Abstract
Acute Myeloid Leukemia (AML) is a set of related diseases characterized by the immortalization and uncontrolled expansion of myeloid precursors. Core therapy for AML has remained unchanged for nearly 30 years, and survival rates remain unsatisfactory. However, advances in the immunotherapy of AML have created opportunities for improved outcomes. Enforcing a tumor-specific immune response through the re-direction of the adaptive immune system, which links remarkable specificity with potent cytotoxic effector functions, has proven particularly compelling. This may be coupled with immune checkpoint blockade and conventional therapies for optimal effect. Engineered antibodies are currently in use in AML and the repertoire of available therapeutics will expand. NK cells have shown effectiveness in this disease. New methods to optimize the targeting and activation of AML cells show potential. Most significantly, adoptive immunotherapy with tumor-specific T cells, and particularly T cells re-directed using genetically introduced TCR or chimeric antigen receptors, have particular promise. Each of these approaches has unique benefits and challenges that we explore in this review.
Keywords: Acute Myeloid Leukemia, T lymphocyte, Chimeric Antigen Receptor, Adoptive Immunotherapy
Introduction
In 2000, Weinberg and Hanahan described the hallmarks of cancer as growth self-sufficiency, resistance to growth inhibitory and apoptotic signals, limitless replication, ability to acquire nutrition by promoting angiogenesis, and invasion and metastasis. In 2011, they refined this, recognizing that immune evasion is an additional core attribute of cancer cells (Hanahan and Weinberg, 2011). This fundamental feature of cancer is particularly salient in acute myeloid leukemia (AML).
Myeloid cells play central roles in immunity. Dendritic cells, macrophages, granulocytes, and platelets are critical in activating and sustaining adaptive and innate immunity. Mature and immature myeloid cells, the latter of which in many respects resemble AML blasts, can also suppress immunity. Myeloid cells, and particularly dendritic cells, localize to sites of B and T cell development where they tolerize lymphocyte precursors that recognize myeloid cell-associated antigens (Klein et al., 2014; Rowland et al., 2013). Immature and unactivated myeloid cells also possess potent veto and suppressive activities, and are key mediators of mature lymphocyte tolerance (Gur et al., 2002; De et al., 2014).
The strong natural interactions of myeloid cells with the immune system provide unique opportunities for immunotherapeutic exploitation so as to target leukemic blasts. Immunotherapy for AML is in its infancy, but proceeding along multiple fronts (Fig. 1). New findings have raised the significant promise that in the near future it will occupy a prominent place in the armamentarium against this difficult disease.
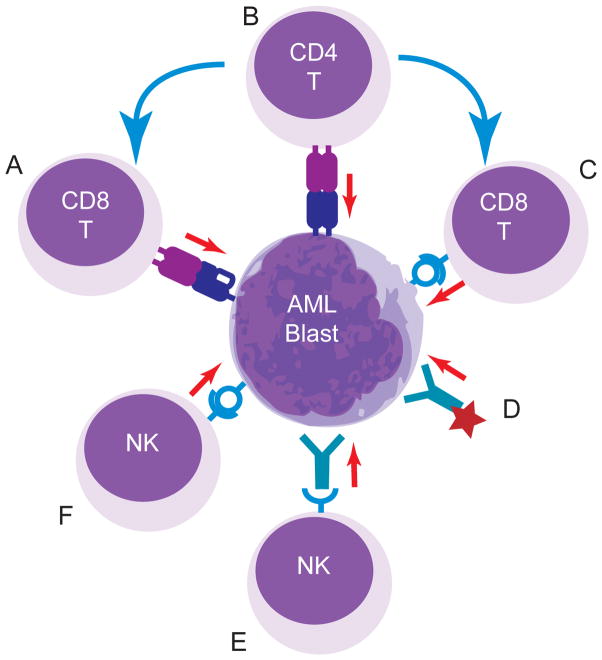
Figure 1. Adoptive immunotherapy of AML.
Depiction of methods to target AML, including: (A) Infusion of AML-antigen-specific cytolytic T lymphocytes. These can be generated through the in vitro expansion of tumor antigen-specific lines or transduction of T cells with tumor-specific TCR; (B) Infusion of tumor antigen-specific CD4+ T cells. These may promote direct tumor lysis or act through their ability to support CD8+ T cell expansion and memory; (C) transfer of CAR-modified CD8+ T cells re-directed through a scFv-TCR hybrid specific for lineage or tumor antigens expressed by AML blasts; (D) infusion of AML-specific antibodies modified to enhance their cytolytic potential through conjugated therapeutics; (E) transfer of NK cells that are activated and target AML in response to FcR binding, AML-specific antibodies or other activating receptors. NK cells can be infused fresh after collection or activated and expanded in vitro; (F) transfer of NK cells modified to express activating CAR. Blue arrows indicate direction of helper activities, red arrows indicate cytotoxic activities.
Current treatment of AML
Over thirty years ago, the “3 + 7” regimen (three days of daunorubicin plus 7 days of cytarabine) was shown to induce remission in approximately 60% of AML patients and became standard induction therapy for children and adults with this disease. Clinical trials conducted in the 1990s demonstrated the benefit of intensive postremission therapy, which included high-dose cytarabine-based chemotherapy or hematopoietic stem cell transplantation (HSCT). Although remission and overall survival rates for children with AML are now greater than 90% and 60%, respectively, all contemporary treatment regimens are still based on anthracyclines, nucleoside analogues, and intensive postremission therapy (Rubnitz et al., 2010a; Gamis et al., 2014). Efforts to improve the outcome of patients with AML have included the replacement of daunorubicin with idarubicin or mitoxantrone (Creutzig et al., 2013); the intensification of cytarabine (Rubnitz et al., 2010a) or daunorubicin during induction; and the addition of maintenance therapy (Perel et al., 2002). With the exception of maintenance therapy, which was associated with an inferior survival, most regimen modifications have had modest effects and most randomized trials have shown no significant difference in outcome between treatment arms. Despite these disappointing results, excellent supportive care, adaptation of therapy on the basis of each patient’s response, and the selective use of HSCT have all contributed to improved treatment results.
The graft-versus-leukemia effects that are elicited after HSCT suggest that anti-tumor immunity may be potent at eradicating leukemia and preventing subsequent relapse. Indeed, many studies have demonstrated that HSCT is associated with lower rates of relapse compared to chemotherapy (Niewerth et al., 2010). However, because HSCT has high rates of treatment-related mortality and morbidity, the indications for performing HSCT in first remission remain controversial (Niewerth et al., 2010). In general, study groups in the United States recommend HSCT for a larger proportion of patients than do European investigators (Horan et al., 2008; Niewerth et al., 2010). In recent years, improvements in supportive care and more comprehensive HLA and NK typing have contributed to greater benefits and fewer side effects among HSCT treated patients (Leung et al., 2011). An important predictor of outcome after HSCT is the level of leukemia at the time of transplant, indicating the importance of inducing deep remissions prior to transplant (Leung et al., 2012). One strategy that has been used is the incorporation of tyrosine kinase inhibitors (TKIs) into the chemotherapy backbone. For example, internal tandem duplications of the FLT3 gene (FLT3 ITD) occur in approximately 15% of pediatric and 30% of adult AML cases and are associated with a poor outcome, particularly in cases with high ratios of FLT3-ITD to wild-type FLT3 (Staffas et al., 2011). Sorafenib, sunitinib, and other FLT3 inhibitors are highly active in patients with FLT3 mutations, but prolonged use of these agents is associated with the development of resistance, most commonly caused by acquired D835 or F691 kinase domain point mutations (Baker et al., 2013). Crenolanib, a novel tyrosine kinase inhibitor, is active in sorafenib-resistant AML mouse models that contain these mutations, suggesting that this agent may extend clinical benefit (Zimmerman et al., 2013). Although TKIs represent a distinct approach to AML therapy, target validation remains slow and new therapeutic strategies are needed.
Antibody-based therapies
Multiple antigens, including CD33, CD123, and CD47, represent potential targets for antibody-based AML therapy. Most efforts have focused on CD33 (Gasiorowski et al., 2014). The activity of gemtuzumab ozogamicin (GO), a humanized anti-CD33 antibody conjugated to calicheamicin, in patients with relapsed AML led to its approval in 2000 (Bross et al., 2001). Randomized trials conducted in adults (Petersdorf et al., 2013; Burnett et al., 2011; Castaigne et al., 2012) and children (Gamis et al., 2014) with newly diagnosed AML suggest that the addition of GO to conventional chemotherapy reduces the risk of relapse, improves event-free survival, and may improve overall survival. Meta-analyses demonstrate that the benefit of GO is greatest among low-risk patients, with only modest benefits in intermediate-risk patients; patients with high-risk AML did not benefit from this agent (Hourigan and Karp, 2013).
Because of limitations related to toxicity and drug resistance, investigators have developed a novel anti-CD33 conjugate (SGN-CD33A) by replacing calicheamicin with a synthetic pyrrolobenzodiazepine (Kung Sutherland et al., 2013). SGN-CD33A, which is more potent than GO at inducing apoptosis in AML cell lines, primary samples, and mouse models, is now being evaluated in Phase I clinical trials (NCT02326584, NCT01902329). An alternative approach to enhancing the efficacy of CD33-directed therapy is the development of CD33/CD3-directed bispecific T-cell engager (BiTE) antibodies, such as AMG 330 (Laszlo et al., 2014; Krupka et al., 2014). By bridging tumor antigens with T cell receptors (TCR), these can direct T cell effector functions, including cytoloysis, against tumor cells. In preclinical models, AMG 330 was able to recruit T cells, resulting in potent CD33-dependent cytotoxicity. Analogous to BiTE antibodies, bispecific killer cell engagers (BiKE) target CD16 on NK cells and tumor-specific antigens, such as CD33. CD16xCD33 BiTEs and CD16xCD33xCD123 trispecific engagers have been recently developed and shown to induce NK cell function and eliminate CD33+ AML cells in preclinical models (Singer et al., 2010; Kugler et al., 2010; Gleason et al., 2014). It is likely that BiTE and BiKE antibodies will soon be tested in clinical trials for patients with relapsed AML.
Natural killer cell therapy
Natural killer (NK) cells can target and kill leukemia cells without prior exposure to those cells (Leung, 2014). The beneficial effects of killer inhibitory receptor (KIR)-mismatched donor NK cells in the setting of allogeneic HSCT for AML was first demonstrated in 2002 (Ruggeri et al., 2002) and have subsequently been confirmed in many studies (Velardi et al., 2012; Venstrom et al., 2012; Cooley et al., 2014). These observations led to interest in the use of allogeneic NK cells in the non-HSCT setting (Miller et al., 2005; Rubnitz et al., 2010b). We performed a pilot study in which we demonstrated that infusions of haploidentical NK cells in patients with AML were well tolerated and associated with transient engraftment, expansion of donor NK cells, minimal toxicity, and no graft-versus-host disease (Rubnitz et al., 2010b). Although these results suggest that treatment with haploidentical mismatched NK cells is a safe and potentially valuable approach to reduce the risk of relapse in patients with AML, clinical trials are required to investigate its benefits. In addition, it is likely that enhancement of NK cell activity will be required to provide optimal antileukemic effects. Potential methods to increase NK cell numbers and activity include the expansion of activated NK cells (Fujisaki et al., 2009) and the addition of RXRγ agonists or lineage-specific antibodies, such as anti-CD33 (Leung et al., 2013; Chan et al., 2012). Another method to enhance NK activity is the use of anti-KIR antibodies to block inhibitory KIRs; this approach was recently shown to be safe in patients with AML (Vey et al., 2012). The depletion of host regulatory T cells (Treg), which may inhibit the proliferation of donor NK cells, may also improve the efficacy of NK cell therapy. A recent clinical trial demonstrated that depletion of host regulatory T cells by an IL-2 diphtheria toxin fusion protein was associated with increased NK cell expansion and higher response rates in adults with relapsed AML (Bachanova et al., 2014). As mentioned above, bispecific killer cell engagers are alternatives to enhance the antileukemic effects of NK cells (Gleason et al., 2014).
T cell recognition of AML
The normal role of myeloid cells in tolerizing the immune system to self antigens would be expected to constrain the immune repertoire’s capacity to recognize AML blasts. Nevertheless, tolerance to AML is incomplete, and elements of a specific adaptive T cell response are detectable against proteinase 3, WT1, TERT, mutated nucleophosmin, and other antigens expressed by AML cells (Greiner et al., 2012; Rezvani et al., 2012). Many documented responses are observed in the context of allogeneic HSCT, though anti-tumor responses have also been detected in patients only receiving conventional chemotherapy (Montagna et al., 2006; Norde et al., 2009). Further, some data indicates that the extent of T cell responses positively correlates with response to therapy (Montagna et al., 2006). Such findings must be interpreted cautiously and may reflect differences in the immunogenicity of tumors with otherwise different biologies or other confounding influences rather than a causal protective role of specific T cell responses in AML progression.
In the context of HSCT, where rapid expansion of pre-existing and newly formed allogeneic lymphocytes in the setting of lymphopenia promotes T cell reactivity, donor-derived T cells protect against relapse, establishing their anti-leukemic activity (Kolb, 2008; Rezvani et al., 2012). Little is known about whether and how the immune system normally shapes the development and evolution of AML. A clearer understanding of this will be important to optimize disease-specific immunotherapies.
Targeting AML with adoptive T cell immunotherapy
Efforts to enhance AML-specific T cell responses through tumor antigen vaccination using a variety of formats have met with mixed successes (Van Tendeloo et al., 2010; Uttenthal et al., 2014; Schmitt et al., 2009; Kuball et al., 2011). An alternative approach to T cell immunotherapy is to expand tumor specific T cells ex-vivo, and transfer these into the host (Ma et al., 2010; Distler et al., 2008; Stromnes et al., 2014). The infusion of activated antigen-specific T cells obviates the need for in situ immune stimulation. To ensure the availability of large numbers of T cells of desired specificity, these lymphocytes can be genetically modified to enforce the expression of pre-selected TCR (Spranger et al., 2012; Udyavar and Geiger, 2010). Identification of such receptors has been time consuming in the past, though new approaches that expedite the isolation of patient and tumor-specific TCR are being refined (Kobayashi et al., 2014). Expanded re-directed T cells have already shown clinical promise in melanoma and other cancers (Blankenstein et al., 2015; Robbins et al., 2011; Johnson et al., 2009). Data is more limited in AML (Spranger et al., 2012; Stauss et al., 2008). The potential here is appealing, particularly considering the normal concentration of T cells within the bone marrow and other hematologic organs where AML resides.
Due to developmental tolerance in the thymus, many native AML-specific TCR may be of low affinity and poorly capable of targeting AML blasts. However, TCR may be developed or modified to possess enhanced TCR reactivity (Schmitt et al., 2013; Stone and Kranz, 2013). Xenogeneic TCR formed by immunizing HLA-transgenic mice or other species may allow the production of higher affinity TCR, though these have the potential to themselves induce specific immunity due to their non-human origins. Alternatively, we have identified using structural analyses and molecular dynamics the presence of an amino acid hot spot, mutation of which, by stabilizing hypervariable TCR CDR3 loops, increases TCR binding free energy and affinity (Alli et al., 2011). Others have similarly used computational design or in vitro evolution techniques to generate high affinity TCR (Pierce et al., 2014; Weber et al., 2005; Holler et al., 2003; Li et al., 2005). Caution is necessary when using modified TCR (Morgan et al., 2013; Parkhurst et al., 2011). TCR mutations can confer new self reactivities, increasing the potential for undesirable responses (Zhong et al., 2013; Holler et al., 2003; Udyavar et al., 2009). Indeed, the majority of the free energy of binding of TCR to peptide-MHC complexes is directed at the MHC rather than specific antigen, and any generic increase in MHC affinity would be expected to shift the recognition profile of TCR, converting subthreshold interactions into effective ligand engagements. Thorough analysis of the safety profile of modified receptors prior to human application is therefore essential.
TCRs are generally dependent on CD4 or CD8 co-receptors to recognize antigen-MHC complexes. As affinity increases, the TCR may become co-receptor independent (Stone et al., 2009). One advantage to the affinity maturation of therapeutic TCR is that this can allow the adaptation of a TCR for both CD4 and CD8 T cells. Although most work on T cell immunotherapy has focused on CD8 effector cells, CD4 T cells have in cases been found to be potent against individual tumors, and are able to support CD8 persistence (Gattinoni et al., 2006; Frankel et al., 2010). Co-transfer of both cell types will likely prove advantageous.
AML inhibition of T cell responses
The utility of TCR-retargeted T cells for immunotherapy may be limited by the immunodulatory properties of AML cells themselves. Many cases of AML are PD-L1 positive, and this is upregulated by T cell cytokines, particularly IFN-γ. PD-L1 binds to PD-1 on T cells, and downregulates signaling through the recruitment of the SHP-2 phosphatase (Berthon et al., 2010; Kronig et al., 2014). AML may express IDO, another IFN-γ induced gene, which through the depletion of tryptophan and generation of degradation byproducts suppresses cellular immunity and can promote regulatory T cell (Treg) formation (Curti et al., 2007). Large numbers of Treg may be present in the bone marrow microenvironment, these may be increased in the context of AML and serve as an additional source for the immunosuppression of therapeutically administered T cells through the expression of CTLA-4, production of pericellular adenosine, and other mechanisms (Cohen et al., 2005; Ustun et al., 2011; Lichtenegger et al., 2014). Considering the multiple inhibitory signals suppressing adoptively transferred T cells, combination immunotherapy will likely be important. PD-1 blockade with nivolumumab is now being tested in AML (clinical trials.gov; NCT02275533). Any effectiveness will likely to be magnified in the setting of adoptive immunotherapy where large numbers of tumor–specific T cells are being transferred. Likewise, blockade of CTLA-4 with ipilimumab and the administration of adenosine antagonists and IDO inhibitors under development may further create a tumor environment that enhances the effectiveness of cellular immunotherapy (Ohta and Sitkovsky, 2014)(clinical trials.gov; NCT01757639).
Successful cellular therapies have the potential to generate prolonged T cell engraftment. The kinetics of therapeutic T cell expansion, tumor cytolysis, and clinical effect may therefore differ substantially from that of conventional pharmacologics or biologics, whose activities follow more readily defined pharmacokinetic and pharmacodynamic parameters. As most current T cell epitopes observed in AML are not tumor specific, the risk of prolonged myelosuppression accompanies any successful cellular immunotherapeutic intervention. Approaches to eliminate these cells should they prove toxic are essential, and may include the incorporation of ‘suicide genes’, such as HSV-TK and dimerizable caspase 9 in therapeutic constructs or therapeutic treatment prior to immunoablative therapy, as may occur pre-HSCT (Jones et al., 2014).
Retargeting T lymphocytes with chimeric antigen receptors
As an alternative to re-targeting T cells through TCR gene transfer, these cells can be redirected using chimeric antigen receptors (CARs). CARs are engineered cell surface molecules that link a target cell ligand-recognition domain to signaling regions from the TCR. For targeting tumors, the most common ligand recognition domain is a single chain antibody Fv (scFv) that recognizes a tumor or lineage-specific cell surface molecule (Eshhar et al., 1993). This is linked to a stalk extending the scFv from the cell surface, transmembrane domain, and intracytoplasmic signaling domains. First generation CARs typically incorporated the TCRζ cytoplasmic domain, which includes 3 ITAM motifs, or other ITAM-bearing CD3 signaling regions for transducing a signal mimicking that of the TCR (Geiger et al., 1999). We and others demonstrated that tandem signaling domains incorporating TCR along with costimulatory or co-receptor signals provide more robust stimulation. This can enhance receptor-modified T cell survival, expansion, and activity (Geiger et al., 2001; Finney et al., 1998). A variety of supplemental signaling domains have now been successfully tested in these second and third generation CARs (Sadelain et al., 2013). No single optimal design, though, has been identified.
The clinical effectiveness of CAR-modified T cells was first established in B cell malignancies. A CD19-41BB–ζ CAR, originally developed by Imai and Campana and clinically applied by June and colleagues, and a CD19-CD28-ζ CAR developed by Brentjens and Sadelain have found particular utility in refractory acute lymphoblastic leukemia and more mature B cell leukemias/lymphomas (Gill and June, 2015; Imai et al., 2004; Brentjens et al., 2013). This has raised the possibility that myeloid leukemias may also be effectively targeted with CAR-modified T cells.
The challenges to extending the success with B cell specific CARs to AML are significant. Except for the initial success of CD19 CARs, other CAR types that have been clinically tested have yet to show utility. Indeed, reports of significant adverse reactions to some CARs, potentially secondary to lineage infidelity, anti-CAR antibody development, and cytokine release syndrome prompt caution and indicate the essential role of CAR design and pre-clinical testing in generating effective receptor structures (Magee and Snook, 2014).
Of foremost importance in CAR design is the recognition domain. AML comprises a diverse array of myeloid cancers, and it is unlikely that a single target will be effective for all subtypes. Moreover, AML is believed to be comprised of a less mature population of cancer stem cells embedded within a more mature leukemic population. Effective therapy will require eradication of these transformed stem cells as well as their more mature descendants.
We and others have focused on CD33 as a CAR target in AML (O’Hear et al., 2015; Pizzitola et al., 2014; Kenderian et al., 2015). Pre-clinical analyses in vitro and in NOD-SCID models support the utility of CD33 CAR-modified T cells. CD33’s widespread expression on leukemic cells and absence of downmodulation after GO treatment indicates that it is a robust target. In a pilot study, a single patient was treated with a CD33-specific CAR (Wang et al., 2015). Suggestions of a beneficial effect were present. Whether leukemic stem cells uniformly express CD33 remains to be established. CD33’s absence from some multipotent myeloid progenitors may limit its effectiveness in some cases of AML (Walter et al., 2012). However, AML stem cell populations remain poorly characterized, and may be fixed at different stages of maturity even within a single subtype of AML. It appears that leukemic stem populations are CD33 positive in some tumors. Several additional AML antigens have been proposed and/or studied as alternatives to CD33, including CD44v6, CD123, and LeY (Ritchie et al., 2013; Gill et al., 2014; Casucci et al., 2013; Pizzitola et al., 2014). Comparative analyses will be necessary to establish the relative utilities of these different targets.
Beyond identifying an optimal target antigen, many of the same obstacles relevant to T cell immunotherapy, will be expected with CAR-modified T cells. This includes the effects of PD-L1, IDO, or other inhibitory molecules in disabling the therapeutic response, and localization and persistence of adoptively transferred therapeutic T cells with AML blasts. In addition, the biology of different chimeric signaling domains remains poorly resolved. Optimal signaling may depend on the tumor environment, coordinate positive and negative signals from tumor cells, and the differentiation status of the infused T cells. In this regards, studies have varied in their identification of optimal therapeutic cell type, though modified naïve or central memory cells may prove more efficacious than effector T cells (Klebanoff et al., 2012; Chan et al., 2015; Terakura et al., 2012).
Conclusions
Fundamental approaches to the treatment of AML have remained largely unchanged over the past several decades. Whereas some improvements in survival have been observed with advances in ancillary care and intensification, a shift in how this often refractory class of tumors is approached is required. Immunotherapy, by attacking the tumor based on its antigenic structure, has the potential to complement current therapies that take advantage of altered tumor biochemistry. Of particular interest are newly developed cellular therapies. Significant progress has been made in the application and redirection of T and NK cells against AML that will be increasingly vetted in patients in upcoming years. These therapies show substantial potential in model systems. Fully realizing their capabilities will entail optimizing their function, minimizing their toxicity, and establishing how conventional and immunologic therapies can be combined in patients with AML.
Reference List
- Alli R, Zhang ZM, Nguyen P, Zheng JJ, Geiger TL. Rational design of T cell receptors with enhanced sensitivity for antigen. PLoS One. 2011;6(3):e18027. doi: 10.1371/journal.pone.0018027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bachanova V, Cooley S, DeFor TE, Verneris MR, Zhang B, McKenna DH, Curtsinger J, Panoskaltsis-Mortari A, Lewis D, Hippen K, McGlave P, Weisdorf DJ, Blazar BR, Miller JS. Clearance of acute myeloid leukemia by haploidentical natural killer cells is improved using IL-2 diphtheria toxin fusion protein. Blood. 2014;123(25):3855–3863. doi: 10.1182/blood-2013-10-532531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker SD, Zimmerman EI, Wang YD, Orwick S, Zatechka DS, Buaboonnam J, Neale GA, Olsen SR, Enemark EJ, Shurtleff S, Rubnitz JE, Mullighan CG, Inaba H. Emergence of polyclonal FLT3 tyrosine kinase domain mutations during sequential therapy with sorafenib and sunitinib in FLT3-ITD-positive acute myeloid leukemia. Clin Cancer Res. 2013;19(20):5758–5768. doi: 10.1158/1078-0432.CCR-13-1323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berthon C, Driss V, Liu J, Kuranda K, Leleu X, Jouy N, Hetuin D, Quesnel B. In acute myeloid leukemia, B7-H1 (PD-L1) protection of blasts from cytotoxic T cells is induced by TLR ligands and interferon-gamma and can be reversed using MEK inhibitors. Cancer Immunol Immunother. 2010;59(12):1839–1849. doi: 10.1007/s00262-010-0909-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blankenstein T, Leisegang M, Uckert W, Schreiber H. Targeting cancer-specific mutations by T cell receptor gene therapy. Curr Opin Immunol. 2015;33C:112–119. doi: 10.1016/j.coi.2015.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brentjens RJ, Davila ML, Riviere I, Park J, Wang X, Cowell LG, Bartido S, Stefanski J, Taylor C, Olszewska M, Borquez-Ojeda O, Qu J, Wasielewska T, He Q, Bernal Y, Rijo IV, Hedvat C, Kobos R, Curran K, Steinherz P, et al. CD19-targeted T cells rapidly induce molecular remissions in adults with chemotherapy-refractory acute lymphoblastic leukemia. Sci Transl Med. 2013;5(177):177ra38. doi: 10.1126/scitranslmed.3005930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bross PF, Beitz J, Chen G, Chen XH, Duffy E, Kieffer L, Roy S, Sridhara R, Rahman A, Williams G, Pazdur R. Approval summary: gemtuzumab ozogamicin in relapsed acute myeloid leukemia. Clin Cancer Res. 2001;7(6):1490–1496. [PubMed] [Google Scholar]
- Burnett AK, Hills RK, Milligan D, Kjeldsen L, Kell J, Russell NH, Yin JA, Hunter A, Goldstone AH, Wheatley K. Identification of patients with acute myeloblastic leukemia who benefit from the addition of gemtuzumab ozogamicin: results of the MRC AML15 trial. J Clin Oncol. 2011;29(4):369–377. doi: 10.1200/JCO.2010.31.4310. [DOI] [PubMed] [Google Scholar]
- Castaigne S, Pautas C, Terre C, Raffoux E, Bordessoule D, Bastie JN, Legrand O, Thomas X, Turlure P, Reman O, de RT, Gastaud L, de GN, Contentin N, Henry E, Marolleau JP, Aljijakli A, Rousselot P, Fenaux P, Preudhomme C, et al. Effect of gemtuzumab ozogamicin on survival of adult patients with de-novo acute myeloid leukaemia (ALFA-0701): a randomised, open-label, phase 3 study. Lancet. 2012;379(9825):1508–1516. doi: 10.1016/S0140-6736(12)60485-1. [DOI] [PubMed] [Google Scholar]
- Casucci M, Nicolis di RB, Falcone L, Camisa B, Norelli M, Genovese P, Gentner B, Gullotta F, Ponzoni M, Bernardi M, Marcatti M, Saudemont A, Bordignon C, Savoldo B, Ciceri F, Naldini L, Dotti G, Bonini C, Bondanza A. CD44v6-targeted T cells mediate potent antitumor effects against acute myeloid leukemia and multiple myeloma. Blood. 2013;122(20):3461–3472. doi: 10.1182/blood-2013-04-493361. [DOI] [PubMed] [Google Scholar]
- Chan WK, Kung SM, Li Y, Zalevsky J, Schell S, Leung W. Antibody-dependent cell-mediated cytotoxicity overcomes NK cell resistance in MLL-rearranged leukemia expressing inhibitory KIR ligands but not activating ligands. Clin Cancer Res. 2012;18(22):6296–6305. doi: 10.1158/1078-0432.CCR-12-0668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan WK, Suwannasaen D, Throm RE, Li Y, Eldridge PW, Houston J, Gray JT, Pui CH, Leung W. Chimeric antigen receptor-redirected CD45RA-negative T cells have potent antileukemia and pathogen memory response without graft-versus-host activity. Leukemia. 2015;29(2):387–395. doi: 10.1038/leu.2014.174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen CJ, Zheng Z, Bray R, Zhao Y, Sherman LA, Rosenberg SA, Morgan RA. Recognition of fresh human tumor by human peripheral blood lymphocytes transduced with a bicistronic retroviral vector encoding a murine anti-p53 TCR. J Immunol. 2005;175(9):5799–5808. doi: 10.4049/jimmunol.175.9.5799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooley S, Weisdorf DJ, Guethlein LA, Klein JP, Wang T, Marsh SG, Spellman S, Haagenson MD, Saeturn K, Ladner M, Trachtenberg E, Parham P, Miller JS. Donor killer cell Ig-like receptor B haplotypes, recipient HLA-C1, and HLA-C mismatch enhance the clinical benefit of unrelated transplantation for acute myelogenous leukemia. J Immunol. 2014;192(10):4592–4600. doi: 10.4049/jimmunol.1302517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Creutzig U, Zimmermann M, Bourquin JP, Dworzak MN, Fleischhack G, Graf N, Klingebiel T, Kremens B, Lehrnbecher T, von NC, Ritter J, Sander A, Schrauder A, von SA, Stary J, Reinhardt D. Randomized trial comparing liposomal daunorubicin with idarubicin as induction for pediatric acute myeloid leukemia: results from Study AML-BFM 2004. Blood. 2013;122(1):37–43. doi: 10.1182/blood-2013-02-484097. [DOI] [PubMed] [Google Scholar]
- Curti A, Pandolfi S, Valzasina B, Aluigi M, Isidori A, Ferri E, Salvestrini V, Bonanno G, Rutella S, Durelli I, Horenstein AL, Fiore F, Massaia M, Colombo MP, Baccarani M, Lemoli RM. Modulation of tryptophan catabolism by human leukemic cells results in the conversion of. Blood. 2007;109(7):2871–2877. doi: 10.1182/blood-2006-07-036863. [DOI] [PubMed] [Google Scholar]
- De VK, Van VE, Lahmar Q, Geeraerts X, De BE, Menu E, Van RI, Vanderkerken K, Van Ginderachter JA. Myeloid-derived suppressor cells as therapeutic target in hematological malignancies. Front Oncol. 2014;4:349. doi: 10.3389/fonc.2014.00349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Distler E, Wolfel C, Kohler S, Nonn M, Kaus N, Schnurer E, Meyer RG, Wehler TC, Huber C, Wolfel T, Hartwig UF, Herr W. Acute myeloid leukemia (AML)-reactive cytotoxic T lymphocyte clones rapidly expanded from CD8(+) CD62L((high)+) T cells of healthy donors prevent AML engraftment in NOD/SCID IL2Rgamma(null) mice. Exp Hematol. 2008;36(4):451–463. doi: 10.1016/j.exphem.2007.12.011. [DOI] [PubMed] [Google Scholar]
- Eshhar Z, Waks T, Gross G, Schindler DG. Specific activation and targeting of cytotoxic lymphocytes through chimeric single chains consisting of antibody-binding domains and the gamma or zeta subunits of the immunoglobulin and T-cell receptors. Proc Natl Acad Sci U S A. 1993;90(2):720–724. doi: 10.1073/pnas.90.2.720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finney HM, Lawson AD, Bebbington CR, Weir AN. Chimeric receptors providing both primary and costimulatory signaling in T cells from a single gene product. J Immunol. 1998;161(6):2791–2797. [PubMed] [Google Scholar]
- Frankel TL, Burns WR, Peng PD, Yu Z, Chinnasamy D, Wargo JA, Zheng Z, Restifo NP, Rosenberg SA, Morgan RA. Both CD4 and CD8 T cells mediate equally effective in vivo tumor treatment when engineered with a highly avid TCR targeting tyrosinase. J Immunol. 2010;184(11):5988–5998. doi: 10.4049/jimmunol.1000189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujisaki H, Kakuda H, Shimasaki N, Imai C, Ma J, Lockey T, Eldridge P, Leung WH, Campana D. Expansion of highly cytotoxic human natural killer cells for cancer cell therapy. Cancer Res. 2009;69(9):4010–4017. doi: 10.1158/0008-5472.CAN-08-3712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gamis AS, Alonzo TA, Meshinchi S, Sung L, Gerbing RB, Raimondi SC, Hirsch BA, Kahwash SB, Heerema-McKenney A, Winter L, Glick K, Davies SM, Byron P, Smith FO, Aplenc R. Gemtuzumab ozogamicin in children and adolescents with De Novo acute myeloid leukemia improves event-free survival by reducing relapse risk: results from the randomized phase III Children’s Oncology Group trial AAML0531. J Clin Oncol. 2014;32(27):3021–3032. doi: 10.1200/JCO.2014.55.3628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gasiorowski RE, Clark GJ, Bradstock K, Hart DN. Antibody therapy for acute myeloid leukaemia. Br J Haematol. 2014;164(4):481–495. doi: 10.1111/bjh.12691. [DOI] [PubMed] [Google Scholar]
- Gattinoni L, Ranganathan A, Surman DR, Palmer DC, Antony PA, Theoret MR, Heimann DM, Rosenberg SA, Restifo NP. CTLA-4 dysregulation of self/tumor-reactive CD8+ T-cell function is CD4+ T-cell dependent. Blood. 2006;108(12):3818–3823. doi: 10.1182/blood-2006-07-034066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geiger TL, Leitenberg D, Flavell RA. The TCR zeta-chain immunoreceptor tyrosine-based activation motifs are sufficient for the activation and differentiation of primary T lymphocytes. J Immunol. 1999;162(10):5931–5939. [PubMed] [Google Scholar]
- Geiger TL, Nguyen P, Leitenberg D, Flavell RA. Integrated src kinase and costimulatory activity enhances signal transduction through single-chain chimeric receptors in T lymphocytes. Blood. 2001;98(8):2364–2371. doi: 10.1182/blood.v98.8.2364. [DOI] [PubMed] [Google Scholar]
- Gill S, June CH. Going viral: chimeric antigen receptor T-cell therapy for hematological malignancies. Immunol Rev. 2015;263(1):68–89. doi: 10.1111/imr.12243. [DOI] [PubMed] [Google Scholar]
- Gill S, Tasian SK, Ruella M, Shestova O, Li Y, Porter DL, Carroll M, Danet-Desnoyers G, Scholler J, Grupp SA, June CH, Kalos M. Preclinical targeting of human acute myeloid leukemia and myeloablation using chimeric antigen receptor-modified T cells. Blood. 2014;123(15):2343–2354. doi: 10.1182/blood-2013-09-529537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gleason MK, Ross JA, Warlick ED, Lund TC, Verneris MR, Wiernik A, Spellman S, Haagenson MD, Lenvik AJ, Litzow MR, Epling-Burnette PK, Blazar BR, Weiner LM, Weisdorf DJ, Vallera DA, Miller JS. CD16xCD33 bispecific killer cell engager (BiKE) activates NK cells against primary MDS and MDSC CD33+ targets. Blood. 2014;123(19):3016–3026. doi: 10.1182/blood-2013-10-533398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greiner J, Ono Y, Hofmann S, Schmitt A, Mehring E, Gotz M, Guillaume P, Dohner K, Mytilineos J, Dohner H, Schmitt M. Mutated regions of nucleophosmin 1 elicit both CD4(+) and CD8(+) T-cell responses in patients with acute myeloid leukemia. Blood. 2012;120(6):1282–1289. doi: 10.1182/blood-2011-11-394395. [DOI] [PubMed] [Google Scholar]
- Gur H, Krauthgamer R, Berrebi A, Klein T, Nagler A, Tabilio A, Martelli MF, Reisner Y. Tolerance induction by megadose hematopoietic progenitor cells: expansion of veto cells by short-term culture of purified human CD34(+) cells. Blood. 2002;99(11):4174–4181. doi: 10.1182/blood.v99.11.4174. [DOI] [PubMed] [Google Scholar]
- Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144(5):646–674. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- Holler PD, Chlewicki LK, Kranz DM. TCRs with high affinity for foreign pMHC show self-reactivity. Nat Immunol. 2003;4(1):55–62. doi: 10.1038/ni863. [DOI] [PubMed] [Google Scholar]
- Horan JT, Alonzo TA, Lyman GH, Gerbing RB, Lange BJ, Ravindranath Y, Becton D, Smith FO, Woods WG. Impact of disease risk on efficacy of matched related bone marrow transplantation for pediatric acute myeloid leukemia: the Children’s Oncology Group. J Clin Oncol. 2008;26(35):5797–5801. doi: 10.1200/JCO.2007.13.5244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hourigan CS, Karp JE. Minimal residual disease in acute myeloid leukaemia. Nat Rev Clin Oncol. 2013;10(8):460–471. doi: 10.1038/nrclinonc.2013.100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imai C, Mihara K, Andreansky M, Nicholson IC, Pui CH, Geiger TL, Campana D. Chimeric receptors with 4-1BB signaling capacity provoke potent cytotoxicity against acute lymphoblastic leukemia. Leukemia. 2004;18(4):676–684. doi: 10.1038/sj.leu.2403302. [DOI] [PubMed] [Google Scholar]
- Johnson LA, Morgan RA, Dudley ME, Cassard L, Yang JC, Hughes MS, Kammula US, Royal RE, Sherry RM, Wunderlich JR, Lee CC, Restifo NP, Schwarz SL, Cogdill AP, Bishop RJ, Kim H, Brewer CC, Rudy SF, VanWaes C, Davis JL, et al. Gene therapy with human and mouse T-cell receptors mediates cancer regression and targets normal tissues expressing cognate antigen. Blood. 2009;114(3):535–546. doi: 10.1182/blood-2009-03-211714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones BS, Lamb LS, Goldman F, Di SA. Improving the safety of cell therapy products by suicide gene transfer. Front Pharmacol. 2014;5:254. doi: 10.3389/fphar.2014.00254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kenderian SS, Ruella M, Shestova O, Klichinsky M, Aikawa V, Morrissette JJ, Scholler J, Song D, Porter DL, Carroll M, June CH, Gill S. CD33 Specific Chimeric Antigen Receptor T Cells Exhibit Potent Preclinical Activity against Human Acute Myeloid Leukemia. Leukemia. 2015 doi: 10.1038/leu.2015.52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klebanoff CA, Gattinoni L, Restifo NP. Sorting through subsets: which T-cell populations mediate highly effective adoptive immunotherapy? J Immunother. 2012;35(9):651–660. doi: 10.1097/CJI.0b013e31827806e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein L, Kyewski B, Allen PM, Hogquist KA. Positive and negative selection of the T cell repertoire: what thymocytes see (and don’t see) Nat Rev Immunol. 2014;14(6):377–391. doi: 10.1038/nri3667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobayashi E, Kishi H, Muraguchi A. A novel system for cloning human TCRs: Cutting short the way to TCR-based anticancer therapy. Oncoimmunology. 2014;3(1):e27258. doi: 10.4161/onci.27258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolb HJ. Graft-versus-leukemia effects of transplantation and donor lymphocytes. Blood. 2008;112(12):4371–4383. doi: 10.1182/blood-2008-03-077974. [DOI] [PubMed] [Google Scholar]
- Kronig H, Kremmler L, Haller B, Englert C, Peschel C, Andreesen R, Blank CU. Interferon-induced programmed death-ligand 1 (PD-L1/B7-H1) expression increases on human acute myeloid leukemia blast cells during treatment. Eur J Haematol. 2014;92(3):195–203. doi: 10.1111/ejh.12228. [DOI] [PubMed] [Google Scholar]
- Krupka C, Kufer P, Kischel R, Zugmaier G, Bogeholz J, Kohnke T, Lichtenegger FS, Schneider S, Metzeler KH, Fiegl M, Spiekermann K, Baeuerle PA, Hiddemann W, Riethmuller G, Subklewe M. CD33 target validation and sustained depletion of AML blasts in long-term cultures by the bispecific T-cell-engaging antibody AMG 330. Blood. 2014;123(3):356–365. doi: 10.1182/blood-2013-08-523548. [DOI] [PubMed] [Google Scholar]
- Kuball J, de BK, Wagner E, Wattad M, Antunes E, Weeratna RD, Vicari AP, Lotz C, van DS, Hol S, Greenberg PD, Heit W, Davis HL, Theobald M. Pitfalls of vaccinations with WT1-, Proteinase3- and MUC1-derived peptides in combination with MontanideISA51 and CpG7909. Cancer Immunol Immunother. 2011;60(2):161–171. doi: 10.1007/s00262-010-0929-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kugler M, Stein C, Kellner C, Mentz K, Saul D, Schwenkert M, Schubert I, Singer H, Oduncu F, Stockmeyer B, Mackensen A, Fey GH. A recombinant trispecific single-chain Fv derivative directed against CD123 and CD33 mediates effective elimination of acute myeloid leukaemia cells by dual targeting. Br J Haematol. 2010;150(5):574–586. doi: 10.1111/j.1365-2141.2010.08300.x. [DOI] [PubMed] [Google Scholar]
- Kung Sutherland MS, Walter RB, Jeffrey SC, Burke PJ, Yu C, Kostner H, Stone I, Ryan MC, Sussman D, Lyon RP, Zeng W, Harrington KH, Klussman K, Westendorf L, Meyer D, Bernstein ID, Senter PD, Benjamin DR, Drachman JG, McEarchern JA. SGN-CD33A: a novel CD33-targeting antibody-drug conjugate using a pyrrolobenzodiazepine dimer is active in models of drug-resistant AML. Blood. 2013;122(8):1455–1463. doi: 10.1182/blood-2013-03-491506. [DOI] [PubMed] [Google Scholar]
- Laszlo GS, Gudgeon CJ, Harrington KH, Dell’Aringa J, Newhall KJ, Means GD, Sinclair AM, Kischel R, Frankel SR, Walter RB. Cellular determinants for preclinical activity of a novel CD33/CD3 bispecific T-cell engager (BiTE) antibody, AMG 330, against human AML. Blood. 2014;123(4):554–561. doi: 10.1182/blood-2013-09-527044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leung W. Infusions of allogeneic natural killer cells as cancer therapy. Clin Cancer Res. 2014;20(13):3390–3400. doi: 10.1158/1078-0432.CCR-13-1766. [DOI] [PubMed] [Google Scholar]
- Leung W, Campana D, Yang J, Pei D, Coustan-Smith E, Gan K, Rubnitz JE, Sandlund JT, Ribeiro RC, Srinivasan A, Hartford C, Triplett BM, Dallas M, Pillai A, Handgretinger R, Laver JH, Pui CH. High success rate of hematopoietic cell transplantation regardless of donor source in children with very high-risk leukemia. Blood. 2011;118(2):223–230. doi: 10.1182/blood-2011-01-333070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leung W, Pui CH, Coustan-Smith E, Yang J, Pei D, Gan K, Srinivasan A, Hartford C, Triplett BM, Dallas M, Pillai A, Shook D, Rubnitz JE, Sandlund JT, Jeha S, Inaba H, Ribeiro RC, Handgretinger R, Laver JH, Campana D. Detectable minimal residual disease before hematopoietic cell transplantation is prognostic but does not preclude cure for children with very-high-risk leukemia. Blood. 2012;120(2):468–472. doi: 10.1182/blood-2012-02-409813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leung WH, Vong QP, Lin W, Janke L, Chen T, Leung W. Modulation of NKG2D ligand expression and metastasis in tumors by spironolactone via RXRgamma activation. J Exp Med. 2013;210(12):2675–2692. doi: 10.1084/jem.20122292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Moysey R, Molloy PE, Vuidepot AL, Mahon T, Baston E, Dunn S, Liddy N, Jacob J, Jakobsen BK, Boulter JM. Directed evolution of human T-cell receptors with picomolar affinities by phage display. Nat Biotechnol. 2005;23(3):349–354. doi: 10.1038/nbt1070. [DOI] [PubMed] [Google Scholar]
- Lichtenegger FS, Lorenz R, Gellhaus K, Hiddemann W, Beck B, Subklewe M. Impaired NK cells and increased T regulatory cell numbers during cytotoxic maintenance therapy in AML. Leuk Res. 2014;38(8):964–969. doi: 10.1016/j.leukres.2014.05.014. [DOI] [PubMed] [Google Scholar]
- Ma Q, Wang C, Jones D, Quintanilla KE, Li D, Wang Y, Wieder ED, Clise-Dwyer K, Alatrash G, Mj Y, Munsell MF, Lu S, Qazilbash MH, Molldrem JJ. Adoptive transfer of PR1 cytotoxic T lymphocytes associated with reduced leukemia burden in a mouse acute myeloid leukemia xenograft model. Cytotherapy. 2010;12(8):1056–1062. doi: 10.3109/14653249.2010.506506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magee MS, Snook AE. Challenges to chimeric antigen receptor (CAR)-T cell therapy for cancer. Discov Med. 2014;18(100):265–271. [PMC free article] [PubMed] [Google Scholar]
- Miller JS, Soignier Y, Panoskaltsis-Mortari A, McNearney SA, Yun GH, Fautsch SK, McKenna D, Le C, DeFor TE, Burns LJ, Orchard PJ, Blazar BR, Wagner JE, Slungaard A, Weisdorf DJ, Okazaki IJ, McGlave PB. Successful adoptive transfer and in vivo expansion of human haploidentical NK cells in patients with cancer. Blood. 2005;105(8):3051–3057. doi: 10.1182/blood-2004-07-2974. [DOI] [PubMed] [Google Scholar]
- Montagna D, Maccario R, Locatelli F, Montini E, Pagani S, Bonetti F, Daudt L, Turin I, Lisini D, Garavaglia C, Dellabona P, Casorati G. Emergence of antitumor cytolytic T cells is associated with maintenance of hematologic remission in children with acute myeloid leukemia. Blood. 2006;108(12):3843–3850. doi: 10.1182/blood-2006-05-021535. [DOI] [PubMed] [Google Scholar]
- Morgan RA, Chinnasamy N, Abate-Daga D, Gros A, Robbins PF, Zheng Z, Dudley ME, Feldman SA, Yang JC, Sherry RM, Phan GQ, Hughes MS, Kammula US, Miller AD, Hessman CJ, Stewart AA, Restifo NP, Quezado MM, Alimchandani M, Rosenberg AZ, et al. Cancer regression and neurological toxicity following anti-MAGE-A3 TCR gene therapy. J Immunother. 2013;36(2):133–151. doi: 10.1097/CJI.0b013e3182829903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niewerth D, Creutzig U, Bierings MB, Kaspers GJ. A review on allogeneic stem cell transplantation for newly diagnosed pediatric acute myeloid leukemia. Blood. 2010;116(13):2205–2214. doi: 10.1182/blood-2010-01-261800. [DOI] [PubMed] [Google Scholar]
- Norde WJ, Overes IM, Maas F, Fredrix H, Vos JC, Kester MG, van d V, Jedema I, Falkenburg JH, Schattenberg AV, de Witte TM, Dolstra H. Myeloid leukemic progenitor cells can be specifically targeted by minor histocompatibility antigen LRH-1-reactive cytotoxic T cells. Blood. 2009;113(10):2312–2323. doi: 10.1182/blood-2008-04-153825. [DOI] [PubMed] [Google Scholar]
- O’Hear C, Heiber JF, Schubert I, Fey G, Geiger TL. Anti-CD33 chimeric antigen receptor targeting of acute myeloid leukemia. Haematologica. 2015;100(3):336–344. doi: 10.3324/haematol.2014.112748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohta A, Sitkovsky M. Extracellular adenosine-mediated modulation of regulatory T cells. Front Immunol. 2014;5:304. doi: 10.3389/fimmu.2014.00304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parkhurst MR, Yang JC, Langan RC, Dudley ME, Nathan DA, Feldman SA, Davis JL, Morgan RA, Merino MJ, Sherry RM, Hughes MS, Kammula US, Phan GQ, Lim RM, Wank SA, Restifo NP, Robbins PF, Laurencot CM, Rosenberg SA. T cells targeting carcinoembryonic antigen can mediate regression of metastatic colorectal cancer but induce severe transient colitis. Mol Ther. 2011;19(3):620–626. doi: 10.1038/mt.2010.272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perel Y, Auvrignon A, Leblanc T, Vannier JP, Michel G, Nelken B, Gandemer V, Schmitt C, Lamagnere JP, de Lumley L, Bader-Meunier B, Couillaud G, Schaison G, Landman-Parker J, Thuret I, Dalle JH, Baruchel A, Leverger G. Impact of addition of maintenance therapy to intensive induction and consolidation chemotherapy for childhood acute myeloblastic leukemia: results of a prospective randomized trial, LAME 89/91. Leucamie Aique Myeloide Enfant. J Clin Oncol. 2002;20(12):2774–2782. doi: 10.1200/JCO.2002.07.300. [DOI] [PubMed] [Google Scholar]
- Petersdorf SH, Kopecky KJ, Slovak M, Willman C, Nevill T, Brandwein J, Larson RA, Erba HP, Stiff PJ, Stuart RK, Walter RB, Tallman MS, Stenke L, Appelbaum FR. A phase 3 study of gemtuzumab ozogamicin during induction and postconsolidation therapy in younger patients with acute myeloid leukemia. Blood. 2013;121(24):4854–4860. doi: 10.1182/blood-2013-01-466706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierce BG, Hellman LM, Hossain M, Singh NK, Vander Kooi CW, Weng Z, Baker BM. Computational design of the affinity and specificity of a therapeutic T cell receptor. PLoS Comput Biol. 2014;10(2):e1003478. doi: 10.1371/journal.pcbi.1003478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pizzitola I, Anjos-Afonso F, Rouault-Pierre K, Lassailly F, Tettamanti S, Spinelli O, Biondi A, Biagi E, Bonnet D. Chimeric antigen receptors against CD33/CD123 antigens efficiently target primary acute myeloid leukemia cells in vivo. Leukemia. 2014;28(8):1596–1605. doi: 10.1038/leu.2014.62. [DOI] [PubMed] [Google Scholar]
- Rezvani K, Yong AS, Mielke S, Savani BN, Jafarpour B, Eniafe R, Le RQ, Musse L, Boss C, Childs R, John BA. Lymphodepletion is permissive to the development of spontaneous T-cell responses to the self-antigen PR1 early after allogeneic stem cell transplantation and in patients with acute myeloid leukemia undergoing WT1 peptide vaccination following chemotherapy. Cancer Immunol Immunother. 2012;61(7):1125–1136. doi: 10.1007/s00262-011-1187-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ritchie DS, Neeson PJ, Khot A, Peinert S, Tai T, Tainton K, Chen K, Shin M, Wall DM, Honemann D, Gambell P, Westerman DA, Haurat J, Westwood JA, Scott AM, Kravets L, Dickinson M, Trapani JA, Smyth MJ, Darcy PK, et al. Persistence and efficacy of second generation CAR T cell against the LeY antigen in acute myeloid leukemia. Mol Ther. 2013;21(11):2122–2129. doi: 10.1038/mt.2013.154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robbins PF, Morgan RA, Feldman SA, Yang JC, Sherry RM, Dudley ME, Wunderlich JR, Nahvi AV, Helman LJ, Mackall CL, Kammula US, Hughes MS, Restifo NP, Raffeld M, Lee CC, Levy CL, Li YF, El-Gamil M, Schwarz SL, Laurencot C, et al. Tumor regression in patients with metastatic synovial cell sarcoma and melanoma using genetically engineered lymphocytes reactive with NY-ESO-1. J Clin Oncol. 2011;29(7):917–924. doi: 10.1200/JCO.2010.32.2537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowland SL, Tuttle K, Torres RM, Pelanda R. Antigen and cytokine receptor signals guide the development of the naive mature B cell repertoire. Immunol Res. 2013;55(1–3):231–240. doi: 10.1007/s12026-012-8366-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubnitz JE, Inaba H, Dahl G, Ribeiro RC, Bowman WP, Taub J, Pounds S, Razzouk BI, Lacayo NJ, Cao X, Meshinchi S, Degar B, Airewele G, Raimondi SC, Onciu M, Coustan-Smith E, Downing JR, Leung W, Pui CH, Campana D. Minimal residual disease-directed therapy for childhood acute myeloid leukaemia: results of the AML02 multicentre trial. Lancet Oncol. 2010a;11:543–552. doi: 10.1016/S1470-2045(10)70090-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubnitz JE, Inaba H, Ribeiro RC, Pounds S, Rooney B, Bell T, Pui CH, Leung W. NKAML: a pilot study to determine the safety and feasibility of haploidentical natural killer cell transplantation in childhood acute myeloid leukemia. J Clin Oncol. 2010b;28(6):955–959. doi: 10.1200/JCO.2009.24.4590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruggeri L, Capanni M, Urbani E, Perruccio K, Shlomchik WD, Tosti A, Posati S, Rogaia D, Frassoni F, Aversa F, Martelli MF, Velardi A. Effectiveness of donor natural killer cell alloreactivity in mismatched hematopoietic transplants. Science. 2002;295(5562):2097–2100. doi: 10.1126/science.1068440. [DOI] [PubMed] [Google Scholar]
- Sadelain M, Brentjens R, Riviere I. The basic principles of chimeric antigen receptor design. Cancer Discov. 2013;3(4):388–398. doi: 10.1158/2159-8290.CD-12-0548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmitt M, Casalegno-Garduno R, Xu X, Schmitt A. Peptide vaccines for patients with acute myeloid leukemia. Expert Rev Vaccines. 2009;8(10):1415–1425. doi: 10.1586/erv.09.90. [DOI] [PubMed] [Google Scholar]
- Schmitt TM, Aggen DH, Stromnes IM, Dossett ML, Richman SA, Kranz DM, Greenberg PD. Enhanced-affinity murine T-cell receptors for tumor/self-antigens can be safe in gene therapy despite surpassing the threshold for thymic selection. Blood. 2013;122(3):348–356. doi: 10.1182/blood-2013-01-478164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singer H, Kellner C, Lanig H, Aigner M, Stockmeyer B, Oduncu F, Schwemmlein M, Stein C, Mentz K, Mackensen A, Fey GH. Effective elimination of acute myeloid leukemic cells by recombinant bispecific antibody derivatives directed against CD33 and CD16. J Immunother. 2010;33(6):599–608. doi: 10.1097/CJI.0b013e3181dda225. [DOI] [PubMed] [Google Scholar]
- Spranger S, Jeremias I, Wilde S, Leisegang M, Starck L, Mosetter B, Uckert W, Heemskerk MH, Schendel DJ, Frankenberger B. TCR-transgenic lymphocytes specific for HMMR/Rhamm limit tumor outgrowth in vivo. Blood. 2012;119(15):3440–3449. doi: 10.1182/blood-2011-06-357939. [DOI] [PubMed] [Google Scholar]
- Staffas A, Kanduri M, Hovland R, Rosenquist R, Ommen HB, Abrahamsson J, Forestier E, Jahnukainen K, Jonsson OG, Zeller B, Palle J, Lonnerholm G, Hasle H, Palmqvist L, Ehrencrona H. Presence of FLT3-ITD and high BAALC expression are independent prognostic markers in childhood acute myeloid leukemia. Blood. 2011;118(22):5905–5913. doi: 10.1182/blood-2011-05-353185. [DOI] [PubMed] [Google Scholar]
- Stauss HJ, Thomas S, Cesco-Gaspere M, Hart DP, Xue SA, Holler A, King J, Wright G, Perro M, Pospori C, Morris E. WT1-specific T cell receptor gene therapy: improving TCR function in transduced T cells. Blood Cells Mol Dis. 2008;40(1):113–116. doi: 10.1016/j.bcmd.2007.06.018. [DOI] [PubMed] [Google Scholar]
- Stone JD, Chervin AS, Kranz DM. T-cell receptor binding affinities and kinetics: impact on T-cell activity and specificity. Immunology. 2009;126(2):165–176. doi: 10.1111/j.1365-2567.2008.03015.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stone JD, Kranz DM. Role of T cell receptor affinity in the efficacy and specificity of adoptive T cell therapies. Front Immunol. 2013;4:244. doi: 10.3389/fimmu.2013.00244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stromnes IM, Schmitt TM, Chapuis AG, Hingorani SR, Greenberg PD. Re-adapting T cells for cancer therapy: from mouse models to clinical trials. Immunol Rev. 2014;257(1):145–164. doi: 10.1111/imr.12141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terakura S, Yamamoto TN, Gardner RA, Turtle CJ, Jensen MC, Riddell SR. Generation of CD19-chimeric antigen receptor modified CD8+ T cells derived from virus-specific central memory T cells. Blood. 2012;119(1):72–82. doi: 10.1182/blood-2011-07-366419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Udyavar A, Alli R, Nguyen P, Baker L, Geiger TL. Subtle affinity-enhancing mutations in a myelin oligodendrocyte glycoprotein-specific TCR alter specificity and generate new self-reactivity. J Immunol. 2009;182(7):4439–4447. doi: 10.4049/jimmunol.0804377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Udyavar A, Geiger TL. Rebalancing immune specificity and function in cancer by T-cell receptor gene therapy. Arch Immunol Ther Exp (Warsz ) 2010;58(5):335–346. doi: 10.1007/s00005-010-0090-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ustun C, Miller JS, Munn DH, Weisdorf DJ, Blazar BR. Regulatory T cells in acute myelogenous leukemia: is it time for immunomodulation? Blood. 2011;118(19):5084–5095. doi: 10.1182/blood-2011-07-365817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uttenthal B, Martinez-Davila I, Ivey A, Craddock C, Chen F, Virchis A, Kottaridis P, Grimwade D, Khwaja A, Stauss H, Morris EC. Wilms’ Tumour 1 (WT1) peptide vaccination in patients with acute myeloid leukaemia induces short-lived WT1-specific immune responses. Br J Haematol. 2014;164(3):366–375. doi: 10.1111/bjh.12637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Tendeloo VF, Van d V, Van DA, Cools N, Anguille S, Ladell K, Gostick E, Vermeulen K, Pieters K, Nijs G, Stein B, Smits EL, Schroyens WA, Gadisseur AP, Vrelust I, Jorens PG, Goossens H, de Vries IJ, Price DA, Oji Y, et al. Induction of complete and molecular remissions in acute myeloid leukemia by Wilms’ tumor 1 antigen-targeted dendritic cell vaccination. Proc Natl Acad Sci U S A. 2010;107(31):13824–13829. doi: 10.1073/pnas.1008051107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Velardi A, Ruggeri L, Mancusi A. Killer-cell immunoglobulin-like receptors reactivity and outcome of stem cell transplant. Curr Opin Hematol. 2012;19(4):319–323. doi: 10.1097/MOH.0b013e32835423c3. [DOI] [PubMed] [Google Scholar]
- Venstrom JM, Pittari G, Gooley TA, Chewning JH, Spellman S, Haagenson M, Gallagher MM, Malkki M, Petersdorf E, Dupont B, Hsu KC. HLA-C-dependent prevention of leukemia relapse by donor activating KIR2DS1. N Engl J Med. 2012;367(9):805–816. doi: 10.1056/NEJMoa1200503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vey N, Bourhis JH, Boissel N, Bordessoule D, Prebet T, Charbonnier A, Etienne A, Andre P, Romagne F, Benson D, Dombret H, Olive D. A phase 1 trial of the anti-inhibitory KIR mAb IPH2101 for AML in complete remission. Blood. 2012;120(22):4317–4323. doi: 10.1182/blood-2012-06-437558. [DOI] [PubMed] [Google Scholar]
- Walter RB, Appelbaum FR, Estey EH, Bernstein ID. Acute myeloid leukemia stem cells and CD33-targeted immunotherapy. Blood. 2012;119(26):6198–6208. doi: 10.1182/blood-2011-11-325050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang QS, Wang Y, Lv HY, Han QW, Fan H, Guo B, Wang LL, Han WD. Treatment of CD33-directed chimeric antigen receptor-modified T cells in one patient with relapsed and refractory acute myeloid leukemia. Mol Ther. 2015;23(1):184–191. doi: 10.1038/mt.2014.164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber KS, Donermeyer DL, Allen PM, Kranz DM. Class II-restricted T cell receptor engineered in vitro for higher affinity retains peptide specificity and function. Proc Natl Acad Sci U S A. 2005;102(52):19033–19038. doi: 10.1073/pnas.0507554102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhong S, Malecek K, Johnson LA, Yu Z, Vega-Saenz de ME, Darvishian F, McGary K, Huang K, Boyer J, Corse E, Shao Y, Rosenberg SA, Restifo NP, Osman I, Krogsgaard M. T-cell receptor affinity and avidity defines antitumor response and autoimmunity in T-cell immunotherapy. Proc Natl Acad Sci U S A. 2013;110(17):6973–6978. doi: 10.1073/pnas.1221609110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmerman EI, Turner DC, Buaboonnam J, Hu S, Orwick S, Roberts MS, Janke LJ, Ramachandran A, Stewart CF, Inaba H, Baker SD. Crenolanib is active against models of drug-resistant FLT3-ITD-positive acute myeloid leukemia. Blood. 2013;122(22):3607–3615. doi: 10.1182/blood-2013-07-513044. [DOI] [PMC free article] [PubMed] [Google Scholar]