Abstract
The silkworm silk glands are powerful secretory organs that can produce and secrete proteins at high levels. As such, it has been suggested that the biosynthetic and secretory power of the silk gland can be harnessed to produce and secrete recombinant proteins in tight or loose association with silk fibers. However, the utility of the silkworm platform is constrained by the fact that it has a relatively primitive protein N-glycosylation pathway, which produces relatively simple insect-type, rather than mammalian-type N-glycans. In this study, we demonstrate for the first time that the silk gland protein N-glycosylation pathway can be glycoengineered. We accomplished this by using a dual piggyBac vector encoding two distinct mammalian glycosyltransferases under the transcriptional control of a posterior silk gland (PSG)-specific promoter. Both mammalian transgenes were expressed and each mammalian N-glycan processing activity was induced in transformed silkworm PSGs. In addition, the transgenic animals produced endogenous glycoproteins containing significant proportions of mammalian-type, terminally galactosylated N-glycans, while the parental animals produced none. This demonstration of the ability to glycoengineer the silkworm extends its potential utility as a recombinant protein production platform.
Keywords: Transgenic silkworm, glycoengineering, glycosylation, piggyBac
Graphical abstract
1. Introduction
The domesticated silkworm, Bombyx mori, has been used to produce silk fibers for thousands of years (Maeda, 1989; Tomita, 2011) and is emerging as a potential platform for recombinant protein production. This began with the use of the silkworm as a host for baculovirus expression vectors (reviewed in Choudary et al., 1995; Kato et al., 2010; Maeda, 1989), which introduced a foreign gene encoding the protein of interest, induced its expression, and initiated recombinant protein production during the very late phase of infection. Like interferon alpha, which was the first human protein produced in baculovirus-infected silkworms (Maeda et al., 1985), recombinant proteins of interest can be secreted into the hemolymph, which facilitates downstream purification. But, the baculovirus-infected silkworm is a binary and transient recombinant protein production system. Thus, various investigators began to genetically transform silkworms with DNA constructs encoding the protein of interest under the transcriptional control of either whole body (Tamura et al., 2000) or silk gland-specific promoters (Adachi et al., 2006; Hino et al., 2006; Iizuka et al., 2009; Iizuka et al., 2013; Kojima et al., 2007; Kurihara et al., 2007; Kuwana et al., 2014; Long et al., 2015; Ogawa et al., 2007; Royer et al., 2005; Seong et al., 2011; Teule et al., 2012; Tomita et al., 2007; Tomita et al., 2003; Wang et al., 2014; Wen et al., 2010; Xu, 2014; Yanagisawa et al., 2007; reviewed in Tomita, 2011). This new, single component approach provided a stable, rather than transient source of recombinant proteins. In addition, the use of silk gland-specific promoters exploited the power of the silk gland for recombinant protein production and secretion in tight or loose association with silk fibers, which permits non-invasive collection and further simplifies downstream purification of recombinant protein products. More recently, efforts have been undertaken to enhance the utility of the silkworm silk gland as a potential bioreactor. In one prominent example, zinc finger nuclease technology was used to edit the endogenous silkworm fibroin heavy chain gene and isolate transgenic silkworms with “empty” silk glands that could produce recombinant proteins at higher levels than the parental strain (Ma et al., 2014).
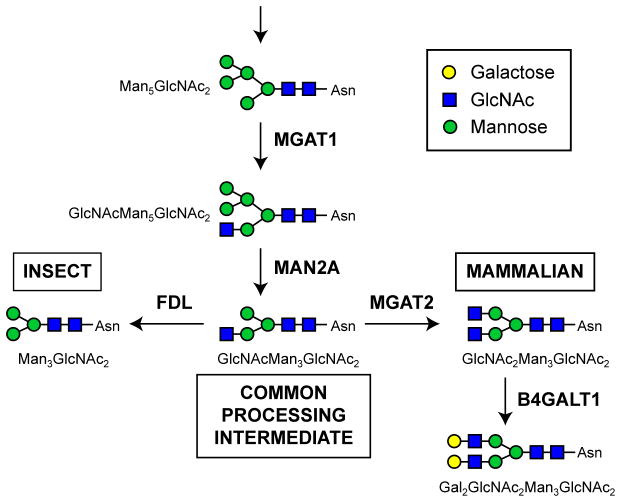
As a eukaryotic organism, the silkworm has the cellular machinery needed to process newly synthesized proteins in various ways, including glycosylation. This study focuses on enhancing protein N-glycosylation, which is a co- and post-translational modification involving the transfer of oligosaccharides to select asparagine residues in a polypeptide, followed by a series of enzymatic processing reactions that initially trim, and then elongate those N-glycans (Kornfeld and Kornfeld, 1985). However, the protein N-glycosylation pathway is not identical in all eukaryotes. Mammals have the most extensive transfer, trimming, and elongation functions, whereas insects have simpler pathways that include the transfer and trimming functions, an extra trimming function (FDL; Geisler et al., 2008; Leonard et al., 2006), and only minimal elongation functions (Fig. 1; reviewed in Geisler and Jarvis, 2009; Harrison and Jarvis, 2006; Marchal et al., 2001; Marz et al., 1995; Shi and Jarvis, 2007). Thus, the major N-glycans observed on silkworm-derived glycoproteins are trimmed structures with terminal mannose residues (Iizuka et al., 2009; Kulakosky et al., 1998; Park et al., 2009; Sasaki et al., 2009). This constrains the utility of the silkworm as a glycoprotein production platform, especially for therapeutic glycoprotein production, because elongated, mammalian-type N-glycan structures are often required for the clinical efficacy of these products (Sola and Griebenow, 2011).
Fig. 1.
N-glycan processing pathways. Relevant steps in the protein N-glycosylation pathways of insect and mammalian cell systems emphasizing differences between the two. Both pathways include the initial transfer of a preassembled N-glycan to a nascent protein, which is not shown, followed by enzymatic removal of terminal sugars (trimming steps), which produce an intermediate common to both systems (GlcNAcMan3GlcNAc2). The major processed N-glycans in insect systems are produced by FDL, which removes the terminal N-acetylglucosamine residue from the common intermediate (Geisler et al., 2008; Leonard et al., 2006) to form paucimannosidic end products (e.g. Man3GlcNAc2). In contrast, the major processed N-glycans in mammalian systems are produced by various glycosyltransferases and other machinery, which elongate the common intermediate to form complex, terminally galactosylated (Gal2GlcNAc2Man3GlcNAc2) or even more extensively processed end products.
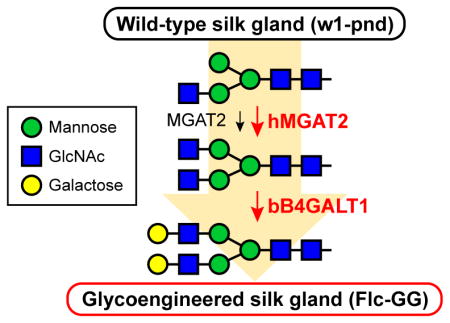
Glycoengineering approaches have been used to address this limitation in baculovirus-insect cell expression systems (reviewed in Geisler and Jarvis, 2009; Geisler et al., 2015; Harrison and Jarvis, 2006; Jarvis, 2009; Shi and Jarvis, 2007). These efforts have yielded new insect cell lines and baculovirus vectors that can be used to produce recombinant glycoproteins with fully elongated, mammalian-type N-glycans. We hypothesized that an analogous approach involving transformation with mammalian N-acetylglucosaminyltransferase II (MGAT2) and β1,4-galactosyltransferase (B4GALT1) could be used to glycoengineer the silkworm and extend its potential utility for recombinant glycoprotein production (Fig. 1). However, previous glycoengineering efforts had only been applied to insect cell lines and we anticipated that introducing mammalian functions into a whole animal would elongate endogenous N-glycans, with potentially serious phenotypic consequences. In fact, in a previous study, we obtained no transgenic offspring when we attempted to glycoengineer the Drosophila melanogaster N-glycosylation pathway using piggyBac vectors encoding mammalian glycosyltransferase genes under the control of whole body promoters (unpublished data). Thus, we designed a tissue-specific glycoengineering approach that focused on extending the N-glycosylation pathway in the posterior silk gland (PSG). This approach yielded phenotypically normal, transgenic silkworms that expressed both mammalian genes, had elevated levels of both MGAT2 and B4GALT1 activities, and produced endogenous PSG glycoproteins with terminally galactosylated N-glycans. These results are the first to demonstrate that the silkworm can be glycoengineered to enhance its potential utility as a recombinant protein production platform.
2. Materials and methods
2.1. Plasmid constructions
pXLBacII-MGAT2/fibL-wi-fibL/B4GALT1/DsRed1, which encodes mammalian MGAT2 and B4GALT1 under the control of B. mori fibL promoters (Fig. S1), is a new dual piggyBac vector constructed for this study. Briefly, the fibL promoter sequence was PCR-amplified using genomic DNA extracted from B. mori larvae as the template and Flc promoter-Fw and Flc promoter-Rv as the primers (Table S1). The amplification product was gel-purified and subcloned into pCR®2.1-TOPO® (Life Technologies, Gaithersburg, MD). After DNA sequence verification, the fibL promoter was excised from the resulting plasmid (pCR2.1TOPO-Flc) with EcoRI and inserted into the EcoRI site of pGEM-WIZ (Bao and Cagan, 2006) to produce pGEM-WIZ-Flc. A second copy of the fibL promoter sequence was then excised from pCR2.1TOPO-Flc with EcoRI, blunted with T4 DNA polymerase (New England BioLabs, Ipswich, MA), and inserted into pGEM-WIZ-Flc digested with AvrII and blunted with T4 DNA polymerase. Restriction mapping and DNA sequencing verified that the resulting plasmid, pGEM-Flc-wi-Flc, had dual fibL promoters in back-to-back orientation separated by the D. melanogaster white intron 2 (wi). Finally, the dual fibL promoter cassette (fibL-wi-fibL) was excised from pGEM-Flc-wi-Flc with SpeI, blunted with T4 DNA polymerase, and used to replace the dual baculovirus ie1 promoter cassette in pXLBacII-GnTII/GalT-DsRed1-LTR (Shi et al., 2007), which had been excised with PmeI and NruI.
2.2. Silkworm transformation
Transformed silkworms were isolated as described previously (Teule et al., 2012), using w1-pnd, a white eye-color, non-diapausing mutant strain of the diapausing B. mori strain w1-c. Briefly, eggs were collected 1 h after being laid by w1-pnd (Tamura et al., 2000) silkworms, arranged on a microscope slide, and the pre-blastoderm embryos were microinjected with 1–5 nL of a DNA mixture consisting of a helper plasmid encoding the piggyBac transposase (pHA3PIG; Tamura et al., 2000) and pXLBacII-MGAT2/fibL-wi-fibL/B4GALT1/DsRed1 dissolved in injection buffer (0.1 mM sodium phosphate, 5 mM KCl, pH 6.8) at a final concentration of 0.2 μg/uL. Microinjections were performed using a World Precision Instruments PV820 pressure regulator, Suruga Seiki M331 micromanipulator, and Narishige HD-21 double-pipette holder. The punctured eggs were sealed with Helping Hand Super Glue gel (The Faucet Queens), and then incubated in a growth chamber at 25°C with 70% humidity. After hatching, the larvae were reared on an artificial diet (Nihon Nosan Company) and subsequent generations were obtained by mating siblings within the same lines. Transgenic progeny were screened by looking for the presence of the DsRed1 eye marker using an Olympus SXZ12 microscope with filters between 450 and 700 nm. Fluorescent eye color-positive insects were inbred over more than eight generations, which formally established homozygosity (Falconer, 1960), and by the eight generation, only marker-positive progeny were obtained (see Results).
2.3. Reverse Transcription Polymerase Chain Reaction (RT-PCR) assays
PSGs were isolated from the w1-pnd, Flc-GG#1, and Flc-GG#2 silkworms on the 5th day of 5th instar and total RNA was extracted using the TRI Reagent (Life Technologies) according to the manufacturer’s instructions. The RNA preparations were then treated with DNaseI Amplification Grade (Life Technologies) and 3 μg samples were reverse transcribed at 50°C for 90 min with ThermoScript™ Reverse Transcriptase (Life Technologies) and oligo(dT)31-VN (Table S1). The resulting cDNA preparations were treated with RNase-H (Life Technologies) and then used for PCRs with Crimson Taq DNA Polymerase (New England BioLabs). The PCR conditions included an initial denaturation step at 95°C for 30 sec, followed by 33 cycles of denaturation for 15 sec at 95°C, annealing for 20 sec at 50°C (hMGAT2-Fw, hMGAT2-Rv, bB4GALT1-Fw, and bB4GALT1-Rv) or 65°C (BmRPL3), and extension for 30 sec at 68°C. The sequences of all the primers used for the RT-PCRs are given in Table S1.
2.4. Splinkerette PCR assays
The piggyBac insertion sites in Flc-GG#1 and Flc-GG#2 silkworms were determined by using a minor modification of a previously described splinkerette PCR method (Horn et al., 2007; Potter and Luo, 2010). A detailed description of the splinkerette PCR method used in this study is described in the Supplementary Material linked to this manuscript.
2.5. Glycosyltransferase assays
PSGs were isolated from 5th day of 5th instar w1-pnd, Flc-GG#1, and Flc-GG#2 silkworm larvae and stored frozen at −80°C. After thawing, the PSGs were homogenized with a plastic pestle in either MGAT2 (50 mM PIPES, pH 6.7, 150 mM NaCl, 20 mM MnCl2, 0.5% Triton X-100) or B4GALT1 (10 mM HEPES, pH 7.4, 140 mM NaCl, 20 mM MnCl2, 0.5% Nonidet P-40) assay buffer. Prior to performing the assays, the extracts were clarified in a microcentrifuge and total protein concentrations were determined using a commercial bicinchoninic acid assay (Pierce, Rockford, IL) with BSA as the standard. For MGAT2 assays, replicate samples of each extract containing 100 μg of total protein were incubated at 37°C for 1 h in 100 μl of MGAT2 buffer containing 0.3 μCi of uridine diphosphate [6-3H]-N-acetylglucosamine (36 Ci/mmol; New England Nuclear, Boston, MA) and 50 μg of mouse IgG2a-Fc purified from Sf9 cells co-infected with Acp6.9-mIgG2a-Fc and AcP(+)IE1-hMGAT1. The major N-glycan structure on the mouse IgG2a-Fc produced by these cells is GlcNAcMan3GlcNAc2+Fuc (m/z = 1590.8), which is the acceptor substrate for MGAT2. The reactions were quenched with 0.5 mL of ice-cold MGAT2 buffer, spotted onto glass fiber filters (Whatman GF/D; Hillsboro, OR), and the filters were dried, washed once with cold 10% (w/v) trichloroacetic acid, once with cold 5% (w/v) trichloroacetic acid, and twice with cold 95% (v/v) ethanol. The filters were then re-dried, placed in vials containing liquid scintillation cocktail (Packard UltimaGold F; Meriden, CT), and radioactivity was measured in a liquid scintillation counter (Beckman model LS6000-IC; Fullerton, CA). The B4GALT1 assays were performed using an analogous filter-based acid-precipitable isotopic method, as described previously (Hollister et al., 1998).
2.6. N-glycosylation profiles
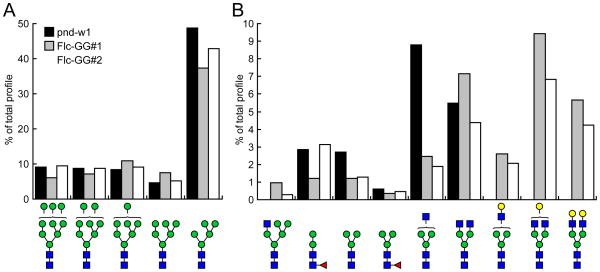
PSGs were isolated from 5th day of 5th instar w1-pnd, Flc-GG#1, and Flc-GG#2 silkworm larvae, stored at −80°C, thawed, homogenized with a plastic pestle in homogenization buffer (10 mM Tris-HCl, pH 7.4, 140 mM NaCl, 1 mM EDTA, 0.5% Nonidet P40), clarified in a microcentrifuge, and total protein concentrations determined using a commercial bicinchoninic acid assay (Pierce, Rockford, IL) with BSA as the standard. The clarified homogenates were used directly as the source of total glycoproteins for N-glycan profiling, as described previously (Mabashi-Asazuma et al., 2014). Samples containing 1.2 mg total protein were reduced with 10 mM dithiothreitol for 1 h at 37°C, alkylamidated with 50 mM iodoacetamide for 1 h at room temperature, and then digested with trypsin overnight at 37°C. The trypsinized glycopeptides were acidified with 0.01% trifluoroacetic acid and then applied to SampliQ C18 cartridges (Agilent Technologies, Santa Clara, CA) that had been washed with 100% acetonitrile and preconditioned with 0.05% trifluoroacetic acid. After the cartridges were washed with 0.05% trifluoroacetic acid, the glycopeptides were eluted with 60% isopropanol and evaporated in a speedvac. The glycopeptides were re-dissolved in 50 mM ammonium bicarbonate, pH 8.5, and then the N-glycans were enzymatically released by exhaustive digestion with PNGase-F (New England BioLabs). The spent reactions were applied to pre-conditioned C18 SepPak cartridges (Waters Corp., Milford, MA) and the flow-through plus one 5% (v/v) aqueous acetic acid wash were pooled, evaporated, and permethylated, as described previously (Dell et al., 1994). The permethylated N-glycan derivatives extracted into chloroform with several aqueous washes were re-evaporated, resuspended in acetonitrile, mixed 1:1 with 2,5-dihydroxybenzoic acid matrix (10 mg/ml in 50% acetonitrile in water), and then samples were spotted onto the MALDI-TOF target plate. Data acquisition was performed manually on a Model 4700 Proteomics Analyzer equipped with an Nd:YAG laser (Applied Biosystems, Framingham, MA) and 1,000 shots were accumulated in the reflectron positive ion mode. The MALDI-TOF MS profiles were analyzed and peaks with signal to noise ratios >3 were assigned to specific N-glycan structures using the CFG database and Glycoworkbench 2.0 software (Ceroni et al., 2008). These peaks were labeled in the profiles and their relative prevalence was calculated and presented in the bar graphs shown in Fig. 5.
Fig. 5.
Quantitative distribution of N-glycans. The proportion of various high mannose (A) and other (B) types of N-glycans on the PSGs isolated from w1-pnd, Flc-GG#1, and Flc-GG#2 silkworms are presented as percentages of the total N-glycans, including only those structures represented by peaks with signal to noise ratios of >3 in Fig. 4.
3. Results
3.1. Silkworm transformation
As noted above, our glycoengineering approach targeted the PSG in order to exploit and enhance its powerful protein production and secretion capacity, while avoiding the potentially lethal effect of indiscriminate endogenous N-glycan elongation in the whole animal. By constructing a new, dual piggyBac vector encoding MGAT2 and B4GALT1 under the control of B. mori fibroin light chain (fibL) promoters (Fig. S1), we expected both transgenes would be expressed in the PSG’s. We also expected MGAT2 would initiate elongation of the upper branch of the paucimannosidic N-glycan intermediates on PSG glycoproteins and B4GALT1 would cap those elongated intermediates with terminal galactose residues to produce mammalian-type N-glycans (Fig. 1). Fresh eggs from the parental silkworm strain (w1-pnd) were injected with a mixture of the silk gland-specific piggyBac vector plus a helper plasmid encoding the piggyBac transposase, as described in Materials and methods. A total of 105 silkworms was hatched from 480 microinjected pre-blastoderm embryos. The hatched larvae produced 71 moths that were mated to generate 34 independent batches of eggs. Putative F1 transformants identified by the red eye phenotype resulting from expression of the 3xP3-DsRed1 marker (Horn et al., 2000) in the piggyBac vector (Fig. S1) were obtained from two egg batches. These animals were used to establish transgenic silkworm lines designated Flc-GG#1 and Flc-GG#2 by in-breeding, with screening in each generation for expression of the eye color marker. Only DsRed1-positive adults were mated to produce the next generation and, by generation eight, all of the silkworm progeny in each line expressed DsRed1. Based upon the historical definition allowed by our in-breeding protocol (Falconer, 1960) and the absence of any adults lacking the fluorescent eye color marker by the eight generation, we concluded that Flc-GG#1 and Flc-GG#2 were both highly likely to be homozygous for at least one transgene. These silkworms were phenotypically normal and had no obvious reproductive, developmental, or growth defects, which validated our tissue-specific glycoengineering strategy.
3.2. Transgene expression
PSGs were dissected from 5th day of 5th instar w1-pnd, Flc-GG#1, and Flc-GG#2 silkworm larvae and total RNA was isolated and used for RT-PCR assays, as described in Materials and methods. Gene-specific primers were used to examine expression of the mammalian MGAT2 and B4GALT1 transgenes. RNA from w1-pnd silkworms was used as a negative control and primers specific for an endogenous B. mori ribosomal protein L3 (BmRPL3) gene were used as positive and loading controls. Duplicate RT-PCR assays were performed in parallel with and without RT to assess possible DNA contamination. No amplification products were observed in any RT-PCRs performed without either RT (Fig. 2, RT−) or template DNA (Fig. 2, no template). In contrast, correctly sized amplification products were observed in all RT-PCRs performed with RT and BmRPL3-specific primers (Fig. 2, RT+; BmRPL3), which validated our RT-PCR method and provided an internal standard for the assays. No amplimers were observed in RT-PCRs containing MGAT2- or B4GALT1-specific primers and total RNA from the w1-pnd silkworms (Fig. 2, RT+; w1-pnd). In contrast, correctly sized amplimers were observed in RT-PCRs containing MGAT2- or B4GALT1-specific primers and total RNA from Flc-GG#1 and Flc-GG#2 (Fig. 2, RT+; Flc-GG#1, Flc-GG#2). These results indicated each transgene was expressed in the PSGs of both transgenic silkworm lines. The MGAT2 and B4GALT1 amplimers obtained with Flc-GG#1 RNA were stronger than those obtained with Flc-GG#2 RNA, indicating Flc-GG#1 expressed both transgenes at higher levels than Flc-GG#2. Splinkerette PCR assays revealed that Flc-GG#1 included three, whereas Flc-GG#2 included two genomic copies of the dual piggyBac vector, with each insert located at a distinct site (Table 1). Thus, it is possible that the observed differences in transgene expression levels can be explained by differences in copy number, position effects, or both.
Fig. 2.
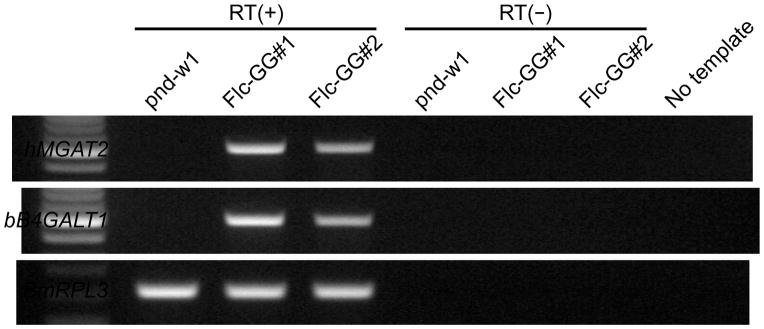
Transgene expression. Total RNA was isolated from the PSGs dissected from 5th instar w1-pnd or transgenic (Flc-GG#1 and Flc-GG#2) silkworm larvae and used for RT-PCRs, as described in Materials and methods. Each reaction was performed with (RT+) and without (RT−) reverse transcriptase to assess DNA contamination of the RNA preparations. One reaction was performed with no template to assess DNA contamination of our other reagents. The primer sets used for these RT-PCRs were specific for the endogenous BmRPL3 or human MGAT2 or bovine B4GALT1 genes, as indicated on the left-hand side of the panel.
Table 1.
piggyBac integration sites in Flc-GG1 and Flc-GG2 genome.
| 5′-flanking sequence | 3′-flanking sequence | Chr. no. | ||
|---|---|---|---|---|
| Flc-GG1-1 | …GTTATTGGATTATTAT | TTAA-piggyBac-TTAA | TCATCATCAGTCCACT… | 5 |
| Flc-GG1-2 | …GTAAAATTGTTTTTAT | TTAA-piggyBac-TTAA | GTAAAGTTGATATGTG… | 10 |
| Flc-GG1-3 | …GATTATCTTTCTAGGG | TTAA-piggyBac-TTAA | GAAACTCGCACTAAGC… | N.D. |
| Flc-GG2-1 | TTAA-piggyBac-TTAA | AACTGCACCAGTGGAA… | N.D. | |
| Flc-GG2-2 | …AGATTGCCGGTTGATA | TTAA-piggyBac-TTAA | AACGTCCACGTCAGGG… | 4 |
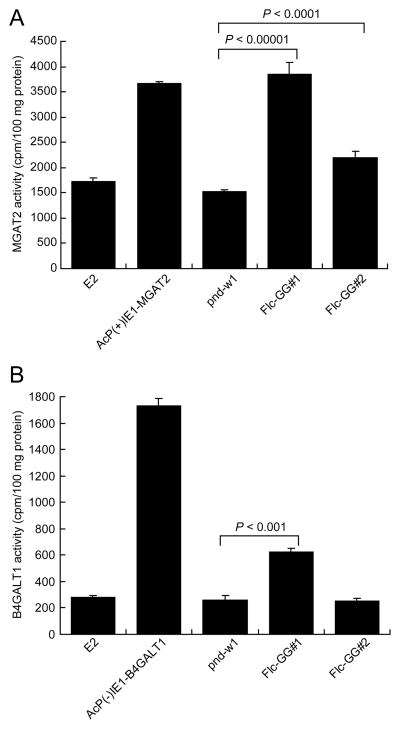
3.3. Glycosyltransferase activities
PSG extracts were prepared and used for MGAT2 and B4GALT1 activity assays to determine if transgene expression at the RNA level induced the expected functions in the transformed silkworms. These assays were validated using extracts from insect cells infected with wild-type baculovirus (Fig. 3, E2) or recombinant baculoviruses encoding either MGAT2 (Fig. 3A, AcP(+)IE1-MGAT2) or B4GALT1 (Fig. 3B, AcP(−)IE1-B4GALT1) as negative and positive controls. The former defined background and the latter demonstrated our assays could detect each transferase activity. PSG extracts isolated from Flc-GG#1 and Flc-GG#2 silkworms both contained significantly more MGAT2 activity than the w1-pnd controls, as expected (Fig. 3A). The Flc-GG#1 extracts had higher levels of MGAT2 activity than the Flc-GG#2 extracts, which was consistent with the fact that Flc-GG#1 had higher levels of MGAT2 RNA (Fig. 2, MGAT2). In contrast, only the PSGs from Flc-GG#1 had significantly more B4GALT1 activity than the w1-pnd controls (Fig. 3B, Flc-GG#1). The absence of higher levels of B4GALT1 activity in the PSGs from Flc-GG#2 (Fig. 3B, Flc-GG#2) was inconsistent with the presence of B4GALT1 RNA in the PSGs from this strain (Fig. 2, B4GALT1: Flc-GG#2). Thus, we considered that the PSG extract might interfere with the B4GALT1 enzyme activity assay. To test this hypothesis, we performed control B4GALT1 assays with known B4GALT1-positive baculovirus-infected insect cell lysates that were spiked or not spiked with w1-pnd PSG extract. The results showed the addition of PSG extract reduced B4GALT1 activity, indicating the assay is less sensitive when used to measure B4GALT1 activity in PSG lysates (data not shown). We concluded that the Flc-GG#2 PSGs likely have B4GALT1 activity, but at lower levels than Flc-GG#1, and at levels that were not detected in our assay due to interference by an unknown substance in PSG extract. This conclusion was verified by the subsequent finding that Flc-GG#2 PSG glycoproteins have terminally-galactosylated N-glycans (see below).
Fig. 3.
Glycosyltransferase activities. PSGs from the parental (w1-pnd) or transgenic (Flc-GG#1 and Flc-GG#2) silkworm strains were extracted and assayed for the presence of MGAT2 (A) and B4GALT1 (B) activities, as described in Materials and methods. Activities were determined as the average cpm of tritiated N-acetylglucosamine or galactose transferred to the acceptor substrate/100 μg protein. The results are presented as the mean ± standard deviation obtained in three independent measurements. The statistical significance of the differences in MGAT2 or B4GALT1 activities observed in the transgenic silkworms, relative to the w1-pnd controls were determined by one-way ANOVA analysis.
3.4. N-glycosylation of PSG glycoproteins
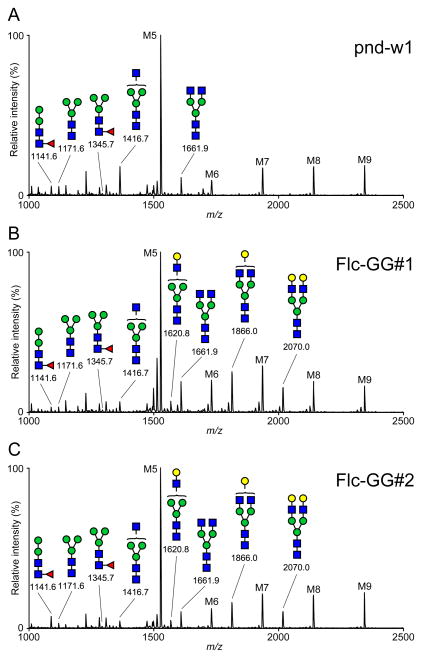
To examine the impact of MGAT2 and B4GALT1 expression on protein N-glycosylation in the PSG, we isolated total proteins from w1-pnd, Flc-GG#1, and Flc-GG#2 silkworm PSGs, prepared tryptic peptide digests, enzymatically released the N-glycans, and examined their structures using MALDI-TOF-MS, as described in Materials and methods. The results showed that the major N-glycan on the PSG glycoproteins from all three strains was M5 (Man5GlcNAc2; Fig. 4). The PSG glycoproteins from all three strains also included high mannose- (M6, M7, M8, and M9), paucimannose- (M3), hybrid- (GlcNAcMan5GlcNAc2), and complex-type (GlcNAc2Man5GlcNAc2) structures with <1% core fucosylation. Most importantly, while the w1-pnd PSG glycoproteins had no detectable terminally galactosylated N-glycans, those from the Flc-GG#1 and Flc-GG#2 silkworms had three different terminally galactosylated N-glycan structures: GalGlcNAcMan3GlcNAc2, GalGlcNAc2Man3GlcNAc2, and Gal2GlcNAc2Man3GlcNAc2 (Fig. 4). A quantitative analysis of the relative signal intensities observed for each N-glycan structure represented by an MS peak with a signal to noise ratio of >3 showed the PSG glycoproteins from the parental and glycoengineered silkworms had similar proportions of high mannose structures, with M5 predominant, as noted above (Fig. 5A). However, the glycoengineered silkworms had lower proportions of paucimannose and hybrid structures, as compared to w1-pnd, and this correlated with the appearance of terminally galactosylated structures in these insects (Fig. 5B). The total proportions of complex, terminally galactosylated structures produced by Flc-GG#1 and Flc-GG#2 were 17.7% and 13.1%, respectively. Hence, the MALDI-TOF-MS results demonstrated that the PSG N-glycosylation pathway was successfully glycoengineered in these two silkworm strains.
Fig. 4.
N-glycosylation profiles. PSGs from (A), Flc-GG#1 (B), and Flc-GG#2 (C) silkworms were extracted, the extracts were used to produce total tryptic glycopeptides, and then total N-glycans were enzymatically released, permethylated, and analyzed by MALDI-TOF-MS, as described in Materials and methods. All molecular ions were detected as [M+Na]+, and peaks with signal to noise ratios of >3 were assigned and annotated with the standard cartoon symbolic representations.
4. Discussion
The silkworm has been used as a host for baculovirus-mediated recombinant protein production for the past 25 years. More recently, various investigators have isolated transgenic silkworms that can constitutively produce recombinant proteins in selected tissues. Thus, the silkworm is emerging as a new recombinant protein production platform with attributes such as the silk glands, which have the capacity for high-level protein production and secretion. However, the silkworm has relatively primitive protein glycosylation pathways, which cannot produce mammalian-type protein glycosylation patterns (Geisler and Jarvis, 2009; Harrison and Jarvis, 2006; Iizuka et al., 2009; Kulakosky et al., 1998; Marchal et al., 2001; Marz et al., 1995; Park et al., 2009; Sasaki et al., 2009; Shi and Jarvis, 2007). This is a significant limitation because most therapeutic glycoproteins require mammalian-type glycosylation patterns for clinical efficacy (Sola and Griebenow, 2011). In previous studies, we have used various glycoengineering approaches to address this problem in the baculovirus-insect cell system (reviewed in Geisler and Jarvis, 2009; Geisler et al., 2015; Harrison and Jarvis, 2006; Jarvis, 2009; Shi and Jarvis, 2007). These efforts yielded novel baculovirus-insect cell systems with extended N-glycosylation pathways that can produce mammalian-type N-glycans, including terminally galactosylated and sialylated structures. However, there have been no reports of any successful attempt to engineer the protein N-glycosylation pathway in the silkworm.
In related studies, we obtained no viable transgenic offspring in efforts to use mammalian glycotransferase genes expressed under the control of constitutive promoters to extend the protein N-glycosylation pathway of D. melanogaster (unpublished data). Thus, we concluded that untargeted glycoengineering in a whole animal, such as the silkworm, might be complicated by adverse phenotypic impact(s) resulting from elongation of endogenous N-glycoprotein glycans. To circumvent this potential problem, we focused our new glycoengineering effort on the silk glands. We considered this to be a useful approach because the silk glands are often targeted for tissue-specific foreign gene expression to take advantage of their high-level recombinant protein production and secretion capacity, as noted above.
A new, dual piggyBac vector encoding MGAT2 and B4GALT1 under the transcriptional control of B. mori flc promoters (Fig. S1) was constructed and used for this purpose. We expected this vector would induce expression of these genes in the posterior silk gland, MGAT2 would elongate the processing intermediate on endogenous PSG glycoproteins to produce GlcNAc2Man3GlcNAc2, and B4GALT1 would further elongate this structure to produce GalGlcNAc2Man3GlcNAc2 and/or Gal2GlcNAc2Man3GlcNAc2 (Fig. 1). We recognized that endogenous FDL (Fig. 1) might compete with these heterologous elongation functions. However, the paucimannose N-glycan produced by FDL is not a “dead-end” product, but rather, can be used as a substrate by the endogenous MGAT1 in insect systems (Geisler and Jarvis, 2012). Thus, FDL would only hinder our new glycoengineering effort if the PSGs had significantly more FDL than MGAT1 activity. Because this was not a problem in our previous, insect cell-based glycoengineering projects, we presumed MGAT1 would outcompete FDL in the silkworm PSG, as well. In the final analysis, we knew we could clearly assess the impact of our new glycoengineering effort because previous studies had shown that native PSGs produce no detectable terminally galactosylated N-glycans (Iizuka et al., 2009; Kulakosky et al., 1998; Park et al., 2009; Sasaki et al., 2009). Thus, we knew that if we observed these structures in transgenic insect PSGs, this would indicate the PSG protein N-glycosylation pathway had been successfully glycoengineered.
As expected, we were able to isolate transgenic silkworms that expressed both mammalian transgenes and their enzymatically active products in the PSGs. In addition, the transgenic silkworms produced PSG glycoproteins with significant proportions of terminally galactosylated N-glycans, which were not detected in the parental controls. Interestingly, our results also showed that w1-pnd silkworms produced low levels of GlcNAc2Man3GlcNAc2, indicating this tissue has endogenous MGAT2 activity. This finding was consistent with a previous report indicating that the middle silk gland of native silkworms can produce endogenous glycoproteins and a recombinant antibody containing GlcNAc2Man3GlcNAc2 (Iizuka et al., 2009). In fact, these results suggest that the middle silk gland might be a better glycoengineering target because it seems to have a higher capacity to produce glycoproteins with N-glycans containing terminal N-acetylglucosamine residues, which would serve as acceptors for terminal galactosylation. However, neither the middle (Iizuka et al., 2009) nor the posterior silk gland (this study) of native silkworms can produce any detectable terminally galactosylated N-glycans. Thus, glycoengineering is required to drive further processing, even in tissues capable of producing hybrid or complex structures with terminal N-acetylglucosamine residues.
The results of this study document the first successful example of glycoengineering in the silkworm, which is emerging as a recombinant protein production platform. They show that mammalian enzymes can be effectively used for this purpose, set the stage for additional glycoengineering for the production of terminally sialylated N-glycoproteins, and pave the way for engineering protein O-glycosylation and other protein processing pathways in the silk gland. Finally, the results obtained in this and a previous study (Iizuka et al., 2009) suggest that therapeutic antibodies produced in the silkworm silk gland would likely have extremely low levels of core fucosylated Fc N-glycans. This is important because core fucosylation of the Fc N-glycan represses antibody effector functions, such as antibody-dependent cell cytotoxicity (Shinkawa et al., 2003). Thus, the demonstration that the silkworm silk gland can be glycoengineered to produce terminally galactosylated N-glycans and that this tissue normally produces low levels of core fucosylated structures is an important step towards solidifying the status of the silkworm as an emerging recombinant protein production platform.
Supplementary Material
Fig. S1. piggyBac vector. This new dual piggyBac vector encodes both human MGAT2 and bovine B4GALT1 under the transcriptional control of the Bombyx mori fibL promoter, with each expression cassette separated by the Drosophila white intron 2 sequence. The vector also includes DsRed1 under the control of the neural-specific synthetic promoter, 3xP3, as an eye color marker. PBAS: piggyBac activation sequence, IR: inverted repeat, TR: terminal repeat.
Supplementary Table S1. Sequences of primers used in this study.
HIGHLIGHTS.
Isolation of transgenic silkworms encoding mammalian protein glycosylation functions.
Targeting mammalian glycogene expression to the silk gland.
Induction of mammalian N-glycan processing enzyme activities in the silk gland.
Induction of mammalian-type N-glycan biosynthesis in the silk gland.
Acknowledgments
This work was supported by Award Numbers R01GM080672 and R01GM49734 from the National Institute of General Medical Sciences, National Institutes of Health. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute of General Medical Sciences or the National Institutes of Health. The MS data were acquired at the Core Facilities for Protein Structural Analysis at Academia Sinica, supported under the Taiwan National Core Facility Program for Biotechnology, NSC Grant Number 100-2325-B-001-029. We gratefully acknowledge Dr. Joe Hull for contributions to this project, including isolation of the fibL promoter sequence from B. mori genomic DNA.
Appendix A
Supplementary data related to this article can be found at…
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Adachi T, Tomita M, Shimizu K, Ogawa S, Yoshizato K. Generation of hybrid transgenic silkworms that express Bombyx mori prolyl-hydroxylase alpha-subunits and human collagens in posterior silk glands: Production of cocoons that contained collagens with hydroxylated proline residues. J Biotechnol. 2006;126:205–219. doi: 10.1016/j.jbiotec.2006.04.035. [DOI] [PubMed] [Google Scholar]
- Bao S, Cagan R. Fast cloning inverted repeats for RNA interference. RNA. 2006;12:2020–2024. doi: 10.1261/rna.258406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ceroni A, Maass K, Geyer H, Geyer R, Dell A, Haslam SM. GlycoWorkbench: a tool for the computer-assisted annotation of mass spectra of glycans. J Proteome Res. 2008;7:1650–1659. doi: 10.1021/pr7008252. [DOI] [PubMed] [Google Scholar]
- Choudary PV, Kamita SG, Maeda S. Expression of foreign genes in Bombyx mori larvae using baculovirus vectors. Meth Mol Biol. 1995;39:243–264. doi: 10.1385/0-89603-272-8:243. [DOI] [PubMed] [Google Scholar]
- Dell A, Reason AJ, Khoo KH, Panico M, McDowell RA, Morris HR. Mass spectrometry of carbohydrate-containing biopolymers. Meth Enzymol. 1994;230:108–132. doi: 10.1016/0076-6879(94)30010-0. [DOI] [PubMed] [Google Scholar]
- Falconer DS. Introduction to Quantitative Genetics. Ronald Press Company; New York: 1960. [Google Scholar]
- Geisler C, Aumiller JJ, Jarvis DL. A fused lobes gene encodes the processing beta-N-acetylglucosaminidase in Sf9 cells. J Biol Chem. 2008;283:11330–11339. doi: 10.1074/jbc.M710279200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geisler C, Jarvis DL. Insect cell glycosylation patterns in the context of biopharmaceuticals. In: Walsh B, editor. Post-translational Modification of Protein Biopharmaceuticals. Wiley-Blackwell; Weinheim: 2009. pp. 165–191. [Google Scholar]
- Geisler C, Jarvis DL. Substrate specificities and intracellular distributions of three N-glycan processing enzymes functioning at a key branch point in the insect N-glycosylation pathway. J Biol Chem. 2012;287:7084–7097. doi: 10.1074/jbc.M111.296814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geisler C, Mabashi-Asazuma H, Jarvis DL. An overview and history of glyco-engineering in insect expression systems. Meth Mol Biol. 2015;1321:131–152. doi: 10.1007/978-1-4939-2760-9_10. [DOI] [PubMed] [Google Scholar]
- Harrison RL, Jarvis DL. Protein N-glycosylation in the baculovirus-insect cell expression system and engineering of insect cells to produce “mammalianized” recombinant glycoproteins. Adv Virus Res. 2006;68:159–191. doi: 10.1016/S0065-3527(06)68005-6. [DOI] [PubMed] [Google Scholar]
- Hino R, Tomita M, Yoshizato K. The generation of germline transgenic silkworms for the production of biologically active recombinant fusion proteins of fibroin and human basic fibroblast growth factor. Biomaterials. 2006;27:5715–5724. doi: 10.1016/j.biomaterials.2006.07.028. [DOI] [PubMed] [Google Scholar]
- Hollister JR, Shaper JH, Jarvis DL. Stable expression of mammalian beta 1,4-galactosyltransferase extends the N-glycosylation pathway in insect cells. Glycobiology. 1998;8:473–480. doi: 10.1093/glycob/8.5.473. [DOI] [PubMed] [Google Scholar]
- Horn C, Jaunich B, Wimmer EA. Highly sensitive, fluorescent transformation marker for Drosophila transgenesis. Dev Genes Evol. 2000;210:623–629. doi: 10.1007/s004270000111. [DOI] [PubMed] [Google Scholar]
- Iizuka M, Ogawa S, Takeuchi A, Nakakita S, Kubo Y, Miyawaki Y, Hirabayashi J, Tomita M. Production of a recombinant mouse monoclonal antibody in transgenic silkworm cocoons. FEBS J. 2009;276:5806–5820. doi: 10.1111/j.1742-4658.2009.07262.x. [DOI] [PubMed] [Google Scholar]
- Iizuka T, Sezutsu H, Tatematsu KI, Kobayashi I, Yonemura N, Uchino K, Nakajima K, Kojima K, Takabayashi C, Machii H, Yamada K, Kurihara H, Asakura T, Nakazawa Y, Miyawaki A, Karasawa S, Kobayashi H, Yamaguchi J, Kuwabara N, Nakamura T, Yoshii K, Tamura T. Colored fluorescent silk made by transgenic silkworms. Adv Funct Mat. 2013;23:5232–5239. [Google Scholar]
- Jarvis DL. Baculovirus-insect cell expression systems. Meth Enzymol. 2009;463:191–222. doi: 10.1016/S0076-6879(09)63014-7. [DOI] [PubMed] [Google Scholar]
- Kato T, Kajikawa M, Maenaka K, Park EY. Silkworm expression system as a platform technology in life science. Appl Microbiol Biotechnol. 2010;85:459–470. doi: 10.1007/s00253-009-2267-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kojima K, Kuwana Y, Sezutsu H, Kobayashi I, Uchino K, Tamura T, Tamada Y. A new method for the modification of fibroin heavy chain protein in the transgenic silkworm. Biosci Biotechnol Biochem. 2007;71:2943–2951. doi: 10.1271/bbb.70353. [DOI] [PubMed] [Google Scholar]
- Kornfeld R, Kornfeld S. Assembly of asparagine-linked oligosaccharides. Annu Rev Biochem. 1985;54:631–664. doi: 10.1146/annurev.bi.54.070185.003215. [DOI] [PubMed] [Google Scholar]
- Kulakosky PC, Hughes PR, Wood HA. N-linked glycosylation of a baculovirus-expressed recombinant glycoprotein in insect larvae and tissue culture cells. Glycobiology. 1998;8:741–745. doi: 10.1093/glycob/8.7.741. [DOI] [PubMed] [Google Scholar]
- Kurihara H, Sezutsu H, Tamura T, Yamada K. Production of an active feline interferon in the cocoon of transgenic silkworms using the fibroin H-chain expression system. Biochem Biophys Res Commun. 2007;355:976–980. doi: 10.1016/j.bbrc.2007.02.055. [DOI] [PubMed] [Google Scholar]
- Kuwana Y, Sezutsu H, Nakajima K, Tamada Y, Kojima K. High-toughness silk produced by a transgenic silkworm expressing spider (Araneus ventricosus) dragline silk protein. PLoS One. 2014;9:e105325. doi: 10.1371/journal.pone.0105325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leonard R, Rendic D, Rabouille C, Wilson IB, Preat T, Altmann F. The Drosophila fused lobes gene encodes an N-acetylglucosaminidase involved in N-glycan processing. J Biol Chem. 2006;281:4867–4875. doi: 10.1074/jbc.M511023200. [DOI] [PubMed] [Google Scholar]
- Long D, Lu W, Zhang Y, Bi L, Xiang Z, Zhao A. An efficient strategy for producing a stable, replaceable, highly efficient transgene expression system in silkworm, Bombyx mori. Sci Rep. 2015;5:8802. doi: 10.1038/srep08802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma S, Shi R, Wang X, Liu Y, Chang J, Gao J, Lu W, Zhang J, Zhao P, Xia Q. Genome editing of BmFib-H gene provides an empty Bombyx mori silk gland for a highly efficient bioreactor. Sci Rep. 2014;4:6867. doi: 10.1038/srep06867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mabashi-Asazuma H, Kuo CW, Khoo KH, Jarvis DL. A novel baculovirus vector for the production of nonfucosylated recombinant glycoproteins in insect cells. Glycobiology. 2014;24:325–340. doi: 10.1093/glycob/cwt161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maeda S. Expression of foreign genes in insects using baculovirus vectors. Annu Rev Entomol. 1989;34:351–372. doi: 10.1146/annurev.en.34.010189.002031. [DOI] [PubMed] [Google Scholar]
- Maeda S, Kawai T, Obinata M, Fujiwara H, Horiuchi T, Saeki Y, Sato Y, Furusawa M. Production of human alpha-interferon in silkworm using a baculovirus vector. Nature. 1985;315:592–594. doi: 10.1038/315592a0. [DOI] [PubMed] [Google Scholar]
- Marchal I, Jarvis DL, Cacan R, Verbert A. Glycoproteins from insect cells: sialylated or not? Biol Chem. 2001;382:151–159. doi: 10.1515/BC.2001.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marz L, Altmann F, Staudacher E, Kubelka V. Protein glycosylation in insects. In: Montreuil J, Vliegenthart JFG, Schachter H, editors. Glycoproteins. Elsevier; Amsterdam: 1995. pp. 543–563. [Google Scholar]
- Ogawa S, Tomita M, Shimizu K, Yoshizato K. Generation of a transgenic silkworm that secretes recombinant proteins in the sericin layer of cocoon: production of recombinant human serum albumin. J Biotechnol. 2007;128:531–544. doi: 10.1016/j.jbiotec.2006.10.019. [DOI] [PubMed] [Google Scholar]
- Park EY, Ishikiriyama M, Nishina T, Kato T, Yagi H, Kato K, Ueda H. Human IgG1 expression in silkworm larval hemolymph using BmNPV bacmids and its N-linked glycan structure. J Biotechnol. 2009;139:108–114. doi: 10.1016/j.jbiotec.2008.09.013. [DOI] [PubMed] [Google Scholar]
- Royer C, Jalabert A, Da Rocha M, Grenier AM, Mauchamp B, Couble P, Chavancy G. Biosynthesis and cocoon-export of a recombinant globular protein in transgenic silkworms. Transgenic Res. 2005;14:463–472. doi: 10.1007/s11248-005-4351-4. [DOI] [PubMed] [Google Scholar]
- Sasaki K, Kajikawa M, Kuroki K, Motohashi T, Shimojima T, Park EY, Kondo S, Yagi H, Kato K, Maenaka K. Silkworm expression and sugar profiling of human immune cell surface receptor, KIR2DL1. Biochem Biophys Res Commun. 2009;387:575–580. doi: 10.1016/j.bbrc.2009.07.065. [DOI] [PubMed] [Google Scholar]
- Seong J, Kim MJ, Kim HS, Kim SA, Jeon HW, Sung SH, Kim KC, Suh DS. Generation of transgenic silkworms for production of erythropoietin in Bombyx mori. Genes & genomics. 2011;33:237–243. [Google Scholar]
- Shi X, Harrison RL, Hollister JR, Mohammed A, Fraser MJ, Jr, Jarvis DL. Construction and characterization of new piggyBac vectors for constitutive or inducible expression of heterologous gene pairs and the identification of a previously unrecognized activator sequence in piggyBac. BMC Biotechnol. 2007;7:5. doi: 10.1186/1472-6750-7-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi X, Jarvis DL. Protein N-glycosylation in the baculovirus-insect cell system. Curr Drug Targ. 2007;8:1116–1125. doi: 10.2174/138945007782151360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shinkawa T, Nakamura K, Yamane N, Shoji-Hosaka E, Kanda Y, Sakurada M, Uchida K, Anazawa H, Satoh M, Yamasaki M, Hanai N, Shitara K. The absence of fucose but not the presence of galactose or bisecting N-acetylglucosamine of human IgG1 complex-type oligosaccharides shows the critical role of enhancing antibody-dependent cellular cytotoxicity. J Biol Chem. 2003;278:3466–3473. doi: 10.1074/jbc.M210665200. [DOI] [PubMed] [Google Scholar]
- Sola RJ, Griebenow K. Glycosylation of therapeutic proteins: an effective strategy to optimize efficacy. BioDrugs. 2011;24:9–21. doi: 10.2165/11530550-000000000-00000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamura T, Thibert C, Royer C, Kanda T, Abraham E, Kamba M, Komoto N, Thomas JL, Mauchamp B, Chavancy G, Shirk P, Fraser M, Prudhomme JC, Couble P. Germline transformation of the silkworm Bombyx mori L. using a piggyBac transposon-derived vector. Nat Biotechnol. 2000;18:81–84. doi: 10.1038/71978. [DOI] [PubMed] [Google Scholar]
- Teule F, Miao YG, Sohn BH, Kim YS, Hull JJ, Fraser MJ, Jr, Lewis RV, Jarvis DL. Silkworms transformed with chimeric silkworm/spider silk genes spin composite silk fibers with improved mechanical properties. Proc Natl Acad Sci USA. 2012;109:923–928. doi: 10.1073/pnas.1109420109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomita M. Transgenic silkworms that weave recombinant proteins into silk cocoons. Biotechnol Lett. 2011;33:645–654. doi: 10.1007/s10529-010-0498-z. [DOI] [PubMed] [Google Scholar]
- Tomita M, Hino R, Ogawa S, Iizuka M, Adachi T, Shimizu K, Sotoshiro H, Yoshizato K. A germline transgenic silkworm that secretes recombinant proteins in the sericin layer of cocoon. Transgenic Res. 2007;16:449–465. doi: 10.1007/s11248-007-9087-x. [DOI] [PubMed] [Google Scholar]
- Tomita M, Munetsuna H, Sato T, Adachi T, Hino R, Hayashi M, Shimizu K, Nakamura N, Tamura T, Yoshizato K. Transgenic silkworms produce recombinant human type III procollagen in cocoons. Nat Biotechnol. 2003;21:52–56. doi: 10.1038/nbt771. [DOI] [PubMed] [Google Scholar]
- Wang F, Xu H, Wang Y, Wang R, Yuan L, Ding H, Song C, Ma S, Peng Z, Peng Z, Zhao P, Xia Q. Advanced silk material spun by a transgenic silkworm promotes cell proliferation for biomedical application. Acta Biomat. 2014;10:4947–4955. doi: 10.1016/j.actbio.2014.06.031. [DOI] [PubMed] [Google Scholar]
- Wen H, Lan X, Zhang Y, Zhao T, Wang Y, Kajiura Z, Nakagaki M. Transgenic silkworms (Bombyx mori) produce recombinant spider dragline silk in cocoons. Mol Biol Rep. 2010;37:1815–1821. doi: 10.1007/s11033-009-9615-2. [DOI] [PubMed] [Google Scholar]
- Xu H. The advances and perspectives of recombinant protein production in the silk gland of silkworm Bombyx mori. Transgenic Res. 2014;23:697–706. doi: 10.1007/s11248-014-9826-8. [DOI] [PubMed] [Google Scholar]
- Yanagisawa S, Zhu Z, Kobayashi I, Uchino K, Tamada Y, Tamura T, Asakura T. Improving cell-adhesive properties of recombinant Bombyx mori silk by incorporation of collagen or fibronectin derived peptides produced by transgenic silkworms. Biomacromolecules. 2007;8:3487–3492. doi: 10.1021/bm700646f. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig. S1. piggyBac vector. This new dual piggyBac vector encodes both human MGAT2 and bovine B4GALT1 under the transcriptional control of the Bombyx mori fibL promoter, with each expression cassette separated by the Drosophila white intron 2 sequence. The vector also includes DsRed1 under the control of the neural-specific synthetic promoter, 3xP3, as an eye color marker. PBAS: piggyBac activation sequence, IR: inverted repeat, TR: terminal repeat.
Supplementary Table S1. Sequences of primers used in this study.