Abstract
Background
Cogent evidence has shown that schizophrenia vulnerability is enhanced by psychosocial stress in adolescence, yet the underpinnings of this phenomenon remain elusive. One of the animal models that best capture the relationship between juvenile stress and schizophrenia is isolation rearing (IR). This manipulation, which consists in subjecting rats to social isolation from weaning through adulthood, results in neurobehavioral alterations akin to those observed in schizophrenia patients. In particular, IR-subjected rats display a marked reduction of the prepulse inhibition (PPI) of the startle reflex, which are posited to reflect imbalances in dopamine neurotransmission in the nucleus accumbens (NAcc). We recently documented that the key neurosteroidogenic enzyme 5α-reductase (5αR) plays an important role in the dopaminergic regulation of PPI; given that IR leads to a marked down-regulation of this enzyme in the NAcc, the present study was designed to further elucidate the functional role of 5αR in the regulation of PPI of IR-subjected rats.
Methods
We studied the impact of the prototypical 5αR inhibitor finasteride (FIN) on the PPI deficits and NAcc steroid profile of IR-subjected male rats, in comparison with socially reared (SR) controls.
Results
FIN (25–100 mg/kg, i.p.) dose-dependently countered IR-induced PPI reduction, without affecting gating integrity in SR rats. The NAcc and striatum of IR-subjected rats displayed several changes in neuroactive steroid profile, including a reduction in pregnenolone in both SR and IR-subjected groups, as well as a decrease in allopregnanolone content in the latter group; both effects were significantly opposed by FIN.
Conclusions
These results show that 5αR inhibition counters the PPI deficits induced by IR, possibly through limbic changes in pregnenolone and/or allopregnanolone concentrations.
Keywords: 5α-reductase, isolation rearing, sensorimotor gating, neurosteroids, schizophrenia, prepulse inhibition
1. INTRODUCTION
Ample empirical evidence has shown greater schizophrenia vulnerability in individuals chronically exposed to psychosocial stressors throughout early developmental stages (Nuechterlein et al., 1992; Norman and Malla, 1993; Walker et al., 2008). Although several studies have focused on the pathophysiological link between juvenile stress and psychosis onset (Corcoran et al., 2003), the neurobiological underpinnings of this phenomenon remain elusive.
One of the best tools to explore the molecular bases of the stress-diathesis model of schizophrenia is afforded by isolation rearing (IR), an experimental manipulation consisting in subjecting rodents to prolonged social deprivation from weaning through adulthood. This manipulation results in an array of neurobehavioral aberrations reminiscent of core phenotypes observed in psychotic patients (Fone and Porkess, 2008), such as the disruption of sensorimotor gating, measured via the prepulse inhibition (PPI) of the acoustic startle reflex (Geyer et al., 1993). In comparison with other behavioral alterations induced by IR, PPI deficits feature several operational advantages, including their correspondence with similar impairments in schizophrenia patients (Braff et al., 1992) and their sensitivity to antipsychotic agents (Bakshi et al., 1999; Binder et al., 2001). Recent findings have shown that the PPI deficits and other behavioral changes in IR-subjected rats are underpinned by alterations in dopamine (DA) (Jones et al., 1992; Hall et al., 1998; Roncada et al., 2009), one of the key neurotransmitters implicated in the pathophysiology of psychotic disorders. In particular, IR has been shown to lead to PPI disruption through DAergic imbalances in the nucleus accumbens (NAcc) (Powell et al., 2003), the terminal of the mesolimbic DA system. Indeed, ample evidence has shown that stimulation of DAergic receptors in the NAcc disrupts PPI in rodents (for a review, see Geyer et al., 2001).
Several lines of evidence suggest that neuroactive steroids may contribute to the pathogenic role of psychosocial stress in schizophrenia (Walker et al., 2008). First, psychosocial stress leads to alterations in the levels of circulating steroids synthesized in the adrenal glands and gonads, which trigger multiple functional changes in the brain (Johnson et al., 1992; Huether, 1996; Kajantie and Phillips, 2006). Secondly, stress causes changes in the synthesis and metabolism of neurosteroids (i.e. steroids synthesized de novo in the brain; see Baulieu, 1998), which in turn play an important role in the orchestration of the behavioral response to stress (Fig. 1) (Purdy et al., 1991; Barbaccia et al., 1996, 2001; Dong et al., 2001; Agis-Balboa et al., 2007; Sanchez et al., 2008, 2009). Thirdly, neurosteroids and circulating neuroactive steroids have been shown to play a key role in the regulation of DA neurotransmission and signaling (Di Paolo, 1994; Sanchez et al., 2010). Finally, schizophrenia patients have been shown to feature abnormalities of neuroactive steroid profiles (Shirayama et al., 2002; Ritsner and Strous, 2010; Bicikova et al., 2013); in particular, Marx and colleagues (2006) have documented high concentrations of pregnenolone and dehydroepiandrosterone (DHEA) in the posterior cingulate and parietal cortex of schizophrenia patients. Notably, these neurosteroids may be effective adjunctive therapies for the management of cognitive and negative symptoms (Strous et al., 2003; Marx et al., 2009; Marx et al., 2011).
Fig. 1.
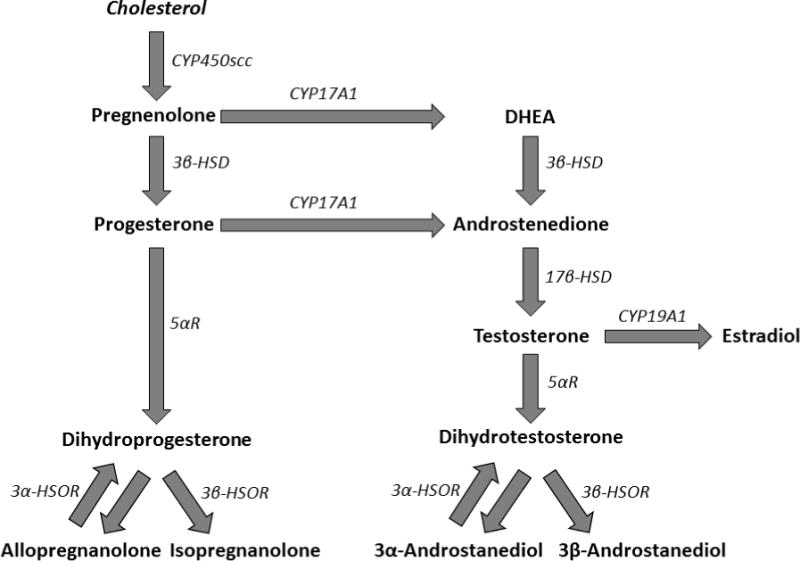
Schematization of the major biosynthetic pathways of neurosteroids. Cholesterol is converted to pregnenolone by cytochrome P450 side-chain cleavage (scc). Pregnenolone is then processed either to progesterone by 3β-hydroxysteroid dehydrogenase (3β-HSD) or to dehydroepiandrosterone (DHEA) by 17-hydroxylase/17,20 lyase (CYP17A1). The conversion of progesterone to dihydroprogesterone is catalyzed by 5α-reductase (5αR). Dihydroprogesterone can be metabolized by the reversible enzyme 3α-hydroxysteroid oxidoreductase (3α-HSOR) and 3β-hydroxysteroid oxidoreductase (3β-HSOR) for the synthesis of allopregnanolone and isopregnanolone, respectively. DHEA is metabolized to androstenedione, which is then converted to testosterone by 17β -hydroxysteroid dehydrogenase (17β-HSD). Testosterone is the converted into estradiol by aromatase (CYP19A1); alternatively, the combined metabolic reactions of 5αR, 3α- and 3β-HSOR metabolize testosterone into its androgenic derivatives dihydrotestosterone (DHT), 3α- and 3β-androstanediol, respectively.
Building on these premises, our group has recently studied the implication of neuroactive steroids in the modulation of behavioral responses induced by DAergic agonists relevant to schizophrenia-associated phenotypes. In particular, we focused on 5α-reductase (5αR), the enzyme catalyzing the rate-limiting step of neurosteroidogenesis as well as the conversion of testosterone into its major androgenic metabolite 5α-dihydrotestosterone (DHT) (Paba et al., 2011). Notably, we found that 5αR inhibitors oppose the PPI deficits induced by DAergic agonists in rodents, likely through the involvement of postsynaptic DA receptors in the NAcc (Bortolato et al., 2008; Paba et al., 2011; Devoto et al., 2012; Frau et al., 2013). Furthermore, the prototypical 5αR inhibitor finasteride (FIN), which is already approved for clinical use as a therapy for benign prostatic hyperplasia and androgenic alopecia, was found to have antipsychotic effects in a treatment-refractory case of chronic schizophrenia (Koethe et al., 2008). The specific molecular mechanisms by which FIN and other 5αR inhibitors exert anti-DAergic properties, however, remain elusive to date.
Conversely to these premises, we recently showed that IR results in a profound reduction in 5αR expression in the NAcc (Bortolato et al., 2011). This finding, however, did not qualify whether this down-regulation is etiologically relevant with respect to the PPI deficits observed in IR-subjected rats, or rather represent a compensatory adaptive response aimed at balancing other molecular changes associated with those impairments. To address this question, in the present study we examined the functional role of 5αR in IR-induced PPI deficits by testing the impact of FIN on the PPI deficits and steroid profile in the NAcc of IR-subjected rats, as compared with socially reared (SR) counterparts. Specifically, we predicted that, if the IR-induced PPI deficits are caused by the reduction in 5αR expression, they should be exacerbated by FIN administration; vice versa, if FIN elicited antipsychotic-like effects in IR-subjected rats, this scenario would likely signify that the down-regulation of 5αR in these animals does not lead to sensorimotor gating impairments.
2. MATERIALS AND METHODS
2.1 Animals and isolation procedure
Sprague–Dawley dams (Harlan Italy, S. Pietro al Natisone, Italy) were mated with sires and single-housed for the whole duration of their pregnancy. Following delivery, litters were culled to 6 pups. At postnatal day 22, rats were weaned and males were randomly assigned to either IR or SR groups. To avoid litter effects, no more than two SR littermates were placed together in the same cage. IR-subjected rats were reared individually in plastic cages, while SR rats were housed four per cage. The sizes of the cages for IR and SR rats were 41 × 26 × 20 cm and 52 × 32 × 20 cm, respectively. Animals were disturbed only for cleaning purposes, which consisted of changing the cage (once a week for IR-subjected rats and twice a week for SR controls). Both groups were housed in the same room so that IR rats maintained visual, auditory, and olfactory contact with the other animals. The room was kept under standard conditions of temperature and humidity, and food and water was available ad libitum. Artificial light was on from 8 PM to 8 AM. Experiments were conducted during the light-off phase of the day, under red light.
2.2 Drugs
Finasteride (FIN) and haloperidol (HAL) were used in this study. FIN (Polichimica, Bologna, Italy) was suspended in Tween 80 and diluted with 0.9% saline solution (1% Tween 80/saline; 1:9 vol:vol). HAL (Sigma Aldrich, Milan, Italy; Catalog N. H1512) was dissolved in a single drop of 1 M HCl and diluted with saline. Both drugs were administered intraperitoneally (i.p.) in an injection volume of 2 ml/kg, 40 min before testing. All experimental procedures were approved by the local ethics committee and carried out in strict accordance with the guidelines for experimental animals care (EEC Council 86/609; Italian D.L. 27/01/92, No. 116).
2.3 Startle reflex and PPI
Startle testing was performed as described in Bortolato et al. (2005). Briefly, the apparatus used for detection of startle reflexes (Med Associates, St Albans, VT, USA) consisted of four standard cages placed in sound-attenuated chambers with fan ventilation. Each cage consisted of a Plexiglas cylinder of 9 cm diameter, mounted on a piezoelectric accelerometric platform connected to an analog-digital converter. Two separate speakers conveyed background noise and acoustic bursts, each one properly placed so as to produce a variation of sound within 1 dB across the startle cage. Both speakers and startle cages were connected to a main PC, which detected and analyzed all chamber variables with specific software. Before each testing session, acoustic stimuli and mechanical responses were calibrated via specific devices supplied by Med Associates. After 8 weeks of IR manipulation (at 80 days of age), IR- and SR-subjected rats were injected with FIN (25–100 mg/kg, i.p.), HAL (0.1 mg/kg, i.p., as positive control) or their vehicles. Each treatment group consisted of 8-10 rats (for a total of 108 rats). Thirty-five min after treatment, rats were placed in the testing cages, for a 5-min acclimatization period with a 70 dB white noise background that continued for the remainder of the session. Each session lasted approximately 25 min, and consisted of three consecutive sequences of trials (periods). Unlike the first and the third period, during which rats were presented with only five pulse-alone trials of 115 dB, the second period consisted of a pseudorandom sequence of 50 trials, including 12 pulse-alone trials, 30 trials of pulse preceded by 74, 78, or 82 dB pre-pulses (10 for each level of pre-pulse loudness), and eight no stimulus trials, where only the background noise was delivered. Inter-trial intervals were selected randomly between 10 and 15 s. Percent PPI was calculated using the following formula: 100−[(mean startle amplitude for pre-pulse pulse trials/mean startle amplitude for pulse alone trials) × 100]. As no significant interactions between prepulse levels and treatment were found in the statistical analysis, the % PPI values were collapsed to represent average PPI.
2.4. Liquid chromatography-tandem mass spectrometry analyses
A separate set of 80-day old male IR-subjected rats and SR controls were treated with either FIN (100 mg/kg, IP) or its vehicle (n=10–11/treatment group, for a total of 43 rats) and sacrificed 60 min after treatment. NAcc samples were harvested according to the indication of the atlas of Paxinos and Watson (1998). Given the low size of this region, analyses were performed on a broader area of the brain, also including the ventral and mediodorsal striatum, in order to achieve a critical content of neurosteroids that may be detected by LC-MS/MS analysis. Sample extraction and purification were performed as previously described (Caruso et al., 2008). Briefly, samples were added with internals standards, homogenized in 2 ml of MeOH/acetic acid (99:1, v/v) using a tissue lyser (Qiagen, Italy). After an overnight extraction at 4 °C, samples were centrifuged at 12,000 rpm for 5 min and the pellet was extracted twice with 1 ml of MeOH/acetic acid (99:1, v/v). The organic phases were combined and dried with a gentle stream of nitrogen in a 40 C water bath. The samples were resuspended with 3 ml of MeOH/H2O (10:90, v/v) and passed through a SPE cartridges, previously activated with MeOH (5 ml) and MeOH:H2O 1:9 (v/v) (5 ml), the steroids were eluted in MeOH, concentrated and transferred in autosampler vials before the LC–MS/MS analysis.
Positive atmospheric pressure chemical ionization (APCI+) experiments were performed with a linear ion trap-mass spectrometer (LTQ, ThermoFisher Co., San Jose, CA, USA) using nitrogen as sheath, auxiliary and sweep gas. The instrument was equipped with a Surveyor liquid chromatography (LC) Pump Plus and a Surveyor Autosampler Plus (ThermoFisher Co., San Jose, CA, USA). The mass spectrometer was employed in MS/MS mode using helium as collision gas. The LC mobile phases were (A) H2O/0.1% formic acid and (B) methanol (MeOH)/0.1% formic acid. The gradient (flow rate 0.5 ml/min) was as follows: T0 70%A, T1.5 70%A, T2 55%A, T3 55%A, T35 36%A, T40 25%A, T41 1%A, T45 1%A, T45.2 70%A, T55 70%A. The split valve was set at 0–6.99 min to waste, 6.99–43.93 min to source and 43.93–55 to waste. The Hypersil Gold column (100 mm × 3 mm, 3 μm; ThermoFisher Co., San Jose, CA, USA) was maintained at 40 C. The injection volume was 25μl and the injector needle was washed with MeOH/water 1/1 (v/v). Peaks of the LC–MS/MS were evaluated using a Dell workstation by means of the software Excalibur® release 2.0 SR2 (ThermoFisher Co., San Jose, CA, USA). Quantitative analyses were performed on the basis of calibration curves prepared and analyzed using internal standards. Analyses targeted the following steroids: pregnenolone, progesterone, 5α-dihydroprogesterone, 3α,5α-tetrahydroprogesterone (allopregnanolone), DHEA, testosterone, DHT, 3α,5α-androstanediol (3α-diol), and 17β-estradiol. A synoptic representation of these steroids and their main metabolic pathways is provided in Fig. 1. Calibration curves were extracted and analyzed as described above for samples. Limits of detection, precision, and accuracy have been previously reported (Caruso et al., 2008).
2.5 Statistical analyses
Normality and homogeneity of variance of data distribution were verified by using the Kolmogorov-Smirnov and Bartlett’s tests. Statistical analyses were performed with one-way or two-way ANOVAs, as appropriate. Post-hoc comparisons were performed by Tukey’s test for factorial designs and Dunnett’s test for repeated measures. Significance threshold was set at 0.05.
3. RESULTS
3.1 Effects of FIN and HAL on IR-induced changes in startle amplitude and PPI
The effects of FIN on startle magnitude were analyzed by a two-way ANOVA design, with treatment and rearing condition as factors (Fig. 2A). While no effect of rearing conditions was identified [F(1,63)=2.38, ns], ANOVA detected a significant main effect for FIN treatment [F(3,63)=2.79, P<0.05], which was found to reflect a significant difference between the dose of 100 mg/kg (i.p.) and the vehicle (P<0.05, Tukey’s test). Furthermore, a significant interaction between rearing conditions and treatment was found [F(1,63)=3.69, P<0.05]. Post-hoc comparisons assessed that this effect reflected a significant difference between SR rats treated with the dose of 100 mg/kg and vehicle (P<0.01).
Fig. 2.
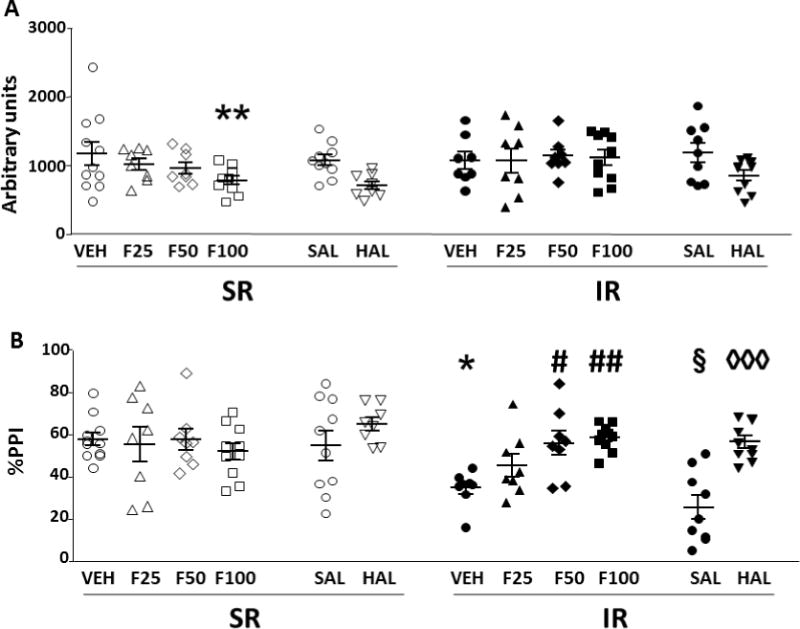
Effects of finasteride (F) and haloperidol (HAL) on startle magnitude (A) and %PPI (B) in isolation-reared (IR) and socially-reared (SR) rats. Values represent mean ± SEM for each experimental group. Treatments are indicated below the horizontal axis. All doses are given in mg/kg (IP). VEH, vehicle of finasteride. SAL, saline (vehicle of HAL). * P<0.05, ** P<0.01 in comparison to SR-VEH group; # P<0.05, ## P<0.01, in comparison to IR-VEH group. § P<0.05 in comparison to SR-SAL group; ◊◊◊ P<0.001 in comparison to IR-SAL group. For further details, see results section.
Analyses of %PPI values, performed with the same statistical design, (Fig. 2B), disclosed that IR-subjected rats displayed a significant reduction in this index in comparison with SR controls [F(1,63)=4.62, P<0.05]. While no main effects were found for the treatment [F(1,63)=2.20, ns], a significant rearing condition × treatment interaction was detected [F(3,63)=4.06, P<0.05]. Tukey’s test revealed that IR-induced PPI disruption (P<0.05 for IR-VEH vs SR-VEH comparison) was reversed by both 50 mg/kg (P<0.05) and 100 mg/kg (P<0.01) FIN doses.
It should be noted that, in a separate set of observations, none of the doses of FIN induced catalepsy in either IR or SR rats [F(3,63)=0.32, ns].
In a parallel set of studies, we verified the effects of HAL on IR-induced changes in startle amplitude and PPI (Fig. 2). HAL reduced startle amplitude [Main treatment effect: F(1,33)=7.18, P<0.05]. Conversely, no significant main effects for rearing condition or significant interactions between factors were found. PPI analyses detected significant differences between SR and IR-subjected rats [F(1,33)=26.69, P<0.001]. Furthermore, ANOVA found a main effect for treatment [F(1,33)=22.56, P<0.001], as well as a significant rearing condition × treatment interaction [F(1,33)=5.89, P<0.05]. Post-hoc analyses revealed that HAL significantly reversed the PPI deficit induced by IR condition (P<0.001).
3.2 Effects of FIN on IR-induced changes in steroid profile in the NAcc
We then evaluated the effect of FIN (100 mg/kg, i.p.) on the steroid profile in the NAcc and striatum of IR-subjected rats and their controls. As shown in Fig. 3A, IR resulted in a profound reduction in the concentration of pregnenolone. [Main effect for condition: F(1,36)=11.55, P<0.05]. Furthermore, FIN led to a marked enhancement in the concentration of this steroid [Main effect for treatment: F(1,36)=25.10; P<0.0001]. No interactions between condition and treatment were found [F(1,36)=7.63, ns]. Analyses of progesterone levels (Fig. 3B) found that this neuroactive steroid was not influenced by IR [[Main effect for condition: F(1,36)=1.42, ns], FIN treatment [Main effect for treatment: F(1,36)=0.45, ns] or their interaction [F(1,36)=1.00, ns]. In line with these results, the analysis of progesterone/pregnenolone ratio disclosed a significant enhancement in IR-subjected rats (Main effect: IR vs SR: P<0.05) and a dramatic reduction caused by FIN treatment (Main effect: FIN vs vehicle: P<0.0001) (Fig. 3H). As shown in Fig. 3C, we found that 5α-dihydroprogesterone levels were significantly reduced by IR [Main effect for condition: F(1,38)=4.25, P<0.05], but not by FIN [Main effect for treatment: F(1,38)=0.98, ns] or by condition × treatment interactions [F(1,38)=1.49, ns]. In addition, the 5α-dihydroprogesterone / progesterone ratio (Fig. 3I) was found to be reduced by IR (Main effect for rearing condition: F(1,32)=5.07, P<0.05). However, this parameter was not affected by FIN [Main effect for treatment: F(1,32)=0.32, ns; rearing × treatment interaction: F(1,32)=0.72, ns]. The analyses of allopregnanolone levels revealed no main effects for either rearing condition [F(1,36)=0.11, ns] or treatment [F(1,36)=0.34, ns]; however, a significant interaction of rearing condition and treatment was found [F(1,36)=7.86, P<0.01]. Post-hoc analyses revealed that, while vehicle-treated IR-subjected rats exhibited a significant reduction in allopregnanolone levels in comparison with their SR counterparts (P<0.05), FIN treatment induced a paradoxical enhancement in allopregnanolone levels in IR rats (P<0.05) (Fig. 3D). These results were paralleled by the changes in allopregnanolone/5α-dihydroprogesterone ratio, which revealed a positive rearing × treatment interaction [F(1,36)=9.14; P<0.01]. Indeed, SR vehicle-treated rats were found to display higher ratios than either IR vehicle-treated (P<0.05) or SR FIN-treated (P<0.01) rats. Furthermore, in IR rats FIN enhanced this ratio (P<0.01) in comparison with vehicle-treated counterparts (Fig. 3J).
Fig. 3.
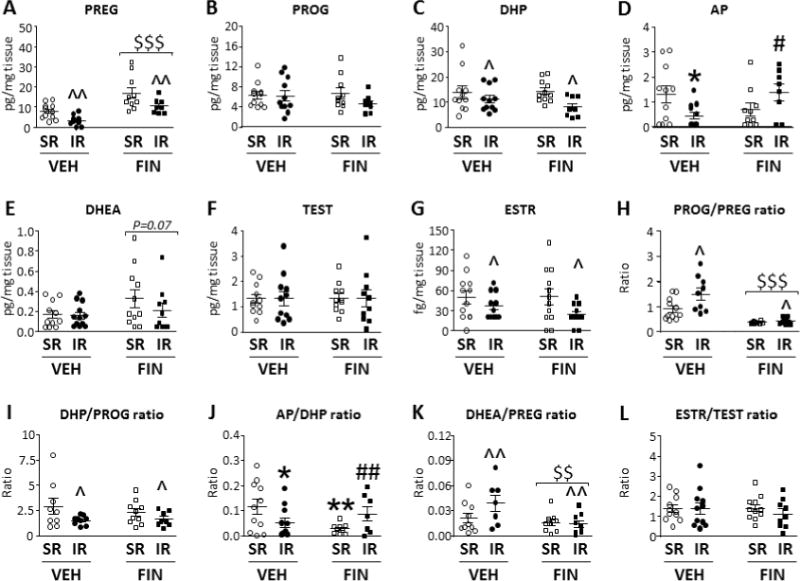
Effects of finasteride (FIN; 100 mg/kg, i.p.) and its vehicle (VEH) on the levels of neuroactive steroids in the Nucleus Accumbens and striatum of isolation-reared (IR) and socially-reared (SR) rats. Treatments are indicated below the horizontal axis. $$, P<0.01; $$$, P<0.001 in comparison with vehicle-treated rats (main effect for treatment); ^, P<0.05; ^^, P<0.01 in comparison with SR rats (main effect for rearing condition); * P<0.05; **, P<0.01 in comparison to VEH-SR group (rearing condition × treatment interaction); # P<0.05, ## P<0.01 in comparison to VEH-IR group (rearing condition × treatment interaction). VEH, vehicle; FIN, finasteride; PREG, pregnenolone; PROG, Progesterone; DHP, 5α-dihydroprogesterone; AP, allopregnanolone; DHEA, dehydroepiandrosterone; TEST, Testosterone; ESTR, 17β-estradiol. For further details, see results section.
While DHEA levels were not affected by IR [Main effect for condition: F(1,39)=0.77, P<0.05], we found a statistical trend for a reduction induced by FIN [Main effect for treatment: F(1,39)=3.43, P=0.07], but no condition × treatment interactions (Fig. 3E). The DHEA/Pregnenolone ratio was found to be increased by IR and reduced by FIN (Main effects: Ps<0.01) (Fig. 3K). While IR failed to affect testosterone levels (Fig. 3F), it caused a reduction in estradiol levels [Main effect for condition: F(1,38)=5.51, P<0.05] (Fig. 3G); conversely, FIN failed to affect the levels of either steroid (Figs. 3F–G). No significant differences were found in the analysis of the estradiol/testosterone ratio (Fig. 3L). Levels of DHT and 3α-diol could not be compared reliably, as their concentrations remained under detection limits in more than 70% of samples.
4. DISCUSSION
The main finding of the present study is that the potent 5αR inhibitor FIN dose-dependently countered the deficits in PPI caused by IR, a well-characterized neurodevelopmental model of juvenile psychosocial stress leading to schizophrenia-related alterations. Similarly to previous experiments (Bortolato et al., 2008), the effects of FIN were generally similar to those of the benchmark antipsychotic HAL; in spite of this analogy, the 5αR blocker failed to induce catalepsy or other overt extrapyramidal manifestations. This result confirms and extends our previous observations on the ameliorative effects of FIN and other 5αR inhibitors on psychosis-related alterations elicited by direct and indirect DAergic agonists in rodents (Bortolato et al., 2008; Devoto et al., 2012; Frau et al., 2013). Furthermore, these data support preliminary clinical evidence on the possible therapeutic potential of FIN in schizophrenia (Koethe et al., 2008), as well as other disorders featuring DAergic alterations, including Tourette syndrome (Bortolato et al., 2007; Muroni et al., 2011) and impulsive behaviors induced by DAergic agonists in susceptible patients (Bortolato et al., 2012).
In agreement with our previous findings (Bortolato et al., 2008; Devoto et al., 2012), startle reflex was significantly reduced by FIN in SR animals. This phenomenon, however, was not observed in IR-exposed animals, suggesting that IR may reduce sensitivity to the effects of 5αR inhibitors with respect to startle reactivity. Notably, the lack of a selective effects of FIN on startle amplitude in IR animals also rules out possible confounds in PPI analysis due to alterations in this parameter (Swerdlow et al., 2000).
We previously showed that the effects of FIN on the regulation of PPI are likely supported by changes in the signaling of the postsynaptic DA receptors in the NAcc (Devoto et al, 2009). Furthermore, several studies have shown that the gating deficits induced by IR are primarily mediated by this region (Powell et al., 2003; Leng et al., 2004). Our results showed that IR leads to significant reductions in pregnenolone, 5α-dihydroprogesterone, allopregnanolone and estradiol in the NAcc. These data are in substantial agreement with previous findings from our group and others, indicating that IR leads to an overall reduction in steroid levels in the cortex (Serra et al., 2000; Bortolato et al., 2011). We also documented no significant changes in progesterone, DHEA and testosterone levels, suggesting that the generalized reduction in neurosteroid biosynthetic pathways may be partially offset by the down-regulation of catabolic enzymes and/or the activation of alternative anabolic processes. The significant changes in steroid ratios are in keeping with the previously documented down-regulation of 5αR in IR-subjected rats (as indicated by the reduction in 5α-dihydroprogesterone / progesterone ratio); furthermore, our data suggest that this manipulation may lead to functional alterations of other neurosteroidogenic enzymes, such as a potential enhancement of the activity of 3β-hydroxysteroid dehydrogenase (3β-HSD), which catalyze the conversion of pregnenolone into progesterone (see Fig. 4).
Fig. 4.
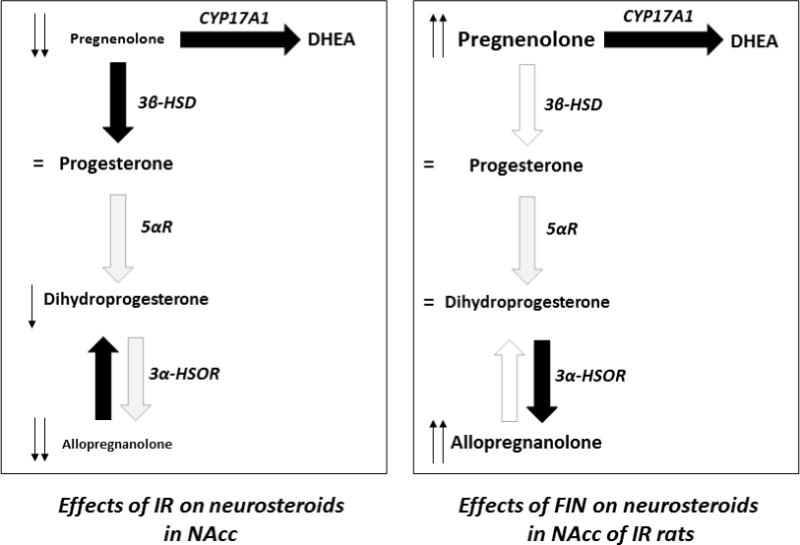
Synoptic schematization of the observed effects of isolation rearing (IR) and finasteride (FIN) on neurosteroid levels in the nucleus accumbens (NAcc). Black and white arrows represent increased and decreased enzyme activities, respectively. CYP17A1, 17-hydroxylase/17,20 lyase; 3β-HSD, 3β-hydroxysteroid dehydrogenase; 5αR, 5α-reductase; 3α-HSOR, 3α-hydroxysteroid oxidoreductase; DHEA, dehydroepiandrosterone. For further details, see text.
Our results indicated that IR led to a significant reduction in 17β-estradiol, but not testosterone, in IR-subjected rats. Both steroids have been shown to be potentially relevant in the control of negative symptoms in schizophrenia (Shirayama et al., 2002; Rao and Kolsch, 2003; Akhondzadeh et al., 2006). Due to the relatively small size of our biological samples, we were unable to obtain a reliable measurement of 5α-reduced androgens, such as DHT and 3α-diol, or testosterone precursors, such as androstenedione; thus, we cannot fully evaluate whether the lack of variations in testosterone levels were reflective of changes in its synthesis or 5αR-mediated metabolism. Nevertheless, it is worth mentioning that, while we did not find any significant changes in aromatase activity (as estimated by the testosterone / estradiol ratio), IR-exposed rats exhibited a significant reduction in estradiol levels, which may have been caused by an enhancement in the metabolic pathways of this steroid, namely its conversion to estrone by 17β-hydroxysteroid dehydrogenase (Labrie et al., 1997), or its 16α-hydroxylation to estriol, which is primarily mediated by several cytochromes P450 (Badawi et al, 2001). Further studies are warranted to assess the effects of sex steroids on the behavioral and neurochemical sequelae of IR, and to study the implications of this manipulation on testosterone and estradiol-metabolic enzymes.
The behavioral effects of FIN were paralleled by a marked increase in the concentrations of pregnenolone and a statistical trend for an enhancement of DHEA levels in the NAcc and striatum. The increased content of these 3β-hydroxy-Δ5-steroids may signify their accumulation in response to the inhibition of 5αR, given that they are the precursors of the two main 5αR substrates, i.e. progesterone and testosterone. The possibility that the antipsychotic-like actions of FIN may be contributed by the enhancement of pregnenolone (and, possibly, DHEA) levels is in keeping with emerging evidence supporting the therapeutic potential of these neurosteroids for cognitive and negative symptoms in schizophrenia (Strous et al., 2003; Marx et al., 2009; Marx et al., 2011; Ritsner et al., 2014). Furthermore, pregnenolone was recently shown to rescue the spontaneous PPI deficits in DA transporter knockout mice, which are also reflective of DA receptor hyperactivation in the NAcc (Wong et al., 2012). As mentioned in the introduction, alterations in levels of brain-regional pregnenolone and DHEA have been documented in schizophrenia (Marx et al., 2006). Furthermore, changes in plasma concentrations of DHEA and pregnenolone (as well as their sulfoconjugated derivatives) have also been documented in schizophrenia patients (Harris et al., 2001; Strous et al, 2004; di Michele et al, 2005; Silver et al., 2005; Gallagher et al, 2007; Ritsner et al., 2006, 2007; Ritsner and Strous, 2010; Bicikova et al., 2013). In particular, changes in the concentrations of circulating neuroactive steroids and their ratios may be related to specific symptomatic aspects of schizophrenia, such as cognitive symptoms, anxiety and stress responsiveness (Ritsner et al., 2007; Ritsner and Strous, 2010). Although the biological actions of pregnenolone and DHEA are not completely understood, they act as potent agonists of σ1 receptors (Maurice et al., 2001), which have been shown to be essential for the modulation of DA D1 receptor signaling (Navarro et al., 2010). Notably, we recently showed that the PPI-ameliorating properties of FIN are due to the suppression of D1 receptor signaling (Frau et al., 2013). In addition, pregnenolone and DHEA have been shown to exert neuroprotective properties against apoptosis and oxidative damage (for a review, see Ritsner, 2010), which may play an important role in shaping the pathophysiology of schizophrenia (Yao et al., 2001; Jarskog, 2006). Future studies will be needed to elucidate whether the effects of FIN in IR-subjected rats are mediated by pregnenolone and DHEA, and substantiate the role of σ1 receptors on the regulation of PPI and other schizophrenia-related endophenotypes.
We found that, while FIN reduced allopregnanolone levels and allopregnanolone / 5α-dihydroprogesterone ratio in SR rats, this drug had surprisingly opposite effects in IR-subjected rats. This paradoxical phenomenon may have been caused by functional changes in 3α-hydroxysteroid oxidoreductase (3α-HSOR), the reversible enzyme involved in the inter-conversion of allopregnanolone and 5α-dihydroprogesterone. Indeed, this enzyme has been shown to serve both the synthesis and degradation of allopregnanolone, depending on the prevalence of its cytosolic or membrane-bound isoform (Mellon and Vaudry, 2001). Depending on the changes induced by IR on the expression and activity of this enzyme, the acute 5αR inhibition may have altered the inter-regulatory cross-talk between these two enzymes, leading to a reduction of the conversion of allopregnanolone into 5α-dihydroprogesterone.
Alternatively, the increase in allopregnanolone may reflect an altered mechanism of action of FIN, possibly due to putative alterations in 5αR structure and intracellular localization in IR-subjected rats. In order to inhibit 5αR, FIN needs to be accepted as a substrate of this enzyme and reduced to dihydroFIN; in turn, this metabolite forms an adduct with NADP, which is then covalently bound to the enzyme (Bull et al., 1996). Thus, the down-regulation of 5αR may limit its transformation into dihydroFIN and unmask other secondary mechanisms of this drug, such as the inhibition of 5β-reductase (Drury et al., 2009), which may paradoxically enhance the synthesis of allopregnanolone and other 5α-reduced neurosteroids.
The enhancement in allopregnanolone in IR rats may play a key contributory role in the effects of FIN. Allopregnanolone is known to activate GABA-A receptors, whose action may indeed interact with potential changes in DA receptor signaling in DAergic regions. Interestingly, allopregnanolone has been shown to counter the enhancement of aggression and fear-related memory in isolated mice (Pinna et al., 2008). Future studies will be needed to test the potential role of allopregnanolone in the modulation of PPI by FIN in IR-subjected rats.
Although our results may provide critical element of insight into the role of 5αR in the regulation of PPI, several limitations need to be acknowledged. Our neuroactive steroid analyses could not be limited to NAcc, in view of the small size of this region, and incorporated also the striatum; however, it is possible that the two regions, albeit organized similarly, may different significantly with respect to steroid profiles. In addition, our studies failed to test whether the changes in neuroactive steroids in the NAcc may be directly conducive to changes in PPI. Future studies with systemic and intra-accumbal infusion of pregnenolone and allopregnanolone will be needed to confirm the potential involvement of these steroids in the therapeutic actions of FIN.
Another important limitation of our study lies in the fact that the alterations in steroid profile in the NAcc were tested in a separate group of rats that did not undergo PPI testing. Given that startle testing is stressful in rats (Engelmann et al., 1996), we cannot exclude that the effects of FIN in PPI regulation may target other unknown changes in neurosteroids specifically induced by behavioral testing. This possibility will need to be accurately evaluated in future studies.
Our study did not include any analysis on the steroid profiles in either plasma or other potentially relevant regions in the DAergic system, including the midbrain. Although previous studies have documented that the PPI deficits induced by IR are primarily dependent on the NAcc, functional alterations in the ventral tegmental areas may lead to changes in DAergic neurotransmission. This interesting possibility awaits further experimental confirmation in future investigations.
Finally, our findings did not qualify the 5αR isoform implicated in the antipsychotic-like effects of FIN in IR-subjected rats. The two major types of 5αR, 1 and 2, are both expressed in the NAcc and down-regulated by IR (Bortolato et al., 2011; Castelli et al., 2013). In particular, the involvement of 5αR2 in the observed effects of FIN may be particularly meaningful from a translational perspective, given that FIN has a higher affinity for this isoenzyme in humans (Paba et al., 2011). Furthermore, given the importance of 5αR2 in the synthesis of androgens, its implication in the anti-DAergic mechanisms of FIN may account for recent findings from our group on PPI-ameliorating properties of abiraterone, the main androgen-synthetic enzyme, in rats (Frau et al., 2014). The involvement of androgens in the effects of FIN may also provide a mechanistic frame to account for the well-documented sex differences in schizophrenia and their relation to DAergic signaling (Godar and Bortolato, 2014).
Irrespective of these limitations, our findings have confirmed FIN’s antipsychotic-like properties, by documenting its ability to correct the gating alterations associated with IR, a neurodevelopmental model of schizophrenia with high face, construct and predictive validity. The actions of FIN are accompanied by changes in neuroactive steroid levels, such as an increase in pregnenolone and allopregnanolone, which countered the effects of IR. These data further confirm previous evidence pointing to 5αR as a promising target for the development of novel treatments for schizophrenia and other neuropsychiatric disorders featuring stress-related gating impairments, such as Tourette syndrome and obsessive-compulsive disorder.
Acknowledgments
We are grateful to Alessandra Pardu, Romina Pes and Silvia Fanni for their assistance in the manuscript preparation.
Role of the Funding Source: This work was supported by grants from the National Institute of Mental Health (NIH R01 MH104603), National Institute of General Medical Sciences (NIH P20 GM103638), Bank of Sardinia Foundation and Autonomous Region of Sardinia, Tourette Syndrome Association and Kansas Strategic Initiative Grant. The authors are indebted to the EU COST Action CM1103 “Structure-based drug design for diagnosis and treatment of neurological diseases: dissecting and modulating complex function in the monoaminergic systems of the brain” for supporting their international collaboration. None of the institutions had any further role in the decision to submit the paper for publication.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributors
Roberto Frau performed the behavioral studies and wrote the first draft of the manuscript. Valentina Bini and Alberto Casti performed the isolation rearing and helped Dr. Frau with the execution of the behavioral studies. Federico Abbiati and Donatella Caruso performed the analyses of steroid levels. Paola Devoto undertook the statistical analyses and performed brain dissections. Marco Bortolato designed the study and wrote the final version of the manuscript. All authors have approved the final manuscript.
Conflict of interest
The authors certify that there is no actual or potential conflict of interest in relation to this article.
References
- Agis-Balboa RC, Pinna G, Pibiri F, Kadriu B, Costa E, Guidotti A. Down-regulation of neurosteroid biosynthesis in corticolimbic circuits mediates social isolation-induced behavior in mice. Proc Natl Acad Sci USA. 2007;104:18736–18741. doi: 10.1073/pnas.0709419104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akhondzadeh S, Rezaei F, Larijani B, Nejatisafa AA, Kashani L, Abbasi SH. Correlation between testosterone, gonadotropins and prolactin and severity of negative symptoms in male patients with chronic schizophrenia. Schizophr Res. 2006;84:2–3. 405–10. doi: 10.1016/j.schres.2006.02.008. [DOI] [PubMed] [Google Scholar]
- Badawi AF, Cavalieri EL, Rogan EG. Role of human cytochrome P450 1A1, 1A2, 1B1, and 3A4 in the 2-, 4-, and 16alpha-hydroxylation of 17beta-estradiol. Metabolism. 2001;50:1001–3. doi: 10.1053/meta.2001.25592. [DOI] [PubMed] [Google Scholar]
- Bakshi VP, Geyer MA. Ontogeny of isolation rearing-induced deficits in sensorimotor gating in rats. Physiol Behav. 1999;67:385–392. doi: 10.1016/s0031-9384(99)00082-7. [DOI] [PubMed] [Google Scholar]
- Barbaccia ML, Roscetti G, Trabucchi M, Purdy RH, Mostallino MC, Perra C. Isoniazid-induced inhibition of GABAergic transmission enhances neurosteroid content in the rat brain. Neuropharmacology. 1996;35:1299–1305. doi: 10.1016/s0028-3908(96)00067-6. [DOI] [PubMed] [Google Scholar]
- Barbaccia ML, Serra M, Purdy RH, Biggio G. Stress and neuroactive steroids. Int Rev Neurobiol. 2001;46:243–272. doi: 10.1016/s0074-7742(01)46065-x. [DOI] [PubMed] [Google Scholar]
- Baulieu EE. Neurosteroids: a novel function of the brain. Psychoneuroendocrinology. 1998;23:963–87. doi: 10.1016/s0306-4530(98)00071-7. [DOI] [PubMed] [Google Scholar]
- Bicikova M, Hill M, Ripova D, Mohr P, Hampl R. Determination of steroid metabolome as a possible tool for laboratory diagnosis of schizophrenia. J Steroid Biochem Mol Biol. 2013;133:77–83. doi: 10.1016/j.jsbmb.2012.08.009. [DOI] [PubMed] [Google Scholar]
- Binder EB, Kinkead B, Owens MJ, Nemeroff CB. The role of neurotensin in the pathophysiology of schizophrenia and the mechanism of action of antipsychotic drugs. Biol Psychiatry. 2001;50:856–872. doi: 10.1016/s0006-3223(01)01211-2. [DOI] [PubMed] [Google Scholar]
- Bortolato M, Aru GN, Fà M, Frau R, Orrù M, Salis P, Casti A, Luckey GC, Mereu G, Gessa GL. Activation of D1, but not D2 receptors potentiates dizocilpine-mediated disruption of prepulse inhibition of the startle. Neuropsychopharmacology. 2005;30:561–74. doi: 10.1038/sj.npp.1300547. [DOI] [PubMed] [Google Scholar]
- Bortolato M, Cannas A, Solla P, Bini V, Puligheddu M, Marrosu F. Finasteride attenuates pathological gambling in patients with Parkinson disease. J Clin Psychopharmacology. 2012;32(3):424–425. doi: 10.1097/JCP.0b013e3182549c2a. [DOI] [PubMed] [Google Scholar]
- Bortolato M, Devoto P, Roncada P, Frau R, Flore G, Saba P, Pistritto G, Soggiu A, Pisanu S, Zappala A, Ristaldi MS, Tattoli M, Cuomo V, Marrosu F, Barbaccia ML. Isolation rearing-induced reduction of brain 5α-reductase expression: relevance to dopaminergic impairments. Neuropharmacology. 2011;60:1301–8. doi: 10.1016/j.neuropharm.2011.01.013. [DOI] [PubMed] [Google Scholar]
- Bortolato M, Frau R, Orru M, Bourov Y, Marrosu F, Mereu G, Devoto P, Gessa GL. Antipsychotic-like properties of 5 alpha-reductase inhibitors. Neuropsychopharmacology. 2008;33:3146–3156. doi: 10.1038/npp.2008.39. [DOI] [PubMed] [Google Scholar]
- Bortolato M, Muroni A, Marrosu F. Treatment of Tourette’s syndrome with finasteride. Am J Psychiatry. 2007;164:1914–1915. doi: 10.1176/appi.ajp.2007.07060978. [DOI] [PubMed] [Google Scholar]
- Braff DL, Grillon C, Geyer MA. Gating and habituation of the startle reflex in schizophrenic patients. Arch Gen Psychiatry. 1992;49:206–215. doi: 10.1001/archpsyc.1992.01820030038005. [DOI] [PubMed] [Google Scholar]
- Bull HG, Garcia-Calvo M, Anderson S, Baginsky WF, Chan HK, Ellsworth DE, Miller RR, Stearns RA, Bakshi RK, Rasmusson GH, Tolman RL, Myers RW, Kozarich JW, Harris GS. Mechanism-based inhibition of human steroid since 5α-Reductase by finasteride: Enzyme catalyzed formation of NADP-dihydrofinasteride, a potent bisubstrate analog inhibitor. J Am Chem Soc. 1996;118:2359–2365. [Google Scholar]
- Caruso D, Scurati S, Maschi O, De Angelis L, Roglio I, Giatti S, Garcia-Segura LM, Melcangi RC. Evaluation of neuroactive steroid levels by liquid chromatography-tandem mass spectrometry in central and peripheral nervous system: effect of diabetes. Neurochem Int. 2008;52:560–8. doi: 10.1016/j.neuint.2007.06.004. [DOI] [PubMed] [Google Scholar]
- Castelli MP, Casti A, Casu A, Frau R, Bortolato M, Spiga S, Ennas MG. Regional distribution of 5α-reductase type 2 in the adult rat brain: an immunohistochemical analysis. Psychoneuroendocrinology. 2013;38:281–293. doi: 10.1016/j.psyneuen.2012.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corcoran C, Walker E, Huot R, Mittal V, Tessner K, Kestler L, Malaspina D. The stress cascade and schizophrenia: etiology and onset. Schizophr Bull. 2003;29:671–92. doi: 10.1093/oxfordjournals.schbul.a007038. [DOI] [PubMed] [Google Scholar]
- Devoto P, Frau R, Bini V, Pillola G, Saba P, Flore G, Corona M, Marrosu F, Bortolato M. Inhibition of 5 alpha-reductase in the nucleus accumbens counters sensorimotor gating deficits induced by dopaminergic activation. Psychoneuroendocrinology. 2012;37:1630–1645. doi: 10.1016/j.psyneuen.2011.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- di Michele F, Caltagirone C, Bonaviri G, Romeo E, Spalletta G. Plasma dehydroepiandrosterone levels are strongly increased in schizophrenia. J Psychiatr Res. 2005;39:267–73. doi: 10.1016/j.jpsychires.2004.08.005. [DOI] [PubMed] [Google Scholar]
- Di Paolo T. Modulation of brain dopamine transmission by sex steroids. Rev Neurosci. 1994;5:27–41. doi: 10.1515/revneuro.1994.5.1.27. [DOI] [PubMed] [Google Scholar]
- Dong E, Matsumoto K, Uzunova V, Sugaya I, Takahata H, Nomura H, Watanabe H, Costa E, Guidotti A. Brain 5alpha-dihydroprogesterone and allopregnanolone synthesis in a mouse model of protracted social isolation. Proc Natl Acad Sci USA. 2001;98:2849–2854. doi: 10.1073/pnas.051628598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drury JE, Di Costanzo L, Penning TM, Christianson DW. Inhibition of human steroid 5beta-reductase (AKR1D1) by finasteride and structure of the enzyme inhibitor complex. J Biol Chem. 2009;284(30):19786–19790. doi: 10.1074/jbc.C109.016931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engelmann M, Thrivikraman KV, Su Y, Nemeroff CB, Montkowski A, Landgraf R, Holsboer F, Plotsky PM. Endocrine and behavioral effects of airpuff-startle in rats. Psychoneuroendocrinology. 1996;21:391–400. doi: 10.1016/0306-4530(96)00006-6. [DOI] [PubMed] [Google Scholar]
- Fone KC, Porkess MV. Behavioural and neurochemical effects of post-weaning social isolation in rodents-relevance to developmental neuropsychiatric disorders. Neurosci Biobehav Rev. 2008;32:1087–1102. doi: 10.1016/j.neubiorev.2008.03.003. [DOI] [PubMed] [Google Scholar]
- Frau R, Bini V, Pes R, Pillola G, Saba P, Devoto P, Bortolato M. Inhibition of 17 alpha-hydroxylase/C17,20 lyase reduces gating deficits consequent to dopaminergic activation. Psychoneuroendocrinology. 2014;39:204–213. doi: 10.1016/j.psyneuen.2013.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frau R, Pillola G, Bini V, Tambaro S, Devoto P, Bortolato M. Inhibition of 5 alpha-reductase attenuates behavior effects of D1-, but not D2-like receptor agonists in C57BL/6 mice. Psychoneuroendocrinology. 2013;38(4):542–551. doi: 10.1016/j.psyneuen.2012.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallagher P, Watson S, Smith MS, Young AH, Ferrier IN. Plasma cortisol-dehydroepiandrosterone (DHEA) ratios in schizophrenia and bipolar disorder. Schizophr Res. 2007;90:258–65. doi: 10.1016/j.schres.2006.11.020. [DOI] [PubMed] [Google Scholar]
- Geyer MA, Krebs-Thomson K, Braff DL, Swerdlow NR. Pharmacological studies of prepulse inhibition models of sensorimotor gating deficits in schizophrenia: a decade in review. Psychopharmacology. 2001;156:117–154. doi: 10.1007/s002130100811. [DOI] [PubMed] [Google Scholar]
- Geyer MA, Wilkinson LS, Humby T, Robbins TW. Isolation rearing of rats produces a deficit in prepulse inhibition of acoustic startle similar to that in schizophrenia. Biol Psychiatry. 1993;34:361–372. doi: 10.1016/0006-3223(93)90180-l. [DOI] [PubMed] [Google Scholar]
- Godar SC, Bortolato M. Gene-sex interactions in schizophrenia: focus on dopamine neurotransmission. Front Behav Neurosci. 2014;8:71. doi: 10.3389/fnbeh.2014.00071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall FS, Wilkinson LS, Humby T, Inglis W, Kendall DA, Marsden CA, Robbins TW. Isolation rearing in rats: pre- and postsynaptic changes in striatal dopaminergic systems. Pharmacol Biochem Behav. 1998;59:859–72. doi: 10.1016/s0091-3057(97)00510-8. [DOI] [PubMed] [Google Scholar]
- Harris DS, Wolkowitz OM, Reus VI. Movement disorder, memory, psychiatric symptoms and serum DHEA levels in schizophrenic and schizoaffective patients. World J Biol Psychiatry. 2001;2:99–102. doi: 10.3109/15622970109027500. [DOI] [PubMed] [Google Scholar]
- Huether G. The central adaptation syndrome: psychosocial stress as a trigger for adaptive modifications of brain structure and brain function. Prog Neurobiol. 1996;48:569–612. doi: 10.1016/0301-0082(96)00003-2. [DOI] [PubMed] [Google Scholar]
- Jarskog LF. Apoptosis in schizophrenia: pathophysiologic and therapeutic considerations. Curr Opin Psychiatry. 2006;19:307–12. doi: 10.1097/01.yco.0000218603.25346.8f. [DOI] [PubMed] [Google Scholar]
- Johnson EO, Kamilaris TC, Chrousos GP, Gold PW. Mechanisms of stress: a dynamic overview of hormonal and behavioral homeostasis. Neurosci Biobehav Rev. 1992;16:115–30. doi: 10.1016/s0149-7634(05)80175-7. [DOI] [PubMed] [Google Scholar]
- Jones GH, Hernandez TD, Kendall DA, Marsden CA, Robbins TW. Dopaminergic and serotonergic function following isolation rearing in rats: study of behavioural responses and postmortem and in vivo neurochemistry. Pharmacol Biochem Behav. 1992;43:17–35. doi: 10.1016/0091-3057(92)90635-s. [DOI] [PubMed] [Google Scholar]
- Kajantie E, Phillips DI. The effects of sex and hormonal status on the physiological response to acute psychosocial stress. Psychoneuroendocrinology. 2006;31:151–78. doi: 10.1016/j.psyneuen.2005.07.002. [DOI] [PubMed] [Google Scholar]
- Koethe D, Bortolato M, Piomelli D, Leweke FM. Improvement of general symptoms in a chronic psychotic patient treated with finasteride: case report. Pharmacopsychiatry. 2008;41:115–116. doi: 10.1055/s-2008-1058110. [DOI] [PubMed] [Google Scholar]
- Labrie F, Luu-The V, Lin SX, Labrie C, Simard J, Breton R, Bélanger A. The key role of 17 beta-hydroxysteroid dehydrogenases in sex steroid biology. Steroids. 1997;62:148–58. doi: 10.1016/s0039-128x(96)00174-2. [DOI] [PubMed] [Google Scholar]
- Leng A, Feldon J, Ferger B. Long-term social isolation and medial prefrontal cortex: dopaminergic and cholinergic neurotransmission. Pharmacol Biochem Behav. 2004;77:371–379. doi: 10.1016/j.pbb.2003.11.011. [DOI] [PubMed] [Google Scholar]
- Marx CE, Bradford DW, Hamer RM, Naylor JC, Allen TB, Lieberman JA, Strauss JL, Kilts JD. Pregnenolone as a novel therapeutic candidate in schizophrenia: emerging preclinical and clinical evidence. Neuroscience. 2011;191:78–90. doi: 10.1016/j.neuroscience.2011.06.076. [DOI] [PubMed] [Google Scholar]
- Marx CE, Keefe RS, Buchanan RW, Hamer RM, Kilts JD, Bradford DW, Strauss JL, Naylor JC, Payne VM, Lieberman JA, Savitz AJ, Leimone LA, Dunn L, Porcu P, Morrow AL, Shampine LJ. Proof-of-concept trial with the neurosteroid pregnenolone targeting cognitive and negative symptoms in schizophrenia. Neuropsychopharmacology. 2009;34:1885–903. doi: 10.1038/npp.2009.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marx CE, Stevens RD, Shampine LJ, Uzunova V, Trost WT, Butterfield MI, Massing MW, Hamer RM, Morrow AL, Lieberman JA. Neuroactive steroids are altered in schizophrenia and bipolar disorder: relevance to pathophysiology and therapeutics. Neuropsychopharmacology. 2006;31:1249–63. doi: 10.1038/sj.npp.1300952. [DOI] [PubMed] [Google Scholar]
- Maurice T, Urani A, Phan VL, Romieu P. The interaction between neuroactive steroids and the sigma1 receptor function: behavioral consequences and therapeutic opportunities. Brain Res Brain Res Rev. 2001;37:116–32. doi: 10.1016/s0165-0173(01)00112-6. [DOI] [PubMed] [Google Scholar]
- Mellon SH, Vaudry H. Biosynthesis of neurosteroids and regulation of their synthesis. Int Rev Neurobiol. 2001;46:33–78. doi: 10.1016/s0074-7742(01)46058-2. [DOI] [PubMed] [Google Scholar]
- Muroni A, Paba S, Puligheddu M, Marrosu F, Bortolato M. A preliminary study of finasteride in Tourette syndrome. Mov Disord. 2011;26(11):2146–2147. doi: 10.1002/mds.23810. [DOI] [PubMed] [Google Scholar]
- Navarro G, Moreno E, Aymerich M, Marcellino D, McCormick PJ, Mallol J, Cortés A, Casadó V, Canela EI, Ortiz J, Fuxe K, Lluís C, Ferré S, Franco R. Direct involvement of sigma-1 receptors in the dopamine D1 receptor-mediated effects of cocaine. Proc Natl Acad Sci. 2010;107:18676–81. doi: 10.1073/pnas.1008911107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norman RM, Malla AK. Stressful life events and schizophrenia. II: Conceptual and methodological issues. Br J Psychiatry. 1993;162:166–74. doi: 10.1192/bjp.162.2.166. [DOI] [PubMed] [Google Scholar]
- Nuechterlein KH, Dawson ME, Gitlin M, Ventura J, Goldstein MJ, Snyder KS, Yee CM, Mintz J. Developmental Processes in Schizophrenic Disorders: longitudinal studies of vulnerability and stress. Schizophr Bull. 1992;18:387–425. doi: 10.1093/schbul/18.3.387. [DOI] [PubMed] [Google Scholar]
- Paba S, Frau R, Godar SC, Devoto P, Marrosu F, Bortolato M. Steroid 5α-reductase as a novel therapeutic target for schizophrenia and other neuropsychiatric disorders. Curr Pharm Des. 2011;17:151–167. doi: 10.2174/138161211795049589. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The rat brain in stereotaxic coordinates. 3rd. Academic Press; San Diego, California: 1998. [Google Scholar]
- Pinna G, Agis-Balboa RC, Pibiri F, Nelson M, Guidotti A, Costa E. Neurosteroid biosynthesis regulates sexually dimorphic fear and aggressive behavior in mice. Neurochem Res. 2008;33:1990–2007. doi: 10.1007/s11064-008-9718-5. [DOI] [PubMed] [Google Scholar]
- Powell SB, Geyer MA, Preece MA, Pitcher LK, Reynolds GP, Swerdlow NR. Dopamine depletion of the nucleus accumbens reverses isolation-induced deficits in prepulse inhibition in rats. Neuroscience. 2003;119:233–240. doi: 10.1016/s0306-4522(03)00122-2. [DOI] [PubMed] [Google Scholar]
- Purdy RH, Morrow AL, Moore PH, Jr, Paul SM. Stress-induced elevations of gamma-aminobutyric acid type A receptor-active steroids in the rat brain. Proc Natl Acad Sci USA. 1991;88:4553–4557. doi: 10.1073/pnas.88.10.4553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao ML, Kölsch H. Effects of estrogen on brain development and neuroprotection–implications for negative symptoms in schizophrenia. Psychoneuroendocrinology. 2003;28(Suppl 2):83–96. doi: 10.1016/s0306-4530(02)00126-9. [DOI] [PubMed] [Google Scholar]
- Ritsner M, Gibel A, Maayan R, Ratner Y, Ram E, Modai I, Weizman A. State and trait related predictors of serum cortisol to DHEA(S) molar ratios and hormone concentrations in schizophrenia patients. Eur Neuropsychopharmacol. 2007;17:257–64. doi: 10.1016/j.euroneuro.2006.09.001. [DOI] [PubMed] [Google Scholar]
- Ritsner M, Maayan R, Gibel A, Strous RD, Modai I, Weizman A. Elevation of the cortisol/dehydroepiandrosterone ratio in schizophrenia patients. Eur Neuropsychopharmacol. 2004;14:267–73. doi: 10.1016/j.euroneuro.2003.09.003. [DOI] [PubMed] [Google Scholar]
- Ritsner MS, Bawakny H, Kreinin A. Pregnenolone treatment reduces severity of negative symptoms in recent-onset schizophrenia: an 8-week, double-blind, randomized add-on two-center trial. Psychiatry Clin Neurosci. 2014;68:432–40. doi: 10.1111/pcn.12150. [DOI] [PubMed] [Google Scholar]
- Ritsner MS, Strous RD. Neurocognitive deficits in schizophrenia are associated with alterations in blood levels of neurosteroids: a multiple regression analysis of findings from a double-blind, randomized, placebo-controlled, crossover trial with DHEA. J Psychiatr Res. 2010;44:75–80. doi: 10.1016/j.jpsychires.2009.07.002. [DOI] [PubMed] [Google Scholar]
- Roncada P, Bortolato M, Frau R, Saba P, Flore G, Soggiu A, Pisanu S, Amoresano A, Carpentieri A, Devoto P. Gating deficits in isolation-reared rats are correlated with alterations in protein expression in nucleus accumbens. J Neurochem. 2009;108:611–620. doi: 10.1111/j.1471-4159.2008.05806.x. [DOI] [PubMed] [Google Scholar]
- Sánchez MG, Bourque M, Morissette M, Di Paolo T. Steroids-dopamine interactions in the pathophysiology and treatment of CNS disorders. CNS Neurosci Ther. 2010;16:e43–71. doi: 10.1111/j.1755-5949.2010.00163.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanchez P, Torres JM, Gavete P, Ortega E. Effects of swim stress on mRNA and protein levels of steroid 5alpha-reductase isozymes in prefrontal cortex of adult male rats. Neurochem Int. 2008;52:426–431. doi: 10.1016/j.neuint.2007.07.019. [DOI] [PubMed] [Google Scholar]
- Sanchez P, Torres JM, Olmo A, O’Valle F, Ortega E. Effects of environmental stress on mRNA and protein expression levels of steroid 5alpha-Reductase isozymes in adult rat brain. Horm Behav. 2009;56:348–353. doi: 10.1016/j.yhbeh.2009.07.001. [DOI] [PubMed] [Google Scholar]
- Serra M, Pisu MG, Littera M, Papi G, Sanna E, Tuveri F. Social isolation-induced decreases in both the abundance of neuroactive steroids and GABA(A) receptor function in rat brain. J Neurochem. 2000;75:732–740. doi: 10.1046/j.1471-4159.2000.0750732.x. [DOI] [PubMed] [Google Scholar]
- Shirayama Y, Hashimoto K, Suzuki Y, Higuchi T. Correlation of plasma neurosteroid levels to the severity of negative symptoms in male patients with schizophrenia. Schizophr Res. 2002;58:69–74. doi: 10.1016/s0920-9964(01)00367-x. [DOI] [PubMed] [Google Scholar]
- Silver H, Knoll G, Isakov V, Goodman C, Finkelstein Y. Blood DHEAS concentrations correlate with cognitive function in chronic schizophrenia patients: a pilot study. J Psychiatr Res. 2005;39:569–75. doi: 10.1016/j.jpsychires.2005.01.008. [DOI] [PubMed] [Google Scholar]
- Strous RD, Maayan R, Lapidus R, Goredetsky L, Zeldich E, Kotler M, Weizman A. Increased circulatory dehydroepiandrosterone and dehydroepiandrosterone-sulphate in first-episode schizophrenia: relationship to gender, aggression and symptomatology. Schizophr Res. 2004;71:427–34. doi: 10.1016/j.schres.2004.03.005. [DOI] [PubMed] [Google Scholar]
- Strous RD, Maayan R, Lapidus R, Stryjer R, Lustig M, Kotler M, Weizman A. Dehydroepiandrosterone augmentation in the management of negative, depressive, and anxiety symptoms in schizophrenia. Arch Gen Psychiatry. 2003;60:133–41. doi: 10.1001/archpsyc.60.2.133. [DOI] [PubMed] [Google Scholar]
- Swerdlow NR, Braff DL, Geyer MA. Animal models of deficient sensorimotor gating: what we know, what we think we know, and what we hope to know soon. Behav Pharmacol. 2000;11:185–204. doi: 10.1097/00008877-200006000-00002. [DOI] [PubMed] [Google Scholar]
- Walker E, Mittal V, Tessner K. Stress and the hypothalamic pituitary adrenal axis in the developmental course of schizophrenia. Annu Rev Clin Psychol. 2008;4:189–216. doi: 10.1146/annurev.clinpsy.4.022007.141248. [DOI] [PubMed] [Google Scholar]
- Wong P, Chang CC, Marx CE, Caron MG, Wetsel WC, Zhang X. Pregnenolone rescues schizophrenia-like behavior in dopamine transporter knockout mice. PLoS One. 2012;7:51455. doi: 10.1371/journal.pone.0051455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao JK, Reddy RD, van Kammen DP. Oxidative damage and schizophrenia: an overview of the evidence and its therapeutic implications. CNS Drugs. 2001;15:287–310. doi: 10.2165/00023210-200115040-00004. [DOI] [PubMed] [Google Scholar]


