Abstract
Background:
Postoperative pain is a common side effect following surgery that can significantly reduce surgical quality and patient’s satisfaction. Treatment options are morphine and buprenorphine. We aimed to compare the efficacy of a single dose of intravenous morphine with sublingual buprenorphine in postoperative pain control following closed reduction surgery.
Methods:
This triple blind clinical trial was conducted on 90 patients referred for closed reduction orthopedic surgery. They were older than 18 years and in classes I and II of the American Society of Anesthesiologists (ASA) with an operation time of 30-90 minutes. Patients were divided into two groups of buprenorphine (4.5µg/kg sublingually) and morphine (0.2mg/kg intravenously). Baseline characteristics, vital signs, pain score, level of sedation and pharmacological side effects were recorded in the recovery room (at 0 and 30 minutes), and in the ward (at 3, 6 and 12 hours). SPSS version 19 software was used for data analysis and the significance level was set at P<0.05.
Results:
Ninety patients were studied, 60 males and 30 females with a mean age of 37.7±16.2 years. There was no significant difference between the two groups in terms of baseline characteristics. Pain score in the morphine group was significantly higher than the buprenorphine group with an average score of 2.5 (P<0.001). Postoperative mean heart rate in the buprenorphine group was four beats lower than the morphine group (P<0.001). Also, in the buprenorphine 48.6% and in the morphine group 86.7% of cases were conscious in recovery (P=0.001) with a higher rate of pruritus in the latter group (P=0.001).
Conclusion:
Sublingual buprenorphine administration before anesthesia induction in closed reduction surgery can lead to better postoperative pain control in comparison to intravenous morphine. Due to simple usage and longer postoperative sedation, sublingual buprenorphine is recommended as a suitable drug in closed reduction surgery.
Keywords: Intravenous morphine, Orthopedic surgery, Patient satisfaction, Post-operative pain, Sublingual buprenorphine
Introduction
Despite the attempts of practitioners, postoperative pain is a common side effect after surgeries that can significantly reduce the quality of surgery and patient satisfaction (1); hence, it is imperative to control this pain. Postoperative pain also seems to be associated with increased hospitalization time and costs and has undesirable effects on the patient’s physical and mental activities (2). Hypercoagulability risk due to stasis, which may lead to deep vein thrombosis and catecholamine release due to pain leading to myocardial ischemia, is another important reason why postoperative pain should be controlled (2).
Various methods have been used for controlling postoperative pain, among them are spinal and epidural injection, patient controlled analgesia (PCA), peripheral nerve blockers, sedative oral drugs and acupuncture. Patients tend to prefer intravenous PCA over intravenously, intramuscularly, or subcutaneously administered PRN opioids. Albeit, the majority of these methods need skilled individuals and certain equipment such as PCA pumps (3). The other option for controlling postoperative pain which physicians most often recommend or prescribe is using a single dose of a drug such as morphine, pethidine, methadone or buprenorphine. Morphine is an alkaloid opium which has sedative effects and it increases the pain threshold while decreasing the psychological effects of pain. Long-term effects of intravenous morphine last up to 3 to 4 hours (4). Morphine can prevent the release of ACTH and causes the release of histamine and sympathoadrenal activation. Several studies have reported a depressant effect of morphine on respiratory mucus transport, which is one of the most important defense mechanisms against respiratory tract infections. However, morphine has shown no impact on the beating frequency of nasal cilia in vitro (5). Among its common adverse effects are pruritius, urinary retention (epidural/spinal), nausea and vomiting, constipation, headache, and somnolence.
Another drug similar to morphine qualitatively is buprenorphine. Mixed agonist/antagonists (buprenorphine, butorphanol, nalbuphine, pentazocine) may act as agonists at low doses and as antagonists (at the same or different receptor type) at higher doses. Such compounds typically exhibit ceiling effects for analgesia, and they may elicit an acute withdrawal syndrome when administered together with a pure agonist (6). Buprenorphine is a thebaine derivative, µ-receptor partial agonist and similar in structure to morphine, but approximately 33 times more potent. Buprenorphine has higher affinity and its effect takes much longer (half-life of 166 minutes). The onset action of buprenorphine is slow, its peak effect may not occur until 3 hours, but its effect duration is prolonged (<10 hours). The volume of distribution of buprenophine is 2.8 L/kg and its clearance is 20 mL/kg/min. The metabolites of bupprenorphine, buprenorphine-3-glucuronide and norbuprenorphine are significantly less potent and have lower affinity for the µ-receptor. Its side effects are: nausea, vomiting, dizziness, urinary retention and reduced ventilation (in doses higher than 3µg/kg) (5). The analgesic effects of morphine and buprenorphine have been compared in a few studies and most of these studies have introduced buprenorphine as a more effective drug with fewer side effects (7).
As the prescription of oral medications is easier and safer than the ones requiring injection, the aim of this study was to find a safe, reliable and highly effective technique with minimal side effects for the prevention and management of acute postoperative pain; Therefore, the efficacy of sublingual buprenorphine, as an oral drug, was compared with a single dose of intravenous morphine following closed reduction orthopedic surgeries.
Material and methods
Study design
This is a triple blind clinical trial performed in a two-month (July-August) interval in 2014 on patients referred to Imam Reza Hospital, Mashhad, Iran to undergo closed reduction orthopedic surgery.
Study population
One hundred and two patients who were candidates for closed reduction orthopedic surgery were assessed for eligibility. Type of fractures included in our study were fractures of the wrist and forearm that could be repaired by closed reduction and were based on a single orthopedic surgeons’s opinion. Inclusion criteria were as follows: older than 18 years of age, belonging to class I or II of the American Society of Anesthesiologists (ASA), and having an operation time between 30-90 minutes. Patients with kidney and liver diseases; severe hypertension; addiction to narcotics, alcohol or benzodiazepines; pregnant or lactating women; and patients with previous sensitivity to buprenorphine or morphine were excluded from the study. Hence, 90 patients were enrolled into our study and their data were recorded.
Interventions
The enrolled patients were divided into two groups of buprenorphine and morphine by using the table of random numbers. The method used in allocating patients was numbered envelopes containing buprenorphine tablets with a placebo syringe (normal saline) or a syringe with morphine solution and placebo tablets (which was isoshape and isoform with buprenorphine). The patients were blind to the content of the solution and tablets. Patients in the morphine group received 0.2mg/kg of intravenous morphine (up to10mg) (Daropakhsh Company, Iran) with a placebo before induction of anesthesia. In the buprenorphine group, 4.5µg/kg buprenorphine (up to 1mg) was given sublingually with 10 cc normal saline half an hour before anesthesia induction.
In addition, 0.03mg/kg midazolam and 1-2µcg/kg fentanyl were administered to all patients as pre-anesthetic drugs. Anesthesia was induced by thiopental (3-5mg/kg) and atracorium 0.5 mg/kg (if required). For anesthesia maintanance, a mixture of 50% oxygen, nitrous oxide (N2O) and propofol (50-150µcg/kg/min) was used whenever appropriate (i.e., elongated surgery). Finally, to reverse the effect of the muscle relaxant a combination of neostigmine (0.04mg/kg) and atropine (0.02mg/kg) was administered.
Study Variables
Before induction of anesthesia the patients’ baseline characteristics including: age, gender, vital signs and pain score were recorded by a single nurse who was blind to the allocation groups. Heart rate and mean arterial pressure were recorded at the time of entering the recovery room and 30 minutes afterward. The same data were once again recorded in the ward by a single nurse at 3, 6 and 12 hours.
Level of sedation was recorded by observing the patients’ consciousness status at the time of entering the recovery room and 30 minutes afterward and 3, 6 and 12 hours later. Level of sedation consisted of three scores as follows: If the patient was fully awake and conscious (aware and gave appropriate response to stimulations and obeyed orders) - one score; sleepy patients (awoke by calling and opend eyes and relatively obeyed orders) - two scores and asleep patients who did not awake by calling and painful stimulations (did not open eyes or obey orders) - three scores.
Pain score was recorded with direct questioning of the patients based on the Visual Analogue Scale (VAS) (0 score for no pain and 10 scores for severe pain) at the time of entering recovery, 30 minutes afterward and 3, 6 and 12 hours after entering the ward. Furthermore, 10-25 mg intravenous pethidine was injected for patients who complained of pain and the need for analgesic injections during this time period were also recorded.
Moreover, pharmacological side effects including nausea, vomiting, pruritus and urinary retention were recorded by a single nurse at the mentioned studied time points.
Ethics
The study protocol was approved by the Ethics Committee of the Mashhad University of Medical Sciences and all patients signed an informed consent before entering the study. Confidentiality of the data was also considered, so patients’ data were encoded for the statistical analysis programs and published as a general result (IRCT Code: IRCT2012101311102N1).
Statistical analysis
Data normality was studied using the one-sample Kolmogorov-Smirnov test. Repeated measures of the ANOVA and t test were used to investegate quantitative variable changes in each group and between groups, respectively. Fisher’s exact and Chi-square tests were used wherever appropriate. Also, SPSS version 19 software was used for data analyses and the significance level was set at P<0.05.
Results
Baseline data
Initially 102 patients were assessed for eligibility, whereas 12 were excluded due to not meeting the inclusion criteria (n=3), declining to participate (n=1), lactating women (n=2), kidney disease (n=1), liver diseases (n=1), and severe hypertension (n=4)[Figure 1].
Figure 1.
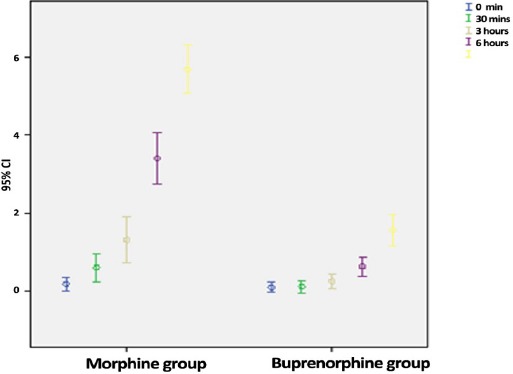
Mean and confidence level for participants’ postoperative pain at various times after surgery.
Hence, 90 patients (60 males and 30 females) with the mean age of 37.7±16.2 years in two individual groups of morphine (n=45) and buprenorphine (n=45) completed the study. The demographic characteristics of both groups are presented in Table 1, which shows no significant difference between the two groups regarding major variables. Mean and range of age, surgical time, heart rate (HR) and mean arterial pressure (MAP) showed no significant differences between the two groups at baseline [Figure 2].
Table 1.
Demographic variables of participants in morphine(M) and buprenorphine(B) groups
| Variable | Group B | Group M | P-Value | ||
|---|---|---|---|---|---|
| Age (Mean±SD) | 39.7(±18.0) | 35.8 (±15.2) | 0.27 | ||
| Surgery duration (Mean±SD) | 72.6(±16.2) | 70.1(±18.0) | 0.48 | ||
| Preoperative Pulse Rate (Mean±SD) | 84.7(±14.4) | 84.9(±13.6) | 0.96 | ||
| Preoperative MAP (Mean±SD) | 107.3(±9.2) | 107.2(±8.3) | 0.91 | ||
| Preoperative VAS (Mean±SD) | 0.01(±0.1) | 0.2(±0.3) | 0.09 | ||
| Gender (female/male) | 31.1% | 65.9% | 64.4% | 35.6% | 0.65 |
Figure 2.
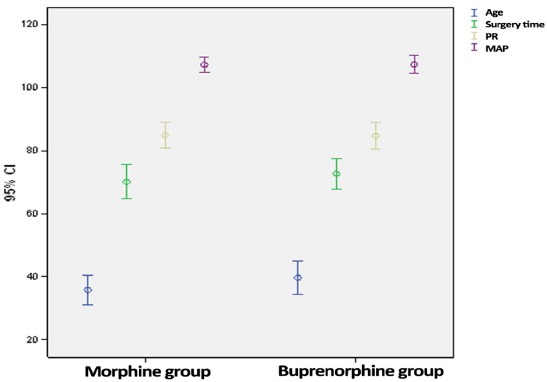
Mean and confidence range of age, surgery duration, Heart Rate and MAP in both groups.
Postoperative pain
Repeated measures of the ANOVA showed significantly higher pain scores in the morphine group compared to the bupernorphine group, with an mean score of 2.5 (P<0.001). Table 2 shows the mean pain score for both groups at different postoperative time points. Figure 2 shows the mean and confidence interval of pain score for both groups at the same postoperative time points. Pethidine (10-25 mg) was administered to 19 patients in the morphine group and to 8 in the buprenorphine group.
Table 2.
Comparing pain rate in participants in morphine group with buprenorphine in postoperative times
| Time | Group B (Mean±SD) | Group M (Mean±SD) | P-Value |
|---|---|---|---|
| Entering into recovery | 0.09(±0.04) | 0.1(±0.05) | 0.09 |
| 30 Minutes after recovery | 0.1(±0.1) | 0.6(±0.1) | 0.004 |
| 3 Hours after recovery | 0.3(±0.2) | 1.3(±1.1) | 0.001 |
| 6 Hours after recovery | 0.8(±0.6) | 3.4(±2.2) | <0.001 |
| 12 Hours after recovery | 1.5(±1.3) | 5.6(±2.1) | <0.001 |
Postoperative Vital Signs
Repeated measures of the ANOVA showed that the postoperative pulse rate in the buprenorphine group was four beats lower than the morphine group on average (P<0.001). There was no significant difference between the two groups in terms of MAP in the postoperative period (105.8 mmHg in the buprenorphine group and 106.3mmHg in the morphine group) (P=0. 6).
Postoperative sedation
Consciousness level at the time of entering the recovery room was significantly different between the two groups (P=0.001). In the buprenorphine group 48.9% of the cases and in the morphine group 86.7% of the cases were conscious at recovery entrance. In addition, the level of sedation in the buprenorphine group was higher 30 minutes after entering the recovery room, while 84.4% of the buprenorphine group and 97.8% of the morphine group were conscious (P=0.02). Level of sedation and consciousness at 3, 6 and 12 hours after entering the ward showed no significant difference between the two groups and patients in both groups were fully conscious at these mentioned time points.
Postoperative pharmaceutical side effects
Among the four major drug side effects considered in this study, the Chi-square test showed that only the rate of pruritus in the recovery room was significantly different between the two groups, with a higher rate in the morphine group (P=0.01). Table 3 shows the frequency distribution of postoperative drug side effects in both groups.
Table 3.
Comparison of distribution frequency of postoperative drugs side effects in both groups
| Variables | Time of entering into recovery (Hour) | Morphine group (n=45) | Buprenorphine group (n=45) | P-Value* |
|---|---|---|---|---|
| Pruritus (%) | 0 | %26.7 | %6.7 | 0.01 |
| 0.5 | %8.9 | %6.7 | 0.5 | |
| 3 | %2.2 | %2.2 | 0.7 | |
| 6 | %0 | %0 | - | |
| 12 | %0 | %0 | - | |
| Nausea (%) | 0 | %24.4 | %28.6 | 0.06 |
| 0.5 | %15.6 | %28.9 | 0.1 | |
| 3 | %2.2 | %4.4 | 0.5 | |
| 6 | %0 | %0 | - | |
| 12 | %0 | %0 | - | |
| Vomiting (%) | 0 | %8.9 | %11.1 | 0.5 |
| 0.5 | %0 | %2.2 | 0.5 | |
| 3 | %0 | %0 | - | |
| 6 | %0 | %0 | - | |
| 12 | %0 | %0 | - | |
| Urinary retention (%) | 0 | %0 | %0 | - |
| 0.5 | %0 | %0 | - | |
| 3 | %6.7 | %4.2 | 0.3 | |
| 6 | %2.2 | %0 | 0.5 | |
| 12 | %0 | %0 | - |
Discussion
Painlessness
The results of this study showed that using sublingual buprenorphine half an hour before surgery resulted in higher postoperative pain reduction compared to intravenous morphine following induction of anesthesia and this is consistent with other similar studies. In Oifa et al.’s study the pain score was compared between buprenorphine infusion (BUP-i) + BUP bolus (BUP-b) with morphine infusion (MO-i)+ (BUP-b) in patients having undergone abdominal surgery (8). The results showed a lower pain score during the post-op hours of 3 to 12 in the buprenorphine infusion group. An important point of this study was that no significant difference in pain score in the first 2 hours after surgery was recorded. This is consistent with our findings, in which no significant difference in pain scores was observed in the initial 30 minutes after surgery. In the study by van den Berg et al. on pain intensity after otorhinolaryngology surgery, postoperative and recovery pain in patients using intravenous buprenorphine was lower than other sedatives like diclofenac, fentanyl and morphine (9). In Maunuksela et al.’s. study on 4-8-year-old children who underwent orthopedic surgery, sublingual buprenorphine shhowed longer-term effects than intravenous morphine (P=0.03) (3). In another study the analgesic effects of sublingual buprenorphine were reported equivalent to intramuscular morphine in pain relief in the postoperative period (10). In contrast, in two other studies sublingual buprenorphine compared to morphine infusion and intramuscular buprenorphine compared to intramuscular morphine resulted in the same analgesic effects after surgery (11,12). Taken together, it seems that using buprenorphine in either form has a sedation effect no less than morphine infusion, while using other non-invasive forms of buprenorphine such as oral or sublingual types due to simple usage leads to a higher compliance from patients. From the pharmacology point of view, the strength and effect duration of buprenorphine is higher than morphine. Various sources have reported the mean morphine effect time as 3-4 hours, while it is 10 hours for buprenorphine and this was further confirmed by our findings (4).
Level of Sedation and consciousness
The results of this study showed that the level of sedation by sublingual buprenorphine compared to intravenous morphine was significantly higher 30 minutes after entering recovery, while after 3 hours there was no significant difference between the level of patients’ consciousness. Risbo et.al’s study also showed that using sublingual buprenorphine when compared to intravenous morphine exhibited a higher sedation level in the first hour after surgery (10); and similar to our study no significant difference was reported one hour after surgery between the two groups. Results of Capogna’s study showed that buprenorphine leads to a higher sedation level than morphine in cesarean postoperative pain (13); similar to the results of van den Berg et al. (9). None of these studies confirmed a higher sedation effect for buprenorphine and there was no significant difference in the sedation rate between the two groups of a single study (8). Furthermore, one animal study showed that 30 minutes after arthrotomy in dogs, the sedation level of intravenous morphine was significantly higher than intramuscular buprenorphine, but after 30 minutes, the effect was the same.
Pharmaceutical side effects
An important aspect in selecting any drug is its pharmaceutical adverse effects. One of the known side effects of morphine is itching. The results of this study showed that the rate of itching in the morphine group was higher than the buprenorphine group. However, this significant difference was only seen at entering the recovery room and after this time, no such difference was recorded regarding itching. In other similar studies, this aspect was not taken into consideration.
The present study showed no significant difference between the two groups in the incidence of nausea and vomiting at different time points, despite the rate of postoperative nausea and vomiting being higher in the buprenorphine group than the morphine group. Other studies have also shown no significant difference in adults regarding postoperative nausea and vomiting (8,10,14,15). However, one study conducted on children reported a higher rate of postoperative nausea and vomiting in the buprenorphine group following orthopedic surgeries than the morphine group (3). The final studied effect was urinary retention which revealed no significant difference between the two groups. No other study has investigated this effect to date. Treatment-related complications were treated symptomatically; 4 mg ondansetron was administered for nausea and vomiting, nelaton catheterization was done for urinary retention while pruritus was treated with 1.25 mg of intravenous droperidol.
Injectable buprenorphine inaccessibility was one of our study limitations. It is suggested that broad studies with larger samples be conducted for comparing the pharmaceutical adverse effects of such drugs.
Sublingual buprenorphine before induction of anesthesia can lead to higher postoperative pain relief than intravenous morphine in orthopedic surgeries.
On the other hand, buprenorphine has higher sedation effects than morphine. Side effects of both drugs are trivial; however, patients in the morphine group experienced more itching in the recovery room. Based on this study results and due to the ease of use and longer postoperative sedation and analgesia effects, sublingual buprenorphine is recommended as an appropriate drug in orthopedic surgeries.
Acknowledgments
The authors would like to thank Mrs. Samaneh Minoee Moghadam, Mrs. Fahime Abbasi and Mr. Mohammad Ali Rouhi for their kind assistance.
References
- 1.Perkins FM, Kehlet H. Chronic pain as an outcome of surgery. A review of predictive factors. Anesthesiology. 2000;93(4):1123–33. doi: 10.1097/00000542-200010000-00038. [DOI] [PubMed] [Google Scholar]
- 2.Wu CL, Fleisher LA. Outcomes research in regional anesthesia and analgesia. Anesth Analg. 2000;91(5):1232–42. doi: 10.1097/00000539-200011000-00035. [DOI] [PubMed] [Google Scholar]
- 3.Maunuksela EL, Korpela R, Olkkola KT. Comparison of buprenorphine with morphine in the treatment of postoperative pain in children. Anesth Analg. 1988;67(3):233–9. [PubMed] [Google Scholar]
- 4.Sarton E, Olofsen E, Romberg R, den Hartigh J, Kest B, Nieuwenhuijs D, et al. Sex differences in morphine analgesia: An experimental study in healthy volunteers. Anesthesiology. 2000;93(5):1245–54. doi: 10.1097/00000542-200011000-00018. [DOI] [PubMed] [Google Scholar]
- 5.Kaziuhiko F. Opioids. In: Miller RD, Eriksson LI, editors. Miller’s Anesthesia. 7th ed. Philadelphia: Churchill Livingstone; 2010. [Google Scholar]
- 6.Hurley RW, Wu CL. Acute Postoperative Pain. In: Miller RD, Eriksson LI, editors. Miller’s Anesthesia. 7th ed. Philadelphia: Churchill Livingstone; 2010. [Google Scholar]
- 7.Moa G, Zetterström H. Sublingual buprenorphine as postoperative analgesic: a double-blind comparison with pethidine. Acta Anaesthesiol Scand. 1990;34(1):68–71. doi: 10.1111/j.1399-6576.1990.tb03044.x. [DOI] [PubMed] [Google Scholar]
- 8.Oifa S, Sydoruk T, White I, Ekstein MP, Marouani N, Chazan S, et al. Effects of intravenous patient-controlled analgesia with buprenorphine and morphine alone and in combination during the first 12 postoperative hours: a randomized, double-blind, four-arm trial in adults undergoing abdominal surgery. Clin Ther. 2009;31(3):527–41. doi: 10.1016/j.clinthera.2009.03.018. [DOI] [PubMed] [Google Scholar]
- 9.van den Berg AA, Honjol NM, Prabhu NV, Datta S, Rozario CJ, Muraleedaran R, et al. Analgesics and ENT surgery. A clinical comparison of the intraoperative, recovery andpostoperative effects of buprenorphine, diclofenac, fentanyl, morphine, nalbuphine, pethidineand placebo given intravenously with induction of anaesthesia. Br J Clin Pharmacol. 1994;38(6):533–43. doi: 10.1111/j.1365-2125.1994.tb04395.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Risbo A, Chraemmer Jørgensen B, Kolby P, Pedersen J, Schmidt JF. Sublingual buprenorphine for premedication and postoperative pain relief in orthopaedic surgery. Acta Anaesthesiol Scand. 1985;29(2):180–2. doi: 10.1111/j.1399-6576.1985.tb02181.x. [DOI] [PubMed] [Google Scholar]
- 11.Brodbelt DC, Taylor PM, Stanway GW. A comparison of preoperative morphine and buprenorphine for postoperative analgesia forarthrotomy in dogs. J Vet Pharmacol Ther. 1997;20(4):284–9. doi: 10.1046/j.1365-2885.1997.00060.x. [DOI] [PubMed] [Google Scholar]
- 12.Gaitini L, Moskovitz B, Katz E, Vaisberg A, Vaida S, Nativ O. Sublingual buprenorphine compared to morphine delivered by a patient-controlled analgesia system as postoperative analgesia after prostatectomy. Urol Int. 1996;57(4):227–9. doi: 10.1159/000282920. [DOI] [PubMed] [Google Scholar]
- 13.Capogna G, Celleno D, Sebastiani M, Costantino P, Reggio S. [Continuous intravenous infusion with patient-controlled anesthesia for postoperative analgesia in cesarean section: morphine versus buprenorphine] Minerva Anestesiol. 1989;55(1-2):33–8. [PubMed] [Google Scholar]
- 14.Bradley JP. A comparison of morphine and buprenorphine for analgesia after abdominal surgery. Anaesth Intensive Care. 1984;12(4):303–10. doi: 10.1177/0310057X8401200403. [DOI] [PubMed] [Google Scholar]
- 15.Dingus DJ, Sherman JC, Rogers DA, DiPiro JT, May R, Bowden TA., Jr Buprenorphine versus morphine for patient-controlled analgesia after cholecystectomy. Surg Gynecol Obstet. 1993;177(1):1–6. [PubMed] [Google Scholar]


