Abstract
Background
The incidence of small vessel-type (lacunar) ischemic strokes is greater in African-Americans compared to whites. The chronic inflammatory changes that result from lacunar stroke are poorly understood. To elucidate these changes, we measured serum inflammatory and thrombotic biomarkers in African-Americans at least 6 weeks post-stroke compared to control individuals.
Methods
Cases were African-Americans with lacunar stroke (n=30), and controls were age-matched African-Americans with no history of stroke or other major neurologic disease (n=37). Blood was obtained >6 weeks post-stroke and analyzed for inflammatory biomarkers. Freshly isolated peripheral blood mononuclear cells were stimulated with lipopolysaccharide (LPS) to assess immune responsiveness in a subset of cases (n=5) and controls (n=4).
Results
After adjustment for covariates, the pro-inflammatory biomarkers, soluble vascular cadherin adhesion molecule-1 (sVCAM-1) and thrombin anti-thrombin (TAT), were independently associated with lacunar stroke. Immune responsiveness to LPS challenge was abnormal in cases compared to controls.
Conclusions
African-Americans with lacunar stroke had elevated blood levels of VCAM-1 and TAT and an abnormal response to acute immune challenge >6 weeks post-stroke, suggesting a chronically compromised systemic inflammatory response.
Keywords: small vessel disease, lacunar stroke, African-Americans, chronic inflammation, race-ethnic disparities, biomarkers
Background
About 795,000 people in the U.S. have a stroke each year with African-Americans having twice the risk of stroke as whites, especially at younger ages [1, 2]. African-Americans are also more likely to have silent lacunar infarcts on MRI compared to whites and are three times more likely to have lacunar strokes [3]. The reasons for this disparity are not fully explained by differences in common risk factors, nor are the underlying mechanisms known. Peripheral inflammatory processes are implicated as a causative factor in small vessel-type cerebrovascular disease, of which lacunar stroke is one phenotype [4–6].
There are at least two major gaps in current data related to the relationship between inflammation and lacunar stroke. Several studies report inflammatory biomarker data in the acute phase after stroke, thus restricting analysis to the immediate damage responses after stroke. Few studies have reported long term, chronic post-stroke changes in inflammatory markers. Second, the majority of studies have been done in whites, yet African-Americans are more likely to have clinical and subclinical small vessel-type cerebrovascular disease. Importantly, there are race-ethnic differences in inflammation at baseline after stroke with African-Americans having an increased systemic inflammatory state, as reflected by increased levels of C reactive protein (CRP) compared to whites. In addition to traditional small vessel risk factors, this increase in CRP identifies patients as being at higher stroke risk [7].
The goal of this study was to determine whether African-American patients have chronic immune dysfunction at >6 weeks post-lacunar stroke. We measured serum inflammatory, endothelial, and thrombotic biomarkers in African-Americans after lacunar stroke compared with age-matched African-American controls. Peripheral immune cells taken from blood samples of patients and control individuals were further evaluated for responsiveness to a pro-inflammatory stimulus (treatment with lipopolysaccharide (LPS). We hypothesized that there would be prolonged immune activity and higher pro-thrombotic marker levels in African-Americans after lacunar stroke, and that subjects with stroke would have immune dysregulation as assessed by LPS stimulation compared with controls.
Materials and Methods
Subjects
Cases
Patients were enrolled at Wake Forest Baptist Medical Center (WFBMC), Duke University Medical Center (DUMC), Carolinas Medical Center—Northeast, and University of California, Davis. All subjects were self-reported to be Black or African-American in order to be eligible. Potential subjects were identified during a hospital admission for acute stroke and their medical records screened for clinical symptoms/signs, imaging results, and history. Patients were approached regarding study participation either during the index hospitalization or in the outpatient setting. Individuals with ischemic stroke (focal neurological deficit of vascular origin) with symptoms lasting greater than 24 hours or those with symptoms lasting less than 24 hours but having neuroimaging (computerized tomography (CT) or magnetic resonance (MR) imaging) evidence of a small vessel distribution (i.e. lacunar) infarction were selected for study. Lacunar ischemic stroke subtype was defined by the presence of a clinical syndrome (pure motor hemiparesis, pure sensory, mixed sensorimotor, ataxic hemiparesis, or dysarthria-clumsy hand) or CT or MR imaging evidence of an infarct in a small vessel distribution. Patients with cardioembolic, large vessel carotid or vertebral artery causes (greater than 50% stenosis by ultrasound, CT, or MR angiogram in the relevant artery) or non-atherothrombotic cause were excluded.
Controls
Control subjects self-reported as Black or African-American were recruited from the community via advertising and direct mailings at 2 of the sites (WFBMC and DUMC) to match the age frequency of cases. To be eligible, controls had no prior history of stroke (screened using the stroke-free questionnaire) [8], or other major neurologic diseases (Parkinson’s disease, vascular or Alzheimer’s dementia, multiple sclerosis, normal pressure hydrocephalus, traumatic brain injury with loss of consciousness greater than 30 minutes, epilepsy, or mental retardation).
Blood Collection
Subjects had a fasting phlebotomy at least 6-weeks after the stroke using standard procedures. The blood was immediately centrifuged at 1200g for 15 minutes, and plasma (from citrated tubes) and serum (from non-citrated tubes) aliquots were stored at −20°C until analysis.
Biomarker quantitation
All cytokine or other biomarker analyses were carried out by custom Quantibody array multiplex analysis performed by RayBiotech (Norcross, GA). Briefly, 50 μl of each sample was diluted in sample diluent and incubated with target cytokine antibodies arrayed on a solid surface, followed by incubation with a biotin-labeled detection antibody. The cytokine-antibody-biotin complex was then visualized through the addition of a streptavidin-labeled Cy3 equivalent dye and detected using a laser scanner. Cytokine standards were run in parallel with all samples. The limits of detection (pg/ml) for cytokines measured using the custom Quantibody array were: interferon-γ (IFNγ: 16.8–30,000); S-100b (464.5–1,500,000); plasminogen activator inhibitor-1 (PAI-1: 112.8–150,000); tumor necrosis factor-α (TNFα: 7.0–3,000); vascular adhesion molecule-1 (VCAM-1: 1639–1,500,000); interleukin-1 receptor antagonist (IL-1ra: 23.0 – 6,000); interleukin-4 (IL-4: 1.6 – 3,000); interleukin-6 (IL-6: 5.9–3,000); interleukin-8 (IL-8: 1.3 – 3,000); and D-Dimer (0.08- 60pg/ml). Thrombin-Anti Thrombin complex (TAT: 1.48 – 120 ng/ml) was measured by ELISA (AbCam, Cambridge MA).
To measure immune cell response to a pro-inflammatory stimulus, fresh whole blood aliquots containing mixed peripheral blood mononuclear cells (PMBCs) was collected from a random subset of the stroke patients (n=5) and age-race matched controls (n=4). These subjects were randomly recruited from the case and control participants enrolled at WFBMC. Participants signed an additional consent form for this portion of the study. PBMCs were stimulated with either LPS (E. coli O111:B4; 100 ng/ml) or saline vehicle for 3 hours at 37°C. Following treatment, blood samples were centrifuged (for 10 minutes, 1100g) and the separated plasma was stored in aliquots at −20°C. Non-stimulated and LPS-stimulated samples were then assayed for cytokine levels as described above.
Statistical analysis
The distributions of the biomarkers were assessed for skew and log-transformed as appropriate, except if the majority of values were below the level of detection (i.e., zero). Odds ratios were estimated to determine the association of each biomarker with stroke status before and after adjustment for baseline characteristics that differed between groups in the descriptive analysis using logistic regression. Wilcoxon rank sum tests were used to compare medians of markers between stroke cases and controls, and chi square tests were used to compare proportions in these two groups. Spearman rank correlation tests were performed to determine correlations amongst the biomarkers. In the LPS study, data were analyzed using a paired Student’s t-test (two-tailed) with p<0.05 considered significant.
Results
Study population characteristics are given in Table 1. A total of 30 stroke cases and 37 controls were enrolled and included in the analysis. Controls were more likely to have high school education, whereas higher proportions of stroke subjects had hypertension, hyperlipidemia, and a history of substance abuse. There was no difference in the history of diabetes or cigarette smoking in stroke cases compared to controls. The median NIH Stroke Scale score among stroke cases at the time of enrollment was 1, with a range of 0 to 4. Table 2 shows differences in median log-transformed levels of biomarkers between cases and controls. Stroke cases had significantly elevated levels of sVCAM-1, IL-1ra, TAT, and IL-6 compared to age-matched controls. In contrast, D-dimer levels were significantly higher in controls than in stroke cases.
Table 1.
Baseline characteristics of cases and controls.a
| Variable | Total (n=67) | Case (n=30) | Control (n=37) | P value |
|---|---|---|---|---|
| Age, yrs, median (IQR) | 59 (53–66) | 57.5 (50–62) | 60 (55–67) | 0.093 |
| Female, n (%) | 43 (64) | 16 (53) | 27 (73) | 0.096 |
| High school education, n (%) | 59 (89.4) | 23 (79.3) | 36 (97.3) | 0.018 |
| Hypertension, n (%) | 48 (71.6) | 25 (83.3) | 23 (62.2) | 0.056 |
| Diabetes, n (%) | 19 (28.4) | 10 (33.3) | 9 (24.3) | 0.416 |
| Hyperlipidemia, n (%) | 29 (43.3) | 18 (60) | 11 (29.7) | 0.013 |
| Tobacco smoker, n (%) | 34 (50.8) | 17 (56.7) | 17 (46.0) | 0.383 |
| Substance abuse, n (%) | 6 (9) | 5 (16.7) | 1 (2.7) | 0.046 |
| BMI, kg/m2 (IQR) | 31.0 (28.3–35.3) | 29.6 (27.7–32.8) | 32.0 (28.9–37.4) | 0.065 |
Numbers in parentheses refer to either interquartile range (IQR) or the percentage of individuals in each group (%).
Table 2.
Comparison of biomarkers between stroke patients and controls using Wilcoxon rank sum test.a
| Biomarker (log-transformed) | Stroke pg/ml, median (IQR) | Control pg/ml, median (IQR) | P value |
|---|---|---|---|
| IL-1ra | 2.074 (1.793–2.274) | 1.427 (−0.301–1.961) | 0.001 |
| IL-6 | 1.286 (1.017–1.529) | 1.022 (−0.301–1.254) | 0.009 |
| IL-8 | 1.285 (0.922–1.477) | 0.888 (.719–1.160) | 0.017 |
| IL-10 | 0.347 (−0.301–0.684) | −0.301 (−0.301–0.114) | 0.027 |
| VCAM-1 | 6.018 (5.902–6.084) | 5.797 (5.686–5.958) | <0.001 |
| IFNγ | 0.478 (−0.301–2.211) | 0.756 (−0.301–2.010) | 0.507 |
| TNF-α* | 0.000 (0.000–6.766) | 0.000 (0.000–8.102) | 0.914 |
| TAT | 1.191 (0.736–1.619) | 0.638 (0.534–0.722) | <0.001 |
| D-Dimer | 2.606 (2.348–2.784) | 2.971 (2.576–3.406) | 0.038 |
TNF-α was not log transformed due to prevalence of zero values, and not included in the correlation analyses below.
The associations between biomarkers and small vessel-type stroke, both unadjusted and adjusted for the variables that were different between cases and controls (age, sex, hyperlipidemia, and hypertension) are given in Table 3. Although IL-1ra, IL-6, IL-10, VCAM-1, and TAT were all higher in the unadjusted models, only VCAM-1 and TAT were independently associated with stroke after covariate adjustment. Table 4 shows correlations between individual biomarkers, with the most significant positive correlations between IL-1ra and IL-10 and IL-6 and IFNγ. Other highly significant correlations were between IL-1ra and IL-6, IL-1ra and VCAM-1, IL-1ra and TAT, IL-6 and IL-8, IL-10 and VCAM-1, and IL-10 and TAT.
Table 3.
Association between biomarkers and prior small vessel stroke in African-Americans.
| Biomarker | Unadjusted | Adjusteda | ||
|---|---|---|---|---|
| Odds Ratio | P value | Odds Ratio | P value | |
| IL-1ra | 3.024 (1.419–6.442) | 0.004 | 1.932 (0.868–4.301) | 0.107 |
| IL-6 | 3.655 (1.378–9.698) | 0.009 | 2.741 (0.810–9.269) | 0.105 |
| IL-8 | 1.987 (0.883–4.470) | 0.097 | 1.841 (0.788–4.301) | 0.158 |
| IL-10 | 2.597 (1.018–6.629) | 0.046 | 1.720 (0.571–5.179) | 0.335 |
| IFNγ | 1.040 (0.685–1.579) | 0.853 | 0.741 (0.435–1.262) | 0.270 |
| VCAM-1 | 274.710 (10.559–>999,999) | >0.001 | 722.715 (14.457–>999.999) | 0.001 |
| TAT | 6.399 (1.904–21.506) | 0.003 | 3.573 (1.010–12.638) | 0.048 |
Adjusted for age (continuous), sex, history of hyperlipidemia, and hypertension
Table 4.
Correlation (Spearman r coefficients) amongst serum cytokine and thrombosis markers in the entire cohort (cases and controls, n=67)a.
| IL-6 | IL-8 | IL-10 | IFNγ | VCAM-1 | TAT | D-Dimer | |
|---|---|---|---|---|---|---|---|
| IL-1ra | 0.419† | 0.121 | 0.464* | 0.322‡ | 0.409† | 0.466† | −0.202 |
| IL-6 | 0.465‡ | 0.229 | 0.508* | 0.346† | 0.262‡ | −0.069 | |
| IL-8 | 0.178 | 0.114 | 0.314‡ | 0.347† | 0.062 | ||
| IL-10 | −0.002 | 0.445† | 0.390† | −0.289‡ | |||
| IFNγ | 0.023 | 0.036 | 0.089 | ||||
| VCAM-1 | 0.386† | −0.276‡ | |||||
| TAT | −0.221‡ |
Statistical significance of correlations is indicated as:
p<0.0005,
p<0.005,
p<0.05
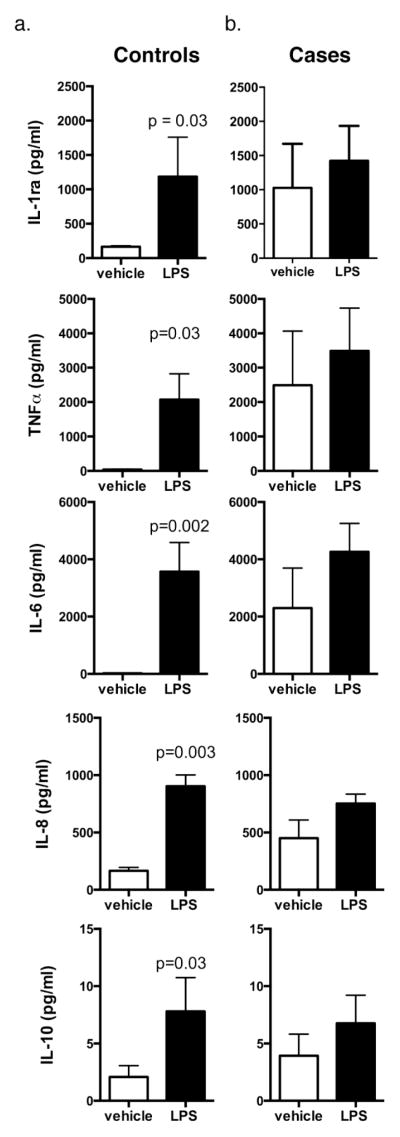
The final part of this study addressed if acute immune function >6 weeks post-stroke was compromised in cases compared to controls. Ex vivo stimulation of PBMCs with LPS in a randomly selected subset of patients and controls resulted in significantly elevated cytokine production compared with vehicle stimulation for IL-1ra, TNFα, IL-6, IL-8, and IL-10 in samples from controls (Fig 1a). In contrast, both vehicle- and LPS-stimulated blood from stroke cases produced equivalent, elevated levels of the same cytokines (Fig 1b).
Fig. 1. Ex vivo LPS stimulation of PBMCs from stroke cases and age-matched controls.
PBMCs were stimulated with saline vehicle or LPS (100 ng/ml) followed by cytokine analysis. A. LPS stimulation significantly increased cytokine levels compared to vehicle in controls. B. The same cytokines were similarly elevated with no significant difference between vehicle and LPS-stimulated samples from cases. Data=mean±SEM; n = 4–5 individuals, with p <0.05 as significant
Discussion
We measured changes in inflammatory and thrombotic biomarkers in African-Americans during the chronic phase (>6wks) after small vessel-type ischemic (lacunar) strokes compared with age-matched, stroke-free African-Americans. Circulating levels of two of these markers, sVCAM-1 and TAT, were independently associated with the chronic phase of lacunar stroke. Soluble vCAM-1 (CD106) is an established marker of inflammation and endothelial dysfunction, whereas TAT is an established marker of thrombin/coagulation activation. To our knowledge, there are no other studies that have evaluated either inflammatory or endothelial biomarkers and none measured plasma levels of sVCAM-1 in the chronic phase after lacunar stroke in African-Americans [9]. These later time points are likely to be important for repair after stroke and to the potential for re-occurrence of stroke.
The increased blood sVCAM-1 levels we observed in this study suggest prolonged changes in the vascular endothelium. VCAM is an important component of the vascular endothelium where it plays critical roles in immune surveillance of tissues, immune signaling and transmigration of neutrophils, monocytes, dendritic cells (DCs) and other immune or tumor cells [10, 11]. Activation of VCAM–mediated signaling pathways varies depending on the type of vascular injury and has been noted as an early pathological characteristic of endothelial dysfunction found in atherosclerotic lesions [12] or in focal cerebral ischemia in rodent models [13]. Unlike the relationship of intracellular cell adhesion molecule (ICAM), however, the relationship of sVCAM-1 to ischemic stroke is more tenuous [9, 14, 15]. Kozuka et al. [16] detected changes in sVCAM-1 and other endothelial factors as part of an acute phase in lacunar infarctions. However, a systemic review concluded that data on sVCAM-1 was insufficient to establish a firm relationship between sVCAM-1 and acute or chronic lacunar stroke. [9]
Our study of a group of African-Americans found a significant relationship between sVCAM-1 and lacunar stroke after adjustment for co-variables that included age, sex, hyperlipidemia, and hypertension. One explanation for the association between sVCAM-1 and stroke has been that sVCAM-1 is also associated with vascular risk factors such as hypertension, older age and hyperlipidemia. For example, patients with essential and acute hypertension have elevated plasma levels of soluble adhesion molecules, such as sVCAM-1 and sICAM-1 [17]. VCAM-1 could reflect the cumulative impact of these risk factors. But, after controlling for differences in baseline risk factors between cases and controls, we found that sVCAM-1 remained associated with chronic stroke suggesting the relationship may not be entirely related to these established stroke risk factors. Thus, although our study was not designed to measure plasma levels of either inflammatory or vascular endothelial factors prior to stroke, we speculate that, after controlling for baseline risk factors, the elevation of sVCAM-1 levels was more likely to be increased as a result of the stroke rather than a pre-existing condition.
We also found an association between higher TAT levels, a measure of the amount of thrombin inhibition (and hence indirectly of coagulation and fibrinolysis) and lacunar stroke after adjustment for potential confounders. The increased post-stroke TAT levels implies long-lasting changes in thrombin production after the index lacunar stroke. Vascular endothelial activation is proposed to change the blood vessel wall leading to platelet activation and TAT formation, providing a possible mechanism by which VCAM-1-mediated endothelial activation and immune signaling may contribute to coagulation within small vessels [18]. Thus, VCAM-1 and TAT may interact to contribute to the apparent prolonged endothelial damage found in African-Americans with lacunar stroke. A meta-analysis of measurements of plasma levels of tissue plasminogen activator (tPA), plasminogen activator inhibitor (PAI) and fibrinogen, however, showed no association between these coagulation and fibrinolysis markers and lacunar infarction [9]. Unfortunately, TAT was not included in this meta-analysis. Other studies show that TAT is increased in Binswanger’s disease, which is characterized by dementia in the setting of severe leukoaraiosis and lacunar strokes [19]. The results of our study, therefore, are consistent with an association between TAT and small vessel disease.
The ex vivo assay of immune activation in a random subset of cases and controls in our study provides important insights into the immune phenotype after lacunar stroke. Cytokine measurement following ex vivo stimulation of whole blood with LPS is a validated, cost-effective surrogate assessment of monocytic, systemic cytokine production and immune responsiveness [20, 21]. PBMCs from non-stroke controls responded with a typical increase in cytokine biomarker production when challenged with LPS. In contrast, stroke cases had cytokine levels in vehicle- and LPS-stimulated samples that were not significantly different from those in LPS-stimulated control samples. Significantly elevated markers included canonical pro-inflammatory cytokines such as TNF-α and IL-6 as well as anti-inflammatory cytokines such as IL-1Ra and IL-10. The observation that baseline levels of cytokines were much higher in cases compared to controls and the failure of LPS to significantly increase cytokine production is indicative of a “ceiling effect,” which suggests that these PBMCs have maximized their immune capacity in the chronic stroke phase. Taken together, these results suggest a lack of immune resolution and may reflect a maintained high inflammatory state after stroke.
Several biomarkers were highly correlated (Table 4), which could suggest additional mechanistic targets for further study in the chronic phase of lacunar stroke. Cytokines, particularly IL-1 and TNF-α, have pivotal roles in regulating inflammatory and innate immune responses to acute infection, but also regulate cellular pathways that are important in acute ischemic stroke [22, 23]. In general, TNF-α and IL-6 blood levels are elevated in acute lacunar stroke and associated with larger infarct volumes and poorer outcomes [24, 9]. Acute pro-inflammatory processes are followed at varying times by anti-inflammatory immune activation that leads to release of cytokines associated with repair and resolution of the immune response to injury. IL-1ra is an anti-inflammatory protein that reversibly blocks the pro-inflammatory actions of IL-1, and increased blood levels of IL-1Ra reduced the extent of acute ischemic injury in experimental models [25]. High blood IL-1ra levels immediately after stroke increase the risk of infection and may thereby lead to poorer outcomes [26]. Interestingly, abnormally elevated levels of IL-1Ra were found up to 6 months after ischemic stroke [27]. We also found highly significant correlations between IL-1Ra and many proinflammatory cytokines. Overall, the results of these studies are consistent with our ex vivo data suggesting the presence of a dysregulated inflammatory response after lacunar stroke.
Our study was limited to a relatively small cohort (30 individuals with stroke, 37 control individuals) and was homogenous in terms of race/ethnicity, age and specific stroke subtype. All subjects with stroke had careful stroke subtype characterization and non-stroke controls were carefully age-matched. Thus, our intriguing findings in an African-American population may not be generalizable to other race-ethnic groups or other populations. We also specifically designed the study to evaluate candidate biomarkers that represented endothelial, inflammatory and coagulation/thrombin-based factors thought to be associated with lacunar stroke. Other biomarkers not assessed in our study may also be important. Importantly, we have examined an understudied but critical phase of stroke recovery, that is, the chronic phase, which may reveal unresolved stroke-based sequelae. Chronic elevation of VCAM-1 and TAT, markers of downstream inflammation, endothelial dysfunction, and thrombin generation, were associated with small vessel stroke in African-Americans. Further research comparing race/ethnic groups, and other stroke types is required to fully substantiate the pathophysiology of chronic changes in VCAM-1 and TAT levels and the changed immune response in our study population. In addition, further research using these biomarkers and immune challenges could have clinical implications for personalized medicine, such as identifying patients at risk of recurrent stroke, identifying at-risk immune phenotypes prior to the onset of first stroke, or identifying novel drug targets for prevention.
Conclusions
Chronic elevations of VCAM-1 and TAT are associated with small vessel stroke in African-Americans. Our data suggest that elevation of immune factors represents a failure to repair and resolve immune responses in the chronic phase after stroke when compared to stroke-free controls in this population.
Acknowledgments
The authors thank Ms. Patricia Frazier and Ms. Catherine Brewer for technical assistance
Funding: This study was funded by American Stroke Association/Bugher Foundation Center for Stroke Prevention Research.
Footnotes
Conflict of Interest: The authors declare that they have no conflict of interest.
Compliance with Ethical Standards:
Research involving Human Participants and/or Animals – All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent – Informed consent was obtained from all individual participants included in the study.
References
- 1.Kissela B, Schneider A, Kleindorfer D, Khoury J, Miller R, Alwell K, et al. Stroke in a biracial population: the excess burden of stroke among blacks. Stroke. 2004;35(2):426–31. doi: 10.1161/01.STR.0000110982.74967.39. [DOI] [PubMed] [Google Scholar]
- 2.Stansbury JP, Jia H, Williams LS, Vogel WB, Duncan PW. Ethnic disparities in stroke: epidemiology, acute care, and postacute outcomes. Stroke. 2005;36(2):374–86. doi: 10.1161/01.STR.0000153065.39325.fd. [DOI] [PubMed] [Google Scholar]
- 3.Gottesman RF, Cummiskey C, Chambless L, Wu KK, Aleksic N, Folsom AR, et al. Hemostatic factors and subclinical brain infarction in a community-based sample: the ARIC study. Cerebrovasc Dis. 2009;28(6):589–94. doi: 10.1159/000247603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Castellanos M, Castillo J, Garcia MM, Leira R, Serena J, Chamorro A, et al. Inflammation-mediated damage in progressing lacunar infarctions: a potential therapeutic target. Stroke. 2002;33(4):982–7. doi: 10.1161/hs0402.105339. [DOI] [PubMed] [Google Scholar]
- 5.Hassan A, Hunt BJ, O’Sullivan M, Parmar K, Bamford JM, Briley D, et al. Markers of endothelial dysfunction in lacunar infarction and ischaemic leukoaraiosis. Brain. 2003;126(Pt 2):424–32. doi: 10.1093/brain/awg040. [DOI] [PubMed] [Google Scholar]
- 6.Rouhl RP, Damoiseaux JG, Lodder J, Theunissen RO, Knottnerus IL, Staals J, et al. Vascular inflammation in cerebral small vessel disease. Neurobiol Aging. 2012;33(8):1800–6. doi: 10.1016/j.neurobiolaging.2011.04.008. [DOI] [PubMed] [Google Scholar]
- 7.Cushman M, McClure LA, Howard VJ, Jenny NS, Lakoski SG, Howard G. Implications of increased C-reactive protein for cardiovascular risk stratification in black and white men and women in the US. Clin Chem. 2009;55(9):1627–36. doi: 10.1373/clinchem.2008.122093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Meschia JF, Brott TG, Chukwudelunzu FE, Hardy J, Brown RD, Jr, Meissner I, et al. Verifying the stroke-free phenotype by structured telephone interview. Stroke. 2000;31(5):1076–80. doi: 10.1161/01.str.31.5.1076. [DOI] [PubMed] [Google Scholar]
- 9.Wiseman S, Marlborough F, Doubal F, Webb DJ, Wardlaw J. Blood markers of coagulation, fibrinolysis, endothelial dysfunction and inflammation in lacunar stroke versus non-lacunar stroke and non-stroke: systematic review and meta-analysis. Cerebrovasc Dis. 2014;37(1):64–75. doi: 10.1159/000356789. [DOI] [PubMed] [Google Scholar]
- 10.Cook-Mills JM, Marchese ME, Abdala-Valencia H. Vascular cell adhesion molecule-1 expression and signaling during disease: regulation by reactive oxygen species and antioxidants. Antioxid Redox Signal. 2011;15(6):1607–38. doi: 10.1089/ars.2010.3522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Schlesinger M, Bendas G. Vascular cell adhesion molecule-1 (VCAM-1)-An increasing insight into its role in tumorigenicity and metastasis. Int J Cancer. 2014 doi: 10.1002/ijc.28927. [DOI] [PubMed] [Google Scholar]
- 12.Gawaz M, Langer H, May AE. Platelets in inflammation and atherogenesis. J Clin Invest. 2005;115(12):3378–84. doi: 10.1172/JCI27196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhou W, Liesz A, Bauer H, Sommer C, Lahrmann B, Valous N, et al. Postischemic brain infiltration of leukocyte subpopulations differs among murine permanent and transient focal cerebral ischemia models. Brain Pathol. 2013;23(1):34–44. doi: 10.1111/j.1750-3639.2012.00614.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tuttolomondo A, Di Sciacca R, Di Raimondo D, Renda C, Pinto A, Licata G. Inflammation as a therapeutic target in acute ischemic stroke treatment. Curr Top Med Chem. 2009;9(14):1240–60. doi: 10.2174/156802609789869619. [DOI] [PubMed] [Google Scholar]
- 15.Tuttolomondo A, Pinto A, Corrao S, Di Raimondo D, Fernandez P, Di Sciacca R, et al. Immuno-inflammatory and thrombotic/fibrinolytic variables associated with acute ischemic stroke diagnosis. Atherosclerosis. 2009;203(2):503–8. doi: 10.1016/j.atherosclerosis.2008.06.030. [DOI] [PubMed] [Google Scholar]
- 16.Kozuka K, Kohriyama T, Nomura E, Ikeda J, Kajikawa H, Nakamura S. Endothelial markers and adhesion molecules in acute ischemic stroke--sequential change and differences in stroke subtype. Atherosclerosis. 2002;161(1):161–8. doi: 10.1016/s0021-9150(01)00635-9. [DOI] [PubMed] [Google Scholar]
- 17.Murray KN, Buggey HF, Denes A, Allan SM. Systemic immune activation shapes stroke outcome. Mol Cell Neurosci. 2013;53:14–25. doi: 10.1016/j.mcn.2012.09.004. [DOI] [PubMed] [Google Scholar]
- 18.Ataga KI. Hypercoagulability and thrombotic complications in hemolytic anemias. Haematologica. 2009;94(11):1481–4. doi: 10.3324/haematol.2009.013672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tomimoto H, Akiguchi I, Ohtani R, Yagi H, Kanda M, Shibasaki H, et al. The coagulation-fibrinolysis system in patients with leukoaraiosis and Binswanger disease. Arch Neurol. 2001;58(10):1620–5. doi: 10.1001/archneur.58.10.1620. [DOI] [PubMed] [Google Scholar]
- 20.Damsgaard CT, Lauritzen L, Calder PC, Kjaer TM, Frokiaer H. Whole-blood culture is a valid low-cost method to measure monocytic cytokines - a comparison of cytokine production in cultures of human whole-blood, mononuclear cells and monocytes. J Immunol Methods. 2009;340(2):95–101. doi: 10.1016/j.jim.2008.10.005. [DOI] [PubMed] [Google Scholar]
- 21.Thurm CW, Halsey JF. Measurement of cytokine production using whole blood. Curr Protoc Immunol. 2005;Chapter 7(Unit 7):18B. doi: 10.1002/0471142735.im0718bs66. [DOI] [PubMed] [Google Scholar]
- 22.Allan SM, Tyrrell PJ, Rothwell NJ. Interleukin-1 and neuronal injury. Nat Rev Immunol. 2005;5(8):629–40. doi: 10.1038/nri1664. [DOI] [PubMed] [Google Scholar]
- 23.Lambertsen KL, Biber K, Finsen B. Inflammatory cytokines in experimental and human stroke. J Cereb Blood Flow Metab. 2012;32(9):1677–98. doi: 10.1038/jcbfm.2012.88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tuttolomondo A, Di Raimondo D, Pecoraro R, Arnao V, Pinto A, Licata G. Inflammation in ischemic stroke subtypes. Curr Pharm Des. 2012;18(28):4289–310. doi: 10.2174/138161212802481200. [DOI] [PubMed] [Google Scholar]
- 25.Banwell V, Sena ES, Macleod MR. Systematic review and stratified meta-analysis of the efficacy of interleukin-1 receptor antagonist in animal models of stroke. J Stroke Cerebrovasc Dis. 2009;18(4):269–76. doi: 10.1016/j.jstrokecerebrovasdis.2008.11.009. [DOI] [PubMed] [Google Scholar]
- 26.Tanzi P, Cain K, Kalil A, Zierath D, Savos A, Gee JM, et al. Post-stroke infection: a role for IL-1ra? Neurocrit Care. 2011;14(2):244–52. doi: 10.1007/s12028-010-9490-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Becker KJ, Dankwa D, Lee R, Schulze J, Zierath D, Tanzi P, et al. Stroke, IL-1ra, IL1RN, infection and outcome. Neurocrit Care. 2014;21(1):140–6. doi: 10.1007/s12028-013-9899-x. [DOI] [PMC free article] [PubMed] [Google Scholar]