Abstract
Craniofacial sutures govern the shape of the craniofacial skeleton during postnatal development. The differentiation of suture mesenchymal cells to osteoblasts is precisely regulated in part by signaling through cell surface receptors that interact with extracellular proteins. Here we report that fibulin-5, a key extracellular matrix protein, is important for craniofacial skeletal development in mice. Fibulin-5 is deposited as a fibrous matrix in cranial neural crest-derived mesenchymal tissues, including craniofacial sutures. Fibulin-5-null mice show decreased premaxillary bone outgrowth during postnatal stages. While premaxillo-maxillary suture mesenchymal cells in fibulin-5-null mice were capable of differentiating into osteoblasts, suture cells in mutant mice were less proliferative. Our study provides the first evidence that fibulin-5 is indispensable for the regulation of facial suture mesenchymal cell proliferation required for craniofacial skeletal morphogenesis.
Keywords: Craniofacial development, Neural crest cells, Fibulin-5, Suture, Premaxillary bone
Introduction
Morphogenesis of the craniofacial skeleton begins during embryonic development, and continues after birth. During postnatal development, bone remodeling in sutures at the osteogenic front accounts for the additional size and shape changes of the craniofacial bones [1,2]. The osteoblasts in the sutures are derived from suture mesenchymal stem cells [3]. Proliferation and differentiation of mesenchymal cells are regulated in part by signaling through cell surface receptors that interact with extracellular proteins. However, it remains unclear how the extracellular matrix proteins regulate both proliferation and differentiation in the suture mesenchymal cells.
Fibulin-5 is an extracellular matrix protein secreted by various cell types, including vascular smooth muscle cells [4,5]. Mice deficient for the fibulin-5 exhibit loose skin, tortuous aorta, and emphysematous lung, demonstrating that fibulin-5 is essential for elastic fiber assembly [6,7,8]. Fibulin-5 is also critical for vaginal stromal tissue homeostasis by suppressing proteases that degrade extracellular matrix [9]. In addition, fibulin-5-null mice exhibit increased angiogenesis after wound healing, suggesting that fibulin-5 functions as an inhibitor of angiogenesis [10]. Thus, fibulin-5 regulates diverse cellular functions in a tissue- and context-specific manner.
Among seven fibulin family proteins, fibulin-1and fibulin-5 are reported to be expressed in mouse neural crest derivatives, including craniofacial mesenchymal tissues [4,5,11,12]. While fibulin-1 is critical for cranial bone formation [13,14], the importance of fibulin-5 in craniofacial development has not been unraveled. We report here that fibulin-5 is localized in postnatal craniofacial sutures, and contributes to facial skeletal development. This study provides a unique mouse model of midfacial hypoplasia, and the first evidence that fibulin-5 is required for postnatal craniofacial morphogenesis.
Materials and methods
Mice
The Fbln5+/− mice [6] were maintained on a C57BL/6J background. Mice from Fbln5+/− × Fbln5+/− crosses were genotyped by PCR using the forward primers pgk-s1 (5’-CTGCTAAAGCGCATGCTCCAGACTG-3’) and A55Gs1 (5’-CGCTTTGGGTATCAGATGGATGAAGG-3’), specific for the mutated and wild-type allele, respectively. The common reverse primer A55Ga2 (5’- AATGAGGTTGGTCACCAATGAGATCC-3’) was also used for genotyping. R26R-EYFP [15] and Wnt1-Cre [16] mice were obtained from The Jackson Laboratory. All mice were maintained in the Animal Facility of The University of Texas Medical School at Houston. The experimental protocol was reviewed and approved by the Animal Welfare Committee, the Institutional Animal Care and Use Committee of the University of Texas Health Science Center at Houston.
Skeletal preparations and measurement
Staining of bone and cartilage in postnatal mice with alizarin red/alcian blue was carried out as described previously [17,18]. The images were captured by an Olympus DP71 digital camera attached to a SZX16 microscope (Olympus). The lengths between landmarks were measured using cellSence Standard 1.13 (Olympus).
Histological analysis, immunohistochemistry, and immunofluorescence
Mice were fixed in 10% formalin at 4°C overnight, followed by decalcification for 2 weeks with 10% EDTA, then embedded in paraffin. For histological studies, mice were serially sectioned (7 µm thickness). Sections were kept on Superfrost Plus glass slides (Fisher Scientific). The paraffin sections were deparaffinized in xylene, and rehydrated in a descending series of ethanol solutions. Hematoxylin-eosin staining was performed according to standard protocols. Elastica van Gieson staining was performed using an Elastin Stain Kit (Sigma-Aldrich, HT25A).
For immunohistochemical staining, sections were subjected to antigen retrieval by the use of Antigen Unmasking Solution (Vector Laboratories, H-3300) for 20 min at 100°C. Slides were then washed in water, permeabilized with 0.1% TritonX-100 in PBS (PBST) for 15 min, and blocked by incubation with 5% sheep serum in PBST for 30 min. Slides were incubated overnight at 4°C with antibodies against osterix (1:500, Abcam, ab22552) and Ki67 (1:100, Abcam, ab16667). After washing three times in PBST for 5 min each time, slides were incubated with biotinylated secondary antibodies, followed by the addition of preformed ABC reagent (Santa Cruz, sc-2018). Immunoreactive cells were visualized using 3,3-diaminobenzidine substrate solutioin (Sigma-Aldrich, D4168) as a chromogen, and counterstained with hematoxylin.
For frozen sections, tissues were embedded in Tissue-Plus O.C.T. Compound (Fisher Scientific) and serially sectioned (10 µm thickness). Sections were kept on Superfrost Plus glass slides (Fisher Scientific). Slides were washed in water, permeabilized with PBST for 15 min, and then blocked by incubation with 5% sheep serum in PBST for 30 min. The slides were incubated overnight at 4°C with antibodies against fibulin-5 (1:100, previously described [8]), and EYFP (1:500, Abcam, ab13970). After washing three times in PBST for 5 min each time, slides were incubated with Alexa Fluor 568- or Alexa Fluor 488-conjugated secondary antibodies (Life Technologies), followed by staining with Hoechst 33342 (Life Technologies). Slides were viewed with an Olympus FluoView FV1000 laser scanning confocal microscope by using the FV10-ASW Viewer (Ver. 3.1).
RNA in situ hybridization
Paraffin sections were hybridized as previously described [19]. Antisense probes were used for detecting Col1a1 [20].
Statistical analysis
A Student’s t-test was applied for statistical analysis. A P-value of less than 0.05 was considered statistically significant.
Results
Fibulin-5-null mice exhibit postnatal facial anomaly
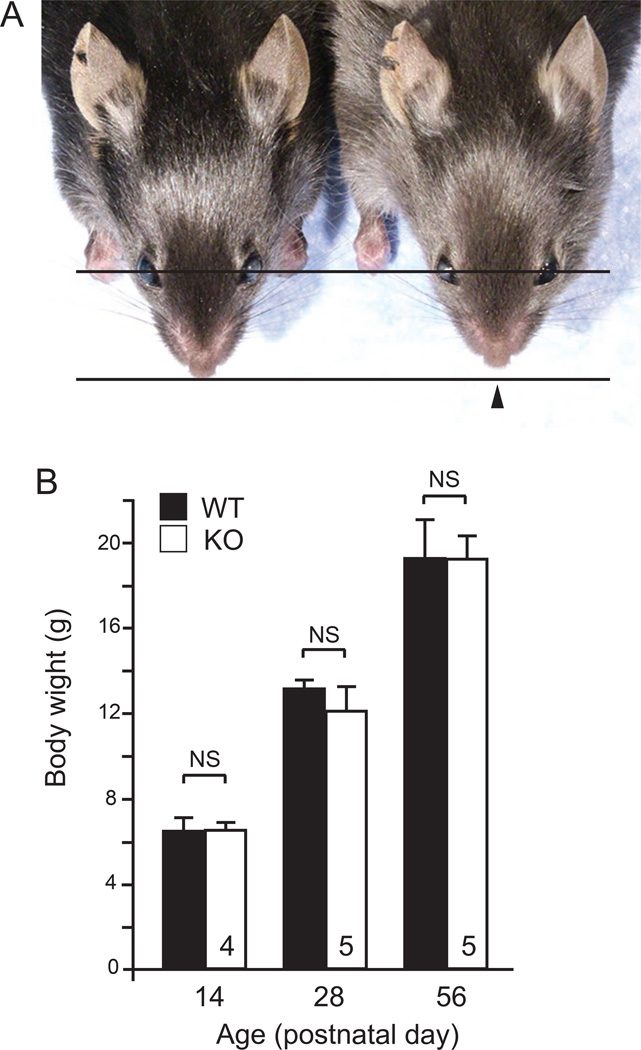
To study the impact of fibulin-5 deficiency during craniofacial development, we examined the facial phenotypes of wild-type (WT) and fibulin-5-null (KO) mice from postnatal day (P) 0 to P84. While no overt morphological defects were seen until around three weeks of age, KO mice had shortened snouts compared to WT mice at P28 (Fig. 1A). To examine the possibility that the shortened snout is due to smaller stature of mutant mice, we measured body weights. The body weights were comparable between WT and KO mice at P14, P28, and P56 (Fig. 1B). In addition, the facial abnormality of KO mice did not increase in severity during development past P28, suggesting that fibulin-5 may play a critical role in the facial development during the early postnatal period.
Fig. 1. Fibulin-5-null mice have abnormal facial development.
(A) Dorsal views of a male fibulin-5-null (KO) mouse (right) and a wild-type (WT) littermate (left) at P28. Black arrowhead indicates shortened snout in KO mouse. (B) Measurement of body weight of female mice at different time points. Analyzed mouse numbers are indicated in the bars. Error bars indicate standard deviation. NS, not significant.
Fibulin-5-null mice have a defect in premaxillary bone elongation
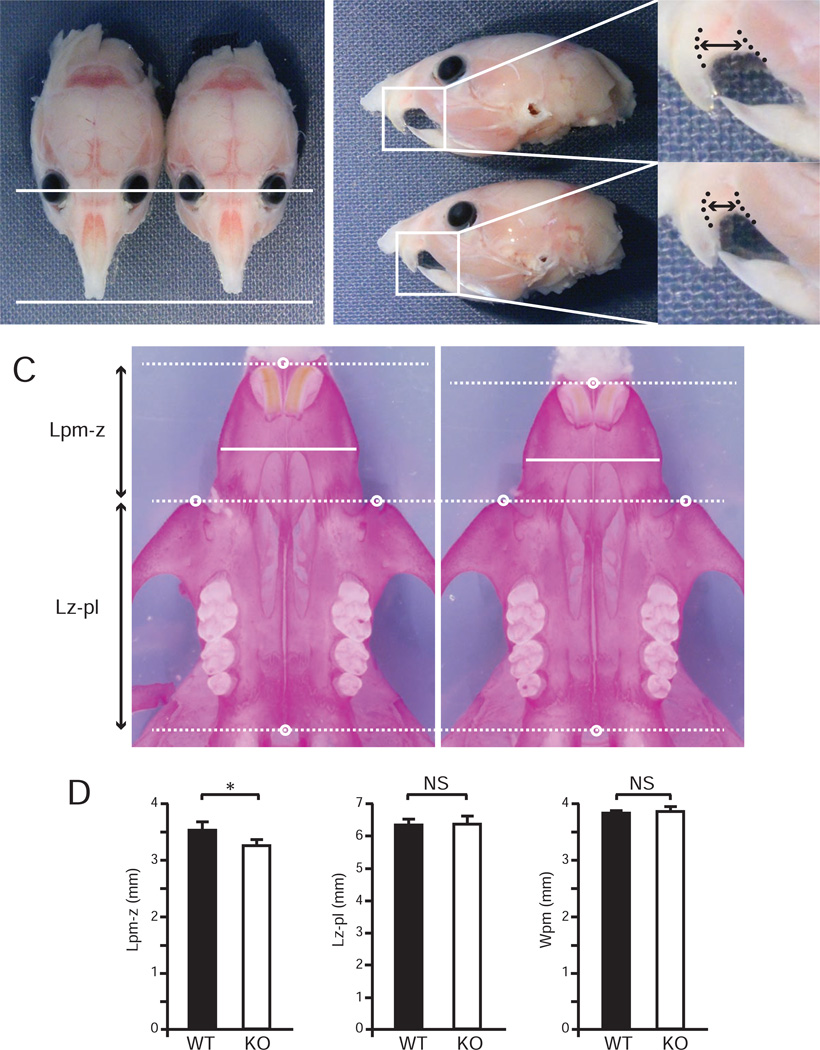
To determine which craniofacial elements are affected in KO mice, we harvested the craniofacial skeletons of WT and KO mice at P28. The bony skeletons of the anterior part of the face in KO mice were shortened relative to WT littermates (Fig. 2A, B). Skeletal staining revealed there was neither obvious synostosis of craniofacial sutures, nor abnormality of cranial base synchondroses in KO mice. Interestingly, KO mice showed a 7.4% decrease in the length of premaxillary bone (p = 0.003), but the length of maxillary bone and the width of premaxillary bone did not differ significantly from WT (Fig. 2C, D). These results suggest that the facial malformation of KO mice is due to decreased longitudinal growth of the premaxillary bone.
Fig. 2. Fibulin-5-null mice have a defect in premaxillary bone formation.
(A, B) Dorsal (A) and lateral (B) views of WT and KO mice at P28 after removing skin and tongue. White arrowhead indicates shortened snout in KO mouse. Inset boxes are magnified in right panels. Length between upper incisor and masseter muscle (two-headed arrow between dotted lines) in KO mouse appeared to be shortened. (C) Ventral views of WT and KO facial bones stained with alizarin red. Landmarks: pm, ventral anterior midpoint of premaxillary bone; z, anterior-most point of zygomatic spine; pl, ventral posterior midpoint of palatal bone. (D) Comparison of the lengths between landmark pm and landmark z (Lpm-z), the lengths between landmark z and landmark pl (Lz-pl), and the widths of premaxillary bone (Wpm) of WT and KO mice (n = 6 for each group). Lpm-z was significantly shorter in KO than WT mice (Student’s t test, *p = 0.003), whereas Lz-pl and Wpm were comparable in both groups. Error bars indicate standard deviation. NS, not significant.
Fibulin-5 is expressed in premaxillo-maxillary suture (PMMS) during the early postnatal period
To address the function of fibulin-5 in postnatal development of the craniofacial skeleton, we examined the expression pattern of fibulin-5 in the craniofacial region of WT mice. It has been demonstrated that Fbln5 is expressed in cranial neural crest-derived tissues [4,5]. Therefore, we used neural crest-specific Cre driver mice (Wnt1-Cre) and reporter mice (R26R-EYFP) to understand the relationship between fibulin-5 localization and cranial neural crest-derived tissues in newborn mice. Co-immunostaining of fibulin-5 and EYFP demonstrated that robust deposition of fibulin-5 was observed in cranial neural crest-derived mesenchymal tissues where EYFP was strongly expressed (Supplementary Fig. S1A).
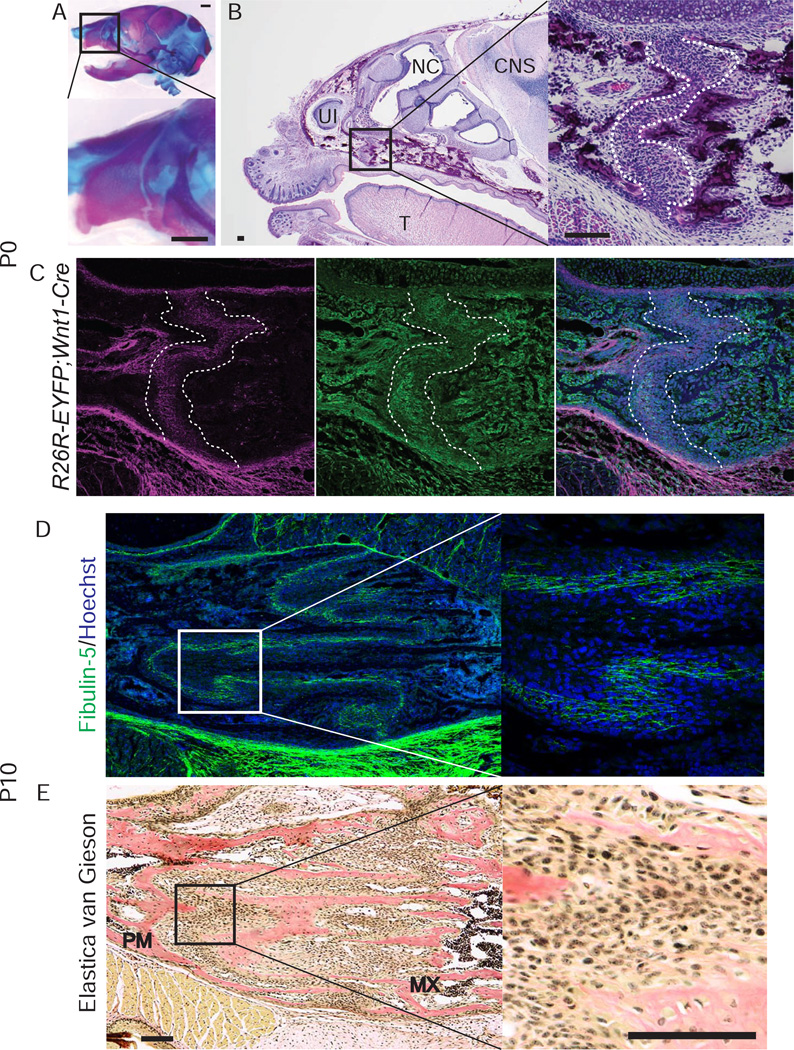
To further investigate the contribution of fibulin-5 to premaxillary bone development, we focused on the premaxillo-maxillary suture (PMMS) and nasal septal cartilage, both of which determine anterior facial growth [21,22,23,24,25,26]. At P0, fibulin-5 was localized in PMMS (Fig. 3A–C, Supplementary Fig. S2), but not in the nasal septal cartilage (Supplementary Fig. S1A). Fibulin-5 was still produced in the PMMS at P10, when PMMS has become highly convoluted (Fig. 3D). Since fibulin-5 plays an essential role in elastic fiber assembly [6], we investigated whether elastic fibers are formed in PMMS and nasal septal cartilage. Elastica van Gieson staining showed that elastic fibers were neither present in PMMS nor in septal cartilage (Fig. 3E, Supplementary Fig. S1B). This suggests that fibulin-5 may not act as an organizer for elastic fiber assembly in PMMS, but instead forms an extracellular niche for suture mesenchymal stem cells when the premaxillary bone is elongating.
Fig. 3. Localization of fibulin-5 in premaxillo-maxillary suture (PMMS).
(A) Alizarin red and alcian blue staining of the head of WT mouse at P0. Inset box is magnified in lower panel. Arrowheads indicate the PMMS. (B) Hematoxylin-eosin staining of a parasagittal section of the anterior head region of WT mouse at P0. Inset box is magnified in right panel. The PMMS is identified as the cellular area between the edges of premaxillary bone and maxillary bone (white dotted lines). (C) Co-immunofluorescent staining of fibulin-5 (magenta) and EYFP (green) of a parasagittal section of neural crest cell (NCC) reporter mouse (R26R-EYFP;Wnt1-Cre) at P0. (D) Immunofluorescent staining of fibulin-5 (green) in PMMS of WT mouse at P10. (E) Elastica van Gieson staining in PMMS of WT mouse at P10. No elastic fibers were detected in PMMS (see also Supplementary Fig. S1B). CNS, central nervous system; MX, maxillary bone; NC, nasal cavity; NVB, neurovascular bundle; PM, premaxillary bone; T, tongue; UI, upper incisor. Scale bars: 1 mm in A; 100 µm in B–E.
PMMS cells in fibulin-5-null mice exhibit a proliferation defect during the early postnatal period
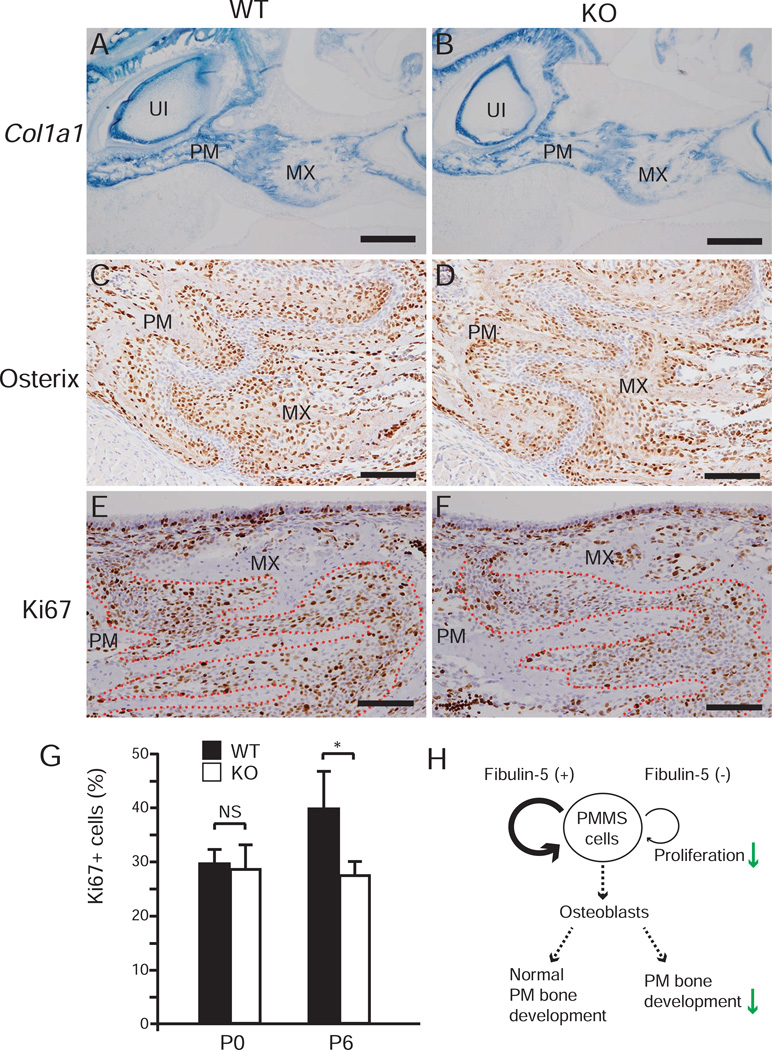
The localization of fibulin-5 in PMMS led us to hypothesize that fibulin-5 plays a role in the regulation of PMMS cells. Therefore, we examined whether KO mice had premature PMMS fusion, since facial suture synostosis is observed in the mouse model of midfacial hypoplasia [21]. Histological analysis demonstrated that premature fusion of PMMS did not occur in KO mice (Supplementary Fig. S3). Next, we analyzed whether there was a decrease in osteoblasts in the premaxillary bone or PMMS of KO mice. mRNA expression of the osteoblast marker Col1a1 in KO mice was comparable to that of WT mice (Fig. 4A, B). Immunohistochemical staining of osterix, a preosteoblast marker, showed that preosteoblasts were present in almost equal levels for both WT and KO mice at the edges of the premaxillary and maxillary bones (Fig. 4C, D). We then explored cell proliferation in PMMS by Ki67 immunostaining. Whereas the percentage of Ki67-positive cells in PMMS of KO mice was equivalent to that of WT mice at P0, a significant reduction of Ki67-positive cells in KO mice was observed at P6 (Fig. 4E–G). These data suggest that fibulin-5 deficiency has little effect on skeletogenic differentiation, but has significant impact on the proliferation of PMMS cells. Taken together, the premaxillary bone elongation defect seen in fibulin-5-null mice might be caused by the reduced cell proliferation in PMMS (Fig. 4H).
Fig. 4. Reduced proliferation of PMMS cells in fibulin-5-null mice.
(A, B) Section in situ hybridization for Col1a1 mRNA on parasagittal sections of WT (A) and KO (B) mice at P6. (C–F) Immunohistochemical staining for osterix (C, D) and Ki67 (E, F) on parasagittal sections of WT (C, E) and KO (D, F) mice at P6. (G) Percentage of Ki67 positive cells (Ki67+ cells) in PMMS at P0 and P6 (n = 3 for each group in both at P0 and P6, Student’s t test, *P = 0.037). Error bars indicate standard deviation. NS, not significant. (H) A summary of this study is shown in H. MX, maxillary bone; PM, premaxillary bone; UI, upper incisor. Scale bars: 500 µm in A and B; 100 µm in C–F.
Discussion
The cranial neural crest is a multipotent cell population that gives rise to craniofacial components, including bone and cartilage [27]. Previous studies demonstrating that Fbln5 mRNA is expressed in cranial neural crest-derived tissues [4,5] led us to investigate the function of fibulin-5 during craniofacial development. Using reporter mice to trace neural crest cells, we successfully showed that fibulin-5 protein is deposited robustly in head mesenchymal tissues, which are mainly derived from cranial neural crest cells. Consistent with the pattern of Fbln5 expression in head mesenchyme, we found that disruption of Fbln5 results in a craniofacial anomaly demonstrating a unique role of fibulin-5 in morphogenesis of cranial neural crest-derived tissues.
We demonstrated that fibulin-5 is indispensable for normal premaxillary bone elongation during the postnatal stage. As the nasal septum cartilage is involved in the lengthening of the anterior part of head [24,25,26], we investigated whether fibulin-5 is localized in septal cartilage. However, fibulin-5 did not exist in septal cartilage, suggesting it is not likely that the defect in premaxillary bone elongation seen in KO mice is due to shortened and/or malformed nasal cartilage. Therefore, we focused on PMMS, which is responsible for premaxillary bone development [21,22,23]. We showed that fibulin-5 is expressed not only in PMMS, but also in other facial sutures including the metopic, coronal, and sagittal sutures (data not shown). One possible explanation for the reason why the phenotypes of KO mice were observed only in PMMS and premaxillary bone is that other extracellular matrix proteins including fibulin family proteins may compensate for the role of fibulin-5 in other parts of the craniofacial sutures and cranial bones.
We also identified that PMMS cells were less proliferative in KO mice than in WT mice. A recent study has shown that Gli1-expressing cells in craniofacial sutures are the mesenchymal stem cell population that provides osteogenic cells in postnatal craniofacial bone [3]. Ablation of Gli1 positive cells in one-month-old mice leads to growth arrest and osteoporosis of craniofacial bones. While the molecular relationship between Gli1 and fibulin-5 is unknown, we speculate that the decreased proliferation of PMMS cells in KO mice may lead to a reduction in the mesenchymal stem cell population, resulting in defective premaxillary bone elongation.
It has been reported that fibulin-5 has diverse biological functions that are tissue- and context-specific [28]. Among all, fibulin-5 plays an essential role in the assembly of elastic fiber, an important component of the extracellular matrix [6,7,8]. We demonstrated that fibulin-5 is localized in PMMS, and elastic fibers are not formed. Fibulin-5 may contribute to proper extracellular matrix organization, which is critical for growth factor restoration and activation, since the extracellular matrix acts not only as a structural support, but also as a regulator of cellular function through interacting directly with adhesion receptors or controlling soluble growth factor presentation to the cells [29]. Previous studies have shown that fibrillin-1, the component of microfibril that interacts with fibulin-5 [30], controls osteoblast maturation and differentiation through the regulation of TGF-β/BMP signals [31]. However, our study indicates that osteogenic commitment of suture mesenchymal stem cells in PMMS was not affected in KO mice. Rather, cell proliferation in PMMS was reduced in KO mice. Further study is required to identify the growth factor and the signaling pathway compromised in PMMS of fibulin-5-null mice.
Supplementary Material
Fibulin-5 is deposited in cranial neural crest-derived mesenchymal tissues.
Fibulin-5-null mice show decreased premaxillary bone growth during postnatal stage.
Fibulin-5 is indispensable for facial suture mesenchymal cell proliferation.
Acknowledgments
We thank Drs. Yingzi Yang, Ernestina Schipani, and Wei Hsu for providing us with in situ plasmids, and Dr. Bridget DeLay for critical reading of this manuscript. This work was supported by a grant from the NIDCR/NIH R00DE021054 to YK, and by a fellowship from The Uehara Memorial Foundation to KN.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Opperman LA. Cranial sutures as intramembranous bone growth sites. Dev Dyn. 2000;219:472–485. doi: 10.1002/1097-0177(2000)9999:9999<::AID-DVDY1073>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- 2.Morriss-Kay GM, Wilkie AO. Growth of the normal skull vault and its alteration in craniosynostosis: insights from human genetics and experimental studies. J Anat. 2005;207:637–653. doi: 10.1111/j.1469-7580.2005.00475.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zhao H, Feng J, Ho TV, Grimes W, Urata M, Chai Y. The suture provides a niche for mesenchymal stem cells of craniofacial bones. Nat Cell Biol. 2015;17:386–396. doi: 10.1038/ncb3139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nakamura T, Ruiz-Lozano P, Lindner V, Yabe D, Taniwaki M, Furukawa Y, Kobuke K, Tashiro K, Lu Z, Andon NL, Schaub R, Matsumori A, Sasayama S, Chien KR, Honjo T. DANCE, a novel secreted RGD protein expressed in developing, atherosclerotic, and balloon-injured arteries. J Biol Chem. 1999;274:22476–22483. doi: 10.1074/jbc.274.32.22476. [DOI] [PubMed] [Google Scholar]
- 5.Kowal RC, Richardson JA, Miano JM, Olson EN. EVEC, a novel epidermal growth factor-like repeat-containing protein upregulated in embryonic and diseased adult vasculature. Circ Res. 1999;84:1166–1176. doi: 10.1161/01.res.84.10.1166. [DOI] [PubMed] [Google Scholar]
- 6.Nakamura T, Lozano PR, Ikeda Y, Iwanaga Y, Hinek A, Minamisawa S, Cheng CF, Kobuke K, Dalton N, Takada Y, Tashiro K, Ross J, Jr, Honjo T, Chien KR. Fibulin-5/DANCE is essential for elastogenesis in vivo. Nature. 2002;415:171–175. doi: 10.1038/415171a. [DOI] [PubMed] [Google Scholar]
- 7.Yanagisawa H, Davis EC, Starcher BC, Ouchi T, Yanagisawa M, Richardson JA, Olson EN. Fibulin-5 is an elastin-binding protein essential for elastic fibre development in vivo. Nature. 2002;415:168–171. doi: 10.1038/415168a. [DOI] [PubMed] [Google Scholar]
- 8.Hirai M, Ohbayashi T, Horiguchi M, Okawa K, Hagiwara A, Chien KR, Kita T, Nakamura T. Fibulin-5/DANCE has an elastogenic organizer activity that is abrogated by proteolytic cleavage in vivo. J Cell Biol. 2007;176:1061–1071. doi: 10.1083/jcb.200611026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Budatha M, Roshanravan S, Zheng Q, Weislander C, Chapman SL, Davis EC, Starcher B, Word RA, Yanagisawa H. Extracellular matrix proteases contribute to progression of pelvic organ prolapse in mice and humans. J Clin Invest. 2011;121:2048–2059. doi: 10.1172/JCI45636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sullivan KM, Bissonnette R, Yanagisawa H, Hussain SN, Davis EC. Fibulin-5 functions as an endogenous angiogenesis inhibitor. Lab Invest. 2007;87:818–827. doi: 10.1038/labinvest.3700594. [DOI] [PubMed] [Google Scholar]
- 11.de Vega S, Iwamoto T, Yamada Y. Fibulins: multiple roles in matrix structures and tissue functions. Cell Mol Life Sci. 2009;66:1890–1902. doi: 10.1007/s00018-009-8632-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhang HY, Timpl R, Sasaki T, Chu ML, Ekblom P. Fibulin-1 and fibulin-2 expression during organogenesis in the developing mouse embryo. Dev Dyn. 1996;205:348–364. doi: 10.1002/(SICI)1097-0177(199603)205:3<348::AID-AJA13>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]
- 13.Cooley MA, Kern CB, Fresco VM, Wessels A, Thompson RP, McQuinn TC, Twal WO, Mjaatvedt CH, Drake CJ, Argraves WS. Fibulin-1 is required for morphogenesis of neural crest-derived structures. Dev Biol. 2008;319:336–345. doi: 10.1016/j.ydbio.2008.04.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cooley MA, Harikrishnan K, Oppel JA, Miler SF, Barth JL, Haycraft CJ, Reddy SV, Scott Argraves W. Fibulin-1 is required for bone formation and Bmp-2-mediated induction of Osterix. Bone. 2014;69:30–38. doi: 10.1016/j.bone.2014.07.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Srinivas S, Watanabe T, Lin CS, William CM, Tanabe Y, Jessell TM, Costantini F. Cre reporter strains produced by targeted insertion of EYFP and ECFP into the ROSA26 locus. BMC Dev Biol. 2001;1:4. doi: 10.1186/1471-213X-1-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chai Y, Jiang X, Ito Y, Bringas P, Jr, Han J, Rowitch DH, Soriano P, McMahon AP, Sucov HM. Fate of the mammalian cranial neural crest during tooth and mandibular morphogenesis. Development. 2000;127:1671–1679. doi: 10.1242/dev.127.8.1671. [DOI] [PubMed] [Google Scholar]
- 17.Ovchinnikov D. Alcian blue/alizarin red staining of cartilage and bone in mouse. Cold Spring Harb Protoc. 2009;2009:pdb prot5170. doi: 10.1101/pdb.prot5170. [DOI] [PubMed] [Google Scholar]
- 18.Nagy A, Gertsenstein M, Vintersten K, Behringer R. Alizarin red staining of post-natal bone in mouse. Cold Spring Harb Protoc. 2009;2009:pdb prot5171. doi: 10.1101/pdb.prot5171. [DOI] [PubMed] [Google Scholar]
- 19.Komatsu Y, Kishigami S, Mishina Y. In situ hybridization methods for mouse whole mounts and tissue sections with and without additional beta-galactosidase staining. Methods Mol Biol. 2014;1092:1–15. doi: 10.1007/978-1-60327-292-6_1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Maruyama T, Jiang M, Hsu W. Gpr177, a novel locus for bone mineral density and osteoporosis, regulates osteogenesis and chondrogenesis in skeletal development. J Bone Miner Res. 2013;28:1150–1159. doi: 10.1002/jbmr.1830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Marks SC, Jr, Lundmark C, Wurtz T, Odgren PR, MacKay CA, Mason-Savas A, Popoff SN. Facial development and type III collagen RNA expression: concurrent repression in the osteopetrotic (Toothless,tl) rat and rescue after treatment with colony-stimulating factor-1. Dev Dyn. 1999;215:117–125. doi: 10.1002/(SICI)1097-0177(199906)215:2<117::AID-DVDY4>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]
- 22.Kolpakova-Hart E, McBratney-Owen B, Hou B, Fukai N, Nicolae C, Zhou J, Olsen BR. Growth of cranial synchondroses and sutures requires polycystin-1. Dev Biol. 2008;321:407–419. doi: 10.1016/j.ydbio.2008.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Purushothaman R, Cox TC, Maga AM, Cunningham ML. Facial suture synostosis of newborn Fgfr1(P250R/+) and Fgfr2(S252W/+) mouse models of Pfeiffer and Apert syndromes. Birth Defects Res A Clin Mol Teratol. 2011;91:603–609. doi: 10.1002/bdra.20811. [DOI] [PubMed] [Google Scholar]
- 24.Wexler MR, Sarnat BG. Rabbit snout growth. Effect of injury to septovomeral region. Arch Otolaryngol. 1961;74:305–313. doi: 10.1001/archotol.1961.00740030312013. [DOI] [PubMed] [Google Scholar]
- 25.Hans MG, Scaletta L, Occhino JC. The effects of antirat nasal septum cartilage antisera on facial growth in the rat. Am J Orthod Dentofacial Orthop. 1996;109:607–615. doi: 10.1016/s0889-5406(96)70072-0. [DOI] [PubMed] [Google Scholar]
- 26.Holton NE, Franciscus RG, Marshall SD, Southard TE, Nieves MA. Nasal septal and premaxillary developmental integration: implications for facial reduction in Homo. Anat Rec (Hoboken) 2011;294:68–78. doi: 10.1002/ar.21288. [DOI] [PubMed] [Google Scholar]
- 27.Sandell LL, Trainor PA. Neural crest cell plasticity. size matters. Adv Exp Med Biol. 2006;589:78–95. doi: 10.1007/978-0-387-46954-6_5. [DOI] [PubMed] [Google Scholar]
- 28.Yanagisawa H, Schluterman MK, Brekken RA. Fibulin-5, an integrin-binding matricellular protein: its function in development and disease. J Cell Commun Signal. 2009;3:337–347. doi: 10.1007/s12079-009-0065-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hynes RO. The extracellular matrix: not just pretty fibrils. Science. 2009;326:1216–1219. doi: 10.1126/science.1176009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Freeman LJ, Lomas A, Hodson N, Sherratt MJ, Mellody KT, Weiss AS, Shuttleworth A, Kielty CM. Fibulin-5 interacts with fibrillin-1 molecules and microfibrils. Biochem J. 2005;388:1–5. doi: 10.1042/BJ20050368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nistala H, Lee-Arteaga S, Smaldone S, Siciliano G, Carta L, Ono RN, Sengle G, Arteaga-Solis E, Levasseur R, Ducy P, Sakai LY, Karsenty G, Ramirez F. Fibrillin-1 and-2 differentially modulate endogenous TGF-beta and BMP bioavailability during bone formation. J Cell Biol. 2010;190:1107–1121. doi: 10.1083/jcb.201003089. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.