Abstract
Behçet’s disease is a chronic multisystem inflammatory disorder characterized mainly by recurrent oral ulcers, ocular involvement, genital ulcers, and skin lesions, presenting with remissions and exacerbations. It is thought that both environmental and genetic factors contribute to its onset and development. Although the etiology of Behçet’s disease remains unclear, recent immunogenetic findings are providing clues to its pathogenesis. In addition to the positive association of HLA-B*51, which was identified more than four decades ago, and which has since been confirmed in multiple populations, recent studies report additional independent associations in the major histocompatibility complex class I region. HLA-B*15, -B*27, -B*57, and -A*26 are independent risk factors for Behçet’s disease, while HLA-B*49 and – A*03 are independent class I alleles that are protective for Behçet’s disease. Genome-wide association studies have identified associations with genome-wide significance (P < 5 × 10−8) in the IL23R–IL12RB2, IL10, STAT4, CCR1-CCR3, KLRC4, ERAP1, TNFAIP3, and FUT2 loci. In addition, targeted next-generation sequencing has revealed the involvement of rare nonsynonymous variants of IL23R, TLR4, NOD2, and MEFV in Behçet’s disease pathogenesis. Significant differences in gene function or mRNA expression associated with the risk alleles of the disease susceptibility loci suggest which genes in a disease-associated locus influence disease pathogenesis. These genes encompass both innate and adaptive immunity and confirm the importance of the predominant polarization towards helper T cell (Th) 1 versus Th2 cells, and the involvement of Th17 cells. In addition, epistasis observed between HLA-B*51 and the risk coding haplotype of the endoplasmic reticulum-associated protease, ERAP1, provides a clue that an HLA class I-peptide presentation-based mechanism contributes to this complex disease.
Keywords: Behçet’s disease, GWAS, HLA-B*51, ERAP1, disease-associated genetic variants
1. Introduction
Behçet’s disease (BD) is a chronic multisystem inflammatory disorder characterized mainly by recurrent oral ulcers, ocular involvement, genital ulcers, and skin lesions, presenting with remissions and exacerbations. Arthritis, gastrointestinal lesions, vasculitis, epididymitis, and central nervous system lesions are also frequently observed disease manifestations in BD patients [1, 2]. BD is relatively prevalent in countries located between 30 and 45 degrees north latitude through the Mediterranean basin, the Middle East, China, and Japan along the ancient Silk Route [3].
BD has aspects of both autoimmune disease and autoinflammatory disease. Effectiveness of classical immunosuppressives such as azathioprine and cyclosporine [4], and the suggested role of the candidate autoantigen, human heat-shock protein 60 (HSP60) [5], are features of autoimmunity. On the other hand, the lack of significant high-titer auto-antibodies or antigen-specific T-cells, strong involvement of major histocompatibility complex (MHC) class I molecules, clinical episodes of unprovoked recurrent inflammation, mainly caused by neutrophils [6], and the disease relationship with familial Mediterranean fever (FMF), such as the contribution of the M694V MEFV mutation to BD susceptibility and the therapeutic effectiveness of colchicine, are features of autoinflammation. Although the etiology of BD remains unclear, recent immunogenetic findings have increased our understanding of pathogenesis. Here we review current knowledge with a focus on the immunogenetics of BD.
2. Genetic Factors
2.1. Geoepidemiology
Although it is thought that common environmental factors such as infections or exposures to toxins or to specific immunogens contribute to BD, development of disease is believed to occur only in genetically predisposed hosts. The wide range of disease prevalence observed among different geographic locales is likely a result of differences in both environment and genetics. Disease prevalence in Turkey, the country with the highest reported prevalence, is estimated from 80 to 420 per 100,000 [1, 7–9]. Other studies report a high prevalence of BD with 13.5 per 100,000 in Japan, or 14.0 per 100,000 in China [10]. On the other hand, a relatively low prevalence is reported in Northern and Western Europe and the United States [10]. The high prevalence areas can be partly explained by the high frequency of HLA-B*51 allele carriage (see below) in these regions [3]. Differences in disease prevalence among recent migrants compared with those residing in their home country help establish a role of the environment, while differences in disease prevalence among individuals of different ancestries residing in the same region reflect the role of genetics in disease susceptibility. The prevalence of BD is reduced among Turks who recently immigrated to Germany (15.1 per 100,000) compared with those residing in Turkey (80 – 420 per 100,000), but is nevertheless high compared with individuals of German ancestry who live in Germany (0.30 per 100,000) [11]. These findings from geoepidemiological studies indicate the importance of both genetic and environmental factors in BD pathogenesis.
2.2. Familial aggregation
Familial aggregation of BD also supports the involvement of genetic factors in pathogenesis. Although BD usually occurs sporadically, familial aggregation and a higher prevalence in siblings and parents of BD patients has been observed [12]. Familial aggregation of BD also varies among populations. In Turks (18.2%), Koreans (15.4%), and Jews (13.2%) familial aggregation is higher than in the Chinese (2.6%), Japanese (2.2%), and various European populations (0–4.5%) [13–16]. Stronger familial aggregation was observed among early onset BD patients [12] compared with individuals with disease onset in adulthood. Analysis of BD pediatric patient families suggested an autosomal recessive mode of inheritance by segregation analysis [17]. However, no particular Mendelian inheritance patterns were shown from analysis of BD including all ages of onset [17, 18]. A study in the Turkish population reported a high sibling recurrence (4.2%), and estimated a high sibling recurrence risk ratio (λs) (11.4 – 52.5), which is the ratio between the risk of being affected among siblings of patients to the disease risk in the general population, a widely used indicator for familial aggregation [19, 20].
3. MHC region
3.1. HLA-B*51
The MHC region on chromosome 6p21 contains human leukocyte antigen (HLA) and other essential genes in the immune response. Ohno et al. reported association of the HL-A5 antigen in the Japanese population in 1973. The antigen was later renamed HLA-B5, a designation that subsumes HLA-B*51 and several other specificities [21]. This study provided the earliest genetic evidence for BD. Among more than 250 subtypes of HLA-B*51 defined by the protein sequence (IPD - IMGT/HLA, a database for sequences of the human HLA), HLA-B*51:01 is the major subtype that has been associated with BD in multiple populations [22–38]. A sequence study of the full gene region of HLA-B*51:01 from 24 cases and 13 healthy controls from the Japanese, Turkish, Jordanian, and Iranian populations confirmed that all individuals carried HLA-B*51:01:01 with no variation in the exons, introns, or 5’-flanking region, suggesting the association of HLA-B*51 represents the association of HLA-B*51:01:01 itself [39].
The geographically pooled prevalences for HLA-B5/B*51 reported in a systematic review and meta-analysis including 4,800 BD patients and 16,289 controls from 80 independent studies are 55.0 – 63.5% in BD cases and 16.8 – 21.7% in controls for populations in East Asia, Middle East/North Africa, Southern Europe, and Northern/Eastern Europe [40]. The pooled overall odds ratio (OR) (95% confidence interval [CI]) was 5.78 (5.00 – 6.67) for HLA-B5/B*51 and 5.90 (4.87 – 7.16) for HLA-B*51 type carriage [40] and the population attributable risk (PAR) of HLA-B5/B*51 was estimated to be 52.2 % for BD patients in Southern Europe, 49.9 % in Middle East/North Africa, 44.4 % in East Asia, and 31.7 % in Northern Europe [40]. In a family-based study, Gül et al. estimated a lower contribution of HLA-B to the overall genetic susceptibility to BD, in the range of 12 – 19 % [41]. Large studies should produce accurate estimates of the HLA-B*51 effect size in specific populations, but differences in analytic techniques make the results difficult to compare. For example, a recent HLA typing study of 300 patients and 300 matched healthy controls from Japan reported an allelic OR (95% CI) of 5.50 for HLA-B*51:01 [42], whereas a recent study from Turkey, with 1,190 cases and 1,257 matched healthy controls reported for an additive model, an OR (95% CI) of 3.0 (2.6 – 3.4) for HLA-B*51 per allele [43]. Regardless, although there is a strong consensus that HLA-B*51 is associated with BD risk, there has been an ongoing controversy about whether the disease association with HLA-B*51 is attributed to a role of this MHC class I variant itself or if the association is found because of its linkage disequilibrium (LD) with another variant in the region.
Hughes et al. recently reported an association study in two independent cohorts from Turkey (503 cases and 504 controls) and Italy (144 cases and 1,270 controls), genotyped on the Immunochip platform, which has very good single nucleotide polymorphism (SNP) coverage of the MHC region [44, 45]. Meta-analysis of imputed MHC region markers using the 1000 Genomes Project haplotypes as the reference and imputed MHC types using 2,512 individuals from the British Birth Cohort and HapMap CEU data as the reference revealed the strongest association signal for a SNP, rs116799036, located approximately 24 kb upstream of HLA-B and 18 kb upstream of MICA with genome-wide significance. To determine whether the genetic effect of rs116799036 could be explained by HLA-B*51:01, and vice versa, Hughes et al. performed conditional analyses. Surprisingly, a genome-wide significant association of rs116799036 remained after conditioning on HLA-B*51:01. On the other hand, the genome-wide significant association of HLA-B*51:01 completely disappeared after conditioning on rs116799036. These findings suggested HLA-B*51 itself may not underlie its association with Behçet’s disease.
A contrary result, however, was recently reported by Ombrello et al. from a large association study of of Behçet’s disease performed in 1,190 cases and 1,257 controls from Turkey using experimentally ascertained as well as imputed classical HLA types and imputed SNP and amino acid variants determined from previous GWAS genotyping data and a reference panel of 5,225 ethnically diverse European individuals with SNP and HLA-type information. Conditional analysis of the HLA-B region, adjusting for either the lead SNP (rs79556279) or HLA-B*51, which is in strong LD with the lead SNP, revealed no additional genome-wide significant associations for markers in the HLA-B – MICA region, but an independently significant association in the HLA-A – HLA-F region remained (see Section 3.2 below). Association of rs116799036 identified by Hughes et al. was found with genome-wide significance, but it was seven orders of magnitude weaker than that of HLA-B*51. Furthermore, the association of HLA-B*51 with Behçet’s disease remained significant even after conditioning for the effect of rs116799036. These findings support the involvement of HLA-B*51 itself in the pathogenesis of Behçet’s disease.
3.2. Other MHC class I genes
Although other MHC class I types, and even other HLA-B types have been reported to be associated with BD, the strong LD within the MHC region and inadequate sample size has made it difficult to reveal other HLA associations with genome-wide significance that are truly independent from HLA-B*51. Recent large studies that either perform analyses conditioning for the effect of HLA-B*51 or that are performed in HLA-B*51 non-carriers have permitted confident identification of additional MHC class I associations with BD. Ombrello et al. performed stepwise conditional analysis of HLA class I types in BD cases and controls from Turkey and revealed independent genetic associations of HLA-B*51, -B*15, and -B*27 as risk, and HLA-A*03 and -B*49 as protective types. HLA-B*57 reached near significance in HLA-B*51 non-carriers after conditioning on -B*15, and -B*49 and this result, combined with a study in the Spanish population [46], indicates that it is also a disease risk type. Similarly, Ombrello et al. found that after conditioning on HLA-B*51, -A*03, and -B*15; HLA-A*26 reached near significance in the Turkish population and this result, combined with an analysis performed in Japanese HLA-B*51 non-carriers by Meguro et al. [42], firmly establishes HLA-A*26 as a BD risk type.
3.3. Association of HLA-B variant amino acids with BD
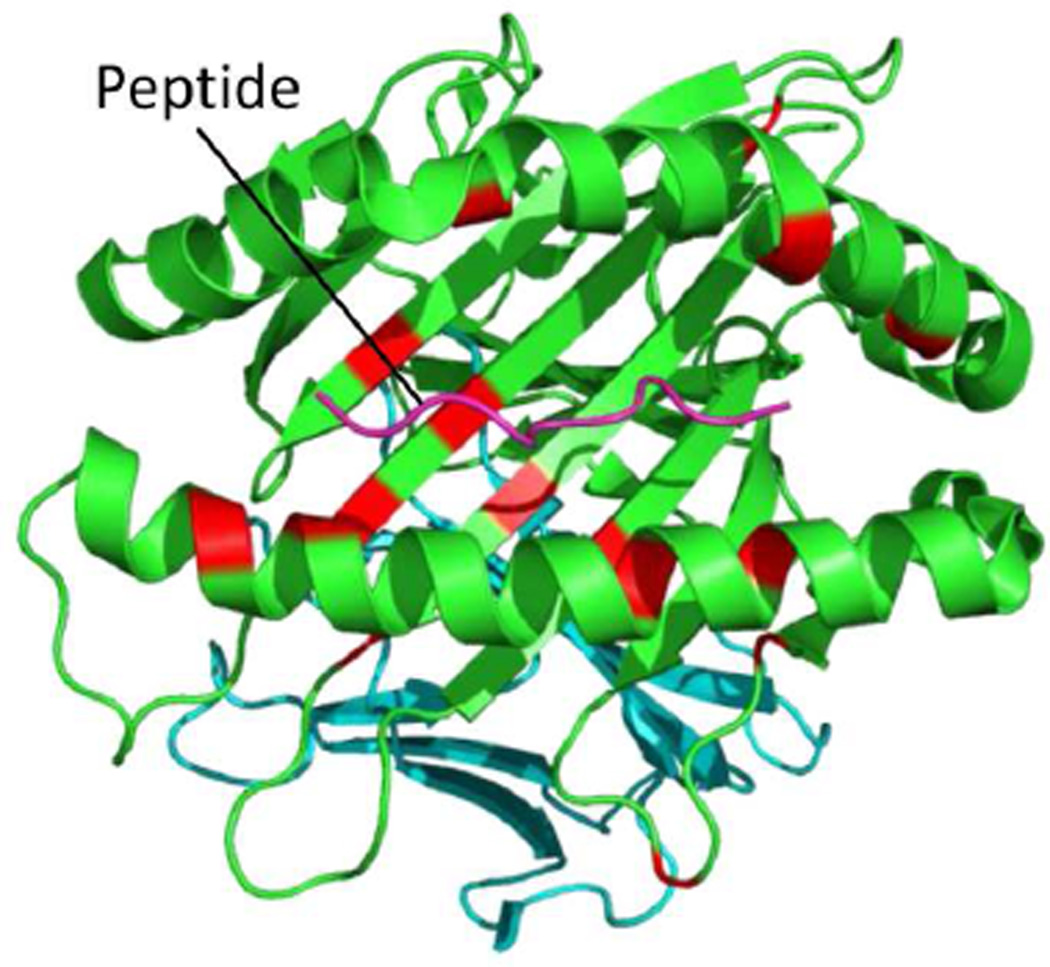
In their study Ombrello et al. also evaluated disease association of the individual variant amino acids of the HLA Class I types and found genome-wide significant associations at 16 of the 69 variant amino acid positions in the HLA-B mature protein (Table 1). These disease-associated amino acids are mainly located within the antigen-binding grove of the HLA-B molecule (Figure 1). None of the amino acid associations was more significant than the association of HLA-B*51 itself, suggesting that no single amino acid variant better encompasses disease risk or protection than the most strongly associated HLA type. Interestingly, the HLA-B risk types -B*51, -B*15, and -B*57, bear 16, 7, and 8 of the 16 disease risk-associated amino acids, respectively, but -B*27 bears only two of the risk associated amino acids (Table 1), suggesting it binds structurally distinct peptides.
Table 1. HLA-B types and BD-associated amino acids.
| HLA-type | Amino acid |
||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Pos. | 9 | 12 | 24 | 41 | 45 | 63 | 67 | 77 | 80 | 95 | 97 | 103 | 116 | 152 | 163 | 171 | |
| HLA-B*15 | Tyr | Met | Ala | Ala | Met | Glu† | Ser† | Ser† | Asn† | Leu† | Arg† | Val | Ser | Glu | Leu | Tyr† | |
| HLA-B*27 | His† | Val† | Thr† | Ala | Glu | Glu† | Cys | Asp | Thr | Leu† | Asn | Val | Asp | Val† | Glu | Tyr† | |
| HLA-B*49† | His† | Met | Thr† | Thr† | Lys† | Glu† | Ser† | Asn | Ile | Trp | Arg† | Leu† | Leu‡ | Glu | Leu | Tyr† | |
| HLA-B*51 | Tyr | Met | Ala | Ala | Thr | Asn | Phe | Asn | Ile | Trp | Thr | Val | Tyr | Glu | Leu | His | |
| HLA-B*57 | Tyr | Met | Ala | Ala | Met | Glu† | Met | Asn | Ile | Ile | Val | Val | Ser | Val† | Leu | Tyr† | |
Bold indicates the amino acids with genome-wide significant association (P < 5 × 10−8) with disease; protective HLA types and amino acids marked with †.
Protective amino acid with significant association after conditioning on positions 97, 152, and 67 [43].
Figure 1. The MHC class I molecule, HLA-B, showing BD-associated amino acid positions.
A 3D model of the HLA-B molecule drawn by PyMol using 1E27, protein data of HLA-B*51:01 from the Protein Data Bank. The pink line shows a peptide antigen in the antigen-binding groove of the HLA-B molecule. Red indicates amino acids with genome-wide significant association (P < 5 × 10−8) with BD from [43].
Stepwise conditional analysis revealed independent associations of several polymorphic amino acids located in HLA-B, with the HLA-B*51 amino acids, threonine at position 97, glutamic acid at 152, and phenylalanine at 67, associated with risk and the HLA-B*49 amino acid, leucine at 116, associated with protection. Additionally, within HLA-A, two amino acids present in HLA-A*03, aspartic acid at position 161 and isoleucine at 97, were protective. Most of these disease-associated amino acid residues of the HLA-B and HLA-A molecules are located within the antigen binding grooves, which are involved in both peptide binding and interactions between MHC class I molecules and receptors on cytotoxic T lymphocytes (CTLs) and natural killer (NK) cells [43]. Interestingly, four amino acid positions of the HLA-B signal peptide were also found to have genome-wide significance, with glycine at position −10 found as the amino acid most significantly associated with disease risk in an analysis of HLA-B*51 non-carriers [43]. Amino acids at positions 67 and 116, and to a lesser extent, 97, critically affect the interactions between the antigen-binding groove of the HLA-B molecule and KIR3DL1/KIR3DS1, which regulate/activate NK cells and CTLs [47]. Furthermore, variants of the HLA-B signal peptide also independently regulate CTL and NK cell activation through binding HLA-E and interactions with C-type lectin heterodimeric receptors, CD94/NKG2 [48–50]. Taken together with the association of the endoplasmic reticulum amino peptidase, ERAP1 (see below), the MHC region associations implicate both peptide-MHC class I binding and regulation of CTL and NK cell activation in BD pathogenesis.
3.4. HLA association with disease manifestations
Some studies have reported the association between MHC class I alleles and specific disease manifestations. A meta-analysis based on 72 studies in 74 study populations revealed moderate association of HLA-B5/-B*51 with male gender, high prevalence of eye involvement, skin involvement, and genital ulcers, and low prevalence of gastrointestinal involvement [51]. Kang et al. reported the association of HLA-B*51 with early onset uveitis and of HLA-A*26 with high prevalence of posterior uveitis in Korean BD patients [52]. They also reported that HLA-A*02:07, -A*26:01 and -A*30:04 were associated with skin lesions and arthritis, with uveitis, and with vascular lesions, genital ulcers, and a positive pathergy test, respectively, by a meta-analysis of the Korean and Japanese populations [53]. HLA-A*26:01 was also associated with poor visual prognosis in Japanese BD patients with uveitis [54]. These findings suggest MHC class I alleles reflect clinical manifestations and prognosis, indicating the possibility of a clinical use as a biomarker for diagnostic or prognostic classification of BD patients.
4. Genome-wide Association Studies
4.1. Background
Genome-wide association studies (GWAS) can provide unbiased catalogs of genes conferring disease susceptibility, using high-throughput genotyping platforms and analysis of SNPs that constitute much of the 0.1% genetic difference in human genomes [55]. To date, 8 SNP-based GWAS for BD have been performed in multiple ethnic groups, including the Turkish, Japanese, Chinese, Korean, and Iranian populations [56–63]. In one study, performed using DNA pooling technology, no SNPs, not even those located within the MHC, reached the genome-wide significance level (P < 5.0 × 10−8) [58]. Results from the other studies identified lead SNPs for loci that meet the criteria of P < 5.0 × 10−8, which is generally used as the threshold for genome-wide significance. These significantly-associated loci are reviewed in this section (Table 2).
Table 2. Summary of lead SNPs associated with genome-wide significance for Behçet’s disease susceptibility.
The pairwise LD is 0.63 for r2 between rs1495965 and rs924080, 0.95 between rs1518111 and rs1800871, 0.90 between rs7574070 and rs897200, and 0.23 between rs7616215 and rs13092160 in the European origin from 1000 Genomes Pilot 1 (SNAP).
| Variant | Gene | Location | Risk Allele |
OR | Population |
Function of the risk allele | Ref. | |
|---|---|---|---|---|---|---|---|---|
| Discovery | Replication | |||||||
| rs1495965 | IL23R,IL12RB2 | Intergenic | G | 1.35 | Japanese | Turkish | [56] | |
| rs924080 | IL23R,IL12RB2 | Intergenic | A | 1.28 | Turkish, Japanese |
[57] | ||
| rs1518111 | IL10 | Intron | A | 1.45 | Turkish | Greek, UK, Iranian, Middle Eastern Arab, Japanese, Han Chinese |
Reduces expression in monocytes | [57, 64] |
| rs1800871 | IL10 | Promoter | T | 1.45 | Japanese | Turkish, Korean, Han Chinese |
[56, 64] | |
| rs9494885 | TNFAIP3 | Intergenic | C | 1.81 | Han Chinese | No difference in expression in PBMCs | ||
| rs7574070 | STAT4 | Intron | A | 1.27 | Turkish | Japanese | Increases expression | [59] |
| rs897200 | STAT4 | Intergenic | A | 1.45 | Han Chinese | Increases expression of STAT4 and IL17 | [61] | |
| rs7616215 | CCR1 | Intergenic | T | 1.39 | Turkish | Japanese | Decreases expression in monocytes Reduces monocyte chemotaxis |
[59] |
| rs13092160 | CCR1, CCR3 | Intergenic | T | 3.13 | Han Chinese | Decreases expression in PBMCs | [100] | |
| rs2617170 | KLRC4 | Missense | C | 1.28 | Turkish | Japanese | [59] | |
| M694V | MEFV | Missense | V | 2.65 | Turkish | Increases response to LPS | [69] | |
| rs17482078 | ERAP1 | Missense | TT2 | 4.56 | Turkish | [59] | ||
| rs681343 | FUT2 | Synonymous | T | 1.30 | Iranian, Turkish | r2 = 1 with a nonsecretor allele (rs601338) | [57, 62] | |
| rs17810546 | IL12A | Intergenic | A/G3 | 1.66 | Turkish, Mixed populations |
[59, 63] | ||
| R381Q, G149R1 |
IL23R | Missense | Protective | Turkish, Japanese |
Reduces IL-23 dependent IL-17 (R381Q) | [69] | ||
| D299G, T399I1 | TLR4 | Missense | Protective | Turkish, Japanese |
Reduces response to LPS Hyporesponsiveness to endotoxin |
[69] | ||
| R702W, G908R L1007fs1 |
NOD2 | Missense Frame shift |
Protective | Turkish, Japanese |
Reduces response to MDP | [69] | ||
Variants from targeted sequencing study without single variant based genome-wide significance.
Homozygotes of rs17482078 showed genome wide significance in BD patients with uveitis.
The reported disease risk allele is different between studies.
4.2. IL10
IL10 was one of the first two identified BD susceptibility loci with genome-wide significance outside the MHC from two GWA studies of Turkish and Japanese populations. Remmers et al. demonstrated an association with an intronic variant, rs1518111, in the Turkish population, and Mizuki et al. found an association with rs1800871 and rs1800872, located in promoter region of IL10 [56, 57]. The SNP rs1518111 was replicated in Middle Eastern Arab, Greek, British, and Korean samples, and rs1800872 replicated in Turkish and Korean samples [56, 57]. Later study showed replication of rs1518111 and rs1800871 from 407 BD patients and 679 healthy controls in the Han Chinese population [64]. Another group also replicated the rs1518111 association in the Iranian population [65]. HapMap Project data show that all three of these variants are in high LD (r2 > 0.9 for all pairs) in both European and Asian ancestries (HaploReg). The disease risk allele A of rs1518111, the lead SNP in the Turkish GWAS, is associated with decreased IL10 expression in monocytes by 35% compared with the non-risk allele G, determined by measuring the allelic imbalance in heterozygous individuals.
IL10 encodes interleukin (IL)-10, which suppresses the production of proinflammatory cytokines such as IL-1, IL-6, IL-12, tumor necrosis factor (TNF), and interferon gamma (IFN-γ), and inhibits the costimulatory activity of macrophages for T cell and NK cell activation [66, 67]. Homozygosity for the risk allele A of rs1518111 was found to be associated with lower amounts of IL-10 protein in monocytes from healthy controls stimulated with Toll-like-receptor ligands, such as lipopolysaccharide (LPS) or the lipoprotein Pam3Cys and muramyl dipeptide (MDP) [57]. A recent study reported that IL-10 serum levels in Behçet’s disease patients were lower than in healthy controls [68].
4.3. IL23R-IL12RB2
The IL23R–IL12RB2 locus was the second locus with genome-wide significance identified by two early GWA studies. Mizuki et al. identified association with rs1495965, the lead SNP in this locus in the Japanese GWAS, which is located in the intergenic region between IL23R and IL12RB2 [56]. In the Turkish GWAS Remmers et al. identified association with rs924080, located similarly in the intergenic region between IL23R and IL12RB2, and obtained genome-wide significance after meta-analysis with the Japanese samples. However, these variants in the IL23R–IL12RB2 locus were not replicated in smaller Korean, Middle Eastern Arab, Greek, and British [57] sample collections. A recent study additionally replicated the susceptibility of the major allele of rs924080 in the Iranian population [65]. These two SNPs are in moderate LD (r2 = 0.63, Table 2) and may detect the same functional variant in both populations. Interestingly, low frequency missense variants of the IL23R (p.Arg381Gln in the Turkish population and p.Gly149Arg in the Japanese population) that reduce its ability to respond to IL-23 stimulation have been associated with protection from BD [69], as well as from ankylosing spondylitis (AS) [70, 71], psoriasis [72, 73], Crohn’s disease [74], ulcerative colitis [75] and inflammatory bowel disease (IBD) [76, 77], suggesting that the intergenic disease-associated common non-coding variants might be associated with increased expression of IL23R compared with the disease-protective minor alleles.
IL23R encodes a subunit of the IL-23 receptor that is expressed on the surface of Th17 cells and macrophages [78]. IL-23 is a heterodimeric proinflammatory cytokine composed of a p19 subunit and a p40 subunit, which is shared with IL-12. IL-23 promotes Th17 cell development and induces the production of proinflammatory cytokines such as IL-1, IL-6, IL-17 and TNF. Th17 cells are known to play a key role in neutrophil inflammation and in autoimmune diseases via IL-17 production and the disease-associated alleles could either increase IL-23 receptor expression or signaling compared with the disease-protective alleles [79]. Although the evidence that the disease-associated variants influence BD susceptibility through their influence on the IL23R is strong, an alternative or additional role for the variants to influence expression of the other nearby gene, IL12RB2, cannot be excluded. IL12RB2 encodes IL-12 receptor beta2, a subunit of IL-12 receptor. IL-12 plays an important role in Th1 responses, T cell and NK cell cytotoxicity, and IFN-γ production by T cells and NK cells. IL12RB2 has been reported to be essential for high-affinity IL-12 binding and IL-12 dependent signaling, and has a crucial role in Th1 cell differentiation [80]. Although no IL12RB2 or IL23R expression quantitative trait loci (eQTL) data have been reported for these non-coding variants, the possibility remains that their effects are exhibited in only in a specific cell type or under certain conditions.
4.4. STAT4
Hou et al. demonstrated the association of BD with rs897200, located 1.8K bp upstream of the STAT4 gene, by GWAS with genome-wide significance in the Han Chinese population, including 149 BD cases and 951 controls with replication in an additional 554 cases and 1,159 controls [61]. An imputation study of Turkish GWAS data also reported a genome-wide significant association of the STAT4 intronic SNP, rs7574070, which is in strong LD with rs897200 (r2 = 0.90, Table 2), by meta-analysis of the Turkish GWAS, a Turkish replication cohort, and Japanese samples [59].
STAT4, a signal transducer and activator of transcription, is activated by the signaling pathway of proinflammatory cytokines, such as IL-12 and IL-23, and is involved in the differentiation of naïve T cells into Th1 and Th17 cells [81–84]. The risk allele A of rs7574070 is associated with increased STAT4 gene expression [59] and the risk allele A of rs897200 is associated with up-regulated expression of the STAT4 gene, increased transcription and protein expression of IL-17, and higher clinical severity score of BD, suggesting the risk allele contributes to the development of BD through the up-regulation of the Th17 pathway instead of the Th1 pathway [61].
4.5. TNFAIP3
Li et al. genotyped 5 non-coding SNPs located in the TNFAIP3 locus in 722 Han Chinese BD patients and 1,415 unrelated matched controls and reported susceptibility of rs9494885 with genome-wide significance [85]. TNFAIP3 encodes the ubiquitin-modifying enzyme A20, which plays a critical role in the regulation of the NF-κB signaling pathway, and is induced by TNF, toll like receptors (TLRs), interleukin 1 receptor (IL-1R), and nucleotide-binding oligomerization domain containing 2 (NOD2) signaling [86–89]. However, Li et al. showed no difference in TNFAIP3 expression among rs9494885 genotypes in peripheral blood mononuclear cells (PBMCs) from 16 healthy individuals [85]. TNFAIP3 polymorphisms have been reported as susceptibility loci for several other complex genetic autoimmune diseases with genome-wide significance, such as rheumatoid arthritis (RA) [90, 91], multiple sclerosis [92], systemic lupus erythematosus (SLE) [93], psoriasis[94], ulcerative colitis, and IBD [95]. A20 (Tnfaip3) deficient mice show several stereotypical phenotypes of human immune-related diseases, such as lymphocyte-dependent colitis, seronegative ankylosing arthritis, and enthesitis for IBD [96], ds-DNA antibodies, nephritis, the antiphospholipid syndrome, and lymphosplenomegary for SLE [97, 98], and polyarthritis for RA [99].
4.6. CCR1-CCR3
Kirino et al. demonstrated an association between BD susceptibility and a common SNP, rs7616215 (0.27 allele frequency in Turkish cases and 0.34 in Turkish controls), located 3’ of the CCR1 gene, from an analysis of imputed GWAS data in the Turkish population [59]. The same SNP allele was also found at a lower frequency in Japanese cases (0.13) than in Japanese controls (0.16), and a meta-analysis yielded a genome-wide significant result and suggested that the higher frequency allele is associated with BD risk. Hou et al. preformed a two-stage candidate gene association study in Han Chinese for the CCR1-CCR3 locus and identified the association of 3 low frequency SNPs, rs13084057, rs13092160, and rs13075270 (~0.02 frequency in Han Chinese cases and 0.06 in Han Chinese controls with genome-wide significance). According to HaploReg all three SNPs are in strong LD in Asians (pairwise r2 ≥ 0.89) and despite their lower allele frequency, they are also in strong LD with rs7616215 (pairwise D’ ≥ 0.96). rs13092160 and rs13075270 are located between and 5’ of both the CCR1 and CCR3 genes and rs13084057 and rs7616215 are located 3’ of CCR1 . The major alleles of all four SNPs are also associated with disease risk [59, 100].
CCR1 and CCR3 encode C-C motif chemokine receptor (CCR) family members, CCR1 and CCR3, respectively, which are composed of 7-transmembrane structures and couple to G- proteins for signal transduction within cells and serve as key regulators of leukocyte trafficking and immune system homeostasis [101, 102]. An expression study in human primary monocytes from healthy controls showed the risk allele T of rs7616215 was associated with lower expression of CCR1 and this result was replicated in an eQTL database. In addition, a migration assay of monocytes in response to the CCR1 ligand, MIP1-α, demonstrated reduced monocyte chemotaxis associated with the BD risk allele T of rs7616215 [59]. Hou et al. reported that the risk allele T of rs13092160, located between CCR1 and CCR3, was associated with reduced expression of both CCR1 and CCR3 in peripheral blood mononuclear cells (PBMCs) from healthy controls [100]. These functional studies provide a new insight into BD pathogenesis by suggesting that genetically-encoded host responses associated with impaired clearance of microbial pathogens are also associated with increased BD risk.
4.7. KLRC4
Kirino and colleagues identified the association of rs2617170, a missense variant of KLRC4 (p.Asn104Ser) with BD by meta-analysis of data from Turkish and Japanese populations. KLRC4 is located in the natural killer complex gene region at 12p13.2-p12.3, which includes the CD94/NKG2 receptor family and the killer cell lectin-like receptor family genes. This region exhibited the strongest linkage peak described in a whole genome linkage analysis of 28 Turkish multicase families including 83 BD patients [103].
KLRC4 encodes killer cell lectin-like receptor subfamily C member 4, expressed on natural killer cells, the function of which has yet to be well described. The haplotype bearing the BD-risk allele C of rs2617170 was reported to be associated with higher natural cytotoxic activity of peripheral blood cells than the haplotype with the BD-protective allele, suggesting an involvement of MHC class I regulated cytotoxicity in BD pathogenesis [104].
4.8. ERAP1
Kirino et al. reported a recessive association of rs17482078, a nonsynonymous coding variant of ERAP1 p.Arg725Gln, in the Turkish population, but the variant was not sufficiently polymorphic for recessive evaluation in the Japanese population [59]. This association is an example of a strong interaction or epistasis between two genes, as the ERAP1 effect is limited to individuals with the HLA-B*51 type. The risk allele has a large effect with an odds ratio of 3.78 in HLA-B*51 carriers.
ERAP1 encodes endoplasmic reticulum aminopeptidase 1, which trims the N-terminus of proteasome-derived peptides to an optimal length for loading into the antigen-binding groove of MHC class I molecules [105]. Epistatic associations of ERAP1 p.Arg725Gln with MHC class I molecules were also observed for psoriasis in HLA-C*06 carriers and for AS in HLA-B*27 carriers [106, 107]. Interestingly, the rs17482078 missense (Gln) variant of ERAP1 is protective for psoriasis and AS but is associated with risk for BD. The BD-associated ERAP1 p.Arg725Gln variant is found on a haplotype along with several other protein coding variants [108]. This haplotype has been associated with reduced peptide trimming activity [109], thus altering the peptides available for MHC class I binding. The difference between risk and protection conferred by the 725Gln haplotype among these three diseases may be explained by different binding affinities among specific peptides trimmed or not trimmed by the ERAP1 variant isoforms for different MHC class I molecules. Furthermore, these differences suggest the presence of different disease-associated trimmed peptide antigens for each of the MHC class I molecules. The finding that the association of rs17482078 was observed only in HLA-B*51 carriers also supports the notion that HLA-B*51 itself, as opposed to other nearby genes, is directly involved in the pathogenesis of BD.
4.9. FUT2
Xavier et al. performed a GWAS using DNA pooling of 292 Iranian BD cases and 294 age- and sex-matched controls and identified an association of rs681343, located in the FUT2 locus. The association reached genome-wide significance after replication in additional Iranian samples and meta-analysis with Turkish GWAS data [62].
FUT2 encodes fucosyltransferase 2, which plays an important role in the synthesis of H antigen, the precursor of the ABO-histo-blood group antigen in body fluids and on the intestinal mucosa [110]. Variants that result in FUT2 deficiency or inactivity fail to express the ABO-histo blood group antigen in body fluids or in the intestinal mucosa and are thus termed “non-secretor” alleles. A coding variant, rs601338, which encodes a stop codon at position 143 of the FUT2 protein, has an allele frequency of 0.43 in individuals of European ancestry and homozygosity for this allele is the most common explanation for non-secretor status in individuals of European ancestry. This non-secretor allele is in strong LD with the BD-associated SNP rs681343 (r2 = 1 in European ancestry), and thus the non-secretor allele is also associated with BD risk. Secretor status contributes to the development of immune responses and is associated with the composition of intestinal bacterial flora [111]. FUT2 non-secretor associated alterations in mucosal glycosylation and in gut microbiome composition could increase susceptibility to BD, because non-secretors experience more limited antigenic stimulation early in life, but alternatively, increased disease risk could be due to effects of altered oral and gastrointestinal tract flora on local and systemic inflammation.
4.10. IL12A
Kirino et al. reported suggestive association of rs1780546, located in the intergenic region near IL12A, in the Turkish cohort from their imputation study, but the association did not reach the level of genome-wide significance, and rs1780546 was not polymorphic in the Japanese cohort [59]. Recently, Kappen et al. conducted a GWAS on 336 cases and 5,843 controls in cohorts of mixed ethnicity and analyzed their data using linear mixed models to correct for ancestry differences and family structure and/or cryptic relationships [63]. They also found association of rs1780546 with BD susceptibility and demonstrated genome-wide association after meta-analysis with previous Turkish GWAS imputation data. Although no functional study of rs1780546 was reported, IL12A encodes IL-12p35, a subunit of the heterodimer of IL-12, which plays a crucial role in polarization of the Th1 pathway through differentiation from naïve CD4+ T cells [80].
In addition, Kappen et al. reported two potential novel associations with genome-wide significance for two SNPs with low minor allele frequency (MAF), rs8187722 on chromosome 6, a synonymous variant in SLC22A3, and rs17087141, which maps to a region containing an uncharacterized non-coding miscRNA LOC400655 on chromosome 18. We omitted these loci from Table 2 because strong associations of these loci were not reported in any other GWAS and the replication study MAFs were similar to those reported in healthy individuals.
5. Rare Variants
Generally, genetic association studies focus on common variants with MAF greater than 5%, while low frequency (1% ≤ MAF < 5%) and rare variants (MAF < 1%) are excluded from the association test, because of inadequate power to evaluate the effects in studies of a few thousand individuals. However, unlike disease-associated, non-coding, common SNPs, whose functional effects and roles in disease susceptibility are difficult to explain, rare coding variants are likely to be enriched for variants that influence protein structure and function.
Kirino et al. performed a targeted deep exonic resequencing study to analyze nonsynonymous coding variants using pooled DNAs from independent Turkish and Japanese populations including 384 BD cases and 384 controls of each population, then validated the variants in 2,461 BD cases and 2,458 controls. Results of gene-wise burden tests revealed association of the combined rare and low frequency nonsynonymous variants of IL23R, TLR4 and NOD2 with BD susceptibility. The variant of IL23R, p.Arg381Gln, observed in the Turkish population partially explained the protective effect of the minor allele variant of the reported common SNP rs924080 association in the Turkish population (r2 = 0.07 and D’ = 0.90), while the protective p.Gly149Arg variant observed in the Japanese population is independent of the previously reported disease-associated common variant rs1495965 in the Japanese population. Two of the identified TLR4 variants, p.Asp299Gly and p.Thr399Ile, were previously reported associated with hyporesponsiveness to endotoxin and [112]. Three of the identified NOD2 coding variants, p.Arg702Trp, p.Gly908Arg, and p.Leu10007fs are associated with Crohn’s disease and are predicted to reduce response to MDP by computational protein analysis [69].
The familial Mediterranean fever mutation, MEFV p.Met694Val, by itself achieved genome-wide significance (P = 1.79 × 10−12) in its association with BD in the Turkish population [69]. This variant was not seen in the Japanese population, nor was an association of the combined MEFV rare and low frequency variants found in Japanese BD. MEFV encodes pyrin, which regulates IL-1β production activated by caspase-1 through the activation of an inflammasome. FMF is a systemic autoinflammatory disorder, caused by recessively inherited mutations in MEFV. It shares some clinical characteristics with BD, such as unprovoked episodes of inflammation, recurrence, and good response to colchicine [113]. Heterozygosity for the MEFV Met694Val mutation is significantly associated with BD risk in the Turkish population [69].
6. Genetic risk sharing among immune related diseases
Disease susceptibility loci have been identified by GWAS performed in several immune-related diseases (Table 3). Although the ability to detect loci that exceed genome-wide significance varies according to the number of individuals included in the study and the allele frequencies of the disease-associated variants, there is a striking overlap of disease susceptibility loci. The overlap of susceptibility genes and the direction of the variant effect can help to elucidate the pathogenesis of both BD and other immune-related diseases. The MHC region shows the strongest association among immune-related diseases. Seropositive diseases such as RA, Kawasaki disease, Sjögren syndrome, systemic sclerosis, systemic lupus erythematosus, and anti-neutrophil cytoplasmic antibody-associated vasculitis, show a strong association with MHC class II but not with MHC class I. On the other hand, seronegative diseases such as AS, psoriasis, psoriatic arthritis, Takayasu’s arthritis, and seronegative RA; and the non-rheumatic inflammatory diseases, such as Crohn’s disease, show strong association with MHC class I but not with MHC class II [95]. BD can also be considered one of the sero-negative diseases with a strong MHC class I (HLA-B*51) association. HLA-B*27, which is well known as the major MHC class I susceptibility molecule for AS [114] and for inflammatory bowel disease and psoriasis when accompanied by spondylitis [115, 116], is also associated with BD [43].
Table 3. The overlap of susceptibility genes for Behçet’s disease with other immune related diseases.
Susceptibility loci for immune related diseases that showed associations with genome-wide significance (P < 5.0 × 10−8) in original studies are shown in the table [56, 57, 59, 62, 63, 69, 85, 92–94, 117, 123–135].
| BD | IBD | CD | UC | Pso | AS | CeD | MS | SLE | PBC | RA | SSc | SJO | T1D | JIA | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| MHC Class I | + | + | + | + | + | ||||||||||
| STAT4 | + | + | + | + | + | + | + | + | + | + | + | ||||
| IL12A | + | + | + | + | + | + | |||||||||
| TNFAIP3 | + | + | + | + | + | + | + | + | |||||||
|
IL23R- IL12RB2 |
+ | + | + | + | + | + | + | ||||||||
| IL10 | + | + | + | + | + | + | |||||||||
| ERAP1 | + | + | + | + | + | ||||||||||
| FUT2 | + | + | + | + | |||||||||||
| CCR1-CCR3 | + | + | |||||||||||||
| KLRC4 | + | ||||||||||||||
| MEFV | + |
BD, Behçet’s disease; IBD, inflammatory bowel disease; CD Crohn’s disease; UC, ulcerative colitis; Pso, psoriasis; AS, ankylosing spondylitis; CeD, celiac disease; MS, multiple sclerosis; SLE, systemic lupus erythematosus; PBC, primary biliary cirrhosis; RA, rheumatoid arthritis; SSc, systemic sclerosis; SJO, Sjögren’s syndrome; T1D, type 1 diabetes; JIA, juvenile idiopathic arthritis
Among the reported susceptibility genes for BD, STAT4 is the most shared among immune- related diseases, although different independent variants are associated with the different immune- related diseases [59]. At the other extreme, there are no other immune-related diseases with a reported association with KLRC4 and MEFV at genome-wide significance so far [91, 117]. Smaller candidate gene studies have provided, however, suggestive evidence of MEFV as a susceptibility locus for AS [118] and inflammatory bowel disease [119] in the Turkish population in which FMF mutations are common. BD shares susceptibility genes such as STAT4, TNFAIP3, IL23R, IL12RB2, IL10, ERAP1, and FUT2 with IBD. These diseases also share many clinical manifestations such as oral ulcers, erythema nodosum, uveitis, arthritis, and ulcers of the colonic and ileocecal mucosa, as well as effective therapeutic agents [120].
Interestingly, among immune-related diseases, the effects of some shared susceptibility variants are known to be associated with the opposite effect on disease risk. Nonsynonymous variants of TLR4, p.Asp299Gly and p.Tr399Ile, predicted to reduce response to LPS, and of NOD2, p.Arg702Trp, p.Gly908Arg, and p.Leu1007fs, predicted to reduce response to MDP, are protective for BD, but are associated with risk for Crohn’s disease [69, 121, 122]. A similar discrepancy is also seen in the interaction between MHC class I and the coding variant of ERAP1, p.Arg725Gln, which increases the risk for BD, but is protective for AS and psoriasis, as described in 4.8. [59, 106, 107]. These findings suggest the causative peptides for each disease are different among MHC class I associated diseases.
7. Conclusion
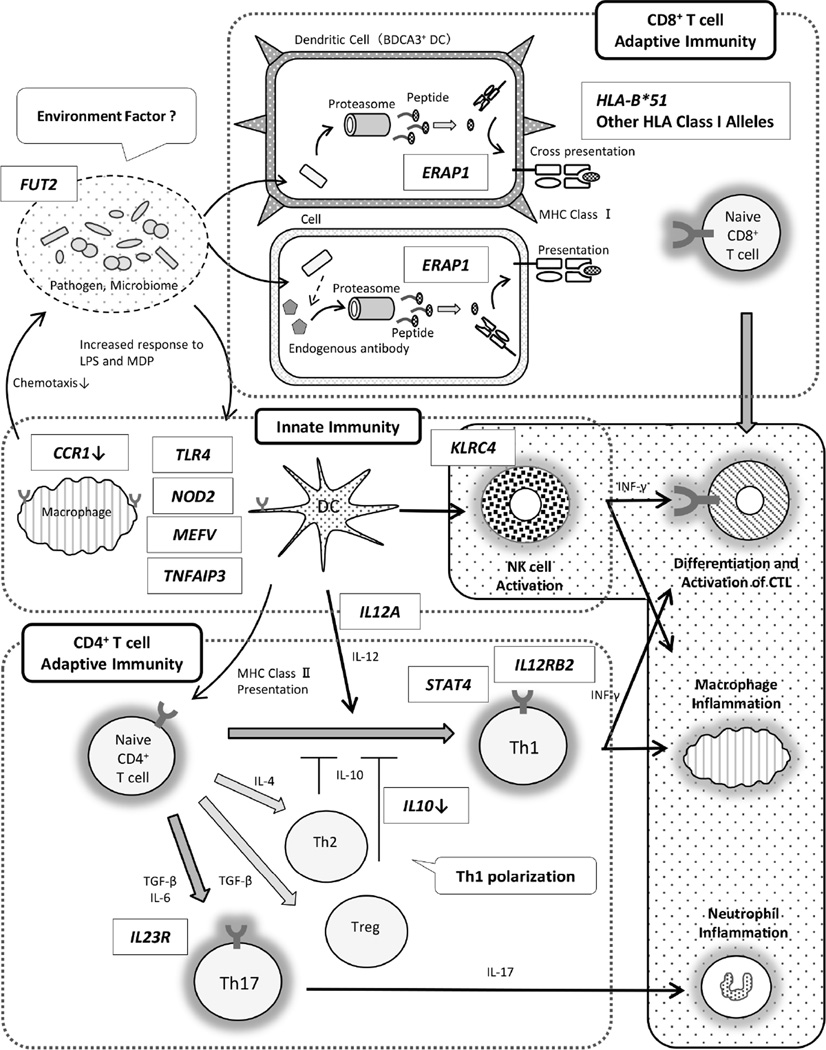
Since the association of HLA-B*51 (HL-A5) was first described more than four-decades ago, many susceptibility genes for BD have been added to the list, largely owing to the development of genomic strategies. The genes identified are involved in both innate and adaptive immunity and support the idea that polarization in Th1/Th17 pathway plays a critical role in BD pathogenesis (Figure 2). Commonalities among susceptibility genes may explain some shared features of immune related diseases. In addition, recent studies suggest the interaction between genetic factors and environmental factors such as the immune response to invasive pathogens and the gut microbiome composition may help explain the role of environmental factors. Therefore, further immunogenetic studies are expected to elucidate BD pathogenesis and also to contribute to the development of more targeted therapies and biomarkers.
Figure 2. Immunogenetic findings and the pathogenesis of Behçet’s disease.
Reported susceptibility genes and functions of risk alleles were described [42, 43, 56, 57, 59, 62, 63, 85].
CTL: cytotoxic T cell, DC: dendritic cell, NK: natural killer cell, Treg: regulatory T cell.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference
- 1.Sakane T, Takeno M, Suzuki N, Inaba G. Behcet’s disease. N Engl J Med. 1999;341:1284–1291. doi: 10.1056/NEJM199910213411707. [DOI] [PubMed] [Google Scholar]
- 2.Hatemi G, Silman A, Bang D, Bodaghi B, Chamberlain AM, Gul A, et al. EULAR recommendations for the management of Behcet disease. Ann Rheum Dis. 2008;67:1656–1662. doi: 10.1136/ard.2007.080432. [DOI] [PubMed] [Google Scholar]
- 3.Verity DH, Marr JE, Ohno S, Wallace GR, Stanford MR. Behcet’s disease, the Silk Road and HLA-B51: historical and geographical perspectives. Tissue Antigens. 1999;54:213–220. doi: 10.1034/j.1399-0039.1999.540301.x. [DOI] [PubMed] [Google Scholar]
- 4.Evereklioglu C. Current concepts in the etiology and treatment of Behcet disease. Surv Ophthalmol. 2005;50:297–350. doi: 10.1016/j.survophthal.2005.04.009. [DOI] [PubMed] [Google Scholar]
- 5.Direskeneli H, Saruhan-Direskeneli G. The role of heat shock proteins in Behcet’s disease. Clin Exp Rheumatol. 2003;21:44–48. [PubMed] [Google Scholar]
- 6.Stojanov S, Kastner DL. Familial autoinflammatory diseases: genetics, pathogenesis and treatment. Curr Opin Rheumatol. 2005;17:586–599. doi: 10.1097/bor.0000174210.78449.6b. [DOI] [PubMed] [Google Scholar]
- 7.Azizlerli G, Kose AA, Sarica R, Gul A, Tutkun IT, Kulac M, et al. Prevalence of Behcet’s disease in Istanbul, Turkey. Int J Dermatol. 2003;42:803–806. doi: 10.1046/j.1365-4362.2003.01893.x. [DOI] [PubMed] [Google Scholar]
- 8.Yurdakul S, Gunaydin I, Tuzun Y, Tankurt N, Pazarli H, Ozyazgan Y, et al. The prevalence of Behcet’s syndrome in a rural area in northern Turkey. J Rheumatol. 1988;15:820–822. [PubMed] [Google Scholar]
- 9.Idil A, Gurler A, Boyvat A, Caliskan D, Ozdemir O, Isik A, et al. The prevalence of Behcet’s disease above the age of 10 years The results of a pilot study conducted at the Park Primary Health Care Center in Ankara, Turkey. Ophthalmic Epidemiol. 2002;9:325–331. doi: 10.1076/opep.9.5.325.10338. [DOI] [PubMed] [Google Scholar]
- 10.Piga M, Mathieu A. Genetic susceptibility to Behcet’s disease: role of genes belonging to the MHC region. Rheumatology (Oxford) 2011;50:299–310. doi: 10.1093/rheumatology/keq331. [DOI] [PubMed] [Google Scholar]
- 11.Papoutsis NG, Abdel-Naser MB, Altenburg A, Orawa H, Kotter I, Krause L, et al. Prevalence of Adamantiades-Behcet’s disease in Germany and the municipality of Berlin: results of a nationwide survey. Clin Exp Rheumatol. 2006;24:125. [PubMed] [Google Scholar]
- 12.Kone-Paut I, Geisler I, Wechsler B, Ozen S, Ozdogan H, Rozenbaum M, et al. Familial aggregation in Behcet’s disease: high frequency in siblings and parents of pediatric probands. J Pediatr. 1999;135:89–93. doi: 10.1016/s0022-3476(99)70333-1. [DOI] [PubMed] [Google Scholar]
- 13.Fietta P. Behcet’s disease: familial clustering and immunogenetics. Clin Exp Rheumatol. 2005;23:96–105. [PubMed] [Google Scholar]
- 14.Zouboulis CC. Epidemiology of Adamantiades-Behcet’s disease. Ann Med Interne (Paris) 1999;150:488–498. [PubMed] [Google Scholar]
- 15.Akpolat T, Koc Y, Yeniay I, Akpek G, Gullu I, Kansu E, et al. Familial Behcet’s disease. Eur J Med. 1992;1:391–395. [PubMed] [Google Scholar]
- 16.Zouboulis CC, Kotter I, Djawari D, Kirch W, Kohl PK, Ochsendorf FR, et al. Epidemiological features of Adamantiades-Behcet’s disease in Germany and in Europe. Yonsei Med J. 1997;38:411–422. doi: 10.3349/ymj.1997.38.6.411. [DOI] [PubMed] [Google Scholar]
- 17.Molinari N, Kone Paut I, Manna R, Demaille J, Daures JP, Touitou I. Identification of an autosomal recessive mode of inheritance in paediatric Behcet’s families by segregation analysis. Am J Med Genet A. 2003;122:115–118. doi: 10.1002/ajmg.a.20136. [DOI] [PubMed] [Google Scholar]
- 18.Bird Stewart JA. Genetic analysis of families of patients with Behcet’s syndrome: data incompatible with autosomal recessive inheritance. Ann Rheum Dis. 1986;45:265–268. doi: 10.1136/ard.45.4.265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gul A, Inanc M, Ocal L, Aral O, Konice M. Familial aggregation of Behcet’s disease in Turkey. Ann Rheum Dis. 2000;59:622–625. doi: 10.1136/ard.59.8.622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gul A, Ohno S. HLA-B*51 and Behcet Disease. Ocul Immunol Inflamm. 2012;20:37–43. doi: 10.3109/09273948.2011.634978. [DOI] [PubMed] [Google Scholar]
- 21.Ohno S, Aoki K, Sugiura S, Nakayama E, Itakura K. Letter: HL-A5 and Behcet’s disease. Lancet. 1973;2:1383–1384. doi: 10.1016/s0140-6736(73)93343-6. [DOI] [PubMed] [Google Scholar]
- 22.Mizuki N, Inoko H, Ando H, Nakamura S, Kashiwase K, Akaza T, et al. Behcet’s disease associated with one of the HLA-B51 subantigens, HLA-B* 5101. Am J Ophthalmol. 1993;116:406–409. doi: 10.1016/s0002-9394(14)71396-0. [DOI] [PubMed] [Google Scholar]
- 23.Mizuki N, Inoko H, Ohno S. Molecular genetics (HLA) of Behcet’s disease. Yonsei Med J. 1997;38:333–349. doi: 10.3349/ymj.1997.38.6.333. [DOI] [PubMed] [Google Scholar]
- 24.Mizuki N, Inoko H, Ohno S. Pathogenic gene responsible for the predisposition of Behcet’s disease. Int Rev Immunol. 1997;14:33–348. doi: 10.3109/08830189709116843. [DOI] [PubMed] [Google Scholar]
- 25.Mizuki N, Ohno S, Ando H, Chen L, Palimeris GD, Stavropoulos-Ghiokas E, et al. A strong association between HLA-B*5101 and Behcet’s disease in Greek patients. Tissue Antigens. 1997;50:57–60. doi: 10.1111/j.1399-0039.1997.tb02835.x. [DOI] [PubMed] [Google Scholar]
- 26.Gonzalez-Escribano MF, Rodriguez MR, Walter K, Sanchez-Roman J, Garcia-Lozano JR, Nunez-Roldan A. Association of HLA-B51 subtypes and Behcet’s disease in Spain. Tissue Antigens. 1998;52:78–80. doi: 10.1111/j.1399-0039.1998.tb03027.x. [DOI] [PubMed] [Google Scholar]
- 27.Koumantaki Y, Stavropoulos C, Spyropoulou M, Messini H, Papademetropoulos M, Giziaki E, et al. HLA-B*5101 in Greek patients with Behcet’s disease. Hum Immunol. 1998;59:250–255. doi: 10.1016/s0198-8859(98)00011-1. [DOI] [PubMed] [Google Scholar]
- 28.Kera J, Mizuki N, Ota M, Katsuyama Y, Pivetti-Pezzi P, Ohno S, et al. Significant associations of HLA-B*5101 and B*5108, and lack of association of class II alleles with Behcet’s disease in Italian patients. Tissue Antigens. 1999;54:565–571. doi: 10.1034/j.1399-0039.1999.540605.x. [DOI] [PubMed] [Google Scholar]
- 29.Mizuki N, Ota M, Katsuyama Y, Yabuki K, Ando H, Goto K, et al. Association analysis between the MIC-A and HLA-B alleles in Japanese patients with Behcet’s disease. Arthritis Rheum. 1999;42:1961–1966. doi: 10.1002/1529-0131(199909)42:9<1961::AID-ANR23>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- 30.Verity DH, Wallace GR, Vaughan RW, Kondeatis E, Madanat W, Zureikat H, et al. HLA and tumour necrosis factor (TNF) polymorphisms in ocular Behcet’s disease. Tissue Antigens. 1999;54:264–272. doi: 10.1034/j.1399-0039.1999.540307.x. [DOI] [PubMed] [Google Scholar]
- 31.Yabuki K, Mizuki N, Ota M, Katsuyama Y, Palimeris G, Stavropoulos C, et al. Association of MICA gene and HLA-B*5101 with Behcet’s disease in Greece. Invest Ophthalmol Vis Sci. 1999;40:1921–1926. [PubMed] [Google Scholar]
- 32.Yabuki K, Ohno S, Mizuki N, Ando H, Tabbara KF, Goto K, et al. HLA class I and II typing of the patients with Behcet’s disease in Saudi Arabia. Tissue Antigens. 1999;54:273–277. doi: 10.1034/j.1399-0039.1999.540308.x. [DOI] [PubMed] [Google Scholar]
- 33.Kotter I, Gunaydin I, Stubiger N, Yazici H, Fresko I, Zouboulis CC, et al. Comparative analysis of the association of HLA-B*51 suballeles with Behcet’s disease in patients of German and Turkish origin. Tissue Antigens. 2001;58:166–170. doi: 10.1034/j.1399-0039.2001.580304.x. [DOI] [PubMed] [Google Scholar]
- 34.Mizuki N, Ota M, Katsuyama Y, Yabuki K, Ando H, Shiina T, et al. HLA-B*51 allele analysis by the PCR-SBT method and a strong association of HLA-B*5101 with Japanese patients with Behcet’s disease. Tissue Antigens. 2001;58:181–184. doi: 10.1034/j.1399-0039.2001.580306.x. [DOI] [PubMed] [Google Scholar]
- 35.Mizuki N, Ota M, Katsuyama Y, Yabuki K, Ando H, Yoshida M, et al. HLA class I genotyping including HLA-B*51 allele typing in the Iranian patients with Behcet’s disease. Tissue Antigens. 2001;57:457–462. doi: 10.1034/j.1399-0039.2001.057005457.x. [DOI] [PubMed] [Google Scholar]
- 36.Mizuki N, Yabuki K, Ota M, Verity D, Katsuyama Y, Ando H, et al. Microsatellite mapping of a susceptible locus within the HLA region for Behcet’s disease using Jordanian patients. Hum Immunol. 2001;62:186–190. doi: 10.1016/s0198-8859(00)00246-9. [DOI] [PubMed] [Google Scholar]
- 37.Paul M, Klein T, Krause I, Molad Y, Narinsky R, Weinberger A. Allelic distribution of HLA-B*5 in HLA-B5-positive Israeli patients with Behcet’s disease. Tissue Antigens. 2001;58:185–186. doi: 10.1034/j.1399-0039.2001.580307.x. [DOI] [PubMed] [Google Scholar]
- 38.Pirim I, Atasoy M, Ikbal M, Erdem T, Aliagaoglu C. HLA class I and class II genotyping in patients with Behcet’s disease: a regional study of eastern part of Turkey. Tissue Antigens. 2004;64:293–297. doi: 10.1111/j.1399-0039.2004.00280.x. [DOI] [PubMed] [Google Scholar]
- 39.Takemoto Y, Naruse T, Namba K, Kitaichi N, Ota M, Shindo Y, et al. Re-evaluation of heterogeneity in HLA-B*510101 associated with Behcet’s disease. Tissue Antigens. 2008;72:347–353. doi: 10.1111/j.1399-0039.2008.01111.x. [DOI] [PubMed] [Google Scholar]
- 40.de Menthon M, Lavalley MP, Maldini C, Guillevin L, Mahr A. HLA-B51/B5 and the risk of Behcet’s disease: a systematic review and meta-analysis of case-control genetic association studies. Arthritis Rheum. 2009;61:1287–1296. doi: 10.1002/art.24642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gul A, Hajeer AH, Worthington J, Barrett JH, Ollier WE, Silman AJ. Evidence for linkage of the HLA-B locus in Behcet’s disease, obtained using the transmission disequilibrium test. Arthritis Rheum. 2001;44:239–240. doi: 10.1002/1529-0131(200101)44:1<239::AID-ANR31>3.0.CO;2-X. [DOI] [PubMed] [Google Scholar]
- 42.Meguro A, Inoko H, Ota M, Katsuyama Y, Oka A, Okada E, et al. Genetics of Behcet disease inside and outside the MHC. Ann Rheum Dis. 2010;69:747–754. doi: 10.1136/ard.2009.108571. [DOI] [PubMed] [Google Scholar]
- 43.Ombrello MJ, Kirino Y, de Bakker PI, Gul A, Kastner DL, Remmers EF. Behcet disease-associated MHC class I residues implicate antigen binding and regulation of cell-mediated cytotoxicity. Proc Natl Acad Sci U S A. 2014;111:8867–8872. doi: 10.1073/pnas.1406575111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hughes T, Coit P, Adler A, Yilmaz V, Aksu K, Duzgun N, et al. Identification of multiple independent susceptibility loci in the HLA region in Behcet’s disease. Nat Genet. 2013;45:319–324. doi: 10.1038/ng.2551. [DOI] [PubMed] [Google Scholar]
- 45.Cortes A, Brown MA. Promise and pitfalls of the Immunochip. Arthritis Res Ther. 2011;13:101. doi: 10.1186/ar3204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Montes-Cano MA, Conde-Jaldon M, Garcia-Lozano JR, Ortiz-Fernandez L, Ortego-Centeno N, Castillo-Palma MJ, et al. HLA and non-HLA genes in Behcet’s disease: a multicentric study in the Spanish population. Arthritis Res Ther. 2013;15:145. doi: 10.1186/ar4328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sanjanwala B, Draghi M, Norman PJ, Guethlein LA, Parham P. Polymorphic sites away from the Bw4 epitope that affect interaction of Bw4+ HLA-B with KIR3DL1. J Immunol. 2008;181:6293–6300. doi: 10.4049/jimmunol.181.9.6293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Braud V, Jones EY, McMichael A. The human major histocompatibility complex class Ib molecule HLA-E binds signal sequence-derived peptides with primary anchor residues at positions 2 and 9. Eur J Immunol. 1997;27:1164–1169. doi: 10.1002/eji.1830270517. [DOI] [PubMed] [Google Scholar]
- 49.Rodgers JR, Cook RG. MHC class Ib molecules bridge innate and acquired immunity. Nat Rev Immunol. 2005;5:459–471. doi: 10.1038/nri1635. [DOI] [PubMed] [Google Scholar]
- 50.Merino AM, Sabbaj S, Easlick J, Goepfert P, Kaslow RA, Tang J. Dimorphic HLA-B signal peptides differentially influence HLA-E- and natural killer cell-mediated cytolysis of HIV-1-infected target cells. Clin Exp Immunol. 2013;174:414–423. doi: 10.1111/cei.12187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Maldini C, Lavalley MP, Cheminant M, de Menthon M, Mahr A. Relationships of HLA-B51 or B5 genotype with Behcet’s disease clinical characteristics: systematic review and meta-analyses of observational studies. Rheumatology (Oxford) 2012;51:887–900. doi: 10.1093/rheumatology/ker428. [DOI] [PubMed] [Google Scholar]
- 52.Kang EH, Park JW, Park C, Yu HG, Lee EB, Park MH, et al. Genetic and non-genetic factors affecting the visual outcome of ocular Behcet’s disease. Hum Immunol. 2013;74:1363–367. doi: 10.1016/j.humimm.2013.06.036. [DOI] [PubMed] [Google Scholar]
- 53.Kang EH, Kim JY, Takeuchi F, Kim JW, Shin K, Lee EY, et al. Associations between the HLA-A polymorphism and the clinical manifestations of Behcet’s disease. Arthritis Res Ther. 2011;13:49. doi: 10.1186/ar3292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kaburaki T, Takamoto M, Numaga J, Kawashima H, Araie M, Ohnogi Y, et al. Genetic association of HLA-A*2601 with ocular Behcet’s disease in Japanese patients. Clin Exp Rheumatol. 2010;28:39–44. [PubMed] [Google Scholar]
- 55.Jorde LB, Wooding SP. Genetic variation, classification and ‘race’. Nat Genet. 2004;36:28–33. doi: 10.1038/ng1435. [DOI] [PubMed] [Google Scholar]
- 56.Mizuki N, Meguro A, Ota M, Ohno S, Shiota T, Kawagoe T, et al. Genome-wide association studies identify IL23R–IL12RB2 and IL10 as Behcet’s disease susceptibility loci. Nat Genet. 2010;42:703–706. doi: 10.1038/ng.624. [DOI] [PubMed] [Google Scholar]
- 57.Remmers EF, Cosan F, Kirino Y, Ombrello MJ, Abaci N, Satorius C, et al. Genome-wide association study identifies variants in the MHC class I, IL10, and IL23R–IL12RB2 regions associated with Behcet’s disease. Nat Genet. 2010;42:698–702. doi: 10.1038/ng.625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Fei Y, Webb R, Cobb BL, Direskeneli H, Saruhan-Direskeneli G, Sawalha AH. Identification of novel genetic susceptibility loci for Behcet’s disease using a genome-wide association study. Arthritis Res Ther. 2009;11:66. doi: 10.1186/ar2695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kirino Y, Bertsias G, Ishigatsubo Y, Mizuki N, Tugal-Tutkun I, Seyahi E, et al. Genome-wide association analysis identifies new susceptibility loci for Behcet’s disease and epistasis between HLA-B*51 and ERAP1. Nat Genet. 2013;45:202–207. doi: 10.1038/ng.2520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Lee YJ, Horie Y, Wallace GR, Choi YS, Park JA, Choi JY, et al. Genome-wide association study identifies GIMAP as a novel susceptibility locus for Behcet’s disease. Ann Rheum Dis. 2013;72:1510–1516. doi: 10.1136/annrheumdis-2011-200288. [DOI] [PubMed] [Google Scholar]
- 61.Hou S, Yang Z, Du L, Jiang Z, Shu Q, Chen Y, et al. Identification of a susceptibility locus in STAT4 for Behcet’s disease in Han Chinese in a genome-wide association study. Arthritis Rheum. 2012;64:4104–4113. doi: 10.1002/art.37708. [DOI] [PubMed] [Google Scholar]
- 62.Xavier JM, Shahram F, Sousa I, Davatchi F, Matos M, Abdollahi BS, et al. FUT2: filling the gap between genes and environment in Behcet’s disease? Ann Rheum Dis. 2013 doi: 10.1136/annrheumdis-2013-204475. [DOI] [PubMed] [Google Scholar]
- 63.Kappen JH, Medina-Gomez C, van Hagen PM, Stolk L, Estrada K, Rivadeneira F, et al. Genome-wide association study in an admixed case series reveals IL12A as a new candidate in Behcet disease. PLoS One. 2015;10:0119085. doi: 10.1371/journal.pone.0119085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Wu Z, Zheng W, Xu J, Sun F, Chen H, Li P, et al. IL10 polymorphisms associated with Behcet’s disease in Chinese Han. Hum Immunol. 2014;75:271–276. doi: 10.1016/j.humimm.2013.11.009. [DOI] [PubMed] [Google Scholar]
- 65.Xavier JM, Shahram F, Davatchi F, Rosa A, Crespo J, Abdollahi BS, et al. Association study of IL10 and IL23R–IL12RB2 in Iranian patients with Behcet’s disease. Arthritis Rheum. 2012;64:2761–2772. doi: 10.1002/art.34437. [DOI] [PubMed] [Google Scholar]
- 66.Fiorentino DF, Zlotnik A, Vieira P, Mosmann TR, Howard M, Moore KW, et al. IL-10 acts on the antigen-presenting cell to inhibit cytokine production by Th1 cells. J Immunol. 1991;146:3444–451. [PubMed] [Google Scholar]
- 67.Mosmann TR, Coffman RL. TH1 and TH2 cells: different patterns of lymphokine secretion lead to different functional properties. Annu Rev Immunol. 1989;7:145–73. doi: 10.1146/annurev.iy.07.040189.001045. [DOI] [PubMed] [Google Scholar]
- 68.Talaat RM, Ashour ME, Bassyouni IH, Raouf AA. Polymorphisms of interleukin 6 and interleukin 10 in Egyptian people with Behcet’s disease. Immunobiology. 2014;219:573–582. doi: 10.1016/j.imbio.2014.03.004. [DOI] [PubMed] [Google Scholar]
- 69.Kirino Y, Zhou Q, Ishigatsubo Y, Mizuki N, Tugal-Tutkun I, Seyahi E, et al. Targeted resequencing implicates the familial Mediterranean fever gene MEFV and the toll-like receptor 4 gene TLR4 in Behcet disease. Proc Natl Acad Sci U S A. 2013;110:8134–8139. doi: 10.1073/pnas.1306352110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Rueda B, Orozco G, Raya E, Fernandez-Sueiro JL, Mulero J, Blanco FJ, et al. The IL23R Arg381Gln non-synonymous polymorphism confers susceptibility to ankylosing spondylitis. Ann Rheum Dis. 2008;67:1451–1454. doi: 10.1136/ard.2007.080283. [DOI] [PubMed] [Google Scholar]
- 71.Davidson SI, Jiang L, Cortes A, Wu X, Glazov EA, Donskoi M, et al. Brief report: high-throughput sequencing of IL23R reveals a low-frequency, nonsynonymous single-nucleotide polymorphism that is associated with ankylosing spondylitis in a Han Chinese population. Arthritis Rheum. 2013;65:1747–1752. doi: 10.1002/art.37976. [DOI] [PubMed] [Google Scholar]
- 72.Cargill M, Schrodi SJ, Chang M, Garcia VE, Brandon R, Callis KP, et al. A large-scale genetic association study confirms IL12B and leads to the identification of IL23R as psoriasis-risk genes. Am J Hum Genet. 2007;80:273–290. doi: 10.1086/511051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Tang H, Jin X, Li Y, Jiang H, Tang X, Yang X, et al. A large-scale screen for coding variants predisposing to psoriasis. Nat Genet. 2014;46:45–50. doi: 10.1038/ng.2827. [DOI] [PubMed] [Google Scholar]
- 74.Momozawa Y, Mni M, Nakamura K, Coppieters W, Almer S, Amininejad L, et al. Resequencing of positional candidates identifies low frequency IL23R coding variants protecting against inflammatory bowel disease. Nat Genet. 2011;43:43–47. doi: 10.1038/ng.733. [DOI] [PubMed] [Google Scholar]
- 75.Beaudoin M, Goyette P, Boucher G, Lo KS, Rivas MA, Stevens C, et al. Deep resequencing of GWAS loci identifies rare variants in CARD9, IL23R and RNF186 that are associated with ulcerative colitis. PLoS genetics. 2013;9:1003723. doi: 10.1371/journal.pgen.1003723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Duerr RH, Taylor KD, Brant SR, Rioux JD, Silverberg MS, Daly MJ, et al. A genome-wide association study identifies IL23R as an inflammatory bowel disease gene. Science. 2006;314:1461–1463. doi: 10.1126/science.1135245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Rivas MA, Beaudoin M, Gardet A, Stevens C, Sharma Y, Zhang CK, et al. Deep resequencing of GWAS loci identifies independent rare variants associated with inflammatory bowel disease. Nat Genet. 2011;43:1066–1073. doi: 10.1038/ng.952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Iwakura Y, Ishigame H. The IL-23/IL-17 axis in inflammation. J Clin Invest. 2006;116:1218–1222. doi: 10.1172/JCI28508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Steinman L. Mixed results with modulation of TH-17 cells in human autoimmune diseases. Nat Immunol. 2010;11:41–44. doi: 10.1038/ni.1803. [DOI] [PubMed] [Google Scholar]
- 80.Chang JT, Shevach EM, Segal BM. Regulation of interleukin (IL)-12 receptor beta2 subunit expression by endogenous IL-12: a critical step in the differentiation of pathogenic autoreactive T cells. J Exp Med. 1999;189:969–978. doi: 10.1084/jem.189.6.969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Morinobu A, Gadina M, Strober W, Visconti R, Fornace A, Montagna C, et al. STAT4 serine phosphorylation is critical for IL-12-induced IFN-gamma production but not for cell proliferation. Proc Natl Acad Sci U S A. 2002;99:12281–12286. doi: 10.1073/pnas.182618999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Kim J, Park JA, Lee EY, Lee YJ, Song YW, Lee EB. Imbalance of Th17 to Th1 cells in Behcet’s disease. Clin Exp Rheumatol. 2010;28:16–19. [PubMed] [Google Scholar]
- 83.Watford WT, Hissong BD, Bream JH, Kanno Y, Muul L, O’Shea JJ. Signaling by IL-12 and IL-23 and the immunoregulatory roles of STAT4. Immunol Rev. 2004;202:139–156. doi: 10.1111/j.0105-2896.2004.00211.x. [DOI] [PubMed] [Google Scholar]
- 84.Mathur AN, Chang HC, Zisoulis DG, Stritesky GL, Yu Q, O’Malley JT, et al. Stat3 and Stat4 direct development of IL-17-secreting Th cells. J Immunol. 2007;178:4901–4907. doi: 10.4049/jimmunol.178.8.4901. [DOI] [PubMed] [Google Scholar]
- 85.Li H, Liu Q, Hou S, Du L, Zhou Q, Zhou Y, et al. TNFAIP3 gene polymorphisms confer risk for Behcet’s disease in a Chinese Han population. Hum Genet. 2013;132:293–300. doi: 10.1007/s00439-012-1250-7. [DOI] [PubMed] [Google Scholar]
- 86.Boone DL, Turer EE, Lee EG, Ahmad RC, Wheeler MT, Tsui C, et al. The ubiquitin-modifying enzyme A20 is required for termination of Toll-like receptor responses. Nat Immunol. 2004;5:1052–1060. doi: 10.1038/ni1110. [DOI] [PubMed] [Google Scholar]
- 87.Hitotsumatsu O, Ahmad RC, Tavares R, Wang M, Philpott D, Turer EE, et al. The ubiquitin-editing enzyme A20 restricts nucleotide-binding oligomerization domain containing 2-triggered signals. Immunity. 2008;28:381–390. doi: 10.1016/j.immuni.2008.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Jaattela M, Mouritzen H, Elling F, Bastholm L. A20 zinc finger protein inhibits TNF and IL-1 signaling. J Immunol. 1996;156:1166–1173. [PubMed] [Google Scholar]
- 89.Lee EG, Boone DL, Chai S, Libby SL, Chien M, Lodolce JP, et al. Failure to regulate TNF-induced NF-kappaB and cell death responses in A20-deficient mice. Science. 2000;289:2350–2354. doi: 10.1126/science.289.5488.2350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Eyre S, Bowes J, Diogo D, Lee A, Barton A, Martin P, et al. High-density genetic mapping identifies new susceptibility loci for rheumatoid arthritis. Nat Genet. 2012;44:1336–1340. doi: 10.1038/ng.2462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Okada Y, Wu D, Trynka G, Raj T, Terao C, Ikari K, et al. Genetics of rheumatoid arthritis contributes to biology and drug discovery. Nature. 2014;506:376–381. doi: 10.1038/nature12873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.International multiple Sclerosis Genetics C. Beecham AH, Patsopoulos NA, Xifara DK, Davis MF, Kemppinen A, et al. Analysis of immune-related loci identifies 48 new susceptibility variants for multiple sclerosis. Nat Genet. 2013;45:1353–1360. doi: 10.1038/ng.2770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Gateva V, Sandling JK, Hom G, Taylor KE, Chung SA, Sun X, et al. A large-scale replication study identifies TNIP1, PRDM1, JAZF1, UHRF1BP1 and IL10 as risk loci for systemic lupus erythematosus. Nat Genet. 2009;41:1228–1233. doi: 10.1038/ng.468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Tsoi LC, Spain SL, Knight J, Ellinghaus E, Stuart PE, Capon F, et al. Identification of 15 new psoriasis susceptibility loci highlights the role of innate immunity. Nat Genet. 2012;44:1341–1348. doi: 10.1038/ng.2467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Kirino Y, Remmers EF. Genetic architectures of seropositive and seronegative rheumatic diseases. Nature reviews Rheumatology. 2015 doi: 10.1038/nrrheum.2015.41. [DOI] [PubMed] [Google Scholar]
- 96.Hammer GE, Turer EE, Taylor KE, Fang CJ, Advincula R, Oshima S, et al. Expression of A20 by dendritic cells preserves immune homeostasis and prevents colitis and spondyloarthritis. Nat Immunol. 2011;12:1184–1193. doi: 10.1038/ni.2135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Kool M, van Loo G, Waelput W, De Prijck S, Muskens F, Sze M, et al. The ubiquitin-editing protein A20 prevents dendritic cell activation, recognition of apoptotic cells, and systemic autoimmunity. Immunity. 2011;35:82–96. doi: 10.1016/j.immuni.2011.05.013. [DOI] [PubMed] [Google Scholar]
- 98.Tavares RM, Turer EE, Liu CL, Advincula R, Scapini P, Rhee L, et al. The ubiquitin modifying enzyme A20 restricts B cell survival and prevents autoimmunity. Immunity. 2010;33:181–191. doi: 10.1016/j.immuni.2010.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Matmati M, Jacques P, Maelfait J, Verheugen E, Kool M, Sze M, et al. A20 (TNFAIP3) deficiency in myeloid cells triggers erosive polyarthritis resembling rheumatoid arthritis. Nat Genet. 2011;43:908–912. doi: 10.1038/ng.874. [DOI] [PubMed] [Google Scholar]
- 100.Hou S, Xiao X, Li F, Jiang Z, Kijlstra A, Yang P. Two-stage association study in Chinese Han identifies two independent associations in CCR1/CCR3 locus as candidate for Behcet’s disease susceptibility. Hum Genet. 2012;131:1841–1850. doi: 10.1007/s00439-012-1200-4. [DOI] [PubMed] [Google Scholar]
- 101.Di Marzio P, Dai WW, Franchin G, Chan AY, Symons M, Sherry B. Role of Rho family GTPases in CCR1- and CCR5-induced actin reorganization in macrophages. Biochem Biophys Res Commun. 2005;331:909–916. doi: 10.1016/j.bbrc.2005.04.015. [DOI] [PubMed] [Google Scholar]
- 102.Penido C, Castro-Faria-Neto HC, Vieira-de-Abreu A, Figueiredo RT, Pelled A, Martins MA, et al. LPS induces eosinophil migration via CCR3 signaling through a mechanism independent of RANTES and Eotaxin. Am J Respir Cell Mol Biol. 2001;25:707–716. doi: 10.1165/ajrcmb.25.6.4401. [DOI] [PubMed] [Google Scholar]
- 103.Karasneh J, Gul A, Ollier WE, Silman AJ, Worthington J. Whole-genome screening for susceptibility genes in multicase families with Behcet’s disease. Arthritis Rheum. 2005;52:1836–1842. doi: 10.1002/art.21060. [DOI] [PubMed] [Google Scholar]
- 104.Hayashi T, Imai K, Morishita Y, Hayashi I, Kusunoki Y, Nakachi K. Identification of the NKG2D haplotypes associated with natural cytotoxic activity of peripheral blood lymphocytes and cancer immunosurveillance. Cancer Res. 2006;66:563–570. doi: 10.1158/0008-5472.CAN-05-2776. [DOI] [PubMed] [Google Scholar]
- 105.Kochan G, Krojer T, Harvey D, Fischer R, Chen L, Vollmar M, et al. Crystal structures of the endoplasmic reticulum aminopeptidase-1 (ERAP1) reveal the molecular basis for N-terminal peptide trimming. Proc Natl Acad Sci U S A. 2011;108:7745–7750. doi: 10.1073/pnas.1101262108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Strange A, Capon F, Spencer CC, Knight J, Weale ME, Allen MH, et al. A genome-wide association study identifies new psoriasis susceptibility loci and an interaction between HLA-C and ERAP1. Nat Genet. 2010;42:985–990. doi: 10.1038/ng.694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Evans DM, Spencer CC, Pointon JJ, Su Z, Harvey D, Kochan G, et al. Interaction between ERAP1 and HLA-B27 in ankylosing spondylitis implicates peptide handling in the mechanism for HLA-B27 in disease susceptibility. Nat Genet. 2011;43:761–767. doi: 10.1038/ng.873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Ombrello MJ, Kastner DL, Remmers EF. Endoplasmic reticulum-associated amino-peptidase 1 and rheumatic disease: genetics. Curr Opin Rheumatol. 2015;27:349–356. doi: 10.1097/BOR.0000000000000189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Reeves E, Edwards CJ, Elliott T, James E. Naturally occurring ERAP1 haplotypes encode functionally distinct alleles with fine substrate specificity. J Immunol. 2013;191:35–43. doi: 10.4049/jimmunol.1300598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Ferrer-Admetlla A, Sikora M, Laayouni H, Esteve A, Roubinet F, Blancher A, et al. A natural history of FUT2 polymorphism in humans. Mol Biol Evol. 2009;26:1993–2003. doi: 10.1093/molbev/msp108. [DOI] [PubMed] [Google Scholar]
- 111.Wacklin P, Makivuokko H, Alakulppi N, Nikkila J, Tenkanen H, Rabina J, et al. Secretor genotype (FUT2 gene) is strongly associated with the composition of Bifidobacteria in the human intestine. PLoS One. 2011;6:20113. doi: 10.1371/journal.pone.0020113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Arbour NC, Lorenz E, Schutte BC, Zabner J, Kline JN, Jones M, et al. TLR4 mutations are associated with endotoxin hyporesponsiveness in humans. Nat Genet. 2000;25:187–191. doi: 10.1038/76048. [DOI] [PubMed] [Google Scholar]
- 113.Chae JJ, Aksentijevich I, Kastner DL. Advances in the understanding of familial Mediterranean fever and possibilities for targeted therapy. Br J Haematol. 2009;146:467–478. doi: 10.1111/j.1365-2141.2009.07733.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Schlosstein L, Terasaki PI, Bluestone R, Pearson CM. High association of an HL-A antigen, W27, with ankylosing spondylitis. N Engl J Med. 1973;288:704–706. doi: 10.1056/NEJM197304052881403. [DOI] [PubMed] [Google Scholar]
- 115.Palm O, Moum B, Ongre A, Gran JT. Prevalence of ankylosing spondylitis and other spondyloarthropathies among patients with inflammatory bowel disease: a population study (the IBSEN study) J Rheumatol. 2002;29:511–515. [PubMed] [Google Scholar]
- 116.Bonfiglioli R, Conde RA, Sampaio-Barros PD, Louzada-Junior P, Donadi EA, Bertolo MB. Frequency of HLA-B27 alleles in Brazilian patients with psoriatic arthritis. Clin Rheumatol. 2008;27:709–712. doi: 10.1007/s10067-007-0770-3. [DOI] [PubMed] [Google Scholar]
- 117.Eyre S, Bowes J, Diogo D, Lee A, Barton A, Martin P, et al. High-density genetic mapping identifies new susceptibility loci for rheumatoid arthritis. Nat Genet. 2012;44:1336–1340. doi: 10.1038/ng.2462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Cosan F, Ustek D, Oku B, Duymaz-Tozkir J, Cakiris A, Abaci N, et al. Association of familial Mediterranean fever-related MEFV variations with ankylosing spondylitis. Arthritis Rheum. 2010;62:3232–3236. doi: 10.1002/art.27683. [DOI] [PubMed] [Google Scholar]
- 119.Akyuz F, Besisik F, Ustek D, Ekmekci C, Uyar A, Pinarbasi B, et al. Association of the MEFV gene variations with inflammatory bowel disease in Turkey. J Clin Gastroenterol. 2013;47:23–27. doi: 10.1097/MCG.0b013e3182597992. [DOI] [PubMed] [Google Scholar]
- 120.Hatemi I, Hatemi G, Celik AF, Melikoglu M, Arzuhal N, Mat C, et al. Frequency of pathergy phenomenon and other features of Behcet’s syndrome among patients with inflammatory bowel disease. Clin Exp Rheumatol. 2008;26:91–95. [PubMed] [Google Scholar]
- 121.Shen X, Shi R, Zhang H, Li K, Zhao Y, Zhang R. The Toll-like receptor 4 D299G and T399I polymorphisms are associated with Crohn’s disease and ulcerative colitis: a meta-analysis. Digestion. 2010;81:69–77. doi: 10.1159/000260417. [DOI] [PubMed] [Google Scholar]
- 122.Girardin SE, Boneca IG, Viala J, Chamaillard M, Labigne A, Thomas G, et al. Nod2 is a general sensor of peptidoglycan through muramyl dipeptide (MDP) detection. J Biol Chem. 2003;278:8869–8872. doi: 10.1074/jbc.C200651200. [DOI] [PubMed] [Google Scholar]
- 123.Anderson CA, Boucher G, Lees CW, Franke A, D’Amato M, Taylor KD, et al. Meta-analysis identifies 29 additional ulcerative colitis risk loci, increasing the number of confirmed associations to 47. Nat Genet. 2011;43:246–252. doi: 10.1038/ng.764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Reveille JD, Sims AM, Danoy P, Evans DM, Leo P, Pointon JJ, et al. Genome-wide association study of ankylosing spondylitis identifies non-MHC susceptibility loci. Nat Genet. 2010;42:123–127. doi: 10.1038/ng.513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.International Genetics of Ankylosing Spondylitis C. Cortes A, Hadler J, Pointon JP, Robinson PC, Karaderi T, et al. Identification of multiple risk variants for ankylosing spondylitis through high-density genotyping of immune-related loci. Nat Genet. 2013;45:730–738. doi: 10.1038/ng.2667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Dubois PC, Trynka G, Franke L, Hunt KA, Romanos J, Curtotti A, et al. Multiple common variants for celiac disease influencing immune gene expression. Nat Genet. 2010;42:295–302. doi: 10.1038/ng.543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Trynka G, Hunt KA, Bockett NA, Romanos J, Mistry V, Szperl A, et al. Dense genotyping identifies and localizes multiple common and rare variant association signals in celiac disease. Nat Genet. 2011;43:1193–1201. doi: 10.1038/ng.998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Armstrong DL, Zidovetzki R, Alarcon-Riquelme ME, Tsao BP, Criswell LA, Kimberly RP, et al. GWAS identifies novel SLE susceptibility genes and explains the association of the HLA region. Genes Immun. 2014;15:347–354. doi: 10.1038/gene.2014.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Liu JZ, Almarri MA, Gaffney DJ, Mells GF, Jostins L, Cordell HJ, et al. Dense fine-mapping study identifies new susceptibility loci for primary biliary cirrhosis. Nat Genet. 2012;44:1137–1141. doi: 10.1038/ng.2395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Mayes MD, Bossini-Castillo L, Gorlova O, Martin JE, Zhou X, Chen WV, et al. Immunochip analysis identifies multiple susceptibility loci for systemic sclerosis. Am J Hum Genet. 2014;94:47–61. doi: 10.1016/j.ajhg.2013.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Lessard CJ, Li H, Adrianto I, Ice JA, Rasmussen A, Grundahl KM, et al. Variants at multiple loci implicated in both innate and adaptive immune responses are associated with Sjogren’s syndrome. Nat Genet. 2013;45:1284–1292. doi: 10.1038/ng.2792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Barrett JC, Clayton DG, Concannon P, Akolkar B, Cooper JD, Erlich HA, et al. Genome-wide association study and meta-analysis find that over 40 loci affect risk of type 1 diabetes. Nat Genet. 2009;41:703–707. doi: 10.1038/ng.381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Bradfield JP, Qu HQ, Wang K, Zhang H, Sleiman PM, Kim CE, et al. A genome-wide meta-analysis of six type 1 diabetes cohorts identifies multiple associated loci. PLoS genetics. 2011;7:1002293. doi: 10.1371/journal.pgen.1002293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Onengut-Gumuscu S, Chen WM, Burren O, Cooper NJ, Quinlan AR, Mychaleckyj JC, et al. Fine mapping of type 1 diabetes susceptibility loci and evidence for colocalization of causal variants with lymphoid gene enhancers. Nat Genet. 2015;47:381–386. doi: 10.1038/ng.3245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Hinks A, Cobb J, Marion MC, Prahalad S, Sudman M, Bowes J, et al. Dense genotyping of immune-related disease regions identifies 14 new susceptibility loci for juvenile idiopathic arthritis. Nat Genet. 2013;45:664–669. doi: 10.1038/ng.2614. [DOI] [PMC free article] [PubMed] [Google Scholar]