Abstract
Objectives
Pulmonary edema is a common sign of heart failure and can be quantified by counting vertical artifacts (B-lines) on lung ultrasound (LUS). The primary aim of this study was to compare a pocket-size ultrasound device to high-end ultrasound systems on the measured number of B-lines. We also compared the impact of different length ultrasound clips on the measured number of B-lines.
Methods and Results
We studied 21 hospitalized heart failure patients (81% men, median age 73, 71% Caucasian) who underwent concurrent 8-and 4-zone LUS using both a pocket ultrasound device and a high-end ultrasound system. For the 4-zone scanning method, the median B-line number was 2 (interquartile range 1–4) for the pocket device and 3 (1–5) for the high-end system (p=0.67). For the 8-zone method, the median B-line number was 4 (2–7) for the pocket device and 5 (3–7) for the high-end system (p=0.18). A higher number of B-lines was identified on the 4- vs 2-second LUS clips (P < 0.001 for 4 zones, P = 0.001 for 8 zones), and on the 6- vs 4-second LUS clips (P=0.057 for 4 zones, P=0.018 for 8 zones).
Conclusions
Our findings suggest significant differences based on LUS clip duration rather than the type of ultrasound device used, with respect to the number of B-lines detectable in patients with heart failure. These factors should be considered in the design and reporting of LUS studies and in longitudinal assessments of heart failure patients.
Keywords: Lung ultrasound, pocket ultrasound device, heart failure
Introduction
Pulmonary edema is one of the most common signs of acute heart failure.1 However, assessment of acute heart failure is challenging for both clinicians and researchers due to the lack of a quantitative diagnostic gold standard. Although physical examination and chest x-ray are routinely used in the assessment of patients with known or suspected pulmonary edema, these methods are qualitative and thus intrinsically insensitive for detecting pulmonary congestion.2, 3 Lung ultrasound (LUS) represents a relatively novel tool for non-invasively assessing pulmonary edema via quantification of B-lines (vertical lines on LUS that arise from the pleural line and can be enumerated in an intercostal space).4–7 Compared to the physical examination and chest x-ray, quantification of B-lines in LUS has demonstrated superior sensitivity and specificity in the identification of a cardiogenic etiology in patients presenting with undifferentiated dyspnea. Furthermore, this method could allow for monitoring of dynamic changes resulting from treatment of pulmonary edema.8, 9
Although prior studies have demonstrated good inter- and intra-observer reproducibility of LUS findings in various populations, the impact of technical factors on the number of B-lines detectable via standardized LUS remains unclear.4, 10–12 While considering the potential utility of LUS in the assessment and monitoring of pulmonary edema, an understanding of the technical factors that may impact the number of measurable B-lines on LUS is essential. The use of a pocket-size ultrasound device could allow for rapid non-invasive assessment and serial examinations of pulmonary edema in a variety of clinical settings, including outpatient clinics, emergency department observation units, and inpatient units. However, it is uncertain whether use of such a device might sacrifice reliability of image quality for the sake of convenience when compared to high-end ultrasound machines. Similarly, shorter scanning times involving shorter duration clips would be attractive both for clinicians and researchers; however, it remains unknown if clip duration significantly affects the number of B-lines observed. Therefore, the primary aim of this study was to compare a pocket-size ultrasound device to high-end ultrasound systems on the measured number of B-lines. Our secondary aim was to compare the impact of different length ultrasound clips on the measured number of B-lines.
Methods
Study setting and population
Between May 2013 and September 2014, we enrolled patients with a history of heart failure, age ≥18 years at the time of enrollment, who were scheduled for clinically indicated transthoracic echocardiography during hospitalization. Heart failure was defined as a current or prior diagnosis of heart failure, as document by a physician in the medical record based on the presence of clinical examination (i.e. elevated jugular venous pressure, rales, edema) and diagnostic signs and symptoms regardless of ejection fraction.2 Patients were excluded from the study if any of the following criteria were present: in situ left ventricular assist device; prior heart transplantation; in situ chest drains or current pneumothorax; recent major chest trauma; active pneumonia, lung or pleural cancer; current hemodialysis or peritoneal dialysis; liver failure; pulmonary fibrosis; pregnancy; or, unwilling or unable to provide informed consent. Eligible participants were identified via the daily echocardiography laboratory scheduling system and review of electronic medical records. This was a prospective, observational study designed specifically to investigate the impact of variation in ultrasound system and clip duration on detection of B lines. All study participants provided informed consent and the study protocol was approved by the local Institutional Review Board.
Study protocol
Lung ultrasonography and echocardiography
Immediately before or after routine echocardiography, LUS was performed in 8 chest zones (4 for each hemi-thorax) as previously reported and recommended by an international consensus statement.6 An abbreviated 4-zone protocol was also evaluated as previously described.13 Patient positioning was kept constant between the pocket device and high-end system LUS examination.13 Trained investigators performed the LUS scans (EmP, JP, AM) according to a standardized imaging protocol and using both a pocket-sized ultrasound device (VScan, General Electric) and a high-end ultrasound system (Philips, General Electric, or Siemens) equipped for routine echocardiographic examinations. Phased array transducers (Pocket device: 1.7–3.8 MHz; High-end: 2–5 MHz) were used for image acquisition with both types of ultrasound systems. The pocket device only allows for 2 second ultrasound clip recording, whereas the high-end system allows recording of 6 second ultrasound clips that were then cropped into 2 second, 4 second, and 6 second clips wherever possible. All images were analyzed offline by a trained investigator (AAM) after all study subjects had been enrolled. We grouped LUS images by imaging device type and by clip duration, and each group was analyzed by a blinded reader on separate days, at least 2 days apart, to minimize bias. We were unable to blind the reviewer to type of machine or clip length itself because these features were inherent characteristics of the clips that could not be removed.
For B-line analyses, the highest number of B-lines (vertical lines arising from the pleural line) for a single intercostal space per LUS clip was counted after review of the entire LUS clip (Figure 1). The sum of B-lines in 4 zones (2 apical and 2 inferolateral) as well as in 8 zones was used for the primary analyses (Figure 2). Zones without clearly visualized lung, absent lung sliding, or with pleural effusions were excluded from the analyses (n=12 study subjects). Only patients with LUS data available for all 4 or 8 zones were included in the primary analyses. Interobserver correlation has been previously reported by our group (r=0.92).10 Left ventricular ejection fraction was reported as documented by the attending cardiologist on the same day as the LUS examination.
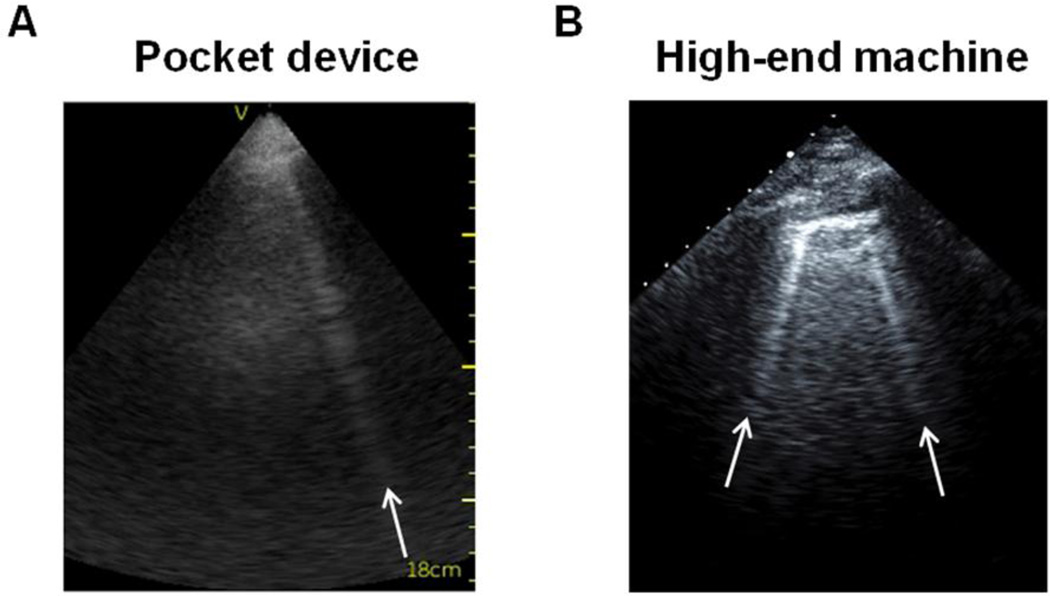
Figure 1.
B-lines on lung ultrasound performed with a pocket device (Panel A) and a high-end ultrasound system (Panel B).
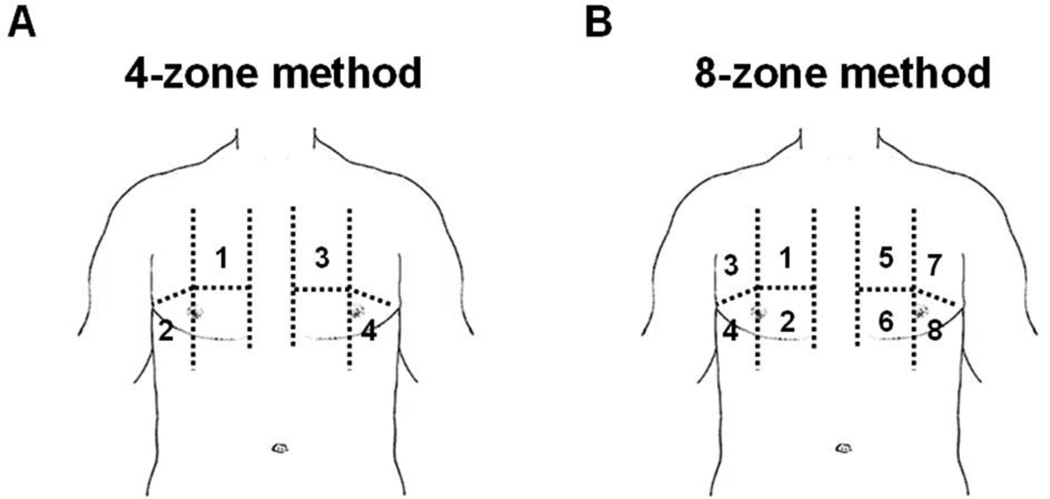
Figure 2.
Lung ultrasound using either the 4-zone (Panel A) or 8-zone (Panel B) assessment method.
Clinical and demographic characteristics
Clinical and demographic data were obtained from electronic medical record review. Body mass index (BMI) was calculated using height and weight documented in the medical record. Laboratory test results were only included in analyses if they were obtained within 7 days of the LUS and documented in the medical record.
Statistical analysis
Continuous variables are presented as medians and interquartile range and categorical variables as counts and percentages. For the primary analyses, the sum of B-lines in 4 and 8 zones was reported for pairwise comparisons between: 1) the pocket device and the high-end ultrasound system, using data collected from 2 second clips; 2) 2 second and 4 second clips, using data collected from the high-end ultrasound system; and, 3) 4 second and 6 second clips, using data collected from the high-end ultrasound system. Wilcoxon rank sum tests were used for pairwise comparisons between the sum of B-lines enumerated for the above described groups. Bland-Altman analyses were used to calculate mean differences and 95% limits of agreement for each pairwise comparison. A two-sided significance level of 0.05 was used for all analyses, and all data were analyzed using Stata SE version 12.1 (StataCorp, Texas 2011).
Funding and role of sponsors
This work was supported by an American Heart Association grant 13CRP14330000 (EP), NHLBI grant R00-HL-107642 (SC), and a grant from the Ellison Foundation (SC). The sponsors had no input or contribution in the development of the research and manuscript.
Results
Of the 37 patients enrolled in the study, 33 had LUS clips recorded at the time of echocardiography and a total of 21 patients had adequate LUS images in all 4 zones and 20 patients had adequate images in all 8 zones. Characteristics for the study sample are shown in Table 1. The median age of study participants was 73 years (range 36–86), 81% were men, 71% had hypertension, and 71% had a reduced left ventricular ejection fraction at the time of LUS. All patients had an active diagnosis of heart failure: 52% had a history of prior heart failure hospitalization, and 62% were hospitalized for acute heart failure during the index admission.
Table 1.
Sample characteristics
| Total Sample (n=21) |
|
|---|---|
| Demographic and clinical characteristics | |
| Age, years | 73 (36–86) |
| Men, n (%) | 17 (81) |
| Race, n (%) | |
| Non-Hispanic white | 15 (71) |
| Non-Hispanic black | 3 (14) |
| Hispanic | 3 (14) |
| Body mass index, kg/m2 | 26 (23–33) |
| Heart rate, beats/minute | 72 (60–82) |
| Systolic blood pressure, mmHg | 118 (100–139) |
| Diastolic blood pressure, mmHg | 65 (60–74) |
| Respiratory rate, breaths/minute | 18 (18–20) |
| Medical history, n (%) | |
| Prior admission for heart failure | 11 (52) |
| Current admission for acute heart failure | 13 (62) |
| Hypertension | 15 (71) |
| Diabetes mellitus | 9 (43) |
| Myocardial infarction | 7 (33) |
| Coronary artery bypass surgery | 8 (38) |
| Atrial fibrillation | 12 (57) |
| Chronic obstructive pulmonary disease | 6 (29) |
| Sleep apnea | 2 (10) |
| Cancer | 2 (10) |
| Laboratory characteristics | |
| Hemoglobin, g/dL | 12.7 (10.4–13.6) |
| Hematocrit, % | 38 (33–41) |
| Sodium, mg/dL | 137 (136–139) |
| Blood urea nitrogen, mg/dL | 27 (20–39) |
| Creatinine, mg/dL | 1.2 (1.1–1.6) |
| NT-proBNP, pg/mL* | 3822 (2099–7238) |
| Echocardiographic characteristics | |
| LV ejection fraction, % | 30 (20–45) |
| Ejection fraction <40% | 15 (71) |
Values are presented as medians (with interquartile range) for continuous variables and counts (with percentages) for categorical variables.
Data shown are for the subset with biomarker measures available (n=10).
Comparison of pocket and high-end ultrasound systems
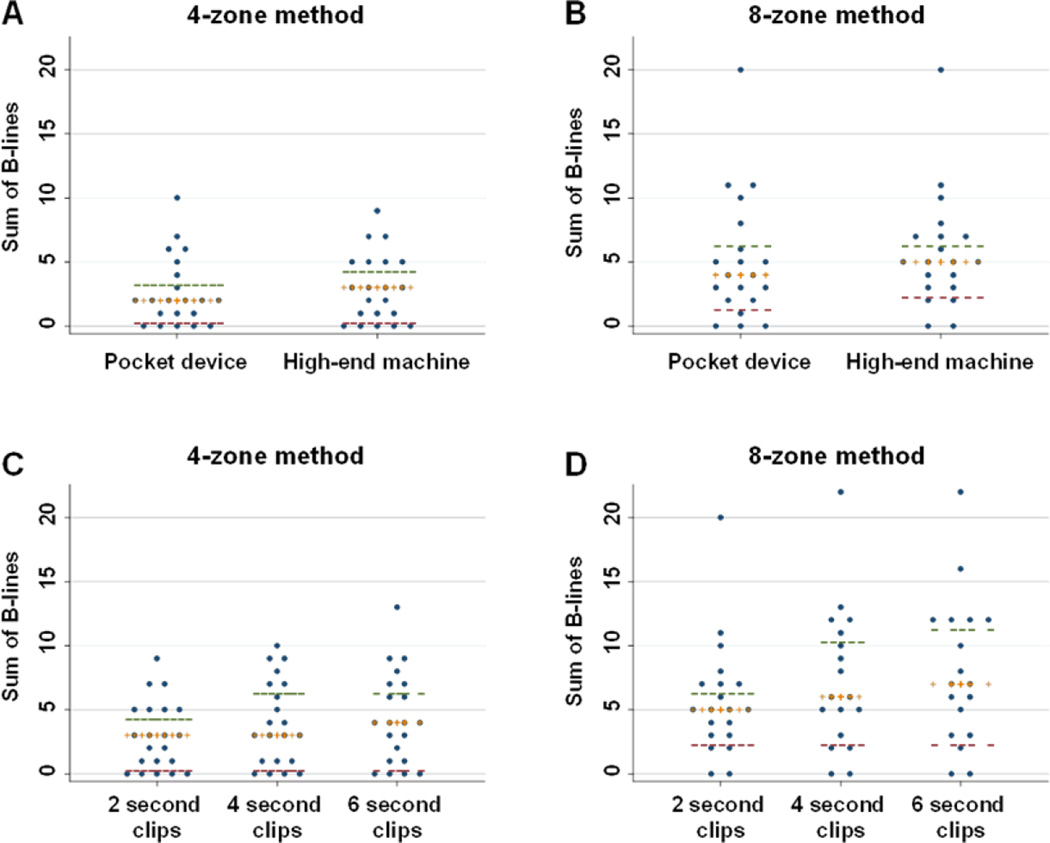
For the 4-zone method, the median B-line number was 2 (interquartile range 1–4) for the pocket device and 3 (1–5) for the high-end system (Table 2, Figure 3). For the 8-zone method, the median B-line number was 4 (2–7) with the pocket device and 5 (3–7) with the high-end system; despite a numeric trend towards greater B-line number with the high-end system, this finding did not reach statistical significance (p=0.67 for 4 zones, p=0.18 for 8 zones). There was a mean difference of 0.3 B-lines (95% limits of agreement: −4.1, 4.7) between the pocket device and high-end ultrasound system for the 4-zone method and a mean difference of 0.6 B-lines (95% limits of agreement: −4.0, 5.3) for the 8-zone method.
Table 2.
Number of B-lines detected by number of zones scanned and by ultrasound device (2 second clips)
| Pocket device | High-end machine | P value* | |
|---|---|---|---|
| Sum of B-lines in 4 zones | 2 (1–4) | 3 (1–5) | 0.67 |
| Sum of B-lines in 8 zones† | 4 (2–7) | 5 (3–7) | 0.18 |
Values shown are median number of B-lines (interquartile range).
Wilcoxon signed-rank test
Subset with 8 zones scanned included n=20.
Figure 3.
The sum of detected B-lines are shown for the same patients scanned with the 4-zone and 8-zone method using the pocket device versus high-end machine (Panels A and B), and using clips of varying duration (Panels C and D). Cross bars represent medians, and horizontal dashes represent upper and lower interquartile range values.
Comparison of different clip lengths on high-end ultrasound system
When assessing number of B-lines by varying clip duration, for the 4-zone method, the median B-line number was 3 (interquartile range 1–5), 3 (1–7), and 4 (1–7) for the 2, 4, and 6 second clips, respectively (Table 3, Figure 3). The number of B-lines was significantly higher for 4 compared to 2 second clips (p<0.001), and the difference between 6 compared to 4 second clips was borderline statistically significant (p=0.057). For the 8-zone method, the median B-line number was 5 (3–7), 6 (3–11), and 7 (3–12) for 2, 4, and 6 second clips, respectively. The number of B-lines was significantly higher for 4 compared to 2 second clips (p=0.001), and for 6 versus 4 second clips (p=0.018).
Table 3.
Number of B-lines by number of zones scanned and by ultrasound clip length (high-end ultrasound machine)
| 2 second clip | 4 second clip | 6 second clip | P value* (2 vs. 4 second) |
P value* (4 vs. 6 second) |
|
|---|---|---|---|---|---|
| Sum of B-lines in 4 zones | 3 (1–5) | 3 (1–7) | 4 (1–7) | <0.001 | 0.057 |
| Sum of B-lines in 8 zones† | 5 (3–7) | 6 (3–11) | 7 (3–12) | 0.001 | 0.018 |
Values shown are median number of B-lines (interquartile range).
Wilcoxon signed-rank test
Subset with 8 zones scanned included n=20.
Discussion
Lung ultrasound has been recognized as useful method for the identification and quantification of pulmonary edema in patients with heart failure. Factors that potentially impact LUS findings in this population are critically important, especially for measuring dynamic changes over time and as part of the clinical assessment of response to heart failure therapy in a variety of clinical settings, including emergency department observation units and inpatient units. Our study findings suggest that the number of B-lines detected by LUS may be impacted by both the type of ultrasound device used and the duration of ultrasound clip recordings.
Prior studies have compared the pocket ultrasound device with high-end ultrasound systems with respect to echocardiographic findings. In general, good agreement has been observed between the two devices for point-of-care assessment of left ventricular ejection fraction, presence of pericardial effusion, inferior vena cava size, and screening for significant valvular disease such as aortic stenosis.14–16 However, limited accuracy was noted for the degree of valvular lesions on B-mode, which may be related to the fact that the current pocket-size devices do not have spectral Doppler capabilities.16 Importantly, LUS findings were not investigated in these prior studies.
Although several studies have investigated the diagnostic utility of LUS in patients presenting with dyspnea and potentially harboring a diagnosis of acute heart failure,17 no prior studies have specifically compared the performance of pocket-size versus high-end ultrasound systems in this patient population. Because B-lines on LUS can reflect the severity of interstitial lung disease as well as pulmonary edema, Cogliati et al. examined such pathologic lung findings on chest computed tomography compared with both high-end and pocket ultrasound systems in 29 patients with known or suspected interstitial lung disease.18 With high-resolution computed tomography considered the imaging gold standard for interstitial lung disease, the pocket device demonstrated similar sensitivity (89%, 95% CI 68–100, vs. 69%, 95% CI 44–94) but lower specificity (50%, 95% CI 28–72, vs. 88%, 95% CI 76–100) when compared to high-end ultrasound. Cohen’s kappa for B-line score between the pocket device and high-end system for LUS was 0.78 in this study. Although our data suggest that fewer B-lines may be detectable using the pocket device versus high-end ultrasound system, we observed that this difference was not statistically significant for both the 4 and 8 zone methods and similar to results reported by prior studies.
In addition to type of device, duration of clip recording is also an important factor that can impact B-line number. We observed a significantly higher number of detectable B-lines when longer ultrasound clips were analyzed. There was on average 1 additional B-line visible on 4 compared to 2 second clips using both the 4 and 8 zone methods, with similar findings for the 6 compared to 4 second clips using the 8 zone method. Although we could not compare the number of Blines to a diagnostic gold standard for pulmonary edema, our findings suggest that 4 and possibly 6 second LUS clips may be preferable over 2 second clips for standardized B-line quantification. This difference between number of B-lines in different length clips should be considered in the study design and reporting of LUS findings in acute heart failure. The impact of clip length could be circumvented in studies were real-time interpretation of LUS clips is performed at the bedside as demonstrated in a recent study of chronic heart failure patients where LUS images acquired with a pocket device were interpreted in real time.19 However, our data suggest that clip length should be considered in studies with offline image analysis blinded to clinical findings. If a pocket device is used in the assessment of patients with known or suspected pulmonary edema, different cut-off values than previously published for high-end systems may need to be used when offline image analysis is employed. In addition, since total B-line number between high-end systems and pocket-devices may not be interchangeable (due to shorter clip duration), longitudinal assessment should likely be performed with the same type of device. Device selection and consistency would be important, for instance, for monitoring the number of B-lines during treatment for acute heart failure.
Limitations
Our findings should be interpreted within the context of the study design. Our study is limited by its small sample size, while conducted in a well characterized cohort of hospitalized patients with previous or current acute heart failure, our findings should be considered hypothesis generating. Because not all study subjects were hospitalized for acute heart failure and since, at the time of the LUS, those with acute heart failure were already receiving treatment for heart failure our cohort likely demonstrated a lower number of B-lines than dyspneic heart failure patients presenting acutely to the Emergency Department. Despite this patient heterogeneity, we were able to still address the overall primary and secondary objectives of this methodological study given the sufficient number of B-lines detected across all patients in this heart failure sample. In addition, not all study subjects had 6 second LUS clips recorded on the high-end ultrasound systems. We were unable to blind the reviewer to type of ultrasound device used or clip length because these features were inherent characteristics of the recorded ultrasound clips. Although we did not formally assess interrater agreement in this study, our group has reported excellent interobserver correlation (r=0.92) for B-line quantification in prior lung ultrasound studies.10 To verify and expand upon our findings, further studies are needed in larger cohorts of patients presenting with either acute or chronic heart failure.
Conclusions
In conclusion, our study results suggest no significant differences among patients with heart failure in the number of detectable B-lines based on whether a pocket device or high-end ultrasound system is used. However, our findings indicate a substantial difference based on LUS clip duration, with a significantly greater number of B-lines detectable in longer compared to shorter clips. Therefore, these factors should be considered in the design and reporting of LUS studies and in longitudinal assessments of patients with heart failure.
Abbreviations
- BMI
Body mass index
- LUS
Lung ultrasound
- LV
Left ventricle
- MHz
Megahertz
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Gheorghiade M, Follath F, Ponikowski P, et al. Assessing and grading congestion in acute heart failure: a scientific statement from the acute heart failure committee of the heart failure association of the European Society of Cardiology and endorsed by the European Society of Intensive Care Medicine. Eur J Heart Fail. 2010;12:423–433. doi: 10.1093/eurjhf/hfq045. [DOI] [PubMed] [Google Scholar]
- 2.McMurray JJ, Adamopoulos S, Anker SD, et al. ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure 2012: The Task Force for the Diagnosis and Treatment of Acute and Chronic Heart Failure 2012 of the European Society of Cardiology. Developed in collaboration with the Heart Failure Association (HFA) of the ESC. Eur Heart J. 2012;33:1787–1847. doi: 10.1093/eurheartj/ehs104. [DOI] [PubMed] [Google Scholar]
- 3.Wang CS, FitzGerald JM, Schulzer M, Mak E, Ayas NT. Does this dyspneic patient in the emergency department have congestive heart failure? JAMA. 2005;294:1944–1956. doi: 10.1001/jama.294.15.1944. [DOI] [PubMed] [Google Scholar]
- 4.Liteplo AS, Marill KA, Villen T, et al. Emergency thoracic ultrasound in the differentiation of the etiology of shortness of breath (ETUDES): sonographic B-lines and N-terminal pro-braintype natriuretic peptide in diagnosing congestive heart failure. Acad Emerg Med. 2009;16:201–210. doi: 10.1111/j.1553-2712.2008.00347.x. [DOI] [PubMed] [Google Scholar]
- 5.Gargani L, Frassi F, Soldati G, Tesorio P, Gheorghiade M, Picano E. Ultrasound lung comets for the differential diagnosis of acute cardiogenic dyspnoea: a comparison with natriuretic peptides. Eur J Heart Fail. 2008;10:70–77. doi: 10.1016/j.ejheart.2007.10.009. [DOI] [PubMed] [Google Scholar]
- 6.Volpicelli G, Elbarbary M, Blaivas M, et al. International evidence-based recommendations for point-of-care lung ultrasound. Intensive Care Med. 2012;38:577–591. doi: 10.1007/s00134-012-2513-4. [DOI] [PubMed] [Google Scholar]
- 7.Platz E, Hempel D, Pivetta E, Rivero J, Solomon SD. Echocardiographic and Lung Ultrasound Characteristics in Ambulatory Patients with Dyspnea or Prior Heart Failure. Echocardiography. 2014;31:133–139. doi: 10.1111/echo.12346. [DOI] [PubMed] [Google Scholar]
- 8.Volpicelli G, Caramello V, Cardinale L, Mussa A, Bar F, Frascisco MF. Bedside ultrasound of the lung for the monitoring of acute decompensated heart failure. Am J Emerg Med. 2008;26:585–591. doi: 10.1016/j.ajem.2007.09.014. [DOI] [PubMed] [Google Scholar]
- 9.Noble VE, Murray AF, Capp R, Sylvia-Reardon MH, Steele DJ, Liteplo A. Ultrasound assessment for extravascular lung water in patients undergoing hemodialysis. Time course for resolution. Chest. 2009;135:1433–1439. doi: 10.1378/chest.08-1811. [DOI] [PubMed] [Google Scholar]
- 10.Platz E, Lattanzi A, Agbo C, et al. Utility of lung ultrasound in predicting pulmonary and cardiac pressures. Eur J Heart Fail. 2012;14:1276–1284. doi: 10.1093/eurjhf/hfs144. [DOI] [PubMed] [Google Scholar]
- 11.Anderson KL, Fields JM, Panebianco NL, Jenq KY, Marin J, Dean AJ. Inter-rater reliability of quantifying pleural B-lines using multiple counting methods. J Ultrasound Med. 2013;32:115–120. doi: 10.7863/jum.2013.32.1.115. [DOI] [PubMed] [Google Scholar]
- 12.Mallamaci F, Benedetto FA, Tripepi R, et al. Detection of pulmonary congestion by chest ultrasound in dialysis patients. JACC Cardiovasc Imaging. 2010;3:586–594. doi: 10.1016/j.jcmg.2010.02.005. [DOI] [PubMed] [Google Scholar]
- 13.Frasure SE, Matilsky DK, Siadecki SD, Platz E, Saul T, Lewiss RE. Impact of patient positioning on lung ultrasound findings in acute heart failure. Eur Heart J Acute Cardiovasc Care. 2014 doi: 10.1177/2048872614551505. [DOI] [PubMed] [Google Scholar]
- 14.Biais M, Carrie C, Delaunay F, Morel N, Revel P, Janvier G. Evaluation of a new pocket echoscopic device for focused cardiac ultrasonography in an emergency setting. Crit Care. 2012;16:R82. doi: 10.1186/cc11340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Abe Y, Ito M, Tanaka C, et al. A novel and simple method using pocket-sized echocardiography to screen for aortic stenosis. J Am Soc Echocardiogr. 2013;26:589–596. doi: 10.1016/j.echo.2013.03.008. [DOI] [PubMed] [Google Scholar]
- 16.Prinz C, Voigt JU. Diagnostic accuracy of a hand-held ultrasound scanner in routine patients referred for echocardiography. J Am Soc Echocardiogr. 2011;24:111–116. doi: 10.1016/j.echo.2010.10.017. [DOI] [PubMed] [Google Scholar]
- 17.Filopei J, Siedenburg H, Rattner P, Fukaya E, Kory P. Impact of pocket ultrasound use by internal medicine housestaff in the diagnosis of Dyspnea. J Hosp Med. 2014 Sep;9(9):594–597. doi: 10.1002/jhm.2219. Epub 2014 Jun 3. [DOI] [PubMed] [Google Scholar]
- 18.Cogliati C, Antivalle M, Torzillo D, et al. Standard and pocket-size lung ultrasound devices can detect interstitial lung disease in rheumatoid arthritis patients. Rheumatology (Oxford) 2014;53:1497–1503. doi: 10.1093/rheumatology/keu033. [DOI] [PubMed] [Google Scholar]
- 19.Gustafsson M, Alehagen U, Johansson P. Imaging congestion with a pocket ultrasound device - prognostic implications in patients with chronic heart failure. J Card Fail. 2015 doi: 10.1016/j.cardfail.2015.02.004. [DOI] [PubMed] [Google Scholar]