Abstract
Introduction
Topical treatments with nasal saline irrigation, topical steroid sprays, or corticosteroid rinses can improve sinonasal symptoms in chronic rhinosinusitis (CRS). However, the impact of these therapies on commensals (Corynebacterium) and on biofilm pathogens associated with CRS (Staphylococcus aureus and Pseudomonas) is not well characterized.
Methods
Paired nasal and sinus swabs were collected endoscopically from 28 controls and 14 CRS patients with nasal polyposis (CRSwNP) who had not received systemic antibiotics or corticosteroids in the previous eight weeks. Total DNA from swab eluents were extracted and analyzed by 16S rRNA gene-based pyrosequencing. A total 359,077 reads were obtained and classified taxonomically. The association of use of topical therapies with sinonasal microbiota composition was assessed by factor and vector-fitting. The proportional abundances of sinonasal bacteria between topical therapy users and non-users were further compared by two-tailed Kolmogorov-Smirnov test among controls and among CRSwNP participants.
Result
Nasal saline irrigation, with or without added budesonide, was not associated with significantly distinct sinonasal microbiota composition or significantly decreased Pseudomonas or S. aureus abundances among either controls or CRSwNP participants. Corynebacterium was slightly lower in controls that reported using saline irrigation than those who did not. No significant association was found between nasal saline irrigation and the proportional abundances of Pseudomonas, S. aureus, and Corynebacterium in CRSwNP participants. However, male CRSwNP patients were noted to have significantly higher Corynebacterium proportional abundances than their female counterparts. The use of topical steroid sprays was associated with a distinct microbiota in control subjects, characterized by higher proportional abundances of Dolosigranulum and Simonsiella and a lower proportional abundance of Campylobacter.
Conclusion
Nasal saline irrigation is not associated with a distinct alteration in the proportional abundance of commensal bacteria or biofilm-forming pathogens in CRSwNP patients. However, use of topical intranasal corticosteroid sprays in control subjects is associated with a distinct sinonasal microbiota.
Keywords: Sinus microbiota, 16S rRNA gene, 16S rRNA gene-based pyrosequencing, Nasal rinse, Irrigation, Nasal saline irrigation, Intranasal steroid spray, Intranasal steroid therapy, Topical Therapy, Chronic Rhinosinusitis, Chronic Sinusitis, Bacteriology, Medical Therapy of Chronic Rhinosinusitis, Steroid therapy
INTRODUCTION
Nasal saline irrigation is common adjuvant therapy for chronic rhinosinusitis patients (CRS), including as a postoperative adjuvant. Clinical trials and meta-analyses have shown irrigation to be effective in reducing CRS symptoms and improving quality of life in both pre-surgical and post-operative periods 1–6. This, together with its low-cost and favorable safety profile, makes nasal saline irrigation a robust choice for the management of sinonasal symptoms.
Nasal saline irrigation is thought to address sinonasal symptoms through several potential mechanisms, including improving mucociliary clearance 7, reducing mucosal inflammation4, maintaining moisture and decreasing postoperative crusting. In addition, nasal saline irrigation is thought to improve symptoms by reducing biofilms and bacterial antigens1, either by direct removal or secondarily by increasing their clearance by the mucociliary mechanism. However, there are limited data regarding the microbiological effects of nasal saline irrigation. This study seeks to determine the association between the sinonasal microbiota and nasal saline irrigation. Specifically, the proportional abundances are examined of Corynebacterium, a common sinonasal bacteria 8,9, and two biofilm-associated pathogens in CRS: Staphylococcus aureus, which has also been implicated as a potential contaminant of nasal rinse, and Pseudomonas, a common waterborne bacteria10–14. If a distinct sinonasal microbiota profile is associated with nasal saline irrigation, this would support the concept that nasal saline irrigation reduces sinonasal symptoms, at least in part, by removal of colonizing or infecting pathogens. Alternatively, if nasal saline irrigation is associated with increased S. aureus or Pseudomonas, this might suggest that nasal lavage results in contamination. To evaluate these possibilities, the sinonasal microbiota was assessed in 28 control subjects and 14 patients with active exacerbation of CRS with nasal polyps (CRSwNP).
MATERIALS AND METHODS
Study design and subjects
Adult patients of both sexes were recruited from the Johns Hopkins Outpatient Rhinology Clinic in 2011. All subjects enrolled had patent maxillary antrostomies following endoscopic sinus surgery greater than one year prior to enrollment. Subjects were excluded if they had used oral antibiotics in the previous eight weeks, or if they had a known history of cystic fibrosis, immunodeficiency, or autoimmune diseases. All participants signed written consent at the time of enrollment. Patients with chronic rhinosinusitis with nasal polyps (CRSwNP) were defined by historical and endoscopic criteria, meeting the definition of the American Academy of Otolaryngology-Head and Neck Surgery Chronic Rhinosinusitis Task Force. Patients had continuous symptoms of rhinosinusitis for greater than 12 consecutive weeks, which was associated with bilateral mucosal disease of the sinuses. The control participants demonstrated normal mucosa on endoscopic exam and had no symptoms or signs of active rhinosinusitis. CRSwNP subjects had endoscopic evidence of polypoid mucosal inflammation.
Each participant’s nasal cavity and maxillary sinus mucosa were sampled separately by calcium alginate swab (MPC, Amarillo, CA) under endoscopic guidance. Each swab was immediately flash frozen on dry ice, then stored at −80°C until analysis. At the time that the samples were taken, information was collected as to the patients’ current self-reported use of topical sinonasal therapies, including saline irrigations and corticosteroids. The study was approved by three institutional review boards: the Northern Arizona University Institutional Review Board (Flagstaff, AZ), the Johns Hopkins Medicine Institutional Review Board (Baltimore, MD), and the Western Institutional Review Board (Olympia, WA), the IRB of record for the Translational Genomics Research Institute.
Sample processing and pyrosequencing analysis
Sample processing and pyrosequencing analysis was performed as previously described 15. Briefly, cell lysis was performed using a combination of enzyme-free chemical and mechanical lysis and the nucleic acid purified using the Qiagen AllPrep Mini Kit (Qiagen, Valencia, CA, USA). Barcoded amplicons of the V3V6 hypervariable region were generated using broad-coverage fusion PCR primers and pooled for sequencing on the Genome Sequencer FLX (Roche Applied Sciences, Branford, USA). The resultant pyrosequences were chimera-checked, demultiplexed, and quality-checked. A total of 407,863 16S rRNA gene sequences underwent taxonomic classification using a custom classifier adapted from the Ribosomal Database Project Naïve Bayesian Classifier16, which was trained with a large curated set of Staphylococcus aureus and Staphylococcus epidermidis sequences to ensure accurate species-level classification of the two species.
Sinonasal microbiota analyses
All sinonasal microbiota comparisons were performed in R version 2.13.117. We calculated proportional abundances as: (Total number of 16S rRNA gene sequences classified as genus A from the sample)/(Total number of 16S rRNA gene sequences classified as bacteria from the sample). All statistical analyses were performed separately in controls and in CRSwNP participants.
We began by evaluating the role of host factors, including nasal saline irrigation on the overall sinonasal microbiota. Using proportional abundance data in Bray-Curtis distance, we visualized the sinonasal microbiota composition by non-metric multidimensional scaling (nMDS) using the R package vegan18. Next, we assessed the association between microbiota composition with host factors (sex, sampling site, and self-reported use of intranasal steroid spray and nasal irrigation) by fitting them as factors or vectors on the nMDS plots18 and plotted 95% confidence intervals. We used indicator analysis to identify the sinonasal bacteria (i.e., the indicator bacteria) uniquely linked to each host factor statistically significantly associated with overall sinonasal microbiota from the factor/vector-fitting analysis. We then examined these indicator bacteria’s proportional abundances using scatterplots.
Net, we assessed the association of nasal saline irrigation with Corynebacterium, Staphylococcus aureus, and Pseudomonas. We compared the proportional abundances of these bacteria in irrigators versus non-irrigators using scatterplots and two-tailed Kolmogorov-Smirnov test with α = 0.05. The prevalence and proportional abundance of all sinonasal bacteria detected were summarized in Tables S1–2.
RESULTS
Participant demographics and clinical history
We enrolled 42 participants with patent maxillary antrostomies, who had not received systemic antibiotics or corticosteroids in the previous eight weeks. Among the 42 participants, 28 were non-diseased controls with normal sinonasal mucosa on endoscopy and 14 were participants with chronic rhinosinusitis with nasal polyposis (CRSwNP) who had endoscopic evidence of polypoid mucosa with inflammation (Table 1).
Table 1.
Demographics and clinical history of study participants
| Controls (n =28) | CRSwNP (n =14) | ||||
|---|---|---|---|---|---|
|
No irrigation (n = 18) |
Any irrigation (n = 10) |
No irrigation (n = 7) |
Any irrigation (n = 7) |
||
| n (%) | |||||
|
Age | |||||
| 21–29 | 0 (0.0) | 1 (10.0) | 1 (14.3) | 0 (0.0) | |
| 30–39 | 2 (11.1) | 0 (0.0) | 1 (14.3) | 2 (28.6) | |
| 40–49 | 5 (27.8) | 3 (30.0) | 2 (28.6) | 0 (0.0) | |
| 50–59 | 5 (27.8) | 3 (30.0) | 1 (14.3) | 5 (71.4) | |
| ≥ 60 | 6 (33.3) | 3 (30.0) | 2 (28.6) | 0 (0.0) | |
|
Previous indication for FESS | |||||
| CRS | 10 (55.6) | 8 (80.0) | 7 (100.0) | 7 (100.0) | |
| non-CRS | 8 (44.4) | 2 (20.0) | 0 (0.0) | 0 (0.0) | |
|
Side sampled | |||||
| Right | 9 (50.0) | 5 (50.0) | 4 (57.1) | 2 (28.6) | |
| Left | 9 (50.0) | 4 (40.0) | 3 (42.9) | 5 (71.4) | |
| Unknown | 0 (0.0) | 1 (10.0) | 0 (0.0) | 0 (0.0) | |
|
Endoscopic findings | |||||
| Normal mucosa Polyps |
18 (100.0) | 10 (100.0) | 0 (0.0) | 0 (0.0) | |
| w/ inflammation only | 0 (0.0) | 0 (0.0) | 3 (42.9) | 0 (0) | |
| w/ mucopurulence | 0 (0.0) | 0 (0.0) | 0 (0.0) | 1 (14.3) | |
| w/ mucin | 0 (0.0) | 0 (0.0) | 4 (57.1) | 6 (85.7) | |
|
Nasal steroid spray use | |||||
| Yes | 5 (27.8) | 5 (50.0) | 3 (42.9) | 6 (85.7) | |
| No | 13 (72.2) | 5 (50.0) | 4 (57.1) | 1 (14.3) | |
The controls comprised of 16 women and 12 men, with a median age of 53.5 years (SD = 12.2, range = 25–77), with 10 who reported using any nasal saline irrigation and 10 who reported using any intranasal steroid spray. Among those who used saline irrigation, 5 concurrently used intranasal steroid spray and one individual used steroid irrigation (Table 1). The CRSwNP group comprised of 11 women and 3 men, with a median age of 52.0 years (SD = 12.4, range = 27–65), with 8 who reported using nasal saline irrigation of any type and 9 who reported using intranasal steroid spray. All CRSwNP participants who used saline irrigation also used some form of topical steroid (i.e., spray or irrigation). We collected and analyzed paired ipsilateral nasal and sinus swabs from each participant.
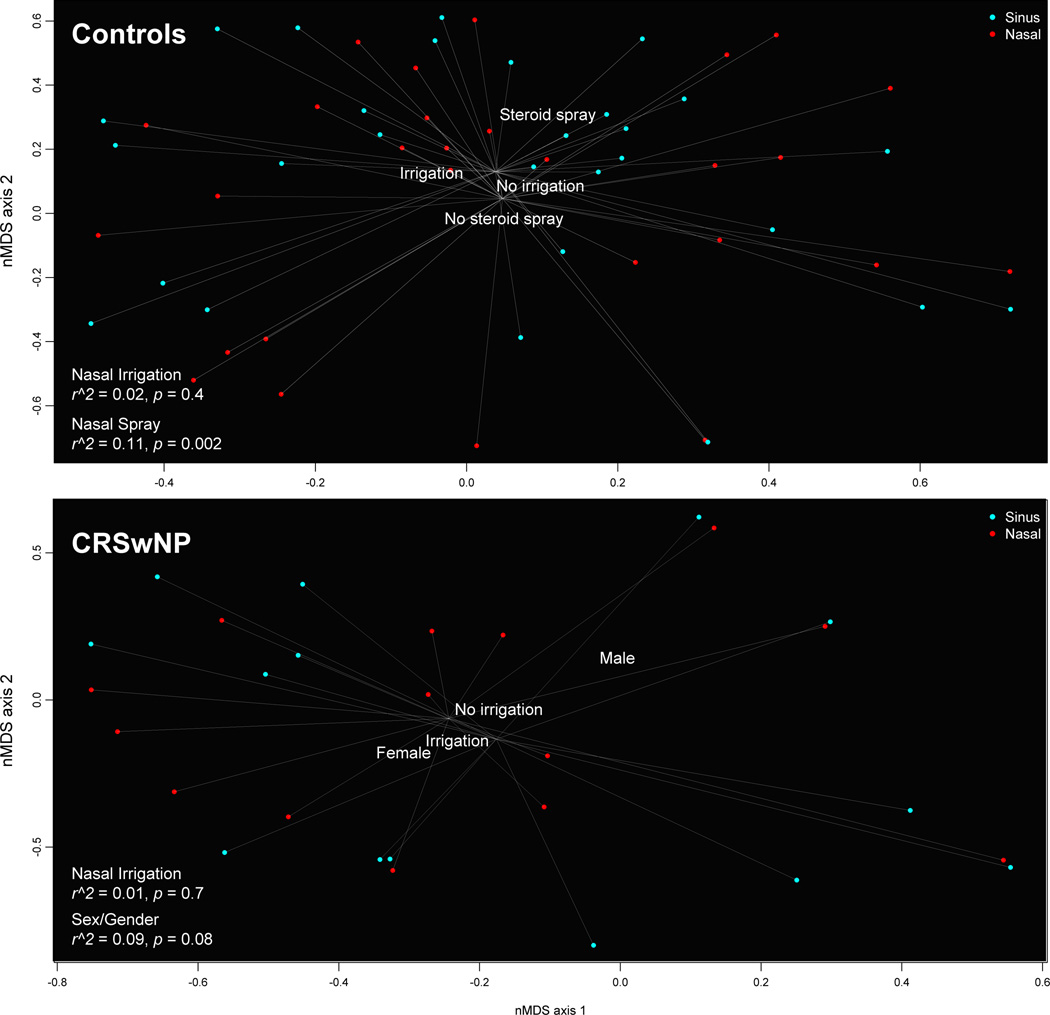
Intranasal steroid spray, but not nasal saline irrigation, was associated with distinct sinonasal microbiota in controls
We found no evidence that nasal saline irrigation—with or without intranasal steroid spray—was associated with distinct sinonasal microbiota in either controls or CRSwNP participants (r2 = 0.02, p = 0.4 in controls; r2 = 0.01, p = 0.7 in CRSwNP); however, we found that any steroid spray use was associated with distinct microbiota in controls (r2 = 0.11, p = 0.002). Further analysis revealed that the difference in sinonasal microbiota were driven by steroid spray users having higher proportional abundances of Dolosigranulum (p < 0.001) and a genetic near neighbor of Simonsiella (p = 0.01) and a lower proportional abundance in Campylobacter (p = 0.05) (Figure S1A–C).
Among CRSwNP participants, we found evidence of sex-specific differences in sinonasal microbiota composition (r2 = 0.09, p = 0.08) (Figure 1). Further analysis showed that the differences in CRSwNP sinonasal microbiota between men and women were driven mostly by the significantly higher proportional abundances of Corynebacterium (p = 0.002) and two other sinonasal bacteria: Serratia (p = 0.003), Finegoldia (p = 0.03) in men (Figure S1D–G).
Figure 1.
A–B. Visualization of association between nasal saline irrigation and sinonasal microbiota composition by non-metric multidimensional scaling in controls and CRSwNP participants. In these two non-metric multidimensional scaling plots, each data point represents the collective nasal/sinus microbiota in a single specimen from a given individual. Data points that are located more closely together represent more similar microbiota compositions than points that are located farther apart. Each data point is also linked to the group’s nasal (red) and sinus (blue) centroid, respectively. Nasal saline irrigation was not associated with a distinct sinonasal microbiota profile, as shown in the controls (Figure 1A) (p = 0.4) and CRSwNP participants (Figure 1B) (p = 0.7). However, intranasal steroid spray users had distinct microbiota than non-users in the controls (Figure 1A) (p = 0.002) and sex-specific differences were seen in CRSwNP participants (Figure 1B) (p = 0.08)
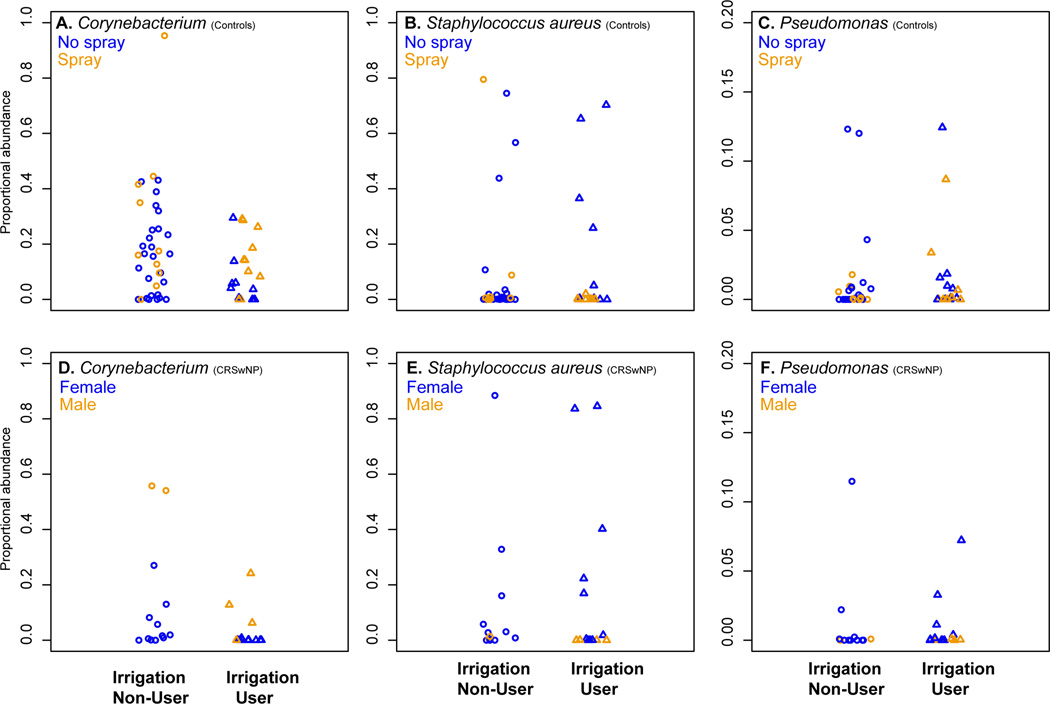
Nasal irrigation was not associated with differences in S. aureus, Pseudomonas, or Corynebacterium abundances in controls or CRSwNP participants
We found that nasal saline irrigation was not associated with lower Pseudomonas or S. aureus abundances in sinonasal microbiota in controls. In addition, the abundance of Corynebacterium was lower in the sinus of controls that reported using any nasal saline irrigation (n = 10) (Mean = 0.09, Range = 0.00 – 0.43) than those who did not (n = 18) (Mean = 0.18, Range = 0.00 – 0.29), but the difference was not statistically significant (p = 0.49) (Figure 2A–C) (Tables S1–2).
Figure 2.
A–F. Scatterplots of sinonasal Corynebacterium, Staphylococcus aureus, and Pseudomonas proportional abundances in controls and CRSwNP participants. This panel of scatterplots depicts the proportional abundances of three sinonasal bacteria (Corynebacterium, S. aureus, and Pseudomonas) in non-irrigators (circles) and irrigators (triangles) among controls (Figure 2A–C) and CRSwNP participants (Figure 2D–F). The host factors shown to be associated significantly with microbiota composition was further denoted in controls (intranasal steroid spray use) and in CRSwNP (male versus female) participants.
Likewise, even though nasal saline irrigation was also associated with lower Corynebacterium abundance in CRSwNP participants, the difference was not statistically significant between CRSwNP irrigators(n = 7) (Mean = 0.02, Range = 0.00 – 0.24) and non-irrigators (n = 7) (Mean = 0.09, Range = 0.00 – 0.56) (p = 0.18). We also found no significant in Pseudomonas abundance between CRSwNP irrigators (Mean = 0.02, Range = 0.00 – 0.07) and non-irrigators (Mean = 0.11, Range = 0.00 – 0.80) (p = 0.9), or in S. aureus abundance between CRSwNP irrigators (Mean = 0.15, Range = 0.00 – 0.85) and non-irrigators (Mean = 0.14, Range = 0.00 – 0.88) (p = 0.9) (Figure 2D–F). The prevalence and proportional abundance of all detected sinonasal bacteria are reported in detail in Tables S1–2.
DISCUSSION
Nasal saline irrigation is thought to reduce sinonasal symptoms partly by removing colonizing or infecting pathogens. In this study, no significant association between nasal saline irrigation was found with overall sinonasal microbiota composition or the nasal bacteria’s proportional abundances. This suggests that the clinical benefits of nasal saline irrigation likely occur through non-microbiological means. An additional implication, however, is that even though high rates of nasal irrigation bottle contamination by S. aureus have been reported 11, nasal irrigation was not associated with higher S. aureus abundances in our participants.
Interestingly, the present study reveals that intranasal corticosteroid spray, a common topical therapy for most sinonasal inflammatory diseases, was associated with a unique microbiota profile in subjects with normal sinonasal mucosa. This may have clinical relevance, since intranasal steroid spray has shown some positive efficacy in adult and pediatric acute bacterial sinusitis 19,20,21 ,22. Thus, the data suggest that, in addition to its well-known anti-inflammatory effects, intranasal steroid spray might have microbiological effects. The clinical implication of the increases in Dolosigranulum and Simonsiella with intranasal steroid spray is still unknown, but Dolosigranulum has been associated with decreased risk for acute otitis media in pediatric patients 23 and characterized primarily in children using culture-independent methods 23,24, whereas Simonsiella has been described as commensal oral bacteria25.
Gender-specific differences in the CRSwNP sinonasal microbiota were also demonstrated in this study. Specifically, men had significantly higher Corynebacterium proportional abundances than did women. Even though Corynebacterium tuberculostearicum has been linked to the development of chronic rhinosinusitis8, most sinonasal studies have shown Corynebacterium to correlate negatively with colonization by CRS pathogens, such as S. aureus 9. Consistent with this relationship, women in the present study were more likely than men to demonstrate S. aureus.
There are several limitations to this study that are primarily attributable to the cross-sectional design and small sample size. Although all subjects were prescribed high-volume high-pressure isotonic saline irrigations, patient compliance was not recorded or accounted for, including whether some patients varied in technique or saline tonicity. Given the small CRSwNP sample, it was not possible to stratify based on disease characteristics such as aspirin sensitivity or asthma. In addition, our cross-sectional design did not allow us to measure the microbiota change associated with nasal saline irrigation by comparing pre- and post-irrigation, which will be important to address in future studies.
CONCLUSION
Saline irrigation of the sinonasal cavities in post-surgical patients is not associated with a distinct sinonasal microbiota. Although nasal lavage seems logically more capable of modifying the microbiome, the present study shows that intranasal topical corticosteroids are associated with distinct alterations in sinonasal bacterial proportional abundances. These findings support the concept that non-microbiological mechanisms, such as improved mucociliary clearance and removal of inflammatory mediators, contribute to the therapeutic benefits of saline. The sinonasal microbial changes associated with topical corticosteroids warrant further exploration, and may suggest unrecognized microbial mechanisms in addition to the known anti-inflammatory effects of these medications.
Supplementary Material
Figure S1A–F. of proportional abundances of sinonasal bacteria associated with intranasal steroid spray use in controls and sex-specific differences in CRSwNP participants. This panel of scatterplot first shows the proportional abundances of indicator sinonasal bacteria that were uniquely prevalent and abundant in control subjects who did (Campylobacteria) or did not (Simonsiella CI < 0.80 and Dolosigranulum) use intranasal steroid spray (Figure S1A–C). Next, among CRSwNP participants, the proportional abundances of four bacteria that are uniquely prevalent and abundant in men (Corynebacterium, Serratia, Finegoldia) (Figure S1D–F) are shown. The individual’s nasal saline irrigation use was further denoted by different symbols in the scatterplots (No irrigation, Circle; Irrigation, Triangle).
Acknowledgments
FUNDING SOURCES: Financial support for this work was provided by the National Institutes of Health (1R15DE021194-01). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
CONFLICT OF INTEREST: The authors have no other financial disclosures or conflict of interest.
REFERENCES
- 1.Wei CC, Adappa ND, Cohen NA. Use of topical nasal therapies in the management of Chronic rhinosinusitis. Laryngoscope. 2013 doi: 10.1002/lary.24066. n/a-n/a. [DOI] [PubMed] [Google Scholar]
- 2.Rudmik L, Hoy M, Schlosser RJ, et al. Topical therapies in the management of chronic rhinosinusitis: an evidence-based review with recommendations. Int Forum Allergy Rhinol. 2013 Apr;3(4):281–298. doi: 10.1002/alr.21096. [DOI] [PubMed] [Google Scholar]
- 3.Rabago D, Pasic T, Zgierska A, Mundt M, Barrett B, Maberry R. The efficacy of hypertonic saline nasal irrigation for chronic sinonasal symptoms. Otolaryngol Head Neck Surg. 2005 Jul;133(1):3–8. doi: 10.1016/j.otohns.2005.03.002. [DOI] [PubMed] [Google Scholar]
- 4.Liang KL, Su MC, Tseng HC, Jiang RS. Impact of pulsatile nasal irrigation on the prognosis of functional endoscopic sinus surgery. J Otolaryngol Head Neck Surg. 2008 Apr;37(2):148–153. [PubMed] [Google Scholar]
- 5.Pynnonen MA, Mukerji SS, Kim HM, Adams ME, Terrell JE. Nasal saline for chronic sinonasal symptoms: a randomized controlled trial. Arch Otolaryngol Head Neck Surg. 2007 Nov;133(11):1115–1120. doi: 10.1001/archotol.133.11.1115. [DOI] [PubMed] [Google Scholar]
- 6.Liang J, Lane AP. Topical Drug Delivery for Chronic Rhinosinusitis. Current otorhinolaryngology reports. 2013 Mar 1;1(1):51–60. doi: 10.1007/s40136-012-0003-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hauptman G, Ryan MW. The effect of saline solutions on nasal patency and mucociliary clearance in rhinosinusitis patients. Otolaryngol Head Neck Surg. 2007 Nov;137(5):815–821. doi: 10.1016/j.otohns.2007.07.034. [DOI] [PubMed] [Google Scholar]
- 8.Abreu NA, Nagalingam NA, Song Y, et al. Sinus Microbiome Diversity Depletion and Corynebacterium tuberculostearicum Enrichment Mediates Rhinosinusitis. Sci Transl Med. 2012 Sep 12;4(151):151ra124. doi: 10.1126/scitranslmed.3003783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yan M, Pamp SJ, Fukuyama J, et al. Nasal microenvironments and interspecific interactions influence nasal microbiota complexity and S. aureus carriage. Cell host & microbe. 2013 Dec 11;14(6):631–640. doi: 10.1016/j.chom.2013.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Prince AA, Steiger JD, Khalid AN, et al. Prevalence of biofilm-forming bacteria in chronic rhinosinusitis. Am J Rhinol. 2008 May-Jun;22(3):239–245. doi: 10.2500/ajr.2008.22.3180. [DOI] [PubMed] [Google Scholar]
- 11.Keen M, Foreman A, Wormald PJ. The clinical significance of nasal irrigation bottle contamination. Laryngoscope. 2010 Oct;120(10):2110–2114. doi: 10.1002/lary.21031. [DOI] [PubMed] [Google Scholar]
- 12.Bendouah Z, Barbeau J, Hamad WA, Desrosiers M. Biofilm formation by Staphylococcus aureus and Pseudomonas aeruginosa is associated with an unfavorable evolution after surgery for chronic sinusitis and nasal polyposis. Otolaryngol Head Neck Surg. 2006 Jun;134(6):991–996. doi: 10.1016/j.otohns.2006.03.001. [DOI] [PubMed] [Google Scholar]
- 13.Foreman A, Wormald PJ. Different biofilms, different disease? A clinical outcomes study. Laryngoscope. 2010 Aug;120(8):1701–1706. doi: 10.1002/lary.21024. [DOI] [PubMed] [Google Scholar]
- 14.Tan NC, Foreman A, Jardeleza C, Douglas R, Tran H, Wormald PJ. The multiplicity of Staphylococcus aureus in chronic rhinosinusitis: correlating surface biofilm and intracellular residence. Laryngoscope. 2012 Aug;122(8):1655–1660. doi: 10.1002/lary.23317. [DOI] [PubMed] [Google Scholar]
- 15.Liu CM, Soldanova K, Nordstrom L, et al. Medical therapy reduces microbiota diversity and evenness in surgically recalcitrant chronic rhinosinusitis. Int Forum Allergy Rhinol. 2013 Oct;3(10):775–781. doi: 10.1002/alr.21195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wang Q, Garrity GM, Tiedje JM, Cole JR. Naive Bayesian classifier for rapid assignment of rRNA sequences into the new bacterial taxonomy. Appl Environ Microbiol. 2007 Aug;73(16):5261–5267. doi: 10.1128/AEM.00062-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.R: A language and environment for statistical computing [computer program] Vienna: R Foundation for Statistical Computing; 2011. [Google Scholar]
- 18.vegan: Community Ecology Package. R package version 1.17-8. 2011 http://CRAN.R-project.org/package=vegan.
- 19.Hayward G, Heneghan C, Perera R, Thompson M. Intranasal Corticosteroids in Management of Acute Sinusitis: A Systematic Review and Meta-Analysis. The Annals of Family Medicine. 2012;10(3):241–249. doi: 10.1370/afm.1338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Barlan IB, Erkan E, Bakir M, Berrak S, Başaran MM. Intranasal Budesonide Spray as an Adjunct to Oral Antibiotic Therapy for Acute Sinusitis in Children. Annals of Allergy, Asthma & Immunology. 1997;78(6):598–601. doi: 10.1016/S1081-1206(10)63223-1. [DOI] [PubMed] [Google Scholar]
- 21.Meltzer EO, Orgel HA, Backhaus JW, et al. Intranasal flunisolide spray as an adjunct to oral antibiotic therapy for sinusitis. The Journal of allergy and clinical immunology. 1993 Dec;92(6):812–823. doi: 10.1016/0091-6749(93)90058-n. [DOI] [PubMed] [Google Scholar]
- 22.Dolor RJ, Witsell DL, Hellkamp AS, et al. Comparison of cefuroxime with or without intranasal fluticasone for the treatment of rhinosinusitis. The CAFFS Trial: a randomized controlled trial. Jama. 2001 Dec 26;286(24):3097–3105. doi: 10.1001/jama.286.24.3097. [DOI] [PubMed] [Google Scholar]
- 23.Pettigrew MM, Laufer AS, Gent JF, Kong Y, Fennie KP, Metlay JP. Upper respiratory tract microbial communities, acute otitis media pathogens, and antibiotic use in healthy and sick children. Appl Environ Microbiol. 2012 Sep;78(17):6262–6270. doi: 10.1128/AEM.01051-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bogaert D, Keijser B, Huse S, et al. Variability and diversity of nasopharyngeal microbiota in children: a metagenomic analysis. PLoS One. 2011;6(2):e17035. doi: 10.1371/journal.pone.0017035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Carandina G, Bacchelli M, Virgili A, Strumia R. Simonsiella filaments isolated from erosive lesions of the human oral cavity. J Clin Microbiol. 1984 Jun;19(6):931–933. doi: 10.1128/jcm.19.6.931-933.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1A–F. of proportional abundances of sinonasal bacteria associated with intranasal steroid spray use in controls and sex-specific differences in CRSwNP participants. This panel of scatterplot first shows the proportional abundances of indicator sinonasal bacteria that were uniquely prevalent and abundant in control subjects who did (Campylobacteria) or did not (Simonsiella CI < 0.80 and Dolosigranulum) use intranasal steroid spray (Figure S1A–C). Next, among CRSwNP participants, the proportional abundances of four bacteria that are uniquely prevalent and abundant in men (Corynebacterium, Serratia, Finegoldia) (Figure S1D–F) are shown. The individual’s nasal saline irrigation use was further denoted by different symbols in the scatterplots (No irrigation, Circle; Irrigation, Triangle).