Abstract
The melanocortin system consists of five receptor subtypes (MC1-5R), endogenous agonists derived from the proopiomelanocortin gene transcript, and the antagonists agouti and agouti-related protein. The Escherichia coli heat shock protein ClpB has previously been described as an antigen mimetic to the endogenous melanocortin agonist α-MSH. Herein, we investigated if a fragment of the ClpB protein could directly signal through the melanocortin receptors. We synthesized a complementary fragment of the ClpB protein that partially aligned with α-MSH. Pharmacological assessment of this fragment resulted in no antagonist activity at the MC3R or the MC4R and no agonist activity at the MC4R. Partial receptor activation was observed for the MC3R and MC5R at 100 μM concentrations. This fragment was shown to be a full micromolar MC1R agonist and may serve as a template for future research into selective MC1R ligands.
Keywords: Melanocortin Receptors, ClpB Heat Shock Protein, NDP-MSH, α-MSH, AlphaScreen® cAMP Assay
Graphical Abstract
The five melanocortin receptors (MC1-5R) are members of the super family of G protein-coupled receptors (GPCR) that signal through the cAMP pathway1 and are postulated to be involved in numerous biological processes. The melanocortin 1 receptor (MC1R) is involved with pigmentation and is primarily expressed in the skin.2,3 The MC2R is expressed in the adrenal cortex and plays a role in steroidogenesis.3 While the MC3R and MC4R are expressed in several tissues, expression of both receptors in the central nervous system has been linked to pathways regulating food intake and energy homeostasis.4–10 The MC5R has been reported to be expressed in many tissues, especially in the periphery,11–13 and is proposed to be involved with exocrine gland function.13 Numerous agonists [α-, β-, γ-melanocyte stimulating hormones (MSH), adrenocorticotropin hormone (ACTH)] for the melanocortin receptor subtypes (MCRs) are derived from the proopiomelanocortin (POMC) gene transcript.14 Additionally, this receptor system contains the only two presently known endogenous antagonists for GPCRs, agouti and agouti-related protein.15–17 A new class of endogenous MCR ligands, the β-defensins, has recently been reported,18 though the exact mechanism of this interaction remains unclear since β-defensins have been described as antagonists,19 weak partial agonists,20 and neutral antagonists21 at the MCRs. The discovery of additional endogenous ligands for the MCRs may allow the development of unique molecular probes and ligands based upon unexplored structural motifs. Due to the involvement of the MC3R and MC4R in food intake and energy homeostasis,6,9,22 novel templates may be developed into therapeutics to help modulate body weight without the reported negative side effects of previously described MC4R specific agonists derived from modifications to POMC-derived ligands.23
The endogenous melanocortin receptor agonist α-MSH has previously been demonstrated to modulate food intake. In rats, direct injection of α-MSH into the central nervous system via intracerebroventricular (i.c.v.) administration inhibited feeding.24 One hypothesis for the development of eating disorders including anorexia nervosa and bulimia nervosa involves dysregulation of melanocortin signaling through interactions of α-MSH with immunoglobulins or autoantibodies.25 In humans, serum from a majority of individuals afflicted with anorexia nervosa or bulimia nervosa contains antibodies that bind α-MSH.25 The plasma level of these antibodies was shown to correlate with the Eating Disorder Inventory-2 assessment in patients with eating disorders.26 The mechanism of how the α-MSH antibodies may result in eating disorders has not been elucidated. It has previously been suggested that these antibodies may: (1) bind and prevent α-MSH induced MCR signaling, (2) bind and still allow α-MSH signaling, protecting the peptide from endopeptidases and prolonging signaling, or (3) bind α-MSH and MCR, followed by activation of the complement immune system, ultimately resulting in neuronal destruction.27
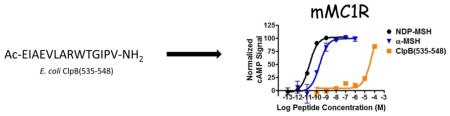
Recently, it has been proposed that the Escherichia coli (E. coli) heat shock disaggregation chaperone protein ClpB is a conformational antigen mimetic of α-MSH and may be responsible for the production of the α-MSH antibodies.28 While this chaperone protein is comprised of 857 residues compared to 13 in α-MSH, a discontinuous five amino acid overlap is observed (Fig. 1). Interestingly this overlap contains half of the canonical melanocortin agonist sequence, His-Phe-Arg-Trp, that is present in all POMC-derived melanocortin agonists.29 The partial overlap with the melanocortin agonist pharmacophore suggests that the ClpB protein may directly interact with the melanocortin receptors. The full length protein could not be assayed due to ClpB intrinsic ATPase activity, which would interfere in the ATP-dependent cAMP signaling pathway.28,30
Figure 1.

Amino acid sequences of NDP-MSH, α-MSH, and ClpB(535-548). Conserved residues are highlighted in green.
In the search of new templates to generate potent and selective melanocortin ligands, it was hypothesized that the ClpB protein may be a ligand for the melanocortin receptors, specifically at the MC3R and MC4R due to the involvement of these receptors in modulating food intake and energy homeostasis. To test this hypothesis, a fourteen residue sequence of ClpB [ClpB(535-548)] was synthesized and pharmacologically characterized at the MC1R, MC3-5R. The MC2R is only stimulated by ACTH and was therefore excluded. The sequence of the ClpB fragment was selected based upon the overlap of ClpB with α-MSH (Fig. 1); similarly, the C-terminus was amidated and the N-terminus was acetylated to correspond to the structural features of α-MSH.
The reported ClpB(535-548) fragment was synthesized manually using standard fluorenylmethoxycarbonyl (Fmoc) methodologies on a rink-amide polystyrene resin.31 Following acetylation of the N-terminal amine, the peptide was cleaved and purified using semi-preparative RP-HPLC. The purity of the peptide (>95%) was assessed by analytical RP-HPLC in two distinct solvent systems and the correct peptide mass was confirmed through ESI mass spectrometry (University of Minnesota Mass Spectrometry Lab). Compounds were assayed for agonist activity using the AlphaScreen® cAMP assay (Perkin Elmer) on stably transfected HEK293 cells according to the manufacturer’s instructions and as previously reported;32 the endogenous α-MSH and more potent synthetic analog NDP-MSH were used as positive control ligands. Since the AlphaScreen® cAMP assay is a competition assay with decreasing signal at higher concentrations, the concentration-activity curves were normalized for illustrative purposes similar to Elster et al.33 The ClpB(535-548) fragment was additionally assessed for antagonist activity at the MC3R and MC4R through a Schild analysis using NDP-MSH as the agonist.34
The purported role of ClpB in eating disorders, the partial sequence overlap of ClpB with α-MSH, and the involvement of the MC3R and MC4R in food intake and energy homeostasis implied that ClpB may interact with the MC3R and MC4R. However, the synthesized fragment of ClpB resulted in no observable agonist activity at the MC4R and only 30% of the maximal agonist response was observed at the MC3R at up to 100 μM concentrations (Table 1, Figure 2). Furthermore, no antagonist activity was observed at either receptor (data not shown). The lack of pharmacological activity with this fragment suggest that the ClpB protein is not a ligand for these receptors.
Table 1.
Agonist Pharmacology of NDP-MSH, α-MSH, and ClpB(535-548) at the Mouse Melanocortin Receptors.a
| Peptide | mMC1R | mMC3R | mMC4R | mMC5R |
|---|---|---|---|---|
|
| ||||
| EC50 (nM) | ||||
|
| ||||
| NDP-MSH | 0.019±0.006 | 0.22±0.03 | 0.34±0.09 | 0.27±0.04 |
| α-MSH | 0.14±0.04 | 0.50±0.06 | 2.9±0.5 | 0.51±0.10 |
| ClpB(535-548) | 41,000±11,000 | 30% @ 100,000 | >100,000 | 50% @ 100,000 |
The indicated errors represents the standard error of the mean determined from at least three independent experiments. >100,000 indicates that the compound was examined but lack agonist and antagonist activity at up to 100 μM concentrations. A percentage indicates the percent maximal stimulatory response observed at 100 μM concentrations but not enough stimulation was observed to determine an EC50 value.
Figure 2.
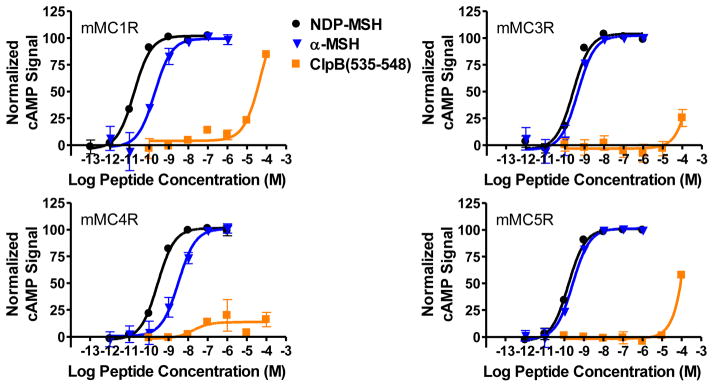
Illustrations of the in vitro agonist pharmacology of NDP-MSH, α-MSH, and ClpB(535-548) at the mouse MC1R, MC3R, MC4R, and MC5R.
At the MC5R, 50% of the maximal stimulatory activity was observed at 100 μM concentrations (Figure 2). A more robust response was observed at the MC1R, where the synthesized ClpB fragment possessed full, micromolar agonist activity (Figure 2). The MC1R is primarily expressed in epithelial cells and is involved with pigmentation. Previous truncation studies have indicated that the minimal melanocortin agonist sequence with activity using the frog Rana pipens29 and the lizard Anolis carolinensis35 skin assays to be Ac-His-Phe-Arg-Trp-NH2. The tripeptide Ac-Phe-Arg-Trp-NH2 was the minimal fragment to show activity at the cloned mouse MCRs, possessing micromolar agonist activity at the mMC1R, minimal activity at the mMC5R, and no activity at the mMC3R or mMC4R.36 Inversion of the Phe to DPhe regained micromolar activity at the mMC4R and mMC5R and minimal activity at the mMC3R.36 This Ac-DPhe-Arg-Trp-NH2 tripeptide has also been shown to possess micromolar agonist activity using the Rana pipens skin assay.37 An alanine scan of α-MSH in murine B16 melanoma cells demonstrated that replacement of Phe, Arg, or Trp with Ala drastically decreased binding affinity of the resulting peptides,38 highlighting the importance of this three residue motif. The synthesized fragment of the ClpB heat shock protein only contained two of the residues previously demonstrated to be required for activation of the MC1R (Arg-Trp), perhaps indicating a truncated pharmacophore that could be utilized in the development of MC1R selective ligands. Additional structure activity relationship studies, alanine scans and truncation experiments may help clarify the key ClpB residues responsible for the observed activity at the MC1R.
In summary, it was hypothesized that the E. coli heat shock protein ClpB may be a ligand for the MCRs. The ClpB(535-548) fragment resulted in negligible agonist and antagonist activity at both the MC3R and MC4R, indicating that this protein may not be able to directly modulate the activity of these receptors. A greater response (50% maximal receptor activation) was observed at the MC5R, while the fragment was able to fully stimulate the MC1R as a micromolar agonist. The ClpB(535-548) fragment could therefore serve as a lead for developing MC1R selective agonists.
Acknowledgments
This work has been supported by NIH Grants R01DK097838 and R01DK091906.
Abbreviations
- ACTH
Adrenocorticotropin Hormone
- Fmoc
Nα 9-fluorenylmethoxycarbonyl
- GPCR
G Protein-Coupled Receptor
- cAMP
cyclic 5′-adenosine monophosphate
- MC1R
Melanocortin-1 Receptor
- MC2R
Melanocortin-2 Receptor
- MC3R
Melanocortin-3 Receptor
- MC4R
Melanocortin-4 Receptor
- MC5R
Melanocortin-5 Receptor
- MCR
Melanocortin Receptor
- MSH
Melanocyte Stimulating Hormone
- POMC
Proopiomelanocortin
- α-MSH
Alpha-Melanocyte Stimulating Hormone
- β-MSH
Beta-Melanocyte Stimulating Hormone
- γ-MSH
Gamma-Melanocyte Stimulating Hormone
- μM
Micromolar
- NDP-MSH (4-Norleucine-7-D-Phenylalanine)
Ac-Ser-Tyr-Ser-Nle-Glu-His-DPhe-Arg-Trp-Gly-Lys-Pro-Val-NH2
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References and Notes
- 1.Haynes RC., Jr J Biol Chem. 1958;233:1220. [PubMed] [Google Scholar]
- 2.Chhajlani V, Wikberg JE. FEBS Lett. 1992;309:417. doi: 10.1016/0014-5793(92)80820-7. [DOI] [PubMed] [Google Scholar]
- 3.Mountjoy KG, Robbins LS, Mortrud MT, Cone RD. Science. 1992;257:1248. doi: 10.1126/science.1325670. [DOI] [PubMed] [Google Scholar]
- 4.Butler AA, Kesterson RA, Khong K, Cullen MJ, Pelleymounter MA, Dekoning J, Baetscher M, Cone RD. Endocrinology. 2000;141:3518. doi: 10.1210/endo.141.9.7791. [DOI] [PubMed] [Google Scholar]
- 5.Chen AS, Marsh DJ, Trumbauer ME, Frazier EG, Guan XM, Yu H, Rosenblum CI, Vongs A, Feng Y, Cao LH, Metzger JM, Strack AM, Camacho RE, Mellin TN, Nunes CN, Min W, Fisher J, Gopal-Truter S, MacIntyre DE, Chen HY, Van der Ploeg LHT. Nat Genet. 2000;26:97. doi: 10.1038/79254. [DOI] [PubMed] [Google Scholar]
- 6.Fan W, Boston BA, Kesterson RA, Hruby VJ, Cone RD. Nature. 1997;385:165. doi: 10.1038/385165a0. [DOI] [PubMed] [Google Scholar]
- 7.Gantz I, Konda Y, Tashiro T, Shimoto Y, Miwa H, Munzert G, Watson SJ, DelValle J, Yamada T. J Biol Chem. 1993;268:8246. [PubMed] [Google Scholar]
- 8.Gantz I, Miwa H, Konda Y, Shimoto Y, Tashiro T, Watson SJ, DelValle J, Yamada T. J Biol Chem. 1993;268:15174. [PubMed] [Google Scholar]
- 9.Huszar D, Lynch CA, Fairchild-Huntress V, Dunmore JH, Fang Q, Berkemeier LR, Gu W, Kesterson RA, Boston BA, Cone RD, Smith FJ, Campfield LA, Burn P, Lee F. Cell. 1997;88:131. doi: 10.1016/s0092-8674(00)81865-6. [DOI] [PubMed] [Google Scholar]
- 10.Roselli-Rehfuss L, Mountjoy KG, Robbins LS, Mortrud MT, Low MJ, Tatro JB, Entwistle ML, Simerly RB, Cone RD. Proc Natl Acad Sci USA. 1993;90:8856. doi: 10.1073/pnas.90.19.8856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gantz I, Shimoto Y, Konda Y, Miwa H, Dickinson CJ, Yamada T. Biochem Biophys Res Commun. 1994;200:1214. doi: 10.1006/bbrc.1994.1580. [DOI] [PubMed] [Google Scholar]
- 12.Griffon N, Mignon V, Facchinetti P, Diaz J, Schwartz JC, Sokoloff P. Biochem Biophys Res Commun. 1994;200:1007. doi: 10.1006/bbrc.1994.1550. [DOI] [PubMed] [Google Scholar]
- 13.Chen W, Kelly MA, Opitz-Araya X, Thomas RE, Low MJ, Cone RD. Cell. 1997;91:789. doi: 10.1016/s0092-8674(00)80467-5. [DOI] [PubMed] [Google Scholar]
- 14.Nakanishi S, Inoue A, Kita T, Nakamura M, Chang AC, Cohen SN, Numa S. Nature. 1979;278:423. doi: 10.1038/278423a0. [DOI] [PubMed] [Google Scholar]
- 15.Blanchard SG, Harris CO, Ittoop OR, Nichols JS, Parks DJ, Truesdale AT, Wilkison WO. Biochemistry. 1995;34:10406. doi: 10.1021/bi00033a012. [DOI] [PubMed] [Google Scholar]
- 16.Fong TM, Mao C, MacNeil T, Kalyani R, Smith T, Weinberg D, Tota MR, VanderPloeg LHT. Biochem Biophys Res Commun. 1997;237:629. doi: 10.1006/bbrc.1997.7200. [DOI] [PubMed] [Google Scholar]
- 17.Ollmann MM, Wilson BD, Yang YK, Kerns JA, Chen YR, Gantz I, Barsh GS. Science. 1997;278:135. doi: 10.1126/science.278.5335.135. [DOI] [PubMed] [Google Scholar]
- 18.Candille SI, Kaelin CB, Cattanach BM, Bin Y, Thompson DA, Nix MA, Kerns JA, Schmutz SM, Millhauser GL, Barsh GS. Science. 2007;318:1418. doi: 10.1126/science.1147880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Swope VB, Jameson JA, McFarland KL, Supp DM, Miller WE, McGraw DW, Patel MA, Nix MA, Millhauser GL, Babcock GF, Abdel-Malek ZA. Journal of Investigative Dermatology. 2012;132:2255. doi: 10.1038/jid.2012.135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Beaumont KA, Smit DJ, Liu YY, Chai E, Patel MP, Millhauser GL, Smith JJ, Alewood PF, Sturm RA. Pigment Cell Melanoma Research. 2012;25:370. doi: 10.1111/j.1755-148X.2012.00990.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nix MA, Kaelin CB, Ta T, Weis A, Morton GJ, Barsh GS, Millhauser GL. Chem Biol. 2013;20:784. doi: 10.1016/j.chembiol.2013.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Irani BG, Xiang ZM, Yarandi HN, Holder JR, Moore MC, Bauzo RM, Proneth B, Shaw AM, Millard WJ, Chambers JB, Benoit SC, Clegg DJ, Haskell-Luevano C. Eur J Pharmacol. 2011;660:80. doi: 10.1016/j.ejphar.2010.10.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Greenfield JR, Miller JW, Keogh JM, Henning E, Satterwhite JH, Cameron GS, Astruc B, Mayer JP, Brage S, See TC, Lomas DJ, O’Rahilly S, Farooqi IS. N Engl J Med. 2009;360:44. doi: 10.1056/NEJMoa0803085. [DOI] [PubMed] [Google Scholar]
- 24.Poggioli R, Vergoni AV, Bertolini A. Peptides. 1986;7:843. doi: 10.1016/0196-9781(86)90104-x. [DOI] [PubMed] [Google Scholar]
- 25.Fetissov SO, Hallman J, Oreland L, Af Klinteberg B, Grenback E, Hulting AL, Hokfelt T. Proc Natl Acad Sci USA. 2002;99:17155. doi: 10.1073/pnas.222658699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fetissov SO, Harro J, Jaanisk M, Jarv A, Podar I, Allik J, Nilsson I, Sakthivel P, Lefvert AK, Hokfelt T. Proc Natl Acad Sci USA. 2005;102:14865. doi: 10.1073/pnas.0507204102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fetissov SO. In: Neuropsychiatric Disorders and Infection. Fatemi SH, editor. Taylor and Francis; Oxfordshire: 2005. pp. 253–262. [Google Scholar]
- 28.Tennoune N, Chan P, Breton J, Legrand R, Chabane YN, Akkermann K, Jarv A, Ouelaa W, Takagi K, Ghouzali I, Francois M, Lucas N, Bole-Feysot C, Pestel-Caron M, do Rego JC, Vaudry D, Harro J, De E, Dechelotte P, Fetissov SO. Translational Psychiatry. 2014;4:e458. doi: 10.1038/tp.2014.98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hruby VJ, Wilkes BC, Hadley ME, Alobeidi F, Sawyer TK, Staples DJ, Devaux AE, Dym O, Castrucci AMD, Hintz MF, Riehm JP, Rao KR. J Med Chem. 1987;30:2126. doi: 10.1021/jm00394a033. [DOI] [PubMed] [Google Scholar]
- 30.Woo KM, Kim KI, Goldberg AL, Ha DB, Chung CH. J Biol Chem. 1992;267:20429. [PubMed] [Google Scholar]
- 31.Carpino LA, Han GY. J Am Chem Soc. 1970;92:5748. [Google Scholar]
- 32.Singh A, Tala S, Flores V, Freeman K, Haskell-Luevano C. ACS Medicinal Chemistry Letters. 2015;6:568. doi: 10.1021/acsmedchemlett.5b00053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Elster L, Elling C, Heding A. Journal of Biomolecular Screening. 2007;12:41. doi: 10.1177/1087057106295895. [DOI] [PubMed] [Google Scholar]
- 34.Schild HO. British Journal of Pharmacology and Chemotherapy. 1947;2:189. doi: 10.1111/j.1476-5381.1947.tb00336.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Castrucci AML, Hadley ME, Sawyer TK, Wilkes BC, Alobeidi F, Staples DJ, Devaux AE, Dym O, Hintz MF, Riehm JP, Rao KR, Hruby VJ. General and Comparative Endocrinology. 1989;73:157. doi: 10.1016/0016-6480(89)90066-x. [DOI] [PubMed] [Google Scholar]
- 36.Haskell-Luevano C, Holder JR, Monck EK, Bauzo RM. J Med Chem. 2001;44:2247. doi: 10.1021/jm010061n. [DOI] [PubMed] [Google Scholar]
- 37.Haskell-Luevano C, Sawyer TK, Hendrata S, North C, Panahinia L, Stum M, Staples DJ, Castrucci AMD, Hadley ME, Hruby VJ. Peptides. 1996;17:995. doi: 10.1016/0196-9781(96)00141-6. [DOI] [PubMed] [Google Scholar]
- 38.Sahm UG, Olivier GWJ, Branch SK, Moss SH, Pouton CW. Peptides. 1994;15:1297. doi: 10.1016/0196-9781(94)90157-0. [DOI] [PubMed] [Google Scholar]


