inflammation shown in the figure are derived from multiple studies described in the main text. IL-27 acts on APCs and decreases the production of pro-inflammatory cytokines- IL-1β, IL-6 and IL-23. IL-27 can directly inhibit Th17 differentiation as well as indirectly inhibit Th17 response by increasing Th1 (IFN-γ) response. Furthermore, IL-27 can increase Treg and IL-10 production, and thereby alter Th17/Treg ratio in favor of immune regulation. IL-27 acts on fibroblasts and other cell types and inhibits the production of MMPs and VEGF, leading to reduced angiogenesis. In addition, IL-27 decreases the production of RANKL from T cells and other cells, and inhibits RANKL/RANK pathway of osteoclast generation. The outcome of these actions of IL-27 is reduced arthritic inflammation. (Induction is shown as a green upward arrow, while inhibition or blockade is shown as a ‘T’. A direct influence is shown as straight line arrow, whereas an indirect influence is shown as a dashed line arrow. APCs, antigen presenting cells; MMPs, matrix metalloproteases; RANK, receptor activator for nuclear factor κB; RANKL, RANK ligand; and VEGF, vascular endothelial growth factor. (*) indicates similar direction of change in all cytokines or bone mediators indicated therein.)
5.1.1. Anti-inflammatory effects of IL-27 in arthritis
The level of IL-27 in the synovial fluid of RA patients was found to be higher than that of osteoarthritis (OA) patients, and IL-27 levels showed a positive correlation with IFN-γ, but a negative correlation with IL-17 levels in RA synovial fluid [71]. The CD14+ mononuclear cells (MNCs) and not fibroblast-like synoviocytes (FLS) were shown to be the major producers of IL-27 in RA joints. Apparently, these MNCs infiltrate into inflamed synovium of the arthritic joints and mediate their anti-inflammatory effects [71]. These effects include inhibition of IL-6 activity; increase in IFN-γ, which in turn inhibits IL-17 response; and reduction of chemokine CCL20, which is chemotactic for CCR6-expresing Th17 cells for their migration into the joints. IL-27 can also suppress osteoclastogenesis as tested in human osteoclasts [69]. This effect was associated with inhibition of NFATc1 induction, RANK expression, and RANKL-induced activation of nuclear factor-kB (NF-kB) and MAPK pathways. The suppressive effect of IL-27 on osteoclastogenesis was much more marked on human osteoclasts than that on murine osteoclasts, which express relatively lower levels of receptor for IL-27. Thus, IL-27 can limit bone erosion in arthritis by inhibiting osteoclastogenesis. Another study revealed that IL-27 suppresses osteoclast differentiation by reducing the expression of RANKL through STAT1-dependent inhibition of c-Fos [26]. IL-27 has also been shown to reduce RANKL expression in T cells [72], further supporting the anti-arthritic activity of this cytokine. IL-27 can also inhibit osteoclastogenesis via reduction of IFN-γ [73].
Studies in the CIA model of RA have unraveled the disease-protective effects of IL-27. For example, the local expression of IL-27 using intra-articular injection of an adenoviral vector in mice with CIA showed reduction in the clinical and histopathological features of arthritis [74]. The level of IL-17 in the serum and joint of these mice was found to be reduced. Accordingly, there was reduction in monocyte recruitment and angiogenesis; both these processes are otherwise facilitated by IL-17 during the course of arthritis. Similarly, IL-27 treatment reduced Th17 but increased Foxp3-expressing Treg, thus altering the Th17/Treg balance towards disease regression in mice with CIA [60]. Furthermore, IL-27 enhanced the suppressive activity of Treg in vivo and in vitro. The IL-27-induced inhibition of Th17 involved suppression of STAT3 phosphorylation and inhibition of RORγt. On the other hand, IL-27 enhanced Foxp3 expression and IL-10 production. Furthermore, the effect of IL-27 in modulating the Th17/Treg balance was also observed in PBMC from RA patients [60]. In another study, treatment with IL-27 reduced the severity of arthritis in mice with CIA. This correlated with reduction in cellular infiltration into the joints, in synovial hyperplasia and bone erosion, in serum IL-6 and collagen-specific IgG2a, and in the production of IFN-γ and IL-17 by peripheral lymphoid cells [70].
Our study in the rat AA model revealed the anti-arthritic activity of IL-27 in Lewis rats [39]. IL-27 controlled arthritic inflammation in these rats by inhibition of Th17 responses, angiogenesis, cell survival, apoptosis, and tissue damage. Th17 cells play an important role in RA pathogenesis, including osteoclastogenesis [75], and we showed that IL-27 inhibits IL-17 response in arthritic rats treated with IL-27. This further explains the protective effect of IL-27 against tissue damage in arthritic joints. The inhibition of Th17 response was attributable in part to the blockade of STAT3 phosphorylation and suppression of RORγt. Furthermore, IL-27 treatment inhibited MMP-9 activity, VEGF secretion, and Akt phosphorylation. In addition, IFN-γ induced the production of IL-27, and both cytokines inhibited Th17 response and arthritis, demonstrating a cooperative interplay between the two cytokines.
5.1.2 Pro-inflammatory effects of IL-27 in arthritis
In contrast to the findings described above, some of the earlier studies have suggested a pro-inflammatory role for IL-27 in RA. IL-27 is found to be elevated in the plasma of RA patients compared with controls [76]. Furthermore, IL-27 was shown to induce ICAM-1, vascular cell adhesion molecule (VCAM)-1, pro-inflammatory cytokine IL-6, chemokines (CCL2, CXCL9, CXCL10), and matrix metalloproteinase-1 (MMP-1) in RA-FLS [76]. These effects of IL-27 were mediated via signaling involving STAT1, PI3k-Akt, c-JNK and JAK. Furthermore, IL-27 showed synergy with TNF-α or IL-1β in its pro-inflammatory effects [76]. In addition, a single nucleotide polymorphism (−924A/G) in the IL-27 gene has been reported to be associated with RA susceptibility [77].
In a study in the rat AA model, neutralization of p28 subunit of IL-27 by autoantibodies was shown to suppress ongoing AA in Lewis rats [78]. Such antibodies could be generated either naturally during the course of AA or experimentally via vaccination with DNA encoding p28. The protective effect of this vaccination approach was attributable in part to suppression of pro-inflammatory cytokine production and inhibition of Th1 polarization, as well as inhibition of antigen-specific effector/memory Th1 cells [78]. In proteoglycan-induced arthritis (PGIA), a Th1-mediated model of RA, the development of the disease was inhibited in IL-27R (TCCR)−/− mice. These results were attributed to a reduction in IFN-γ-producing Th1 cells, but not IL-4- or IL-17-expressing T cells in these mice [79]. Thus, IL-27 contributed to disease development in this model by inducing Th1 cells and increasing IFN-γ production.
5.2. Multiple sclerosis (MS)
MS is an autoimmune disease that affects the brain and the spinal cord, and it is characterized by neuroinflammation as well as neurodegeneration caused by the depletion of myelin in the central nervous system (CNS) [80, 81]. The pathogenesis of MS involves Th1 and Th17 responses to myelin antigens [80, 81]. The level of IL-27 in the plasma/serum from MS patients was found to be significantly lower than that of healthy controls [82-84], and IL-27 level showed an inverse correlation with the level of plasma IL-17 as well as the frequency of Th17 cells in the blood [82]. The patients in these studies included newly diagnosed or progressive MS patients.
Experimental autoimmune encephalomyelitis (EAE) is a well-established animal model of human MS [85]. Although IL-27 has been shown to possess both pro- and anti-inflammatory properties in various immune-mediated conditions, mostly anti-inflammatory activity of IL-27 has been observed in different studies in MS/EAE. For example, IL-27 receptor (WSX-1)-deficient mice were found to be highly susceptible to EAE compared to wild type mice [42]. The increased severity of EAE in these mice was attributable to increased production and activity of Th17 cells in the absence of normal IL-27 signaling. This inference was supported by the finding that IL-27 inhibited the generation of Th17 in vitro, and this effect was STAT1-dependent [42]. Similarly, the level of expression of the IL-27 receptor (WSX-1) and IL-27 subunits in the CNS of mice with relapsing-remitting EAE was found to be the highest at the effector phase of the disease [86]. Furthermore, the disease-regulating role of IL-27 in this EAE model was shown by inhibiting passive EAE induction using potentially encephalitogenic T cells exposed to IL-27 in vitro prior to their adoptive transfer, as well as by suppressing active EAE induction by s.c. injection of IL-27 [86]. However, in one study, IL-27 failed to inhibit IL-17 production from Th17 in vitro and in vivo, and consequently failed to suppress EAE development, showing the inhibitory effect of IL-27 on T cell differentiation but not on committed Th17 cells [38]. In another study, overexpression of the p28 subunit of IL-27 inhibited the initiation and progression of EAE, thus showing the immunoregulatory effect on IL-27 on CNS autoimmunity [87]. This outcome involved reduced generation and activity of both Th1 and Th17 cells.
The results of a study based on multiwalled carbon nanotube (MWCNT), which activate APCs to produce IL-27, support the role of this cytokine in suppressing EAE by inhibiting Th17 response [88]. Wild type APCs could activate antigen-induced T cells into Th1 cells, which were pathogenic as tested in the passive EAE induction model. However, APCs that had been exposed to MWCNT, and thereby produced IL-27, failed to prime an effective Th17 population and these T cells were ineffective in inducing EAE compared to controls. Neutralization of IL-27 in APCs exposed to MWCNT further supported the role of IL-27 in the above-mentioned regulation of EAE.
IFN-β is being used for the treatment of relapsing remitting MS (RRMS) patients [89]. Studies in EAE and MS patients have revealed that IL-27 is involved in mediating the disease-modulating effects of IFN-β. It has been shown that IFN-β suppresses IL-1 and IL-23 production by activated human dendritic cells as well as the ability of these DCs to promote Th17 generation, but it enhances IL-10 production by DCs [90]. These effects of IFN-β are mediated in part via IL-27, as assessed from experiments based on IL-27 neutralization or on IL-27 receptor deficient mice using the EAE model [90]. In regard to MS, the responsiveness of RRMS patients to IFN-β positively correlates with the level of IL-27, such that poor responders to IFN-β have lower level of IL-27 than that of good responders [90]. IFN-β and IL-27 share similar properties in regard to the induction of IL-10, the inhibition of Th17 response, and the suppression of EAE. Furthermore, EAE pathogenesis may involve predominantly either Th1 or Th17 responses. However, IFN-β-mediated suppression of EAE is not dependent on IL-27 signaling [91]. Furthermore, IL-27-mediated control of EAE requires IL-10 in the case of Th1-induced EAE, but not Th17-induced EAE [91].
Additional anti-inflammatory mechanisms of IL-27 in EAE include suppression of the generation of pathogenic effector T cells [92] as well as upregulation of CD39 in DCs, which in turn inhibit the NLRP3 inflammasome activation [92]. In addition, IL-27 can upregulate PD-L1 on naïve T cells, which then can inhibit Th17 differentiation in trans through PD-1-PD-L1 interaction [24]. This pathways can inhibit the activity of encephalitogenic T cells and suppress EAE [24].
Above studies demonstrate the immunoregulatory activity of IL-27. However, IL-27 has also been shown to be involved in disease progression in EAE. Using the approach of DNA vaccination to produce autoantibodies to p28 subunit of IL-27, it was shown that in vivo neutralization of p28 inhibited ongoing EAE in mice [93]. This outcome was attributable in part to inhibition of antigen-specific Th1 cells and production of pro-inflammatory cytokines. The effect on T cells involved inhibition of polarization of naïve T cells to Th1 as well as suppression of antigen-specific activated/memory Th1 cells [93].
5.3. Colitis
Inflammatory bowel disease (IBD) can manifest in two forms, Crohn's disease and ulcerative colitis [94, 95]. Autoimmune effector mechanisms have been implicated in the pathogenesis of IBD. IL-27p28 and EBI3 transcripts were found to be significantly increased in patients with Crohn's disease, but not in ulcerative colitis [96]. Similarly, the expression of these two subunits was also demonstrated in granulomas of Crohn's disease [97]. These findings are of relevance, considering the role of IL-27 in modulation of Th1 and Th17, which are involved in the pathogenesis of Crohn's disease [94, 95]. Colitis can be induced in mice by injection of chemicals such as 2,4,6-trinitrobenzene sulfonic acid (TNBS) [98] or dextran sodium sulphate (DSS) [99], and by adoptive transfer of CD4+CD45RBhi T cells into Rag−/− mice [100]. Furthermore, mice deficient in IL-10 develop spontaneous colitis [12].
5.3.1. IL-27 induces protection against colitis
Subcutaneous injection of a single-chain recombinant human IL-27 attenuated TNBS-induced colitis in mice through suppression of Th17 cell differentiation [98]. This was evident from the gross and histological features of colitis, as well as from inhibition of IL-17 and other pro-inflammatory cytokines couple with reduced frequency of Th17 cells. IL-27 treatment was effective in containing the ongoing colitis as well. Similarly, oral delivery of genetically engineered, IL-27-expressing Lactococcus lactis was shown to suppress enterocolitis induced by T cell transfer and its lethal consequences [100]. In this model, colitis is induced by the adoptive transfer of CD4+CD45RBhi T cells into Rag−/− mice. The beneficial effect of mucosal delivery of IL-27 involved the production of IL-10 by the T cells, and reduction of pro-inflammatory cytokines and Th17 frequency in the gut-associated lymphoid tissue [100]. The delivery of IL-27 via L. Lactis was more effective than either IL-10 administered in a similar fashion or soluble IL-27. Oral delivery of IL-27 was also effective in another model of colitis, DSS-induced colitis. In another study, mice deficient in IL-27 receptor subunit EBI3 showed differential susceptibility to colitis in two different models [101]. IL-27R−/− mice have decreased numbers of invariant natural killer T cells (iNKT), and upon stimulation with their ligand αGalCer in vitro, produce decreased IL-4 and IFN-γ. These mice were protected against oxazolone-induced colitis, whose development requires IL-4 produced by iNKT cells, but displayed typical development of TNBS-induced colitis, whose pathogenesis is dependent on Th1 response. IL-27 also contributes to the protective effect of Treg by enhancing their survival [59].
5.3.2. IL-27 promotes inflammation in colitis
IL-10-deficient mice spontaneously develop intestinal inflammation [12]. Mice deficient in both IL-10 and IL-27 receptor (WSX-1) showed delayed onset of colitis compared with mice deficient in IL-10 only [12]. A pro-inflammatory role of IL-27 has been reported in a colitis model in which the disease is induced by CD4 T cell transfer. IL-27Rα+/+ T cell receptor (TCR)β−/− recipients developed severe colitis, whereas IL-27Rα−/− TCRβ−/− mice were protected against this disease [102]. Gut inflammation in the former recipients involved IL-27-induced enhanced production of IL-6 and IL-1β by APCs and consequently increased Th17 differentiation, which contribute to the development of colitis. In another study, mice deficient in IL-27R (WSX-1) showed markedly reduced severity of DSS-induced colitis compared to controls [99]. Furthermore, protection against colitis in these mice was associated with reduced production of pro-inflammatory cytokines IFN-γ, IL-6, and TNFα by immune cells infiltrating the lamina propria. IL-27 has also been shown to be required for fully manifesting the pathogenicity of T cells in an adoptive transfer model of colitis, and this effect was attributable to the ability of this cytokine to increase the survival of T cells [59].
5.4 Systemic lupus erythematosus (SLE) (Lupus)
Lupus is a systemic autoimmune disease involving the formation of autoantibodies to double-stranded deoxyribonucleic acid (dsDNA) and other self antigens, the generation of immune complexes, and tissue damage in the kidneys and other organs [103]. Type I IFN, IL-17, and other cytokines have been invoked in the pathogenesis of this disease [103, 104]. The testing of serum/plasma or urine levels of IL-27 revealed disparate findings in different studies: reduced IL-27 levels in SLE compared to healthy controls [105] versus increased levels in SLE compared with controls [106]. In the former study, no correlation was found between IL-27 levels and disease activity. However, in the latter study reporting increased IL-27, the level of that cytokine showed correlation with IL-6 and anti-dsDNA antibodies [106]. Furthermore, glucocorticoid treatment led to reduction in IL-27 levels in that study. Increased serum and urine levels of IL-27 were also observed in other studies, but with the difference that IL-27 level showed positive correlation with disease activity in one [107], but inverse correlation with disease in another [108]. However, in both these studies, IL-27 level increased following treatment leading to improvement in disease activity. Taken together, these studies point to pro- versus anti-inflammatory activity of IL-27 in SLE patients.
Mouse models of lupus include spontaneously developing disease (e.g., in MRL/lpr and BWF1 mice) [109] and experimentally-induced disease (e.g., that induced by chronic graft versus host disease) [110]. IL-27 signaling has been associated with the pathogenesis of lupus nephritis in mice [109, 111-113]. MRL/lpr mice develop a Th1-mediated renal pathology that represents diffuse proliferative glomerulonephritis in human SLE. However, the same mouse strain but deficient in IL-27 receptor α (WSX-1) revealed a change in immunopathology from Th1 type in wild mice to a Th2 type, which resembled human membranous glomerulonephritis [109]. Similarly, mice deficient in EBI3 subunit of the cytokine IL-27 showed a shift in cytokine response from Th1 to Th2 and consequent change in immune pathology of glomerulonephritis [111], which was similar to that described above in WSX-1-deficient mice. On the contrary, overexpression of the same receptor (WSX-1) afforded protection against autoimmune lupus nephritis, validating the role of IL-27-mediated Th1 response in regulation of autoimmunity in this disease model [112]. WSX-overexpression also reduced spontaneous lupus-like skin inflammation [114]. Taken together, these results emphasize the role of IL-27 in Th1/Th2 balance and its impact on disease pathogenesis.
5.5. Psoriasis
Psoriasis is a chronic inflammatory skin disease of autoimmune origin. Activated T cells, neutrophils, and other types of leukocytes infiltrate the skin causing skin hyperplasia [115]. Keratinocyte hyperplasia is a feature of the disease process in psoriasis. Mouse models of psoriasis include various spontaneous mutation models, transgenic and knockout models, and xenograft models [116]. IL-27 levels were found to be higher in the serum and the diseased tissue in psoriatic patients compared with controls [117, 118], and this increase correlated with the severity of the disease. IL-27-secreting cells were shown to infiltrate into the psoriatic lesions, but not into other type of dermatitis or normal skin.
The pro-inflammatory cytokines TNFα and IL-18 play an important role in the pathogenesis of psoriasis [119, 120]. TNFα induces chemokine expression in keratinocytes, and these chemokines facilitate the cellular migration into skin lesions in psoriasis. It has been shown that IL-17 and IL-27 have opposite effects on these TNFα-mediated events in human keratinocytes, and thereby on skin inflammation in psoriasis [119]. It has been proposed that IL-27 has a dual role in the pathogenesis of psoriasis such that it might promote the onset of psoriasis by inducing certain chemokines in keratinocytes, but it may also inhibit the spreading of inflammation in the course of disease by suppressing TNFα-induced cytokines and chemokines [118]. Furthermore, IL-18 binding protein (IL-18BP) is a natural endogenous antagonist of IL-18, and IL-27 has been shown to upregulate IL-18BP in human keratinocytes and induce anti-inflammatory activity by neutralizing IL-18 [120]. This effect of IL-27 is STAT1-mediated. The role of IL-27 was tested in a mouse model of psoriasis in which the disease was induced by local application of imiquimod to the skin [121]. IL-27 injected locally s.c. aggravated the disease compared to controls injected with the vehicle. Following IL-27 treatment, the levels of mRNA for IFN-γ, TNFα, and various chemokines in the injected skin were increased. However, the levels of these mediators of inflammation and chemotaxis were reduced along with protection against disease following neutralization of the IL-27 effect [121].
5.6. Type 1 diabetes mellitus (T1D)
T1D is an autoimmune disease characterized by inflammation of pancreatic islets (insulitis) and destruction of pancreatic β-cells. The precise trigger for these events is not clear, but infiltration of the islets of Langerhans by CD4 and CD8 T cells, B cells. and macrophages is an early event in individuals susceptible to T1D [122]. These immune cells attack and damage β-cells leading to reduction in insulin production. The non-obese diabetic (NOD) mouse and streptozotocin (STZ)-induced diabetes are two of the commonly used models of human T1D. There is limited information on the involvement of IL-27 in T1D in humans. Furthermore, in a study on a Brazilian population, a lack of association between IL-27 gene variants and susceptibility to T1D was observed [123].
Mice deficient in IL-27 subunit (EBI3−/−) or IL-27 receptor subunit (WSX-1−/−) mice) treated with STZ for induction of diabetes showed increased blood glucose and islet proinsulin levels as well as enhanced immune cell infiltration into the islets compared to wild type controls [124]. Furthermore, treatment of EBI3−/− and wild type mice with IL-27 led to reduction of these disease-associated parameters, demonstrating the immunoregulatory role of IL-27 in this model of T1D [124].
In contrast to the STZ-induced diabetes model, the NOD mouse model of T1D revealed a disease-propagating role for IL-27 in diabetes [125]. In that study, diabetes was passively induced by the adoptive transfer of diabetogenic splenocytes. The treatment of diabetogenic splenocytes with IL-27 prior to their adoptive transfer accelerated the onset of the disease in the recipients. The IL-27-treated splenocytes showed increased production of pro-inflammatory cytokines coupled with inhibition of anti-inflammatory cytokine production. On the contrary, the blockade of IL-27 caused a marked delay in the onset of diabetes [125].
5.7 Uveitis
Uveitis is an intraocular inflammatory disease of infectious or autoimmune etiology [126, 127]. Th17 cells have been shown to be present in PBMC of patients with uveitis, and the number of these cells increased during acute phase of the disease but reduced as a result of therapy [127]. Experimental autoimmune uveitis (EAU) is an experimental model of human uveitis [128]. Both Th1 and Th17 cells have been shown to be involved in the pathogenesis of uveitis. IL-27 plays a role in uveitis, and depending on the stage of the disease, both pro-inflammatory/pathogenic and anti-inflammatory/protective roles have been assigned to this cytokine.
The role of IL-27 in the disease process was evident in mice deficient in IL-27R (WSX-1), which showed reduced clinical and histopathological features of uveitis in the early phase of the disease compared to wild type mice [129]. This protection was associated with reduction in IFN-γ, chemokines, and pathogenicity of T cells. However, this difference in disease severity was abated in the late of the disease in the two groups of mice. Similar results showing differential effects in the early and late phase of uveitis were obtained in mice deficient in EBI3 subunit of IL-27 [130]. The attenuation of uveitis was associated with decreased Th1 response.
IL-27 was shown to be expressed in retina in mice with uveitis [127]. This cytokine was shown to inhibit Th17 cells in vitro in a STAT1-dependent manner. Furthermore, IFN-γ can induce IL-27, and both these cytokines can inhibit Th17 [127]. Thus, Th1 response emerging in the late phase of uveitis can regulate progression of the disease by Th17 by controlling them directly through IFN-γ as well as via IL-27. Other studies in the EAU model in which mice with uveitis were treated with IL-27 or its variant revealed the disease-regulating attribute of IL-27. Treatment with a recombinant p28/p40 cytokine suppressed uveitis by inhibiting both Th1 and Th17 responses, but promoting Foxp3-expressing, IL-10-producing Treg [131]. The effect of p28/p40 was superior to that of p28 alone. Similarly, the overexpression of IL-27p28 in mice led to attenuated uveitis following the regimen aimed at prevention versus progression of uveitis [87]. The protective effect of IL-27 was attributed to the inhibitory effect of IL-27 on pathogenic Th1 and Th17 responses. We have described above the role of STAT1 in IL-27 signaling. STAT1-deficient mice were shown to develop more severe uveitis than control mice [132], and this disease aggravation was associated with reduced production of IL-27 and IL-10.
6. Conclusion
IL-27 is a regulator of T cell differentiation and function. IL-27 induces Th1 and Tr1, but inhibits Th2, Th17 and Treg differentiation and function. However, under certain conditions, opposite effects on certain T cell subsets have been observed, for example, inhibition of Th1 and enhancement of Th17 or Treg generation and activity. IL-27 can display pro- or anti-inflammatory activity in different autoimmune diseases. The precise conditions that impart dual functional attributes to IL-27 have not yet been fully defined. However, with some exceptions, mostly anti-inflammatory activity of IL-27 has been observed in different experimental models of autoimmunity. These results in turn would help plan and guide additional studies to investigate the role of IL-27 in patients with autoimmunity. Regardless, IL-27 represents a novel, promising therapeutic agent for autoimmune diseases. Pilot clinical trials using IL-27 in patients with autoimmunity in the near future would unravel the translational significance of the basic research summarized in this article.
Take-home messages.
IL-27 generally promotes the differentiation of Th1 and Tr1 cells, but inhibits Th2, Th17, and Treg cells.
IL-27 inhibits the production of pro-inflammatory cytokines IL-1, IL-6 and IL-17, but induces the production of anti-inflammatory cytokine IL-10.
IL-27 is involved in the pathogenesis of autoimmunity, and with some exceptions, mostly anti-inflammatory effects have been described in different diseases.
IL-27 represents a promising new therapeutic agent/target for the control of autoimmune diseases.
Highlights.
* IL-27 is a new cytokine of the IL-12 family and consists of EBI3 and p28 subunits.
* The receptor for IL-27 consists of the orphan receptor WSX-1 and gp130.
* IL-27 modulates the differentiation and activity of various T cell subsets.
* Both pro- and anti-inflammatory effects of IL-27 have been observed in autoimmunity.
* IL-27 is a promising therapeutic target/agent for the control of autoimmune diseases.
Acknowledgements
This work was supported by grants from the National Institutes of Health, Bethesda, MD and the Rheumatology Research Foundation, Atlanta, GA.
Abbreviations
- EBI3
Epstein-Barr virus-induced gene
- Tr1
type 1 regulatory cells
- Foxp3
forkhead box P3
- Tfh
T follicular helper
- gp130
Glycoprotein 130; (gp130)
- c-maf
c-musculoaponeurotic fibrosarcoma oncogene
- Ahr
aryl hydrocarbon receptor
- Egr-2
early growth response gene-2
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interest:
The authors declare no conflict of interest.
References
- 1.Pflanz S, Timans JC, Cheung J, Rosales R, Kanzler H, Gilbert J, Hibbert L, Churakova T, Travis M, Vaisberg E, Blumenschein WM, Mattson JD, Wagner JL, To W, Zurawski S, McClanahan TK, Gorman DM, Bazan JF, de Waal Malefyt R, Rennick D, Kastelein RA. IL-27, a heterodimeric cytokine composed of EBI3 and p28 protein, induces proliferation of naive CD4+ T cells. Immunity. 2002;16:779–90. doi: 10.1016/s1074-7613(02)00324-2. [DOI] [PubMed] [Google Scholar]
- 2.Abbas AK, Murphy KM, Sher A. Functional diversity of helper T lymphocytes. Nature. 1996;383:787–93. doi: 10.1038/383787a0. [DOI] [PubMed] [Google Scholar]
- 3.Bettelli E, Carrier Y, Gao W, Korn T, Strom TB, Oukka M, Weiner HL, Kuchroo VK. Reciprocal developmental pathways for the generation of pathogenic effector TH17 and regulatory T cells. Nature. 2006;441:235–8. doi: 10.1038/nature04753. [DOI] [PubMed] [Google Scholar]
- 4.Stockinger B, Veldhoen M. Differentiation and function of Th17 T cells. Current opinion in immunology. 2007;19:281–6. doi: 10.1016/j.coi.2007.04.005. [DOI] [PubMed] [Google Scholar]
- 5.Hall AO, Silver JS, Hunter CA. The immunobiology of IL-27. Advances in immunology. 2012;115:1–44. doi: 10.1016/B978-0-12-394299-9.00001-1. [DOI] [PubMed] [Google Scholar]
- 6.Hunter CA, Villarino A, Artis D, Scott P. The role of IL-27 in the development of T-cell responses during parasitic infections. Immunological reviews. 2004;202:106–14. doi: 10.1111/j.0105-2896.2004.00213.x. [DOI] [PubMed] [Google Scholar]
- 7.Yoshida S.H. Hiroki, Senaldi Giorgio, Covey Todd, Faggioni Raffaella, Mu Sharon, Xia Min, Wakeham Andrew C, Nishina Hiroshi, Potter Julia, Saris Chris J.M, Mak Tak W. WSX-1 Is Required for the Initiation of Th1 Responses and Resistance to L. major Infection. Immunity. 2001;15:9. doi: 10.1016/s1074-7613(01)00206-0. [DOI] [PubMed] [Google Scholar]
- 8.Villarino L.H. Alejandro, Lieberman Linda, Wilson Emma, Mak Tak, Yoshida Hiroki, Kastelein Robert A., Saris Christiaan, Hunter Christopher A. The IL-27R (WSX-1) Is Required to Suppress T Cell Hyperactivity during Infection. Immunity. 2003;19:645. doi: 10.1016/s1074-7613(03)00300-5. [DOI] [PubMed] [Google Scholar]
- 9.Rosas LE SA, Roth KM, Keiser TL, Barbi J, Hunter C, de Sauvage FJ, Satoskar AR. Interleukin-27R (WSX-1/T-cell cytokine receptor) gene-deficient mice display enhanced resistance to leishmania donovani infection but develop severe liver immunopathology. The American Journal of Pathology. 2006;168:11. doi: 10.2353/ajpath.2006.050013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hölscher C HA, Rückerl D, Yoshimoto T, Yoshida H, Mak T, Saris C, Ehlers S. The IL-27 receptor chain WSX-1 differentially regulates antibacterial immunity and survival during experimental tuberculosis. Journal of Immunology. 2005;174:10. doi: 10.4049/jimmunol.174.6.3534. [DOI] [PubMed] [Google Scholar]
- 11.Robinson CM NG. Interleukin-12 and interleukin-27 regulate macrophage control of Mycobacterium tuberculosis. The Journal of Infectious Disease. 2008;198:7. doi: 10.1086/589774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Villarino AV, Artis D, Bezbradica JS, Miller O, Saris CJ, Joyce S, Hunter CA. IL-27R deficiency delays the onset of colitis and protects from helminth-induced pathology in a model of chronic IBD. International immunology. 2008;20:739–52. doi: 10.1093/intimm/dxn032. [DOI] [PubMed] [Google Scholar]
- 13.Yoshida H, Hunter CA. The immunobiology of interleukin-27. Annual review of immunology. 2015;33:417–43. doi: 10.1146/annurev-immunol-032414-112134. [DOI] [PubMed] [Google Scholar]
- 14.Devergne O, Hummel M, Koeppen H, Le Beau MM, Nathanson EC, Kieff E, Birkenbach M. A novel interleukin-12 p40-related protein induced by latent Epstein-Barr virus infection in B lymphocytes. J Virol. 1996;70:1143–53. doi: 10.1128/jvi.70.2.1143-1153.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pflanz S, Hibbert L, Mattson J, Rosales R, Vaisberg E, Bazan JF, Phillips JH, McClanahan TK, de Waal Malefyt R, Kastelein RA. WSX-1 and glycoprotein 130 constitute a signal-transducing receptor for IL-27. J Immunol. 2004;172:2225–31. doi: 10.4049/jimmunol.172.4.2225. [DOI] [PubMed] [Google Scholar]
- 16.Wang H, Meng R, Li Z, Yang B, Liu Y, Huang F, Zhang J, Chen H, Wu C. IL-27 induces the differentiation of Tr1-like cells from human naive CD4+ T cells via the phosphorylation of STAT1 and STAT3. Immunol Lett. 2011;136:21–8. doi: 10.1016/j.imlet.2010.11.007. [DOI] [PubMed] [Google Scholar]
- 17.Pot C, Apetoh L, Awasthi A, Kuchroo VK. Induction of regulatory Tr1 cells and inhibition of T(H)17 cells by IL-27. Seminars in immunology. 2011;23:438–45. doi: 10.1016/j.smim.2011.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Iwasaki Y, Fujio K, Okamura T, Yanai A, Sumitomo S, Shoda H, Tamura T, Yoshida H, Charnay P, Yamamoto K. Egr-2 transcription factor is required for Blimp-1-mediated IL-10 production in IL-27-stimulated CD4+ T cells. European journal of immunology. 2013;43:1063–73. doi: 10.1002/eji.201242942. [DOI] [PubMed] [Google Scholar]
- 19.Hirahara K, Onodera A, Villarino AV, Bonelli M, Sciume G, Laurence A, Sun HW, Brooks SR, Vahedi G, Shih HY, Gutierrez-Cruz G, Iwata S, Suzuki R, Mikami Y, Okamoto Y, Nakayama T, Holland SM, Hunter CA, Kanno Y, O'Shea JJ. Asymmetric Action of STAT Transcription Factors Drives Transcriptional Outputs and Cytokine Specificity. Immunity. 2015;42:877–89. doi: 10.1016/j.immuni.2015.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Owaki T, Asakawa M, Fukai F, Mizuguchi J, Yoshimoto T. IL-27 induces Th1 differentiation via p38 MAPK/T-bet- and intercellular adhesion molecule-1/LFA-1/ERK1/2-dependent pathways. J Immunol. 2006;177:7579–87. doi: 10.4049/jimmunol.177.11.7579. [DOI] [PubMed] [Google Scholar]
- 21.Vasanthakumar A, Kallies A. IL-27 paves different roads to Tr1. European journal of immunology. 2013;43:882–5. doi: 10.1002/eji.201343479. [DOI] [PubMed] [Google Scholar]
- 22.Iwasaki Y, Fujio K, Okamura T, Yamamoto K. Interleukin-27 in T cell immunity. International journal of molecular sciences. 2015;16:2851–63. doi: 10.3390/ijms16022851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Huber M, Steinwald V, Guralnik A, Brustle A, Kleemann P, Rosenplanter C, Decker T, Lohoff M. IL-27 inhibits the development of regulatory T cells via STAT3. International immunology. 2008;20:223–34. doi: 10.1093/intimm/dxm139. [DOI] [PubMed] [Google Scholar]
- 24.Hirahara K, Ghoreschi K, Yang XP, Takahashi H, Laurence A, Vahedi G, Sciume G, Hall AO, Dupont CD, Francisco LM, Chen Q, Tanaka M, Kanno Y, Sun HW, Sharpe AH, Hunter CA, O'Shea JJ. Interleukin-27 priming of T cells controls IL-17 production in trans via induction of the ligand PD-L1. Immunity. 2012;36:1017–30. doi: 10.1016/j.immuni.2012.03.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ziblat A, Domaica CI, Spallanzani RG, Iraolagoitia XL, Rossi LE, Avila DE, Torres NI, Fuertes MB, Zwirner NW. IL-27 stimulates human NK-cell effector functions and primes NK cells for IL-18 responsiveness. European journal of immunology. 2015;45:192–202. doi: 10.1002/eji.201444699. [DOI] [PubMed] [Google Scholar]
- 26.Furukawa M, Takaishi H, Takito J, Yoda M, Sakai S, Hikata T, Hakozaki A, Uchikawa S, Matsumoto M, Chiba K, Kimura T, Okada Y, Matsuo K, Yoshida H, Toyama Y. IL-27 abrogates receptor activator of NF-kappa B ligand-mediated osteoclastogenesis of human granulocyte-macrophage colony-forming unit cells through STAT1-dependent inhibition of c-Fos. J Immunol. 2009;183:2397–406. doi: 10.4049/jimmunol.0802091. [DOI] [PubMed] [Google Scholar]
- 27.Seder RA, Gazzinelli R, Sher A, Paul WE. Interleukin 12 acts directly on CD4+ T cells to enhance priming for interferon gamma production and diminishes interleukin 4 inhibition of such priming. Proceedings of the National Academy of Sciences of the United States of America. 1993;90:10188–92. doi: 10.1073/pnas.90.21.10188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Takeda A, Hamano S, Yamanaka A, Hanada T, Ishibashi T, Mak TW, Yoshimura A, Yoshida H. Cutting edge: role of IL-27/WSX-1 signaling for induction of T-bet through activation of STAT1 during initial Th1 commitment. J Immunol. 2003;170:4886–90. doi: 10.4049/jimmunol.170.10.4886. [DOI] [PubMed] [Google Scholar]
- 29.Lucas S, Ghilardi N, Li J, de Sauvage FJ. IL-27 regulates IL-12 responsiveness of naive CD4+ T cells through Stat1-dependent and -independent mechanisms. Proceedings of the National Academy of Sciences of the United States of America. 2003;100:15047–52. doi: 10.1073/pnas.2536517100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Batten M, Kljavin NM, Li J, Walter MJ, de Sauvage FJ, Ghilardi N. Cutting edge: IL-27 is a potent inducer of IL-10 but not FoxP3 in murine T cells. J Immunol. 2008;180:2752–6. doi: 10.4049/jimmunol.180.5.2752. [DOI] [PubMed] [Google Scholar]
- 31.Owaki T, Asakawa M, Kamiya S, Takeda K, Fukai F, Mizuguchi J, Yoshimoto T. IL-27 suppresses CD28-mediated [correction of medicated] IL-2 production through suppressor of cytokine signaling 3. J Immunol. 2006;176:2773–80. doi: 10.4049/jimmunol.176.5.2773. [DOI] [PubMed] [Google Scholar]
- 32.Zheng W, Flavell RA. The transcription factor GATA-3 is necessary and sufficient for Th2 cytokine gene expression in CD4 T cells. Cell. 1997;89:587–96. doi: 10.1016/s0092-8674(00)80240-8. [DOI] [PubMed] [Google Scholar]
- 33.Ouyang W, Ranganath SH, Weindel K, Bhattacharya D, Murphy TL, Sha WC, Murphy KM. Inhibition of Th1 development mediated by GATA-3 through an IL-4-independent mechanism. Immunity. 1998;9:745–55. doi: 10.1016/s1074-7613(00)80671-8. [DOI] [PubMed] [Google Scholar]
- 34.Artis D, Villarino A, Silverman M, He W, Thornton EM, Mu S, Summer S, Covey TM, Huang E, Yoshida H, Koretzky G, Goldschmidt M, Wu GD, de Sauvage F, Miller HR, Saris CJ, Scott P, Hunter CA. The IL-27 receptor (WSX-1) is an inhibitor of innate and adaptive elements of type 2 immunity. J Immunol. 2004;173:5626–34. doi: 10.4049/jimmunol.173.9.5626. [DOI] [PubMed] [Google Scholar]
- 35.Gaffen SL, Jain R, Garg AV, Cua DJ. The IL-23-IL-17 immune axis: from mechanisms to therapeutic testing. Nat Rev Immunol. 2014;14:585–600. doi: 10.1038/nri3707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lubberts E. The IL-23-IL-17 axis in inflammatory arthritis. Nat Rev Rheumatol. 2015 Apr 28; doi: 10.1038/nrrheum.2015.128. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 37.Diveu C, McGeachy MJ, Boniface K, Stumhofer JS, Sathe M, Joyce-Shaikh B, Chen Y, Tato CM, McClanahan TK, de Waal Malefyt R, Hunter CA, Cua DJ, Kastelein RA. IL-27 blocks RORc expression to inhibit lineage commitment of Th17 cells. J Immunol. 2009;182:5748–56. doi: 10.4049/jimmunol.0801162. [DOI] [PubMed] [Google Scholar]
- 38.El-behi M, Ciric B, Yu S, Zhang GX, Fitzgerald DC, Rostami A. Differential effect of IL-27 on developing versus committed Th17 cells. J Immunol. 2009;183:4957–67. doi: 10.4049/jimmunol.0900735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rajaiah R, Puttabyatappa M, Polumuri SK, Moudgil KD. Interleukin-27 and interferon-gamma are involved in regulation of autoimmune arthritis. The Journal of biological chemistry. 2011;286:2817–25. doi: 10.1074/jbc.M110.187013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yang J, Yang M, Htut TM, Ouyang X, Hanidu A, Li X, Sellati R, Jiang H, Zhang S, Li H, Zhao J, Ting AT, Mayer L, Unkeless JC, Labadia ME, Hodge M, Li J, Xiong H. Epstein-Barr virus-induced gene 3 negatively regulates IL-17, IL-22 and RORgamma t. European journal of immunology. 2008;38:1204–14. doi: 10.1002/eji.200838145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Stumhofer JS, Laurence A, Wilson EH, Huang E, Tato CM, Johnson LM, Villarino AV, Huang Q, Yoshimura A, Sehy D, Saris CJ, O'Shea JJ, Hennighausen L, Ernst M, Hunter CA. Interleukin 27 negatively regulates the development of interleukin 17-producing T helper cells during chronic inflammation of the central nervous system. Nature immunology. 2006;7:937–45. doi: 10.1038/ni1376. [DOI] [PubMed] [Google Scholar]
- 42.Batten M, Li J, Yi S, Kljavin NM, Danilenko DM, Lucas S, Lee J, de Sauvage FJ, Ghilardi N. Interleukin 27 limits autoimmune encephalomyelitis by suppressing the development of interleukin 17-producing T cells. Nature immunology. 2006;7:929–36. doi: 10.1038/ni1375. [DOI] [PubMed] [Google Scholar]
- 43.Young A, Linehan E, Hams E, O'Hara Hall AC, McClurg A, Johnston JA, Hunter CA, Fallon PG, Fitzgerald DC. Cutting edge: suppression of GM-CSF expression in murine and human T cells by IL-27. J Immunol. 2012;189:2079–83. doi: 10.4049/jimmunol.1200131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sage PT, Sharpe AH. T follicular regulatory cells in the regulation of B cell responses. Trends in immunology. 2015;36:410–8. doi: 10.1016/j.it.2015.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Nurieva RI, Chung Y, Martinez GJ, Yang XO, Tanaka S, Matskevitch TD, Wang YH, Dong C. Bcl6 mediates the development of T follicular helper cells. Science (New York, N.Y. 2009;325:1001–5. doi: 10.1126/science.1176676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bauquet AT, Jin H, Paterson AM, Mitsdoerffer M, Ho IC, Sharpe AH, Kuchroo VK. The costimulatory molecule ICOS regulates the expression of c-Maf and IL-21 in the development of follicular T helper cells and TH-17 cells. Nature immunology. 2009;10:167–75. doi: 10.1038/ni.1690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Batten M, Ramamoorthi N, Kljavin NM, Ma CS, Cox JH, Dengler HS, Danilenko DM, Caplazi P, Wong M, Fulcher DA, Cook MC, King C, Tangye SG, de Sauvage FJ, Ghilardi N. IL-27 supports germinal center function by enhancing IL-21 production and the function of T follicular helper cells. The Journal of experimental medicine. 2010;207:2895–906. doi: 10.1084/jem.20100064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Gringhuis SI, Kaptein TM, Wevers BA, van der Vlist M, Klaver EJ, van Die I, Vriend LE, de Jong MA, Geijtenbeek TB. Fucose-based PAMPs prime dendritic cells for follicular T helper cell polarization via DC-SIGN-dependent IL-27 production. Nature communications. 2014;5:5074. doi: 10.1038/ncomms6074. [DOI] [PubMed] [Google Scholar]
- 49.Pot C, Apetoh L, Kuchroo VK. Type 1 regulatory T cells (Tr1) in autoimmunity. Seminars in immunology. 2011;23:202–8. doi: 10.1016/j.smim.2011.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Awasthi A, Carrier Y, Peron JP, Bettelli E, Kamanaka M, Flavell RA, Kuchroo VK, Oukka M, Weiner HL. A dominant function for interleukin 27 in generating interleukin 10-producing anti-inflammatory T cells. Nature immunology. 2007;8:1380–9. doi: 10.1038/ni1541. [DOI] [PubMed] [Google Scholar]
- 51.Gagliani N, Magnani CF, Huber S, Gianolini ME, Pala M, Licona-Limon P, Guo B, Herbert DR, Bulfone A, Trentini F, Di Serio C, Bacchetta R, Andreani M, Brockmann L, Gregori S, Flavell RA, Roncarolo MG. Coexpression of CD49b and LAG-3 identifies human and mouse T regulatory type 1 cells. Nature medicine. 2013;19:739–46. doi: 10.1038/nm.3179. [DOI] [PubMed] [Google Scholar]
- 52.Apetoh L, Quintana FJ, Pot C, Joller N, Xiao S, Kumar D, Burns EJ, Sherr DH, Weiner HL, Kuchroo VK. The aryl hydrocarbon receptor interacts with c-Maf to promote the differentiation of type 1 regulatory T cells induced by IL-27. Nature immunology. 2010;11:854–61. doi: 10.1038/ni.1912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wu C, Pot C, Apetoh L, Thalhamer T, Zhu B, Murugaiyan G, Xiao S, Lee Y, Rangachari M, Yosef N, Kuchroo VK. Metallothioneins negatively regulate IL-27-induced type 1 regulatory T-cell differentiation. Proceedings of the National Academy of Sciences of the United States of America. 2013;110:7802–7. doi: 10.1073/pnas.1211776110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Stumhofer JS, Silver JS, Laurence A, Porrett PM, Harris TH, Turka LA, Ernst M, Saris CJ, O'Shea JJ, Hunter CA. Interleukins 27 and 6 induce STAT3-mediated T cell production of interleukin 10. Nature immunology. 2007;8:1363–71. doi: 10.1038/ni1537. [DOI] [PubMed] [Google Scholar]
- 55.Shevach EM, Thornton AM. tTregs, pTregs, and iTregs: similarities and differences. Immunological reviews. 2014;259:88–102. doi: 10.1111/imr.12160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Neufert C, Becker C, Wirtz S, Fantini MC, Weigmann B, Galle PR, Neurath MF. IL-27 controls the development of inducible regulatory T cells and Th17 cells via differential effects on STAT1. European journal of immunology. 2007;37:1809–16. doi: 10.1002/eji.200636896. [DOI] [PubMed] [Google Scholar]
- 57.Cox JH, Kljavin NM, Ramamoorthi N, Diehl L, Batten M, Ghilardi N. IL-27 promotes T cell-dependent colitis through multiple mechanisms. The Journal of experimental medicine. 2011;208:115–23. doi: 10.1084/jem.20100410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Hall AO, Beiting DP, Tato C, John B, Oldenhove G, Lombana CG, Pritchard GH, Silver JS, Bouladoux N, Stumhofer JS, Harris TH, Grainger J, Wojno ED, Wagage S, Roos DS, Scott P, Turka LA, Cherry S, Reiner SL, Cua D, Belkaid Y, Elloso MM, Hunter CA. The cytokines interleukin 27 and interferon-gamma promote distinct Treg cell populations required to limit infection-induced pathology. Immunity. 2012;37:511–23. doi: 10.1016/j.immuni.2012.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kim G, Shinnakasu R, Saris CJ, Cheroutre H, Kronenberg M. A novel role for IL-27 in mediating the survival of activated mouse CD4 T lymphocytes. J Immunol. 2013;190:1510–8. doi: 10.4049/jimmunol.1201017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Moon SJ, Park JS, Heo YJ, Kang CM, Kim EK, Lim MA, Ryu JG, Park SJ, Park KS, Sung YC, Park SH, Kim HY, Min JK, Cho ML. In vivo action of IL-27: reciprocal regulation of Th17 and Treg cells in collagen-induced arthritis. Exp Mol Med. 2013;45:e46. doi: 10.1038/emm.2013.89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Do JS, Visperas A, Sanogo YO, Bechtel JJ, Dvorina N, Kim S, Jang E, Stohlman SA, Shen B, Fairchild RL, Baldwin Iii WM, Vignali DA, Min B. An IL-27/Lag3 axis enhances Foxp3 regulatory T cell-suppressive function and therapeutic efficacy. Mucosal immunology. 2015 doi: 10.1038/mi.2015.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Schneider R, Yaneva T, Beauseigle D, El-Khoury L, Arbour N. IL-27 increases the proliferation and effector functions of human naive CD8+ T lymphocytes and promotes their development into Tc1 cells. European journal of immunology. 2011;41:47–59. doi: 10.1002/eji.201040804. [DOI] [PubMed] [Google Scholar]
- 63.Morishima N, Owaki T, Asakawa M, Kamiya S, Mizuguchi J, Yoshimoto T. Augmentation of effector CD8+ T cell generation with enhanced granzyme B expression by IL-27. J Immunol. 2005;175:1686–93. doi: 10.4049/jimmunol.175.3.1686. [DOI] [PubMed] [Google Scholar]
- 64.Yoshimoto T, Okada K, Morishima N, Kamiya S, Owaki T, Asakawa M, Iwakura Y, Fukai F, Mizuguchi J. Induction of IgG2a class switching in B cells by IL-27. J Immunol. 2004;173:2479–85. doi: 10.4049/jimmunol.173.4.2479. [DOI] [PubMed] [Google Scholar]
- 65.Boumendjel A, Tawk L, Malefijt Rde W, Boulay V, Yssel H, Pene J. IL-27 induces the production of IgG1 by human B cells. Eur Cytokine Netw. 2006;17:281–9. [PubMed] [Google Scholar]
- 66.Brennan FM, McInnes IB. Evidence that cytokines play a role in rheumatoid arthritis. The Journal of clinical investigation. 2008;118:3537–45. doi: 10.1172/JCI36389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Zwerina J, Redlich K, Schett G, Smolen JS. Pathogenesis of rheumatoid arthritis: targeting cytokines. Annals of the New York Academy of Sciences. 2005;1051:716–29. doi: 10.1196/annals.1361.116. [DOI] [PubMed] [Google Scholar]
- 68.Asquith DL, Miller AM, McInnes IB, Liew FY. Animal models of rheumatoid arthritis. European journal of immunology. 2009;39:2040–4. doi: 10.1002/eji.200939578. [DOI] [PubMed] [Google Scholar]
- 69.Kalliolias GD, Zhao B, Triantafyllopoulou A, Park-Min KH, Ivashkiv LB. Interleukin-27 inhibits human osteoclastogenesis by abrogating RANKL-mediated induction of nuclear factor of activated T cells c1 and suppressing proximal RANK signaling. Arthritis Rheum. 2010;62:402–13. doi: 10.1002/art.27200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Niedbala W, Cai B, Wei X, Patakas A, Leung BP, McInnes IB, Liew FY. Interleukin 27 attenuates collagen-induced arthritis. Annals of the rheumatic diseases. 2008;67:1474–9. doi: 10.1136/ard.2007.083360. [DOI] [PubMed] [Google Scholar]
- 71.Tanida S, Yoshitomi H, Ishikawa M, Kasahara T, Murata K, Shibuya H, Ito H, Nakamura T. IL-27-producing CD14(+) cells infiltrate inflamed joints of rheumatoid arthritis and regulate inflammation and chemotactic migration. Cytokine. 2011;55:237–44. doi: 10.1016/j.cyto.2011.04.020. [DOI] [PubMed] [Google Scholar]
- 72.Kamiya S, Okumura M, Chiba Y, Fukawa T, Nakamura C, Nimura N, Mizuguchi J, Wada S, Yoshimoto T. IL-27 suppresses RANKL expression in CD4+ T cells in part through STAT3. Immunol Lett. 2011;138:47–53. doi: 10.1016/j.imlet.2011.02.022. [DOI] [PubMed] [Google Scholar]
- 73.Park JS, Jung YO, Oh HJ, Park SJ, Heo YJ, Kang CM, Kwok SK, Ju JH, Park KS, Cho ML, Sung YC, Park SH, Kim HY. Interleukin-27 suppresses osteoclastogenesis via induction of interferon-gamma. Immunology. 2012;137:326–35. doi: 10.1111/j.1365-2567.2012.03622.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Pickens SR, Chamberlain ND, Volin MV, Mandelin AM, 2nd, Agrawal H, Matsui M, Yoshimoto T, Shahrara S. Local expression of interleukin-27 ameliorates collagen-induced arthritis. Arthritis Rheum. 2011;63:2289–98. doi: 10.1002/art.30324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Sato K, Suematsu A, Okamoto K, Yamaguchi A, Morishita Y, Kadono Y, Tanaka S, Kodama T, Akira S, Iwakura Y, Cua DJ, Takayanagi H. Th17 functions as an osteoclastogenic helper T cell subset that links T cell activation and bone destruction. The Journal of experimental medicine. 2006;203:2673–82. doi: 10.1084/jem.20061775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Wong CK, Chen da P, Tam LS, Li EK, Yin YB, Lam CW. Effects of inflammatory cytokine IL-27 on the activation of fibroblast-like synoviocytes in rheumatoid arthritis. Arthritis Res Ther. 2010;12:R129. doi: 10.1186/ar3067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Paradowska-Gorycka A, Raszkiewicz B, Jurkowska M, Felis-Giemza A, Romanowska-Prochnicka K, Manczak M, Olesinska M. Association of single nucleotide polymorphisms in the IL27 gene with rheumatoid arthritis. Scandinavian journal of immunology. 2014;80:298–305. doi: 10.1111/sji.12209. [DOI] [PubMed] [Google Scholar]
- 78.Goldberg R, Wildbaum G, Zohar Y, Maor G, Karin N. Suppression of ongoing adjuvant-induced arthritis by neutralizing the function of the p28 subunit of IL-27. J Immunol. 2004;173:1171–8. doi: 10.4049/jimmunol.173.2.1171. [DOI] [PubMed] [Google Scholar]
- 79.Cao Y, Doodes PD, Glant TT, Finnegan A. IL-27 induces a Th1 immune response and susceptibility to experimental arthritis. J Immunol. 2008;180:922–30. doi: 10.4049/jimmunol.180.2.922. [DOI] [PubMed] [Google Scholar]
- 80.Rostami A, Ciric B. Role of Th17 cells in the pathogenesis of CNS inflammatory demyelination. Journal of the neurological sciences. 2013;333:76–87. doi: 10.1016/j.jns.2013.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Yadav SK, Mindur JE, Ito K, Dhib-Jalbut S. Advances in the immunopathogenesis of multiple sclerosis. Current opinion in neurology. 2015;28:206–19. doi: 10.1097/WCO.0000000000000205. [DOI] [PubMed] [Google Scholar]
- 82.Tang SC, Fan XH, Pan QM, Sun QS, Liu Y. Decreased expression of IL-27 and its correlation with Th1 and Th17 cells in progressive multiple sclerosis. Journal of the neurological sciences. 2015;348:174–80. doi: 10.1016/j.jns.2014.11.035. [DOI] [PubMed] [Google Scholar]
- 83.Babaloo Z, Yeganeh RK, Farhoodi M, Baradaran B, Bonyadi M, Aghebati L. Increased IL-17A but decreased IL-27 serum levels in patients with multiple sclerosis. Iran J Immunol. 2013;10:47–54. [PubMed] [Google Scholar]
- 84.Hasheminia SJ, Tolouei S, Zarkesh-Esfahani SH, Shaygannejad V, Shirzad HA, Torabi R, Hashem Zadeh Chaloshtory M. Cytokines gene expression in newly diagnosed multiple sclerosis patients. Iran J Allergy Asthma Immunol. 2015;14:208–16. [PubMed] [Google Scholar]
- 85.Furlan R, Cuomo C, Martino G. Animal models of multiple sclerosis. Methods Mol Biol. 2009;549:157–73. doi: 10.1007/978-1-60327-931-4_11. [DOI] [PubMed] [Google Scholar]
- 86.Fitzgerald DC, Ciric B, Touil T, Harle H, Grammatikopolou J, Das Sarma J, Gran B, Zhang GX, Rostami A. Suppressive effect of IL-27 on encephalitogenic Th17 cells and the effector phase of experimental autoimmune encephalomyelitis. J Immunol. 2007;179:3268–75. doi: 10.4049/jimmunol.179.5.3268. [DOI] [PubMed] [Google Scholar]
- 87.Chong WP, Horai R, Mattapallil MJ, Silver PB, Chen J, Zhou R, Sergeev Y, Villasmil R, Chan CC, Caspi RR. IL-27p28 inhibits central nervous system autoimmunity by concurrently antagonizing Th1 and Th17 responses. Journal of autoimmunity. 2014;50:12–22. doi: 10.1016/j.jaut.2013.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Moraes AS, Paula RF, Pradella F, Santos MP, Oliveira EC, von Glehn F, Camilo DS, Ceragioli H, Peterlevitz A, Baranauskas V, Volpini W, Farias AS, Santos LM. The suppressive effect of IL-27 on encephalitogenic Th17 cells induced by multiwalled carbon nanotubes reduces the severity of experimental autoimmune encephalomyelitis. CNS Neurosci Ther. 2013;19:682–7. doi: 10.1111/cns.12121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Sormani MP, De Stefano N. Defining and scoring response to IFN-beta in multiple sclerosis. Nature reviews. 2013;9:504–12. doi: 10.1038/nrneurol.2013.146. [DOI] [PubMed] [Google Scholar]
- 90.Sweeney CM, Lonergan R, Basdeo SA, Kinsella K, Dungan LS, Higgins SC, Kelly PJ, Costelloe L, Tubridy N, Mills KH, Fletcher JM. IL-27 mediates the response to IFN-beta therapy in multiple sclerosis patients by inhibiting Th17 cells. Brain Behav Immun. 2011;25:1170–81. doi: 10.1016/j.bbi.2011.03.007. [DOI] [PubMed] [Google Scholar]
- 91.Fitzgerald DC, Fonseca-Kelly Z, Cullimore ML, Safabakhsh P, Saris CJ, Zhang GX, Rostami A. Independent and interdependent immunoregulatory effects of IL-27, IFN-beta, and IL-10 in the suppression of human Th17 cells and murine experimental autoimmune encephalomyelitis. J Immunol. 2013;190:3225–34. doi: 10.4049/jimmunol.1200141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Mascanfroni ID, Yeste A, Vieira SM, Burns EJ, Patel B, Sloma I, Wu Y, Mayo L, Ben-Hamo R, Efroni S, Kuchroo VK, Robson SC, Quintana FJ. IL-27 acts on DCs to suppress the T cell response and autoimmunity by inducing expression of the immunoregulatory molecule CD39. Nature immunology. 2013;14:1054–63. doi: 10.1038/ni.2695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Goldberg R, Zohar Y, Wildbaum G, Geron Y, Maor G, Karin N. Suppression of ongoing experimental autoimmune encephalomyelitis by neutralizing the function of the p28 subunit of IL-27. J Immunol. 2004;173:6465–71. doi: 10.4049/jimmunol.173.10.6465. [DOI] [PubMed] [Google Scholar]
- 94.Kaser A, Zeissig S, Blumberg RS. Inflammatory bowel disease. Annual review of immunology. 2010;28:573–621. doi: 10.1146/annurev-immunol-030409-101225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Weaver CT, Elson CO, Fouser LA, Kolls JK. The Th17 pathway and inflammatory diseases of the intestines, lungs, and skin. Annual review of pathology. 2013;8:477–512. doi: 10.1146/annurev-pathol-011110-130318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Schmidt C, Giese T, Ludwig B, Mueller-Molaian I, Marth T, Zeuzem S, Meuer SC, Stallmach A. Expression of interleukin-12-related cytokine transcripts in inflammatory bowel disease: elevated interleukin-23p19 and interleukin-27p28 in Crohn's disease but not in ulcerative colitis. Inflammatory bowel diseases. 2005;11:16–23. doi: 10.1097/00054725-200501000-00003. [DOI] [PubMed] [Google Scholar]
- 97.Larousserie F, Pflanz S, Coulomb-L'Hermine A, Brousse N, Kastelein R, Devergne O. Expression of IL-27 in human Th1-associated granulomatous diseases. The Journal of pathology. 2004;202:164–71. doi: 10.1002/path.1508. [DOI] [PubMed] [Google Scholar]
- 98.Sasaoka T, Ito M, Yamashita J, Nakajima K, Tanaka I, Narita M, Hara Y, Hada K, Takahashi M, Ohno Y, Matsuo T, Kaneshiro Y, Tanaka H, Kaneko K. Treatment with IL-27 attenuates experimental colitis through the suppression of the development of IL-17-producing T helper cells. Am J Physiol Gastrointest Liver Physiol. 2011;300:G568–76. doi: 10.1152/ajpgi.00329.2010. [DOI] [PubMed] [Google Scholar]
- 99.Honda K, Nakamura K, Matsui N, Takahashi M, Kitamura Y, Mizutani T, Harada N, Nawata H, Hamano S, Yoshida H. T helper 1-inducing property of IL-27/WSX-1 signaling is required for the induction of experimental colitis. Inflammatory bowel diseases. 2005;11:1044–52. doi: 10.1097/01.mib.0000191611.05466.1f. [DOI] [PubMed] [Google Scholar]
- 100.Hanson ML, Hixon JA, Li W, Felber BK, Anver MR, Stewart CA, Janelsins BM, Datta SK, Shen W, McLean MH, Durum SK. Oral delivery of IL-27 recombinant bacteria attenuates immune colitis in mice. Gastroenterology. 2014;146:210–221. e13. doi: 10.1053/j.gastro.2013.09.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Nieuwenhuis EE, Neurath MF, Corazza N, Iijima H, Trgovcich J, Wirtz S, Glickman J, Bailey D, Yoshida M, Galle PR, Kronenberg M, Birkenbach M, Blumberg RS. Disruption of T helper 2-immune responses in Epstein-Barr virus-induced gene 3-deficient mice. Proceedings of the National Academy of Sciences of the United States of America. 2002;99:16951–6. doi: 10.1073/pnas.252648899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Visperas A, Do JS, Bulek K, Li X, Min B. IL-27, targeting antigen-presenting cells, promotes Th17 differentiation and colitis in mice. Mucosal immunology. 2014;7:625–33. doi: 10.1038/mi.2013.82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Borchers AT, Leibushor N, Naguwa SM, Cheema GS, Shoenfeld Y, Gershwin ME. Lupus nephritis: a critical review. Autoimmunity reviews. 2012;12:174–94. doi: 10.1016/j.autrev.2012.08.018. [DOI] [PubMed] [Google Scholar]
- 104.Abdel Galil SM, Ezzeldin N, El-Boshy ME. The role of serum IL-17 and IL-6 as biomarkers of disease activity and predictors of remission in patients with lupus nephritis. Cytokine. 2015 doi: 10.1016/j.cyto.2015.05.007. [DOI] [PubMed] [Google Scholar]
- 105.Li TT, Zhang T, Chen GM, Zhu QQ, Tao JH, Pan HF, Ye DQ. Low level of serum interleukin 27 in patients with systemic lupus erythematosus. J Investig Med. 2010;58:737–9. doi: 10.231/JIM.0b013e3181d88f7b. [DOI] [PubMed] [Google Scholar]
- 106.Qiu F, Song L, Yang N, Li X. Glucocorticoid downregulates expression of IL-12 family cytokines in systemic lupus erythematosus patients. Lupus. 2013;22:1011–6. doi: 10.1177/0961203313498799. [DOI] [PubMed] [Google Scholar]
- 107.Xia LP, Li BF, Shen H, Lu J. Interleukin-27 and interleukin-23 in patients with systemic lupus erythematosus: possible role in lupus nephritis. Scandinavian journal of rheumatology. 2015;44:200–5. doi: 10.3109/03009742.2014.962080. [DOI] [PubMed] [Google Scholar]
- 108.Kwan BC, Tam LS, Lai KB, Lai FM, Li EK, Wang G, Chow KM, Li PK, Szeto CC. The gene expression of type 17 T-helper cell-related cytokines in the urinary sediment of patients with systemic lupus erythematosus. Rheumatology (Oxford, England) 2009;48:1491–7. doi: 10.1093/rheumatology/kep255. [DOI] [PubMed] [Google Scholar]
- 109.Shimizu S, Sugiyama N, Masutani K, Sadanaga A, Miyazaki Y, Inoue Y, Akahoshi M, Katafuchi R, Hirakata H, Harada M, Hamano S, Nakashima H, Yoshida H. Membranous glomerulonephritis development with Th2-type immune deviations in MRL/lpr mice deficient for IL-27 receptor (WSX-1) J Immunol. 2005;175:7185–92. doi: 10.4049/jimmunol.175.11.7185. [DOI] [PubMed] [Google Scholar]
- 110.Rus V, Nguyen V, Puliaev R, Puliaeva I, Zernetkina V, Luzina I, Papadimitriou JC, Via CS. T cell TRAIL promotes murine lupus by sustaining effector CD4 Th cell numbers and by inhibiting CD8 CTL activity. J Immunol. 2007;178:3962–72. doi: 10.4049/jimmunol.178.6.3962. [DOI] [PubMed] [Google Scholar]
- 111.Igawa T, Nakashima H, Sadanaga A, Masutani K, Miyake K, Shimizu S, Takeda A, Hamano S, Yoshida H. Deficiency in EBV-induced gene 3 (EBI3) in MRL/lpr mice results in pathological alteration of autoimmune glomerulonephritis and sialadenitis. Modern rheumatology / the Japan Rheumatism Association. 2009;19:33–41. doi: 10.1007/s10165-008-0117-1. [DOI] [PubMed] [Google Scholar]
- 112.Sugiyama N, Nakashima H, Yoshimura T, Sadanaga A, Shimizu S, Masutani K, Igawa T, Akahoshi M, Miyake K, Takeda A, Yoshimura A, Hamano S, Yoshida H. Amelioration of human lupus-like phenotypes in MRL/lpr mice by overexpression of interleukin 27 receptor alpha (WSX-1) Annals of the rheumatic diseases. 2008;67:1461–7. doi: 10.1136/ard.2007.077537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Pan HF, Tao JH, Ye DQ. Therapeutic potential of IL-27 in systemic lupus erythematosus. Expert opinion on therapeutic targets. 2010;14:479–84. doi: 10.1517/14728221003769911. [DOI] [PubMed] [Google Scholar]
- 114.Kido M, Takeuchi S, Sugiyama N, Esaki H, Nakashima H, Yoshida H, Furue M. T cell-specific overexpression of interleukin-27 receptor alpha subunit (WSX-1) prevents spontaneous skin inflammation in MRL/lpr mice. The British journal of dermatology. 2011;164:1214–20. doi: 10.1111/j.1365-2133.2011.10244.x. [DOI] [PubMed] [Google Scholar]
- 115.Boehncke WH, Schon MP. Psoriasis. Lancet. 2015 doi: 10.1016/S0140-6736(14)61909-7. [DOI] [PubMed] [Google Scholar]
- 116.Gudjonsson JE, Johnston A, Dyson M, Valdimarsson H, Elder JT. Mouse models of psoriasis. J Invest Dermatol. 2007;127:1292–308. doi: 10.1038/sj.jid.5700807. [DOI] [PubMed] [Google Scholar]
- 117.Tojo G, Fujimura T, Kambayashi Y, Aiba S. Systemic Lupus Erythematosus Accompanied by Psoriasis Induces IL-27-Producing Cells in Both Affected Areas of the Skin. Case reports in dermatology. 2012;4:181–5. doi: 10.1159/000342803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Shibata S, Tada Y, Kanda N, Nashiro K, Kamata M, Karakawa M, Miyagaki T, Kai H, Saeki H, Shirakata Y, Watanabe S, Tamaki K, Sato S. Possible roles of IL-27 in the pathogenesis of psoriasis. J Invest Dermatol. 2010;130:1034–9. doi: 10.1038/jid.2009.349. [DOI] [PubMed] [Google Scholar]
- 119.Fujiwara S, Nagai H, Oniki S, Yoshimoto T, Nishigori C. Interleukin (IL)-17 versus IL-27: opposite effects on tumor necrosis factor-alpha-mediated chemokine production in human keratinocytes. Exp Dermatol. 2012;21:70–2. doi: 10.1111/j.1600-0625.2011.01384.x. [DOI] [PubMed] [Google Scholar]
- 120.Wittmann M, Doble R, Bachmann M, Pfeilschifter J, Werfel T, Muhl H. IL-27 Regulates IL-18 binding protein in skin resident cells. PloS one. 2012;7:e38751. doi: 10.1371/journal.pone.0038751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Shibata S, Tada Y, Asano Y, Yanaba K, Sugaya M, Kadono T, Kanda N, Watanabe S, Sato S. IL-27 activates Th1-mediated responses in imiquimod-induced psoriasis-like skin lesions. J Invest Dermatol. 2013;133:479–88. doi: 10.1038/jid.2012.313. [DOI] [PubMed] [Google Scholar]
- 122.Foulis AK. Pancreatic pathology in type 1 diabetes in human. Novartis Found Symp. 2008;292:2–13. discussion 13-8, 122-9, 202-3. [PubMed] [Google Scholar]
- 123.Santos AS, Melo ME, Crisostomo LG, Fukui RT, Matioli SR, Silva ME. Lack of association between IL27 gene variants and type 1 diabetes susceptibility. Cytokine. 2013;61:349–52. doi: 10.1016/j.cyto.2012.12.003. [DOI] [PubMed] [Google Scholar]
- 124.Fujimoto H, Hirase T, Miyazaki Y, Hara H, Ide-Iwata N, Nishimoto-Hazuku A, Saris CJ, Yoshida H, Node K. IL-27 inhibits hyperglycemia and pancreatic islet inflammation induced by streptozotocin in mice. Am J Pathol. 2011;179:2327–36. doi: 10.1016/j.ajpath.2011.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Wang R, Han G, Wang J, Chen G, Xu R, Wang L, Li X, Shen B, Li Y. The pathogenic role of interleukin-27 in autoimmune diabetes. Cell Mol Life Sci. 2008;65:3851–60. doi: 10.1007/s00018-008-8540-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Nussenblatt RB, Fortin E, Schiffman R, Rizzo L, Smith J, Van Veldhuisen P, Sran P, Yaffe A, Goldman CK, Waldmann TA, Whitcup SM. Treatment of noninfectious intermediate and posterior uveitis with the humanized anti-Tac mAb: a phase I/II clinical trial. Proceedings of the National Academy of Sciences of the United States of America. 1999;96:7462–6. doi: 10.1073/pnas.96.13.7462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Amadi-Obi A, Yu CR, Liu X, Mahdi RM, Clarke GL, Nussenblatt RB, Gery I, Lee YS, Egwuagu CE. TH17 cells contribute to uveitis and scleritis and are expanded by IL-2 and inhibited by IL-27/STAT1. Nature medicine. 2007;13:711–8. doi: 10.1038/nm1585. [DOI] [PubMed] [Google Scholar]
- 128.Caspi RR. Understanding autoimmunity in the eye: from animal models to novel therapies. Discovery medicine. 2014;17:155–62. [PMC free article] [PubMed] [Google Scholar]
- 129.Sonoda KH, Yoshimura T, Takeda A, Ishibashi T, Hamano S, Yoshida H. WSX-1 plays a significant role for the initiation of experimental autoimmune uveitis. International immunology. 2007;19:93–8. doi: 10.1093/intimm/dxl125. [DOI] [PubMed] [Google Scholar]
- 130.Takeda A, Hasegawa E, Fukuhara T, Hirakawa S, Yamada H, Yang Y, Yoshimura T, Hisatomi T, Oshima Y, Yoshida H, Sonoda KH, Ishibashi T. EBI3 is pivotal for the initiation of experimental autoimmune uveitis. Experimental eye research. 2014;125:107–13. doi: 10.1016/j.exer.2014.06.004. [DOI] [PubMed] [Google Scholar]
- 131.Wang RX, Yu CR, Mahdi RM, Egwuagu CE. Novel IL27p28/IL12p40 cytokine suppressed experimental autoimmune uveitis by inhibiting autoreactive Th1/Th17 cells and promoting expansion of regulatory T cells. The Journal of biological chemistry. 2012;287:36012–21. doi: 10.1074/jbc.M112.390625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Lee YS, Amadi-Obi A, Yu CR, Egwuagu CE. Retinal cells suppress intraocular inflammation (uveitis) through production of interleukin-27 and interleukin-10. Immunology. 2011;132:492–502. doi: 10.1111/j.1365-2567.2010.03379.x. [DOI] [PMC free article] [PubMed] [Google Scholar]