Summary
Genomic technologies have significantly advanced our understanding of the composition and diversity of host-associated microbial populations. However, their spatial organization and functional interactions relative to the host have been more challenging to study. Here we present a pipeline for the assessment of intestinal microbiota localization within immunofluorescence images of fixed gut cross-sections that includes a flexible software package, BacSpace, for high-throughput quantification of microbial organization. Applying this pipeline to gnotobiotic and human microbiota-colonized mice, we demonstrate that elimination of microbiota accessible carbohydrates (MACs) from the diet results in thinner mucus in the distal colon, increased proximity of microbes to the epithelium, and heightened expression of the inflammatory marker REG3β. Measurements of microbe-microbe proximity reveal that a MAC-deficient diet alters monophyletic spatial clustering. Furthermore, we quantify the invasion of Helicobacter pylori into the glands of the mouse stomach relative to host mitotic progenitor cells, illustrating the generalizability of this approach.
Introduction
Heterogeneous spatial organization is the norm in natural microbial ecosystems ranging from deep-sea methane vents (Pernthaler et al., 2008) to the surface of thermophilic sludge granules (Sekiguchi et al., 1999). Despite the dynamic nature of these environments, intimate and specific physical associations between diverse microbes are ubiquitous in complex consortia, and in some cases are known to facilitate interspecies metabolic exchange (Reeburgh, 2007). Similar heterogeneity and organization appear to exist within the gut microbiota (Nava et al., 2011; Swidsinski et al., 2007). The organizational principles that govern our internal ecosystem remain poorly understood, despite emerging ecological paradigms that have implications for localization of microbes within the gut. Most notably, the inflammatory status of the intestine responds to and regulates the proximity and identity of gut microbes closest to the host epithelium (Johansson et al., 2014; Stecher et al., 2007; Swidsinski et al., 2005). Yet our unrefined understanding of the progression of events that lead to altered localization and how they are influenced by a breadth of inflammatory or colonization states reflects current limitations in imaging as an experimental approach. As numerous other scenarios emerge in which microbial localization is likely to dictate dynamics within the community or between the community and the host (Barr et al., 2013; Dejea et al., 2014; McHardy et al., 2013; Shen et al., 2012), it is necessary to move beyond compositional descriptions toward a spatial understanding of microbiota function and interaction. Quantitative measurements of spatial organization over scales sufficient to attain confidence in conclusions are critical to define the physical associations that underlie our superorganismal emergent properties.
The inflammatory risk of housing microbes within our gastrointestinal tract is evident in the complex feedback between the microbiota and the host immune system (Hooper and Macpherson, 2010). In the colon, an important environmental and immunological feature is the dense, thick, highly glycosylated and hydrated mucus layer, which consists of two distinct domains: the attached, striated inner mucus layer that excludes bacteria from contacting the epithelium and a looser outer mucus layer that provides structural scaffolding and a nutrient source for the gut microbiota (Johansson et al., 2011). Mouse models in which critical components of the mucus have been ablated are predisposed to colitis, which is accompanied by microbial mislocalization to the mucosa (Fu et al., 2011; Johansson et al., 2008), consistent with the observation of epithelial-adherent commensals in biopsies from patients with inflammatory bowel disease (Johansson et al., 2014; Swidsinski et al., 2005). The removal of microbiota-accessible carbohydrates (MACs), which are typically abundant in dietary fiber and serve as a primary metabolic input for the human gut microbiota, shifts the balance of the microbiota toward mucus-consuming bacteria (Sonnenburg and Sonnenburg, 2014). Whether such microbial blooms are localized to the mucus interface, the impact on the thickness of the mucus barrier, and potential large-scale reorganization of the entire community have not been visualized in the quantitative manner necessary for biological conclusions.
A major obstacle to imaging intact gut samples is the diversity of material that needs to be maintained, including host intestinal cells, material undergoing digestion, microbes, and mucus. In particular, the sensitivity of the mucus layer to standard fixation protocols has served as a persistent challenge for imaging (Johansson et al., 2008). Heterogeneous microenvironments exist throughout the gut, and complex spatial patterns spanning scales from cells (micrometer) to tissues (millimeter) can be affected by numerous variables. Therefore, a robust pipeline including mucus-preserving sample preparation, image segmentation, and quantitative analysis tools is required. Such a framework would enable rigorous testing of the statistical significance of changes in microbiota organization longitudinally along the gut, due to diet, and with differences in host physiological or colonization conditions.
Here, we integrate improved histological preservation methods (Johansson and Hansson, 2012), fluorescence in situ hybridization (FISH), and high-resolution microscopy with an image analysis pipeline to study the factors that influence microbial localization in gnotobiotic mice (ex-germ-free mice colonized with defined communities of bacteria), or humanized mice (ex-germ-free mice colonized by a complete human microbiota). These examples of complex host-microbe organization exemplify the power and necessity of high-throughput imaging and analysis of microbial communities for revealing changes in spatial patterning across multiple scales.
Results
A MAC-deficient diet drives thinning of the inner mucus layer
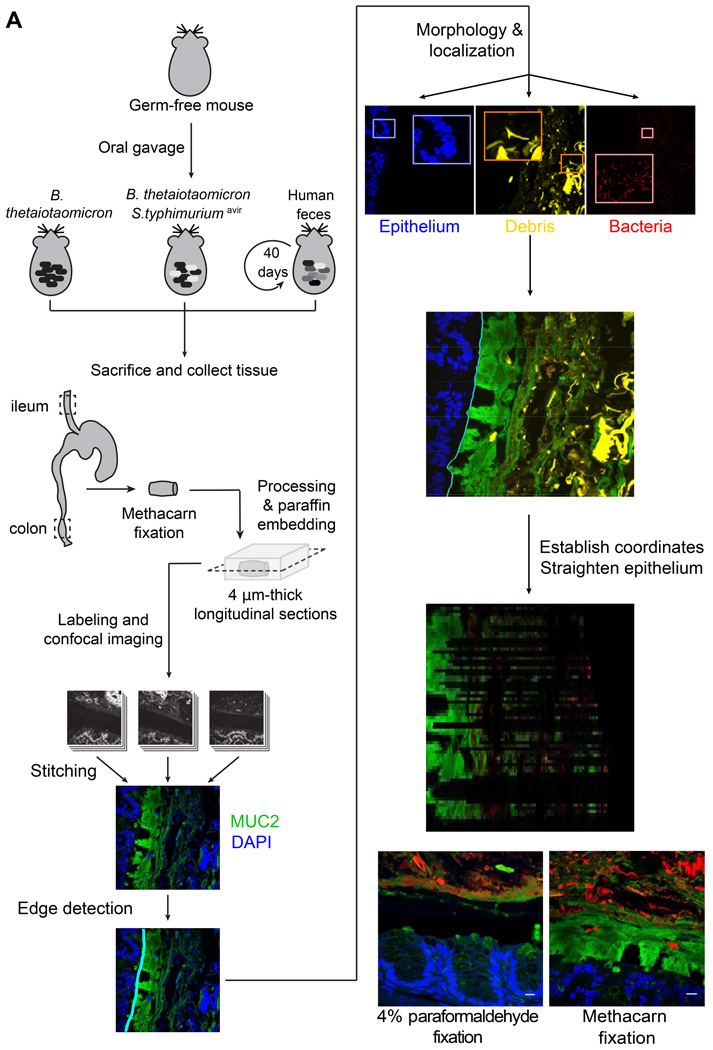
To address how mucus thickness is affected by increased microbial utilization, we fed germ-free mice diets rich (standard) or deficient in MACs and colonized them with Bt, a model gut symbiont known to forage on gut mucus in times of MAC scarcity (Sonnenburg et al., 2005). Ten days after colonization, mice were sacrificed and colon segments were harvested and fixed. These segments were processed into thin sections, labeled with antibodies to the primary intestinal mucin, MUC2, and the DNA dye 4’,6-diamidino-2-phenylindole (DAPI), and imaged with a laser-scanning confocal microscope (Experimental Procedures, Fig. 1A, Fig. S1).
Figure 1. Preservation of the inner mucus layer in histological sections allows for quantitative analysis of the spatial structure of the gut ecosystem.
(A) Schematic of protocol for gnotobiotic mouse experiments, tissue collection and processing, image acquisition, and analysis.
(B,C) Segments of mouse distal colon fixed in (B) 4% paraformaldehyde or (C) methacarn solution. Blue, DAPI staining in the epithelium; red, DAPI staining in lumen of gut, including bacteria and shed host nuclei (luminal debris has not been differentiated in this image); green, antibody staining for MUC2. Scale bars: 10 µm.
In order to enable immunofluorescence imaging of MUC2, we used methacarn fixation, which preserves the mucus layer structure compared to traditional formaldehyde-based fixatives in which the mucus collapses (Johansson and Hansson, 2012) (Fig. 1B vs. 1C, large black areas). By observation of images from standard diet- and MD diet-fed mice, this layer appeared thinner in MD diet-fed mice. We further imaged large sections (∼9 mm perimeter) of the distal colon to assess the variability in mucus thickness. Imaging a section of colon containing an entire fecal pellet required ∼40 fields of view at 20x magnification. Therefore, it was evident that quantitative analysis required computational tools that were specifically adapted for in vivo gastrointestinal images and could automate the quantification of microbial localization within the gut.
To address this need, we developed BacSpace, a user-friendly, flexible, MATLAB-based software platform that enables (i) stitching of overlapping images into one continuous image; (ii) both automated and manual definition of landmarks of interest such as the epithelial boundary; (iii) differentiation of microbial fluorescence signal from highly autofluorescent objects such as diet-derived plant material and shed epithelial cells that are found in mucus; and (iv) measurement of cell-to-cell and cell-to-landmark distance distributions based on a well-defined local coordinate system (Fig. 1A). An instruction manual, the source code for BacSpace, and the analysis of all data in this study are freely available at http://bitbucket.org/gabriel_billings/bacspace/downloads.
Although individual images can be analyzed separately, BacSpace incorporates stitching software (Preibisch et al., 2009) that facilitates visualization and systematic comparisons across large regions. Nonspecific fluorescence of epithelial tissue in the DAPI channel proved bright enough for host tissue identification, hence we identified the epithelial boundary via the spatial gradient of the DAPI signal. In BacSpace, a preliminary boundary is found by thresholding the image at low resolution, and is iteratively refined at successively higher resolutions until a final boundary is identified at the full image resolution (Supplemental Experimental Procedures). This multi-scale approach enables a laptop to process gigabytes of image data in minutes without sacrificing accuracy.
After identification of the epithelial boundary and separation of distinct fluorescence signals, BacSpace computationally straightens the region of the image proximal to the boundary in order to facilitate measurement with a biologically relevant coordinate system. Because we were specifically interested in measurements of distance to the epithelium, we designed a straightening algorithm that preserves distances in the direction normal to the epithelial boundary contour (Supplemental Experimental Procedures). Distance from this contour therefore served as one coordinate; distance along the epithelium served as the other. Due to the curvature of the epithelium, some contour points are not associated with any image pixels as vectors extend further from the epithelium. Thus, the straightened image has gaps in these parts, in which data are absent and therefore do not contribute to subsequent calculations.
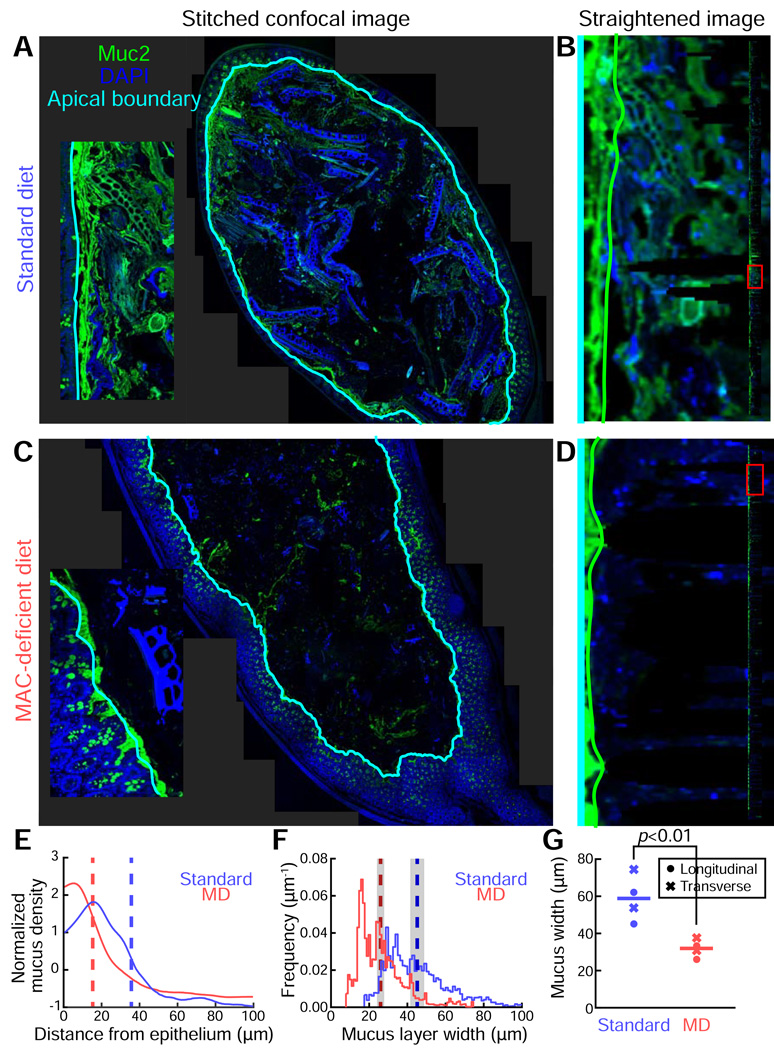
Using BacSpace, we stitched tiled images of distal colon cross-sections from standard diet- and MD diet-fed mice (Fig. 2A,C). The epithelial boundary was identified based on autofluorescence in the DAPI channel as described above, and the image was straightened to allow for accurate measurements of inner mucus thickness along the boundary (Fig. 2B,D). To quantify mucus thickness and to study its variation along the perimeter of the fecal pellet, the outer edge of the inner mucus layer was identified by the gradient of MUC2 immunofluorescence in the direction perpendicular to the epithelium. By computing the mucus fluorescence signal intensity, averaged along the length of the epithelium and normalized as a function of distance from the epithelium out into the lumen (Fig. 2E), we observed an MD diet-induced thinning of the inner mucus layer. To compare changes in mucus thickness between samples, we noted that the thickness varied subtly across space, and hence measurements at nearby locations along the epithelium were not independent. To account for this dependence, we computed the spatial autocorrelation function, and found similar correlation lengths of 21 µm (standard diet) and 16 µm (MD diet) (Fig. S2). These calculations indicate that, for statistical purposes, a conservative estimate is that independent measurements can be obtained every ∼50 µm (Bayley, 1946). Thus, the epithelial perimeter of the standard diet-fed mouse cross-section (∼40 stitched images) of 9.4 mm was equivalent to 180 independent measurements, while that of the MD diet-fed mouse (8.8 mm) represented 176 independent measurements.
Figure 2. Large-scale image reconstruction of the distal colon enables quantification of mucus thickness and variability within a sample and between dietary conditions.
(A,C) Stitched images (∼40) of the fecal pellet-containing distal colons of Bt-monocolonized mice fed a (A) standard diet or (C) an MD diet. Samples were stained with DAPI (blue) and a MUC2 antibody (green), and overlaid with the computationally identified apical boundary (cyan).
(B,D) Detection of the edge of the inner mucus layer (green line) along a short length of the epithelium, corresponding to the insets in (A) and (C), in mice fed (B) a standard diet or (D) an MD diet. Edges were detected via computational straightening of the image. Inset: full straightened image along boundary of an entire gut segment. The red box highlights the portion of the segment visualized in the main panel.
(E) Mucus density versus distance from the epithelium for the colon sections in (A) (blue) and (C) (red), averaged over the length of the epithelium. Curves are mean-subtracted and divided by the standard deviation for normalization. Dashed vertical lines mark the location of the maximum gradient, indicating the edge of the inner mucus layer.
(F) Distribution of mucus layer widths in the colon sections shown in (A) (blue) and (C) (red), measured along the boundary of the entire gut segment. Dashed vertical lines indicate the mean widths, and shaded regions indicate standard error of the mean, accounting for sample autocorrelation.
(G) Average mucus width for mice fed standard and MD diets. Circles indicated measurements taken from longitudinal sections, and crosses indicate measurements taken from transverse sections; each data point represents an individual mouse (n=4 mice per diet). Horizontal lines indicate average width. P-value reflects one-sided t-test.
Having determined that a cross-section of a fecal pellet represents a large number of independent measurements, we investigated the distribution of mucus-layer widths (Fig. 2F). In standard diet-fed mice, the average mucus thickness was 45±1 µm (S.E.M). An MD diet significantly decreased the average mucus thickness to 26±1 µm (one-sided, p<10−30). Interestingly, the overlap between the mucus thickness distributions in the two dietary conditions (Fig. 2F) indicates that there is a reasonable probability (∼9%) that a comparison between single, randomly selected fields of view will indicate thicker mucus in samples derived from an MD diet-fed mouse than in samples from a standard diet-fed mouse, illustrating the critical need for automated analysis of large-scale imaging datasets.
In measuring mucus thickness, we primarily used longitudinal rather than transverse sections, as the approximate cylindrical symmetry of the distal colon suggests that longitudinal sections better sample the organizational diversity of the intestinal tract. However, in the lumen of longitudinal sections, even those near the midplane, the nearest epithelial tissue could potentially lie out of the plane of the section; in such a case our measurements of distance to the epithelium would over-estimate the true distance. To address this possibility, we investigated transverse sections and explored the full range of shifts that could be expected due to three-dimensional curvature. For the typical depths of the longitudinal slices we examined, our transverse measurements placed an upper bound of 25±5% for particular regions of the longitudinal slice with non-optimal out-of-plane curvature (Fig. S2). Heterogeneity in the 3D curvature along the longitudinal slice would ameliorate this source of error, and we found a highly significant (p=0.008, one-sided t-test) difference in mucus thickness across dietary conditions for transverse and longitudinal slices (Fig. 2G).
Bacteria are in closer proximity to the epithelium when diet lacks microbiota accessible carbohydrates
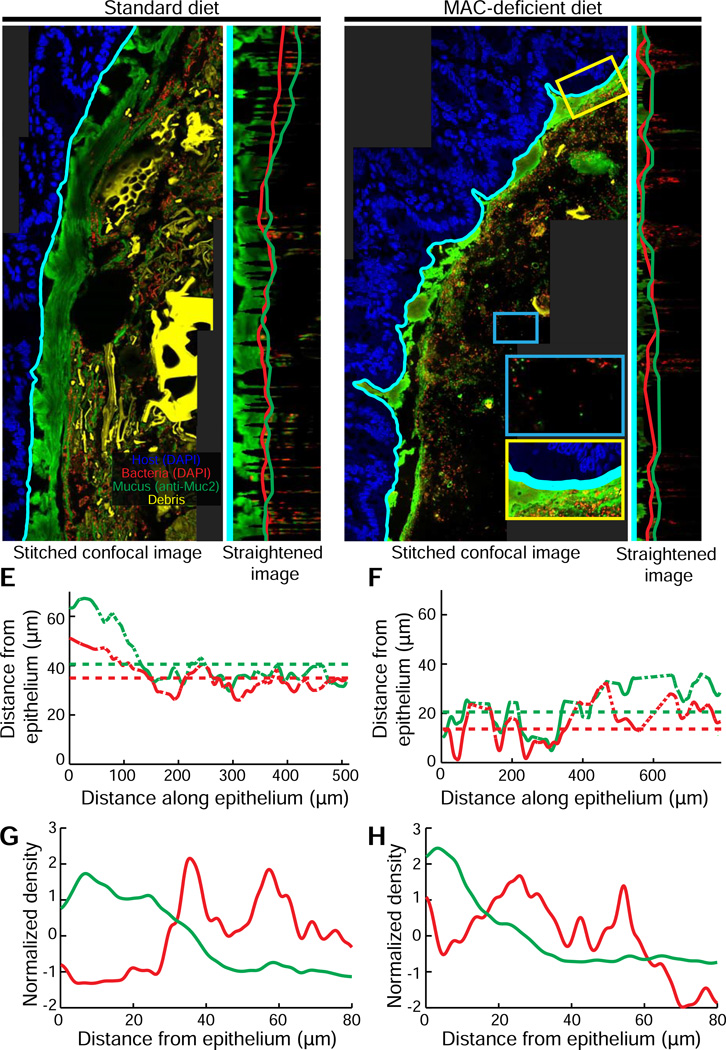
To determine whether bacterial proximity to host tissue is altered in the presence of the thinner mucus that we observed in MD diet-fed mice, we performed confocal imaging of DAPI- and MUC2-labeled tissue at a higher magnification (63x) to enable the detection of individual bacterial cells.
One significant challenge for automated image analysis in the gut is the autofluorescent dietary plant matter in the lumen (Fig. 2A). BacSpace differentiates signal from microbial cells from other luminal autofluorescent material based on size and brightness (Fig. S1). Once non-bacterial signal has been identified, the DAPI image signal is separated into three categories: (i) host cells (non-luminal DAPI signal), (ii) non-microbial autofluorescent material and shed host cells and (iii) microbial cells (remaining luminal DAPI signal), which can be further classified based on other staining methods such as FISH.
Using BacSpace, we obtained stitched tiled colon image sets from standard diet- and MD diet-fed Bt-monocolonized mice (Fig. 3A,C, respectively) and separated the epithelial, bacterial, and luminal autofluorescence signals as described above. We then straightened the images from the MUC2 and DAPI channels to measure the distribution of mucus and bacterial signal as a function of distance from the epithelium (Fig. 3B,D). Consistent with our lower-resolution measurements of whole fecal pellets (Fig. 2), an MD diet resulted in a thinner mucus layer of 20±2 µm compared to the standard mouse diet with thickness 40±3 µm (one-sided t-test, p<0.002) (Fig. 3E,F). Moreover, at higher resolution, we were able to determine that the decreased mucus thickness in MD diet-fed mice was also correlated with increased proximity of bacteria to the epithelium (mean distances: MD diet, 14±2µm; standard diet, 35±2 µm, p<10−5) (Fig. 3E,F). This proximity of Bt to host tissue results from both the thinner mucosal layer in the MD diet condition and from bacterial invasion of this layer (yellow inset in Fig. 3C). In addition, we noted sparser bacterial localization in the lumen in the absence of dietary MACs (blue inset in Fig. 3C; Fig. 3G vs. 3H), consistent with a shift in bacteria localization towards the mucus layer.
Figure 3. Diet change drives a shift in microbiota localization in the distal gut.
(A,C) Higher-resolution stitched images of the distal colon of Bt-monocolonized mice fed (A) a standard diet and or (C) an MD diet (C). Yellow inset (C): bacteria invading mucus layer. Blue inset (C): region far from the epithelium with few bacteria.
(B,D) Computationally straightened versions of the region of images shown in (A) and (C), respectively. Computationally determined edges defining the boundary of the inner mucus layer (green line) and the proximity to host epithelium of bacterial colonization (red line).
(E,F) Variation in width of inner mucus layer (green) and inner boundary of bacterial colonization (red) along the epithelium for mice fed (E) standard or (F) MD diets. Dashed portions of traces indicate regions where interpolation is required due to contour curvature. Dotted horizontal lines indicate the average widths (excluding interpolated points).
(G,H) Density, as determined by fluorescent intensity, of bacteria (red line) and mucus (green line) plotted in distance away from the epithelium for mice fed a (G) standard or (H) MD diet. Curves are mean-subtracted and divided by the standard deviation for normalization.
A MAC-deficient diet alters the spatial organization of a complex microbial community
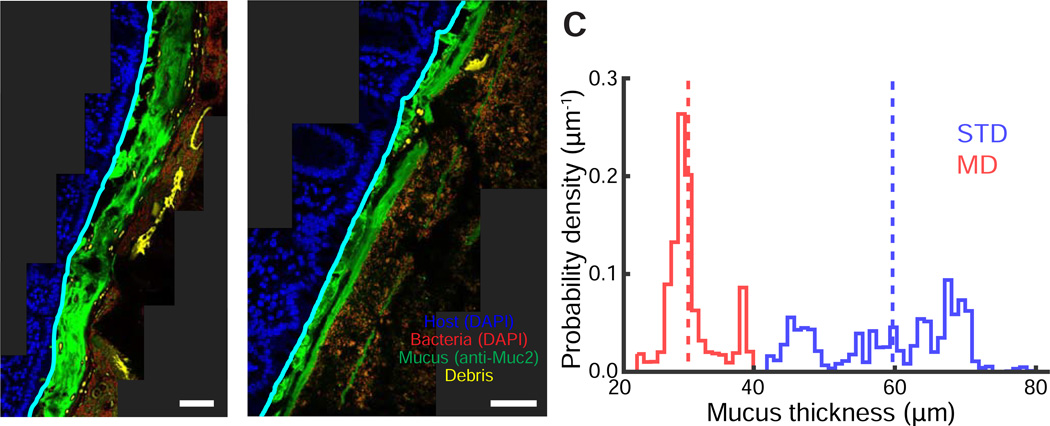
Our observation that an MD diet results in thinning of the inner mucus layer of Bt-monocolonized mice prompted us to query whether this effect would also be observed within complex microbiota-colonized mice harboring diverse mucus-utilizing bacterial species. To address this question, we colonized germ-free mice with human fecal microbiota by oral gavage and allowed the community to equilibrate for 40 days (Marcobal et al., 2013; Turnbaugh et al., 2009). We then performed a 10-day diet shift wherein half the humanized mice were switched to an MD diet. As before, we used BacSpace to stitch MUC2-and DAPI-labeled distal colon images and measured mucus thickness for both standard and MD diet conditions (Fig. 4A–C, Fig. S3). Consistent with our results from Bt-monocolonized mice, mucus thickness was significantly reduced from 59±2 µm (mean±standard error of the mean) on a standard diet to 31±1 µm on an MD diet (one-sided t-test, p<10−6; Fig. 4C).
Figure 4. Diet changes affect the spatial organization of a complex intestinal community.
(A,B) Distal colon sections from humanized mice fed a (A) standard or (B) MD diet. Sections were stained with DAPI (epithelial signal, blue; luminal signal, red) and with MUC2 antibodies (green). Computed epithelial boundary is outlined in cyan, and debris appears in yellow.
(C) Distribution of mucus layer thickness in mice fed a standard (blue) or MD (red) diet. Dashed line represents the mean. Images are representative of two imaged mice per condition; replicates are shown in Fig. S3.
BacSpace enables quantitative analysis of microbial spatial organization
We sought to probe whether cross-feeding interactions influence the proximity of two species in a simplified gut community. To examine this, we bicolonized mice with an avirulent strain of St (Stavir) , which eliminates the inflammatory effects caused by SPI-1- and SPI-2-mediated invasion on localization, and with either wildtype Bt or the sialidase-deficient strain BtΔBT0455 (Ng et al., 2013).
Proximal colon sections were labeled with DAPI and Cy5-conjugated Salmonella and Enterobacteriaceae-specific probes to distinguish St (positive for both Cy5 and DAPI) from Bt (DAPI only) (Fig. S4A,B). After using BacSpace to identify and to subtract epithelial tissue and luminal debris, we used a two-step thresholding process to convert each image into a binary mask. Overlapping regions in the DAPI and Cy5 masks were identified as St cells, while regions of the DAPI mask that did not overlap with the Cy5 mask were identified as Bt. Information about the proximity of Bt to St cells (and vice versa) could be garnered with equal effectiveness by computing the distances between pixels identified as Bt and St cells, as these could be clearly differentiated, making subdivision of cell clusters unnecessary.
To test the proximity of St cells to Bt cells, for each pixel in an St region, we computed the distance to the nearest Bt pixel, and vice versa. When we compared inter-species distances in mice colonized with St and either the wild-type Bt or the mutant Bt, we found few differences between the distributions of distances from St cells to the nearest Bt cell (Fig. S4C) and from Bt cells to the nearest St cell (Fig. S4D) indicating that proximity of the two strains is not dependent upon Bt liberation of sialic acid.
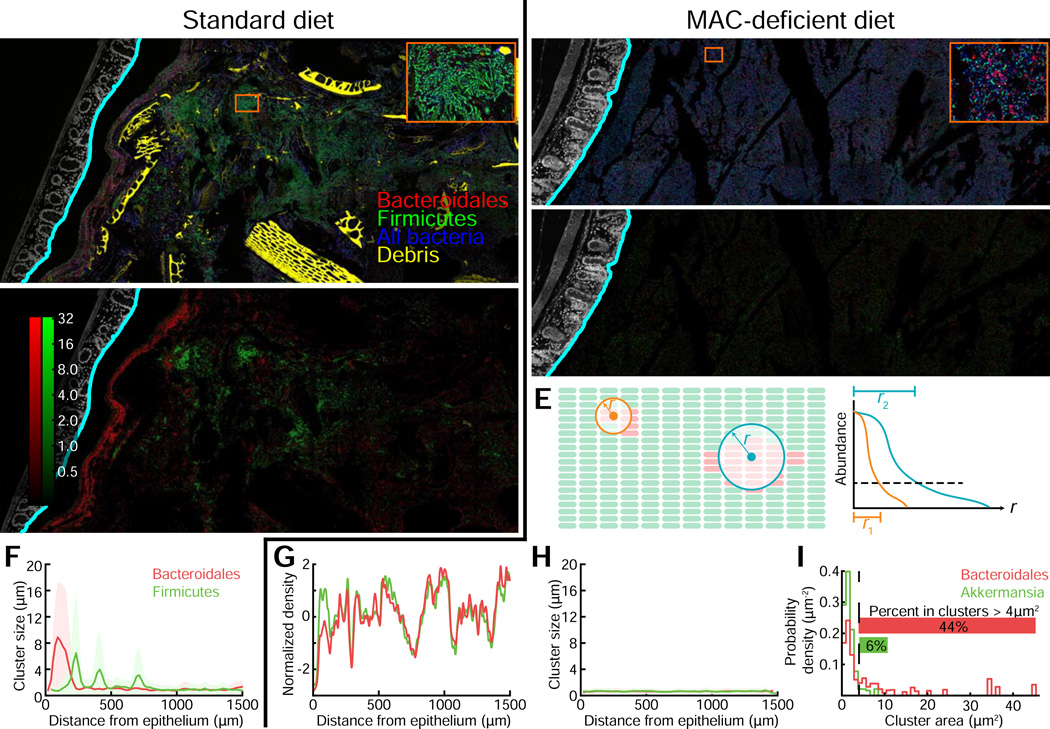
In our monocolonized model, the density of Bt cells increased closer to the epithelium as a result of the shift to an MD diet (Fig. 3), consistent with the role of mucus as both a barrier and a dietary source for bacteria. To explore the role of mucus in driving bacterial localization in more complex communities, we investigated enrichment of bacteria capable of consuming mucin glycans near the mucus in a complex colonization state (Fig. 5). Due to the high abundance of Bacteroides in the gut, we expected that shifts in localization of Bacteroides would be accompanied by altered localization of other abundant taxa within the Firmicutes. To detect diet-driven shifts in localization, we applied FISH probes with specificity for Bacteroidales, Firmicutes, Akkermansia, and Bifidobacterium taxa, as well as DAPI to detect all bacteria, to distal colon sections of both MD and standard diet-fed mice. The Bifidobacterium probe was not included in the final images, as this genus was not detectable in either in vivo condition (Fig. S5A,B).
Figure 5. Diet changes affect clustering and mixing of a complex intestinal community.
(A,B) Distal colon sections from humanized mice fed a (A) standard or (B) MD diet. Sections were stained with DAPI (epithelial signal, white; luminal signal, blue), and FISH probes Bac303 (Bacteroidales, red) and LGC354A–C and Erec482 (Firmicutes, green). Computed epithelial boundary is outlined in cyan, and debris appears in yellow. Insets show a large Firmicutes cluster (A) and small Bacteroidales clusters (B). Images are representative of two imaged mice per condition; results for replicates are shown in Fig. S6.
(C,D) Pixel intensity corresponds to measured cluster size in images (A) and (B), respectively, for Bacteroidales (red) and Firmicutes (green). Intensities are on a log scale for clarity, and the same color scale is used for both images.
(E) Schematic of cluster size calculation: for each probe, the fluorescence signal in neighborhoods of increasing radii was integrated. For a small cluster (orange), the signal decays rapidly with increasing radius. For a large cluster (blue), the signal decays more slowly with increasing radius.
(F,H) Mean cluster size of Bacteroidales (red) and Firmicutes (green), computed as in (C-E), versus distance from epithelium, for mice fed a (F) standard or (H) MD diet. Shaded regions indicate the standard deviation of cluster widths. For computing means, a bin size of 20 µm was used.
(G) Density of Bacteroidales (red) and Firmicutes (green) as a function of distance from epithelium for mice fed an MD diet. Curves were mean-subtracted and divided by the standard deviation for normalization. Solid lines are measured from samples in (A) and (B).
(I) Distribution of Akkermansia and Bacteroidales cell clump sizes in MD-fed humanized mice. An area threshold of 4 µm2 (dotted line) was used to classify clusters vs. single cells.
Under standard diet conditions, co-labeling with Bacteroidales and Firmicutes FISH probes revealed clusters of Bacteroidales that appeared to mostly exclude Firmicutes and clusters of Firmicutes that appeared to mostly exclude Bacteroidales (Fig. 5A, Fig. S6). To quantify and identify clusters, we processed large tiled images with BacSpace and then investigated how the abundance of each taxon within a neighborhood of each pixel scaled with neighborhood size (Fig. 5E). The intensity values in the resulting transformed image correspond to cluster size and support homologous clustering within these groups (Fig. 5C,F, Fig. S6).
After a 10-day shift to an MD diet, however, the large homologous clusters of Bacteroidales or Firmicutes disappeared, and a much more evenly mixed community is evident (Fig. 5B,D). Using BacSpace to measure the density of each taxon, we observed that the abundance of Bacteroidales strongly co-varied with the abundance of Firmicutes (Fig. 5G) and observed few large clusters of either taxon (Fig. 5H), suggesting a well-mixed community. In the standard diet, bacteria labeled with the Akkermansia probe Muc1437 were undetectable. In contrast, after a 10-day shift to an MD diet, Akkermansia were visibly abundant (Fig. S5), consistent with previous sequencing data from humanized mice fed an MD diet (Marcobal et al., 2013). However, this change to reliance on host mucins and the resulting bloom in a prolific mucin-utilizer did not coincide with any spatial enrichment of Akkermansia near the inner mucus (Fig. S5). Another notable organizational phenotype in this diet was the formation of smaller Bacteroidales-specific cell clusters that were composed of ∼10–15 cells (Fig. 5B, inset). We used a two-step thresholding process to convert each image into binary masks defining Bacteroidales and Akkermansia by FISH probe fluorescence. From these masks, we identified cell clusters and quantified the probability density function of being in a cluster of a given size. Forty-four percent of cells identified as Bacteroidales appeared in clusters larger than 4 µm2; in contrast, only 6% of Akkermansia cells were in clusters of this size or larger (Fig. 5I).
Quantitative analysis of the spatial pattern of host protein expression
The close proximity of bacteria to host tissue in the absence of dietary MACs led us to hypothesize that the host tissue would exhibit signs of heightened inflammatory response. We previously reported that the soluble stool proteome provides a sensitive measure of host intestinal physiological status in mice (Lichtman et al., 2013). To identify host proteins secreted into the luminal environment, we conducted a mass spectrometry-based survey of soluble stool proteins from groups of humanized mice maintained on either a standard (n=2) or an MD diet (n=3) for 14 weeks, and a group of germ-free mice (n=3) switched to an MD diet for 14 weeks. In order to identify microbiota- and diet-dependent effects, we identified proteins enriched in humanized mice fed an MD diet compared to both standard diet-fed humanized and MD diet-fed germ-free mice. The antimicrobial lectin regenerating islet-derived protein 3 beta (REG3β) was one of the most enriched proteins (6.1-fold and 5.3-fold enriched in two MD diet-fed humanized mice relative to the average of two standard diet-fed humanized mice; see Table S1 for a complete list of proteins enriched in the MD dietary condition).
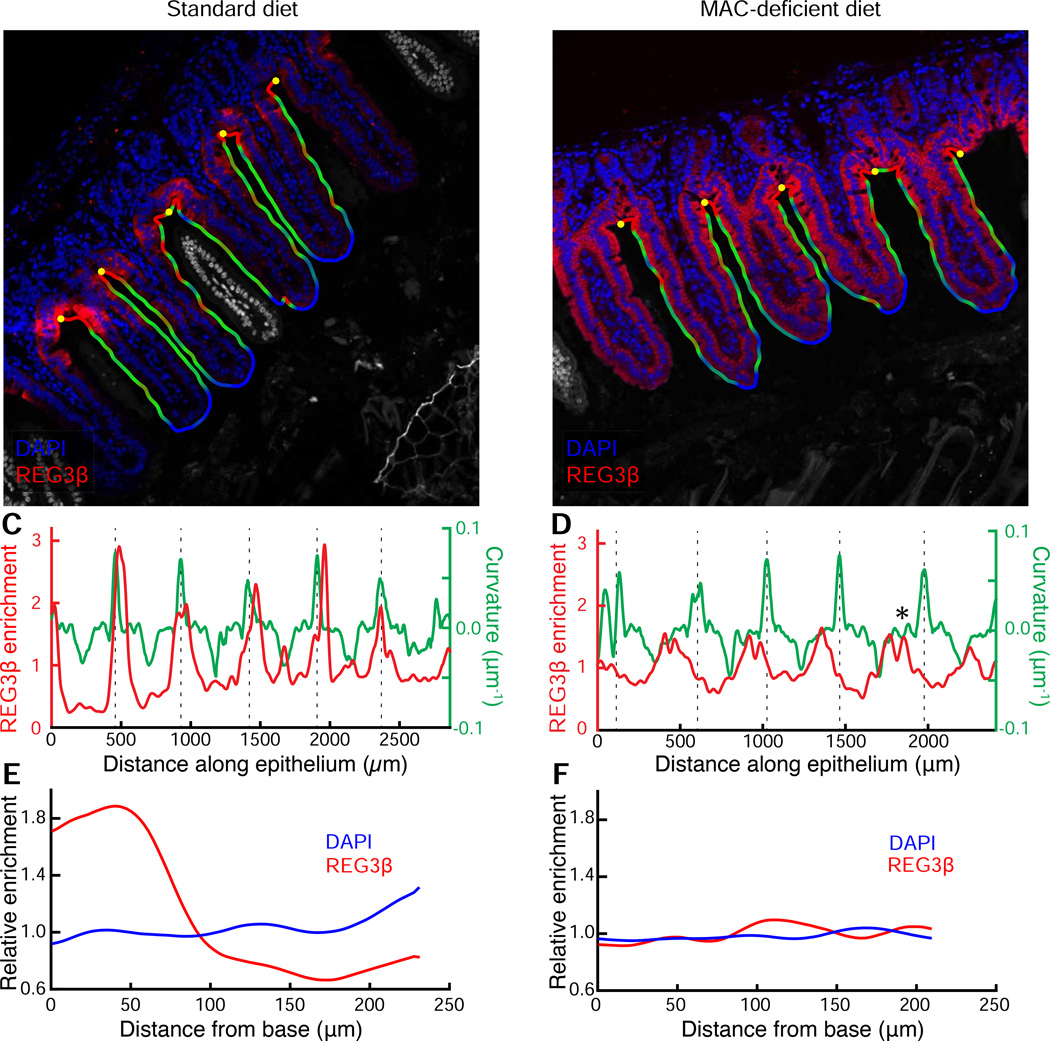
To validate this finding, we used an anti-REG3β antibody to assess expression in the small intestine, where REG3β is predominately expressed (Burger-van Paassen et al., 2012), of standard and MD diet-fed humanized mice and quantified the immunofluorescence spatial patterns using BacSpace, further illustrating the versatility of the software. Supporting our proteomic data, MD diet-fed mice exhibited higher overall fluorescence intensity of REG3β staining in the ileum (Fig. 6A,B). In addition, qualitative differences in the spatial pattern of expression were apparent, with a more uniform REG3(3 distribution along the epithelium of MD diet-fed mice (Fig. 6B). Though the antibody may cross-react with REG3γ, a closely related C-type lectin also expressed in the small intestine, our proteomics data indicate that there is no enrichment in REG3γ in MD diet-fed humanized mice (Table S1). To quantify the fluorescence signal along the length of the epithelium, we modified our previous protocol slightly: using the straightened image generated by BacSpace, we integrated the fluorescence signal from the epithelial boundary to the points with a closest approach of 4 µm into the mucosa (as opposed to out into the lumen as in the analyses in Fig. 2–Fig. 5). To relate REG3β expression to anatomical features, we computed the curvature of the epithelial boundary; the base of the villi corresponded to peaks in curvature. In mice fed a standard diet, sharp peaks in REG3β signal (up to 6-fold enrichment over baseline) colocalized with peaks in curvature (Fig. 6C). In contrast, MD diet-fed mice exhibited less variation in fluorescence signal along the length of the epithelium, and there were maxima in REG3β enrichment that did not correspond to maxima in curvature (Fig. 6D).
Figure 6. Diet change alters the innate immune response in the ileum.
(A, B) Ileum of mice fed a (A) standard or (B) MD diet. Samples were stained with DAPI (blue) and REG3β antibody (red). The computationally identified outline is colored by contour curvature, and the computationally identified base of the villi is shown with yellow dots. The region outside the contour, including villi that are not continuous with the imaged epithelium due to sectioning angle, are colored gray. Images are representative of two imaged mice per condition; results for replicates are shown in Fig. S7, A-F.
(C, D) Variation in enrichment of REG3β normalized to the mean, as determined by fluorescence intensity (red) and curvature of epithelial contour (green) along the length of the epithelium. Dashed lines indicate the base of the villi. Asterisk in (D) indicates an example of a maximum in REG3β signal that is not associated with a maximum of curvature.
(E, F) Distribution of REG3β signal (red) and cell nuclei (DAPI, blue) relative to the base of the villi in a (E) standard or (F) MD diet.
To quantify the difference in REG3β expression patterns, we computationally identified the bases of the villi based on peaks in boundary curvature and measured the variation in REG3β signal as a function of distance along the epithelial boundary from the villi bases. In standard diet-fed mice, REG3β expression was enriched by 2- to 3-fold at the base of the villi relative to the villi tips (Fig. 6E). In contrast, MD diet-fed mice had nearly uniform expression of REG3β that extended from the base to the tip of the villi (Fig. 6F, Fig. S7). Thus, the diet-associated induction of REG3β corresponds to a spatially heterogeneous change in expression correlated with the geometry of the epithelium. Notably, the change in REG3β expression pattern mirrors that in a previous study that reported enhanced expression of REG3β in the small intestine of MUC2(−/−) mice, which have a genetically compromised mucosal barrier, spontaneously develop colitis, and have bacteria in close proximity to the epithelium (Burger-van Paassen et al., 2012). Therefore, bacterial localization changes connected to alterations in the host mucus barrier can drive alterations in small intestinal REG3β expression. These findings also demonstrate that BacSpace can be readily applied to a wide variety of imaging datasets involving proximity of any fluorescence signal to an extended boundary.
Colonization of Helicobacter pylori (Hp) in gastric glands is locally associated with host cell proliferation
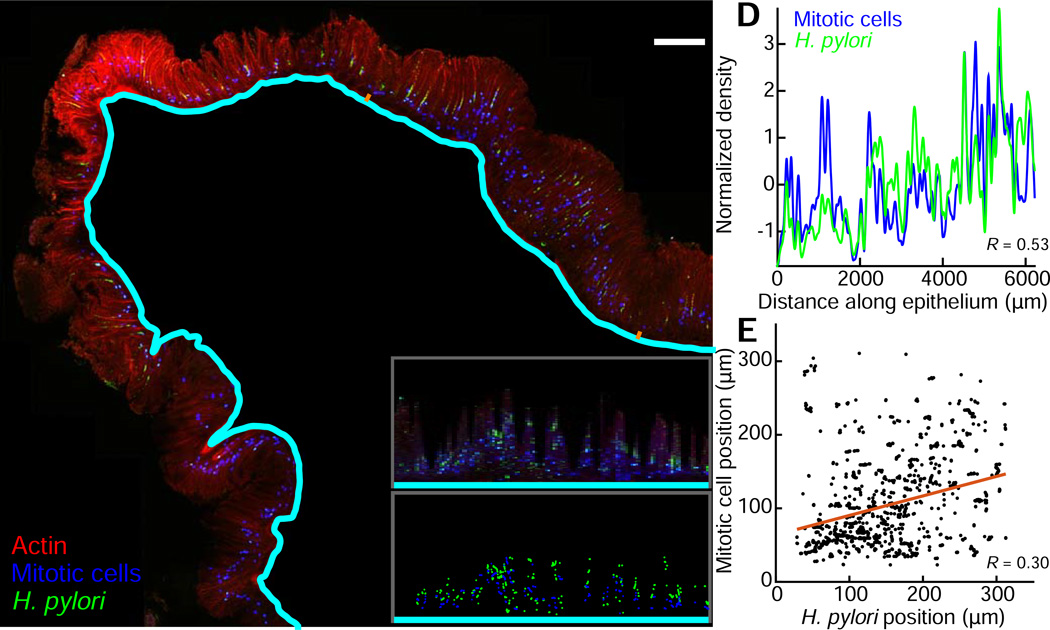
In addition to host protein expression, we applied BacSpace to quantify the spatial distribution of bacteria that reside within the mucosa. A population of Hp, a common member of the stomach-resident microbiota, colonizes not just the mucus layer on the surface of the stomach but also penetrates and forms microcolonies deep in the glands of the antrum (Howitt et al., 2011). More recently, we reported that Hp drives Lgr5+ stem cell proliferation in the stomach glands it colonizes, leading to hyperplasia. We further found that glands that are colonized with Hp contain more mitotic cells than glands without Hp (Sigal et al., 2015). To quantify Hp-driven host cell proliferation, we imaged sections of mouse stomachs labeled with an anti-Hp antibody, an anti-phospho-Histone-H3 antibody indicating mitotic host cells, DAPI, and the actin label phalloidin (Fig. 7A). Using the algorithm employed in Fig. 2–Fig. 6 to identify the apical boundary of the epithelium in DAPI-labeled sections, we used BacSpace to identify the base of the mucosa based on actin enrichment in the submucosa, visualized with fluorescently conjugated phalloidin. Despite the differences in tissue type, imaging modality, and length scale, BacSpace successfully identified the base of the mucosa. We used the straightened image generated from BacSpace to measure the variation in density of Hp and mitotic cells along the length of the longitudinal section (Fig. 7B,D). We found that the density of Hp was correlated with the density of mitotic host cells (Pearson correlation coefficient = 0.53, p<10−10), confirming and expanding on our previous work involving manual counting (Sigal et al., 2015). We then used the power of this computational approach to characterize the depth of Hp colonization relative to the nearest mitotic cells. To test whether host cells that are near Hp bacteria are more proliferative, for each contour point in the straightened image generated by BacSpace we measured the median position of Hp and the median position of mitotic cells, relative to the base of the mucosa (Fig. 7C). For contour points in which we found both Hp and mitotic cells, we found that the position of Hp is correlated with the position of mitotic cells (Fig. 7E, R=0.34, p=0.0007). The results (i) provide additional support for the ability of Hp, which can cause gastric cancer, to locally influence cell division within the stomach, and (ii) illustrate the general utility of our computational approach to a wide variety of biological problems.
Figure 7. BacSpace reveals spatial coordination between H. pylori gastric gland colonization and mitotic progenitor cell division in the stomach.
(A) Longitudinal section of the stomach antrum two weeks post infection with Hp. F-actin is stained with phalloidin (red), and Hp (green) and phospho-Histone-H3 (blue, indicating mitotic cells) are visualized by immunolabeling. BacSpace detection of the mucosal boundary appears in cyan. Images and quantification are representative of two imaged mice; results for replicates are shown in Fig. S7, G-H.
(B) Computationally straightened segment of the section in (A), corresponding to the epithelial region marked by orange dashes in (A).
(C) Median distance from the mucosal boundary of Hp (green dots) and mitotic nuclei (blue dots) for positions in (B) along the epithelium in which both Hp and mitotic cells are detected.
(D) Variation in density (normalized by z-score) of Hp (green) and mitotic cells (blue) along the length of the antrum indicates correlation (Pearson correlation coefficient 0.53) between the Hp and mitotic cell fluorescence in the direction away from the submucosal boundary.
(E) Average distance from submucosal boundary of Hp and mitotic cells at positions along the antrum for which both are present.
Discussion
The gastrointestinal tract is a heterogeneous environment. Within the lumen, microbes can cling to one another, partition within or be excluded from difficult-to-detect biochemical gradients, or attach to scaffolds of food particles, mucus, or sloughed epithelial cells. At the microbial-host interface, local immune responses directed toward gut microbes and differences in mucus expression and structure along the length of the gut affect microbiota community functions. Since single fields of view are generally unable to capture the full scope of this variability across length scales, a pipeline for analyzing large imaging datasets is critical for building an understanding of microbiota localization in an unbiased fashion.
In this study, we combined mucus preservation and labeling methods with confocal microscopy to generate images that visualize the microbiota at single-cell resolution. To facilitate image analyses, we developed computational tools that automate stitching, subtraction of non-specific signal, and bacterial localization measurements in the gastrointestinal environment. This method allows rigorous study of the spatial relationships among various bacterial taxa and the host. It is important to note that fixing and sectioning the gut introduces changes to the native state of the mucus due to its high water content. Methacarn fixation of colonic tissue results in shrinkage of mucus thickness and generation of spaces lacking mucus adjacent to the epithelium (Ermund et al., 2013; Johansson et al., 2008). While the methods we employ appear to preserve much of the spatial arrangement of host tissue, mucus, and luminal contents including microbes, comparative measurements between samples handled in the same manner and inclusion of proper controls are necessary. BacSpace should facilitate aggregation of data over many fields of view for each sample, or across multiple planes through a z-stack (Johansson and Hansson, 2012) to help mitigate error introduced through experimental manipulation.
Underlying the coevolution of humans and their gut commensals has been a diet rich in microbiota-accessible carbohydrates such as plant polysaccharides. A low-fiber diet characteristic of industrialized countries has long been hypothesized to contribute to Western diseases (Burkitt, 1973; Sonnenburg and Sonnenburg, 2014) such as IBD. Although the gut microbiota appears to mislocalize to host tissue and potentiates IBD (Johansson et al., 2014; Swidsinski et al., 2005), whether an interaction between diet and the gut microbiota contributes to the onset of IBD remains to be determined. Our work demonstrates that an MD diet results in thinner mucus, a closer proximity of bacteria to the mucosa, and an increase in expression of the antimicrobial innate immune protein REG3β. Taken together, available data suggest a model in which the loss of dietary MACs results in increased bacterial consumption of mucin glycans and microbial encroachment into the mucus barrier. While diet may also directly change the glycosylation, hydration, or structure of the mucus layer, our data capture dramatic changes in the spatial arrangement of the gut microbiota (Fig. 3,Fig. 5), a potential missing link in the mechanisms connecting diet, IBD, and the microbiota. Further work is needed to determine whether thinner mucus, altered mucus penetrability (Johansson et al., 2014), selection of mucus-degrading bacteria, and increased expression of innate immune defense proteins are precursors of IBD development.
We have also applied cell-to-cell measurements in defined and complex communities. We demonstrate that in a MAC-rich standard diet the intestinal microbiota contains large clusters dominated by related taxa, whereas, in mice fed an MD diet organization appears more homogeneous and small (∼10–15 cell) Bacteroidales-specific clusters occur regularly. Domains enriched in related taxa could arise from multiple factors, such as differential growth rates (e.g., blooms that fail to disperse), or selection within chemical microenvironments. Identification of particular species within the clusters using more specific labeling strategies or creating defined communities in gnotobiotic mice will help to elucidate the factors underlying this organization.
Microscopy and image analysis are underutilized tools in the study of the gut microbiota, but with improved histological methods and the computational tools provided here, we are now poised to address many more questions. Our high-resolution microscopic methods, gnotobiotic and humanized mouse models, and genetically modified microbes, combined with advances in complementary technologies such as imaging mass-spectrometry to determine localization of metabolites (Rath et al., 2012), will help to establish the rules governing localization within this diverse and heterogeneous community. Ultimately, these methods will be critical for defining the significant and biologically meaningful spatial relationships within biopsies from healthy and diseased human gut tissue.
Experimental Procedures
Mouse experiments
For gnotobiotic and humanized mouse experiments, germ-free Swiss-Webster mice were maintained in gnotobiotic isolators and fed either a standard polysaccharide-rich diet (Purina LabDiet 5K67) or a custom MAC-deficient diet composed of 68% glucose (w/v), 18% protein (w/v), and 7% fat (w/v) (Bio-Serv) (Sonnenburg et al., 2010). Mixed-gender mice aged 6–8 weeks were used for all experiments.
For Bt monocolonization diet experiments, mice were gavaged with 108 CFUs Bt, switched to the MD diet the day of colonization, and maintained on this diet for 10 days before sacrifice. For Bt and Salmonella typhimuriumavir (Stavir; SL1344 ΔSPI-1ΔSPI-2 (orgA::Tet, ssaV::Kan) (Broz et al., 2010)) bicolonized mice, 200 µL each of overnight cultures of Bt and Stavir were administered by gavage. Bicolonized mice were maintained on a standard diet, and sacrificed 5 days post-gavage.
For humanized mouse experiments, germ-free mice were colonized with a complete human microbiota by gavage of a fecal sample from a healthy anonymous donor and given 40 days for the microbiota to equilibrate prior to diet shift (Marcobal et al., 2013). After community equilibration, mice were either maintained on the standard diet or shifted to the MD diet for short-term (10 days) or long-term (14 weeks) experiments.
For Hp-infected-mice, six-week-old male C57BL/6 mice were infected with a single oral dose of 108 Hp and sacrificed 2 weeks post infection.
Tissue collection and processing
Mice were sacrificed by CO2 asphyxiation followed by cervical dislocation, and sections of the ileum, proximal colon, and distal colon were collected and placed in cassettes. Tissues were fixed and processed as previously described (Johansson and Hansson, 2012). Briefly, tissues were fixed by immersion in methacarn solution (60% methanol, 30% chloroform, 10% acetic acid) for 48 h, followed by two successive washes each in methanol for 35 min, ethanol for 30 min, and xylene for 25 min. Tissue samples within cassettes were then submerged in melted paraffin at 68 °C for 1 h, removed, and kept at room temperature until sectioning. Paraffin blocks were cut into 4 µm-thick sections and deparaffinized for immunofluorescence and FISH (Johansson and Hansson, 2012).
Immunostaining
After deparaffinization and rehydration, slides were incubated in antigen retrieval solution (10 mM sodium citrate, pH 6.0) at 90 °C for 30 min. Sli des were blocked with blocking buffer (5% bovine serum albumin in PBS) for 30 min at room temperature in a humid chamber. For mucus visualization, a polyclonal rabbit anti-mouse Muc2-specific antibody (Santa Cruz Biotechnology) was diluted 1:100 in blocking buffer, applied to the slide, and incubated at 4 °C for 4–18 h. Slides were washed three time s in PBS. The secondary antibody (Alexa Fluor 488 Affini-pure donkey anti-rabbit IgG, Jackson ImmunoResearch) was diluted 1:100 in blocking buffer with 10 µg/mL DAPI (Sigma), added to the sample, and incubated in the dark at 4 °C for 1 h. REG3β staining was performed similarly with a polyclonal sheep anti-mouse REG3β antibody (R&D Systems) diluted 1:100 in blocking buffer and donkey anti-sheep secondary antibody diluted 1:250 in blocking buffer (Alexa Fluor 594 Affini-pure, Jackson ImmunoResearch). After incubation with the secondary antibody and DAPI, slides were washed three times in PBS, dried, and mounted with Vectashield mounting medium (Vector Labs). Slides were stored at 4 °C in the dark until imaging.
FISH preparation
FISH was performed as previously described (Johansson and Hansson, 2012). Briefly, deparaffinized sections were incubated with FISH probes (Stanford Protein and Nucleic Acids facility) diluted to 10 ng/µL in hybridization buffer (0.9 M NaCl, 20 mM Tris-Hcl (pH 7.4), 0.01% sodium dodecyl sulfate, 10% formamide) at 47 °C for 3–18 h. Slides were incubated with FISH washing buffer (0.9 M NaCl, 20 mM Tris-HCl (pH 7.4)) preheated to 47 °C f or 10 min, and washed three times in PBS. Samples were then incubated with 10 µg/mL DAPI in PBS in the dark for 1 h at 4 °C , washe d three times in PBS, allowed to dry, and mounted in Vectashield mounting medium. The fluorescently labeled DNA probes used in this study are listed in Table S2.
Gnotobiotic and humanized mice imaging
Images were acquired on a Zeiss LSM 700 confocal microscope with the ZEN 2012 (Fig. 5 and S6) or Zen 2009 (all other figures) software (Zeiss). With the exception of the images in Fig. 2 and 7, which were acquired at 20x, samples were imaged with a 63x oil-immersion objective with optical sections taken at 0.29-µm resolution. Images were acquired at a frame size of 2048×2048 with 16-bit depth. For Fig. 5 and S6, tile scans were acquired on a microscope equipped with a mechanical stage. For all other figures, we acquired a collection of overlapping images by manual translation of the microscope stage, with several optical sections for each field of view to ensure that focal drift during stage translation would not preclude stitching of the images.
Detailed methods and additional analyses are available in the Supplementary Experimental Procedures.
Supplementary Material
Acknowledgments
We thank members of the Huang and Sonnenburg labs for helpful discussions, Steven Higginbottom for outstanding gnotobiotic technical support, Pauline Chu for histology expertise, and Jon Lynch for providing BtΔBT0455. Stavir was a gift from Petr Broz and Denise Monack of Stanford. Preliminary work on this project was performed by Megan Bergkessel, Sarah Carden, Katharine Ng, and Anshul Rana as a part of the Spring 2012 BioE 45 course. K.C.H. acknowledges the Stanford Systems Biology Center funded by NIH grant P50 GM107615. This work was funded in part by NSF Graduate Research Fellowships (to G.B. and K.A.E.), a Stanford Graduate Fellowship (to K.A.E.), NSF CAREER Award MCB-1149328 (to K.C.H.), NIH Director’s New Innovator Awards DP2-OD006466 (to K.C.H.) and DP2-OD006515 (to J.L.S.), a MathWorks Curriculum Development Award (to K.C.H. and J.S.), NIH R01-DK085025 (to J.L.S.), an unrestricted gift to the Stanford Microbiome Research Fund from the Samarth Foundation, and an Investigators in the Pathogenesis of Infectious Disease Award from the Burroughs Wellcome Fund (to J.L.S.).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Author Contributions
K.E., G.B., M.S., and J.L. conducted the experiments, K.E., G.B., M.S., J.L., J.E., M.A., K.H., and J.S. designed the experiments, G.B. and K.H designed the software, K.E., G.B., K.H., and J.S. wrote the paper. All authors analyzed data and provided comments on the manuscript.
References
- Barr JJ, Auro R, Furlan M, Whiteson KL, Erb ML, Pogliano J, Stotland A, Wolkowicz R, Cutting AS, Doran KS, et al. Bacteriophage adhering to mucus provide a non-host-derived immunity. Proceedings of the National Academy of Sciences of the United States of America. 2013;110:10771–10776. doi: 10.1073/pnas.1305923110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bayley GVHJM. The “Effective” Number of Independent Observations in an Autocorrelated Time Series. Supplement to the Journal of the Royal Statistical Society. 1946;8:184–197. [Google Scholar]
- Broz P, Newton K, Lamkanfi M, Mariathasan S, Dixit VM, Monack DM. Redundant roles for inflammasome receptors NLRP3 and NLRC4 in host defense against Salmonella. The Journal of experimental medicine. 2010;207:1745–1755. doi: 10.1084/jem.20100257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burger-van Paassen N, Loonen LM, Witte-Bouma J, Korteland-van Male AM, de Bruijn AC, van der Sluis M, Lu P, Van Goudoever JB, Wells JM, Dekker J, et al. Mucin Muc2 deficiency and weaning influences the expression of the innate defense genes Reg3beta, Reg3gamma and angiogenin-4. PloS one. 2012;7:e38798. doi: 10.1371/journal.pone.0038798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burkitt DP. Epidemiology of large bowel disease: the role of fibre. The Proceedings of the Nutrition Society. 1973;32:145–149. doi: 10.1079/pns19730032. [DOI] [PubMed] [Google Scholar]
- Dejea CM, Wick EC, Hechenbleikner EM, White JR, Mark Welch JL, Rossetti BJ, Peterson SN, Snesrud EC, Borisy GG, Lazarev M, et al. Microbiota organization is a distinct feature of proximal colorectal cancers. 2014;111:18321–18326. doi: 10.1073/pnas.1406199111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ermund A, Schutte A, Johansson ME, Gustafsson JK, Hansson GC. Studies of mucus in mouse stomach, small intestine, and colon. I. Gastrointestinal mucus layers have different properties depending on location as well as over the Peyer’s patches. American journal of physiology Gastrointestinal and liver physiology. 2013;305:G341–G347. doi: 10.1152/ajpgi.00046.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu J, Wei B, Wen T, Johansson ME, Liu X, Bradford E, Thomsson KA, McGee S, Mansour L, Tong M, et al. Loss of intestinal core 1-derived O-glycans causes spontaneous colitis in mice. The Journal of clinical investigation. 2011;121:1657–1666. doi: 10.1172/JCI45538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hooper LV, Macpherson AJ. Immune adaptations that maintain homeostasis with the intestinal microbiota. Nature reviews Immunology. 2010;10:159–169. doi: 10.1038/nri2710. [DOI] [PubMed] [Google Scholar]
- Howitt MR, Lee JY, Lertsethtakarn P, Vogelmann R, Joubert LM, Ottemann KM, Amieva MR. ChePep controls Helicobacter pylori Infection of the gastric glands and chemotaxis in the Epsilonproteobacteria. mBio. 2011;2 doi: 10.1128/mBio.00098-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johansson ME, Gustafsson JK, Holmen-Larsson J, Jabbar KS, Xia L, Xu H, Ghishan FK, Carvalho FA, Gewirtz AT, Sjovall H, et al. Bacteria penetrate the normally impenetrable inner colon mucus layer in both murine colitis models and patients with ulcerative colitis. Gut. 2014;63:281–291. doi: 10.1136/gutjnl-2012-303207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johansson ME, Hansson GC. Preservation of mucus in histological sections, immunostaining of mucins in fixed tissue, and localization of bacteria with FISH. Methods in molecular biology (Clifton, NJ) 2012;842:229–235. doi: 10.1007/978-1-61779-513-8_13. [DOI] [PubMed] [Google Scholar]
- Johansson ME, Larsson JM, Hansson GC. The two mucus layers of colon are organized by the MUC2 mucin, whereas the outer layer is a legislator of host-microbial interactions. Proceedings of the National Academy of Sciences of the United States of America. 2011;108(Suppl 1):4659–4665. doi: 10.1073/pnas.1006451107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johansson ME, Phillipson M, Petersson J, Velcich A, Holm L, Hansson GC. The inner of the two Muc2 mucin-dependent mucus layers in colon is devoid of bacteria. Proceedings of the National Academy of Sciences of the United States of America. 2008;105:15064–15069. doi: 10.1073/pnas.0803124105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lichtman JS, Marcobal A, Sonnenburg JL, Elias JE. Host-centric proteomics of stool: a novel strategy focused on intestinal responses to the gut microbiota. Molecular & cellular proteomics : MCP. 2013;12:3310–3318. doi: 10.1074/mcp.M113.029967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marcobal A, Kashyap PC, Nelson TA, Aronov PA, Donia MS, Spormann A, Fischbach MA, Sonnenburg JL. A metabolomic view of how the human gut microbiota impacts the host metabolome using humanized and gnotobiotic mice. The ISME journal. 2013;7:1933–1943. doi: 10.1038/ismej.2013.89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McHardy IH, Goudarzi M, Tong M, Ruegger PM, Schwager E, Weger JR, Graeber TG, Sonnenburg JL, Horvath S, Huttenhower C, et al. Integrative analysis of the microbiome and metabolome of the human intestinal mucosal surface reveals exquisite inter-relationships. Microbiome. 2013;1:17. doi: 10.1186/2049-2618-1-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nava GM, Friedrichsen HJ, Stappenbeck TS. Spatial organization of intestinal microbiota in the mouse ascending colon. The ISME journal. 2011;5:627–638. doi: 10.1038/ismej.2010.161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ng KM, Ferreyra JA, Higginbottom SK, Lynch JB, Kashyap PC, Gopinath S, Naidu N, Choudhury B, Weimer BC, Monack DM, et al. Microbiota-liberated host sugars facilitate post-antibiotic expansion of enteric pathogens. Nature. 2013;502:96–99. doi: 10.1038/nature12503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pernthaler A, Dekas AE, Brown CT, Goffredi SK, Embaye T, Orphan VJ. Diverse syntrophic partnerships from deep-sea methane vents revealed by direct cell capture and metagenomics. Proceedings of the National Academy of Sciences of the United States of America. 2008;105:7052–7057. doi: 10.1073/pnas.0711303105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Preibisch S, Saalfeld S, Tomancak P. Globally optimal stitching of tiled 3D microscopic image acquisitions. Bioinformatics. 2009;25:1463–1465. doi: 10.1093/bioinformatics/btp184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rath CM, Alexandrov T, Higginbottom SK, Song J, Milla ME, Fischbach MA, Sonnenburg JL, Dorrestein PC. Molecular analysis of model gut microbiotas by imaging mass spectrometry and nanodesorption electrospray ionization reveals dietary metabolite transformations. Analytical chemistry. 2012;84:9259–9267. doi: 10.1021/ac302039u. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reeburgh WS. Oceanic methane biogeochemistry. Chemical reviews. 2007;107:486–513. doi: 10.1021/cr050362v. [DOI] [PubMed] [Google Scholar]
- Sekiguchi Y, Kamagata Y, Nakamura K, Ohashi A, Harada H. Fluorescence in situ hybridization using 16S rRNA-targeted oligonucleotides reveals localization of methanogens and selected uncultured bacteria in mesophilic and thermophilic sludge granules. Applied and environmental microbiology. 1999;65:1280–1288. doi: 10.1128/aem.65.3.1280-1288.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen Y, Giardino Torchia ML, Lawson GW, Karp CL, Ashwell JD, Mazmanian SK. Outer membrane vesicles of a human commensal mediate immune regulation and disease protection. Cell host & microbe. 2012;12:509–520. doi: 10.1016/j.chom.2012.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sigal M, Rothenberg ME, Logan CY, Lee JY, Honaker RW, Cooper RL, Passarelli B, Camorlinga M, Bouley DM, Alvarez G, et al. Helicobacter pylori Activate and Expand Lgr5+ Stem Cells Through Direct Colonization of the Gastric Glands. Gastroenterology. 2015 doi: 10.1053/j.gastro.2015.02.049. [DOI] [PubMed] [Google Scholar]
- Sonnenburg ED, Sonnenburg JL. Starving our Microbial Self: The Deleterious Consequences of a Diet Deficient in Microbiota-Accessible Carbohydrates. Cell metabolism. 2014 doi: 10.1016/j.cmet.2014.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sonnenburg ED, Zheng H, Joglekar P, Higginbottom SK, Firbank SJ, Bolam DN, Sonnenburg JL. Specificity of polysaccharide use in intestinal bacteroides species determines diet-induced microbiota alterations. Cell. 2010;141:1241–1252. doi: 10.1016/j.cell.2010.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sonnenburg JL, Xu J, Leip DD, Chen CH, Westover BP, Weatherford J, Buhler JD, Gordon JI. Glycan foraging in vivo by an intestine-adapted bacterial symbiont. Science (New York, NY) 2005;307:1955–1959. doi: 10.1126/science.1109051. [DOI] [PubMed] [Google Scholar]
- Stecher B, Robbiani R, Walker AW, Westendorf AM, Barthel M, Kremer M, Chaffron S, Macpherson AJ, Buer J, Parkhill J, et al. Salmonella enterica serovar typhimurium exploits inflammation to compete with the intestinal microbiota. PLoS biology. 2007;5:2177–2189. doi: 10.1371/journal.pbio.0050244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swidsinski A, Sydora BC, Doerffel Y, Loening-Baucke V, Vaneechoutte M, Lupicki M, Scholze J, Lochs H, Dieleman LA. Viscosity gradient within the mucus layer determines the mucosal barrier function and the spatial organization of the intestinal microbiota. Inflammatory bowel diseases. 2007;13:963–970. doi: 10.1002/ibd.20163. [DOI] [PubMed] [Google Scholar]
- Swidsinski A, Weber J, Loening-Baucke V, Hale LP, Lochs H. Spatial organization and composition of the mucosal flora in patients with inflammatory bowel disease. Journal of clinical microbiology. 2005;43:3380–3389. doi: 10.1128/JCM.43.7.3380-3389.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turnbaugh PJ, Ridaura VK, Faith JJ, Rey FE, Knight R, Gordon JI. The effect of diet on the human gut microbiome: a metagenomic analysis in humanized gnotobiotic mice. Science translational medicine. 2009;1:6ra14. doi: 10.1126/scitranslmed.3000322. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.