Abstract
Costimulation blockade with the B7-CD28 pathway-specific agent belatacept is now used in clinical kidney transplantation, but its efficacy remains imperfect. Numerous alternate costimulatory pathways have been proposed as targets to synergize with belatacept, one of which being the inducible costimulator (ICOS)-ICOS ligand pathway. Combined ICOS-ICOS-L and CD28-B7 blockade has been shown to prevent rejection in mice, but has not been studied in primates. We therefore tested a novel ICOS-Ig human Fc-fusion protein in a nonhuman primate kidney transplant model alone and in combination with belatacept. ICOS-Ig did not prolong rejection-free survival as a monotherapy or in combination with belatacept. In ICOS-Ig alone treated animals, most graft-infiltrating CD4+ and CD8+ T cells expressed ICOS, and ICOS+ T cells were present in peripheral blood to a lesser degree. Adding belatacept reduced the proportion of graft-infiltrating ICOS+ T cells and virtually eliminated their presence in peripheral blood. Graft-infiltrating T cells in belatacept-resistant rejection were primarily CD8+CD28−, but importantly, very few CD8+CD28− T cells expressed ICOS. We conclude that ICOS-Ig, alone or combined with belatacept, does not prolong renal allograft survival in nonhuman primates. This may relate to selective loss of ICOS with CD28 loss.
Introduction
Costimulation blockade (CoB) with the B7-specific fusion protein belatacept has emerged as an alternative to treatment with calcineurin inhibitors (CNIs) for maintenance therapy after kidney transplantation. Use of belatacept is associated with similar 5-year graft survival, better renal function, and improved side effect profiles compared to CNIs (1, 2). However, belatacept also is associated with an increased early acute rejection rate, indicating that adjuvant agents are needed to control CoB-resistant rejection mediated by CoB-resistant T cell populations (3).
Numerous costimulatory pathways exist beyond those that are dependent on the B7-CD28 pathway. Preclinical data suggest that blockade of these alternative costimulatory signals may have synergistic effects, and an alternate costimulatory pathway of interest is the inducible costimulator (ICOS)-ICOS ligand pathway. ICOS is a CD28 homologue that is not expressed by naïve T cells, but is rapidly upregulated upon T cell activation and is constitutively expressed by some resting memory T cells (4, 5). ICOS exclusively binds ICOS ligand (ICOS-L, B7h, B7RP-1), which is expressed on B cells, macrophages, and dendritic cells, and can be induced by inflammatory signals on a variety of non-lymphoid cells (6, 7). These patterns of expression suggest ICOS-ICOS-L interactions may be important for T cell effector responses. ICOS costimulation has been shown to enhance T cell activation, differentiation, proliferation and effector functions, and T cell-dependent humoral responses such as germinal center formation and isotype switching (4, 5, 8–10). ICOS-ICOS-L interaction is critical for IL-4 and IL-10, but not IL-2, production (8).
In transplantation, ICOS+ T cells have been detected in rejected allografts from rodents and nonhuman primates (11–13), and in human renal allograft biopsies (14). CD8+ memory T cells, thought to be critical mediators of CoB-resistant rejection, have been shown to infiltrate the allograft within 24 hours after reperfusion (15) and upregulate ICOS within the first 72 hours as they divide within the allograft (16). Consistent with their known relative costimulation-independent nature, TCR engagement alone without CD28-B7 signaling is sufficient to induce ICOS upregulation on CD8+ memory T cells (16). In mice ICOS costimulation has been shown to augment contact hypersensitivity at secondary challenge more profoundly than at sensitization, further confirming the functional role of ICOS signaling in memory T cells and secondary effector responses (5). Given these findings, blocking ICOS-ICOS-L interactions may specifically target memory T cells, particularly alloreactive populations that are resistant to belatacept.
Many studies have examined the effect of ICOS blockade alone or paired with other costimulation blockade agents. ICOS blockade alone has yielded modest improvement in graft survival (11, 17), while combined ICOS and CD40-CD154 blockade demonstrated prolonged graft survival in rodent models (13, 18). Several reports highlight the effect of ICOS signaling in the CD28-deficient murine setting. ICOS is upregulated upon TCR engagement and CD28 costimulation in naïve T cells, but ICOS costimulation is able to induce proliferation in T cells from mice deficient in CD28, indicating that ICOS signaling can provide adequate costimulation independent of CD28 (5). Furthermore, ICOS blockade significantly prolongs cardiac allograft survival in CD28-deficient mice (19).
Interestingly, combined CD28-B7 and ICOS blockade has yielded mixed results in animal studies. Combined CTLA4-Ig and ICOS blockade led to long-term allograft survival in murine cardiac and islet transplant models (13, 17). In a rat cardiac allograft model, ICOS-Ig alone had no effect on mean graft survival and abrogated the beneficial effect of CTLA4-Ig when used in combination (20). B7RP-1 ligation with ICOS-Ig treatment was found to decrease B7 expression on antigen presenting cells (APCs) in vivo, potentially accounting for these results. Additionally, administration of ICOS blockade in an early or delayed fashion produced dichotomous results, with results favoring delayed ICOS blockade (19–21). To date, there are no published studies testing ICOS pathway blockade in a large animal model. Additionally, given the problems surrounding clinical translation of CD40-CD154 pathway blockade, we chose to pair ICOS-ICOSL blockade with the clinically available CD28-B7 costimulation blocker, belatacept, in a MHC-mismatched nonhuman primate model of kidney transplantation. In this study, ICOS-ICOS-L disruption with ICOS-Ig, a human Fc-fusion protein, did not prolong renal allograft survival when given alone or in combination with belatacept.
Materials and Methods
Donor-recipient pair selection and kidney transplantation
All experiments described herein were performed in compliance with the principles set forth in “The Guide for the Care and Use of Laboratory Animals” (Institute of Laboratory Animal Resources, National Research Council, DHHS). Outbred rhesus monkeys (Macaca mulatta) ranging between 3–5 years old were obtained from AlphaGenesis, Inc. (Yemassee, SC, USA) and Yerkes National Primate Research Center (Lawrenceville, GA, USA). Donor-recipient pairs were chosen to maximize genetic disparity at both MHC class I and class II alleles based on 454 deep sequencing analysis (University of Wisconsin, Madison, WI, USA). Kidney transplantation was performed using standard microvascular techniques (22). Animals were heparinized (100 Units/kg) during organ procurement and implantation. Left native nephrectomy was performed at least 3 weeks prior to transplantation, and a completion right native nephrectomy was performed at the time of transplantation. All transplanted animals were monitored with daily clinical assessment and serial laboratory evaluations, including complete blood count and serum chemistry.
NHP experimental groups and immunomodulation
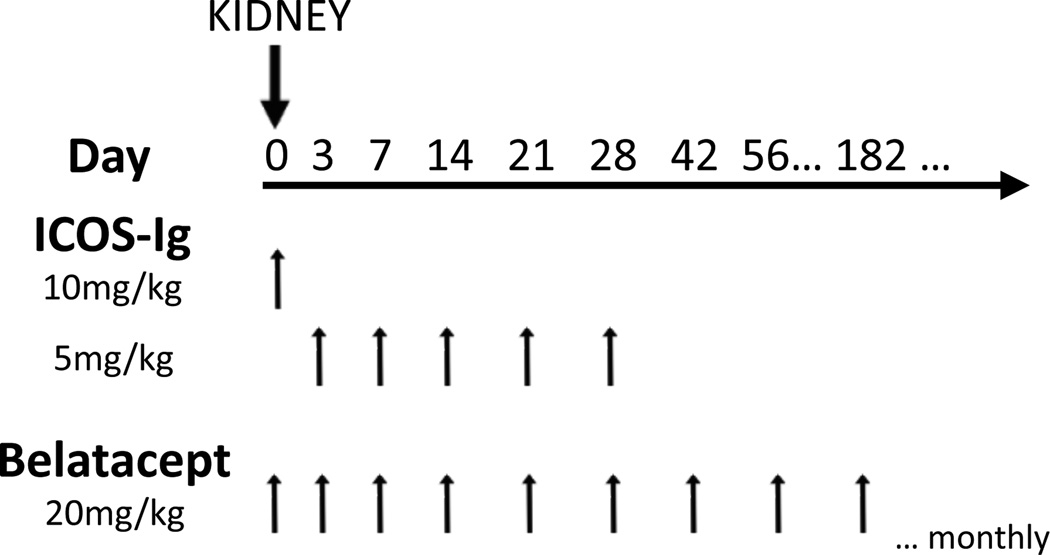
A total of 9 rhesus monkeys in 3 experimental groups were transplanted in this study. Three animals per group received maintenance therapy with either ICOS-Ig alone (Perseid Therapeutics, Redwood, CA, USA), belatacept alone (Nulojix; Bristol-Myers Squibb, New York, NY, USA), or combination ICOS-Ig and belatacept. ICOS-Ig was given at 10 mg/kg IV on day 0, followed by 5 mg/kg on days 3, 7, 14, 21, and 28. Belatacept (20 mg/kg IV) was given on days 0, 3, 7, and 14, every two weeks for 6 months, and then monthly thereafter (Figure 1). All animals received a single dose of methylprednisolone (15 mg/kg) at the time of transplantation. ICOS-Ig dosing was based on preliminary dosing and receptor occupancy studies (Supplemental Figure). Data from experimental groups were compared to historical untreated controls. ICOS-Ig was provided by Perseid Therapeutics, and belatacept was provided by Bristol-Myers Squibb.
Figure 1. Maintenance immunodulation regimen after kidney transplantation.
Histopathology and immunohistochemistry
All kidney allograft specimens were prepared by a histopathologist (MS) and scored by a nephropathologist (ABF). Kidneys were preserved in 10% formalin and embedded in paraffin. Standard hematoxylin and eosin staining was performed on all nephrectomy specimens. Samples were graded for rejection based on modified 1997 Banff scoring criteria (23). Immunohistochemistry was performed on rejected allografts to detect expression of ICOS and ICOS-L. Briefly, tissues sections were deparaffinized and labeled with rabbit anti-human ICOS antibody (Abcam, Cambridge, MA, USA) or ICOS-Ig (Perseid Therapeutics) and then visualized using the LSAB+ labeled Streptavidin-Biotin kit (DAKO, Carpinteria, CA, USA). Nuclei were counterstained with hematoxylin.
Isolation of graft-infiltrating lymphocytes
To isolate graft-infiltrating lymphocytes, rejected allografts were mechanically disrupted and filtered through 70µM cell strainers. Cell suspensions were separated by Ficoll-Paque density gradient centrifugation (GE Healthcare Life Sciences, Sweden) to isolate peripheral blood mononuclear cells (PBMCs). PBMCs were washed twice and counted prior to antibody staining.
Antibodies and flow cytometric analysis
Flow cytometric analysis was performed pre-transplant and serially post-transplant to characterize peripheral blood immune cell phenotypes. Total T cells and T cell subsets were quantified by complete blood cell count and flow cytometry. Fresh PBMCs were isolated by ficoll density gradient centrifugation (BD Biosciences, Franklin Lakes, NJ, USA). PBMCs were stained with the following monoclonal antibodies: CD3 PacBlue, CD3 APC-Cy7, CD4 PerCP-Cy5.5, CD8 V500, CD28 PE-Cy7, CD127 PE-Cy7, PD-1 APC, ICOS PE, LFA-1 APC, CD45RA PE-Cy7, CD20 APC-Cy7 (all BD Biosciences), CD95 PacBlue, CD69 FITC (Invitrogen, Grand Island, NY, USA), and CD25 PE (Miltenyi Biotech, Germany). PBMCs (1.5 × 106) were incubated with appropriately titered antibodies for 15 minutes at 4°C and washed twice. For intracellular staining, cells were fixed and permeabilized using the BD Cytofix/Cytoperm kit (BD Biosciences) according to the manufacturer’s instructions. Intracellular staining was performed with FoxP3 Alexa488 (Biolegend, San Diego, CA, USA) to detect regulatory T (TReg) cells. Samples were acquired immediately on a BD LSR II multicolor flow cytometer, and data were analyzed using FlowJo software (Tree Star, San Carlos, CA, USA). For the stimulation assay, 1.5 × 106 PBMCs were cultured in RPMI 1640 (Corning cellgro, Manassas, VA, USA) supplemented with 10% fetal bovine serum and stimulated with 10 µM phorbol 12-myristate 13-acetate (PMA) and 200 nM ionomycin (Sigma-Aldrich, St. Louis, MO, USA) for 24 hours. PBMCs were washed twice prior to antibody staining and data acquisition.
Statistics
Survival statistics were calculated using a log-rank test. T cell frequencies were compared using a nonparametric Mann-Whitney U test. Data were analyzed using Prism 6 (GraphPad Software, La Jolla, CA, USA). A two-tailed p-value of <0.05 was considered statistically significant.
Results
ICOS is expressed on resting and activated memory T cells in rhesus macaques
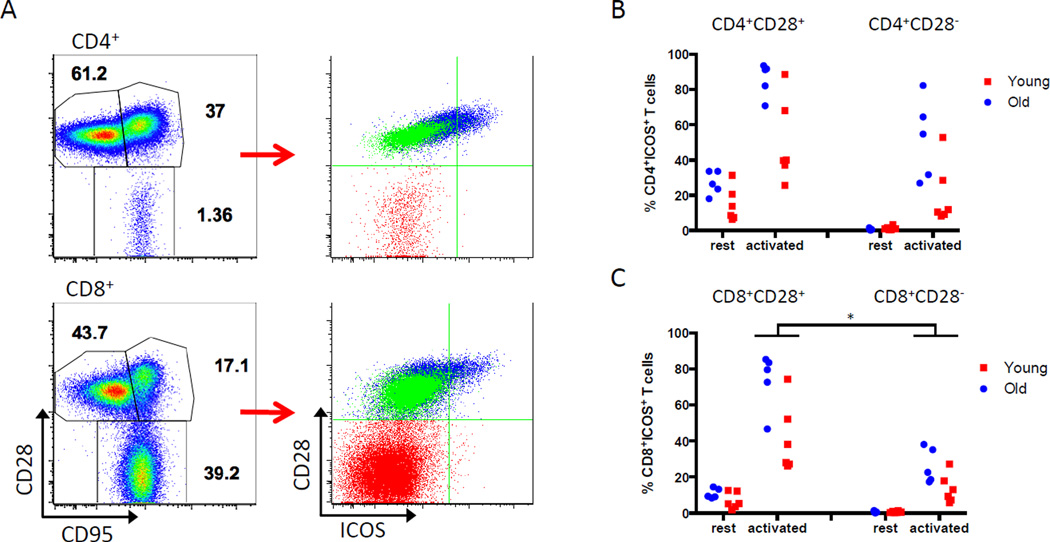
Previous studies have shown that ICOS is constitutively expressed on some resting memory T cells in aged mice (5). To investigate the expression of ICOS on resting and activated T cells in nonhuman primates, flow cytometric analysis was performed on PBMCs from 11 healthy rhesus macaques. Six young (1–3 years old) and 5 older (6–12 years old) monkeys were chosen to represent a range of antigen experience and immune repertoires. Memory T cells were defined by CD28 and CD95 expression (24). A subset of resting CD4+ and CD8+CD28+ CD95hi central memory T (TCM) cells expressed ICOS. However, resting CD28+CD95lo naïve T (TN) cells and CD28−CD95hi effector memory T (25) cells lacked ICOS expression (Figure 2A).
Figure 2. Expression of ICOS in resting and activated memory T cell subsets.
PBMCs from 11 healthy, young (n=6) and older (n=5) rhesus monkeys were analyzed for ICOS expression by flow cytometry. (A) Memory subsets were defined using CD28 and CD95 expression (left) to derive naïve T cells (CD28+CD95lo; TN); central memory T cells (CD28+CD95hi; TCM); and effector memory T cells (CD28−CD95hi; TEM). Memory subsets were re-defined by CD28 and ICOS expression and overlaid (right). Resting TCM cells (blue overlay) express ICOS, while TN cells (green) and TEM cells (red) do not express ICOS at rest. Representative data from a young monkey is shown. (B, C) PBMCs were stimulated for 24 hours with PMA/ionomycin. CD4+ (B) and CD8+ (C) CD28+ and CD28− subsets were analyzed for ICOS expression. Data is expressed as % ICOS+ T cells in CD4+CD28+, CD4+CD28−, CD8+CD28+ or CD8+CD28− subsets. Activated CD8+CD28− T cells expressed significantly less ICOS than CD8+CD28+ T cells (* p=0.0001).
To assess the magnitude of ICOS upregulation after activation, resting PBMCs were activated using PMA/ionomycin and analyzed for ICOS expression. CD4+ and CD8+ CD28+ T cells readily upregulated ICOS upon activation (Figure 2B, 2C). Only older monkeys consistently had significant CD4+CD28− populations at baseline (15–71% of total CD4+ T cells; data not shown), and up to 80% of this population upregulated ICOS. CD8+CD28− T cells comprised 28–52% of all CD8+ T cells in young animals and 64–95% of all CD8+ T cells in older monkeys (data not shown). Compared to the CD8+CD28+ subset, CD8+CD28− T cells in both young and old monkeys demonstrated a decreased ability to upregulate ICOS after activation (p=0.0001). These data characterize the variability of ICOS expression with changes in age, CD28 status, and activation state.
ICOS and ICOS-L expressing cells infiltrate the allograft in belatacept-resistant rejection
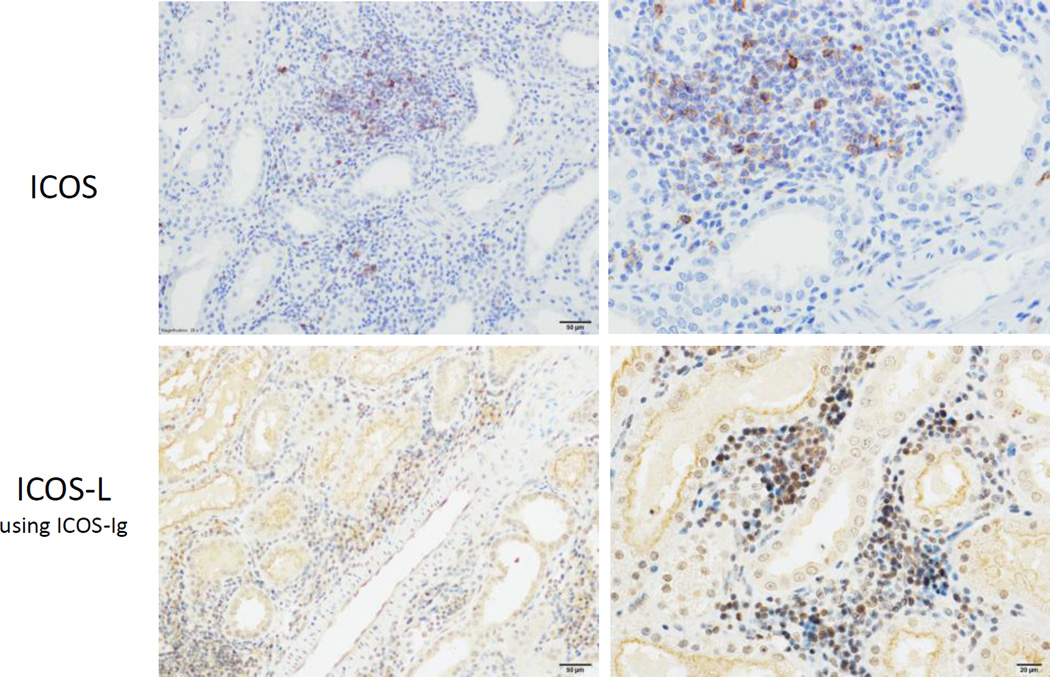
To determine the presence of ICOS and ICOS-L in belatacept-resistant rejection, immunohistochemistry was performed on renal allografts from animals that rejected on belatacept monotherapy. Rejected allografts were stained with anti-ICOS (Figure 3, top row) or ICOS-Ig to detect ICOS-L (Figure 3, bottom row). Both ICOS and ICOS-L were identified in interstitial infiltrates in allografts with belatacept-resistant rejection. These expression patterns verify this pathway is a potential avenue for therapeutic intervention in belatacept-resistant rejection and can be targeted by ICOS-Ig in this model.
Figure 3. Expression of ICOS and ICOS-L in belatacept-resistant rejection.
Immunohistochemical staining was performed on rejected allografts from animals treated with belatacept monotherapy to detect expression of ICOS (top row) and ICOS-L (bottom row). ICOS-Ig was used to detect expression of ICOS-L. Representative images at 20× (left) and 40× (right) magnification are shown.
ICOS-Ig does not enhance renal allograft survival when given alone or in combination with belatacept
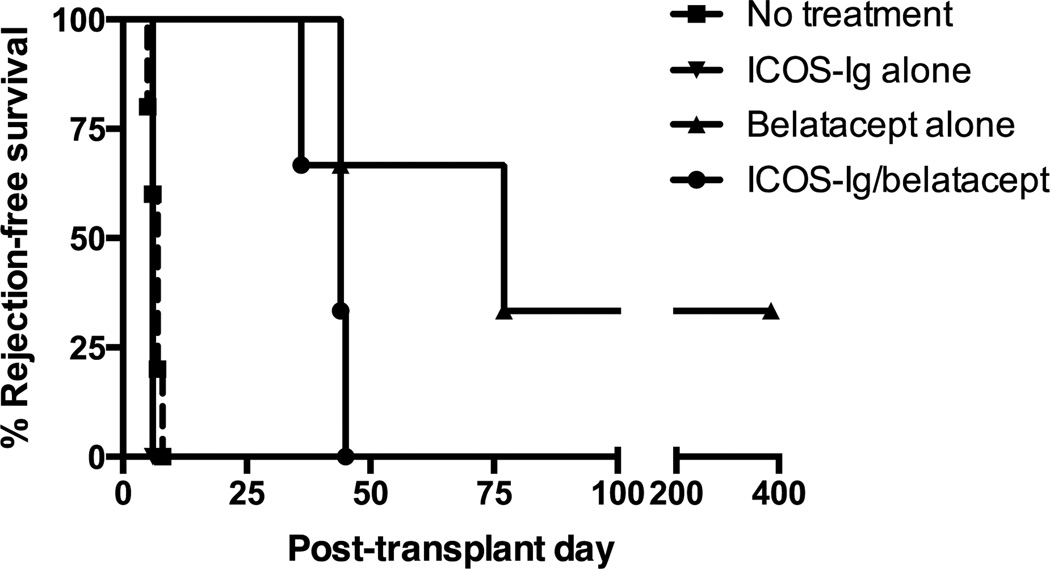
Studies in rodents have shown mixed results when pairing ICOS-ICOS-L and CD28-B7 blockade, and no studies have been done in large animal models. Therefore, we tested combined ICOS-ICOS-L and CD28-B7 blockade in a robust nonhuman primate model. Nine rhesus monkeys underwent MHC-mismatched renal allotransplantation and received maintenance therapy with ICOS-Ig alone, belatacept alone or combination ICOS-Ig and belatacept (Figure 4). All 3 animals that received ICOS-Ig alone acutely rejected their allografts by post-transplant day 6, similar to untreated controls. Animals receiving belatacept monotherapy had improved graft survival (44, 71, 385+ days). One animal in the belatacept alone group was sacrificed over 1 year after transplant with a functioning allograft. Three animals received combined ICOS-Ig and belatacept treatment and rejection-free survivals were 35, 44 and 45 days (Table 1).
Figure 4. ICOS-Ig does not promote rejection-free renal allograft survival.
Animals (n=3 per treatment group) received ICOS-Ig alone, belatacept alone, or combined ICOS-Ig and belatacept maintenance therapy after transplantation. Rejection-free survival is shown. Historical data from untreated controls are shown. ICOS-Ig did not prolong rejection-free renal allograft survival, either as monotherapy or in combination with belatacept.
Table 1.
Rejection-free survival
| Treatment | Rejection-free survival (d) |
p-value |
|---|---|---|
| No treatment | 5, 6, 7, 7, 8 | --- |
| ICOS-Ig alone | 6, 6, 6 | 0.281 |
| Belatacept alone | 44, 71, 385* | --- |
| ICOS-Ig/belatacept | 36, 44, 45 | 0.132 |
Sacrificed with normal graft function
compared to no treatment group
compared to belatacept alone group
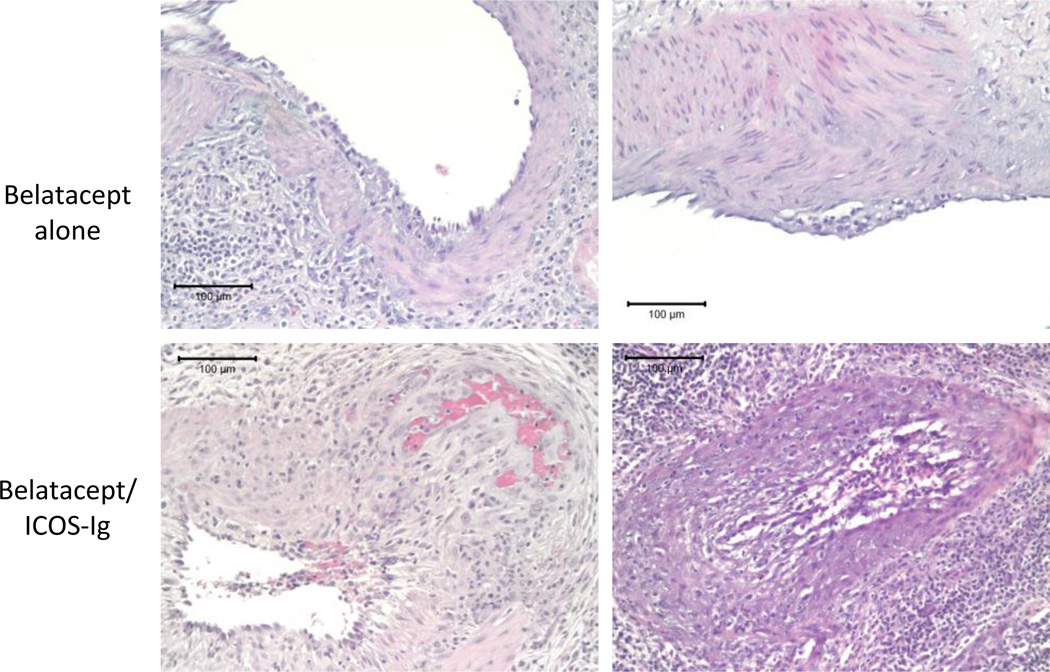
Standard hematoxylin and eosin staining was performed on all allograft specimens. Rejected allografts were characterized by dense lymphocytic infiltrates, extensive tubulitis, and endarteritis (Figure 5). All rejections were acute cellular mediated rejections, ranging from Banff IIA to III. Two belatacept alone animals experienced Banff IIA rejection. However, rejected allografts from all 3 animals receiving ICOS-Ig and belatacept demonstrated more severe pathology, including arteritis with fibrinoid change and transmural arteritis, and these rejections were graded Banff III. We concluded that the addition of ICOS-Ig did not prolong renal allograft survival, alone or in combination with belatacept. Although ICOS-Ig did not clearly abrogate the beneficial effect of CD28-B7 blockade with belatacept in this study, as demonstrated by a previous report (20), there was a trend toward decreased rejection-free survival and more severe rejection pathology with combined ICOS-Ig and belatacept therapy.
Figure 5. Combined ICOS-Ig and belatacept treatment resulted in more severe rejection pathology.
Eight of 9 animals experienced acute rejection, and rejected allografts were evaluated with standard H&E staining. Animals that received belatacept monotherapy demonstrated Banff IIA rejection (top row), while all 3 combined ICOS-Ig/belatacept treated animals experienced Banff III rejection (bottom row). Representative images demonstrating arteritis with fibrinoid change (bottom left) and transmural arteritis (bottom right) characteristic of more severe pathology are shown. All images are shown at 20× magnification.
CD4+ and CD8+ ICOS+ T cells infiltrate rejected allografts
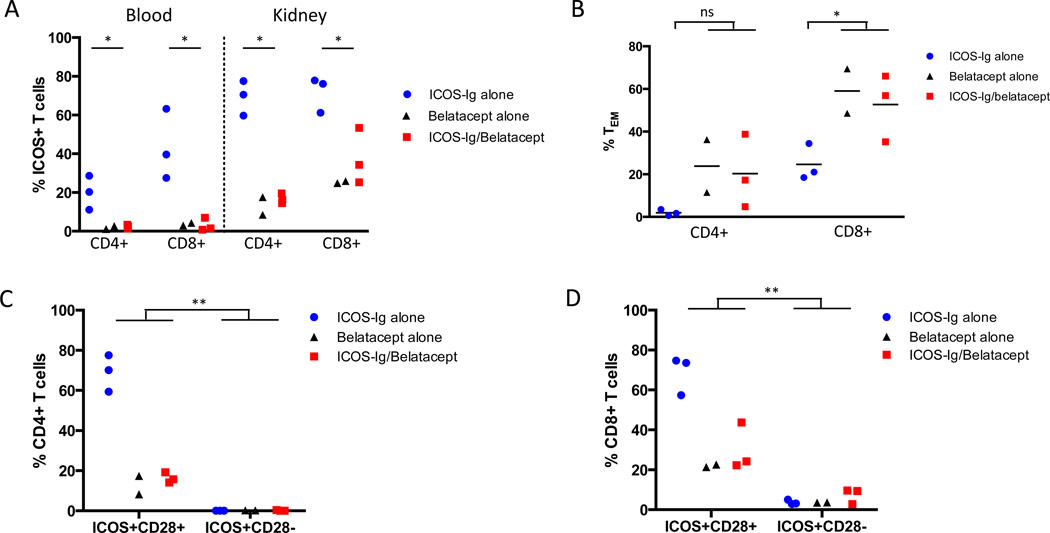
PBMCs and graft-infiltrating lymphocytes from rejected kidneys were isolated and flow cytometric analysis was performed to examine expression of ICOS and CD28. In animals treated with ICOS-Ig alone, ICOS was widely upregulated on CD4+ and CD8+ T cells in both peripheral blood and in the allograft (Figure 6A). Animals that received belatacept, with or without ICOS-Ig, showed distinctly different T cell phenotypes in peripheral blood and the allograft. Belatacept reduced the frequency of ICOS+ T cells, such that peripheral blood was almost devoid of CD4+ or CD8+ ICOS+ cells. CD4+ and CD8+ICOS+ T cells were isolated from the allograft, albeit at a lower frequency than the ICOS-Ig alone group. Since the belatacept alone and combined belatacept/ICOS-Ig animals displayed similar T cell phenotypes, these groups were combined in subsequent statistical analyses. There was a significant diminution in peripheral blood and graft-infiltrating ICOS+ T cells in animals that received belatacept, with or without ICOS-Ig, compared to those that received ICOS-Ig alone (p=0.03; Figure 6A).
Figure 6. Belatacept-resistant CD28− T cells do not express ICOS.
(A) PBMCs and graft-infiltrating lymphocytes were isolated from animals at the time of rejection and analyzed for ICOS expression by flow cytometry. Animals treated with belatacept, with or without ICOS-Ig, had significantly lower frequencies of CD4+ and CD8+ICOS+ T cells compared to the ICOS-Ig monotherapy group in both peripheral blood and the allograft (* p=0.03). (B) Graft-infiltrating lymphocytes were analyzed for memory phenotypes. CD4+ and CD8+ TEM cells were present in higher proportions in belatacept-resistant rejection compared to rejection on ICOS-Ig monotherapy (* p=0.03). (C) CD4+ and (D) CD8+ graft-infiltrating T cells were segregated into CD28+ICOS+ and CD28−ICOS+ subsets. CD4+ and CD8+ CD28− T cells showed significantly reduced ICOS expression compared to CD28+ T cells across all treatment groups (** p=0.002).
CD28− graft-infiltrating T cells lack ICOS expression
Alloreactive TEM cells are characterized by diminished requirements for costimulation and the ability to traffic rapidly to the allograft and exert effector function (26, 27). Therefore, graft-infiltrating lymphocytes were analyzed for memory phenotypes and ICOS expression. The frequency of graft-infiltrating CD4+ and CD8+ CD28−CD95hi TEM cells was increased in animals that received belatacept compared to ICOS-Ig alone animals (p=0.03; Figure 6B), supporting the integral role of these populations in belatacept-resistant rejection. In contrast, ICOS-Ig alone treated animals expressed higher percentages of graft-infiltrating CD28+ T cells. While belatacept can control alloreactive CD28+ naïve T cell responses effectively, it appears that ICOS-Ig did not prevent this population from rapidly proliferating and promoting rejection.
Since CD28− T cells are presumed to escape belatacept control, we focused on the co-expression patterns of CD28 and ICOS on graft-infiltrating T cells. In the ICOS-Ig alone group, the majority of CD4+ICOS+ (Figure 6C) and CD8+ICOS+ graft-infiltrating T cells (Figure 6D) co-expressed CD28. Belatacept significantly decreased the frequency of ICOS+CD28+ T cells compared to the ICOS-Ig alone group. Among CD4+ graft-infiltrating T cells, ICOS+CD28− T cells were barely detectable (0–0.4%; Figure 6C). Although the majority of belatacept-resistant, graft-infiltrating CD8+ T cells were CD28−, ICOS+CD28− T cells represented only 2.8–9.6% of total CD8+ graft-infiltrating T cells (Figure 6D). The frequency of ICOS+CD28− T cells was significantly lower than ICOS+CD28+ T cells when comparing animals across all treatment groups in both CD4+ and CD8+ populations (p=0.002). Therefore, most of the CD28− T cells that are presumed to escape belatacept inhibition do not express ICOS and would not be targeted by ICOS-ICOS-L blockade, underscoring the costimulation-independent nature of this population.
Discussion
ICOS is an activation-induced, CD28-related costimulatory molecule that has garnered attention as a potential target for therapeutic intervention in transplantation. Previous studies in rodent models suggested that ICOS blockade might address specific cell populations that are important in CoB-resistant allograft rejection. Our initial data in nonhuman primates demonstrated that ICOS was widely expressed on activated T cells, and both ICOS and ICOS-L were identified in renal allografts from animals with belatacept-resistant rejection. Therefore, we tested the effect of ICOS-ICOS-L blockade in a rigorous MHC-mismatched nonhuman primate kidney transplant model. We found that ICOS-ICOS-L blockade with ICOS-Ig failed to prolong renal allograft survival when used as monotherapy or in combination with CD28-B7 inhibition with belatacept.
In this study, rhesus monkeys received immediate ICOS blockade beginning the day of transplantation. However, several studies in naïve rodent models have shown beneficial effects of delayed, and not immediate, ICOS blockade after transplantation (19–21). Similarly, in a model of experimental allergic encephalomyelitis where disease is mediated by Th1 cells, ICOS blockade during the T cell priming phase exacerbated disease, while delayed ICOS blockade during the effector phase alleviated it (28). ICOS costimulation is involved in both Th1 and Th2 cell differentiation, though it appears to be more critical for Th2 development (8, 29). One hypothesis is that early ICOS blockade during the T cell priming phase may skew T cell differentiation toward a Th1 phenotype. However, studies have shown inconsistent results in terms of Th1/Th2 cytokine deviation after ICOS pathway blockade (20, 28).
Alternatively, ICOS has been implicated in the maintenance and development of key regulatory cell populations. ICOS-deficient mice showed reduced CD4+FoxP3+ regulatory T (TReg) cells, indicating a role for ICOS in TReg homeostasis (30). In an allergen-induced airway hyperreactivity model, ICOS-ICOS-L interactions were necessary to promote the development and function of antigen-specific, IL-10-producing TReg cells (31). ICOS-ICOS-L disruption has also been shown to interfere with the generation and suppressive quality of induced TReg cells (32). Therefore, early ICOS blockade could abrogate the development of these important regulatory populations. In our study, CD4+CD25hiFoxP3+ TReg cells were equally reduced in quantity in both combined ICOS-Ig/belatacept and belatacept alone animals (data not shown), consistent with our previous work showing a reduction in TReg cells with belatacept treatment in this model (33). However, we did not perform functional analysis of these cell populations to assess potential alterations in their suppressive capabilities.
Regardless of the cause, the beneficial effects of delayed ICOS blockade in naïve rodent models may not be reproduced in large, outbred animal models, such as nonhuman primates, which are equipped with significantly more antigen-experienced immune repertoires than naïve rodents. Among our typical rhesus transplant recipients, memory T cells can account for over half the T cell repertoire, a composition that is more reminiscent of humans and accentuates the relevance of this preclinical transplant model. We studied baseline ICOS expression in healthy rhesus monkeys and found that up to 20% of CD4+ and 3.5% of CD8+ resting memory T cells express ICOS. In vitro stimulation for 24 hours resulted in dramatic ICOS upregulation, particularly in CD28+ T cells. We hypothesized that immediate ICOS blockade would be necessary to control these populations after transplantation. In mice, ICOS blockade has been shown to reduce allograft infiltration of recently activated effectors (18), and reduce effector function, but not infiltration or proliferation, of allospecific CD8+ memory T cells (16). However, in this study ICOS-Ig alone treated animals rejected with the same kinetics as untreated controls, indicating that if there was a decrease in migration or effector function of CD8+ T cells it was insufficient to delay clinical rejection in this robust model.
Combined ICOS and CD28 pathway blockade did not synergize to enhance graft survival in this study; in fact, there was a trend toward decreased allograft survival and more severe rejection when both costimulatory pathways were inhibited. Previous work has demonstrated that the addition of ICOS blockade abrogated the beneficial effects of CTLA4-Ig in a rat cardiac allograft model (20). Ligation of B7RP1 with ICOS-Ig was shown to decrease expression of B7 molecules on APCs in vivo, but not in vitro, indicating that the disruption of ICOS-ICOS-L interactions is necessary for B7 downregulation. Thus, the lack of synergy between belatacept and ICOS-ICOS-L blockade in this study could be attributed in part to an ICOS-Ig-induced diminution in the number of B7 targets for both belatacept blockade and CTLA4 inhibitory signaling. Alternatively, we demonstrated a decrease in the frequency of ICOS+ T cells in belatacept treated animals in this study. In a murine model, both CTLA4-Ig and selective CD28 blockade resulted in impaired upregulation of ICOS after alloactivation (34). Therefore, treatment with CD28-B7 costimulation blockade appears to hinder ICOS upregulation and may also explain the lack of efficacy of combined belatacept and ICOS-Ig in this study.
Alloreactive CD8+CD28− TEM cells are hypothesized to be critical mediators of belatacept-resistant rejection, as they can function with relative independence from CD28 costimulation and thus may escape belatacept control (25). In this study the majority of graft-infiltrating lymphocytes at rejection were CD8+CD28− T cells, but few of these cells expressed ICOS. In fact, ICOS+ graft-infiltrating T cells demonstrated a striking co-expression with CD28. This difference in ICOS expression between CD28+ and CD28− cells was even more pronounced in the context of transplantation than observed in our initial in vitro stimulation studies, and the reduction of ICOS expression in CD28− T cells was demonstrated in all treatment groups. These data suggest that ICOS expression in T cells may be more intimately linked to CD28 expression than previous elaborated or that CD28− T cells can function independent of ICOS costimulation. Habicht, et al used CD4−CD28− and CD8−CD28− mice to investigate mechanisms of CD28-independent rejection and found that blockade of CD40-CD154 and CD134-CD134L interactions, but not the ICOS-ICOS-L pathway, prolonged allograft survival (35). Similarly, these data show that ICOS-ICOS-L blockade was not able to overcome belatacept-resistant, CD28-independent allograft rejection. Taken together, our results suggest that the ICOS-ICOS-L pathway is not the ideal target to prevent belatacept-resistant rejection.
Supplementary Material
To determine an appropriate therapeutic dose of ICOS-Ig, cynomolgus monkeys were given a single intravenous dose of ICOS-Ig (0.1 mg/kg or 1.0 mg/kg), and ICOS-L occupancy on B cells was detected via receptor competition assay using an ICOS-L monoclonal antibody. Two animals were tested per dose. A single dose of ICOS-Ig at 1.0 mg/kg was able to saturate ICOS-L for 7 days. In contrast, receptor occupancy rates rapidly decreased after IV administration of ICOS-Ig at 0.1 mg/kg.
Acknowledgments
This study was funded from support by NIH grant 2U19AI051731, Yerkes National Primate Research Center (P51OD011132), and the Georgia Research Alliance. The authors would like to thank the veterinary staff at Yerkes National Primate Research Center for their contributions to this study.
Abbreviations
- CoB
costimulation blockade
- CNI
calcineurin inhibitor
- ICOS
inducible costimulator
- ICOS-L
inducible costimulator ligand
- PBMCs
peripheral blood mononuclear cells
- TCM cells
central memory T cells
- TEM cells
effector memory T cells
- TN cells
naïve T cells
- TReg cells
regulatory T cells
Footnotes
Disclosures
The authors of this manuscript have conflicts of interest to disclose as described by the American Journal of Transplantation. S. Chapin, B. Devens, and E. Karrer were employed by Perseid Therapeutics during the time of this study. The remaining authors have no conflicts of interest to disclose.
Supplementary Information
Additional Supporting Information may be found in the online version of this article.
References
- 1.Rostaing L, Vincenti F, Grinyo J, Rice KM, Bresnahan B, Steinberg S, et al. Long-term belatacept exposure maintains efficacy and safety at 5 years: results from the long-term extension of the BENEFIT study. Am J Transplant. 2013;13(11):2875–2883. doi: 10.1111/ajt.12460. [DOI] [PubMed] [Google Scholar]
- 2.Vanrenterghem Y, Bresnahan B, Campistol J, Durrbach A, Grinyo J, Neumayer HH, et al. Belatacept-based regimens are associated with improved cardiovascular and metabolic risk factors compared with cyclosporine in kidney transplant recipients (BENEFIT and BENEFIT-EXT studies) Transplantation. 2011;91(9):976–983. doi: 10.1097/TP.0b013e31820c10eb. [DOI] [PubMed] [Google Scholar]
- 3.Vincenti F, Charpentier B, Vanrenterghem Y, Rostaing L, Bresnahan B, Darji P, et al. A phase III study of belatacept-based immunosuppression regimens versus cyclosporine in renal transplant recipients (BENEFIT study) Am J Transplant. 2010;10(3):535–546. doi: 10.1111/j.1600-6143.2009.03005.x. [DOI] [PubMed] [Google Scholar]
- 4.Hutloff A, Dittrich AM, Beier KC, Eljaschewitsch B, Kraft R, Anagnostopoulos I, et al. ICOS is an inducible T-cell co-stimulator structurally and functionally related to CD28. Nature. 1999;397(6716):263–266. doi: 10.1038/16717. [DOI] [PubMed] [Google Scholar]
- 5.Yoshinaga SK, Whoriskey JS, Khare SD, Sarmiento U, Guo J, Horan T, et al. T-cell co-stimulation through B7RP-1 and ICOS. Nature. 1999;402(6763):827–832. doi: 10.1038/45582. [DOI] [PubMed] [Google Scholar]
- 6.Aicher A, Hayden-Ledbetter M, Brady WA, Pezzutto A, Richter G, Magaletti D, et al. Characterization of human inducible costimulator ligand expression and function. J Immunol. 2000;164(9):4689–4696. doi: 10.4049/jimmunol.164.9.4689. [DOI] [PubMed] [Google Scholar]
- 7.Swallow MM, Wallin JJ, Sha WC. B7h, a novel costimulatory homolog of B7.1 and B7.2, is induced by TNFalpha. Immunity. 1999;11(4):423–432. doi: 10.1016/s1074-7613(00)80117-x. [DOI] [PubMed] [Google Scholar]
- 8.Coyle AJ, Lehar S, Lloyd C, Tian J, Delaney T, Manning S, et al. The CD28-related molecule ICOS is required for effective T cell-dependent immune responses. Immunity. 2000;13(1):95–105. doi: 10.1016/s1074-7613(00)00011-x. [DOI] [PubMed] [Google Scholar]
- 9.McAdam AJ, Greenwald RJ, Levin MA, Chernova T, Malenkovich N, Ling V, et al. ICOS is critical for CD40-mediated antibody class switching. Nature. 2001;409(6816):102–105. doi: 10.1038/35051107. [DOI] [PubMed] [Google Scholar]
- 10.Tafuri A, Shahinian A, Bladt F, Yoshinaga SK, Jordana M, Wakeham A, et al. ICOS is essential for effective T-helper-cell responses. Nature. 2001;409(6816):105–109. doi: 10.1038/35051113. [DOI] [PubMed] [Google Scholar]
- 11.Ozkaynak E, Gao W, Shemmeri N, Wang C, Gutierrez-Ramos JC, Amaral J, et al. Importance of ICOS-B7RP-1 costimulation in acute and chronic allograft rejection. Nat Immunol. 2001;2(7):591–596. doi: 10.1038/89731. [DOI] [PubMed] [Google Scholar]
- 12.Azimzadeh AM, Pfeiffer S, Wu G, Schroder C, Zorn GL, 3rd, Kelishadi SS, et al. Alloimmunity in primate heart recipients with CD154 blockade: evidence for alternative costimulation mechanisms. Transplantation. 2006;81(2):255–264. doi: 10.1097/01.tp.0000190099.62847.e6. [DOI] [PubMed] [Google Scholar]
- 13.Nanji SA, Hancock WW, Anderson CC, Adams AB, Luo B, Schur CD, et al. Multiple combination therapies involving blockade of ICOS/B7RP-1 costimulation facilitate long-term islet allograft survival. Am J Transplant. 2004;4(4):526–536. doi: 10.1111/j.1600-6143.2004.00384.x. [DOI] [PubMed] [Google Scholar]
- 14.Akalin E, Dikman S, Murphy B, Bromberg JS, Hancock WW. Glomerular infiltration by CXCR3+ ICOS+ activated T cells in chronic allograft nephropathy with transplant glomerulopathy. Am J Transplant. 2003;3(9):1116–1120. doi: 10.1034/j.1600-6143.2003.00151.x. [DOI] [PubMed] [Google Scholar]
- 15.Schenk AD, Nozaki T, Rabant M, Valujskikh A, Fairchild RL. Donor-reactive CD8 memory T cells infiltrate cardiac allografts within 24-h posttransplant in naive recipients. Am J Transplant. 2008;8(8):1652–1661. doi: 10.1111/j.1600-6143.2008.02302.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Schenk AD, Gorbacheva V, Rabant M, Fairchild RL, Valujskikh A. Effector functions of donor-reactive CD8 memory T cells are dependent on ICOS induced during division in cardiac grafts. Am J Transplant. 2009;9(1):64–73. doi: 10.1111/j.1600-6143.2008.02460.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kosuge H, Suzuki J, Gotoh R, Koga N, Ito H, Isobe M, et al. Induction of immunologic tolerance to cardiac allograft by simultaneous blockade of inducible co-stimulator and cytotoxic T-lymphocyte antigen 4 pathway. Transplantation. 2003;75(8):1374–1379. doi: 10.1097/01.TP.0000061601.26325.82. [DOI] [PubMed] [Google Scholar]
- 18.Zhang QW, Rabant M, Schenk A, Valujskikh A. ICOS-Dependent and -independent functions of memory CD4 T cells in allograft rejection. Am J Transplant. 2008;8(3):497–506. doi: 10.1111/j.1600-6143.2007.02096.x. [DOI] [PubMed] [Google Scholar]
- 19.Harada H, Salama AD, Sho M, Izawa A, Sandner SE, Ito T, et al. The role of the ICOS-B7h T cell costimulatory pathway in transplantation immunity. J Clin Invest. 2003;112(2):234–243. doi: 10.1172/JCI17008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Salama AD, Yuan X, Nayer A, Chandraker A, Inobe M, Uede T, et al. Interaction between ICOS-B7RP1 and B7-CD28 costimulatory pathways in alloimmune responses in vivo. Am J Transplant. 2003;3(4):390–395. doi: 10.1034/j.1600-6143.2003.00085.x. [DOI] [PubMed] [Google Scholar]
- 21.Kashizuka H, Sho M, Nomi T, Ikeda N, Kuzumoto Y, Akashi S, et al. Role of the ICOS-B7h costimulatory pathway in the pathophysiology of chronic allograft rejection. Transplantation. 2005;79(9):1045–1050. doi: 10.1097/01.tp.0000161665.35243.21. [DOI] [PubMed] [Google Scholar]
- 22.Kirk AD, Harlan DM, Armstrong NN, Davis TA, Dong Y, Gray GS, et al. CTLA4-Ig and anti-CD40 ligand prevent renal allograft rejection in primates. Proc Natl Acad Sci U S A. 1997;94(16):8789–8794. doi: 10.1073/pnas.94.16.8789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Racusen LC, Solez K, Colvin RB, Bonsib SM, Castro MC, Cavallo T, et al. The Banff 97 working classification of renal allograft pathology. Kidney Int. 1999;55(2):713–723. doi: 10.1046/j.1523-1755.1999.00299.x. [DOI] [PubMed] [Google Scholar]
- 24.Pitcher CJ, Hagen SI, Walker JM, Lum R, Mitchell BL, Maino VC, et al. Development and homeostasis of T cell memory in rhesus macaque. J Immunol. 2002;168(1):29–43. doi: 10.4049/jimmunol.168.1.29. [DOI] [PubMed] [Google Scholar]
- 25.Lo DJ, Weaver TA, Stempora L, Mehta AK, Ford ML, Larsen CP, et al. Selective targeting of human alloresponsive CD8+ effector memory T cells based on CD2 expression. Am J Transplant. 2011;11(1):22–33. doi: 10.1111/j.1600-6143.2010.03317.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chalasani G, Dai Z, Konieczny BT, Baddoura FK, Lakkis FG. Recall and propagation of allospecific memory T cells independent of secondary lymphoid organs. Proc Natl Acad Sci U S A. 2002;99(9):6175–6180. doi: 10.1073/pnas.092596999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dengler TJ, Pober JS. Human vascular endothelial cells stimulate memory but not naive CD8+ T cells to differentiate into CTL retaining an early activation phenotype. J Immunol. 2000;164(10):5146–5155. doi: 10.4049/jimmunol.164.10.5146. [DOI] [PubMed] [Google Scholar]
- 28.Rottman JB, Smith T, Tonra JR, Ganley K, Bloom T, Silva R, et al. The costimulatory molecule ICOS plays an important role in the immunopathogenesis of EAE. Nat Immunol. 2001;2(7):605–611. doi: 10.1038/89750. [DOI] [PubMed] [Google Scholar]
- 29.Sperling AI, Bluestone JA. ICOS costimulation: It's not just for TH2 cells anymore. Nat Immunol. 2001;2(7):573–574. doi: 10.1038/89709. [DOI] [PubMed] [Google Scholar]
- 30.Burmeister Y, Lischke T, Dahler AC, Mages HW, Lam KP, Coyle AJ, et al. ICOS controls the pool size of effector-memory and regulatory T cells. J Immunol. 2008;180(2):774–782. doi: 10.4049/jimmunol.180.2.774. [DOI] [PubMed] [Google Scholar]
- 31.Akbari O, Freeman GJ, Meyer EH, Greenfield EA, Chang TT, Sharpe AH, et al. Antigen-specific regulatory T cells develop via the ICOS-ICOS-ligand pathway and inhibit allergen-induced airway hyperreactivity. Nat Med. 2002;8(9):1024–1032. doi: 10.1038/nm745. [DOI] [PubMed] [Google Scholar]
- 32.Zheng J, Chan PL, Liu Y, Qin G, Xiang Z, Lam KT, et al. ICOS regulates the generation and function of human CD4+ Treg in a CTLA-4 dependent manner. PLoS One. 2013;8(12):e82203. doi: 10.1371/journal.pone.0082203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lo DJ, Anderson DJ, Weaver TA, Leopardi F, Song M, Farris AB, et al. Belatacept and sirolimus prolong nonhuman primate renal allograft survival without a requirement for memory T cell depletion. Am J Transplant. 2013;13(2):320–328. doi: 10.1111/j.1600-6143.2012.04342.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Liu D, Krummey SM, Badell IR, Wagener M, Schneeweis LA, Stetsko DK, et al. 2B4 (CD244) induced by selective CD28 blockade functionally regulates allograft-specific CD8+ T cell responses. J Exp Med. 2014;211(2):297–311. doi: 10.1084/jem.20130902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Habicht A, Najafian N, Yagita H, Sayegh MH, Clarkson MR. New insights in CD28-independent allograft rejection. Am J Transplant. 2007;7(8):1917–1926. doi: 10.1111/j.1600-6143.2007.01886.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
To determine an appropriate therapeutic dose of ICOS-Ig, cynomolgus monkeys were given a single intravenous dose of ICOS-Ig (0.1 mg/kg or 1.0 mg/kg), and ICOS-L occupancy on B cells was detected via receptor competition assay using an ICOS-L monoclonal antibody. Two animals were tested per dose. A single dose of ICOS-Ig at 1.0 mg/kg was able to saturate ICOS-L for 7 days. In contrast, receptor occupancy rates rapidly decreased after IV administration of ICOS-Ig at 0.1 mg/kg.