Abstract
Background and Aims
Little is known about the change in risk conferred by family history of colorectal cancer (CRC) as a person ages. We evaluated the effect of family history on CRC incidence and mortality after age 55 y, when the risk of early onset cancer had passed.
Methods
We collected data from participants in the randomized, controlled Prostate, Lung, Colorectal and Ovarian cancer screening trial of flexible sigmoidoscopy vs usual care (55–74 y old, no history of CRC), performed at 10 US centers from 1993 to 2001. A detailed family history of colorectal cancer was obtained at enrollment and subjects were followed for CRC incidence and mortality for up to 13 years.
Results
Among 144,768 participants, 14,961 (10.3%) reported a family of CRC. Of 2090 incident cases, 273 had a family history of CRC (13.1%); among 538 deaths from CRC, 71 (13.2%) had a family history of CRC. Overall, family history of CRC was associated with an increased risk of CRC incidence (hazard ratio [HR], 1.30; 95% confidence interval [CI], 1.10–1.50; P<.0001) and increased mortality (HR, 1.31; 95% CI, 1.02–1.69; P=.03). Subjects with 1 first-degree relative (FDR) with CRC (n=238; HR, 1.23; 95% CI, 1.07–1.42) or ≥2 FDRs with CRC (n=35; HR, 2.04; 95% CI, 1.44–2.86) were at increased risk for incident CRC. However, among individuals with 1 FDR with CRC, there was no difference in risk based on the age at diagnosis in the FDR (for FDR age <60 y: HR, 1.27; 95% CI, 0.97–1.63; for FDR age 60–70 y: HR, 1.33; 95% CI, 1.06–1.62; for FDR >70 y: HR, 1.14; 95% CI, 0.93–1.45; Ptrend=.59).
Conclusion and Relevance
After an age of 55 y, subjects with 1 FDR with CRC had only a modest increase in risk for CRC incidence and death; age of onset in the FDR was not significantly associated with risk. Individuals with ≥2 FDRs with CRC had continued increased risk in older age. Guidelines and clinical practice for subjects with a family history of CRC should be modified to align CRC testing to risk. Clinical Trials.gov number, NCT00002540
Keywords: Colon Cancer, screening, genetic, risk factors, adenomatous polyps
INTRODUCTION
A family history (FH) of colorectal cancer (CRC) in a first degree relative (parents, siblings or children) has long been identified as a risk factor for CRC 1,2 Approximately 5–10% of the population have at least 1 affected first degree relative (FDR) with CRC 1,3. The increased risk of CRC conferred by having a family history is thought to be determined by the number of affected relatives, the age of disease onset in the affected relative, and the closeness or degree of relation4–6. The lifetime risk of CRC is approximately 2-fold increased in those with an affected first degree relative with CRC1,2,7–10. The risk increases to a greater degree in individuals with multiple affected first degree relatives or when the first degree relative is diagnosed before age 502,5,7–11.
Little is known about how the risk of CRC in individuals with an affected first degree relative manifests as a subject ages. Current screening recommendations for CRC presume ongoing, increased risk for subjects with ≥2 affected first degree relatives or a first degree relative diagnosed before age 60. Guidelines advise these subjects to undergo colonoscopy screening at age 40 or 10 years before the youngest affected first degree relative, with indefinite, repeated colonoscopy every 5 years 12,13.
A family history of CRC is also often used to justify more intensive screening and surveillance colonoscopy, albeit with uncertain yield 14,15. In the Clinical Outcomes Research Initiative database for example, among subjects with no findings at baseline colonoscopy and a repeat colonoscopy examination within 1 – 5 years (N=7372), 30.1% of exams were performed because of a family history of CRC, and significant lesions were detected infrequently 15.
Our aim was to evaluate the effect of a family history of CRC on incidence and mortality to CRC in later age, in a cohort where the risk of early onset cancer had passed.
METHODS
The Prostate, Lung, Colorectal and Ovarian (PLCO) cancer screening trial of flexible sigmoidoscopy enrolled men and women aged 55 to 74 years with no prior history of CRC at ten screening centers from 1993 to 2001. Individuals were randomized to an intervention or usual care arm. Intervention arm subjects received flexible sigmoidoscopy at baseline and again at year 3 (for those randomized before April, 1995) or year 5. Intervention arm subjects also received annual chest-radiograph, prostate-specific antigen (men only), digital rectal exam (men only), CA125 (women only), and trans-vaginal ultrasound (women only) for 4–6 years. Exclusion criteria included a history of prostate, lung, colorectal or ovarian cancer. Beginning in 1995, subjects with a colonoscopy, flexible sigmoidoscopy or barium enema within the prior 3 years were ineligible for enrollment. Details of the trial have been previously published 16,17.
Demographics and medical history, including family history of cancer, were ascertained via a baseline questionnaire administered at enrollment. With respect to family history, the questionnaire asked: “Have your parents, children, brothers, sisters, half-brothers or half-sisters ever been diagnosed as having any type of cancer (Do not include basal-cell skin cancer)?” For those responding yes, a chart was provided to document the relationship of the relative, the type of cancer, and the age that the relative was diagnosed with that cancer.
Incident cancers and deaths were ascertained, primarily by means of a mailed Annual Study Update (ASU) questionnaire. Medical records pertaining to diagnosed cancers were reviewed and data on the stage, histology and grade of cancers were abstracted by certified tumor registrars. Information on vital status was supplemented by periodic linkage to the National Death Index. Cause of death was reviewed blinded to study arm, in a formal adjudication process 18. Subjects were followed for 13 years, to December 31, 2009, death, or loss to follow-up, whichever came first. Screening centers obtained written informed consent from each participant and the institutional review board approved the PLCO protocol at each center.
Surveillance colonoscopy utilization and outcome were assessed in a randomly selected subset of subjects in the intervention arm. The details of that investigation have been described previously14,19. The authors had access to the study data and reviewed and approved the final manuscript.
Statistical Analysis
Family history of CRC was defined as a first-degree relative (FDR), i.e., a parent, full sibling or child, with CRC. Subjects not completing the family history section of the baseline questionnaire were excluded. CRC incidence rates per 10,000 person years (PY) of follow-up were computed by CRC family history status; in addition, for subjects with a family history, CRC incidence rates were computed according to the youngest age at diagnosis of the FDR with CRC (< 60, 60–70, >70) and the number of FDRs with CRC. Cox proportional hazards models were utilized to examine the hazard ratio (HR) for incident CRC with a family history of CRC and for characteristics of the family history; covariates included trial arm, gender, age, history of lower endoscopy or fecal-occult blood test (FOBT) in the 3 years preceding enrollment, BMI, and use of non-steroidal anti-inflammatory drugs (NSAIDs) or aspirin. To examine a possible interaction of family history with age (< 65, ≥65), age was treated as a time-varying covariate in the Cox model; thus, we examined whether family history had a differential HR for incident CRC diagnosed in the age range 55–64 versus incident CRC diagnosed in the age range ≥65. We also examined interactions of family history with trial arm and gender. Similar analyses were performed for mortality from CRC.
RESULTS
A total of 154,900 subjects were enrolled in PLCO, of which 144,768 were included on the basis of completed family history information. Of the 144,768 evaluable individuals, 14,961 (10.3%) reported a family history of CRC in at least 1 FDR. Baseline characteristics of study participants with and without a family history are shown in Table 1. Subjects reporting a family history were slightly older, more likely to be women, and more likely to have undergone endoscopic testing and fecal occult blood testing in the 3 years prior to trial enrollment. Subjects with a FH of CRC were evenly distributed into the intervention and control arms of the trial (51.1% vs. 48.9%) and did not differ from subjects without a family history in other demographic categories (age, race, education). Median length of follow-up was 12.5 and 11.8 years for those with and without a family history, respectively.
Table 1.
Baseline Subject Characteristics
| Characteristic | No Family History of CRC (N=129808) | Family History of CRC (N=14961) |
|---|---|---|
| Gender | ||
| Male | 64103 (49.4%) | 6566 (43.9%) |
| Female | 65705 (50.6%) | 8395 (56.1%) |
| Age (yrs) | ||
| 55–59 | 43805 (33.8%) | 4525 (30.3%) |
| 60–64 | 39919 (30.8%) | 4643 (31.0%) |
| 65–69 | 29037 (22.4%) | 3568 (23.9%) |
| 70–74 | 17047 (13.3%) | 2225 (14.9%) |
| Race/Ethnicity | ||
| White (non-Hispanic) | 114660 (88.3%) | 13584 (90.8%) |
| Black (non-Hispanic) | 6715 (5.2%) | 542 (3.6%) |
| Hispanic | 2450 (1.9%) | 215 (1.4%) |
| Asian | 4854 (3.7%) | 540 (3.6%) |
| Other/Unknown | 1129 (0.9%) | 80 (0.5%) |
| Education | ||
| High school grad or less | 38822 (29.9%) | 4649 (31.0%) |
| Some college | 44555 (34.3%) | 5252 (35.1%) |
| College grad | 46202 (35.6%) | 5039 (33.7%) |
| Unknown | 229 (0.2%) | 21 (0.1%) |
| Prior FOBT | ||
| Yes | 50865 (39.2%) | 6364 (42.5%) |
| No | 74814 (57.6%) | 8164 (54.6%) |
| Unknown | 4129 (3.2%) | 433 (2.9%) |
| Prior Lower GI Endoscopy | ||
| Yes | 16420 (12.7%) | 2550 (17.0%) |
| No | 111264 (85.7%) | 12209 (81.6%) |
| Unknown | 2124 (1.6%) | 202 (1.4%) |
| Either prior FOBT or Lower GI Endoscopy* | ||
| Yes | 55990 (43.1%) | 7116 (47.6%) |
| No | 73333 (56.5%) | 7802 (52.2%) |
| Unknown | 485 (0.4%) | 43 (0.3%) |
| NSAID ≥3–4 days/week | ||
| Yes | 56687 (43.7%) | 6457 (43.2%) |
| No | 73011 (56.3 %) | 8491 (56.8%) |
| Unknown | 110 (0.08%) | 13 (0.09%) |
| Trial Arm | ||
| Intervention | 65301 (50.3%) | 7641 (51.1%) |
| Control | 64507 (49.7%) | 7320 (48.9%) |
FOBT or prior sigmoidoscopy, colonoscopy, or barium enema within 3 years of study entry
During screening and follow-up there were 2090 incident cases of CRC. Of these, 273 individuals (13.1%) had a FH of CRC. Overall, a family history of CRC compared with those without a family history was associated with an increased risk of CRC incidence (hazard ratio [HR], 1.30; 95% confidence interval [CI], 1.10–1.50; P<.0001). CRC risk increased with a greater number of affected first degree relatives. Subjects with 1 first-degree relative (FDR) with CRC (n=238; HR, 1.23; 95% CI, 1.07–1.42) or ≥2 FDRs with CRC (n=35; HR, 2.04; 95% CI, 1.44–2.86) were at increased risk for incident CRC (Table 2). The HR based on the age at diagnosis in the affected FDR, including subjects with ≥2 FDR with CRC, was 1.46 (95%CI 1.17–1.81), 1.33 (95%CI 1.09–1.63) and 1.15 (95%CI 0.92–1.44) for subjects with an FDR diagnosed at age <60, 60–70 and > 70 years, respectively. There was no statistically significant trend (p=0.18) towards an increasing risk of CRC with a younger age at diagnosis in the affected FDR.
Table 2.
Relationship Between Family History of CRC and Incident CRC
| # Cases of CRC (N=2090) | Person-years | Rate (per 10,000 PY) | MV1 adjusted Hazard Ratio (95% CI) | p-value | |
|---|---|---|---|---|---|
| Family history of CRC2 | |||||
| No | 1817 | 1423420 | 12.8 | 1.00 (ref) | <0.0001 |
| Yes | 273 | 165057 | 16.5 | 1.30 (1.10–1.50) | |
| # of affected FDRs | |||||
| 0 (No FH) | 1817 | 1423420 | 12.8 | 1.00 (ref) | 0.0083 |
| 1 FDR | 238 | 151995 | 15.7 | 1.23 (1.07–1.42) | |
| ≥2 FDR | 35 | 13062 | 26.8 | 2.04 (1.44–2.86) | |
| Age at diagnosis of affected FDR4 | |||||
| No FH | 1817 | 1423420 | 12.8 | 1.00 (ref) | 0.185 |
| FDR diagnosed at >70 | 88 | 59047 | 14.9 | 1.15 (0.92–1.44) | |
| FDR diagnosed 60–70 | 97 | 57008 | 17.0 | 1.33 (1.09–1.63) | |
| FDR diagnosed <60 | 81 | 45368 | 17.9 | 1.46 (1.17–1.81) | |
Multivariate adjustment including trial arm, age, gender, prior FOBT, prior lower GI endoscopy, NSAID use and BMI
Defined as positive FH in a first degree relative
P-value is p-trend for increasing number of affected FDRs among those with a family history of CRC
When there are more than 1 affected FDR, the age at diagnosis is the youngest affected FDR. In 7 subjects, the age at diagnosis of CRC in the FDR was unknown.
P-value is p-trend for increasing age at diagnosis of FDR. These estimates include subjects with ≥2 FDRs with CRC.
There was no significant interaction of family history with subjects’ age for CRC incidence; family history HR’s were 1.56 for the age range 55–64 versus 1.25 for the age range ≥65 (p=0.13 for interaction).
Among 538 deaths to CRC, 71 (13.2%) had a FH of CRC. As with CRC incidence, CRC mortality was similarly significantly increased among those with a FH of CRC (HR 1.31; 95%CI 1.02–1.69, p=0.03) (Table 3). There was no statistically significantly increased risk of CRC mortality among those with ≥2 affected FDRs compared to those with only one (HR 1.53; 95%CI (0.7–3.3, ptrend=0.68), but there were only 7 deaths among subjects with ≥2 affected FDRs (Table 3). There was no statistically significant trend (p=0.81) towards an increasing risk of mortality to CRC with a younger age at diagnosis in the affected FDR.
Table 3.
Relationship Between Family History of CRC and Mortality to CRC
| # Deaths to CRC (N=538) | Person-years | Rate (per 10,000 PY) | MV1 adjusted Hazard Ratio (95% CI) | p-value | |
|---|---|---|---|---|---|
| Family history of CRC2 | |||||
| No | 467 | 1458514 | 3.20 | 1.00 (ref) | 0.03 |
| Yes | 71 | 169349 | 4.19 | 1.31 (1.02–1.69) | |
| # of affected FDRs | |||||
| 0 (No FH) | 467 | 1458514 | 3.20 | 1.00 (ref) | 0.683 |
| 1 FDR | 64 | 155967 | 4.10 | 1.29 (1.00–1.70) | |
| ≥2 FDR | 7 | 13383 | 5.23 | 1.53 (0.7–3.3) | |
| Age at diagnosis of affected FDR4 | |||||
| No FH | 467 | 1458514 | 3.20 | 1.00 (ref) | 0.815 |
| FDR diagnosed at >70 | 28 | 60443 | 4.6 | 1.50 (1.02–2.2) | |
| FDR diagnosed 60–70 | 19 | 58466 | 3.2 | 1.04 (0.7–1.6) | |
| FDR diagnosed <60 | 24 | 46705 | 5.1 | 1.66 (1.1–2.5) | |
Multivariate adjustment including trial arm, age, gender, prior FOBT, prior lower GI endoscopy, NSAID use and BMI
Defined as positive FH in a first degree relative
P-value is p-trend for increasing number of affected FDRs among those with a family history of CRC
When there are more than 1 affected FDR, the age at diagnosis is the youngest affected FDR
P-value is p-trend for increasing age at diagnosis of FDR. These estimates include subjects with ≥2 FDRs with CRC.
FH of CRC was significantly associated with an increased risk of CRC in both men (RR 1.26; 95%CI 1.05–1.50, p=0.012) and women (HR 1.35; 95%CI 1.12–1.63, p=0.002) (Table 4). Similar point estimates, though not statistically significant for men, were observed for the association of family history and CRC mortality in men (N=307 deaths, HR 1.20; 95%CI 0.8–1.7, p=0.3) and women (N=231 deaths, HR 1.44; 95%CI 1.01–2.0, p=0.04) (Supplemental Table 1). Both men (HR 1.79; 95%CI 1.07–3.00) and women (RR 2.27; 95%CI 1.44–3.57) had a significantly increased risk of CRC incidence with ≥2 affected FDRs. Men and women also had a similar increased risk of CRC with 1 affected FDR (men: HR 1.21; 95%CI 1.00–1.47, women: RR 1.26; 95%CI 1.03–1.54) (Table 4). Similar trends in men and women were observed for the relationship between the age at diagnosis of the affected FDR and the risk of incident CRC (Table 4).
Table 4.
Relationship Between Family History of CRC and Incident CRC by Gender
| # Cases (N) | Person-years | Rate (per 10,000 PY) | MV1 adjusted Hazard Ratio (95% CI) | p-value | |
|---|---|---|---|---|---|
| Men (N = 1204 CRC cases) | |||||
| Family history of CRC | |||||
| No | 1067 | 697453 | 15.3 | 1.00 (Ref) | 0.012 |
| Yes | 137 | 71640 | 19.1 | 1.26 (1.05–1.50) | |
| # of affected FDRs | |||||
| 0 (No FH) | 1067 | 697453 | 15.3 | 1.00 (Ref) | 0.162 |
| 1 | 122 | 66365 | 18.4 | 1.21 (1.00–1.47) | |
| ≥ 2 | 15 | 5276 | 28.4 | 1.79 (1.07–3.00) | |
| Age at diagnosis of affected FDR3 | |||||
| 0 (No FH) | 1067 | 697453 | 15.3 | 1.00 (ref) | 0.302 |
| > 70 | 46 | 24872 | 18.5 | 1.19 (0.90–1.60) | |
| 60–70 | 47 | 26249 | 17.9 | 1.18 (0.87–1.59) | |
| < 60 | 43 | 19074 | 22.5 | 1.52 (1.11–2.08) | |
| Unknown Age | 1 | ||||
| Women (N = 886 CRC cases) | |||||
| Family history of CRC | |||||
| No | 750 | 725967 | 10.3 | 1.00 (ref) | 0.002 |
| Yes | 136 | 93417 | 14.6 | 1.35 (1.12–1.63) | |
| # of affected FDRs | |||||
| 0 (No FH) | 750 | 725967 | 10.3 | 1.00 (ref) | 0.022 |
| 1 | 116 | 85631 | 13.5 | 1.26 (1.03–1.54) | |
| ≥ 2 | 20 | 7786 | 25.7 | 2.27 (1.14–3.57) | |
| Age at diagnosis of affected FDR3 | |||||
| 0 (No FH) | 750 | 725967 | 10.3 | 1.00 (ref) | 0.282 |
| > 70 | 42 | 34175 | 12.3 | 1.10 (0.80–1.52) | |
| 60–70 | 50 | 30759 | 16.3 | 1.52 (1.14–2.04) | |
| < 60 | 38 | 26294 | 14.5 | 1.38 (1.00–1.92) | |
| Unknown Age | 6 | ||||
Multivariate adjustment including trial arm, age, FOBT, prior lower GI endoscopy, NSAID use and BMI
P-value for trend
When there are more than 1 affected FDR, the age at diagnosis is the youngest affected FDR
Among those with a FH of CRC, there were 56 cases of rectal and 217 cases of colon cancer. FH of CRC in a FDR was significantly associated with the risk of colon cancer (HR 1.31; 95%CI 1.41–1.50, p=0.0003); a similar HR was observed for rectal cancer, although it was not statistically significant (HR 1.27; 95%CI 0.95–1.69, p=0.10) (Supplemental Table 2). There was no difference in the association of FH with proximal (HR 1.24; 95%CI 1.03–1.48) as opposed to distal CRC (HR 1.36; 95%CI 1.13–1.64) (Supplemental Table 2).
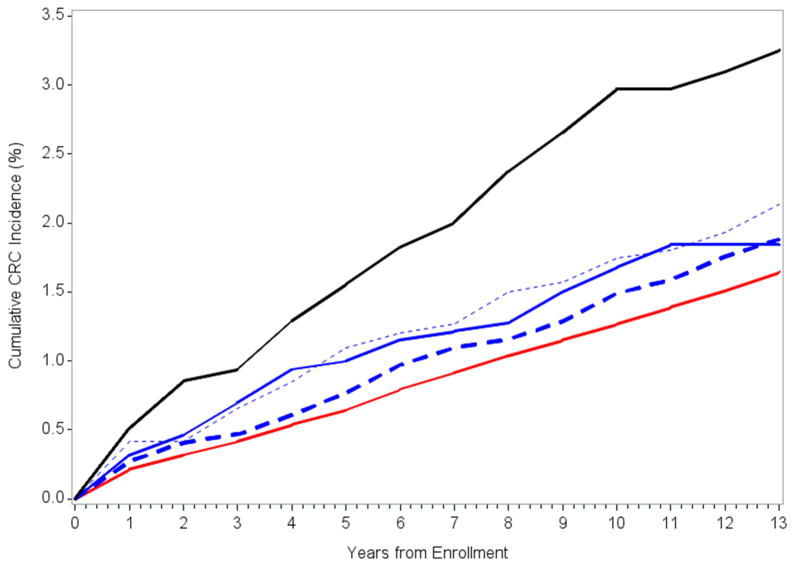
The risk of incident CRC by the age at diagnosis in the FDR was assessed after excluding individuals with ≥ 2 affected FDRs, as they were at higher risk (Table 5). Among individuals with 1 FDR with CRC, there was no difference in risk based on the age at diagnosis in the FDR (HR 1.27; 95%CI 0.97–1.63 for subjects with a FDR diagnosed at age <60, HR 1.33; 95%CI 1.06–1.62 for subjects with FDR diagnosed between 60–70 years, HR 1.14; 95%CI 0.93–1.45 for subjects with FDR diagnosed at age >70; p trend = 0.59). Figure 1 demonstrates the cumulative risk of FH-associated CRC over time, stratified by FH risk group. The absolute increase in CRC incidence was 0.33% (95% CI: 0.10–0.56%) for those with 1 affected FDR and 1.6% (95% CI: 0.6–2.6%) for those with ≥2 affected FDRs.
Table 5.
Relationship Between Family History of CRC and Incident CRC Risk
| Family History Traits | # CRCs (N=2090) | Person Years | Rate (per 10,000 PY) | HR (95% CI) | p-value |
|---|---|---|---|---|---|
| No FH | 1817 | 1423420 | 12.8 | 1.00 (ref) | |
| ≥ 2 affected FDRs | 35 | 13062 | 26.8 | 2.04 (1.44–2.86) | <0.0001 |
| Only 1 affected FDR, FDR < 60 yrs at diagnosis | 59 | 38175 | 15.5 | 1.27 (0.97–1.63) | 0.071 |
| Only 1 affected FDR, FDR 60–70 yrs at diagnosis | 89 | 52764 | 16.9 | 1.33 (1.06–1.62) | 0.0091 |
| Only 1 affected FDR, FDR > 70 yrs at diagnosis | 85 | 57565 | 14.8 | 1.14 (0.93–1.45) | 0.261 |
P trend = 0.59, for subjects with 1 affected FDR
Figure 1.
Cumulative CRC incidence by family history status. Red line is no family history of CRC, black line is ≥2 FDRs with a family history of CRC; blue lines are 1 FDR with a family history classified by the age of onset of CRC in the FDR: age > 70 (blue dashed line), age 60–70 (blue dotted line), age < 60 (blue solid line).
The family history HRs for both CRC incidence and CRC mortality were similar within each trial arm; 1.27 (95% CI: 1.04–1.5) and 1.53 (95% CI: 1.1–2.2) for incidence and mortality, respectively, in the intervention arm versus 1.33 (95% CI: 1.1–1.6) and 1.15 (95% CI: 0.8–1.6) for incidence and mortality in the usual care arm (p-value for interaction of FH by trial arm equals 0.84 for incidence and 0.73 for mortality ). Nor did we identify a statistically significant interaction between FH of CRC and screening history (FOBT or endoscopy) prior to enrollment and CRC incidence, p=0.16.
Because a difference in utilization of surveillance colonoscopy after screening in subjects with compared to those without a family history of CRC could have affected CRC incidence and mortality, surveillance colonoscopy utilization was examined in a randomly selected cohort of subjects (N=3594) in the intervention arm of the PLCO trial 14,19. The use of surveillance colonoscopy by family history and by baseline adenoma findings is depicted in Table 6. At 5 years after baseline colonoscopy, surveillance colonoscopy was utilized by 7.2% more subjects with a family history of CRC compared to those without a family history (53.9% vs. 46.7%). At 7 years the difference was 8.8% and at 10 years the difference was 6.6%.
Table 6.
Use of Surveillance Colonoscopy by Family History and Baseline Adenoma Status (N=3954)
| % with Surveillance Exam After Baseline Colonoscopy | |||||
|---|---|---|---|---|---|
| Family History | Baseline Adenoma Status | N (%) | Within 5 years | Within 7 years | Within 10 years |
| No | 3438 | 46.7 | 61.6 | 67.8 | |
| None | 1191 (34.6) | 37.9 | 54.2 | 61.0 | |
| Non-advanced | 1001 (29.1) | 45.2 | 61.9 | 68.4 | |
| Advanced | 1246 (36.2) | 56.3 | 68.5 | 73.8 | |
| Yes | 516 | 53.9 | 70.4 | 74.4 | |
| None | 186 (36.1) | 43.0 | 62.9 | 68.3 | |
| Non-advanced | 135 (26.2) | 50.4 | 68.9 | 73.3 | |
| Advanced | 195 (37.8) | 66.7 | 78.5 | 81.0 | |
DISCUSSION
In our prospective study, we observed a modest 30% increased risk in CRC incidence and mortality in subjects over age 55 with a family history of CRC in a first degree relative (FDR). Subjects with two first degree relatives with CRC were identified as a high risk group, with a 2-fold increased risk of incident CRC. Within our age cohort, after excluding subjects with ≥2 FDR, a young age of onset in the FDR (<60 years at time of diagnosis) was not associated with a differential increased risk in CRC incidence or mortality compared to subjects with first degree relatives affected at older ages (Table 5). We observed no difference in the risk relationships between a family history of CRC and incident CRC in men compared to women. Nor did we observe a stronger relationship between a family history of colorectal cancer and proximal as opposed to distal cancer or colon as opposed to rectal cancer.
A family history of CRC is used to justify more intensive surveillance colonoscopy in subjects with adenomatous polyps, at times in excess of recommended guidelines,14,15 though evidence of an increased yield in subjects with a family history is unproven20. Given that our data indicate a relatively small increase in cancer incidence or mortality in subjects after age 55 with a family history of CRC, more aggressive surveillance colonoscopy in subjects with a family history of CRC and a history of adenomatous polyps is unlikely to substantially contribute to cancer prevention.
Because CRC incidence as an outcome is potentially subject to lead time and over diagnosis bias, we also evaluated the relationship of CRC mortality to family history of CRC. CRC mortality occurred in 25.7% of incident cases (538/2090), limiting statistical power relative to cancer incidence. A family history of CRC was associated with an increase in CRC mortality (HR=1.31), similar in magnitude to the increased risk observed for CRC incidence, suggesting a limited impact of lead time or over diagnosis bias to our conclusions.
These data derive from a cancer screening trial, with subsequent colonoscopy surveillance provided by local providers. Our estimates of only a modest difference in CRC incidence and mortality between subjects with a family history of CRC compared to those without could be affected if either the effectiveness of screening or surveillance, or the utilization of surveillance after screening were significantly different in subjects with compared to those without a family history of CRC. We did not observe a significant difference in the benefit of screening in subjects with a family history of CRC, as the hazard ratios for CRC incidence and mortality were similar in those with and without a family history. We evaluated surveillance colonoscopy in a randomly selected cohort of nearly 4000 subjects in the screening arm of the trial. In clinical practice, one would expect subjects with a family history of CRC to undergo more surveillance colonoscopy. We observed a small increase in surveillance colonoscopy among those with a family history, ranging from 6.6 – 8.2% more utilization at 5 – 10 years after baseline colonoscopy, an amount that is unlikely to have significantly altered the CRC incidence rates among subjects with a family history in comparison to those without. Furthermore, the benefit of post-polypectomy surveillance colonoscopy on CRC incidence and mortality has not been determined. While randomized trials of CRC screening with stool testing or flexible sigmoidoscopy demonstrate a significant reduction in CRC incidence and mortality 21, the contribution of surveillance colonoscopy has not been evaluated in a clinical trial22. It is even less certain whether post-polypectomy surveillance has a greater effect on outcome in subjects with a family history compared to those without, and in a pooled analysis of multiple trials, there was no difference in detection of advanced adenoma or cancer in subjects with compared to those without a family history20.
Advantages of these data compared to what has been previously available should be acknowledged. Most investigations exploring family history-associated CRC risk are retrospective, case-control studies 2,8,9. In PLCO, individuals were queried about their family history at enrollment, so recall bias regarding the presence of a FH of cancer was minimized. Incident cancers in PLCO were verified by obtaining confirmatory pathologic documentation. The only other prospective study on family history and incident CRC comes from the combined Nurses‘ Health Study and Health Professionals Follow-up Study 1, which followed subjects beginning at an age as young as 30 years old. In that cohort, a family history of CRC in a FDR was associated with a 1.7-fold increased risk of CRC. Only 73 subjects with a family history and CRC were included compared to 273 subjects evaluated here, and the former study included only 45 FH-associated CRC cases over age 55, whereas all of our cases were age 55 or more at enrollment. Thus, the PLCO cohort is by far the largest prospective study on FH associated CRC and is particularly informative of CRC risk amongst middle aged and older subjects.
Our data do not address the need or utility of screening subjects prior to age 55 who have a family history of CRC, since the PLCO trial only enrolled subjects age 55 or older and excluded subjects with a prior history of CRC. Many studies demonstrate a higher risk of colorectal cancer at a young age in subjects with first degree relatives with CRC diagnosed prior to age 501,2,7,8,10. Screening these subjects at young ages, such as 10 years prior to age at diagnosis in the FDR, is recommended by guidelines. In the Nurses‘ Health Study and Health Professionals Follow-up Study prospective cohort study1 for example, there was a marked increase in CRC risk in younger subjects with a family history of CRC. Subjects < 45 years of age with a FH of CRC had a 5-fold increased risk of CRC (N=5, RR 5.37; 95%CI 1.98–14.6), compared to those without a FH of CRC.
Our data address the ongoing risk of incident CRC once the subject has reached age 55, where early onset disease, reflecting a highly penetrant genetic component, has passed. The risk estimate for incident CRC in our cohort (HR=1.3) differs from that in the Nurses and Health Professionals study cohort (RR=1.7) and to the preponderance of retrospective studies included in meta-analyses 2,7. Our lower hazard ratio is likely attributable to the exclusion in our cohort of young onset cancer cases (occurring prior to age <55), and because retrospective studies may be affected by the selection of controls at lower risk of CRC compared to cases, and due to recall bias which may inflate the recollection of a family history of cancer among cases compared to controls.
A recent population-based, colonoscopy based, case control study in Utah demonstrated an overall increased risk of CRC among FDRs compared to controls (HR=1.79) and noted a statistically significant difference in risk in a case-case analysis between cases with FDR’s diagnosed at age <60 (HR=2.11) versus cases with FDR’s diagnosed at age >60 (HR=1.77) (HR 1.5; 95%CI 1.19–1.89 for the comparison between the two)23. Data from Utah also suggested the increased risk with a family history extended beyond FDR to second degree relatives and first cousins23. These data include CRC cases diagnosed at younger ages and selection bias could also account for some of the observed difference. Our prospective results differ, and suggest that as subjects with a FH of CRC age, the likelihood of a highly penetrant, heritable cancer risk is low and screening can be more like that of an average risk individual. A recent prospective evaluation of colonoscopy effectiveness in the Nurses’ Health Study and Health Professionals Follow-up Study demonstrated a reduced incidence of CRC in subjects with a FH of CRC who underwent a colonoscopy within 5 years (n=43, HR 0.44; 95%CI 0.30–0.66) compared to those with colonoscopy more than 5 years ago (n=26, HR 0.91; 95%CI 0.55–1.51), and to subjects with a FH of CRC who did not undergo any prior colonoscopy24. However, one cannot derive recommendations for the optimal timing of colonoscopy from these findings without accounting for the number of affected FDRs, the age of the individuals, and the age of onset in the affected relatives.
Additional limitations of our study include that the family history information was obtained by self-report and not verified. However, previous investigations have demonstrated accuracy in self-reported family history of CRC 25–27. The PLCO population is generally well-educated and predominantly Caucasian, so generalization of these findings to minorities and low income groups may be limited.
In conclusion, individuals with ≥2 FDR with CRC remain at increased risk for CRC into later age. In contrast, after age 55, subjects with 1 FDR with CRC have only a modest increased risk of CRC incidence and mortality compared to those without a FH. Furthermore, after age 55, there was no difference in risk based on the age at diagnosis in the FDR. Our data suggest that increased screening and surveillance colonoscopy in subjects with a FH of CRC in 1 FDR is unlikely to contribute considerably to cancer prevention after age 55. These findings do not impact decisions on when to begin screening, but do suggest that FH-associated CRC risk is only modestly increased once the risk of early onset cancer has passed. Guidelines and clinical practice for subjects with a FH of CRC should be modified to align CRC testing to risk.
Supplementary Material
Acknowledgments
This work was supported by a contract from the Division of Cancer Prevention, National Cancer Institute N01-CN-25511 to the University of Pittsburgh Cancer Institute.
Footnotes
PLCO cancer screening trial registered as: NCT00002540
Disclosures: No authors report any conflict of interest.
Author Contribution:
RES: Study Concept and Design; Acquisition of Data; Analysis and Interpretation of Data; Drafting of the Manuscript; Critical Revision of the Manuscript for Important Intellectual Content; Obtained Funding; Study Supervision
AR: Analysis and Interpretation of Data; Drafting of the Manuscript; Critical Revision of the Manuscript for Important Intellectual Content
KJU: Analysis of Interpretation of Data; Critical Revision of the Manuscript for Important Intellectual Content; Statistical Analysis
SIB: Analysis and Interpretation of Data; Critical Revision of the Manuscript for Important Intellectual Content
KF: Critical Revision of the Manuscript for Important Intellectual Content
TLR: Analysis and Interpretation of Data; Critical Revision of the Manuscript for Important Intellectual Content; Statistical Analysis; Administrative Technical or Material Support
PFP: Study Concept and Design; Analysis and Interpretation of Data; Critical Revision of the Manuscript for Important Intellectual Content; Statistical Analysis; Study Supervision
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Fuchs CS, Giovannucci EL, Colditz GA, et al. A prospective study of family history and the risk of colorectal cancer [see comments] New England Journal of Medicine. 1994;331:1669–74. doi: 10.1056/NEJM199412223312501. [DOI] [PubMed] [Google Scholar]
- 2.Johns LE, Houlston RS. A systematic review and meta-analysis of familial colorectal cancer risk. American Journal of Gastroenterology. 2001;96:2992–3003. doi: 10.1111/j.1572-0241.2001.04677.x. [DOI] [PubMed] [Google Scholar]
- 3.Ramsey SD, Yoon P, Moonesinghe R, Khoury MJ. Population-based study of the prevalence of family history of cancer: implications for cancer screening and prevention. Genetics in Medicine. 2006;8:571–5. doi: 10.1097/01.gim.0000237867.34011.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kune GA, Kune S, Watson LF The Melbourne Colorectal Cancer Study. Characterization of patients with a family history of colorectal cancer. Diseases of the Colon & Rectum. 1987;30:600–6. doi: 10.1007/BF02554806. [DOI] [PubMed] [Google Scholar]
- 5.St John DJ, McDermott FT, Hopper JL, Debney EA, Johnson WR, Hughes ES. Cancer risk in relatives of patients with common colorectal cancer. Annals of Internal Medicine. 1993;118:785–90. doi: 10.7326/0003-4819-118-10-199305150-00005. [DOI] [PubMed] [Google Scholar]
- 6.Stephenson BM, Finan PJ, Gascoyne J, Garbett F, Murday VA, Bishop DT. Frequency of familial colorectal cancer. British Journal of Surgery. 1991;78:1162–6. doi: 10.1002/bjs.1800781005. [DOI] [PubMed] [Google Scholar]
- 7.Butterworth AS, Higgins JPT, Pharoah P. Relative and absolute risk of colorectal cancer for individuals with a family history: a meta-analysis. European Journal of Cancer. 2006;42:216–27. doi: 10.1016/j.ejca.2005.09.023. [DOI] [PubMed] [Google Scholar]
- 8.Hemminki K, Li X. Familial colorectal adenocarcinoma from the Swedish Family-Cancer Database. International Journal of Cancer. 2001;94:743–8. doi: 10.1002/ijc.1533. [DOI] [PubMed] [Google Scholar]
- 9.Slattery ML, Kerber RA. Family history of cancer and colon cancer risk: the Utah Population Database [published erratum appears in J Natl Cancer Inst 1994 Dec 7;86(23):1802] Journal of the National Cancer Institute. 1994;86:1618–26. doi: 10.1093/jnci/86.21.1618. [DOI] [PubMed] [Google Scholar]
- 10.Andrieu N, Launoy G, Guillois R, Ory-Paoletti C, Gignoux M. Familial relative risk of colorectal cancer: a population-based study. European Journal of Cancer. 2003;39:1904–11. doi: 10.1016/s0959-8049(03)00420-9. [DOI] [PubMed] [Google Scholar]
- 11.Rex DK, Johnson DA, Lieberman DA, Burt RW, Sonnenberg A. Colorectal cancer prevention 2000: screening recommendations of the American College of Gastroenterology. American College of Gastroenterology American Journal of Gastroenterology. 2000;95:868–77. doi: 10.1111/j.1572-0241.2000.02059.x. [DOI] [PubMed] [Google Scholar]
- 12.Rex DK, Johnson DA, Anderson JC, et al. American College of Gastroenterology guidelines for colorectal cancer screening 2009 [corrected].[Erratum appears in Am J Gastroenterol. 2009 Jun;104(6):1613] American Journal of Gastroenterology. 2009;104:739–50. doi: 10.1038/ajg.2009.104. [DOI] [PubMed] [Google Scholar]
- 13.Levin B, Lieberman DA, McFarland B, et al. Screening and surveillance for the early detection of colorectal cancer and adenomatous polyps, 2008: a joint guideline from the American Cancer Society, the US Multi-Society Task Force on Colorectal Cancer, and the American College of Radiology. Gastroenterology. 2008;134(5):1570–95. doi: 10.1053/j.gastro.2008.02.002. [Review] [210 refs] [DOI] [PubMed] [Google Scholar]
- 14.Schoen RE, Pinsky PF, Weissfeld JL, et al. Utilization of surveillance colonoscopy in community practice. Gastroenterology. 2010;138:73–81. doi: 10.1053/j.gastro.2009.09.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lieberman DA, Holub JL, Morris CD, Logan J, Williams JL, Carney P. Low rate of large polyps (>9 mm) within 10 years after an adequate baseline colonoscopy with no polyps. Gastroenterology. 2014;147:343–50. doi: 10.1053/j.gastro.2014.04.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Schoen RE, Pinsky PF, Weissfeld JL, et al. Colorectal-cancer incidence and mortality with screening flexible sigmoidoscopy. New England Journal of Medicine. 2012;366:2345–57. doi: 10.1056/NEJMoa1114635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Prorok PC, Andriole GL, Bresalier RS, et al. Design of the Prostate, Lung, Colorectal and Ovarian (PLCO) Cancer Screening Trial. Controlled Clinical Trials. 2000;21:Suppl-309S. doi: 10.1016/s0197-2456(00)00098-2. [DOI] [PubMed] [Google Scholar]
- 18.Miller AB, Yurgalevitch S, Weissfeld JL. Death review process in the Prostate, Lung, Colorectal and Ovarian (PLCO) Cancer Screening Trial. Controlled Clinical Trials. 2000;21:Suppl-406S. doi: 10.1016/s0197-2456(00)00095-7. [DOI] [PubMed] [Google Scholar]
- 19.Pinsky PF, Schoen RE, Weissfeld JL, et al. The yield of surveillance colonoscopy by adenoma history and time to examination. Clinical Gastroenterology & Hepatology. 2009;7(1):86–92. doi: 10.1016/j.cgh.2008.07.014. [DOI] [PubMed] [Google Scholar]
- 20.Martinez ME, Baron JA, Lieberman DA, et al. A pooled analysis of advanced colorectal neoplasia diagnoses after colonoscopic polypectomy. Gastroenterology. 2009;136:832–41. doi: 10.1053/j.gastro.2008.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Weinberg DS, Schoen RE. Screening for colorectal cancer. Annals of Internal Medicine. 2014;160:6. doi: 10.7326/0003-4819-160-9-201405060-01005. [DOI] [PubMed] [Google Scholar]
- 22.Hassan C, Quintero E, Dumonceau J-M, et al. Post-polypectomy colonoscopy surveillance: European Society of Gastrointestinal Endoscopy (ESGE) Guideline. Endoscopy. 2013;45:842–51. doi: 10.1055/s-0033-1344548. [DOI] [PubMed] [Google Scholar]
- 23.Samadder NJ, Curtin K, Tuohy TMF, et al. Increased risk of colorectal neoplasia among family members of patients with colorectal cancer: a population-based study in utah. Gastroenterology. 2014;147:814–21. e5. doi: 10.1053/j.gastro.2014.07.006. [DOI] [PubMed] [Google Scholar]
- 24.Nishihara R, Wu K, Lochhead P, et al. Long-term colorectal-cancer incidence and mortality after lower endoscopy. New England Journal of Medicine. 2013;369:1095–105. doi: 10.1056/NEJMoa1301969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Aitken J, Bain C, Ward M, Siskind V, MacLennan R. How accurate is self-reported family history of colorectal cancer? American Journal of Epidemiology. 1995;141:863–71. doi: 10.1093/oxfordjournals.aje.a117522. [DOI] [PubMed] [Google Scholar]
- 26.Kerber RA, Slattery ML. Comparison of self-reported and database-linked family history of cancer data in a case-control study. American Journal of Epidemiology. 1997;146:244–8. doi: 10.1093/oxfordjournals.aje.a009259. [DOI] [PubMed] [Google Scholar]
- 27.Ziogas A, Anton-Culver H. Validation of family history data in cancer family registries. American Journal of Preventive Medicine. 2003;24:190–8. doi: 10.1016/s0749-3797(02)00593-7. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.