Abstract
Objective
Although health behavior theories postulate that risk perception should motivate colorectal cancer (CRC) screening, this relationship is unclear. This meta-analysis aims to examine the relationship between CRC risk perception and screening behavior, while considering potential moderators and study quality.
Method
A search of six databases yielded 58 studies (63 effect sizes) that quantitatively assessed the relationship between CRC risk perception and screening behavior.
Results
Most included effect sizes (75%) reported a positive association between CRC risk perception and screening behavior. A random effects meta-analysis yielded an overall effect size of z=0.13 (95% CI 0.10–0.16), which was heterogeneous (I2=99%, τ2=0.01). Effect sizes from high-quality studies were significantly lower than those from lower quality studies (z=0.02 vs. 0.16).
Conclusions
We found a small, positive relationship between CRC risk perception and reported screening behavior, with important identified heterogeneity across moderators. Future studies should focus on high quality study design.
Keywords: Meta-Analysis, Perceived Risk, Colorectal Neoplasms, Early Detection of Cancer, Patient-Reported Outcomes
Multiple professional societies have recommended routine colorectal cancer (CRC) screening starting at age 50 (American Cancer Society, 2015; U. S. Preventive Services Task Force, 2008) due to strong evidence that screening reduces mortality (Zauber et al., 2012) from this extremely common malignancy in both men and women (American Cancer Society, 2015). Multiple screening methods are available, including annual fecal occult blood testing (FOBT), flexible sigmoidoscopy (FS) every five years with FOBT every three years, and colonoscopy every ten years. Colonoscopy has become a standard of care (U. S. Preventive Services Task Force, 2008), and is widely covered by public and private health insurance policies, given the potential of colonoscopy to identify and remove both cancerous and precancerous adenomas as the time of the colonoscopy (Rex et al., 2002). Despite the demonstrated clinical benefit of routine screening for CRC, screening rates have stalled at around 65% nationwide (Centers for Disease Control and Prevention, 2012).
Efforts to increase CRC screening rates often focus on increasing adults’ perceptions of risk for developing CRC. Perceived illness risk involves a belief about their potential likelihood of developing illness (Weinstein, 2000). Most theories of health behavior change propose that heightened perceptions of illness risk encourage self-protective actions (Beck & Frankel, 1981; Cummings, Becker, & Maile, 1980; Janz & Becker, 1984; Leventhal & Cameron, 1987; Weinstein, 1988). Addressing risk perception for CRC might be particularly important to increase screening rates, because CRC risk perception is quite low in the general population (Clipp et al., 2004; J.L. Hay, Coups, & Ford, 2006; Vernon, Myers, Tilley, & Li, 2001). Further, CRC often develops to an advanced stage in the absence of symptoms, suggesting that risk appreciation may be an important impetus for screening among asymptomatic individuals. Based in part on this theoretical and empirical groundwork, interventions to encourage CRC screening frequently include components that aim to increase CRC risk perception (Rawl, Menon, Burness, & Breslau, 2012). While there is evidence that individual-level interventions effectively promote CRC screening (Holden, Jonas, Porterfield, Reuland, & Harris, 2010; Sabatino et al., 2012), a recent Cochrane review indicates that the inclusion of personalized risk information in these interventions does not consistently lead to higher CRC screening rates (Edwards et al., 2013). For example, in a general population sample of 50–70 year-old individuals who were non-adherent with screening guidelines, Vernon and colleagues (Vernon et al., 2011) found no effect for a tailored intervention approach to increasing CRC screening compared to a general information website alone; the tailored approach increased risk perception, but this did not translate to improved screening rates. Similarly, Schroy and colleagues (Schroy et al., 2012) found that among screening-nonadherent individuals aged 50–79, adding a personalized risk assessment to a shared decision aid did not improve screening rates over use of the decision aid alone.
These findings lead us to an important question: Should CRC screening interventions abandon efforts to change CRC risk perception? If risk perception is not consistently related to screening adherence, then a shift in intervention content may be warranted, either overall, for certain CRC screening tests, or for some specific population subgroups that may be less responsive to CRC interventions that highlight risk perception. To address this, we applied systematic review and meta-analytic techniques to the body of research examining the association between CRC risk perception and screening behavior (i.e., FOBT, FS, colonoscopy, or overall adherence). We hypothesized that this overall relationship would be characterized by a small, significant effect size, consistent with what has been found regarding risk perception and other behaviors (r = 0.10 – 0.25; (Brewer et al., 2007; Floyd, Prentice-Dunn, & Rogers, 2000; Harrison, Mullen, & Green, 1992; McCaul, Branstetter, Schroeder, & Glasgow, 1996; Milne, Sheeran, & Orbell, 2000), and that the relationship between CRC risk perception and screening behavior would differ based on type of screening test. In a seminal 1997 narrative review paper, Vernon found that CRC risk perception was more consistently related to FS than FOBT (Vernon, 1997), but the literature has grown and clinical practice has changed substantially since that time.
We also aimed to examine whether the association of CRC risk perception and screening behavior differs systematically based on whether CRC risk perception and screening are assessed at the same time in a cross-sectional study design, or whether CRC risk perception is assessed prior to subsequent adoption of screening in a prospective study design. A larger effect for prospective studies may indicate that CRC risk perception does indeed promote screening across time, because cross-sectional studies may confound the effect of CRC risk perception on screening behavior and the subsequent effect of screening completion on CRC risk perception (Brewer, Weinstein, Cuite, & Herrington, 2004).
Finally, we examine whether the association between CRC risk perception and screening behavior differs systematically based on other potential moderators of the effect size, including whether participants were at higher or average risk for CRC (Edwards et al., 2013), as well as demographic factors, including educational attainment, racial and gender study composition, and whether the studies were conducted in the United States or internationally. These findings may indicate whether interventions targeted to specific sub-populations should include personalized risk information. We also evaluated the impact of risk perception item format (i.e., social comparative, verbal absolute, numerical absolute, (Brewer et al., 2007)), study screening rate, year of publication, as well as four study quality indicators (i.e., study recruitment rate, whether the screening outcome variable excluded tests provided in the context of symptoms, whether CRC risk perception information used single or multiple items, given that the use of a multiple item measure increases measurement reliability, and whether the screening outcome variable was self-reported or medical chart-confirmed). We expected a more consistent effect will be found in higher quality studies of the association of CRC risk perception and screening behavior.
Method
Search Strategy
We searched English language journal articles using EMBASE (1947 – March 2015), Thomson Reuters Web of Knowledge (1955 – March 2015), PubMed (1966 – March 2015), PsycINFO (1967 – March 2015), and SciVerse Scopus (1996 – March 2015). The search terms were: (perceived risk OR perceived risks OR risk perception OR risk perceptions OR perception of risk OR perception of risks OR perceived vulnerability OR perceived susceptibility OR perceived likelihood OR subjective risk OR subjective risks) AND (colonoscopy OR sigmoidoscopy OR FOBT OR FOBTs OR fecal occult blood test OR fecal occult blood tests OR barium enema OR barium enemas OR colorectal cancer screening OR colorectal cancer screenings OR colorectal cancer screens OR colon cancer screening OR colon cancer screenings OR colon cancer screens OR diagnostic bowel test OR diagnostic bowel tests OR bowel test OR bowel tests OR bowel screening OR bowel screenings OR bowel screens OR Fecal Immunochemical Test OR CT colonography OR stool DNA test OR stool DNA tests OR sDNA). An additional grey literature search was completed to identify unpublished dissertations and abstracts from conference proceedings.
Selection Strategy
We deemed studies were eligible for inclusion if they: (1) included an original report of a quantitative assessment of the relationship between CRC risk perceptions and patient self-reported, physician-reported, or medical chart-documented CRC screening using any test, and (2) included participants without a CRC history ages 40 or older.
Screening Process
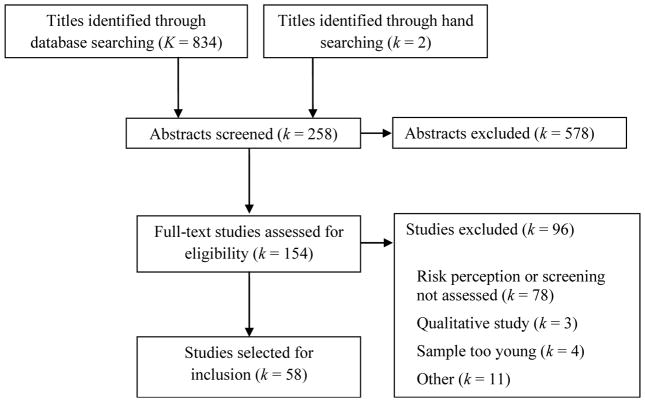
First, two co-authors independently reviewed each title for eligibility, with discrepancies resolved in discussion. Second, each potentially eligible article was randomly assigned to a pair of co-authors for full abstract screening. Articles moved forward for full-text review if both coauthors agreed on eligibility. In instances of disagreement, a third co-author arbitrated the article. Third, we randomly assigned each article to a pair of co-authors for full-text review. This included a primary reviewer and a secondary reviewer for the purposes of verification and quality assurance. Both reviewers independently completed standardized coding forms to extract the pre-determined data from each potentially eligible article. Reviewers then met as a group and compared full-text article reviews to resolve any potential discrepancies between reviewers and make final decisions regarding article inclusion. Following final selection, each author searched references from included articles to determine whether they should be considered for inclusion. We screened potentially eligible articles for eligibility using the same process as articles identified through database searches (Figure 1).
Figure 1.
PRISMA Flow Chart
Data abstraction
Two co-authors independently abstracted data on CRC screening test, perceived risk measures, potential moderators, and the association of risk perception and screening behavior for each effect size in each study. For each relationship between perceived CRC risk and the outcome measure, the data abstractor documented bivariate statistics. If the original study authors only reported multivariate statistics, and if bivariate statistics could not be obtained after two attempts to contact the authors, abstractors documented multivariate statistics. For instances where null findings were explicitly presented but neither multivariate nor bivariate statistics were reported, we imputed the effect size was as zero, with the standard error calculated based on the sample size (Higgins, White, & Wood, 2008). For the purposes of analysis, we transformed all effect sizes to a z statistic (Rosenthal, 1984).
We deemed the following variables potential moderators a priori, with cutoffs empirically defined when possible, and otherwise defined by an equal split of the data at the mean: screening test modality (FOBT, FS in combination with FOBT, colonoscopy, or a combination of screening modalities); research design (prospective or cross-sectional); risk status of the study population (e.g., first-degree family members of patients with CRC were coded as “high risk;” unselected samples consisting of the general population coded as “average risk”); high versus low educational attainment (≤ 50% high school graduate), majority versus minority of white participants in study sample; and high (≥ 50%) versus low proportion of males; United States versus international study sample; study screening prevalence (above or below 64.5%, (Joseph et al., 2012)); the potential impact of publication year (continuous); and whether a reported effect size was used versus an imputed standard deviation for a reported null effect. We also examined potential differences related to the type of perceived risk measure scale used in a given study. Frequently used options included social comparison scales, and absolute likelihood scales with verbal (e.g., “not very likely”) or numerical (e.g., 30% risk) anchors.
Four potential moderators served as indicators of study quality: recruitment rate (≥ 60% of eligible participants or lower); whether the screening outcome variable excluded tests provided in the context of symptoms (yes/no); whether the screening outcome was based on patient self-report or medical chart abstraction; and whether studies differed based on whether single or multiple items were used to assess CRC risk perception.
Statistical Analysis
We conducted a random-effects meta-analysis to determine association between CRC risk perception and screening behavior (Thompson & Higgins, 2002). We examined presence and degree of heterogeneity using the I2 and τ2 statistics (Higgins, Thompson, Deeks, & Altman, 2003). To investigate sources of potential heterogeneity and the role of moderators, we conducted a multivariate meta-regression (Thompson & Higgins, 2002), with moderators entered into the meta-regression one at a time. Additionally, we completed a meta-regression to determine whether a composite study quality variable (i.e., studies that met at least three of the four quality indicators) significantly affected the relationship between CRC risk perception and screening behavior. We examined publication bias through examination of a funnel plot (Begg & Mazumdar, 1994; Sterne et al., 2011). As an additional indicator of publication bias, we examined whether a given study prioritized risk perception as a specific research aim impacted the relationship between CRC risk perception and screening behavior. We conducted this and all other analyses using the metaerg, metan, and metafunnel functions of Stata v.11.1.
Results
Search Results
The initial literature search yielded a total of 834 titles. Two titles were identified through additional hand searching. Following sequential title screening, two of the primary authors reviewed each of the 258 unique article abstracts, with 154 retained for full text review. Reasons for article exclusion during the full text review included: personal risk perception was not assessed, CRC screening behavior was not assessed, CRC screening intentions were assessed rather than actual behavior, the sample was primarily younger than age 40, qualitative study design, and the effect size had been reported in the same dataset in a prior publication. Additionally, abstracts from conference proceedings were excluded if we received no response from study authors after two e-mail requests for additional information. A total of 58 articles (describing 58 unique studies and 63 effect sizes) met eligibility criteria (Figure 1) and were included in this review. Interrater agreement was high (Cohen’s κ = 0.84).
Study Characteristics (K = 58 studies)
Table 1 provides study demographics and clinical characteristics of included studies. Half of the studies (k = 29) used current adherence with any screening test modality (i.e., FOBT, FS, or colonoscopy), rather than use of one specific screening modality, as the primary outcome. Twelve studies (21%) used a prospective design. Fourteen included studies (24%) addressed a high-risk population such as first-degree family members of colorectal cancer patients. Of 42 studies that reported educational attainment, 22 (52%) included samples with a majority of individuals who did not complete high school. Of 44 studies that reported racial composition, 27 (61%) had a study sample consisting of at least half non-white participants. Most (72%) were conducted in the United States. Twenty of the studies (34%) were published prior to 2005. Of 53 studies that reported the percent of individuals in the sample screened, only 13 (25%) had a percentage greater than 64.5%. CRC risk perception measures employed by these studies were comprised of the following scale formats: 21 social comparative, 21 verbal absolute, 4 numeric absolute, and 12 used a combination of these types.
Table 1.
Demographic and Clinical Characteristics of Studies (K = 58) by Study Quality
| Study | N | Screening Test | Design | Risk Level | % ≤ HS | % Non-White | USA/Int. | Recruit. Rate | Exclude Test/Sx | PRM | #PR Items | Data Source |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| (Bazargan, Ani, Bazargan-Hejazi, Baker, & Bastani, 2009)± | 302 | FOBT, FS, Colonoscopy | Pros. | General | NR | 96% | USA | 97% | Yes | SC | 1 | Chart |
| (Dear, Scott, Chambers, Corbett, & Taupin, 2008)± | 404 | Colonoscopy | Cross | General | NR | NR | USA | 65% | Yes | NuA | 1 | Chart |
| (Gorin, 2005)± | 950 | FOBT | Pros. | General | 99% | 100% | USA | 79% | Yes | SC | 1 | Chart |
| (K. Kim, Chapman, & Vallina, 2012)± | 111 | FOBT | Cross | General | NR | NR | USA | 69% | No | VA | 2 | Chart |
| (Kremers, Mesters, Pladdet, van den Borne, & Stockbrugger, 2000)± | 131 | FS | Pros. | General | NR | NR | Int. | 75% | No | VA, SC | 4 | Chart |
| (Lipkus et al., 2000)± | 435 | FOBT | Pros. | General | 65% | 79% | USA | 76% | Yes | VA, SC | 2 | Patient |
| (Madlensky, Esplen, Gallinger, McLaughlin, & Goel, 2003)± | 368 | FOBT, FS, Colonoscopy | Cross | High | 100% | NR | Int. | 69% | Yes | VA | 1 | Chart |
| (Manne et al., 2002)± | 504 | FOBT, FS, Colonoscopy | Cross | High | 29% | 7% | USA | 66% | Yes | NuA | 5 | Chart |
| (Matthews, Nattinger, Venkatesan, Shaker, & Anderson, 2007)± | 149 | FOBT, FS, Colonoscopy | Cross | General | 60% | 64% | USA | 87% | No | SC | 2 | Chart |
| (W.Y. Sun, 2010)± | 288 | FOBT, FS, Colonoscopy | Cross | General | 53% | 100% | USA | 86% | No | VA | 5 | Chart |
| (Taniguchi et al., 2014)± | 56 | Colonoscopy | Cross | General | 68% | 100% | Int. | 65% | No | NuA, SC | 6 | Chart |
| (Taouqi, Ingrand, Beauchant, Migeot, & Ingrand, 2010)± | 172 | Colonoscopy | Cross | General | 47% | NR | Int. | 69% | Yes | VA | 5 | Patient |
|
| ||||||||||||
| (Azaiza & Cohen, 2008) | 472 | FOBT, FS, Colonoscopy | Cross | General | NR | NR | Int. | 43% | No | SC | 2 | Patient |
| (Bae, Park, & Lim, 2014) | 237 | FOBT | Cross | General | 24% | 100% | Int. | 89% | No | VA | 5 | Patient |
| (Blalock, DeVellis, Afifi, & Sandler, 1990) | 295 | FOBT | Pros. | High | NR | 40% | USA | 57% | No | VA, SC | 2 | Chart |
| (Bleiker et al., 2005) | 132 | FS, Colonoscopy | Cross | High | NR | NR | Int. | 84% | No | NuA | 1 | Chart |
| (Brawarsky, Brooks, Mucci, & Wood, 2004) | 716 | FOBT, FS, Colonoscopy | Cross | General | 36% | 9% | USA | 48% | No | SC | 1 | Patient |
| (Brenes & Paskett, 2000) | 202 | FS | Cross | General | 29% | 77% | USA | 76% | No | SC | 1 | Patient |
| (Choi et al., 2013) | 2004 | FS, Colonosocpy | Cross | General | 47% | 100% | Int. | 67% | No | NuA | 1 | Patient |
| (Codori et al., 2001) | 1074 | FS, Colonoscopy | Cross | High | NR | 3% | USA | 43% | No | VA | 1 | Patient |
| (Costanza et al., 2005) | 1253 | FOBT, FS, Colonoscopy | Cross | General | 26% | 6% | USA | 69% | No | VA, SC | 2 | Patient |
| (Cronan, Devoscomby, Villalta, & Gallagher, 2008) | 158 | FOBT, FS, Colonoscopy | Cross | General | NR | 68% | USA | NR | No | VA | 1 | Patient |
| (Fernandez et al., 2015) | 544 | FOBT, FS, Colonoscopy | Cross | General | 84% | NR | USA | 91% | No | VA, SC | 2 | Patient |
| (Flander et al., 2014) | 26 | Colonoscopy | Cross | High | NR | NR | Int. | 19% | No | NuA | 1 | Patient |
| (Frank, Swedmark, & Grubbs, 2004) | 49 | FOBT, FS, Colonoscopy | Cross | General | NR | 100% | USA | 35% | No | SC | 3 | Patient |
| (Friedman, Webb, Richards, & Plon, 1999) | 171 | FOBT | Cross | High | 6% | 0% | USA | 51% | No | VA | 1 | Chart |
| (Friedman, Everett, Peterson, Ogbonnaya, & Mendizabal, 2001) | 160 | FOBT | Pros. | General | 76% | 88% | USA | NR | No | VA | 1 | Chart |
| (Gimeno Garcia, Quintero, Nicolas Perez, Hernandez, & JimenezSosa, 2011) | 334 | FOBT, FS, Colonoscopy | Cross | High | 65% | NR | Int. | NR | No | SC | 1 | Patient |
| (Glenn et al., 2011) | 378 | FOBT, FS, Colonoscopy | Pros. | High | 37% | 70% | USA | 71% | No | VA | 1 | Patient |
| (Griffith, 2009) | 492 | FOBT, FS, Colonoscopy | Cross | General | 51% | 100% | USA | NR | Yes | VA | 1 | Patient |
| (J. L. Hay et al., 2003) | 280 | FOBT, FS, Colonoscopy | Cross | General | 19% | 24% | USA | 83% | Yes | SC | 1 | Patient |
| (Javadzade et al., 2012) | 98 | FOBT | Cross | General | NR | NR | Int. | NR | No | VA | 4 | Patient |
| (Kelly & Shank, 1992) | 180 | FS | Pros. | General | 82% | 3% | USA | 47% | Yes | VA | 1 | Chart |
| (S. E. Kim et al., 2008) | 1160 | Colonoscopy | Cross | General | 50% | 71% | USA | 42% | No | VA | 1 | Patient |
| (Koo et al., 2010) | 342 | FOBT, FS, Colonoscopy | Cross | General | NR | NR | Int. | 90% | No | VA, SC | 1 | Patient |
| (Lawsin, DuHamel, Weiss, Rakowski, & Jandorf, 2007) | 107 | FS, FOBT | Cross | General | 51% | 100% | USA | 54% | No | SC | 1 | Patient |
| (Lewis & Jensen, 1996) | 236 | FS | Cross | General | 35% | 10% | USA | 86% | No | SC | 1 | Patient |
| (Longacre, Cramer, & Gross, 2006) | 12132 | FS, Colonoscopy | Cross | General | 51% | 31% | USA | 90% | No | VA | 1 | Patient |
| (Lubetkin, Santana, Tso, & Jia, 2008) | 344 | FOBT, FS, Colonoscopy | Cross | General | 64% | 100% | USA | 71% | Yes | SC | 1 | Patient |
| (Mack et al., 2009) | 356 | FOBT, FS, Colonoscopy | Cross | High | 28% | NR | Int. | 48% | No | SC | 1 | Patient |
| (Mandelson et al., 2000) | 890 | FOBT | Cross | General | 36% | 11% | USA | 80% | Yes | SC | 1 | Patient |
| (McQueen et al., 2007) | 597 | FOBT, FS, Colonoscopy | Pros. | High | 41% | 100% | USA | 58% | No | VA | 3 | Chart |
| (Menon, Szalacha, Prabhughate, & Kue, 2014) | 275 | FOBT, FS, Colonoscopy | Cross | General | 65% | 87% | USA | 83% | No | VA | 1 | Patient |
| (Moser et al., 2007)† | 6035 | FOBT, FS, Colonoscopy | Cross | General | 50% | 15% | USA | 33% | No | VA | 2 | Patient |
| (Myers et al., 1994) | 501 | FOBT | Pros. | General | NR | 22% | USA | 78% | No | VA | 1 | Chart |
| (Palmer et al., 2007) | 481 | FOBT, FS, Colonoscopy | Cross | High | 18% | 27% | USA | 28% | No | VA, SC | 3 | Chart |
| (Palmer, Chhabra, & McKinney, 2011) | 504 | FOBT, FS, Colonoscopy | Cross | General | 51% | 100% | USA | 58% | No | VA, SC | 1 | Patient |
| (Paskett et al., 1997) * | 216 | FOBT, FS | Cross | General | 100% | 79% | USA | 75% | No | VA | 1 | Patient |
| (Santos, Lourenco, & Rossi, 2011) | 116 | Colonoscopy | Cross | High | 58% | 100% | Int. | 65% | No | NuA, SC, VA | 2 | Patient |
| (Shokar, Carlson, & Weller, 2008) | 560 | FOBT, FS, Colonoscopy | Cross | General | 52% | 64% | USA | 56% | No | SC | 1 | Patient |
| (Stark, Bertone-Johnson, Costanza, & Stoddard, 2006) | 1646 | FOBT, FS, Colonoscopy | Cross | General | 24% | 6% | USA | 67% | No | VA, SC | 2 | Patient |
| (W. Y. Sun, Basch, Wolf, & Li, 2004) | 203 | FOBT, FS | Cross | General | 78% | 100% | USA | 89% | No | SC | 3 | Patient |
| (Sutton et al., 2000) | 2632 | FS | Pros. | General | NR | 3% | Int. | 74% | No | SC | 1 | Chart |
| (Teng et al., 2006) † | 262 | FOBT, FS, Colonoscopy | Cross | General | 49% | 100% | USA | 89% | No | VA | 1 | Patient |
| (Tessaro, Mangone, Parkar, & Pawar, 2006) | 802 | FOBT, FS, Colonoscopy | Cross | General | 38% | 52% | USA | 67% | No | SC | 1 | Patient |
| (Wardle, Miles, & Atkin, 2005) | 5462 | FS | Pros. | General | NR | NR | Int. | 69% | No | SC | 1 | Chart |
| (Weinberg, Turner, Wang, Myers, & Miller, 2004) | 406 | FOBT, FS, Colonoscopy | Cross | General | 49% | 14% | USA | 52% | Yes | SC | 1 | Patient |
| (Zlot, Silvey, Newell, Coates, & Leman, 2011) | 1657 | FOBT, FS, Colonoscopy | Cross | High | 33% | NR | USA | 56% | No | VA | 1 | Patient |
Note: FOBT indicates Fecal Occult Bloot Test; FS, Flexible Sigmoidoscopy; Cross, Cross-Sectional; Pros., Prospective; NR, Not Reported; ≤ HS, High School degree or less; Int., International; Recruit., Recruitment; Sx, Symptoms; PRM, Perceived Risk Measure; VA, Verbal Absolute; SC, Social Comparative; NuA, Numerical Absolute; #RP Items, Number of Risk Perception Items.
Study reported 2 effect sizes.
Study reported 3 effect sizes.
Study met at least 3 of the 4 quality indicators (recruitment rate ≥ 60%; screening outcome variable excluded tests provided in the context of symptoms; screening outcome was based on medical chart abstraction; and use of multiple items to assess CRC risk perception)
Of the study quality indicators, most (67%) reported an adequate recruitment rate (at least 60%), yet only 22% explicitly excluded from the analysis study participants who underwent testing in the context of a workup for specific gastrointestinal symptoms rather than for asymptomatic screening. Twenty-one studies (36%) used two or more items to assess CRC risk perception. Thirty-eight studies (66%) used patient-reported rather than chart-confirmed screening as the behavioral outcome variable.
Meta-Analytic Findings
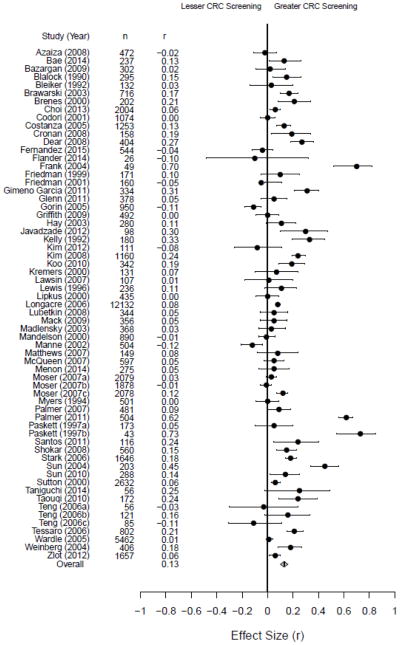
We meta-analyzed the 58 included studies to assess the relationship between CRC. Fifty-five of these studies contained a single effect size, one (Paskett, Rushing, D’Agostino, Tatum, & Velez, 1997) reported two effect sizes (i.e., separate effect sizes for African-American and Caucasian patients), and two (Moser, McCaul, Peters, Nelson, & Marcus, 2007; Teng, Friedman, & Green, 2006) reported three effect sizes each (i.e., separate effect sizes for FOBT, FS, and colonoscopy, with different participants in each analysis); a total of 63 effect sizes were included in the meta-analysis. We imputed an effect size of zero, along with a corresponding imputed standard deviation, for four studies where null findings were reported (Codori, Petersen, Miglioretti, & Boyd, 2001; Griffith, 2009; Lipkus, Lyna, & Rimer, 2000; Myers et al., 1994). In general, 47 of the 63 effect sizes (75%) reflected a positive relationship between CRC risk perception and screening behavior. The pooled effect size was z = 0.13, 95% CI [0.10, 0.16], with a range of −0.28 to 0.93, see Figure 2. The meta-analyzed effect sizes were quite heterogeneous (I2 = 99%, τ2 = 0.01).
Figure 2.
Forest Plot of Meta-Analysis Effect Sizes
Twelve effect sizes satisfied the a priori criteria for study quality (i.e., possessed at least three out of four of the following: recruitment rate ≥ 60%, study excluded tests provided in the context of symptoms, screening behavior was captured via medical chart abstraction rather than from patient self-report, and multiple items were used to measure CRC risk perception). The study quality meta-regression indicated a statistically significant difference between the high quality (n = 11) and lower quality (n = 52) groups (t(62) = −2.00, p = 0.05, Adjusted R2 = 0.05). The pooled effect size for the “high quality” effect sizes was z = 0.02, 95% CI[−0.04, 0.09], with the pooled effect sizes for the lower quality effect sizes being z = 0.16, 95% CI[0.13, 0.19].
Meta-regression analyses separately included each of the four quality indicator variables to determine their individual relationship to CRC risk perception and screening behavior. We found that whether a given study excluded tests provided in the context of symptoms moderated the association between CRC risk perception and screening behavior (t(62) = −2.12, p = 0.04, Adjusted R2 = 0.06). The pooled value for the effect sizes that excluded tests provided in the context of symptom follow-up (n = 13) was z = 0.03, 95% CI[−0.05, 0.10], whereas the pooled value for the effect sizes that did not exclude tests provided in the context of symptoms (n = 50) was z = 0.17, 95% CI[0.14, 0.20]. None of the remaining primary hypothesized variables statistically moderated the relationship between CRC risk perception and screening behavior when individually entered into the meta-regression.
Publication Bias
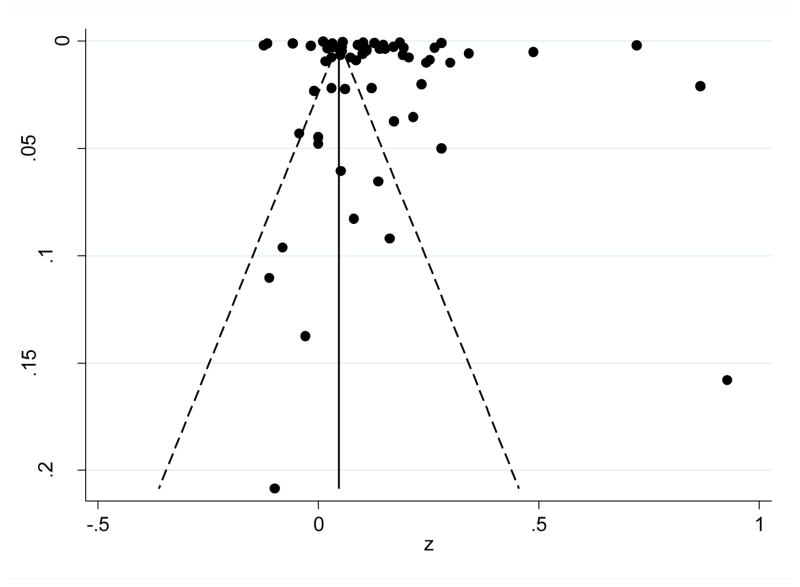
A funnel plot of all studies with pseudo 95% confidence limits is displayed in Figure 3. The funnel plot is asymmetrical and is a potential indicator of the presence of publication bias; however, a contributing factor to the asymmetry could be the large number of studies with an effect size at or around zero (i.e., 36 effect sizes include zero in their respective confidence intervals). Additionally, since there was not a significant relationship between study sample size and effect size (Pearson r = −0.12, p = 0.37), there may be a minimal influence of publication bias in this analysis.
Figure 3.
Funnel Plot with Pseudo 95% Confidence Limits
Given that many studies did not prioritize the assessment of perceived risk as a primary research aim, we also examined publication bias by determining whether prioritization of risk perception in study emphasis (operationalized as mention of the perceived risk-screening relationship as a specific research aim, or not) and CRC screening behavior in the research aims moderated study effect size. Thirty-four studies prioritized risk perception as a study aim, however this was not associated with effect size (p > 0.05).
Discussion
As hypothesized, we observed a small, positive, statistically significant relationship between CRC risk perception and screening adherence, z = 0.13, 95% CI [0.10, 0.16], which falls consistently within what has been found regarding risk perception and other behaviors (z = 0.10 – 0.25); (Brewer et al., 2007; Floyd et al., 2000; Harrison et al., 1992; McCaul et al., 1996; Milne et al., 2000). This supports the idea that CRC risk perception may be a stronger determinant of behavior in combination with other theory-driven factors (e.g., self-efficacy, (Sheeran, Harris, & Epton, 2014)), or structural or physician factors, and may be best tested as moderators or mediators of behavior change, as well as direct effects (McQueen et al., 2010).
We observed a highly heterogeneous relationship between CRC risk perception and screening behavior, and though there was preliminary evidence of publication bias, effect sizes of the studies that prioritized the relationship between CRC risk perception and screening as a primary study aim did not statistically differ from those that explored the relationship as a secondary outcome, nor was there a relationship between sample size and effect size. The complex elements inherent in the assessments of overall colorectal cancer screening adherence could contribute to effect size heterogeneity. For instance, the association of CRC risk perception and screening may vary by test modality as different tests present different behavioral challenges. Unfortunately we could not examine this potential source of heterogeneity because the majority of studies assessed whether participants underwent screening of any modality. Data from the 2012 Behavioral Risk Factor Surveillance System (Klabunde, Joseph, King, White, & Plescia, 2013) indicates that 61.7% of individuals between the ages of 50–75 years had a colonoscopy within 10 years, whereas 10.4% had an FOBT within one year. Future work should include other newly developed screening modalities (e.g., fecal immunochemical testing; FIT), to examine whether CRC risk perception and screening may change as more options become available.
Additionally, most studies in the meta-analysis (k = 40) relied upon self-reporting of screening behavior, which is subject to recall bias. Studies of CRC screening self-report have found a wide range of sensitivity and specificity of self-report, compared to the gold standard of chart review, for each test (Baier et al., 2000; Bastani et al., 2008; Gordon, Hiatt, & Lampert, 1993; Hall et al., 2004; Mandelson, LaCroix, Anderson, Nadel, & Lee, 1999; Montano & Phillips, 1995; Rauscher, Johnson, Cho, & Walk, 2008). Validity of self-reported CRC screening appears to be best with carefully worded questions that describe the specific testing experience (Baier et al., 2000; Hall et al., 2004). Of the 38 studies in the meta-analysis that included self-reported screening behavior, only one provided an explicit description of screening tests, while four studies mentioned that tests were briefly described. The remaining studies that included self-reported screening behavior did not report whether or how screening tests were described to survey respondents. Further, recall issues likely differ based on the screening modality, with more invasive testing being more salient. Recall may also be better for more recent testing. The studies that relied on chart review for assessment of screening behavior are presumably less subject to bias, though heterogeneity was found within these studies as well. Ultimately, the unmeasured sources of heterogeneity arising from outcome measurement may complicate the assessment of relationship between risk perception and screening behavior.
We examined a wide range of variables as potential moderators of CRC risk perception and screening behavior. These included screening outcomes, study design, sample composition, and four study quality indicators (recruitment rate ≥ 60% of eligible participants, whether the screening outcome variable excluded tests for symptoms, whether the screening outcome was based on medical chart abstraction, and whether multiple items were used to assess CRC risk perception, See Table 1, top panel, for the high quality studies). Study quality was a significant moderator of the relationship between CRC risk perception and screening. But contrary to our hypothesis, higher quality studies had lower effect sizes (i.e., 0.16 vs. 0.02). In an examination of each quality indicator in turn, we found the effect size was significantly higher in studies that did not exclude tests for symptoms compared to studies that did exclude tests for symptoms (i.e., z = 0.17 vs. 0.03), suggesting that the observed relationship between CRC risk perception and screening may be inflated by heightened risk perceptions among those who pursue testing in the diagnostic context. Accordingly, risk perceptions may be less important in the asymptomatic screening context than the overall effect size indicates. Of note, most studies reviewed here (78%) did not exclude tests for symptoms. Future studies of the effect of CRC risk perception on screening behavior should only include screening tests among asymptomatic adults.
Another quality indicator, risk perception measure format (i.e., measurement scale type or whether single or multiple items were used), also did not moderate the effect size of the relationship between CRC risk perception and screening behavior, which may have been due to the exclusion of lower quality studies, such as those that assessed CRC screening intentions. Examination of other measurement issues, such as whether CRC risk perceptions were conditional on screening non-adherence or not, or risk within a specific time frame or lifetime, could not be accomplished in this review since risk perception measures were universally not conditional, and largely did not specify a time frame. Finally, the great diversity in risk perception item wording could also contribute to effect size heterogeneity.
Study design did not moderate the association between risk perception and screening. We expected that there would be a significantly stronger relationship between CRC risk perception and screening for prospective studies compared to cross-sectional studies (Brewer et al., 2004), but we found no effect size differences between prospective and cross-sectional studies. Prior meta-analytic findings that have examined study design as a moderator have also not consistently found that prospective studies reveal stronger effect sizes. Brewer and colleagues (Brewer et al., 2007) found that the effect size between disease risk perception and vaccination was smaller for cross-sectional studies versus prospective studies; in contrast, McCaul and colleagues (McCaul et al., 1996) found that the effect size between breast cancer risk perceptions and mammography screening was actually larger for cross-sectional versus prospective studies. Sheeran and colleagues (Sheeran et al., 2014) found stronger effects for risk appraisals on intentions for change rather than subsequent behavioral adoption, proposing that this may reflect more proximal timing and active deliberation inherent in intention formation about behavioral choices. We did not specifically test this aspect in our review, as we excluded studies that measured intentions for CRC screening. Yet, our findings justify continued work to clarify factors that may help explain the role of risk perceptions in different stages of behavioral decision-making and behavioral adoption, both prior to screening offers, as well as after screening has been completed. In future work, a larger pool of prospective intervention studies would allow for direct examination of whether changes in CRC risk perceptions specifically lead to increased screening uptake.
The imputation of zero for four of the effect sizes in this analysis may potentially bias our effect size toward the null; however we found that whether an effect size was imputed did not have a statistically significant relationship with CRC risk perception and thus we chose to retain these imputed studies to prevent additional publication bias. Additionally, it is possible that the cutoffs selected for the meta-regression are not generalizable to all samples (e.g., low educational attainment defined as samples with ≤ 50% high school graduates). In instances where there was no clear empirical cutoff to utilize for the analysis, multiple cutoffs were explored (e.g., 40% high school graduates vs. 60% high school graduates; high (≥ 40%) versus low (< 40%) proportion of males), however this did not substantively alter the results of the analysis.
The goal of the present study was to determine whether CRC screening interventions should abandon efforts to change CRC risk perception. The small, positive relationship between CRC risk perception and screening behavior reflects the importance of interventions that target multiple factors. Our findings highlighted the importance of conducting high-quality studies. In particular, studies should include only asymptomatic adults undergoing routine screening, rather than including adults who are receiving testing to address symptoms. While further research may well reveal lower effect sizes for the CRC risk perceptions and screening relation, given the considerable effect size heterogeneity, before abandoning risk perception as a potentially important predictor of screening, assessments should be standardized to allow for better determination the impact of CRC risk perception on screening behavior in future research.
Footnotes
This project was supported by a National Institutes of Health Research Training Grant (T32 CA009461-25); a National Institutes of Health Support Grant (P30-CA-008748); as well as National Institutes of Health Grants CA137532 and CA133376 (PI: Hay) and R03-CA144682 (PI: Salz). Dr. Salz is also supported by a Scholar in Clinical Research Award from the Leukemia and Lymphoma Society. The authors wish to acknowledge Sarah Jewell for her valuable assistance with the literature searches, Noel Brewer and Kevin McCaul for their helpful feedback, as well as Richard Moser for providing raw data for the analysis and the MSK Behavioral Research Methods Core Facility. A portion of these results were presented at the 2013 Meeting of the Society of Behavioral Medicine in San Francisco, CA.
Ethical Standards
The authors of this article report no conflict of interest. No animal or human studies were carried out by the authors for this article.
Contributor Information
Thomas M. Atkinson, Email: atkinsot@mskcc.org.
Talya Salz, Email: salzt@mskcc.org.
Kaitlin K. Touza, Email: ktouza@iupui.edu.
Yuelin Li, Email: liy12@mskcc.org.
Jennifer L. Hay, Email: hayj@mskcc.org.
References
- American Cancer Society. [Accessed 31 July 2015];Cancer Facts & Figures 2015. Available from: http://www.cancer.org/acs/groups/content/@editorial/documents/document/acspc-044552.pdf.
- Azaiza F, Cohen M. Colorectal cancer screening, intentions, and predictors in Jewish and Arab Israelis: a population-based study. Health Educ Behav. 2008;35:478–493. doi: 10.1177/1090198106297045. [DOI] [PubMed] [Google Scholar]
- Bae N, Park S, Lim S. Factors associated with adherence to fecal occult blood testing for colorectal cancer screening among adults in the Republic of Korea. Eur J Oncol Nurs. 2014;18:72–77. doi: 10.1016/j.ejon.2013.09.001. [DOI] [PubMed] [Google Scholar]
- Baier M, Calonge N, Cutter G, McClatchey M, Schoentgen S, Hines S, Ahnen D. Validity of self-reported colorectal cancer screening behavior. Cancer Epidemiol Biomarkers Prev. 2000;9:229–232. [PubMed] [Google Scholar]
- Bastani R, Glenn BA, Maxwell AE, Ganz PA, Mojica CM, Chang LC. Validation of self-reported colorectal cancer (CRC) screening in a study of ethnically diverse first-degree relatives of CRC cases. Cancer Epidemiol Biomarkers Prev. 2008;17:791–798. doi: 10.1158/1055-9965.EPI-07-2625. [DOI] [PubMed] [Google Scholar]
- Bazargan M, Ani C, Bazargan-Hejazi S, Baker RS, Bastani R. Colorectal cancer screening among underserved minority population: discrepancy between physicians’ recommended, scheduled, and completed tests. Patient Educ Couns. 2009;76:240–247. doi: 10.1016/j.pec.2008.12.005. [DOI] [PubMed] [Google Scholar]
- Beck KH, Frankel A. A conceptualization of threat communications and protective health behavior. Soc Psychol Q. 1981;44:204–217. [PubMed] [Google Scholar]
- Begg CB, Mazumdar M. Operating characteristics of a rank correlation test for publication bias. Biometrics. 1994;50:1088–1101. [PubMed] [Google Scholar]
- Blalock SJ, DeVellis BM, Afifi RA, Sandler RS. Risk perceptions and participation in colorectal cancer screening. Health Psychol. 1990;9:792–806. doi: 10.1037//0278-6133.9.6.792. [DOI] [PubMed] [Google Scholar]
- Bleiker EMA, Menko FH, Taal BG, Kluijt I, Wever LDV, Gerritsma MA, Aaronson NK. Screening behavior of individuals at high risk for colorectal cancer. Gastroenterology. 2005;128:280–287. doi: 10.1053/j.gastro.2004.11.002. [DOI] [PubMed] [Google Scholar]
- Brawarsky P, Brooks DR, Mucci LA, Wood PA. Effect of physician recommendation and patient adherence on rates of colorectal cancer testing. Cancer Detect Prev. 2004;28:260–268. doi: 10.1016/j.cdp.2004.04.006. [DOI] [PubMed] [Google Scholar]
- Brenes GA, Paskett ED. Predictors of stage of adoption for colorectal cancer screening. Prev Med. 2000;31:410–416. doi: 10.1006/pmed.2000.0729. [DOI] [PubMed] [Google Scholar]
- Brewer NT, Chapman GB, Gibbons FX, Gerrard M, McCaul KD, Weinstein ND. Meta-analysis of the relationship between risk perception and health behavior: The example of vaccination. Health Psychology. 2007;26:136–145. doi: 10.1037/0278-6133.26.2.136. [DOI] [PubMed] [Google Scholar]
- Brewer NT, Weinstein ND, Cuite CL, Herrington JE. Risk perceptions and their relation to risk behavior. Ann Behav Med. 2004;27:125–130. doi: 10.1207/s15324796abm2702_7. [DOI] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention. Cancer screening - United States, 2010. Morbidity and Mortality Weekly Report. 2012;61:41–45. [PubMed] [Google Scholar]
- Choi KC, So WK, Chan DN, Shiu AT, Ho SS, Chan HY, Chan CW. Gender differences in the use of colorectal cancer tests among older Chinese adults. Eur J Oncol Nurs. 2013;17:603–609. doi: 10.1016/j.ejon.2013.01.004. [DOI] [PubMed] [Google Scholar]
- Clipp EC, Carver EH, Pollak KI, Puleo E, Emmons KM, Onken J, McBride CM. Age-related vulnerabilities of older adults with colon adenomas: evidence from Project Prevent. Cancer. 2004;100:1085–1094. doi: 10.1002/cncr.20082. [DOI] [PubMed] [Google Scholar]
- Codori AM, Petersen GM, Miglioretti DL, Boyd P. Health beliefs and endoscopic screening for colorectal cancer: potential for cancer prevention. Prev Med. 2001;33:128–136. doi: 10.1006/pmed.2001.0862. [DOI] [PubMed] [Google Scholar]
- Costanza ME, Luckmann R, Stoddard AM, Avrunin JS, White MJ, Stark JR, Rosal MC. Applying a stage model of behavior change to colon cancer screening. Prev Med. 2005;41:707–719. doi: 10.1016/j.ypmed.2004.12.013. [DOI] [PubMed] [Google Scholar]
- Cronan TA, Devoscomby L, Villalta I, Gallagher R. Ethnic Differences in Colorectal Cancer Screening. Journal of Psychosocial Oncology. 2008;26:63–86. doi: 10.1300/J077v26n02_05. [DOI] [PubMed] [Google Scholar]
- Cummings KM, Becker MH, Maile MC. Bringing the models together: an empirical approach to combining variables used to explain health actions. J Behav Med. 1980;3:123–145. doi: 10.1007/BF00844986. [DOI] [PubMed] [Google Scholar]
- Dear K, Scott L, Chambers S, Corbett MC, Taupin D. Perception of Colorectal Cancer Risk does not Enhance Participation in Screening. Therap Adv Gastroenterol. 2008;1:157–167. doi: 10.1177/1756283X08097776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards AG, Naik G, Ahmed H, Elwyn GJ, Pickles T, Hood K, Playle R. Personalised risk communication for informed decision making about taking screening tests. Cochrane Database Syst Rev. 2013;2:CD001865. doi: 10.1002/14651858.CD001865.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandez ME, Savas LS, Wilson KM, Byrd TL, Atkinson J, Torres-Vigil I, Vernon SW. Colorectal cancer screening among Latinos in three communities on the Texas-Mexico border. Health Educ Behav. 2015;42:16–25. doi: 10.1177/1090198114529592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flander L, Speirs-Bridge A, Rutstein A, Niven H, Win AK, Ait Ouakrim D, Jenkins M. Perceived versus predicted risks of colorectal cancer and self-reported colonoscopies by members of mismatch repair gene mutation-carrying families who have declined genetic testing. J Genet Couns. 2014;23:79–88. doi: 10.1007/s10897-013-9614-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Floyd DL, Prentice-Dunn S, Rogers RW. A Meta-Analysis of Research on Protection Motivation Theory. Journal of Applied Social Psychology. 2000;30:407–429. doi: 10.1111/j.1559-1816.2000.tb02323.x. [DOI] [Google Scholar]
- Frank D, Swedmark J, Grubbs L. Colon cancer screening in African American women. ABNF J. 2004;15:67–70. [PubMed] [Google Scholar]
- Friedman LC, Everett TE, Peterson L, Ogbonnaya KI, Mendizabal V. Compliance with fecal occult blood test screening among low-income medical outpatients: a randomized controlled trial using a videotaped intervention. J Cancer Educ. 2001;16:85–88. doi: 10.1080/08858190109528738. [DOI] [PubMed] [Google Scholar]
- Friedman LC, Webb JA, Richards CS, Plon SE. Psychological and behavioral factors associated with colorectal cancer screening among Ashkenazim. Prev Med. 1999;29:119–125. doi: 10.1006/pmed.1999.0508. [DOI] [PubMed] [Google Scholar]
- Gimeno Garcia AZ, Quintero E, Nicolas Perez D, Hernandez M, JimenezSosa A. Colorectal cancer screening in first-degree relatives of colorectal cancer: participation, knowledge, and barriers against screening. Eur J Gastroenterol Hepatol. 2011;23:1165–1171. doi: 10.1097/MEG.0b013e32834a289e. [DOI] [PubMed] [Google Scholar]
- Glenn BA, Herrmann AK, Crespi CM, Mojica CM, Chang LC, Maxwell AE, Bastani R. Changes in risk perceptions in relation to self-reported colorectal cancer screening among first-degree relatives of colorectal cancer cases enrolled in a randomized trial. Health Psychol. 2011;30:481–491. doi: 10.1037/a0024288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon NP, Hiatt RA, Lampert DI. Concordance of self-reported data and medical record audit for six cancer screening procedures. J Natl Cancer Inst. 1993;85:566–570. doi: 10.1093/jnci/85.7.566. [DOI] [PubMed] [Google Scholar]
- Gorin SS. Correlates of Colorectal Cancer Screening Compliance Among Urban Hispanics. Journal of Behavioral Medicine. 2005;28:125–137. doi: 10.1007/s10865-005-3662-5. [DOI] [PubMed] [Google Scholar]
- Griffith KA. Biological, psychological and behavioral, and social variables influencing colorectal cancer screening in African Americans. Nurs Res. 2009;58:312–320. doi: 10.1097/NNR.0b013e3181ac143d. [DOI] [PubMed] [Google Scholar]
- Hall HI, Van Den Eeden SK, Tolsma DD, Rardin K, Thompson T, Hughes Sinclair A, Nadel M. Testing for prostate and colorectal cancer: comparison of self-report and medical record audit. Prev Med. 2004;39:27–35. doi: 10.1016/j.ypmed.2004.02.024. [DOI] [PubMed] [Google Scholar]
- Harrison JA, Mullen PD, Green LW. A meta-analysis of studies of the Health Belief Model with adults. Health Educ Res. 1992;7:107–116. doi: 10.1093/her/7.1.107. [DOI] [PubMed] [Google Scholar]
- Hay JL, Coups E, Ford J. Predictors of perceived risk for colon cancer in a national probability sample in the United States. J Health Commun. 2006;11(Suppl 1):71–92. doi: 10.1080/10810730600637376. [DOI] [PubMed] [Google Scholar]
- Hay JL, Ford JS, Klein D, Primavera LH, Buckley TR, Stein TR, Ostroff JS. Adherence to colorectal cancer screening in mammography-adherent older women. J Behav Med. 2003;26:553–576. doi: 10.1023/a:1026253802962. [DOI] [PubMed] [Google Scholar]
- Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327:557–560. doi: 10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higgins JP, White IR, Wood AM. Imputation methods for missing outcome data in meta-analysis of clinical trials. Clin Trials. 2008;5:225–239. doi: 10.1177/1740774508091600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holden DJ, Jonas DE, Porterfield DS, Reuland D, Harris R. Systematic review: enhancing the use and quality of colorectal cancer screening. Ann Intern Med. 2010;152:668–676. doi: 10.7326/0003-4819-152-10-201005180-00239. [DOI] [PubMed] [Google Scholar]
- Janz NK, Becker MH. The Health Belief Model: a decade later. Health Educ Q. 1984;11:1–47. doi: 10.1177/109019818401100101. [DOI] [PubMed] [Google Scholar]
- Javadzade SH, Reisi M, Mostafavi F, Hasanzade A, Shahnazi H, Sharifirad G. Factors associated with the fecal occult blood testing for colorectal cancer screening based on health belief model structures in moderate risk individuals, Isfahan, 2011. J Educ Health Promot. 2012;1:18. doi: 10.4103/2277-9531.99218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joseph DA, King JB, Miller JW, Richardson LC Centers for Disease, C., & Prevention. Prevalence of colorectal cancer screening among adults--Behavioral Risk Factor Surveillance System, United States, 2010. MMWR Morb Mortal Wkly Rep. 2012;61(Suppl):51–56. [PubMed] [Google Scholar]
- Kelly RB, Shank JC. Adherence to screening flexible sigmoidoscopy in asymptomatic patients. Med Care. 1992;30:1029–1042. doi: 10.1097/00005650-199211000-00006. [DOI] [PubMed] [Google Scholar]
- Kim K, Chapman C, Vallina H. Colorectal cancer screening among Chinese American immigrants. J Immigr Minor Health. 2012;14:898–901. doi: 10.1007/s10903-011-9559-1. [DOI] [PubMed] [Google Scholar]
- Kim SE, Perez-Stable EJ, Wong S, Gregorich S, Sawaya GF, Walsh JM, Kaplan CP. Association between cancer risk perception and screening behavior among diverse women. Arch Intern Med. 2008;168:728–734. doi: 10.1001/archinte.168.7.728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klabunde CN, Joseph DA, King JB, White A, Plescia M. Vital Signs: Colorectal Cancer Screening Test Use United States 2012. [Accessed May 26, 2015];MMWR. 2013 Available from: http://www.cdc.gov/mmwr/preview/mmwrhtml/mm6244a4.htm?s_cid=mm6244a4_w.
- Koo JH, Arasaratnam MM, Liu K, Redmond DM, Connor SJ, Sung JJ, Leong RW. Knowledge, perception and practices of colorectal cancer screening in an ethnically diverse population. Cancer Epidemiol. 2010;34:604–610. doi: 10.1016/j.canep.2010.05.013. [DOI] [PubMed] [Google Scholar]
- Kremers SP, Mesters I, Pladdet IE, van den Borne B, Stockbrugger RW. Participation in a sigmoidoscopic colorectal cancer screening program: a pilot study. Cancer Epidemiol Biomarkers Prev. 2000;9:1127–1130. [PubMed] [Google Scholar]
- Lawsin C, DuHamel K, Weiss A, Rakowski W, Jandorf L. Colorectal cancer screening among low-income African Americans in East Harlem: a theoretical approach to understanding barriers and promoters to screening. J Urban Health. 2007;84:32–44. doi: 10.1007/s11524-006-9126-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leventhal H, Cameron L. Behavioral theories and the problem of compliance. Patient Education and Counseling. 1987;10:117–138. doi: 10.1016/0738-3991(87)90093-0. [DOI] [Google Scholar]
- Lewis SF, Jensen NM. Screening sigmoidoscopy. Factors associated with utilization. J Gen Intern Med. 1996;11:542–544. doi: 10.1007/BF02599602. [DOI] [PubMed] [Google Scholar]
- Lipkus IM, Lyna PR, Rimer BK. Colorectal cancer risk perceptions and screening intentions in a minority population. J Natl Med Assoc. 2000;92:492–500. [PMC free article] [PubMed] [Google Scholar]
- Longacre AV, Cramer LD, Gross CP. Screening colonoscopy use among individuals at higher colorectal cancer risk. J Clin Gastroenterol. 2006;40:490–496. doi: 10.1097/00004836-200607000-00006. [DOI] [PubMed] [Google Scholar]
- Lubetkin EI, Santana A, Tso A, Jia H. Predictors of cancer screening among low-income primary care patients. J Health Care Poor Underserved. 2008;19:135–148. doi: 10.1353/hpu.2008.0001. [DOI] [PubMed] [Google Scholar]
- Mack LA, Cook LS, Temple WJ, Carlson LE, Hilsden RJ, Paolucci EO. Colorectal cancer screening among first-degree relatives of colorectal cancer patients: benefits and barriers. Ann Surg Oncol. 2009;16:2092–2100. doi: 10.1245/s10434-009-0528-z. [DOI] [PubMed] [Google Scholar]
- Madlensky L, Esplen MJ, Gallinger S, McLaughlin JR, Goel V. Relatives of colorectal cancer patients. Am J Prev Med. 2003;25:187–194. doi: 10.1016/s0749-3797(03)00202-2. [DOI] [PubMed] [Google Scholar]
- Mandelson MT, Curry SJ, Anderson LA, Nadel MR, Lee NC, Rutter CM, LaCroix AZ. Colorectal cancer screening participation by older women. Am J Prev Med. 2000;19:149–154. doi: 10.1016/s0749-3797(00)00193-8. [DOI] [PubMed] [Google Scholar]
- Mandelson MT, LaCroix AZ, Anderson LA, Nadel MR, Lee NC. Comparison of self-reported fecal occult blood testing with automated laboratory records among older women in a health maintenance organization. Am J Epidemiol. 1999;150:617–621. doi: 10.1093/oxfordjournals.aje.a010060. [DOI] [PubMed] [Google Scholar]
- Manne S, Markowitz A, Winawer S, Meropol NJ, Haller D, Rakowski W, Jandorf L. Correlates of colorectal cancer screening compliance and stage of adoption among siblings of individuals with early onset colorectal cancer. Health Psychology. 2002;21:3–15. doi: 10.1037//0278-6133.21.1.3. [DOI] [PubMed] [Google Scholar]
- Matthews BA, Nattinger AB, Venkatesan T, Shaker R, Anderson RC. Objective risk, subjective risk, and colorectal cancer screening among a clinic sample. Psychol Health Med. 2007;12:135–147. doi: 10.1080/13548500500429312. [DOI] [PubMed] [Google Scholar]
- McCaul KD, Branstetter AD, Schroeder DM, Glasgow RE. What is the relationship between breast cancer risk and mammography screening? A meta-analytic review. Health Psychology. 1996;15:423–429. doi: 10.1037/0278-6133.15.6.423. [DOI] [PubMed] [Google Scholar]
- McQueen A, Vernon SW, Myers RE, Watts BG, Lee ES, Tilley BC. Correlates and predictors of colorectal cancer screening among male automotive workers. Cancer Epidemiol Biomarkers Prev. 2007;16:500–509. doi: 10.1158/1055-9965.EPI-06-0757. [DOI] [PubMed] [Google Scholar]
- McQueen A, Vernon SW, Rothman AJ, Norman GJ, Myers RE, Tilley BC. Examining the role of perceived susceptibility on colorectal cancer screening intention and behavior. Ann Behav Med. 2010;40:205–217. doi: 10.1007/s12160-010-9215-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menon U, Szalacha L, Prabhughate A, Kue J. Correlates of colorectal cancer screening among South Asian immigrants in the United States. Cancer Nurs. 2014;37:E19–27. doi: 10.1097/NCC.0b013e31828db95e. [DOI] [PubMed] [Google Scholar]
- Milne S, Sheeran P, Orbell S. Prediction and Intervention in Health-Related Behavior: A Meta-Analytic Review of Protection Motivation Theory. Journal of Applied Social Psychology. 2000;30:106–143. doi: 10.1111/j.1559-1816.2000.tb02308.x. [DOI] [Google Scholar]
- Montano DE, Phillips WR. Cancer screening by primary care physicians: a comparison of rates obtained from physician self-report, patient survey, and chart audit. Am J Public Health. 1995;85:795–800. doi: 10.2105/ajph.85.6.795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moser RP, McCaul K, Peters E, Nelson W, Marcus SE. Associations of perceived risk and worry with cancer health-protective actions: data from the Health Information National Trends Survey (HINTS) J Health Psychol. 2007;12:53–65. doi: 10.1177/1359105307071735. [DOI] [PubMed] [Google Scholar]
- Myers RE, Ross E, Jepson C, Wolf T, Balshem A, Millner L, Leventhal H. Modeling adherence to colorectal cancer screening. Prev Med. 1994;23:142–151. doi: 10.1006/pmed.1994.1020. [DOI] [PubMed] [Google Scholar]
- Palmer RC, Chhabra D, McKinney S. Colorectal cancer screening adherence in African-American men and women 50 years of age and older living in Maryland. J Community Health. 2011;36:517–524. doi: 10.1007/s10900-010-9336-4. [DOI] [PubMed] [Google Scholar]
- Palmer RC, Emmons KM, Fletcher RH, Lobb R, Miroshnik I, Kemp JA, Bauer M. Familial risk and colorectal cancer screening health beliefs and attitudes in an insured population. Prev Med. 2007;45:336–341. doi: 10.1016/j.ypmed.2007.07.021. [DOI] [PubMed] [Google Scholar]
- Paskett ED, Rushing J, D’Agostino R, Jr, Tatum C, Velez R. Cancer screening behaviors of low-income women: the impact of race. Womens Health. 1997;3:203–226. [PubMed] [Google Scholar]
- Rauscher GH, Johnson TP, Cho YI, Walk JA. Accuracy of self-reported cancer-screening histories: a meta-analysis. Cancer Epidemiol Biomarkers Prev. 2008;17:748–757. doi: 10.1158/1055-9965.EPI-07-2629. [DOI] [PubMed] [Google Scholar]
- Rawl SM, Menon U, Burness A, Breslau ES. Interventions to promote colorectal cancer screening: an integrative review. Nurs Outlook. 2012;60:172–181. e113. doi: 10.1016/j.outlook.2011.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rex DK, Bond JH, Winawer S, Levin TR, Burt RW, Johnson DA Cancer U. S. M.-S. T. F. o. C. Quality in the technical performance of colonoscopy and the continuous quality improvement process for colonoscopy: recommendations of the U.S. Multi-Society Task Force on Colorectal Cancer. Am J Gastroenterol. 2002;97:1296–1308. doi: 10.1111/j.1572-0241.2002.05812.x. [DOI] [PubMed] [Google Scholar]
- Rosenthal R. Meta-analytic procedures for social research. Beverly Hills, CA: Sage; 1984. [Google Scholar]
- Sabatino SA, Lawrence B, Elder R, Mercer SL, Wilson KM, DeVinney B Community Preventive Services Task F. Effectiveness of interventions to increase screening for breast, cervical, and colorectal cancers: nine updated systematic reviews for the guide to community preventive services. Am J Prev Med. 2012;43:97–118. doi: 10.1016/j.amepre.2012.04.009. [DOI] [PubMed] [Google Scholar]
- Santos EM, Lourenco MT, Rossi BM. Risk perception among Brazilian individuals with high risk for colorectal cancer and colonoscopy. Hered Cancer Clin Pract. 2011;9:4. doi: 10.1186/1897-4287-9-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schroy PC, 3rd, Coe AM, Mylvaganam SR, Ahn LB, Lydotes MA, Robinson PA, Heeren TC. The Your Disease Risk Index for colorectal cancer is an inaccurate risk stratification tool for advanced colorectal neoplasia at screening colonoscopy. Cancer Prev Res (Phila) 2012;5:1044–1052. doi: 10.1158/1940-6207.CAPR-12-0014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheeran P, Harris PR, Epton T. Does heightening risk appraisals change people’s intentions and behavior? A meta-analysis of experimental studies. Psychol Bull. 2014;140:511–543. doi: 10.1037/a0033065. [DOI] [PubMed] [Google Scholar]
- Shokar NK, Carlson CA, Weller SC. Factors associated with racial/ethnic differences in colorectal cancer screening. J Am Board Fam Med. 2008;21:414–426. doi: 10.3122/jabfm.2008.05.070266. [DOI] [PubMed] [Google Scholar]
- Stark JR, Bertone-Johnson ER, Costanza ME, Stoddard AM. Factors associated with colorectal cancer risk perception: the role of polyps and family history. Health Educ Res. 2006;21:740–749. doi: 10.1093/her/cyl049. [DOI] [PubMed] [Google Scholar]
- Sterne JA, Sutton AJ, Ioannidis JP, Terrin N, Jones DR, Lau J, Higgins JP. Recommendations for examining and interpreting funnel plot asymmetry in meta-analyses of randomised controlled trials. BMJ. 2011;343:d4002. doi: 10.1136/bmj.d4002. [DOI] [PubMed] [Google Scholar]
- Sun WY. EdD Dissertation. Teachers College, Columbia University; 2010. Knowledge, beliefs, attitudes, barriers, and acculturation associated with colorectal cancer screening among Chinese Americans. [Google Scholar]
- Sun WY, Basch CE, Wolf RL, Li XJ. Factors associated with colorectal cancer screening among Chinese-Americans. Prev Med. 2004;39:323–329. doi: 10.1016/j.ypmed.2004.04.029. [DOI] [PubMed] [Google Scholar]
- Sutton S, Wardle J, Taylor T, McCaffery K, Williamson S, Edwards R, Atkin W. Predictors of attendance in the United Kingdom flexible sigmoidoscopy screening trial. J Med Screen. 2000;7:99–104. doi: 10.1136/jms.7.2.99. [DOI] [PubMed] [Google Scholar]
- Taniguchi T, Hirai K, Sumi R, Hayashi N, Maeda K, Ito T. Predictors of colonoscopy use one year after colonoscopy: prospective study of surveillance behavior for colorectal cancer. Health Psychol Behav Med. 2014;2:283–295. doi: 10.1080/21642850.2014.889573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taouqi M, Ingrand I, Beauchant M, Migeot V, Ingrand P. Determinants of participation in colonoscopic screening by siblings of colorectal cancer patients in France. BMC Cancer. 2010;10:355. doi: 10.1186/1471-2407-10-355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teng EJ, Friedman LC, Green CE. Determinants of colorectal cancer screening behavior among Chinese Americans. Psychooncology. 2006;15:374–381. doi: 10.1002/pon.958. [DOI] [PubMed] [Google Scholar]
- Tessaro I, Mangone C, Parkar I, Pawar V. Knowledge, barriers, and predictors of colorectal cancer screening in an Appalachian church population. Prev Chronic Dis. 2006;3:A123. [PMC free article] [PubMed] [Google Scholar]
- Thompson SG, Higgins JP. How should meta-regression analyses be undertaken and interpreted? Stat Med. 2002;21:1559–1573. doi: 10.1002/sim.1187. [DOI] [PubMed] [Google Scholar]
- U. S. Preventive Services Task Force. Screening for colorectal cancer: U.S. Preventive Services Task Force recommendation statement. Ann Intern Med. 2008;149:627–637. doi: 10.7326/0003-4819-149-9-200811040-00243. [DOI] [PubMed] [Google Scholar]
- Vernon SW. Participation in colorectal cancer screening: a review. J Natl Cancer Inst. 1997;89:1406–1422. doi: 10.1093/jnci/89.19.1406. [DOI] [PubMed] [Google Scholar]
- Vernon SW, Bartholomew LK, McQueen A, Bettencourt JL, Greisinger A, Coan SP, Myers RE. A randomized controlled trial of a tailored interactive computer-delivered intervention to promote colorectal cancer screening: sometimes more is just the same. Ann Behav Med. 2011;41:284–299. doi: 10.1007/s12160-010-9258-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vernon SW, Myers RE, Tilley BC, Li SH. Factors associated with perceived risk in automotive employees at increased risk of colorectal cancer. Cancer Epidemiology Biomarkers & Prevention. 2001;10:35–43. [PubMed] [Google Scholar]
- Wardle J, Miles A, Atkin W. Gender differences in utilization of colorectal cancer screening. J Med Screen. 2005;12:20–27. doi: 10.1258/0969141053279158. [DOI] [PubMed] [Google Scholar]
- Weinberg DS, Turner BJ, Wang H, Myers RE, Miller S. A survey of women regarding factors affecting colorectal cancer screening compliance. Prev Med. 2004;38:669–675. doi: 10.1016/j.ypmed.2004.02.015. [DOI] [PubMed] [Google Scholar]
- Weinstein ND. The precaution adoption process. Health Psychol. 1988;7:355–386. doi: 10.1037//0278-6133.7.4.355. [DOI] [PubMed] [Google Scholar]
- Weinstein ND. Perceived probability, perceived severity, and health-protective behavior. Health Psychology. 2000;19:65–74. doi: 10.1037/0278-6133.19.1.65. [DOI] [PubMed] [Google Scholar]
- Zauber AG, Winawer SJ, O’Brien MJ, Lansdorp-Vogelaar I, van Ballegooijen M, Hankey BF, Waye JD. Colonoscopic polypectomy and long-term prevention of colorectal-cancer deaths. N Engl J Med. 2012;366:687–696. doi: 10.1056/NEJMoa1100370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zlot AI, Silvey K, Newell N, Coates RJ, Leman R. Family History of Colorectal Cancer: Clinicians’ Preventive Recommendations and Patient Behavior. Preventing Chronic Disease. 2011 doi: 10.5888/pcd9.100254. [DOI] [PMC free article] [PubMed] [Google Scholar]