Abstract
Current anticoagulation strategies do not eliminate thromboembolic stroke or limb loss during neonatal extracorporeal membrane oxygenation (ECMO), a form of cardiopulmonary bypass (CPB). In adults, CPB surgery generates prothrombotic platelet-derived microparticles (PMPs), submicron membrane vesicles released from activated platelets. However, information on PMP generation in neonatal ECMO systems is lacking. The objective of this study was to compare PMP generation in five different neonatal ECMO systems, using a simulated circuit with swine blood at 300 ml/min for 4 hours. Systems were composed of both newer components (centrifugal pump and hollow-fiber oxygenator) and traditional components (roller-head pump and silicone membrane oxygenator). Free plasma hemoglobin levels were measured as an indicator of hemolysis and flow cytometry-measured PMP. Hemolysis generated in all ECMO systems was similar to that observed in noncirculated static blood (p = 0.48). There was no difference in net PMP levels between different oxygenators with a given pump. In contrast, net PMP generation in ECMO systems with a centrifugal pump was at least 2.5 times greater than in roller-head pump systems. This was significant when using either a hollow-fiber (p < 0.005) or a silicone membrane (p < 0.05) oxygenator. Future studies are needed to define the relationship between pump-generated PMP and thrombosis.
Keywords: cardiopulmonary bypass, ECMO, flow cytometry, cell-derived microparticles, blood platelets, hemolysis
Over the past 60 years, support by cardiopulmonary bypass (CPB) systems has decreased the mortality and morbidity of children, especially those who require surgery for life-threatening anatomical or heart defects.1,2 Cardiopulmonary bypass support outside the operating room, also known as extracorporeal membrane oxygenation (ECMO), has led to a decrease in mortality for children with severe cardiac or respiratory failure.3,4 Although thousands of neonates are supported with ECMO every year, frequent thromboembolic complications result in significant mortality, neurological morbidity, and loss of limb.5,6 A 2012 survey of ECMO centers revealed that more than 50% of patients supported on ECMO develop a clot in the circuit and 7% develop a thrombus in the patient despite routine anticoagulation.7 Mechanisms that promote thrombosis within a neonatal ECMO system remain unclear.
A neonatal ECMO system consists of two primary components: a blood pump that assists circulation and an oxygenator that assists gas exchange. The extracorporeal blood pump increases the flow rate (ml/min) of circulating blood, which in turn increases the amount of wall shear stress. This shear stress induces platelet activation, which induces rapid and significant thrombocytopenia, increased platelet aggregation, and increased soluble P-selectin concentrations in the plasma.8,9 Previous work by this lab documented no difference in hemolysis or platelet aggregation in an in vitro neonatal ECMO model, using several different combinations of neonatal pumps and oxygenators.10 Moreover, therapies directed toward the inhibition of shear-induced platelet activation have not changed the incidence of thrombus formation.
Recent research has documented that activated platelets may promote thrombosis by the release of platelet-derived microparticles (PMPs).11 Microparticles are small cell-derived membrane vesicles, between 0.1 and 1 micron, which may mediate vascular injury and promote prothrombotic and proinflammatory conditions.11–13 Several studies have described an increased amount of prothrombotic PMPs from adult patients supported by CPB.9,14–16 No studies have examined the generation of PMP in neonatal ECMO systems. The objective of this study was to examine the PMP generated in several different combinations of neonatal pumps and oxygenators, using the latest technology (e.g., centrifugal pump) with more traditional components (e.g., roller-head pump).
Materials and Methods
Five different combinations of neonatal ECMO systems were tested (Figure 1). The systems included the Jostra Rotaflow centrifugal pump (Maquet, Wayne, NJ), the Jostra HL-20 roller pump (Maquet), the Quadrox-D hollow-fiber oxygenator (Maquet), Quadrox-iD pediatric oxygenator (Maquet), and the 0800 ECMO silicone membrane oxygenator (Medtronic, Minneapolis, MN). The five ECMO systems and static blood control are Static (S), Roller-head pump + Silicone Oxygenator (RS), Centrifugal Pump + Silicone Oxygenator (CS), Roller-head pump + Quadrox Oxygenator (RQ), Centrifugal + Quadrox Oxygenator (CQ), and Centrifugal + Pediatric Oxygenator (CPQ). All of the ECMO systems were primed with single donor-derived porcine whole blood, collected by venipuncture in citrate-phosphate-dextrose (LAMPIRE Biologics, Pipersville, PA). Blood was anticoagulated with four additional IU/ml of porcine heparin (Baxter Healthcare, Deerfield, IL). For each experimental run (n = 3) of four different ECMO systems, the primed blood, from a single mature donor, was less than 48 hours old. Immature piglet whole blood was not used as prime because the amount needed to simultaneously fill four neonatal ECMO circuits. A small amount of primed circuit blood, removed before circulation, served as the static control. The static blood was incubated in a water bath at 38°C, the nominal temperature for swine, for the entire duration of the experiment. In separate set of experiments (n = 4), the same methods tested the CPQ system. The Children’s National Medical Center (CNMC) Institutional Animal Care and Use Committee approved all of the studies.
Figure 1.

Neonatal in vitro ECMO model. A graphical depiction of a test circuit adapted from a previous study, which includes each of the in vitro circuit components that include the reservoir bag, pump, and oxygenator. Blood is drained from the reservoir bag, into the pump, pushed through the oxygenator, across an arterial catheter, and returns to the reservoir bag. To simulate the arterial resistance of an actual patient, an arterial catheter connected the oxygenator to the blood reservoir.
Experimental Procedure
The in vitro model used quarter-inch heparin-coated tubing (Medtronic) to connect the pump, oxygenator, and reservoir bag. As per CNMC hospital protocol, the roller-pump raceway used quarter-inch Supertygon tubing.17 A Gish reservoir bag (Medtronic) was the venous reservoir. A neonatal arterial catheter maintained a nominal 240 ± 25 mm Hg arterial pressure by restricting the outflow tubing for four of the ECMO systems. The CPQ system had higher pressures at 310 ± 7 mm Hg. There was no venous catheter from the blood reservoir to the pump inlet because previous studies have documented that the shear stress generated in large cannulas at neonatal flow rates is negligible.18 Each roller pump was properly calibrated before the experimental run, according to previously published procedures and the CNMC hospital protocol.19 A mixture of oxygen and room air ventilated each ECMO system to keep the pH, PCO2, and PO2 within normal range. Blood circulated for 4 hours at a flow rate of 300 ml/min (standard support for a 3 kg neonate at roughly 2/3 cardiac index). A flow meter (Transonic Systems, Ithaca, NY) validated the set flow rate of 300 ml/min for all of the ECMO systems. To measure hematocrit and free plasma hemoglobin (fPH), blood samples were drawn from each system and the static blood at baseline, 30, 60, 120, 180, and 240 minutes of circulation. Platelet-derived microparticles were assessed in a blood sample from each system and the static blood at baseline, 120, and 240 minutes of circulation.
Laboratory Assays
Whole blood flow cytometry, as previously described, quantified PMP concentration.20,21 In brief, aliquots (5 μl) of heparinized whole blood removed from the circuit or the static control were immediately diluted with 1 ml of flow cytometry staining buffer solution (eBioscience, San Diego, CA). The diluted whole blood was then incubated with a saturating concentration of fluorescein isothiocyanate (FITC)-labeled anti-porcine CD61 (Clone: JM2E5, AbD Serotec, Kidlington, UK) for 20 minutes in the dark at room temperature. The CD61 antibody recognizes and immunoprecipitates the GPIIb/IIIa surface glycoprotein on porcine platelets.22 After incubation, a single low speed wash (300g for 10 minutes) removed nonspecific antibody. Samples were analyzed in a multichannel FACSCalibur flow cytometer (BD Bioscience, San Jose, CA). In parallel, a FITC-mouse IgG1 isotype control (BD Bioscience) evaluated nonspecific binding. A fluorescent flow cytometric gate was set to acquire 35,000 cell events in the platelet population. Forward scatter height (FSC-H) defined PMP size by a set size gate, created by a blend of monodisperse fluorescent size beads (Megamix, BioCytex, Marseille, France) of three diameters 0.5, 0.9, and 3 μm. PMPs were defined as within the sizes of 0.5 and 0.9 μm beads and within a significant fluorescent threshold consistent with bound CD61 antibody when compared with the isotype control.
A previously described spectrophotometric assay, modified for porcine blood, was used to detect fPH.23 Drabkin’s reagent (Sigma, Saint Louis, MO) converted all common forms of hemoglobin to cyanmethemoglobin for detection at 550 nm (Molecular Devices, Sunnyvale, CA). Hematocrit and fPH were then used to determine the normalized index of hemolysis (NIH), an industry standard for in vitro system evaluation.24
Statistical Methods
The primary outcomes were longitudinal data for PMP, fPH, and net PMP production by ECMO systems summarized by the linear trapezoid method to calculate the area under the curve (net PMP). The net PMP was computed as the area above baseline (time = 0) and is representative of the net accumulation over time. PMP concentration and net PMP were log-transformed to approximate a Gaussian distribution. For the longitudinal analysis, a mixed model of PMP/fPH was used with time and ECMO system as covariates with a system by time interaction and blood sample time as a random intercept that accounted for the correlation within and between ECMO systems that use the same blood sample. Another mixed model assessed the potential longitudinal dependence of PMP on fPH within the same sample and time. This model determined whether higher fPH correlated with PMP production independently of time. A mixed model also assessed the net PMP values with ECMO system as a covariate and blood sample number as a random intercept to account for correlation between ECMO systems that used the same blood sample. Adjustments were made for multiple comparisons. The NIH was calculated for each ECMO system and sample, and then analyzed using a mixed model to estimate the influence of ECMO system on NIH. All analyses were performed on the R system for statistical computing v3+ (R Foundation, Vienna, Austria). All testing was two-sided with a significance level of alpha = 0.05 with results presented as mean ± standard error (SE). The mixed models were fit with the lme4 and lmerTest R packages.
Results
Hematocrit did not significantly change in the static blood or in samples derived from all five of the ECMO systems during circulation (p = 0.13). The average hematocrit in the static sample was 40 ± 1% (mean ± SE, n = 3 independent experiments). All systems started with a similar baseline amount of fPH, which then progressively increased over time in four ECMO systems and in the static blood (Figure 2). In systems with a silicone membrane oxygenator, the rate of fPH generation was 0.90 ± 0.04 and 1.08 ± 0.02 mg/dl/hr for roller-head (RS) and centrifugal pump (CS), respectively (mean ± SE, n = 3 independent experiments). In systems with the Quadrox-D hollow-fiber oxygenator, the rate of fPH generation was 0.64 ± 0.05 and 1.07 ± 0.05 for roller-head (RQ) and centrifugal pump (CQ), respectively (mean ± SE, n = 3 independent experiments). However, when comparing each system or the static sample, there was no significant difference in fPH generated over time (p = 0.48). The NIH is a standardized measure of biomedical device hemolysis calculated from change in fPH, circulation time, hematocrit, volume of the circuit (600 ml), and a set flow rate of 300 ml/min. The roller-head pump and silicone oxygenator ECMO system (RS) generated the largest NIH at 0.57 ± 0.25 g/L (mean ± SE, n = 3 independent samples, Figure 3). However, there were no significant differences in the NIH for any of the neonatal ECMO systems (p = 0.21).
Figure 2.

Effect of neonatal ECMO systems on free plasma hemoglobin (fPH) levels. A: Silicone oxygenator systems. B: Hollow-fiber oxygenator systems. Data represent the mean ± SE (n = 3 independent experiments).
Figure 3.
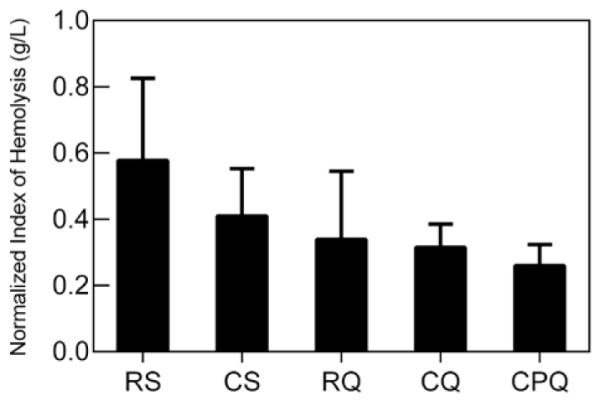
Effect of neonatal ECMO systems on the normalized index of hemolysis (NIH). NIH values of each neonatal ECMO system in g/L. Data represent the mean ± SE (n = 3 independent experiments) except in CPQ system (n = 4 independent experiments). CPQ, Centrifugal + Pediatric Oxygenator.
Platelet-derived microparticles progressively increased during circulation in four of the ECMO systems and static blood (Figure 4). PMP levels were similar in four of the ECMO systems and static blood at baseline. Systems that included a centrifugal pump generated a greater amount of PMP in comparison with systems with a roller pump. In systems with a silicone membrane oxygenator, the rate of PMP generation was 651 ± 22 and 2348 ± 160 PMP/μl/hr for RS and CS, respectively. In systems with a hollow-fiber oxygenator, the rate of PMP generation was 305 ± 128 and 2383 ± 214 for RQ and CQ, respectively. To compare different systems, net PMP was calculated (Table 1). There were no differences in net PMP generation between different oxygenators with a given pump, such as roller-head (p = 0.13) and centrifugal (p = 0.75). in contrast, ECMO systems with a centrifugal pump generated at least 2.5 times more net PMP than roller-head pump systems (Table 2). This was significantly increased with either a hollow-fiber (p < 0.005) or a silicone membrane (p < 0.05) oxygenator. When compared with the static control, net PMP generated by the CQ system was different (p < 0.006); however, the net PMP generated by RQ was not (p = 0.91).
Figure 4.

Effect of neonatal ECMO systems on the generation of platelet-derived microparticles (PMPs). A: Silicone oxygenator systems. B: Hollow-fiber oxygenator systems. Data represent the mean ± SE (n = 3 independent experiments).
Table 1.
Periodic and Total Platelet-Derived Microparticle Concentrations (MP/μl × 103) for the In Vitro Neonatal ECMO System Over Time
| ECMO System |
0 minute |
120 minutes |
240 minutes |
Net PMP |
|||||
|---|---|---|---|---|---|---|---|---|---|
| Mean | SEM | Mean | SEM | Mean | SEM | Mean | SEM | p Value* | |
| Static | 3.02 | 0.22 | 3.92 | 1.05 | 4.30 | 0.46 | 184.23 | 66.49 | x |
| RS | 2.54 | 0.22 | 3.97 | 0.92 | 5.14 | 0.65 | 329.00 | 62.87 | 0.119 |
| CS† | 3.80 | 1.28 | 9.45 | 3.34 | 13.19 | 5.58 | 1244.33 | 286.75 | 0.005 |
| RQ | 3.59 | 1.11 | 3.43 | 0.71 | 4.82 | 1.42 | 84.45 | 8.87 | 0.910 |
| CQ† | 2.92 | 0.23 | 8.97 | 2.36 | 12.45 | 3.73 | 1297.00 | 267.85 | 0.003 |
| CPQ† | 8.39 | 2.05 | 20.66 | 7.86 | 25.74 | 8.20 | 2040.00 | 616.60 | 0.006 |
p value represents the difference in net PMP of the system and the static blood control.
Statistically significant difference when compared with static (p < 0.01).
Table 2.
Significant Differences in Net Platelet-Derived Microparticle Generation (Net PMP) Between Neonatal ECMO Systems
| Roller-Head Pump |
|||
|---|---|---|---|
| Silicone | Quadrox | ||
| Centrifugal pump |
Silicone | (0.00, 1.00) 0.05 |
(0.33, 1.47) 0.0072 |
| Quadrox | (0.07, 1.07) 0.031 |
(0.40, 1.54) 0.005 |
|
| Pediatric Quadrox |
(0.08, 1.50) 0.033 |
(0.46, 1.93) 0.005 |
|
Comparison 95% confidence intervals and p values between centrifiugal pump neonatal and roller-head pump ECMO systems. A mixed statistical model evaluated the effect of ECMO system, blood sample, and time to define comparisons of net PMP between each ECMO system.
PMP, platelet-derived microparticle generation.
In separate experiments, the centrifugal pump with a pediatric Quadrox oxygenator ECMO system (CPQ) was tested (mean ± SE, n = 4 independent experiments). Similar to previous experiments, fPH increased in the CPQ and the static blood control 1.11 ± 0.06 and 0.59 ± 0.05 mg/dl/hr, respectively (p = 0.48). The centrifugal pump and pediatric Quadrox oxygenator ECMO system (CPQ) generated the smallest NIH level, although not significant from other systems (p = 0.21). PMP increased in the CPQ system compared with the static control at 4337 ± 517 and 318 ± 38 PMP/μl/hr, respectively (p = 0.02). This system also generated the greatest net PMP at 203 ± 123 × 103 PMP/μl. In summary, when a neonatal ECMO system included a centrifugal pump, net PMP generation was 2.5 times greater than roller-head pump systems. This difference occurs early at 120 minutes of perfusion (Figure 4).
Discussion
There is a growing need for ECMO support for neonates who suffer from heart failure, especially those who are postoperative from congenital cardiac surgery. Neonates who need cardiac ECMO support have a high rate of life-threatening thrombosis, between 10% and 33%.7 Current laboratory tests such as prothrombin time, partial thromboplastin time, platelet count, fibrinogen level, activated clotting time, or heparin dose are poor predictors of these complications.5 Several alternatives can assess hemostasis on ECMO including thrombin generation, antifactor Xa activity, heparin levels, antithrombin levels, and thromboelastography, but use of these assays has not proven to reduce thrombotic complications for neonatal patients on ECMO. Additional studies are required to explore the biological mechanisms that lead to ECMO-induced thrombosis a condition that can result in stroke, loss of limb, or death.
The American Society for Testing Materials international standard for continuous flow blood pumps states that blood trauma caused by a pump (e.g., roller pump or centrifugal pump) is best assessed by the amount of hemolysis generated over time.24 Hemolysis is a predictor of ECMO-induced renal failure and subsequent mortality.25 However, advancements in pump design and materials have reduced this risk significantly. The results in this study confirm no significant differences in fPH or NIH in any of the five neonatal ECMO circuits over time. Instead, this study documents an increase in pump-dependent PMP concentration, which may be representative marker of blood trauma caused by a pump.
It has been proposed that the amount of deformability time experienced by a circulating cell correlates to the amount of microparticles produced.26 Centrifugal pumps work by reverse vortex that applies a dynamic shear stress, which deforms cells. Roller-head pumps work by peristaltic action that applies a constant shear stress. Engineering models of continuous flow blood pumps have shown that dynamic in comparison with constant shear stress promoted a larger amount of platelet activation.27 The dynamic nature of the centrifugal pump may lead to a greater increase in platelet activation that over time generates a greater net PMP. The increase in revolutions per minute of the centrifugal pump in comparison with the roller-head pump may create larger amounts of PMP with each pass through the pump.
Various pediatric conditions have increased concentrations of PMP including cyanotic congenital heart disease, sepsis, and prematurity.28–30 Of all microparticles, PMPs are the most numerous and promote thrombosis by exposure of membrane phosphatidylserine, a strong promoter of the contact-dependent coagulation pathway.11 A few studies document an increase in PMP over time in adult patients supported with an artificial blood pump.14,31 Chung et al.32 observed an increase in tissue factor bearing PMP in adults during CPB surgery. Diehl et al.9 observed an increase in circulating blood cell-derived microparticles in patients with ventricular assist devices; of note, these increases correlated with an activation of the parent circulating cells. Each of these clinical studies used adult devices and flow rates. The current study is the first to document an increase in PMP in neonatal ECMO systems in vitro. Future studies will investigate whether constant or dynamic shear stress is the source of the increased PMP. Future clinical studies will also examine the role that PMPs play in pump-induced thrombotic complications.
This study has several limitations because of the in vitro nature of the neonatal ECMO model. For instance, in the limited time to collect samples, the in vitro model may not replicate current neonatal support. Further, porcine blood may generate different results than human blood. Nevertheless, because of similarities between human and porcine platelets, porcine blood was the best choice for testing.33 Prior in vitro studies have used porcine blood to test ECMO systems. Additionally, the use of porcine-specific antibodies, maintaining a nominal swine temperature during perfusion and maintaining a static blood control for comparison, reduced the potential for false results. The lack of coagulation tests, functional assays of thrombosis, and clot formation limits this study. This study also has significant deficits in PMP detection, which includes the use of a signal cell-specific antibody compared with other studies that use two antibodies to detect PMP (e.g., cell-specific CD61 and phostidylserine [Annexin V]). These results are still novel as they controlled for errors by rigorous collection methods (e.g., analyzed samples within an hour) and used size-specific beads to establish proper size gates for analysis.
Conclusions
Neonatal ECMO systems with a centrifugal pump generated more PMP compared with roller-head pump systems. This study suggests that although centrifugal pumps do not increase hemolysis, they induce platelets to release PMP in comparison with roller-head pumps. This is concerning because a recent study on left ventricular assist devices documents that increased phosphatidylserine + MPs are associated with adverse clinical outcomes.34 Therefore, this study confirms a new biological consequence that may promote thrombosis in biomedical devices. Future studies will use human blood, improved microparticle enumeration methods, correlation to clinically relevant thrombosis assays, and clinical studies on neonatal patients supported with extracorporeal blood pumps.
Acknowledgments
Supported by the Children’s National Lady Board of Visitors and the Teh Shah Memorial Fund for ECMO Research.
Footnotes
Disclosure: The authors have no conflicts of interest to report.
References
- 1.Haines NM, Rycus PT, Zwischenberger JB, Bartlett RH, Undar A. Extracorporeal Life Support Registry Report 2008: Neonatal and pediatric cardiac cases. ASAIO J. 2009;55:111–116. doi: 10.1097/MAT.0b013e318190b6f7. [DOI] [PubMed] [Google Scholar]
- 2.Groom RC, Froebe S, Martin J, et al. Update on pediatric perfusion practice in North America: 2005 survey. J Extra Corpor Technol. 2005;37:343–350. [PMC free article] [PubMed] [Google Scholar]
- 3.Thiagarajan RR, Laussen PC, Rycus PT, Bartlett RH, Bratton SL. Extracorporeal membrane oxygenation to aid cardiopulmonary resuscitation in infants and children. Circulation. 2007;116:1693–1700. doi: 10.1161/CIRCULATIONAHA.106.680678. [DOI] [PubMed] [Google Scholar]
- 4.Zabrocki LA, Brogan TV, Statler KD, Poss WB, Rollins MD, Bratton SL. Extracorporeal membrane oxygenation for pediatric respiratory failure: Survival and predictors of mortality. Crit Care Med. 2011;39:364–370. doi: 10.1097/CCM.0b013e3181fb7b35. [DOI] [PubMed] [Google Scholar]
- 5.Reed RC, Rutledge JC. Laboratory and clinical predictors of thrombosis and hemorrhage in 29 pediatric extracorporeal membrane oxygenation nonsurvivors. Pediatr Dev Pathol. 2010;13:385–392. doi: 10.2350/09-09-0704-OA.1. [DOI] [PubMed] [Google Scholar]
- 6.Zwischenberger JB, Nguyen TT, Upp JR, Jr, et al. Complications of neonatal extracorporeal membrane oxygenation. Collective experience from the Extracorporeal Life Support Organization. J Thorac Cardiovasc Surg. 1994;107:838–48. discussion 848. [PubMed] [Google Scholar]
- 7.Paden ML, Conrad SA, Rycus PT, Thiagarajan RR. ELSO Registry: Extracorporeal Life Support Organization Registry Report 2012. ASAIO J. 2013;59:202–210. doi: 10.1097/MAT.0b013e3182904a52. [DOI] [PubMed] [Google Scholar]
- 8.Cheung Py, Sawicki G, Salas E, Etches PC, Schulz R, Radomski MW. The mechanisms of platelet dysfunction during extracorporeal membrane oxygenation in critically ill neonates. Crit Care Med. 2000;28:2584–2590. doi: 10.1097/00003246-200007000-00067. [DOI] [PubMed] [Google Scholar]
- 9.Diehl P, Aleker M, Helbing T, et al. Enhanced microparticles in ventricular assist device patients predict platelet, leukocyte and endothelial cell activation. Interact Cardiovasc Thorac Surg. 2010;11:133–137. doi: 10.1510/icvts.2010.232603. [DOI] [PubMed] [Google Scholar]
- 10.Meyer AD, Wiles AA, Rivera O, et al. Hemolytic and thrombocytopathic characteristics of extracorporeal membrane oxygenation systems at simulated flow rate for neonates. Pediatr Crit Care Med. 2012;13:e255–e261. doi: 10.1097/PCC.0b013e31823c98ef. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nomura S, Ozaki y, ikeda y. Function and role of microparticles in various clinical settings. Thromb Res. 2008;123:8–23. doi: 10.1016/j.thromres.2008.06.006. [DOI] [PubMed] [Google Scholar]
- 12.Mastronardi ML, Mostefai HA, Meziani F, Martínez MC, Asfar P, Andriantsitohaina R. Circulating microparticles from septic shock patients exert differential tissue expression of enzymes related to inflammation and oxidative stress. Crit Care Med. 2011;39:1739–1748. doi: 10.1097/CCM.0b013e3182190b4b. [DOI] [PubMed] [Google Scholar]
- 13.Owens AP, 3rd, Mackman N. Microparticles in hemostasis and thrombosis. Circ Res. 2011;108:1284–1297. doi: 10.1161/CIRCRESAHA.110.233056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Abrams CS, Ellison N, Budzynski AZ, Shattil SJ. Direct detection of activated platelets and platelet-derived microparticles in humans. Blood. 1990;75:128–138. [PubMed] [Google Scholar]
- 15.Nieuwland R, Berckmans RJ, Rotteveel-Eijkman RC, et al. Cell-derived microparticles generated in patients during cardiopulmonary bypass are highly procoagulant. Circulation. 1997;96:3534–3541. doi: 10.1161/01.cir.96.10.3534. [DOI] [PubMed] [Google Scholar]
- 16.Biró E, Sturk-Maquelin KN, Vogel GM, et al. Human cell-derived microparticles promote thrombus formation in vivo in a tissue factor-dependent manner. J Thromb Haemost. 2003;1:2561–2568. doi: 10.1046/j.1538-7836.2003.00456.x. [DOI] [PubMed] [Google Scholar]
- 17.Lawson DS, ing R, Cheifetz iM, et al. Hemolytic characteristics of three commercially available centrifugal blood pumps. Pediatr Crit Care Med. 2005;6:573–577. doi: 10.1097/01.pcc.0000163282.63992.13. [DOI] [PubMed] [Google Scholar]
- 18.Van Meurs KP, Mikesell GT, Seale WR, Short BL, Rivera O. Maximum blood flow rates for arterial cannulae used in neonatal ECMO. ASAIO Trans. 1990;36:M679–M681. [PubMed] [Google Scholar]
- 19.Tamari y, Lee-Sensiba K, Leonard EF, Tortolani AJ. A dynamic method for setting roller pumps nonocclusively reduces hemolysis and predicts retrograde flow. ASAIO J. 1997;43:39–52. [PubMed] [Google Scholar]
- 20.Christersson C, Johnell M, Siegbahn A. Evaluation of microparticles in whole blood by multicolour flow cytometry assay. Scand J Clin Lab Invest. 2013;73:229–239. doi: 10.3109/00365513.2013.769278. [DOI] [PubMed] [Google Scholar]
- 21.Robert S, Poncelet P, Lacroix R, et al. Standardization of platelet-derived microparticle counting using calibrated beads and a Cytomics FC500 routine flow cytometer: a first step towards multicenter studies? J Thromb Haemost. 2009;7:190–197. doi: 10.1111/j.1538-7836.2008.03200.x. [DOI] [PubMed] [Google Scholar]
- 22.Perez de la Lastra JM, Moreno A, Perez J, Llanes D. Characterization of the porcine homologue to human platelet glycoprotein IIb-IIIa (CD41/CD61) by a monoclonal antibody. Tissue Antigens. 1997;49:588–94. doi: 10.1111/j.1399-0039.1997.tb02806.x. [DOI] [PubMed] [Google Scholar]
- 23.Malinauskas RA. Plasma hemoglobin measurement techniques for the in vitro evaluation of blood damage caused by medical devices. Artif Organs. 1997;21:1255–1267. doi: 10.1111/j.1525-1594.1997.tb00486.x. [DOI] [PubMed] [Google Scholar]
- 24.ASTM F1841-97(2013) Standard Practice for Assessment of Hemolysis in Continuous Flow Blood Pumps. ASTM international; West Conshohocken, PA: 2013. www.astm.org. [Google Scholar]
- 25.Gbadegesin R, Zhao S, Charpie J, Brophy PD, Smoyer WE, Lin JJ. Significance of hemolysis on extracorporeal life support after cardiac surgery in children. Pediatr Nephrol. 2009;24:589–595. doi: 10.1007/s00467-008-1047-z. [DOI] [PubMed] [Google Scholar]
- 26.Reininger AJ, Heijnen HF, Schumann H, Specht HM, Schramm W, Ruggeri ZM. Mechanism of platelet adhesion to von Willebrand factor and microparticle formation under high shear stress. Blood. 2006;107:3537–3545. doi: 10.1182/blood-2005-02-0618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sheriff J, Soares JS, Xenos M, Jesty J, Slepian MJ, Bluestein D. Evaluation of shear-induced platelet activation models under constant and dynamic shear stress loading conditions relevant to devices. Ann Biomed Eng. 2013;41:1279–1296. doi: 10.1007/s10439-013-0758-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Horigome H, Hiramatsu y, Shigeta O, Nagasawa T, Matsui A. Overproduction of platelet microparticles in cyanotic congenital heart disease with polycythemia. J Am Coll Cardiol. 2002;39:1072–1077. doi: 10.1016/s0735-1097(02)01718-7. [DOI] [PubMed] [Google Scholar]
- 29.Michelson AD, Rajasekhar D, Bednarek FJ, Barnard MR. Platelet andplatelet-derived microparticle surface factor V/Va bind-ing in whole blood: differences between neonates and adults. Thromb Haemost. 2000;84:689–694. [PubMed] [Google Scholar]
- 30.Nieuwland R, Berckmans RJ, McGregor S, et al. Cellular origin and procoagulant properties of microparticles in meningococcal sepsis. Blood. 2000;95:930–935. [PubMed] [Google Scholar]
- 31.Daniel L, Fakhouri F, Joly D, et al. Increase of circulating neutrophil and platelet microparticles during acute vasculitis and hemodialysis. Kidney Int. 2006;69:1416–1423. doi: 10.1038/sj.ki.5000306. [DOI] [PubMed] [Google Scholar]
- 32.Chung J, Suzuki H, Tabuchi N, Sato K, Shibamiya A, Koyama T. Identification of tissue factor and platelet-derived particles on leukocytes during cardiopulmonary bypass by flow cytometry and immunoelectron microscopy. Thromb Haemost. 2007;98(2):368–74. [PubMed] [Google Scholar]
- 33.Delgado AV, Alexander SL, McManus AT, Pusateri AE. Antibodies against human cell receptors, CD36, CD41a, and CD62P cross-react with porcine platelets. Cytometry B Clin Cytom. 2003;56:62–67. doi: 10.1002/cyto.b.10042. [DOI] [PubMed] [Google Scholar]
- 34.Nascimbene A, Hernandez R, George JK, et al. Association between cell-derived microparticles and adverse events in patients with nonpulsatile left ventricular assist devices. J Heart Lung Transplant. 2014;33:470–477. doi: 10.1016/j.healun.2014.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]


