Abstract
Objective
To investigate the effect of reducing spasticity via onabotulinumtoxin A (Obtx-A) injection on cerebellar activation after chronic stroke during unilateral gripping.
Design
Pre-post, case series.
Setting
Outpatient spasticity clinic.
Participants
Individuals with chronic spasticity (N = 4).
Interventions
Upper-limb Obtx-A injection.
Main Outcome Measures
Functional magnetic resonance imaging (fMRI) was used to measure changes in cerebellar activation before and after upper-limb Obtx-A injection. During fMRI testing, participants performed the same motor task before and after injection, which was 15% and 30% of maximum voluntary isometric gripping measured before Obtx-A injection.
Results
After Obtx-A injection, cerebellar activation increased bilaterally during gripping with the paretic hand and during rest. During both pre- and postinjection scans, the paretic hand showed larger cerebellar activation during gripping compared with the nonparetic hand. Cerebellar activation during gripping with the nonparetic hand did not change significantly after Obtx-A injection.
Conclusions
Reducing spasticity via Obtx-A injection may increase cerebellar activation both during gripping tasks with the paretic hand and during rest. To our knowledge, this is the first study that examines changes in cerebellar activation after spasticity treatment with Obtx-A.
Keywords: Botulinum toxins, type A, Cerebellum, Magnetic resonance imaging, Muscle spasticity, Rehabilitation, Stroke
Stroke and other central nervous system (CNS) injuries can lead to spasticity, a motor disorder characterized by velocity-dependent increase in the tonic stretch reflexes.1 Because spasticity can cause pain and contractures of muscles and joints, it is an important limiting factor for restoring function and improving quality of life.
Onabotulinumtoxin A (Obtx-A) is a Food and Drug Administration–approved medication used in the treatment of dystonia and spasticity. Clinical studies have documented efficacy in relieving muscle spasticity with neurotoxins.2–4 Obtx-A treatments take 2 to 3 days to become effective, with efficacy peaking at 6 weeks and lasting around 3 months in most individuals.2 Obtx-A can inhibit muscle fiber response to motoneuron activity by blocking acetylcholine (ACh) release at the presynaptic nerve terminal.5
Although the primary effects of intramuscular injection of Obtx-A are achieved per ipherally, remote changes in the CNS have been found in previous studies.6–9 For example, Obtx-A appears to enter motoneurons by retrograde axonal transport because significant levels of Obtx-A have been found in the spinal cord after Obtx-A injection.7 Previous studies have also described remote effects on cortical function.6,8,9 Increased intracortical inhibition with transcranial magnetic stimulation was shown 1 month after injecting Obtx-A compared with preinjection status in patients with dystonia.6 During passive movements of paretic and nonparetic hands, functional magnetic resonance imaging (fMRI) activity after botulinum toxin injection for poststroke spasticity was increased bilaterally in the sensorimotor cortex, secondary somatosensory areas, and supplementary motor area predominantly in the contralesional hemisphere when compared with the rest.8 A small study with 4 patients who were poststroke described a significant decrease in activation of the posterior cingulate/precuneus region after Obtx-A treatments.9 However, no studies have examined changes in cerebellar function after Obtx-A injection.
The cerebellum, one of major structures in the CNS, is important for motor control and has been the target of surgical and neuromodulation treatments for spasticity.10–13 Previous studies have shown that modulating cerebellar activation via cerebellar stimulation at anterior or posterior lobes is associated with changes in spasticity.10–12 For example, cerebellar stimulation reduced spasticity in 85% of 600 individuals with cerebral palsy.11 Additionally, reduction of pain associated with muscle tone has been shown after cerebellar stimulation.14 Ebner et al induced changes in spasticity by modulating cerebellar stimulus parameters at the anterior lobe (paravermal region).12
One mechanism by which cerebellar stimulation may affect spasticity is in the regulation of muscle spindle sensitivity. Cerebellar stimulation in anesthetized or decerebrate cats can alter the resting discharge and stretch sensitivity of muscle spindle afferents.15 These results support the notion that the cerebellum plays a role in modulating reflex gain, influencing ascending and descending sensorimotor pathways,16 the proper functioning of which may be disrupted after stroke or other brain injury. Therefore, because spasticity increases muscle spindle sensitivity, the cerebellum may regulate spasticity by a similar mechanism. A clearer understanding of this role is needed to develop effective rehabilitation strategies aimed at enhancing cerebellar function, alleviating spasticity, and recuperating movement skills.
The purpose of this study was to investigate changes in cerebellar activation after reducing spasticity via Obtx-A injections in persons with chronic stroke during unilateral hand gripping. We hypothesized that reduction of spasticity after Obtx-A injection would alter cerebellum activation during a hand-gripping task performed with the paretic (more paretic) hand. We used fMRI to measure blood oxygenation level-dependent (BOLD) signals in the cerebellum during a hand-gripping task performed with the paretic (more paretic) and nonparetic (less paretic) hands. Scans were performed pre- and postinjections of Obtx-A to measure changes in cerebellar activation after treatment.
Methods
Participants
Four individuals with chronic upper-limb spastic hemiparesis (table 1) participated in this pilot study. We recruited a convenience sample from the spasticity clinic. All patients had unilateral spastic hemiparesis for a minimum of 6 months and had at least 2 prior sessions of Obtx-A for spasticity treatment. They were Obtx-A stable and had their most recent botulinum toxin injection at least 3 months before study participation. We selected a 3-month washout period based on the work of de Paiva et al,17 who reported that the motor endplates where botulinum toxin was injected regained function after 3 months and were indistinguishable from endplates where toxin was not injected. At baseline, all patients had preinjection Modified Ashworth Scale (MAS) scores ≥2 in at least 1 of 3 muscle groups (elbow, wrist, or finger flexors). In addition, all patients had to attain at minimum a stage 2 rating on the Chedoke-McMaster Assessment Hand Impairment Scale that includes at least 2 of 3 items: positive Hoffman sign, resistance to passive wrist or finger extension, and facilitated finger flexion. In addition, subjects had to demonstrate the ability to complete at least 1 of the tasks that met criteria for stage 3 (ie, wrist extension more than one half range). The 7-point Chedoke-McMaster Assessment Hand Impairment Scale is reliable and valid to determine the severity of hand impairments.18 These criteria identified participants with minimal residual hand function and excluded those who had no voluntary motion.19 Participants were able to answer questions and follow instructions and did not have severe, fixed joint contractures in the affected arm. Individuals who met the screening criteria were given additional information regarding the study and if interested, provided informed consent. After signing the consent, each participant received unilateral Obtx-A injection on the paretic hand. All protocols were approved by the institutional review board.
Table 1.
Characteristics of the patients
| Participant No. | Age (y) | Sex | Type of Stroke | Affected Hand | Area of Stroke | Years Since Onset | Preinjection MAS Finger/Thumb | Preinjection CMA Hand |
|---|---|---|---|---|---|---|---|---|
| 1 | 53 | M | Thrombotic | L | R temporal lobe | 2.5 | 2/1 | 5 |
| 2 | 66 | M | Thrombotic | L | R frontal-temporal lobe | 3 | 3/3 | 5 |
| 3 | 61 | F | Hemorrhage | L | R frontal-parietal lobe | 2.5 | 4/1 | 4 |
| 4 | 42 | F | Thrombotic | L | R parietal lobe | 3 | 3/2 | 4 |
Abbreviations: CMA, Chedoke-McMaster Assessment Hand Impairment Scale; F, female; L, left; M, male; MAS, Modified Ashworth Scale score; R, right.
Isometric hand-gripping task
Isometric hand-gripping task was used because it has been shown to be a good indicator of upper-limb function after stroke.20,21 In addition, it is a relatively simple task22 for individuals with severe spasticity. While participants lay in the scanner, we measured isometric maximum voluntary contractions (MVCs) during unilateral gripping, which was the maximum of 3 trials23 for each hand. MRI-compatible isometric hand dynamometersa were used to measure grip force. The motor task performed by the participants during fMRI was 15% and 30% of MVC24 unilateral gripping measured at the preinjection testing session. We chose 15% and 30% of MVC based on a previous study.24 These target forces were modest enough to allow participants with moderate to severe spasticity to successfully perform the task repeatedly without fatigue before and after unilateral Obtx-A injection. A real-time LabVIEW-based biofeedback system was used to instruct the target force for each trial. Participants performed 1 grip per 4 seconds (.25Hz).25,26 This rate was chosen to prevent fatigue and excessive motion.
We used a blocked design23,25,26 to separate rest and gripping conditions into distinct blocks in which 2 blocks are presented one after another for the duration of the experimental run. Each participant alternated between 20-second epochs of rest (rest block) and 20-second epochs of .25Hz gripping at 15% or 30% MVC force level (gripping block, 5 grips for each gripping block). Randomized counterbalancing assignment was used to decide which hand to study first. Each participant performed a total of 10 blocks of handgripping trials (5 repetitions per block), with each hand (50 grips for each hand, 100 grips in total) with randomized target force level (5 blocks for each target force). Before scanning, participants practiced gripping until they could comfortably perform the task. To monitor bilateral movements, each participant held identical isometric dynamometers in both hands during unilateral gripping.27
Data acquisition
Magnetic resonance images were acquired using a Siemens Allegra 3.0T scannerb at the Brain Imaging Research Center of Carnegie Mellon University and the University of Pittsburgh. We collected imaging data for each patient within the 7 days before Obtx-A injection and 7 to 10 days postinjection. The time point of 7 to 10 days postinjection was chosen because the effect of Obtx-A starts within 2 to 3 days, with clear decreases in spasticity apparent within 7 to 10 days.2,28 Foam cushioning and tape were used to immobilize the head within the scanner to minimize head motion. Cushioned straps were used to stabilize the hand device. We collected T2-weighted anatomic images (repetition time = 6440ms; echo time = 73ms; flip angle α = 150°; field of view = 205mm; slice thickness = 3.2mm; matrix size = 256×256) and T1-weighted magnetization-prepared rapid gradient-echo images (repetition time = 1630ms; echo time = 2.48ms; flip angle α = 8°; field of view = 205mm; slice thickness = 0.8mm; matrix size = 256×256; voxel size = 0.8×0.8×0.8mm). T2*-weighted image volumes with BOLD contrast were collected using a gradient echo, echo planar imaging (EPI) sequence (repetition time = 2000ms; echo time = 25ms; flip angle α = 79°; field of view = 205mm; slice thickness = 3.2mm contiguous; matrix size = 64×64; voxel size = 3.2×3.2×3.2mm; interslice gap = 1 mm; number of slices = 39), providing coverage of the entire brain. BOLD image collection was preceded by 4 dummy scans to allow for equilibration effects.
Data analysis
We used voxel-based Statistical Parametric Mapping (SPM5)c for image processing and statistical analysis.29,30 To remove subvoxel motion-related signal change, all EPI data were aligned to the first image during spatial realignment. The realigned EPI images were then coregistered to the participant’s anatomic image, resliced, and normalized to a standard EPI template based on the Montreal Neurological Institute reference brain.29 We smoothed all normalized images with an isotropic 8mm full-width half-maximum Gaussian kernel to account for intersubject anatomic differences.29
For the purpose of this study, we focused our analyses on the cerebellum. The PickAtlas software (version 2.4)d was used for cerebellum region of interest analysis.31 A general linear model30 was used to create a statistical parametric map. The unilateral gripping condition data included both 15% and 30% of MVC preinjection hand gripping blocks. The rest condition data contained all of the rest blocks. The threshold of the t statistic (SPM5) for within-subject analyses when comparing between conditions (ie, gripping condition over rest condition) on the same day was P<.001, corrected for multiple comparisons. The threshold was P<.05 (uncorrected)26 for comparisons between postinjection and preinjection (during gripping with paretic hand and during rest) by using a paired t test. To gain fundamental understanding of cerebellar activation differences between unilateral gripping with the more spastic and less spastic hands, we used paired t tests (P<.05, uncorrected)26 to compare between gripping with paretic hand and gripping with the nonparetic hand at pre- and postinjection. Activations are presented overlaid on the brain surface in the neurologic space (ie, right equals right) based on the Montreal Neurological Institute reference.
Results
Clinical data
Patient characteristics and fMRI scans are provided in table 1 and figure 1. Participants did not receive any occupational or physical therapy between pre- and postinjection. Before unilateral Obtx-A injection on the paretic hand, all patients had moderate to severe spastic hemiparesis on the left side. Their preinjection MAS score of the finger was at least ≥2 (see table 1). With Chedoke-McMaster Assessment Hand Impairment Scale preinjection scores of 4 or 5, all patients were able to perform the unilateral gripping adequately. There were no significant differences between pre- and postinjection in isometric MVC on the right and left sides (P>.05, paired t test).
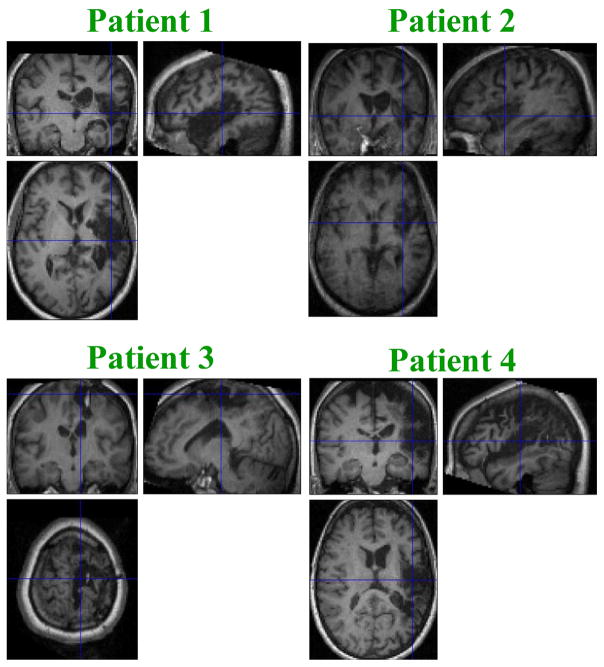
Fig. 1.
Magnetization-prepared rapid gradient-echo fMRI scans for 4 patients. Patient 1 had a right internal carotid artery thrombotic stroke. Patient 2 had a right middle cerebral artery thrombotic stroke. Patient 3 had a right intracerebral hemorrhage. Patient 4 had a right middle cerebral artery thrombotic stroke.
Cerebellar activation differences between unilateral gripping with paretic and nonparetic hands
The cerebellar activation was larger during gripping with the paretic hand versus gripping with the nonparetic hand both before and after Obtx-A injection (shown in figs 2 and 3: see fig 2B vs fig 2E; fig 2C vs fig 2D; and fig 3). When comparing gripping with the paretic hand over the nonparetic hand at the preinjection scan, significant cerebellar activation (79 voxels) occurred in the anterior lobe (lobules IV and V, x = 18, y = −34, z = −24, T = 12.15) and posterior lobe (lobule VIII, x = 36, y = −60, z = −48, T = 7.65; crus I, x = 40, y = −68, z = −32, T = 3.04) (fig 4A). When comparing gripping with the paretic hand over the nonparetic hand at the postinjection scan, significant cerebellar activation (616 voxels) occurred in the posterior lobe (lobule VIII, x = 6, y = −72, z = −38, T = 26.84; crus I, x = 42, y = −70, z = −28, T = 13.33; lobule VI, x = −18, y = −64, z = −16, T = 11.76) (fig 4B).
Fig. 2.
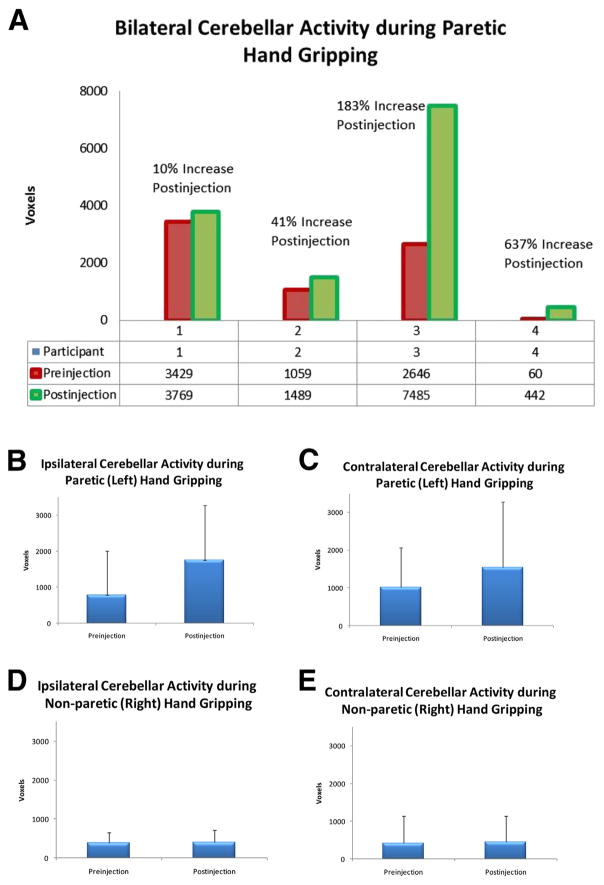
(A) Bilateral (combination of ipsilateral and contralateral sides) cerebellar activation during gripping with the paretic hand at pre- and postinjections for each participant (participants 1–4). The average percentage change on bilateral cerebellar activation during the paretic hand gripping after unilateral Obtx-A injection on the paretic hand was an 83% increase across all participants (participant 1: +10%; participant 2: +41%; participant 3: +183%; participant 4: +637%). Average (B) ipsilateral and (C) contralateral cerebellar activation during gripping with the paretic (left) hand at pre- and postinjections. Average (D) ipsilateral and (E) contralateral cerebellar activation during gripping with the nonparetic (right) hand at pre- and postinjections. (P<.001, family-wise error corrected).
Fig. 3.
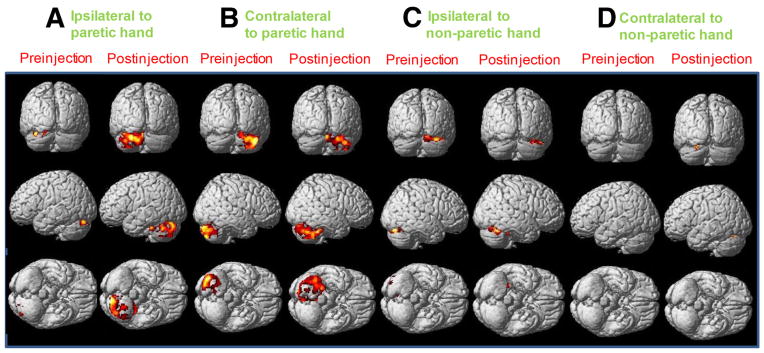
Cerebellar activation at pre- and postinjections during unilateral paretic hand grasping and unilateral nonparetic hand grasping (participant 3). (A) Ipsilateral cerebellar activation during unilateral paretic hand grasping, (B) contralateral cerebellar activation during unilateral paretic hand grasping, (C) ipsilateral cerebellar activation during unilateral nonparetic hand grasping, and (D) contralateral cerebellar activation during unilateral nonparetic hand grasping. (P<.001, family-wise error corrected).
Fig. 4.
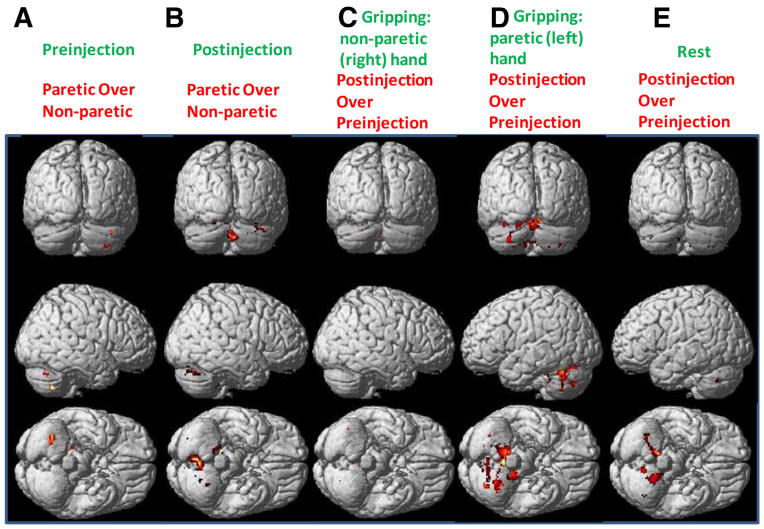
Bilateral cerebellar activation at (A) preinjection and (B) postinjection when comparing gripping with paretic with nonparetic hand, during unilateral gripping with (C) nonparetic (right) and (D) paretic (left) hands, and during (E) rest when comparing postinjection with preinjection. (P<.05, uncorrected, paired t test).
Pre- and postinjection changes in cerebellar activation during gripping
Nonparetic hand gripping was used as an internal control condition because all participants received unilateral Obtx-A injection only on the paretic hand. During gripping with the nonparetic (untreated) hand, cerebellar activation on the ipsilateral (see fig 2D) and contralateral (see fig 2E) sides did not change significantly (fig 4C). This result was expected because the Obtx-A treatment was applied only to the paretic hand. After unilateral Obtx-A injection, all participants demonstrated increased cerebellar activation bilaterally during gripping with the paretic hand (see figs 2, 3, and 4D). For instance, functional imaging for patient 3 showed larger areas of activation in the ipsilateral (see fig 3A) and contralateral cerebellum (see fig 3B) during gripping with the paretic hand postinjection compared with preinjection. The average bilateral cerebellum activation increased 83% after Obtx-A injection (see fig 2A). More specifically, the ipsilateral cerebellar activation increased 123% (see fig 2B) compared with 52% in the contralateral cerebellum after Obtx-A injection (see fig 2C).
When comparing postinjection with preinjection during gripping with the paretic hand, significant activations occurred bilaterally in the cerebellum, including ipsilateral cerebellum anterior lobe (lobules IV and V, x = −6, y = −68, z = −10, T = 41.02; lobule VI, x = −36, y = −48, z = −26, T = 17.63) and contralateral cerebellum posterior lobe (lobule VI, x = 10, y = −78, z = −18, T = 14.24), which has a total volume of 2765 voxels (see fig 4D).
Pre- and postinjection changes in cerebellar activation during rest
Comparison of postinjection with preinjection during rest revealed significant activation in the right cerebellum anterior lobe (lobules IV and V, x = 12, y = −56, z = −10, T = 112.12), left cerebellum anterior lobe (lobules IV and V, x = −14, y = −44, z = −28, T = 52.49), and right cerebellum posterior lobe (lobule IX, x = 12, y = −44, z = −50, T = 26.78), which has a total volume of 517 voxels (fig 4E).
Discussion
To our knowledge, this is the first study to examine changes in cerebellar activation after spasticity treatment with unilateral Obtx-A injection on the paretic hand. Our findings demonstrated the following: (1) larger cerebellar activation both before and after Obtx-A injection during gripping with the paretic hand in comparison with gripping with the nonparetic hand; (2) increased cerebellar activation bilaterally after Obtx-A injection at rest and during gripping with the paretic hand; and (3) no significant change with the nonparetic hand gripping in bilateral cerebellar activation after Obtx-A injection.
Effects of intramuscular Obtx-A injection on the CNS
Our findings introduce new possibilities for investigating the spasticity mechanisms and how Obtx-A injection may impact CNS physiology. Obtx-A injections inhibit ACh release.5 It produces a clinical effect over 3 to 4 months.28 The effects of Obtx-A injection may not be limited to simply inhibiting ACh release in the peripheral nervous system. Our fMRI results provide evidence that cerebellar activation may be increased after spasticity reduction via Obtx-A injection. Indeed, other evidence also supports Obtx-A effects, directly and remotely, on the CNS, including reduced alpha and gamma motoneuron excitability,32–34 retrograde axonal transport,7 increased presynaptic inhibition,35,36 and enlarged intracortical inhibition.6 Animal studies show Obtx-A injection reduces muscle spindle afferent discharge even without any mechanical changes as detected by an isometric muscle tension transducer.34,37 These studies suggest changes in the CNS occur after Obtx-A injection.
Cerebellum and spasticity
The contribution of cerebellum to spasticity has been the subject of many investigations. Our findings showed cerebellar activation was larger during gripping with the paretic hand than the nonparetic hand both before and after Obtx-A was administered. Our interpretation is that overactive motoneurons caused by spasticity may generate stronger afferent input to the cerebellum during movement with the spastic hand than during movement with the nonspastic hand. This result is consistent with the finding of increased cerebellar activation in individuals with overactive motoneurons caused by dystonia.38 Other fMRI studies have demonstrated increased bilateral cerebellar activation during hand tasks with unilateral writer’s cramp, which is a task-specific dystonia, compared with healthy controls.39 Structural abnormalities with decreased gray matter were found in the cerebellum bilaterally in patients with unilateral writer’s cramp compared with healthy controls.40 Therefore, the relation between the cerebellum and overactive motoneurons caused by spasticity41 or dystonia38,39,42–44 may be an important mechanistic link.
The clinical effects of Obtx-A injection on the peripheral nervous system with reduced spasticity are well known, whereas the remote effects of spasticity reduction on the CNS are less understood. Our finding was that after Obtx-A injection, bilateral cerebellar activations were increased during unilateral gripping with the paretic hand and during rest. One potential mechanism of larger bilateral cerebellar activity could be related to increased efforts and complexity in generating and coordinating force production in the paretic hand because of the decreased muscle tone induced by Obtx-A treatment. The second possible explanation is that the reduction in tone and associated afferent activity leads to a reduction in inhibition provided by Purkinje cells, which are modulated by afferent inputs. Overactive afferent inputs caused by peripheral afferent stimulation can evoke modest increases in Purkinje cell firing.45 Furthermore, excitation of Purkinje cells by using surface electric stimulation in the anterior lobe of the cerebellum can modulate muscle tone in decerebrated cats.46 Spasticity reduction after Obtx-A injection may reduce overactive afferent discharges.34,47 Therefore, increased cerebellar activation observed after Obtx-A injection during unilateral gripping with the paretic hand and during rest may be caused by an unmasking of cerebellar activation by reducing inhibition from Purkinje cell activity associated with decreased afferent discharges. This hypothesis is consistent with the finding that blocking Purkinje cell inputs with local microinjections of gamma-aminobutyric acid type A antagonists leads to increased movement-related discharge in the cerebellar nuclei.48 Further studies are needed to investigate these mechanisms.
Our fMRI data suggest that anterior and posterior lobes of the cerebellum (lobules IV–VI, VIII, IX, and crus I) may be involved in spasticity regulation. Indeed, anterior and posterior lobes of the cerebellum have been the target areas for modulating spasticity by using cerebellar stimulation.10–12 Inactivation of lobules VI through VIII with 2% lidocaine has been reported to increase the dynamic sensitivity of Ia afferents in decerebrate cats.49 Cerebellar area contralateral to the moved hand (crus I) is associated with passive movement resistance.41 Some of these spasticity-related cerebellar areas are also linked to therapy-related motor improvement. For example, increased bilateral cerebellar activation (lobule VI and crus I) is correlated with functional hand improvement after 2 weeks of movement therapy.50 Increased bilateral cerebellar activation (lobules VI and VIII) has also been found after repetitive movement therapy.26
Bilateral changes in the cerebellum after unilateral Obtx-A injection
Although Obtx-A was injected in only one arm, our findings indicate that changes in the cerebellum after injection may occur bilaterally both during gripping with the paretic hand and during rest. Typically, there is an ipsilateral association between the cerebellum and limb movement. However, several studies have shown bilateral cerebellar activation while performing unilateral limb movements.51–54 Bilateral limb movement representation can be seen in deep cerebellar nuclei.55 Bilateral cerebellar structure and activation differences are found in patients with unilateral dystonia compared with healthy controls.39,40 Therefore, it is possible that unilateral changes in muscle tone after Obtx-A injection may have bilateral effects on the cerebellum.
Study limitations
Participants with their most recent botulinum toxin injection at least 3 months before study participation may not be sufficient because clinical effects of Obtx-A injection may last 4 to 6 months. Nevertheless, all participants that were tested exhibited significant spasticity as indicated by the MAS score at the preinjection point. It was a clinically meaningful interval because they were injected when spasticity reemerged. Although the MAS score was not recorded immediately after injection, prior studies have shown Obtx-A injection reduced spasticity as measured by the MAS score.2,4,19
This pilot study examined only 4 patients. It limits our ability to generalize the findings to a larger population. The physiologic differences among patients may contribute to differences in the magnitudes of increase in cerebellar activation. The reproducibility of the BOLD signal across visits may be also 1 potential limiting factor. However, our imaging results for the nonparetic hand were consistent across days without significant differences between pre- and postinjections. These limitations have been seen in other fMRI studies.56,57 Our results showed an average 83% increase across all participants on bilateral cerebellar activation during the paretic hand gripping after unilateral Obtx-A injection. Indeed, participants 3 and 4 showed larger percentage increases than participants 1 and 2. The differences in percentage increase among participants could be associated with type of stroke, location of stroke, and preinjection hand function measured by the Chedoke-McMaster Assessment Hand Impairment Scale (Chedoke-McMaster Assessment Hand Impairment Scale score of 4 for both participants 3 and 4 and a score of 5 for both participants 1 and 2). Future studies with larger sample sizes and randomized, double-blind, placebo-controlled designs may help to validate and extend the preliminary findings reported here. Because the cerebellum is highly interconnected with other areas of the brain,58 spasticity may not only be associated with increased cerebellum activity but also with other cortical and subcortical areas.41,59 Future studies are needed to examine remote CNS changes after reducing spasticity.
Increased cerebellar activation has also been shown in relation to the functional improvement after rehabilitative therapy.50,60 Our findings suggest increased cerebellar activation after Obtx-A injection occurred, even without any rehabilitative therapy. It may be possible that increased cerebellar activation after Obtx-A injection can augment the effect of rehabilitative therapy in improving motor function, but this requires further investigation. Because the efficacy of Obtx-A injection peaks at 6 weeks, future studies should include a longitudinal follow-up to monitor changes in cerebellar activation at late time points.
Conclusions
Changes in cerebellar activation can be identified after spasticity treatment with Obtx-A. This pilot study introduces new opportunities for exploring the relation between spasticity management and CNS function, and such studies may help us understand the mechanisms underlying spasticity.
Acknowledgments
Supported by the University of San Francisco Faculty Development Fund, Brain Imaging Research Center pilot grant, National Institutes of Health Post-Doctoral Training grant (Training Rehabilitation Clinicians for Research Careers) (grant no. T32 HD049307), and Allergan.
We thank Peter L. Strick, PhD (Neurobiology, University of Pittsburgh) and Michael Boninger, MD (Physical Medicine and Rehabilitation [PM&R], University of Pittsburgh) for informative discussion and constructive guidance. We thank Katie Russell, PhD (PM&R, University of Pittsburgh), Joaquin Anguera, PhD (Neurology, University of California, San Francisco), Howard J. Aizenstein, MD, PhD (Psychiatry, University of Pittsburgh), and Joseph H. Ricker, PhD (PM&R, University of Pittsburgh) for their suggestions on biomedical imaging. We thank Elizabeth R. Skidmore, PhD, OTR/L (Occupational Therapy, University of Pittsburgh) for recommendations of measurement, James A. Hokanson, PhD (Biomedical Engineering, Duke University) for equipment setup, Wei Wang, MD, PhD (PM&R, University of Pittsburgh) for comments, and 2 occupational therapists, Renee A. McDade and Lynne M. Huber, for data collection.
List of abbreviations
- ACh
acetylcholine
- BOLD
blood oxygenation level-dependent
- CNS
central nervous system
- EPI
echo planar imaging
- fMRI
functional magnetic resonance imaging
- MAS
Modified Ashworth Scale
- MVC
maximum voluntary contraction
- Obtx-A
onabotulinumtoxin A
Footnotes
Hand Clench Dynamometer for MRI; BIOPAC Systems.
Allegra 3.0T scanner; Siemens.
SPM5; Wellcome Department of Imaging Neuroscience. Available at: http://www.fil.ion.ucl.ac.uk/spm/.
PickAtlas software (version 2.4); Wake Forest University.
Disclosures: Chang, Weber, and Munin disclose financial support from Allegran.
References
- 1.Lance JW. The control of muscle tone, reflexes, and movement: Robert Wartenberg Lecture. Neurology. 1980;30:1303–13. doi: 10.1212/wnl.30.12.1303. [DOI] [PubMed] [Google Scholar]
- 2.Brashear A, Gordon MF, Elovic E, et al. Intramuscular injection of botulinum toxin for the treatment of wrist and finger spasticity after a stroke. N Engl J Med. 2002;347:395–400. doi: 10.1056/NEJMoa011892. [DOI] [PubMed] [Google Scholar]
- 3.Bakheit AM, Thilmann AF, Ward AB, et al. A randomized, double-blind, placebo-controlled, dose-ranging study to compare the efficacy and safety of three doses of botulinum toxin type A (Dysport) with placebo in upper limb spasticity after stroke. Stroke. 2000;31:2402–6. doi: 10.1161/01.str.31.10.2402. [DOI] [PubMed] [Google Scholar]
- 4.Simpson DM, Alexander DN, O’Brien CF, et al. Botulinum toxin type A in the treatment of upper extremity spasticity: a randomized, double-blind, placebo-controlled trial. Neurology. 1996;46:1306–10. doi: 10.1212/wnl.46.5.1306. [DOI] [PubMed] [Google Scholar]
- 5.Ray P. Botulinum toxin A inhibits acetylcholine release from cultured neurons in vitro. In Vitro Cell Dev Biol Anim. 1993;29A:456–60. [PubMed] [Google Scholar]
- 6.Gilio F, Curra A, Lorenzano C, Modugno N, Manfredi M, Berardelli A. Effects of botulinum toxin type A on intracortical inhibition in patients with dystonia. Ann Neurol. 2000;48:20–6. [PubMed] [Google Scholar]
- 7.Wiegand H, Erdmann G, Wellhoner HH. 125I-labelled botulinum A neurotoxin: pharmacokinetics in cats after intramuscular injection. Naunyn Schmiedebergs Arch Pharmacol. 1976;292:161–5. doi: 10.1007/BF00498587. [DOI] [PubMed] [Google Scholar]
- 8.Diserens K, Ruegg D, Kleiser R, et al. Effect of repetitive arm cycling following botulinum toxin injection for poststroke spasticity: evidence from FMRI. Neurorehabil Neural Repair. 2010;24:753–62. doi: 10.1177/1545968310372138. [DOI] [PubMed] [Google Scholar]
- 9.Senkarova Z, Hlustik P, Otruba P, Herzig R, Kanovsky P. Modulation of cortical activity in patients suffering from upper arm spasticity following stroke and treated with botulinum toxin A: an fMRI study. J Neuroimaging. 2010;20:9–15. doi: 10.1111/j.1552-6569.2009.00375.x. [DOI] [PubMed] [Google Scholar]
- 10.Cooper IS. Effect of stimulation of posterior cerebellum on neurological disease. Lancet. 1973;1:1321. doi: 10.1016/s0140-6736(73)91338-x. [DOI] [PubMed] [Google Scholar]
- 11.Davis R. Cerebellar stimulation for cerebral palsy spasticity, function, and seizures. Arch Med Res. 2000;31:290–9. doi: 10.1016/s0188-4409(00)00065-5. [DOI] [PubMed] [Google Scholar]
- 12.Ebner TJ, Bloedel JR, Vitek JL, Schwartz AB. The effects of cerebellar stimulation on the stretch reflex in the spastic monkey. Brain. 1982;105:425–42. doi: 10.1093/brain/105.3.425. [DOI] [PubMed] [Google Scholar]
- 13.Heimburger F. The role of the cerebellar nuclei in spasticity. Confin Neurol. 1970;32:105–13. doi: 10.1159/000103403. [DOI] [PubMed] [Google Scholar]
- 14.Harat M, Radziszewski K, Rudas M, Okon M, Galanda M. Clinical evaluation of deep cerebellar stimulation for spasticity in patients with cerebral palsy. Neurol Neurochir Pol. 2009;43:36–44. [PubMed] [Google Scholar]
- 15.Granit R, Holmgren B, Merton PA. The two routes for excitation of muscle and their subservience to the cerebellum. J Physiol. 1955;130:213–24. doi: 10.1113/jphysiol.1955.sp005404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.MacKay WA, Murphy JT. Cerebellar modulation of reflex gain. Prog Neurobiol. 1979;13:361–417. doi: 10.1016/0301-0082(79)90004-2. [DOI] [PubMed] [Google Scholar]
- 17.de Paiva A, Meunier FA, Molgo J, Aoki KR, Dolly JO. Functional repair of motor endplates after botulinum neurotoxin type A poisoning: biphasic switch of synaptic activity between nerve sprouts and their parent terminals. Proc Natl Acad Sci U S A. 1999;96:3200–5. doi: 10.1073/pnas.96.6.3200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gowland C, Stratford P, Ward M, et al. Measuring physical impairment and disability with the Chedoke-McMaster Stroke Assessment. Stroke. 1993;24:58–63. doi: 10.1161/01.str.24.1.58. [DOI] [PubMed] [Google Scholar]
- 19.Chang CL, Munin MC, Skidmore ER, Niyonkuru C, Huber LM, Weber DJ. Effect of baseline spastic hemiparesis on recovery of upper-limb function following botulinum toxin type A injections and postinjection therapy. Arch Phys Med Rehabil. 2009;90:1462–8. doi: 10.1016/j.apmr.2009.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Boissy P, Bourbonnais D, Carlotti MM, Gravel D, Arsenault BA. Maximal grip force in chronic stroke subjects and its relationship to global upper extremity function. Clin Rehabil. 1999;13:354–62. doi: 10.1191/026921599676433080. [DOI] [PubMed] [Google Scholar]
- 21.Sunderland A, Tinson D, Bradley L, Hewer RL. Arm function after stroke. An evaluation of grip strength as a measure of recovery and a prognostic indicator. J Neurol Neurosurg Psychiatry. 1989;52:1267–72. doi: 10.1136/jnnp.52.11.1267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cramer SC, Nelles G, Schaechter JD, Kaplan JD, Finklestein SP, Rosen BR. A functional MRI study of three motor tasks in the evaluation of stroke recovery. Neurorehabil Neural Repair. 2001;15:1–8. doi: 10.1177/154596830101500101. [DOI] [PubMed] [Google Scholar]
- 23.Ward NS, Brown MM, Thompson AJ, Frackowiak RS. Neural correlates of motor recovery after stroke: a longitudinal fMRI study. Brain. 2003;126:2476–96. doi: 10.1093/brain/awg245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ward NS, Newton JM, Swayne OB, et al. The relationship between brain activity and peak grip force is modulated by corticospinal system integrity after subcortical stroke. Eur J Neurosci. 2007;25:1865–73. doi: 10.1111/j.1460-9568.2007.05434.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Schaechter JD, Perdue KL, Wang R. Structural damage to the corticospinal tract correlates with bilateral sensorimotor cortex reorganization in stroke patients. Neuroimage. 2008;39:1370–82. doi: 10.1016/j.neuroimage.2007.09.071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lindberg PG, Schmitz C, Engardt M, Forssberg H, Borg J. Use-dependent up- and down-regulation of sensorimotor brain circuits in stroke patients. Neurorehabil Neural Repair. 2007;21:315–26. doi: 10.1177/1545968306296965. [DOI] [PubMed] [Google Scholar]
- 27.Nelles G, Cramer SC, Schaechter JD, Kaplan JD, Finklestein SP. Quantitative assessment of mirror movements after stroke. Stroke. 1998;29:1182–7. doi: 10.1161/01.str.29.6.1182. [DOI] [PubMed] [Google Scholar]
- 28.Ward AB. Spasticity treatment with botulinum toxins. J Neural Transm. 2008;115:607–16. doi: 10.1007/s00702-007-0833-2. [DOI] [PubMed] [Google Scholar]
- 29.Friston KJ, Ashburner J, Frith CD, Poline JB, Heather JD, Frackowiak RS. Spatial registration and normalization of images. Hum Brain Map. 1995;3:165–89. [Google Scholar]
- 30.Friston KJ, Holmes AP, Worsley KJ, Poline JB, Frith CD, Frackowiak RS. Statistical parametric maps in functional imaging: a general linear approach. Hum Brain Map. 1995;2:189–210. [Google Scholar]
- 31.Maldjian JA, Laurienti PJ, Kraft RA, Burdette JH. An automated method for neuroanatomic and cytoarchitectonic atlas-based interrogation of fMRI data sets. Neuroimage. 2003;19:1233–9. doi: 10.1016/s1053-8119(03)00169-1. [DOI] [PubMed] [Google Scholar]
- 32.Pauri F, Boffa L, Cassetta E, Pasqualetti P, Rossini PM. Botulinum toxin type-A treatment in spastic paraparesis: a neurophysiological study. J Neurol Sci. 2000;181:89–97. doi: 10.1016/s0022-510x(00)00439-1. [DOI] [PubMed] [Google Scholar]
- 33.Gracies JM. Physiological effects of botulinum toxin in spasticity. Mov Disord. 2004;19(Suppl 8):S120–8. doi: 10.1002/mds.20065. [DOI] [PubMed] [Google Scholar]
- 34.Filippi GM, Errico P, Santarelli R, Bagolini B, Manni E. Botulinum A toxin effects on rat jaw muscle spindles. Acta Otolaryngol. 1993;113:400–4. doi: 10.3109/00016489309135834. [DOI] [PubMed] [Google Scholar]
- 35.Priori A, Berardelli A, Mercuri B, Manfredi M. Physiological effects produced by botulinum toxin treatment of upper limb dystonia. Changes in reciprocal inhibition between forearm muscles. Brain. 1995;118:801–7. doi: 10.1093/brain/118.3.801. [DOI] [PubMed] [Google Scholar]
- 36.Modugno N, Priori A, Berardelli A, Vacca L, Mercuri B, Manfredi M. Botulinum toxin restores presynaptic inhibition of group Ia afferents in patients with essential tremor. Muscle Nerve. 1998;21:1701–5. doi: 10.1002/(sici)1097-4598(199812)21:12<1701::aid-mus12>3.0.co;2-k. [DOI] [PubMed] [Google Scholar]
- 37.Rosales RL, Arimura K, Takenaga S, Osame M. Extrafusal and intrafusal muscle effects in experimental botulinum toxin-A injection. Muscle Nerve. 1996;19:488–96. doi: 10.1002/(SICI)1097-4598(199604)19:4<488::AID-MUS9>3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]
- 38.Eidelberg D, Moeller JR, Antonini A, et al. Functional brain networks in DYT1 dystonia. Ann Neurol. 1998;44:303–12. doi: 10.1002/ana.410440304. [DOI] [PubMed] [Google Scholar]
- 39.Odergren T, Stone-Elander S, Ingvar M. Cerebral and cerebellar activation in correlation to the action-induced dystonia in writer’s cramp. Mov Disord. 1998;13:497–508. doi: 10.1002/mds.870130321. [DOI] [PubMed] [Google Scholar]
- 40.Delmaire C, Vidailhet M, Elbaz A, et al. Structural abnormalities in the cerebellum and sensorimotor circuit in writer’s cramp. Neurology. 2007;69:376–80. doi: 10.1212/01.wnl.0000266591.49624.1a. [DOI] [PubMed] [Google Scholar]
- 41.Lindberg PG, Gaverth J, Fagergren A, Fransson P, Forssberg H, Borg J. Cortical activity in relation to velocity dependent movement resistance in the flexor muscles of the hand after stroke. Neurorehabil Neural Repair. 2009;23:800–10. doi: 10.1177/1545968309332735. [DOI] [PubMed] [Google Scholar]
- 42.Pizoli CE, Jinnah HA, Billingsley ML, Hess EJ. Abnormal cerebellar signaling induces dystonia in mice. J Neurosci. 2002;22:7825–33. doi: 10.1523/JNEUROSCI.22-17-07825.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mazziotta JC, Hutchinson M, Fife TD, Woods R. Advanced neuroimaging methods in the study of movement disorders: dystonia and blepharospasm. Adv Neurol. 1998;78:153–60. [PubMed] [Google Scholar]
- 44.Kluge A, Kettner B, Zschenderlein R, et al. Changes in perfusion pattern using ECD-SPECT indicate frontal lobe and cerebellar involvement in exercise-induced paroxysmal dystonia. Mov Disord. 1998;13:125–34. doi: 10.1002/mds.870130124. [DOI] [PubMed] [Google Scholar]
- 45.Holtzman T, Rajapaksa T, Mostofi A, Edgley SA. Different responses of rat cerebellar Purkinje cells and Golgi cells evoked by widespread convergent sensory inputs. J Physiol. 2006;574:491–507. doi: 10.1113/jphysiol.2006.108282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Granit R, Phillips CG. Effects on Purkinje cells of surface stimulation of the cerebellum. J Physiol. 1957;135:73–92. doi: 10.1113/jphysiol.1957.sp005696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Manni E, Bagolini B, Pettorossi VE, Errico P. Effect of botulinum toxin on extraocular muscle proprioception. Doc Ophthalmol. 1989;72:189–98. doi: 10.1007/BF00156709. [DOI] [PubMed] [Google Scholar]
- 48.Holdefer RN, Houk JC, Miller LE. Movement-related discharge in the cerebellar nuclei persists after local injections of GABA(A) antagonists. J Neurophysiol. 2005;93:35–43. doi: 10.1152/jn.00603.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Gorassini M, Prochazka A, Taylor JL. Cerebellar ataxia and muscle spindle sensitivity. J Neurophysiol. 1993;70:1853–62. doi: 10.1152/jn.1993.70.5.1853. [DOI] [PubMed] [Google Scholar]
- 50.Johansen-Berg H, Dawes H, Guy C, Smith SM, Wade DT, Matthews PM. Correlation between motor improvements and altered fMRI activity after rehabilitative therapy. Brain. 2002;125:2731–42. doi: 10.1093/brain/awf282. [DOI] [PubMed] [Google Scholar]
- 51.Nair DG, Purcott KL, Fuchs A, Steinberg F, Kelso JA. Cortical and cerebellar activity of the human brain during imagined and executed unimanual and bimanual action sequences: a functional MRI study. Brain Res Cogn Brain Res. 2003;15:250–60. doi: 10.1016/s0926-6410(02)00197-0. [DOI] [PubMed] [Google Scholar]
- 52.Kawashima R, Matsumura M, Sadato N, et al. Regional cerebral blood flow changes in human brain related to ipsilateral and contralateral complex hand movements–a PET study. Eur J Neurosci. 1998;10:2254–60. doi: 10.1046/j.1460-9568.1998.00237.x. [DOI] [PubMed] [Google Scholar]
- 53.Cui SZ, Li EZ, Zang YF, Weng XC, Ivry R, Wang JJ. Both sides of human cerebellum involved in preparation and execution of sequential movements. Neuroreport. 2000;11:3849–53. doi: 10.1097/00001756-200011270-00049. [DOI] [PubMed] [Google Scholar]
- 54.Ehrsson HH, Kuhtz-Buschbeck JP, Forssberg H. Brain regions controlling nonsynergistic versus synergistic movement of the digits: a functional magnetic resonance imaging study. J Neurosci. 2002;22:5074–80. doi: 10.1523/JNEUROSCI.22-12-05074.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Soteropoulos DS, Baker SN. Bilateral representation in the deep cerebellar nuclei. J Physiol. 2008;586:1117–36. doi: 10.1113/jphysiol.2007.144220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ward NS, Brown MM, Thompson AJ, Frackowiak RS. Longitudinal changes in cerebral response to proprioceptive input in individual patients after stroke: an FMRI study. Neurorehabil Neural Repair. 2006;20:398–405. doi: 10.1177/1545968306286322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Dong Y, Winstein CJ, Albistegui-DuBois R, Dobkin BH. Evolution of FMRI activation in the perilesional primary motor cortex and cerebellum with rehabilitation training-related motor gains after stroke: a pilot study. Neurorehabil Neural Repair. 2007;21:412–28. doi: 10.1177/1545968306298598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Middleton FA, Strick PL. Anatomical evidence for cerebellar and basal ganglia involvement in higher cognitive function. Science. 1994;266:458–61. doi: 10.1126/science.7939688. [DOI] [PubMed] [Google Scholar]
- 59.Steiner I, Argov Z, Gomori JM, Gottlieb D, Melamed E. Immediate spasticity with acute hemiplegia is a sign of basal ganglia hemorrhage. Acta Neurol Scand. 1985;71:168–70. doi: 10.1111/j.1600-0404.1985.tb03183.x. [DOI] [PubMed] [Google Scholar]
- 60.Small SL, Hlustik P, Noll DC, Genovese C, Solodkin A. Cerebellar hemispheric activation ipsilateral to the paretic hand correlates with functional recovery after stroke. Brain. 2002;125:1544–57. doi: 10.1093/brain/awf148. [DOI] [PubMed] [Google Scholar]