Abstract
The epithelial-mesenchymal transition (EMT) program has emerged as a central driver of tumor malignancy. Moreover, the recently uncovered link between passage through an EMT and acquisition of stem-like properties indicates that activation of the EMT programs serves as a major mechanism for generating cancer stem cells, i.e., the cells that are responsible for initiating and propagating the disease. In this review, we summarize the evidence supporting the widespread involvement of the EMT program in tumor pathogenesis and attempt to rationalize the connection between the EMT program and acquisition of stem-cell traits. We propose that the epithelial-mesenchymal plasticity is likely controlled by multiple varients of the core EMT program and foresee the need to resolve the various programs and the molecular mechanisms that underlie them.
Keywords: EMT (epithelial-mesenchymal transition), Cancer stem cells, tumor progression, plasticity
The EMT is a naturally occurring transdifferentiation program
The successes over the past decade in reprogramming the terminally differentiated cells of normal adult tissues into pluripotent stem cells (Takahashi and Yamanaka, 2006; Yu et al., 2007) have led to the widely embraced assumption that almost any type of dedifferentiation or transdifferentiation is possible, if only the proper combination of ectopically expressed transcription factors is introduced into adult cells. These successes in experimental reprogramming raise the question of which changes in cell state actually occur in nature rather than being artifacts of experimentally forced ectopic gene expression.
Foremost among the naturally occurring transdifferentiation programs is the cell-biological program termed the epithelial-mesenchymal transition (EMT), which converts epithelial cells into more mesenchymal derivatives, and the reverse of this program, termed the mesenchymal-epithelial transition (MET) (Nieto, 2013). Evidence accumulated over the past two decades indicates clearly that this program operates during development to ensure the interconversions of cells that are required to form various distinct cell types and thus the tissues and organs of complex metazoans (Nieto, 2013; Thiery et al., 2009). This cell-biological program is orchestrated by a cohort of transcription factors (EMT-TFs), including those of Snail, Twist and Zeb families (Nieto, 2011; Thiery et al., 2009).
Two additional aspects of the EMT program are worthy of detailed examination: In some epithelial tissues the EMT program seems to be linked to residence of cells in stem cell-like states. In addition, versions of the EMT program are coopted by cancer cells, enabling them to acquire cellular traits associated with high-grade malignancy. These ramifications of the EMT program have revealed previously unapparent mechanistic connections between ontogeny and tumor pathogenesis.
The EMT program and epithelial-mesenchymal plasticity
The EMT program, as its name implies, governs changes of cell states along the epithelial versus mesenchymal axis and converts epithelial cells to mesenchymal cells when this program is fully executed. To describe the extremes poles of the epithelial vs. mesenchymal axis (Table 1), one notes that epithelial cells, often with polygonal shapes in monolayer culture, are polarized along their apical-basal axis and are tightly connected to one another laterally via adherens and tight junctions; in vivo these lateral ties ensure the structural integrity of epithelial cell sheets. Fully mesenchymal cells, in stark contrast, exhibit spindle-like morphology with no signs of apical-basal polarity and are loosely attached to the surrounding extracellular matrix through focal adhesions; these features help to explain their heightened motility and invasiveness relative to their epithelial counterparts.
Table 1.
Typical Differences Between Epithelial Cells And Mesenchymal Cells
| Epithelial Cells | Mesenchymal Cells | |
|---|---|---|
| Morphology in 2-D culture | Polygonal and cobble stone-like | Elongated and spindle-like |
| Polarity | Exhibit Apical-Basal Polarity | Exhibit Front-Back Polarity |
| Motility | Non-motile | Motile and Invasive |
| Cytoskeleton | Express Cytokeratins | Express Vimentin |
| Cell-Cell adhesion | Form adherens junctions and tight Junctions with adjacent epithelial cells | Do not form adherens junctions and tight junctions Attach to the extracellular matrix via focal adhesions |
The profound biological distinctions between epithelial cells and mesenchymal cells are determined by differences in their respective transcription programs, which control, among other gene products, the expression of key structural proteins, including those involved in maintaining the cytoskeleton and in forging cell-cell adhesions (Nieto, 2011, 2013; Thiery et al., 2009). Thus, epithelial cells express various types of cytokeratins which form their intermediate filaments, whereas the mesenchymal cells express instead the intermediate filament protein vimentin. The expression of cell-cell adhesion molecules and polarization complexes is generally repressed in mesenchymal cells. A hallmark of EMT is the replacement of E-cadherin by N-cadherin, which results in the formation of far weaker cell-cell adhesions between adjacent cells.
The EMT program can be activated with remarkable rapidity in epithelial cells in response to physiologic signals in both a cell-autonomous and non-cell-autonomous manner. Using gastrulation as an example, the EMT program is activated in the epithelial cells in the epiblast and completely converts epithelial cells to mesenchymal cells of the mesoderm in response to inductive signals, such as FGFs (fibroblast growth factors) and Wnt Signaling pathways (Tam and Behringer, 1997). Similarly, in adult tissues, the EMT program is activated quickly in response to wounding, facilitating rapid closure of the wounds and reestablishment of the epithelial barriers that are essential for protecting the interior of the organs from external insults (Savagner 2005). Such rapid interconversion between the epithelial and mesenchymal states implies plasticity in epithelial cells that render them highly responsive to EMT-inducing signals. Moreover, this plasticity suggests that residence in one of these two states is maintained in a metastable fashion, with complex molecular and cellular mechanisms operating to ensure long-term residence in one state or the other.
The depiction of the EMT program as a binary switch that moves cells from a fully epithelial to a fully mesenchymal state misrepresents the normal actions of this program, which usually moves cells from a fully epithelial state to one that is partially mesenchymal, with retention of certain key epithelial markers (Grunert et al., 2003; Theveneau et al., 2010). Nonetheless, the acquisition of even a subset of mesenchymal traits endows cells that previously resided in a fully epithelial state with a suite of mesenchymal traits that exert profound effects on their biology.
EMT and the normal epithelial stem-cell state
Over the past five years a series of discoveries has converged on the conclusion that after epithelial cells pass, at least partially, through an EMT, they are poised to enter into an epithelial stem-cell state. At least in the context of mammary epithelial cells, this holds true for both normal and neoplastic cells (Guo et al., 2012; Mani et al., 2008; Morel et al., 2012; Morel et al., 2008; Scheel et al., 2011). Given the biological similarities among diverse epithelial cell types (Blanpain et al., 2007), it seems plausible that versions of this scenario are likely to hold true in other epithelial tissues as well.
The stemness of epithelial cells can be demonstrated by their ability to reconstitute their tissue-of-origin after transplantation into a suitable microenvironment (Blanpain et al., 2007). For instance, the stemness of normal mammary epithelial cells can be gauged by implanting candidate cells into cleared mammary stromal fat pads, i.e., the structures from which the incipient mammary ductal trees present in young female mice have been surgically removed. Following implantation of bona fide mammary stem cells, entire mammary ductal trees will grow out over a period of six to eight weeks that are indistinguishable from those formed during normal mammary gland morphogenesis (Shackleton et al., 2006; Stingl et al., 2006). Such success in gland formation provides a rigorous test of stemness, in that candidate stem cells can be shown to create a complete adult organ. In the one case reported to date, forcing a population of normal murine mammary epithelial cells through an EMT, achieved through the combined actions of the transiently expressed Slug and Sox9 EMT-TFs, increased the representation of mammary stem cells within heterogeneous populations of mammary epithelial cells by two orders-of-magnitude, as gauged by this mammary gland reconstitution assay (Guo et al., 2012).
Of additional relevance here is the observation that the Slug EMT-TF is expressed in the basal/abluminal layer of normal murine and human mammary ducts at sites where normal mammary stem cells are proposed to reside (Guo et al., 2012; Nassour et al., 2012). Moreover, qRT-PCR quantification of FACS (fluorescence-activated cell sorting)-enriched mammary stem cells demonstrates that the mammary stem cells residing within normal mammary ducts expresses the Slug EMT-TF at levels that are more than two orders-of-magnitude higher per cell than the differentiated luminal epithelial cell population (Guo et al., 2012). These observations suggest that at least one component of the EMT program operates in normal tissues in the absence of pathology such as wound healing or neoplasia.
The biological rationale of the connection between EMT and stemness is not so easily constructed. It would seem to represent a cell-biological relationship that dates back to the inception of metazoan phyla rather than being a recent invention of mammals. One insight may come from considerations of how wound healing occurs in epithelial tissues. Closure of the wound involves the movement of epithelial cells into the gap, their proliferation, and ultimately their assembling of lateral connections, such as adherens junctions. Cells that are entirely epithelial are thought to be poorly motile in contrast to their mesenchymal counterparts, suggesting that cells at the edges of wounds need to activate, at least partially, an EMT program. Indeed, the experimental scarring of a human keratinocyte monolayer in vitro provokes the expression of the Slug EMT-TFs in the epithelial cells lining the edges of the newly formed gap (Savagner et al., 2005). In mice, Slug expression is elevated in keratinocytes lining the cutaneous wound margins in vivo, and epithelial outgrowth and wound closure is impaired in keratinocytes isolated from Slug knock-out mice (Shirley et al., 2010). These observations suggest that transient expression of an EMT program is intrinsic to epithelial wound healing. At the same time, the cells that are participating in reconstructing a damaged epithelium need to undergo a burst of proliferation in order to expeditiously fill the vacant area. This rapid proliferation may require the generation of new stem cells such as those created by activation of an EMT program. Following migration into wound sites and rapid increases in the sizes of epithelial cell populations, intrinsic epithelial-mesenchymal plasticity allows the cells that have undergone a partial EMT to revert back to an epithelial state via MET in order to successfully reconstruct the epithelial cell layer; such METs are likely to be accompanied by declines in the sizes of stem cell populations. While imprecise and conjectural at present, a rationale such as this one may eventually be found to explain the previously unanticipated connection between the EMT program and epithelial stem-cell properties.
EMT and cancer pathogenesis
Almost 80% of life-threatening human malignancies derive from epithelial tissues, generating a wide array of commonly occurring carcinomas, including tumors of the lung, colon, breast, pancreas, prostate, bladder, ovary, kidney and liver. In each case, the epithelial states of the corresponding normal cells-of-origin dictate that early-stage tumors arising in these tissues continue to express the cytokeratins and E-cadherin that are hallmarks of the epithelial state. In addition, neoplastic cells in these early tumors retain expression of the key biological phenotypes of epithelial cells, such as a lack of motility and an ability to form continuous cell sheets. These traits contrast starkly with those of cells present in advanced carcinomas, which arise as products of a complex succession of steps that is often termed tumor progression. Cells of the highly aggressive primary tumors display mesenchymal features, including motility and invasiveness, the latter of which is associated with metastatic dissemination (Huber et al., 2005; Morel et al., 2012; Rhim et al., 2012; Sarrio et al., 2008). At the mechanistic level, the acquisition of these malignant traits can be explained by the activation within carcinoma cells of previously latent EMT programs during tumor progression.
Taking breast cancer as an example, acquisition of mesenchymal features is positively associated with more aggressive subtypes of this disease and tumor progression (Aleskandarany et al., 2014; Blanco et al., 2002; Blick et al., 2008; Choi et al., 2013). More direct demonstrations of the link between EMT activation and tumor malignancy comes from a large body of loss-of-function and gain-of-function studies in xenograft tumor models. In both human and mouse breast cancer cell lines, depletion of EMT-TFs, such as Twist, Snail and Zeb1, greatly inhibits metastatic dissemination both from sites of primary tumor formation (e.g., the mammary stromal fat pad) and after experimental introduction of carcinoma cells into the venous circulation (i.e., tail-vein injection). Conversely, ectopic activation of the EMT program through forced expression of EMT-TFs can enhance metastatic dissemination of orthotopically implanted human breast cancer cells (Guo et al., 2012; Roy et al., 2014; Wu et al., 2009; Yang et al., 2004; Zhang et al., 2013; Tran et al., 2014).
Besides promoting systemic dissemination, the EMT program also appears to serve as a major driver of drug resistance and subsequent disease recurrence in breast cancer patients (Cheng et al., 2014; Creighton et al., 2009; Oliveras-Ferraros et al., 2012). Similarly, in a mouse model of Her2-induced tumors, the Snail EMT-TF is spontaneously activated in recurrent tumors in vivo and confers on them a highly mesenchymal phenotype (Moody et al., 2005), providing additional support of the causal relationship between EMT activation and tumor relapse. The connection between EMT activation and enhanced tumorigenicity has been confirmed in a variety of human cancer cell lines (Creighton et al., 2010). More recently, inhibition of epithelial-mesenchymal plasticity by blocking activation of the Zeb1 EMT-TF efficiently suppressed transition of carcinoma cells from a weakly tumorigenic into a highly tumorigenic state, i.e., into a state where they exhibit increases in their tumor-initiating capability (Chaffer et al., 2013).
Intriguingly, a number of recent studies have connected activation of the EMT program to acquisition of immunosuppressive capabilities in a variety of cancers. In melanoma, expression of the Snail EMT-TF can simultaneously inhibit differentiation of cytotoxic T cells and induce the formation of immunosuppressive regulatory T cells, the latter effect being mediated through production of thrombospondin (Kudo-Saito et al., 2009). In breast cancer cells, the EMT program enhances resistance of tumor cells to cytotoxic T cell-mediated lysis, at least in part by inducing autophagy (Akalay et al., 2013; Akalay et al., 2015). In lung cancer cells, activation of the EMT-TF Zeb1 has been linked to increases in PD-L1 expression, a known immune-suppressive molecule that can block the attack of tumor-infiltrating lymphocytes (Chen et al., 2014).
Given the pleiotropic functions of EMT activation in driving cancer progression and the growing reports of its association with various types of aggressive carcinoma cells, it is now plausible that essentially all carcinomas develop malignancy-associated traits through activation of an EMT program in their constituent neoplastic cells. However, this statement of widespread association of EMT with various cancers needs to be qualified, as many clinical pathologists dispute the existence of this program and its role in generating high-grade carcinomas (Tarin et al., 2005). A substantial part of this reluctance appears to derive from the fact that at the technical level, the repertoire of markers used to score clinical tissue sections often makes it difficult if not impossible to distinguish bona fide carcinoma cells that have undergone an EMT from adjacent recruited tumor-associated stromal cells derived from normal host tissues, many of which are fibroblasts and myofibroblasts, which naturally express EMT-associated markers. Since carcinoma cells often undergo a partial EMT, clear evidence of the involvement of this program in tumor progression can derive from the detection of cells that co-express acquired mesenchymal traits with certain retained epithelial markers inherited from their fully epithelial precursors. Indeed, recent analyses of human breast cancer specimens by in situ hybridization of pooled epithelial and mesenchymal markers has revealed the existence of tumor cells with both epithelial and mesenchymal features in invasive breast cancers of all subtypes (Yu et al., 2013). Moreover, a significant fraction of circulating tumor cells isolated from the peripheral blood of human patients with advanced prostate and breast cancers also co-express both epithelial and mesenchymal markers (Husemann et al., 2008; Raimondi et al., 2011; Yu et al., 2013).
The apparent widespread involvement of EMT in the pathogenesis of carcinomas raises the question of its contribution to the non-carcinomatous tumors encountered in the clinic. Neuroectodermal tumors, such as gliomas and glioblastomas, often give evidence of having passed through at least a partial EMT by exhibiting certain mesenchymal markers, including expression of a variety of EMT-TFs (Kahlert et al., 2013; Tso et al., 2006). From the perspective of developmental biology, one can rationalize this behavior by citing the distant origins of these tumors from a primitive epithelium – the neuroectoderm. However, the progression of tumors representing the other two major classes of cancers – hematopoietic and mesenchymal/sarcomatous – does not seem to involve activation of EMT programs. Instead, at least in the case of sarcomas, the corresponding normal cells-of-origin – plausibly cells akin to fibroblasts or mesenchymal stem cells (MSCs), express a variety of EMT-TFs constitutively, ostensibly to maintain their residence in a fully mesenchymal state (Chang et al., 2002). When viewed from this perspective, one can propose that ancestors of the normal cells-of-origin of sarcomas underwent EMTs early in ontogeny, long before the inception of tumor development. Indeed, primitive connective tissue cells, i.e., cells similar to MSCs, can be created experimentally by forcing epithelial cells through a complete EMT program that erases all traces of their epithelial origins and replaces them entirely with mesenchymal ones (Battula et al., 2010).
The EMT and entrance into the cancer stem cell state
Cellular heterogeneity has been widely reported in a variety of hematopoietic and solid cancers. One prominent feature of the cellular heterogeneity involves the differing degrees of tumor-initiating potential exhibited by various cancer cells coexisting within the same tumor. These more tumorigenic cells are termed tumor-initiating cells, or cancer stem cells (CSCs), as they resemble normal stem cells in terms of the ability to self-renew and generate more differentiated derivatives. The presence of CSCs is best quantified experimentally by implanting tumor cells at limiting dilutions into appropriate mouse hosts and scoring thereafter for any observed tumor formation. By such measures, forcing bulk populations of human breast cancer cells through an EMT by transient expression of EMT-TFs can increase the frequency of tumor-initiating breast CSCs (Guo et al., 2012; Mani et al., 2008; Morel et al., 2008), indicating that EMT programs play roles in facilitating the entrance of non-stem cells into stem-cell states in both normal and neoplastic mammary tissues. We note that the cited studies, virtually all of which have involved the experimental activation of EMT-TFs, do not take into account the fact that EMT-TFs are often naturally activated in temporal hierarchies and that they cooperate with one another to drive EMT progression in various physiologic settings (Nieto, 2013). This contrasts with the experimental methods reported to date, in which either the temporal sequence or the levels of ectopically expressed EMT-TF have usually deviated strongly from those controlled by normal physiologic regulation. Accordingly, the reported increases in CSC activity following induction of an EMT might actually be far higher if such experiments were performed under more optimal, physiologically appropriate conditions.
Given the multiple successive steps that occur during the tumor progression that leads from normal to highly malignant cells, it seems plausible that each cell population formed during each of the intermediate stages of carcinoma progression (i.e., hyperplastic, dysplastic, adenomatous, etc.) contains its own subpopulation of stem cells. These subpopulations should presumably depend on specific versions of the stem-cell program operating either in the fully normal or in the fully neoplastic cell populations within a specific tissue. In order to equip the tumor cells of each intermediate stage with desired combinations of epithelial and mesenchymal traits and maintain their epithelial-mesenchymal plasticity, these versions of stem-cell programs may potentially engage different combinations of EMT-TFs to achieve different levels of EMT activation. Retention of such plasticity appears to be important, since some have found that constitutive expression of strong EMT-inducers, such as Twist and Prrx1, blocks epithelial-mesenchymal plasticity, thereby precluding the MET that appear to be critical to the ability of carcinoma cells to establish new tumors or metastatic colonies (Ocana et al., 2012; Stankic et al., 2013; Tran et al., 2014; Tsai et al., 2012). In contrast, transient activation of an EMT program enables normal and neoplastic mammary epithelial cells to enter into the stem-cell state, possibly through enhancing their epithelial-mesenchymal plasticity (Schmidt et al., 2015). For these reasons, it appears that carcinoma CSCs (as well as the corresponding normal stem cells) reside in a phenotypic state that combines both epithelial and mesenchymal features and, at the same time, permits these cells to move into alternative states lying along the epithelial-mesenchymal axis. If validated, this would indicate that normal stem cells reside in a state that is quite distinct from that of bona fide mesenchymal cells, which lie at the extreme pole of the epithelial-mesenchymal axis.
Contextual signals inducing an EMT in carcinoma cells
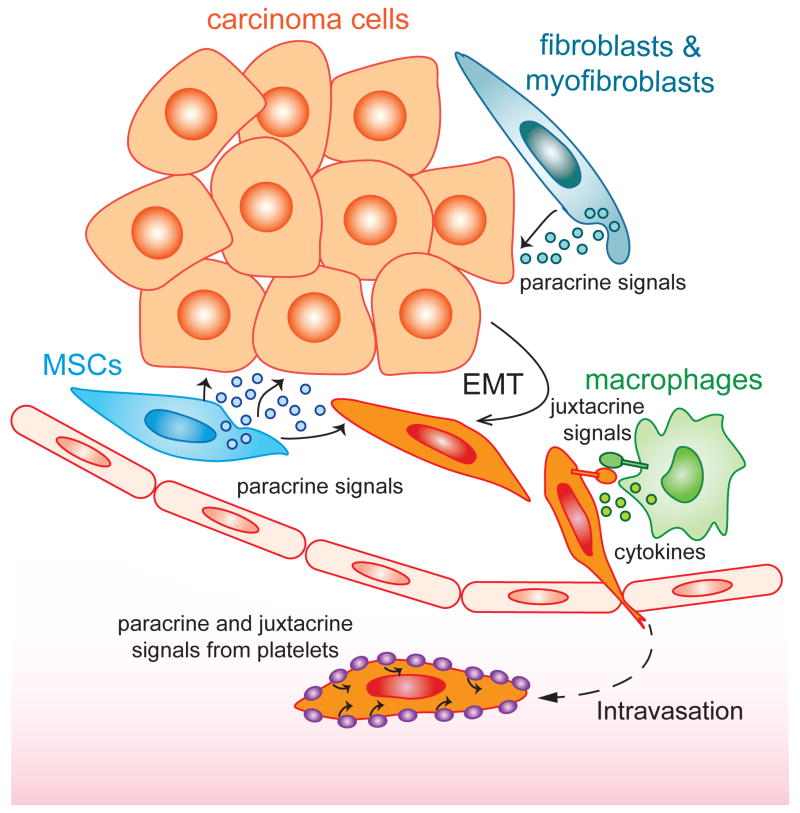
The patterns of activation of EMTs during development dictate that this program must be expressed in response to contextual signals experienced by individual cells in various locations within developing embryos. By extension, related patterns of heterotypic signaling must also operate during wound healing and tumor progression to activate this program. In certain carcinomas, the expression of EMT programs can often be observed among carcinoma cells that are closely apposed to stromal cells, specifically cells forming the “reactive stroma” that is developed late in tumor progression. Included among the cells of the late-stage stroma are not only the dominant fibroblasts and myofibroblasts, but cell types associated with wound healing and inflammation, such as MSCs, macrophages, and lymphocytes.
Once activated, continued expression of an EMT program within a carcinoma cell may depend on continuous paracrine signals received from the stroma; such dependence dictates that carcinoma cells will lapse back into an epithelial state (via an MET) when they no longer experience these inductive signals. Alternatively, carcinoma cells that have activated an EMT program may maintain its continued expression in a cell-autonomous manner, doing so via self-reinforcing, positive-feedback loops (Scheel et al., 2011). Among these heterotypic paracrine signals is the well-described cytokine TGF-β, which has been shown to be released predominantly by myofibroblasts (Oft et al., 1998). In addition, a diverse array of alternative signaling molecules has also been observed to participate in EMT induction, including morphogens such as Wnt, Notch, Shh, cytokines, prostaglandin E2, and growth factors such as EGF, FGF, HGF, PDGF, and VEGF (Thiery et al., 2009). Under many circumstances, the EMT programs are activated in response to several distinct paracrine signals acting in combination (Thiery et al., 2009) to ensure that an EMT is only activated when several distinct prerequisites have been met in individual cells. In the context of normal development, such an arrangement ensures that activation of EMT programs will be confined to discrete sectors within embryos. In the context of tumor progression, a variety of stromal cell-derived signals synergize with one another to induce and maintain EMTs in primary tumors (Fig. 1). Indeed, prostaglandin E2 (PGE2) can be secreted by fibroblasts, myofibroblasts, and MSCs (Li et al., 2012), while CCL18 and Ephrin is presented by monocytes and macrophages (Lu et al., 2014; Su et al., 2014), and TGF-β is released from myofibroblasts and platelets (Labelle et al., 2011). In certain experimental models, inhibition of any of these signals could suppress EMT activation and greatly attenuate tumor initiation and metastases in various xenograft tumor models, reflecting the fact that EMT is a highly coordinated process requiring the combinatorial actions of multiple contextual signals.
Fig. 1. EMT-inducing signals in the tumor microenvironment.
During the process of tumor progression, the EMT program is induced and maintained through concerted actions of a number of stroma-derived paracrine and juxtacrine signals.
The diversity of EMT-inducing signals enumerated above impinge upon and activate an array of EMT-TFs, which, as mentioned, include members of the Snail family, Twist family, Zeb family, FOX family of proteins and others (Thiery et al., 2009). Such diversity suggests that different versions of the EMT program could in theory operate in different tissues during developmental morphogenesis, and that distinct variants of this program may function during the progression of different types of carcinomas. Moreover, a number of posttranscriptional and epigenetic mechanisms are employed to ensure that the EMT program is precisely regulated and robustly executed. For instance, transcription factors of the Zeb family form a double-negative feedback loop with the miR-200 family of microRNAs, causing this regulatory loop to operate as a bi-stable switch between the epithelial and mesenchymal state in a variety of carcinomas (Brabletz and Brabletz, 2010; Burk et al., 2008; Hill et al., 2013). Similarly, Snail represses the expression of miR-34, a miRNA that otherwise can bind to the 3’ UTR of the Snail mRNA and cause its degradation (Brabletz, 2012; Kim et al., 2011). The EMT program also elicits profound changes in the transcriptome within a cell by altering the expression of splicing factors, including that of the ESRP (epithelial splicing regulatory proteins), RBFOX, MBNL (Muscleblind-like), CELF (CUG-BP- and ETR-3-like factors), and hnRNP (heterogeneous nuclear ribonucleoproteins) families, thereby promoting production of mRNA isoforms specific to the mesenchymal state (Shapiro et al., 2011).
Beyond the level of mRNAs, EMT-TFs can alter chromatin configurations to achieve stable, long-term silencing of epithelial genes as cells pass through a complete EMT (reviewed in Tam and Weinberg, 2013). For example, Snail can recruit a series of chromatin-modifying enzymes to the E-cadherin promoter to erase a critical mark of active transcription (trimethylated H3K4) and leave in its stead a trimethylated H3K9 mark. Such a trimethylated H3K9 mark could promote the recruitment of DNA methyltransferases (DNMTs), in turn causing CpG methylation of the promoter and formation of a constitutive heterochromatin that is highly resistant to transcription activation (Lin et al., 2014).
Besides these conventional modes of regulation, a recent report has suggested that metabolic reprogramming serves as an additional self-reinforcing mechanism of the EMT program (Shaul et al., 2014). In particular, induction of the EMT program in human breast cancer cells drove expression of the pyrimidine-degrading enzyme dihydropyrimidine dehydrogenase (DPYD), which led to an accumulation of its enzymatic products, dihydropyrimidines, in cells with mesenchymal features. This metabolic change is required for efficient activation and ongoing expression of an EMT program, because shRNA-mediated silencing of DPYD or reduction of intracellular dihydropyrimidine concentrations attenuated entrance into the mesenchymal state and acquisition of invasive and metastatic features.
EMT, epithelial-mesenchymal plasticity and the invasion-metastasis cascade
The later stages of malignant progression of carcinomas have been portrayed as a succession of steps termed the invasion-metastasis cascade (Fidler, 1978; Fidler, 2003). Thus, carcinoma cells within primary tumors acquire invasiveness, intravasate into microvessels within these tumors, circulate to distant anatomical sites (hematogenous dissemination), lodge in microvessels of such distant tissues, invade through the walls of microvessels into the parenchyma of these distant tissues (extravasation), seed the formation of micrometastatic deposits, and occasionally spawn macroscopic, clinically apparent metastases. This last step – often termed colonization – occurs with low efficiency. A variant of this cascade portrays the lymph nodes that drain sites of primary tumor formation as temporary staging areas in which cancer cells initially collect via the draining lymph ducts of a tumor, proliferate in these sites, and thereafter disseminate hematogenously to more distant tissues.
While a multitude of genetic and heritable epigenetic alterations have been found to play critical roles in driving primary tumor formation, the contributions of these changes to completion of the multiple steps of the invasion-metastasis cascade remain unclear. It is possible that the mutations that are responsible for primary tumor formation do not enable carcinoma cells to complete the various steps of this cascade; instead, additional mutant alleles are required to drive cells into metastatic dissemination. Alternatively the genetic (and heritable epigenetic) alterations carried by primary tumor cells may already suffice to empower these cells to disseminate; according to this second scenario, certain changes in epigenetic programs may, on their own, propel primary carcinoma cells to distant anatomical sites.
If the second scenario were validated, it would imply that carcinoma cells within primary tumors are already genetically equipped to disseminate and thus do not require additional mutations beyond those selected during primary tumor formation. This portrayal becomes increasingly plausible in light of recent evidence. The induced expression of EMT-TFs in primary, otherwise-non-metastatic tumor cells can increase the ability of these cells to seed lung metastases without the need for additional genetic changes (Mani et al., 2008; Morel et al., 2012; Morel et al., 2008). Moreover, and less directly, the incubation time required for colorectal adenomatous polyps to spawn full-blown primary colorectal carcinomas has been estimated to be ~17 years; however, following this, a period of two years or less has been found to suffice before liver metastases of these tumors can be detected (Calabrese et al., 2004; Jones et al., 2008). This finding might suggest that rare mutations are needed for polyp-to-carcinoma progression to occur, explaining the long lag time associated with this progression while, in contrast, certain rapidly acquired epigenetic changes, such as the activation of a latent EMT-TF program, may enable primary colorectal carcinoma cells to spread to the liver. Still, neither of these lines of evidence –both preclinical and clinical – provide rigorous proof that epigenetic activation of a previously silent transcription program, specifically EMT, suffices to enable primary carcinoma cells to disseminate to distant sites.
The last step of the invasion-metastasis cascade –colonization – does not seem to fall within the purview of the EMT program. Thus, this growth of micro- into macroscopic metastases would seem to require, among other things, the adaptation of cells arising in one fully differentiated tissue to the foreign and potentially inhospitable microenvironment of a second, quite distinct differentiated tissue. For example, why should breast carcinoma cells, which arise and develop in the tissue microenvironment of the mammary gland, be equipped to survive and proliferate in the microenvironments of unfamiliar tissues, such as those of the brain, lung, liver or bone marrow? The known functions of the EMT would not seem to confer such adaptive powers, suggesting that disseminated cells must invent such adaptations on their own following their arrival at distant tissues (Klein 2009). Indeed, it is plausible that such an adaptive program contrived by the disseminated carcinoma cells in one patient is unique, being cobbled together on an ad hoc basis by assembling components of various differentiation programs; accordingly, the bone marrow metastases arising in one breast cancer patient may invent a different adaptive program than those formed in another patient suffering the same disease. The fact that these adaptations are difficult to acquire would seem to be suggested by the astronomically low likelihood of successful colonization (Cameron et al., 2000; Chambers et al., 2002; Luzzi et al., 1998).
Although it remains obscure whether the tissue-specific adaptations are acquired through either epigenetic or genetic means, accumulating evidence suggests that METs are required for successful metastatic colonization. Indeed, distant metastases encountered in human carcinoma patients often manifest the epithelial features and even architectural organization of their correspondent tissues-of-origin. Thus, the quasi-mesenchymal cells that serve as the founders of metastatic colonies must be able to spawn large numbers of epithelial progeny to recreate the hierarchical relationships of CSCs and non-CSC that previously operated within the corresponding primary tumors (Malanchi et al., 2012; Thiery, 2002). Moreover, several reports using different tumor models or cell line systems have converged on the conclusion that constitutive activation of strong EMT programs will block MET, and prevent colonization of tumor cells at the metastatic sites (Ocana et al., 2012; Stankic et al., 2013; Tsai et al., 2012). These observations indicate that the epithelial-mesenchymal plasticity lies at the heart of tumor development, and that such plasticity is opportunistically exploited by carcinoma cells to accomplish the last step of the metastatic cascade -- colonization. This is reminiscent of the dynamic inter-conversions between the epithelial and mesenchymal states during embryogenesis, where EMT and subsequent MET is required for the proper development of mesoderm-derived epithelial structures, such as the collecting ducts of the kidney (derived from the intermediate mesoderm) and the mesothelial membranes (derived from the lateral plate mesoderm).
Concluding remarks
The connection made between activation of the EMT program and entrance into a CSC state has placed the EMT program as a central regulator of carcinoma progression. Thus, besides the conventional depiction of the EMT program as a driver of metastatic dissemination, activation of the EMT program can directly enhance tumor-initiation, thereby creating metastatic CSCs. Moreover, intrinsic epithelial-mesenchymal plasticity enables cancer cells to switch between the mesenchymal CSC state and a more differentiated, more rapidly proliferating epithelial state, thereby creating one important dimension of intra-tumoral heterogeneity (Chaffer et al., 2011; Chaffer et al., 2013; Gupta et al., 2011). This epithelial-mesenchymal plasticity implies that tumor cells can reside in a partial mesenchymal state and display various combinations of epithelial and mesenchymal features (Tam and Weinberg, 2013). Moreover, CSCs have been found to exhibit a heightened resistance to a variety of existing treatments, including radio-and chemotherapy in various carcinoma types (Creighton et al., 2009; Singh and Settleman, 2010). This resistance causes enrichment of the more mesenchymal, stem-like cancer cells after initial treatment, which can often give rise to clinical relapse (Gupta et al., 2009). These considerations explain why the EMT and the CSC programs are increasingly attracting the attentions of those involved in finding new ways to clinically treat advanced carcinomas, almost all of which, sooner or later, generate growths that evade treatments and prove lethal to those carrying them.
These metastable, partial mesenchymal states arising in a diverse array of carcinomas are likely controlled by multiple alternative variants of the core EMT program, which differ from one another in EMT-TF usage, epigenetic and metabolic programming, and the paracrine and autocrine signals that are involved in triggering and maintaining them (Caramel et al., 2013; Scheel et al., 2011; Tam and Weinberg, 2013). We foresee that future research will identify additional markers that allow further classification of distinct intermediate epithelial-mesenchymal states, and provide insights into the activation and regulation of these various EMT programs in different tumor types and at distinct stages of tumor development (Outstanding questions). These results will likely open new avenues that should allow development of early detection strategies and more effective therapeutic targeting of malignant solid tumors.
Outstanding Questions.
What are the functionally important, distinct intermediate states along the epithelial-mesenchymal axis that naturally arise under physiological and pathological conditions, in particular during carcinoma progression?
What are the features/markers that would allow identification of these intermediate states?
Which of these states favors the formation of epithelial stem cells?
Which combinations of EMT-associated transcription factors are responsible for organizing these distinct states?
Which types of contextual signals and acting in various combinations serve physiologically to activate expression of EMT-inducing transcription factors and, in turn, entrance into more mesenchymal cell states?
Are the close functional connections between the stem-cell state and the EMT program, which have been most thoroughly documented in the context of the mammary epithelium, also apparent in other epithelial tissues or do other principles govern EMT and stemness in other tissues?
Do still-unidentified cell-biological programs act together with the EMT program to orchestrate entrance into and out of the epithelial stem-cell state?
Is the mesenchymal-epithelial transition (MET) actively induced by specific signals, or is it simply a default process that occurs when EMT-inducing signals are absent?
Why mechanistically is epithelial-mesenchymal plasticity critical for residence in the normal and neoplastic stem-cell states?
How can the EMT program be reversed in order to eliminate therapy-resistance cancer stem cells?
Trends Box.
The Epithelial-mesenchymal transition (EMT) program is a naturally occurring transdifferentiation program that governs changes of cell states along the epithelial versus mesenchymal axis and confers upon epithelial cells epithelial-mesenchymal plasticity.
Activation of the EMT program places normal and neoplastic epithelial cells in states where they are poised to enter into stem cell compartments.
During development and cancer pathogenesis, the EMT program is induced by a number of synergistic contextual signals.
Epithelial-mesenchymal plasticity is critical for carcinoma progression and metastasis. Inhibition of EMT activation and/or epithelial-mesenchymal plasticity may serve as new ways to clinically treat advanced carcinomas.
Acknowledgments
R.A.W. is an American Cancer Society and Ludwig Foundation professor. This research was supported by the Breast Cancer Research Foundation, the Samuel Waxman Cancer Research Foundation, the Ludwig Center for Molecular Oncology at MIT, National Cancer Institute Program P01-CA080111, R01-CA078461, U01-CA184897 (to R.A.W.), K99-CA194160 (to X.Y.), and the Helen Hay Whitney Foundation (to X.Y.).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Acloque H, Adams MS, Fishwick K, Bronner-Fraser M, Nieto MA. Epithelial-mesenchymal transitions: the importance of changing cell state in development and disease. J Clin Invest. 2009;119:1438–1449. doi: 10.1172/JCI38019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akalay I, Janji B, Hasmim M, Noman MZ, Andre F, De Cremoux P, Bertheau P, Badoual C, Vielh P, Larsen AK, et al. Epithelial-to-mesenchymal transition and autophagy induction in breast carcinoma promote escape from T-cell-mediated lysis. Cancer Res. 2013;73:2418–2427. doi: 10.1158/0008-5472.CAN-12-2432. [DOI] [PubMed] [Google Scholar]
- Akalay I, Tan TZ, Kumar P, Janji B, Mami-Chouaib F, Charpy C, Vielh P, Larsen AK, Thiery JP, Sabbah M, et al. Targeting WNT1-inducible signaling pathway protein 2 alters human breast cancer cell susceptibility to specific lysis through regulation of KLF-4 and miR-7 expression. Oncogene. 2015;34:2261–2271. doi: 10.1038/onc.2014.151. [DOI] [PubMed] [Google Scholar]
- Aleskandarany MA, Negm OH, Green AR, Ahmed MA, Nolan CC, Tighe PJ, Ellis IO, Rakha EA. Epithelial mesenchymal transition in early invasive breast cancer: an immunohistochemical and reverse phase protein array study. Breast Cancer Res Treat. 2014;145:339–348. doi: 10.1007/s10549-014-2927-5. [DOI] [PubMed] [Google Scholar]
- Battula VL, Evans KW, Hollier BG, Shi Y, Marini FC, Ayyanan A, Wang RY, Brisken C, Guerra R, Andreeff M, et al. Epithelial-mesenchymal transition-derived cells exhibit multilineage differentiation potential similar to mesenchymal stem cells. Stem cells. 2010;28:1435–1445. doi: 10.1002/stem.467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blanco MJ, Moreno-Bueno G, Sarrio D, Locascio A, Cano A, Palacios J, Nieto MA. Correlation of Snail expression with histological grade and lymph node status in breast carcinomas. Oncogene. 2002;21:3241–3246. doi: 10.1038/sj.onc.1205416. [DOI] [PubMed] [Google Scholar]
- Blanpain C, Horsley V, Fuchs E. Epithelial stem cells: turning over new leaves. Cell. 2007;128:445–458. doi: 10.1016/j.cell.2007.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blick T, Widodo E, Hugo H, Waltham M, Lenburg ME, Neve RM, Thompson EW. Epithelial mesenchymal transition traits in human breast cancer cell lines. Clinical & experimental metastasis. 2008;25:629–642. doi: 10.1007/s10585-008-9170-6. [DOI] [PubMed] [Google Scholar]
- Brabletz S, Brabletz T. The ZEB/miR-200 feedback loop--a motor of cellular plasticity in development and cancer? EMBO Rep. 2010;11:670–677. doi: 10.1038/embor.2010.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brabletz T. MiR-34 and SNAIL: another double-negative feedback loop controlling cellular plasticity/EMT governed by p53. Cell cycle. 2012;11:215–216. doi: 10.4161/cc.11.2.18900. [DOI] [PubMed] [Google Scholar]
- Burk U, Schubert J, Wellner U, Schmalhofer O, Vincan E, Spaderna S, Brabletz T. A reciprocal repression between ZEB1 and members of the miR-200 family promotes EMT and invasion in cancer cells. EMBO Rep. 2008;9:582–589. doi: 10.1038/embor.2008.74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calabrese P, Tavare S, Shibata D. Pretumor progression: clonal evolution of human stem cell populations. Am J Pathol. 2004;164:1337–1346. doi: 10.1016/S0002-9440(10)63220-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cameron MD, Schmidt EE, Kerkvliet N, Nadkarni KV, Morris VL, Groom AC, Chambers AF, MacDonald IC. Temporal progression of metastasis in lung: cell survival, dormancy, and location dependence of metastatic inefficiency. Cancer Res. 2000;60:2541–2546. [PubMed] [Google Scholar]
- Caramel J, Papadogeorgakis E, Hill L, Browne GJ, Richard G, Wierinckx A, Saldanha G, Osborne J, Hutchinson P, Tse G, et al. A switch in the expression of embryonic EMT-inducers drives the development of malignant melanoma. Cancer Cell. 2013;24:466–480. doi: 10.1016/j.ccr.2013.08.018. [DOI] [PubMed] [Google Scholar]
- Chaffer CL, Brueckmann I, Scheel C, Kaestli AJ, Wiggins PA, Rodrigues LO, Brooks M, Reinhardt F, Su Y, Polyak K, et al. Normal and neoplastic nonstem cells can spontaneously convert to a stem-like state. Proc Natl Acad Sci U S A. 2011;108:7950–7955. doi: 10.1073/pnas.1102454108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaffer CL, Marjanovic ND, Lee T, Bell G, Kleer CG, Reinhardt F, D'Alessio AC, Young RA, Weinberg RA. Poised chromatin at the ZEB1 promoter enables breast cancer cell plasticity and enhances tumorigenicity. Cell. 2013;154:61–74. doi: 10.1016/j.cell.2013.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang HY, Chi JT, Dudoit S, Bondre C, van de Rijn M, Botstein D, Brown PO. Diversity, topographic differentiation, and positional memory in human fibroblasts. Proc Natl Acad Sci U S A. 2002;99:12877–12882. doi: 10.1073/pnas.162488599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chambers AF, Groom AC, MacDonald IC. Dissemination and growth of cancer cells in metastatic sites. Nat Rev Cancer. 2002;2:563–572. doi: 10.1038/nrc865. [DOI] [PubMed] [Google Scholar]
- Chen L, Gibbons DL, Goswami S, Cortez MA, Ahn YH, Byers LA, Zhang X, Yi X, Dwyer D, Lin W, et al. Metastasis is regulated via microRNA-200/ZEB1 axis control of tumour cell PD-L1 expression and intratumoral immunosuppression. Nature communications. 2014;5:5241. doi: 10.1038/ncomms6241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng Q, Chang JT, Gwin WR, Zhu J, Ambs S, Geradts J, Lyerly HK. A signature of epithelial-mesenchymal plasticity and stromal activation in primary tumor modulates late recurrence in breast cancer independent of disease subtype. Breast cancer research : BCR. 2014;16:407. doi: 10.1186/s13058-014-0407-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi Y, Lee HJ, Jang MH, Gwak JM, Lee KS, Kim EJ, Kim HJ, Lee HE, Park SY. Epithelial-mesenchymal transition increases during the progression of in situ to invasive basal-like breast cancer. Human pathology. 2013;44:2581–2589. doi: 10.1016/j.humpath.2013.07.003. [DOI] [PubMed] [Google Scholar]
- Creighton CJ, Chang JC, Rosen JM. Epithelial-mesenchymal transition (EMT) in tumor-initiating cells and its clinical implications in breast cancer. J Mammary Gland Biol Neoplasia. 2010;15:253–260. doi: 10.1007/s10911-010-9173-1. [DOI] [PubMed] [Google Scholar]
- Creighton CJ, Li X, Landis M, Dixon JM, Neumeister VM, Sjolund A, Rimm DL, Wong H, Rodriguez A, Herschkowitz JI, et al. Residual breast cancers after conventional therapy display mesenchymal as well as tumor-initiating features. Proc Natl Acad Sci U S A. 2009;106:13820–13825. doi: 10.1073/pnas.0905718106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fidler IJ. Tumor heterogeneity and the biology of cancer invasion and metastasis. Cancer Res. 1978;38:2651–2660. [PubMed] [Google Scholar]
- Fidler IJ. The pathogenesis of cancer metastasis: the 'seed and soil' hypothesis revisited. Nat Rev Cancer. 2003;3:453–458. doi: 10.1038/nrc1098. [DOI] [PubMed] [Google Scholar]
- Grunert S, Jechlinger M, Beug H. Diverse cellular and molecular mechanisms contribute to epithelial plasticity and metastasis. Nat Rev Mol Cell Biol. 2003;4:657–665. doi: 10.1038/nrm1175. [DOI] [PubMed] [Google Scholar]
- Guo W, Keckesova Z, Donaher JL, Shibue T, Tischler V, Reinhardt F, Itzkovitz S, Noske A, Zurrer-Hardi U, Bell G, et al. Slug and Sox9 cooperatively determine the mammary stem cell state. Cell. 2012;148:1015–1028. doi: 10.1016/j.cell.2012.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta PB, Fillmore CM, Jiang G, Shapira SD, Tao K, Kuperwasser C, Lander ES. Stochastic state transitions give rise to phenotypic equilibrium in populations of cancer cells. Cell. 2011;146:633–644. doi: 10.1016/j.cell.2011.07.026. [DOI] [PubMed] [Google Scholar]
- Gupta PB, Onder TT, Jiang G, Tao K, Kuperwasser C, Weinberg RA, Lander ES. Identification of selective inhibitors of cancer stem cells by high-throughput screening. Cell. 2009;138:645–659. doi: 10.1016/j.cell.2009.06.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hay ED. Organization and fine structure of epithelium and mesenchyme in the developing chick embryo. In: Fleischmajer R, Billingham RE, editors. Epithelial-mesenchymal interactions. Baltimore: Williams and Wilkins; 1968. pp. 31–55. [Google Scholar]
- Hill L, Browne G, Tulchinsky E. ZEB/miR-200 feedback loop: at the crossroads of signal transduction in cancer. Int J Cancer. 2013;132:745–754. doi: 10.1002/ijc.27708. [DOI] [PubMed] [Google Scholar]
- Huber MA, Kraut N, Beug H. Molecular requirements for epithelial-mesenchymal transition during tumor progression. Curr Opin Cell Biol. 2005;17:548–558. doi: 10.1016/j.ceb.2005.08.001. [DOI] [PubMed] [Google Scholar]
- Husemann Y, Geigl JB, Schubert F, Musiani P, Meyer M, Burghart E, Forni G, Eils R, Fehm T, Riethmuller G, et al. Systemic spread is an early step in breast cancer. Cancer Cell. 2008;13:58–68. doi: 10.1016/j.ccr.2007.12.003. [DOI] [PubMed] [Google Scholar]
- Jones S, Chen WD, Parmigiani G, Diehl F, Beerenwinkel N, Antal T, Traulsen A, Nowak MA, Siegel C, Velculescu VE, et al. Comparative lesion sequencing provides insights into tumor evolution. Proc Natl Acad Sci U S A. 2008;105:4283–4288. doi: 10.1073/pnas.0712345105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kahlert UD, Nikkhah G, Maciaczyk J. Epithelial-to-mesenchymal(-like) transition as a relevant molecular event in malignant gliomas. Cancer Lett. 2013;331:131–138. doi: 10.1016/j.canlet.2012.12.010. [DOI] [PubMed] [Google Scholar]
- Kim NH, Kim HS, Li XY, Lee I, Choi HS, Kang SE, Cha SY, Ryu JK, Yoon D, Fearon ER, et al. A p53/miRNA-34 axis regulates Snail1-dependent cancer cell epithelial-mesenchymal transition. The Journal of cell biology. 2011;195:417–433. doi: 10.1083/jcb.201103097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein CA. Parallel progression of primary tumours and metastases. Nat Rev Cancer. 2009;9:302–312. doi: 10.1038/nrc2627. [DOI] [PubMed] [Google Scholar]
- Kudo-Saito C, Shirako H, Takeuchi T, Kawakami Y. Cancer metastasis is accelerated through immunosuppression during Snail-induced EMT of cancer cells. Cancer Cell. 2009;15:195–206. doi: 10.1016/j.ccr.2009.01.023. [DOI] [PubMed] [Google Scholar]
- Labelle M, Begum S, Hynes RO. Direct signaling between platelets and cancer cells induces an epithelial-mesenchymal-like transition and promotes metastasis. Cancer Cell. 2011;20:576–590. doi: 10.1016/j.ccr.2011.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li HJ, Reinhardt F, Herschman HR, Weinberg RA. Cancer-stimulated mesenchymal stem cells create a carcinoma stem cell niche via prostaglandin E2 signaling. Cancer discovery. 2012;2:840–855. doi: 10.1158/2159-8290.CD-12-0101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin Y, Dong C, Zhou BP. Epigenetic regulation of EMT: the Snail story. Current pharmaceutical design. 2014;20:1698–1705. doi: 10.2174/13816128113199990512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu H, Clauser KR, Tam WL, Frose J, Ye X, Eaton EN, Reinhardt F, Donnenberg VS, Bhargava R, Carr SA, et al. A breast cancer stem cell niche supported by juxtacrine signalling from monocytes and macrophages. Nat Cell Biol. 2014;16:1105–1117. doi: 10.1038/ncb3041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luzzi KJ, MacDonald IC, Schmidt EE, Kerkvliet N, Morris VL, Chambers AF, Groom AC. Multistep nature of metastatic inefficiency: dormancy of solitary cells after successful extravasation and limited survival of early micrometastases. Am J Pathol. 1998;153:865–873. doi: 10.1016/S0002-9440(10)65628-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malanchi I, Santamaria-Martinez A, Susanto E, Peng H, Lehr HA, Delaloye JF, Huelsken J. Interactions between cancer stem cells and their niche govern metastatic colonization. Nature. 2012;481:85–89. doi: 10.1038/nature10694. [DOI] [PubMed] [Google Scholar]
- Mani SA, Guo W, Liao MJ, Eaton EN, Ayyanan A, Zhou AY, Brooks M, Reinhard F, Zhang CC, Shipitsin M, et al. The epithelial-mesenchymal transition generates cells with properties of stem cells. Cell. 2008;133:704–715. doi: 10.1016/j.cell.2008.03.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Massague J. TGFbeta in Cancer. Cell. 2008;134:215–230. doi: 10.1016/j.cell.2008.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moody SE, Perez D, Pan TC, Sarkisian CJ, Portocarrero CP, Sterner CJ, Notorfrancesco KL, Cardiff RD, Chodosh LA. The transcriptional repressor Snail promotes mammary tumor recurrence. Cancer Cell. 2005;8:197–209. doi: 10.1016/j.ccr.2005.07.009. [DOI] [PubMed] [Google Scholar]
- Morel AP, Hinkal GW, Thomas C, Fauvet F, Courtois-Cox S, Wierinckx A, Devouassoux-Shisheboran M, Treilleux I, Tissier A, Gras B, et al. EMT inducers catalyze malignant transformation of mammary epithelial cells and drive tumorigenesis towards claudin-low tumors in transgenic mice. PLoS Genet. 2012;8:e1002723. doi: 10.1371/journal.pgen.1002723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morel AP, Lievre M, Thomas C, Hinkal G, Ansieau S, Puisieux A. Generation of breast cancer stem cells through epithelial-mesenchymal transition. PLoS One. 2008;3:e2888. doi: 10.1371/journal.pone.0002888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakaya Y, Sheng G. Epithelial to mesenchymal transition during gastrulation: an embryological view. Development, growth & differentiation. 2008;50:755–766. doi: 10.1111/j.1440-169X.2008.01070.x. [DOI] [PubMed] [Google Scholar]
- Nassour M, Idoux-Gillet Y, Selmi A, Come C, Faraldo ML, Deugnier MA, Savagner P. Slug controls stem/progenitor cell growth dynamics during mammary gland morphogenesis. PLoS One. 2012;7:e53498. doi: 10.1371/journal.pone.0053498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nieto MA. The ins and outs of the epithelial to mesenchymal transition in health and disease. Annu Rev Cell Dev Biol. 2011;27:347–376. doi: 10.1146/annurev-cellbio-092910-154036. [DOI] [PubMed] [Google Scholar]
- Nieto MA. Epithelial plasticity: a common theme in embryonic and cancer cells. Science. 2013;342:1234850. doi: 10.1126/science.1234850. [DOI] [PubMed] [Google Scholar]
- Ocana OH, Corcoles R, Fabra A, Moreno-Bueno G, Acloque H, Vega S, Barrallo-Gimeno A, Cano A, Nieto MA. Metastatic colonization requires the repression of the epithelial-mesenchymal transition inducer Prrx1. Cancer Cell. 2012;22:709–724. doi: 10.1016/j.ccr.2012.10.012. [DOI] [PubMed] [Google Scholar]
- Oft M, Heider KH, Beug H. TGFbeta signaling is necessary for carcinoma cell invasiveness and metastasis. Current biology : CB. 1998;8:1243–1252. doi: 10.1016/s0960-9822(07)00533-7. [DOI] [PubMed] [Google Scholar]
- Oliveras-Ferraros C, Corominas-Faja B, Cufi S, Vazquez-Martin A, Martin-Castillo B, Iglesias JM, Lopez-Bonet E, Martin AG, Menendez JA. Epithelial-to-mesenchymal transition (EMT) confers primary resistance to trastuzumab (Herceptin) Cell cycle. 2012;11:4020–4032. doi: 10.4161/cc.22225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raimondi C, Gradilone A, Naso G, Vincenzi B, Petracca A, Nicolazzo C, Palazzo A, Saltarelli R, Spremberg F, Cortesi E, et al. Epithelial-mesenchymal transition and stemness features in circulating tumor cells from breast cancer patients. Breast Cancer Res Treat. 2011;130:449–455. doi: 10.1007/s10549-011-1373-x. [DOI] [PubMed] [Google Scholar]
- Rhim AD, Mirek ET, Aiello NM, Maitra A, Bailey JM, McAllister F, Reichert M, Beatty GL, Rustgi AK, Vonderheide RH, et al. EMT and dissemination precede pancreatic tumor formation. Cell. 2012;148:349–361. doi: 10.1016/j.cell.2011.11.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roy SS, Gonugunta VK, Bandyopadhyay A, Rao MK, Goodall GJ, Sun LZ, Tekmal RR, Vadlamudi RK. Significance of PELP1/HDAC2/miR-200 regulatory network in EMT and metastasis of breast cancer. Oncogene. 2014;33:3707–3716. doi: 10.1038/onc.2013.332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarrio D, Rodriguez-Pinilla SM, Hardisson D, Cano A, Moreno-Bueno G, Palacios J. Epithelial-mesenchymal transition in breast cancer relates to the basal-like phenotype. Cancer Res. 2008;68:989–997. doi: 10.1158/0008-5472.CAN-07-2017. [DOI] [PubMed] [Google Scholar]
- Savagner P, Kusewitt DF, Carver EA, Magnino F, Choi C, Gridley T, Hudson LG. Developmental transcription factor slug is required for effective re-epithelialization by adult keratinocytes. J Cell Physiol. 2005;202:858–866. doi: 10.1002/jcp.20188. [DOI] [PubMed] [Google Scholar]
- Scheel C, Eaton EN, Li SH, Chaffer CL, Reinhardt F, Kah KJ, Bell G, Guo W, Rubin J, Richardson AL, et al. Paracrine and autocrine signals induce and maintain mesenchymal and stem cell states in the breast. Cell. 2011;145:926–940. doi: 10.1016/j.cell.2011.04.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt JM, Panzilius E, Bartsch HS, Irmler M, Beckers J, Kari V, Linnemann JR, Dragoi D, Hirschi B, Kloos UJ, et al. Stem-cell-like properties and epithelial plasticity arise as stable traits after transient Twist1 activation. Cell reports. 2015;10:131–139. doi: 10.1016/j.celrep.2014.12.032. [DOI] [PubMed] [Google Scholar]
- Shackleton M, Vaillant F, Simpson KJ, Stingl J, Smyth GK, Asselin-Labat ML, Wu L, Lindeman GJ, Visvader JE. Generation of a functional mammary gland from a single stem cell. Nature. 2006;439:84–88. doi: 10.1038/nature04372. [DOI] [PubMed] [Google Scholar]
- Shapiro IM, Cheng AW, Flytzanis NC, Balsamo M, Condeelis JS, Oktay MH, Burge CB, Gertler FB. An EMT-driven alternative splicing program occurs in human breast cancer and modulates cellular phenotype. PLoS genetics. 2011;7:e1002218. doi: 10.1371/journal.pgen.1002218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaul YD, Freinkman E, Comb WC, Cantor JR, Tam WL, Thiru P, Kim D, Kanarek N, Pacold ME, Chen WW, et al. Dihydropyrimidine accumulation is required for the epithelial-mesenchymal transition. Cell. 2014;158:1094–1109. doi: 10.1016/j.cell.2014.07.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shirley SH, Hudson LG, He J, Kusewitt DF. The skinny on Slug. Mol Carcinog. 2010;49:851–861. doi: 10.1002/mc.20674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh A, Settleman J. EMT, cancer stem cells and drug resistance: an emerging axis of evil in the war on cancer. Oncogene. 2010;29:4741–4751. doi: 10.1038/onc.2010.215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stankic M, Pavlovic S, Chin Y, Brogi E, Padua D, Norton L, Massague J, Benezra R. TGF-beta-Id1 signaling opposes Twist1 and promotes metastatic colonization via a mesenchymal-to-epithelial transition. Cell reports. 2013;5:1228–1242. doi: 10.1016/j.celrep.2013.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stingl J, Eirew P, Ricketson I, Shackleton M, Vaillant F, Choi D, Li HI, Eaves CJ. Purification and unique properties of mammary epithelial stem cells. Nature. 2006;439:993–997. doi: 10.1038/nature04496. [DOI] [PubMed] [Google Scholar]
- Su S, Liu Q, Chen J, Chen F, He C, Huang D, Wu W, Lin L, Huang W, Zhang J, et al. A positive feedback loop between mesenchymal-like cancer cells and macrophages is essential to breast cancer metastasis. Cancer Cell. 2014;25:605–620. doi: 10.1016/j.ccr.2014.03.021. [DOI] [PubMed] [Google Scholar]
- Takahashi K, Yamanaka S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell. 2006;126:663–676. doi: 10.1016/j.cell.2006.07.024. [DOI] [PubMed] [Google Scholar]
- Tam PP, Behringer RR. Mouse gastrulation: the formation of a mammalian body plan. Mechanisms of development. 1997;68:3–25. doi: 10.1016/s0925-4773(97)00123-8. [DOI] [PubMed] [Google Scholar]
- Tam WL, Weinberg RA. The epigenetics of epithelial-mesenchymal plasticity in cancer. Nat Med. 2013;19:1438–1449. doi: 10.1038/nm.3336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tarin D, Thompson EW, Newgreen DF. The fallacy of epithelial mesenchymal transition in neoplasia. Cancer Res. 2005;65:5996–6000. doi: 10.1158/0008-5472.CAN-05-0699. discussion 6000–5991. [DOI] [PubMed] [Google Scholar]
- Theveneau E, Marchant L, Kuriyama S, Gull M, Moepps B, Parsons M, Mayor R. Collective chemotaxis requires contact-dependent cell polarity. Developmental cell. 2010;19:39–53. doi: 10.1016/j.devcel.2010.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thiery JP. Epithelial-mesenchymal transitions in tumour progression. Nat Rev Cancer. 2002;2:442–454. doi: 10.1038/nrc822. [DOI] [PubMed] [Google Scholar]
- Thiery JP, Acloque H, Huang RY, Nieto MA. Epithelial-mesenchymal transitions in development and disease. Cell. 2009;139:871–890. doi: 10.1016/j.cell.2009.11.007. [DOI] [PubMed] [Google Scholar]
- Tran HD, Luitel K, Kim M, Zhang K, Longmore GD, Tran DD. Transient SNAIL1 expression is necessary for metastatic competence in breast cancer. Cancer Res. 2014;74:6330–6340. doi: 10.1158/0008-5472.CAN-14-0923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai JH, Donaher JL, Murphy DA, Chau S, Yang J. Spatiotemporal regulation of epithelial-mesenchymal transition is essential for squamous cell carcinoma metastasis. Cancer Cell. 2012;22:725–736. doi: 10.1016/j.ccr.2012.09.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tso CL, Shintaku P, Chen J, Liu Q, Liu J, Chen Z, Yoshimoto K, Mischel PS, Cloughesy TF, Liau LM, et al. Primary glioblastomas express mesenchymal stem-like properties. Molecular cancer research : MCR. 2006;4:607–619. doi: 10.1158/1541-7786.MCR-06-0005. [DOI] [PubMed] [Google Scholar]
- Wu Y, Deng J, Rychahou PG, Qiu S, Evers BM, Zhou BP. Stabilization of snail by NF-kappaB is required for inflammation-induced cell migration and invasion. Cancer Cell. 2009;15:416–428. doi: 10.1016/j.ccr.2009.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang J, Mani SA, Donaher JL, Ramaswamy S, Itzykson RA, Come C, Savagner P, Gitelman I, Richardson A, Weinberg RA. Twist, a master regulator of morphogenesis, plays an essential role in tumor metastasis. Cell. 2004;117:927–939. doi: 10.1016/j.cell.2004.06.006. [DOI] [PubMed] [Google Scholar]
- Yu J, Vodyanik MA, Smuga-Otto K, Antosiewicz-Bourget J, Frane JL, Tian S, Nie J, Jonsdottir GA, Ruotti V, Stewart R, et al. Induced pluripotent stem cell lines derived from human somatic cells. Science. 2007;318:1917–1920. doi: 10.1126/science.1151526. [DOI] [PubMed] [Google Scholar]
- Yu M, Bardia A, Wittner BS, Stott SL, Smas ME, Ting DT, Isakoff SJ, Ciciliano JC, Wells MN, Shah AM, et al. Circulating breast tumor cells exhibit dynamic changes in epithelial and mesenchymal composition. Science. 2013;339:580–584. doi: 10.1126/science.1228522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang K, Corsa CA, Ponik SM, Prior JL, Piwnica-Worms D, Eliceiri KW, Keely PJ, Longmore GD. The collagen receptor discoidin domain receptor 2 stabilizes SNAIL1 to facilitate breast cancer metastasis. Nat Cell Biol. 2013;15:677–687. doi: 10.1038/ncb2743. [DOI] [PMC free article] [PubMed] [Google Scholar]