Abstract
Reactive oxygen species (ROS) are important in regulating normal cell physiological functions, but when produced in excess lead to the augmented pathogenesis of various diseases. Among these ischemia reperfusion injury, Alzheimer’s disease and rheumatoid arthritis, are particularly important. Since ROS can be counteracted by a variety of antioxidants, natural and synthetic antioxidants have been developed. However, due to the ubiquitous production of ROS in living systems, poor in vivo efficiency of these agents and lack of target specificity, the current clinical modalities to treat oxidative stress damage are limited. Advances in the developing field of nanomedicine have yielded nanoparticles that can prolong antioxidant activity, and target specificity of these agents. Thus, catalytic antioxidants such as recombinant superoxide dismutase (SOD), in combination with platinum and cerium oxide nanoparticles manifest higher efficacy at smaller doses with potentially lower toxicity. This article reviews recent advances in antioxidant nanoparticles and their applications to manage oxidative stress-mediated diseases.
1. Introduction
Besides their role in normal cell physiological function and cell to cell signaling, free radicals have been implicated in the pathophysiology of numerous disease processes1,2. Overproduction of highly reactive radical species or their precursors leads to oxidative stress, which has been observed in cardiovascular disease3, cerebrovascular stroke4, Alzheimer’s disease5, arthritis6, diabetes7 and cancer8, among others. Considerable research has been performed in bolstering endogenous antioxidant capacity9, administering natural antioxidants10, and synthesizing novel antioxidants11 to combat oxidative damage.
Cardiovascular disease is one of the most prevalent global health conditions, and claims 17.3 million lives annually as estimated by the World Health Organization. Studies indicate that reactive oxygen species (ROS) are implicated in the pathogenesis of various aspects of cardiovascular disease, including ischemic heart disease, ischemia/reperfusion (I/R) injury, atherosclerosis, and congestive heart failure12. Aberrant production of oxidants due to xanthine oxidase, nitric oxide synthase (NOS) and Fe-catalyzed reactions lead to the enhanced intracellular Ca2+ overload, lipid peroxidation, and apoptosis of vascular cells. Similar mechanisms are known to drive the pathogenesis of acute cerebral ischemic stroke, where the oxidative damage causes cerebral swelling and degradation to the blood brain barrier (BBB). Reperfusion after an ischemic stroke, although necessary to restore cellular functions, causes high concentrations of ROS production which often manifest more harm than the initial ischemic injury. The O2·− generated during this process is able to react with nitric oxide (NO) produced from nitric oxide synthases (NOS)13 to form peroxynitrite (ONOO−), which is highly reactive and causes aberrant oxidation and nitration of DNA and proteins. Nitric oxide generated during I/R is helpful in promoting vasodilation, thus O2·− should be targeted to prevent downstream formation more oxidizing ROS, particularly ONOO−.
Alzheimer’s disease (AD) is a neurodegenerative disorder characterized by the accumulation of modified proteins such as amyloid-β (Aβ) plaques that have an affinity for interacting with inorganic cations like Cu2+, Fe3+, and Zn2+ 14. These reactions with macromolecules catalyze the formation of ROS via Fenton reaction to cause neurodegeneration. While the development of oxidative stress may be secondary to AD, the ROS-mediated neuronal damage plays an important role in the disease pathogenesis through progressive decline in neuron function. Rheumatoid arthritis (RA) is an autoimmune disease of the joints. Macrophages and activated T-cells attack healthy tissue partly through oxidative mechanisms via nuclear factor kappa-B (NF-κB), tumor necrosis factor-alpha (TNF-α) and nuclear factor erythroid 2-related factor (NRF2) activation, which leads to an increased inflammatory milieu and progressive joint destruction6.
Oxygen-containing free radical molecules and their precursors formed in biological systems are collectively termed ROS, which includes superoxide (O2·−), hydrogen peroxide (H2O2) and hydroxyl radical (HO·). Additionally, reactive nitrogen species (RNS), such as NO and ONOO−, have similar dynamics and are therefore investigated to understand the pathobiology of oxidative stress related conditions. Superoxide anion is formed in vivo by xanthine oxidase15, NADPH oxidase16, activated immune cells17, or leakage from the electron transport chain of mitochondria18, and is regulated by enzymes such as superoxide dismutase (SOD) and peroxidases, as well as endogenous supplies of antioxidants such as glutathione (GSH). Under the condition of oxidative stress, ROS and RNS are produced in excess leading to lipid peroxidation, protein oxidation and nitration, and DNA fragmentation that ultimately affects cell membrane structures, enzyme functions, and gene expression (Figure 1).
Figure 1.
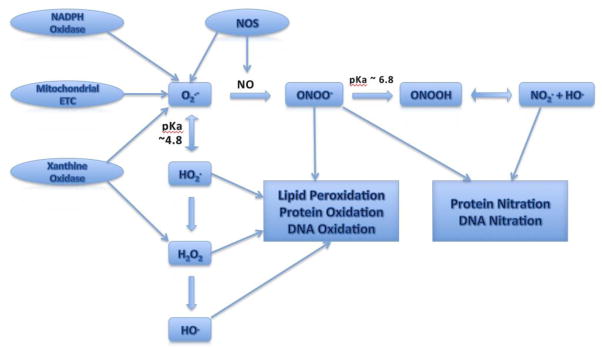
Schematic diagram of ROS and RNS generation during oxidative stress
2. Small Molecule ROS Scavengers
2.1. Edaravone (Radicut®)
Recent advances in research on the role of ROS in disease pathogenesis have propelled therapeutic strategies towards the development of ROS scavengers for clinical application. Currently, the only drug clinically approved as a free radical scavenger is Edaravone (Radicut®) for treatment of cerebral ischemic stroke in Japan since 200119. Edaravone has not been clinically evaluated for its antioxidant capability, however in vivo studies suggest its therapeutic mechanism is through scavenging free radicals, mainly HO· and upregulating endothelial NOS (eNOS) expression in cerebral tissue when administered with a thrombolytic agent during the onset of an acute ischemic stroke20. While increased eNOS activity will generate more NO, the antioxidant property of Edaravone reduces the formation of ONOO− 21. Further in vivo studies suggest that Edaravone prevents the oxidation of low density lipoprotein (LDL), a resource to the formation of atherosclerosis, and a concomitantly scavenges lipid peroxyl radicals22. The combination of these effects has been instrumental in reducing neuronal damage, cerebral edema, and consequently improving patient recovery. Edaravone administration reduced the white matter lesions after hypoperfusion23, and significantly improved the motor function 21 days after treatment in rats24. Edaravone is currently in clinical use only for acute ischemic stroke, although its ROS scavenging properties suggest its possible use for other diseases involving oxidative damage, such as cardiovascular diseases. The clinical use of Edaravone has been limited due to its renal toxicity among other adverse effects such as hepatic/biliary dysfunction and platelet reduction25. Concomitant administration with lipoic acid was shown to reduce these adverse effects while maintaining neuroprotection in a rat model of ischemic stroke26.
2.2. N-Acetylcysteine
Thiols such as endogenous glutathione (GSH) act as cellular antioxidants by reducing ROS generation and ‘repairing’ carbon-centered radicals formed on DNA and proteins during oxidative stress by donating a hydrogen atom. N-acetylcysteine (NAC) is an antioxidant that has been used as a mucolytic agent and treatment for acetaminophen toxicity. Similar to GSH, NAC’s antioxidant property is due to its reduced thiol moiety that allows scavenging HO· and ·CH3 radicals, with a little to no physiological activity towards O2.− 27 or ONOO− 28. Furthermore, NAC is a metal chelator that mediates its antioxidant effects by suppressing Fenton-type reactions of free Fe3+, Cu2+ and Zn2+ ions. In human studies, intravenous NAC administered with nitroglycerin and streptokinase was shown to reduce oxidative damage from I/R injury to the left ventricle of the heart after a myocardial infarction29. This was attributed to HO· scavenging and inhibition of angiotensin converting enzyme by NAC. While some studies show improvement due to the radical scavenging ability of NAC, this molecule is unable to penetrate cell membrane and blood brain barrier (BBB), which reflects as well in its low bioavailability when given orally. Under oxidative stress however, NAC was observed to cross cell membranes30, most likely due to the increased permeability of the membranes under these pathological conditions. NAC has also been shown to be effective in the regulation of chronic ROS overproduction through inhibition of pro-inflammatory cytokines due to its conversion to glutathione. It was observed that NAC down-regulated NO-induced interleukin-1 beta (IL-1β) and tumor necrosis factor-alpha (TNF-α) production in human chondrocytes to reduce apoptosis 7. NAC is currently involved in clinical trials for various diseases involving oxidative stress such as Sickle Cell disease and Parkinson’s disease (Clinicaltrials.gov NCT01849016 and NCT01470027, respectively). In these studies, the antioxidant capacity of NAC will be evaluated by measuring oxidative markers in blood or cerebrospinal fluid as primary or secondary outpoints.
2.3. NXY-059 (Cerovive®)
NXY-059 is an experimental α-phenyl-tert-butyl nitrone (PBN)-derived antioxidant that is capable of trapping free radicals 31, which is characteristic of the nitrone family. This results in the formation of a more stable radical molecule that is easily detected by electron paramagnetic resonance (EPR) spectroscopy, before decomposing to release NO32. In addition to free radical spin-trapping, NXY-059 has been shown to act as a protective agent in ischemic injury through the prevention of mitochondrial dysfunction and reduction of cytochrome c release that resulted in increased cell survival33. NXY-059 has shown great promise in pre-clinical studies, however its use in the SAINT-I and SAINT-II double-blinded placebo controlled clinical trials for the treatment of acute ischemic stroke within 6 hours of onset proved ineffective34. Similarly, the experimental antioxidant drugs Tirilazad35 and Ebselen36 were found ineffective in treating acute ischemic stroke in clinical trials, causing a major setback in the advancement of antioxidants as therapeutics.
2.4. Natural Antioxidants
Several natural antioxidants have been used in pre-clinical and clinical applications as treatments for diseases driven by free radical mediated pathophysiology. These compounds benefit from their ease of use and good bioavailability as most are common in foods or available in over-the-counter formulations. Vitamin E is a lipophilic antioxidant that has been shown to act on lipid radicals to break the chain of peroxidation in cell membranes, and is currently involved in clinical trials for AD, RA, and ischemic stroke (Clinicaltrails.gov: NCT00040378, NCT00399282, and NCT01578629, respectively). The ability of vitamin E to scavenge other radicals is limited, as evidenced by numerous negative clinical experiments (Clinicaltrials.gov: NCT00363129, NCT00117403); however its use in combination with other antioxidants like vitamin C or selenium may overcome this deficiency. Polyphenols such as resveratrol, found in red wine, and catechins such as epigallocatechin-3-gallate (EGCG) found in green tea, are emerging antioxidants that also exhibit anti-inflammatory properties as well, making them attractive candidates for the treatment of RA and other chronic inflammatory diseases 37. EGCG has been shown to scavenge O2·− and HO· radicals, as well as down-regulate pro-inflammatory cytokines and up-regulate antioxidant enzymes like SOD, catalase and glutathione peroxidase, which provides a multi-faceted approach to treat oxidative stress conditions. While natural antioxidants carry less toxicity concerns than synthetic antioxidants, they are limited by their lack of target specificity, and are found to be more effective in concert rather than treatments with a single natural antioxidant38.
2.5. Synthetic Antioxidants
The development of synthetic antioxidants that can be ‘customized’ to have enhanced pharmacological activity is an active field in inflammation research. Chemical substitutions to natural antioxidants can yield products with more cell specificity, enzyme selectivity, and increased reactivity towards upstream ROS like O2·−. Spin-quenching antioxidants or spin trapping agents are compounds that are ‘stable’ radicals that yield non-radical products upon reaction to ROS. Examples of spin-quenching molecules include 2,2,6,6-tetramethylpiperidine-1-oxyl (TEMPO)39, a nitroxyl radical, or trityl radicals such as perchlorotriphenylmethyl (PTM-TC)40. Nitroxyl molecules are able to act as self-replenishing antioxidants to convert O2·− to H2O2, although they have been shown to have short in vivo half-lives and may induce hypotension when in circulation41. The nitroxyl 4-hydroxy-TEMPO (TEMPOL) has also been shown to cause cell-cycle arrest in breast cancer cells by p21 overexpression, leading to apoptosis by DNA fragmentation42. P21 overexpression has been shown to be detrimental in oxidative-stress conditions by suppressing glutathione peroxidase and SOD43, thus application of nitroxyl antioxidants should take caution of this cytotoxic effect. Trityl radicals exhibit considerably high reactivity toward O2·− (as high as 8.3 × 108 M−1s−1 for PTM-TC)40 while retaining some degree of inertness towards other oxido-reductant species44. In contrast, spin-generating synthetic antioxidants are diamagnetic molecules that form paramagnetic species upon reacting with free radicals. Hydroxylamines such as 1-hydroxy-4-phosphono-oxy-2,2,6,6-tetramethylpiperidine (PP-H) contain an N-hydroxy group that is oxidized by O2·− to form a more stable nitroxyl radical and H2O245. The H2O2 formed can then be degraded by catalase, however H2O2 can form more unstable ROS, which poses a problem for the use of hydroxylamines in vivo. Nitrones are a family of antioxidants that are classified as linear, phenyl N-tertiary butyl nitrone (PBN)-derivatives (such as NXY-059), or cyclic, 5,5-dimethyl-1-pyrroline-N-oxide (DMPO)-derivatives (such as 5-diethoxyphosphoryl-5-methyl-1-pyrroline-N-oxide, DEPMPO). Each class is able to react with radical species to form a more stable radical adduct. These molecules are commonly used as radical probes in a technique called spin-trapping, as the radical adducts formed can be detected by EPR spectroscopy which can give spectra that are unique to each radical species formed. Nitrones are able to spin-trap multiple species of ROS, RNS46, and carbon centered radicals, and thus makes them target specific with improved reactivity47 and radical adduct stability48. In addition, nitrones also exhibit anti-inflammatory properties through down regulation of pro-inflammatory mediators. For example, PBN was shown to act on the mitogen-activated protein kinase (MAPK) cascade in human osteoarthritis chondrocytes49, while DMPO was found to downregulate inducible NOS (iNOS) as well as inhibit the phosphorylation of MAPKs10. Currently, nitrones suffer from varying cellular toxicity, and fairly slow reactivity toward O2·−50, and the recent clinical failure of NXY-059 has hindered their application.
3. Nanomedicine ROS Scavengers
Currently, the limitations of small-molecule natural and synthetic antioxidants include low solubility, poor bioavailability, and their lack of specificity. Nanomedicine involves the utilization of molecules ranging from as small as <10 nm (e.g. metal nanocrystals)51, to macromolecules as large as 300 nm52. Polymeric nanoparticles are used to encapsulate or incorporate small molecules to provide protection from degradation or to aid in absorption and distribution of natural antioxidants. Nanoparticles can provide higher solubility to poor water-soluble compounds and enhanced surface functionalization to yield target-specificity. Inorganic metal nanoparticles are also being used for their catalytic properties, which is enhanced by their relatively large surface areas. However, toxicities have been observed as some of the nanoparticles are known to accumulate in tissues such as liver and brain25, and others are known to have pro-oxidant properties26. This can be avoided by using biodegradable carrier molecules, such as albumin or poly(lactic-co-glycolic) acid (PLGA), that can be broken down by lysosomal or hydrolytic degradation of the matrix polymers53.
3.1. Catalytic ROS-Scavenging Nanoparticles
3.1.1. SOD-containing Nanoparticles
The application of nanomedicine in ROS-mediated pathologies has dramatically advanced the strategies to the scavenging of free-radicals under oxidative stress, including target-specificity, increased cell membrane permeability, and the use of catalytic scavengers. The obvious advantage of using a catalytic ROS scavenger is that the compound is not depleted during the reaction and can potentially neutralize numerous ROS molecules, which can elevate higher potency with a lower dose. Endogenously, cells use SOD to catalyze the neutralization of O2·− to O2 and H2O2. Nanoparticles were engineered with recombinant SOD conjugation to allow effective cellular delivery of the enzyme under oxidative stress conditions, while protecting the enzyme and avoiding its degradation in the serum54. The conjugation of SOD to nanoparticles also promotes BBB permeability, which allows the application of these nanoparticles to I/R injury in the brain. Upon reaching the cells, the nanoparticle is endocytosed, and the enzyme is able to catalyze the degradation of O2·−. Reddy et al, used SOD-conjugated poly (D,L-lactic-co-glycolic acid) (PLGA) nanoparticles to treat I/R injury in the brains of rats to achieve sustained SOD delivery that enhanced the rate of survival and improved their neurological function54. An infusion of SOD nanoparticles during reperfusion reduced the infarct size by 65% over a saline control and 40% better than SOD in delivered in solution. Chen et al, recently engineered silica nanoparticles conjugated with recombinant Cu, Zn-SOD containing a His-tag domain for attachment to the nanoparticle, as well as a human immunodeficiency virus (HIV) transactivator protein (TAT) domain which allows enhanced transmembrane delivery55. Using a novel delivery approach, the authors denatured the enzyme while attached to the nanoparticle before its delivery into cells, where it was shown to be re-folded to regain its catalytic activity.
3.1.2. Platinum Nanoparticles
Platinum has been used clinically as a chemotherapeutic, as cisplatin for example, and in chemistry as a catalyst for hydrogenation and oxidation reactions. It has been shown to catalytically convert O2·− to H2O2, and H2O2 to H2O and O2, which makes it an attractive candidate as a SOD/catalase mimetic for the treatment of oxidative stress56. Studies have shown the efficacy for platinum nanoparticles in vitro conditions, where it was shown to scavenge peroxyl radicals as well57. However, it is not yet clear if they are able to scavenge HO·, which are the most potent ROS present under oxidative stress conditions. In a similar study, Kim et al, used HIV TAT-conjugated platinum nanoparticles in an in vivo model of oxidative stress to increase the uptake of the nanoparticles into cells58. It was found that the TAT-conjugated platinum nanoparticles achieved similar antioxidant effects at one hundredth of the dose of non-derived platinum nanoparticles, which allows a much higher bioavailability with less degree of toxicity. The TAT-conjugated platinum nanoparticles were shown to increase survival of C. elegans under both acute oxidative stress, elicited by paraquat exposure, as well as chronic endogenous ROS, thus could be of potential therapeutic value in chronic inflammatory diseases.
3.1.3. Cerium Nanoparticles
Cerium nanoparticles (ceria) possess catalytic properties similar to that of platinum nanoparticles due to their ability to convert O2·− to O2, to generate Ce3+ from Ce4+, and then auto-regenerate Ce4+ from the reduction of Ce3+ or by reaction with HO· 59,60. Ceria were also found to catalyze the degradation of H2O261, which depicts a multi-faceted mechanism to its antioxidant properties. Furthermore, ceria were determined to decrease NO production from macrophages in mouse cells through a down-regulation of iNOS, which creates anti-inflammatory effects62. In another study, ceria exhibited a scavenging activity for ONOO−, a potent RNS generated from the reaction of O2·− and NO63. These properties make ceria particularly useful for chronic ROS-mediated inflammatory diseases like RA, where scavenging of NO generated from iNOS in macrophages can stop further amplification of inflammatory damage. In vitro studies provide conflicting evidence of ceria toxicity in different cell lines, which may partly be attributed to the size and surface area of the ceria particles, with larger particles exhibiting greater toxicity64. Estevez et al, investigated the effects of ceria in an ischemic model of mouse hippocampal brain slices and found an approximately 50% reduction in cell death likely due to a marked decrease in the levels of ROS and ONOO− 11. Ceria can be readily taken up by the cells, however, their tendency to form aggregates in the cytoplasm has partly limited their antioxidant properties65. We postulate that this could potentially be ameliorated by using a carrier polymer to inhibit the aggregation while allowing the ceria to react with ROS.
3.2. Site-Directed Nanoparticles
3.2.1. H2O2-Sensitive Nanoparticles
Due to the ubiquitous production of ROS under normal physiological conditions, a tissue-specific targeted approach to ROS scavenging in oxidative tissue injury can be a valuable tool in an efforts to increase antioxidant efficacy. The production of certain molecules in response to oxidative stress make them a useful target for the delivery of antioxidants. Lee et al, developed an antioxidant nanoparticle insensitive to H2O2, a common ROS produced during oxidative stress conditions66. This copolyoxalate containing vanillyl alcohol (PVAX) particle is structured with peroxalate ester linkages that degrade upon reaction with H2O2. This causes the release of the antioxidant vanillyl alcohol, which reduces ROS production and thus elicits anti-inflammatory effect. Vanillyl alcohol was shown to down-regulate the expression iNOS and cyclooxygenase-2 (COX-2) to reduce inflammation 67, which was markedly improved with the use of the PVAX nanoparticles. The administration of PVAX to a mouse model of I/R injury effectively suppressed oxidative stress-induced damage in both hind-limb I/R and hepatic I/R injury.
3.2.2. Mitochondria-Directed Nanoparticles
Mitochondria play a pivotal role in the production of ROS in living systems. A leakage from the electron transport chain may cause an aberrant superoxide anion production in mitochondria, condition termed as ‘mitochondrial disease’, which has been closely linked to diseases such as type 2 diabetes and AD68. Therefore, targeting mitochondria for the delivery of antioxidants can potentially regulate ROS generation from this cellular source to reduce ROS damage and improve overall cellular functions. In this regard, triphenylphosphonium (TPP) is a molecule known to readily cross cell membranes and accumulate in mitochondria of cells due to its lipophilic cation. Marrache et al, conjugated PLGA-b-poly(ethylene glycol) (PEG) nanoparticles with TPP to allow enhanced protection and improved site-directed delivery of mitochondria-targeting chemotherapeutics69. The researchers loaded the PLGA-b-PEG-TPP nanoparticles with curcumin, a known antioxidant of therapeutic value in AD, to deliver it more effectively to cultured human neuroblastoma cells for enhanced neuroprotection against Aβ than free curcumin. The use of mitochondrial-directed nanoparticles provides a novel approach for site-specific delivery of ROS scavengers to blunt chronic oxidative pathologies by reducing the risk-to-benefit ratio.
3.2.3. pH-Sensitive Nanoparticles
Nitroxyls such as TEMPO are stable radicals that scavenge free radicals to form two non-radical species. They also partially mimic SOD, due to their ability to self-regenerate under oxidative conditions. The in vitro success of nitroxyls could not be replicated in a similar fashion in vivo due to their hypotension inducing effect, which can be detrimental in cardiovascular or cerebrovascular I/R injury, and also due to their susceptibility to reduction by endogenous circulating molecules resulting in deactivation. Marushima et al, have developed a micelle nanoparticle with encapsulated 4-amino-TEMPO that offers in vivo protection to the nitroxyl molecules70. The study showed that the radical containing nanoparticle (RNP) significantly decreased infarct size in a rat model of acute cerebrovascular I/R injury over saline control and free TEMPOL molecules. Interestingly, there was no observed effect on blood pressure by the RNP molecules in contrast to the effect of free TEMPOL that produced a drop in blood pressure. In addition, the RNP molecules showed an in vivo half-life 60-times longer than TEMPOL.
At the site of ischemia, a large reduction in tissue pH occurs, which returns to the normal levels upon reperfusion, a phenomenon commonly referred to as the ‘paradox of reperfusion injury’71. Recent exciting development in the field of nanomedicine has yielded pH-sensitive nanoparticles capable of releasing their contents in response to a drop in pH levels. The micelle RNPs used are sensitive to pH, and are reported to form polymers under mildly acidic conditions to allow leakage of the TEMPO molecules to the ischemic area. This approach was used to design a pH-sensitive ‘redox polymer’ that is persistent enough to be delivered to the brain after oral administration for the treatment of chronic neurodegenerative disease72. The RNP micelle is broken apart in the low pH of the stomach, and polymer molecules with covalently-linked TEMPOL are absorbed. Alternatively, the pH-sensitivity of the polymer was removed to prohibit disruption of the micelle, and to promote accumulation in the colon for the treatment of colitis, a chronic inflammatory condition of the colon73. Overall, the protection and target specificity offered by the micelle encapsulation of the RNPs show great promise for their application as antioxidant therapies for oxidative-stress mediated disease.
Similar to the paradox of reperfusion injury, a drop in pH occurs at the site of bacterial infection74. This characteristic was therapeutically exploited by Radovic-Moreno et al, to develop a nanoparticle capable of ‘charge-switching’ at the site of infections due to a change in pH from 7.4 to 6.075. Structurally, the shell of PLGA nanoparticle was conjugated with poly-L-histidine to form a co-polymer, which was subsequently loaded with an antibiotic to be released at the target site. The nanoparticles were found to selectively bind to gram-negative bacteria due to the positive charge of the bacterial cell wall caused by the low pH, thus allowing the release of antibiotic into the bacterial cell. Using a pH-sensitive approach could facilitate higher accumulation of antioxidants in the cells during reperfusion, a period of significant ROS generation, as opposed to the post-reperfusion period in this process.
4.1. Future Directions / Conclusions
The summarized multi-faceted approaches to the delivery of antioxidants suggest the potential application of nanomedicine-based therapies hold significant advancement over conventional therapies. Due to the ubiquitous production of ROS in living systems, protection of antioxidants and their targeted delivery to the site of oxidative stress can greatly enhance their efficacy. For instance, the possibility of conjugating to the surfaces of nanoparticles to allow sensitivity to the local environment, and thus target specificity, as well as protection of their cargo while in circulation provide great promise and incremental benefit in applying such antioxidant therapies. Catalytic antioxidants like ceria, platinum, and recombinant SOD, as well as partially catalytic antioxidants like nitroxyls may loaded into nanoparticles to afford protection until they reach sites of oxidative stress. Additionally, advances in nanoparticle surface functionalization such as pH- and H2O2-sensitive polymers, organelle-directed molecules, and HIV TAT-conjugation may yield increased target-specificity for their antioxidant contents, a quality that is not seen in endogenous, natural or current clinical antioxidants. Taken together, combinations of antioxidant and surface functionalization can be tailored for specific disease processes and enhanced therapeutic gain. Some suggested antioxidants and recent nanoparticle advances as applied to ROS-mediated pathologies are listed in Table 1. For example, the catalytic ceria, platinum and SOD scavengers may be applied to cardiovascular and cerebrovascular I/R injury to down-regulate acute oxidative stress, while TEMPO and nitrones may provide radical scavenging capabilities along with NO donating properties. These antioxidants may be loaded onto pH- or H2O2-sensitive nanoparticles or mitochondrial-directed nanoparticles to yield greater target specificity, and PLGA coating can ensure BBB permeability. Rheumatoid arthritis and Osteoarthritis are characterized by chronic oxidative and nitrosative stress as well as inflammation, which may potentially be ameliorated by EGCG or nitrones for their scavenging and anti-inflammatory properties, or ceria for their ONOO− diminishing capabilities. Similarly, AD is characterized by chronic oxidative stress, but presents the obstacle of BBB permeability. Encapsulation by PLGA with H2O2-sensitive conjugation can provide permeability and specificity for the antioxidants, while NAC as free radical scavenger can chelate metal ions to prevent Fenton reactions. However, rigorous research is warranted in this area to further validate these promising therapeutic approaches that capitalize the nanotechnology platform for better treatment options and newer generation of drugs.
Table 1.
Selected ROS-Mediated Pathologies with Respective Applicable Antioxidants and Nanomedicine Advances
| Pathology | Antioxidant / Scavenger | NP / Conjugation |
|---|---|---|
Cardiovascular/ Cerebrovascular I/R
|
SOD Platinum NPs Ceria TEMPO Nitrone |
PLGA TPP Poly-L-His / Micelle Peroxylate ester |
RA / Osteoarthritis
|
EGCG Vanillyl Alcohol Ceria Nitrone |
PLGA TAT |
AD
|
NAC TEMPO Nitrone |
PLGA Peroxylate ester |
Acknowledgments
This study was supported by the NIH grant AR063104 (S.A.), The Arthritis Foundation Innovative Research Grant (S.A.), and the Start-up funds from Washington State University.
Footnotes
Conflict of Interest. The authors declare no conflict of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Miller AA, Drummond GR, Sobey CG. Reactive oxygen species in the cerebral circulation: are they all bad? Antioxid Redox Signal. 2006;8:1113–1120. doi: 10.1089/ars.2006.8.1113. [DOI] [PubMed] [Google Scholar]
- 2.Valko M, Leibfritz D, Moncol J, Cronin MT, Mazur M, Telser J. Free radicals and antioxidants in normal physiological functions and human disease. Int J Biochem Cell Biol. 2007;39:44–84. doi: 10.1016/j.biocel.2006.07.001. [DOI] [PubMed] [Google Scholar]
- 3.Tsutsui H, Kinugawa S, Matsushima S. Mitochondrial oxidative stress and dysfunction in myocardial remodelling. Cardiovasc Res. 2009;81:449–456. doi: 10.1093/cvr/cvn280. [DOI] [PubMed] [Google Scholar]
- 4.Zweier JL, Talukder MA. The role of oxidants and free radicals in reperfusion injury. Cardiovasc Res. 2006;70:181–190. doi: 10.1016/j.cardiores.2006.02.025. [DOI] [PubMed] [Google Scholar]
- 5.Sayre LM, Perry G, Smith MA. Oxidative stress and neurotoxicity. Chem Res Toxicol. 2008;21:172–188. doi: 10.1021/tx700210j. [DOI] [PubMed] [Google Scholar]
- 6.Winyard PG, et al. Measurement and meaning of markers of reactive species of oxygen, nitrogen and sulfur in healthy human subjects and patients with inflammatory joint disease. Biochem Soc Trans. 2011;39:1226–1232. doi: 10.1042/BST0391226. [DOI] [PubMed] [Google Scholar]
- 7.Tangvarasittichai S. Oxidative stress, insulin resistance, dyslipidemia and type 2 diabetes mellitus. World J Diabetes. 2015;6:456–480. doi: 10.4239/wjd.v6.i3.456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Benz CC, Yau C. Ageing, oxidative stress and cancer: paradigms in parallax. Nat Rev Cancer. 2008;8:875–879. doi: 10.1038/nrc2522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gorrini C, Harris IS, Mak TW. Modulation of oxidative stress as an anticancer strategy. Nat Rev Drug Discov. 2013;12:931–947. doi: 10.1038/nrd4002. [DOI] [PubMed] [Google Scholar]
- 10.Pattison DJ, Winyard PG. Dietary antioxidants in inflammatory arthritis: do they have any role in etiology or therapy? Nat Clin Pract Rheumatol. 2008;4:590–596. doi: 10.1038/ncprheum0920. [DOI] [PubMed] [Google Scholar]
- 11.Villamena FA, Das A, Nash KM. Potential implication of the chemical properties and bioactivity of nitrone spin traps for therapeutics. Future Med Chem. 2012;4:1171–1207. doi: 10.4155/fmc.12.74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kukreja RC, Hess ML. The oxygen free radical system: from equations through membrane-protein interactions to cardiovascular injury and protection. Cardiovasc Res. 1992;26:641–655. doi: 10.1093/cvr/26.7.641. [DOI] [PubMed] [Google Scholar]
- 13.Khan SA, et al. Neuronal nitric oxide synthase negatively regulates xanthine oxidoreductase inhibition of cardiac excitation-contraction coupling. Proc Natl Acad Sci U S A. 2004;101:15944–15948. doi: 10.1073/pnas.0404136101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rosini M, Simoni E, Milelli A, Minarini A, Melchiorre C. Oxidative stress in Alzheimer’s disease: are we connecting the dots? J Med Chem. 2013 doi: 10.1021/jm400970m. [DOI] [PubMed] [Google Scholar]
- 15.Harrison R. Structure and function of xanthine oxidoreductase: where are we now? Free Radic Biol Med. 2002;33:774–797. doi: 10.1016/s0891-5849(02)00956-5. [DOI] [PubMed] [Google Scholar]
- 16.Lambeth JD. NOX enzymes and the biology of reactive oxygen. Nat Rev Immunol. 2004;4:181–189. doi: 10.1038/nri1312. [DOI] [PubMed] [Google Scholar]
- 17.Winterbourn CC. Reconciling the chemistry and biology of reactive oxygen species. Nat Chem Biol. 2008;4:278–286. doi: 10.1038/nchembio.85. [DOI] [PubMed] [Google Scholar]
- 18.Chen YR, Chen CL, Yeh A, Liu X, Zweier JL. Direct and indirect roles of cytochrome b in the mediation of superoxide generation and NO catabolism by mitochondrial succinate-cytochrome c reductase. J Biol Chem. 2006;281:13159–13168. doi: 10.1074/jbc.M513627200. [DOI] [PubMed] [Google Scholar]
- 19.Edaravone Acute Infarction Study G. Effect of a novel free radical scavenger, edaravone (MCI-186), on acute brain infarction. Randomized, placebo-controlled, double-blind study at multicenters. Cerebrovasc Dis. 2003;15:222–229. doi: 10.1159/000069318. [DOI] [PubMed] [Google Scholar]
- 20.Watanabe T, Yuki S, Egawa M, Nishi H. Protective effects of MCI-186 on cerebral ischemia: possible involvement of free radical scavenging and antioxidant actions. J Pharmacol Exp Ther. 1994;268:1597–1604. [PubMed] [Google Scholar]
- 21.Zhang N, Komine-Kobayashi M, Tanaka R, Liu M, Mizuno Y, Urabe T. Edaravone reduces early accumulation of oxidative products and sequential inflammatory responses after transient focal ischemia in mice brain. Stroke. 2005;36:2220–2225. doi: 10.1161/01.STR.0000182241.07096.06. [DOI] [PubMed] [Google Scholar]
- 22.Yamamoto T, Yuki S, Watanabe T, Mitsuka M, Saito KI, Kogure K. Delayed neuronal death prevented by inhibition of increased hydroxyl radical formation in a transient cerebral ischemia. Brain Res. 1997;762:240–242. doi: 10.1016/s0006-8993(97)00490-3. [DOI] [PubMed] [Google Scholar]
- 23.Miyamoto N, et al. Oxidative stress interferes with white matter renewal after prolonged cerebral hypoperfusion in mice. Stroke. 2013;44:3516–3521. doi: 10.1161/STROKEAHA.113.002813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ueno Y, Zhang N, Miyamoto N, Tanaka R, Hattori N, Urabe T. Edaravone attenuates white matter lesions through endothelial protection in a rat chronic hypoperfusion model. Neuroscience. 2009;162:317–327. doi: 10.1016/j.neuroscience.2009.04.065. [DOI] [PubMed] [Google Scholar]
- 25.Watanabe T, Tahara M, Todo S. The novel antioxidant edaravone: from bench to bedside. Cardiovasc Ther. 2008;26:101–114. doi: 10.1111/j.1527-3466.2008.00041.x. [DOI] [PubMed] [Google Scholar]
- 26.Connell BJ, Saleh MC, Kucukkaya I, Abd-El-Aziz AS, Khan BV, Saleh TM. UPEI-300, a conjugate of lipoic acid and edaravone, mediates neuroprotection in ischemia/reperfusion. Neurosci Lett. 2014;561:151–155. doi: 10.1016/j.neulet.2013.12.060. [DOI] [PubMed] [Google Scholar]
- 27.Benrahmoune M, Therond P, Abedinzadeh Z. The reaction of superoxide radical with N-acetylcysteine. Free Radic Biol Med. 2000;29:775–782. doi: 10.1016/s0891-5849(00)00380-4. [DOI] [PubMed] [Google Scholar]
- 28.Trujillo M, Ferrer-Sueta G, Thomson L, Flohe L, Radi R. Kinetics of peroxiredoxins and their role in the decomposition of peroxynitrite. Subcell Biochem. 2007;44:83–113. doi: 10.1007/978-1-4020-6051-9_5. [DOI] [PubMed] [Google Scholar]
- 29.Sochman J, Vrbska J, Musilova B, Rocek M. Infarct Size Limitation: acute N-acetylcysteine defense (ISLAND trial): preliminary analysis and report after the first 30 patients. Clin Cardiol. 1996;19:94–100. doi: 10.1002/clc.4960190205. [DOI] [PubMed] [Google Scholar]
- 30.Erickson MA, Hansen K, Banks WA. Inflammation-induced dysfunction of the low-density lipoprotein receptor-related protein-1 at the blood-brain barrier: protection by the antioxidant N-acetylcysteine. Brain Behav Immun. 2012;26:1085–1094. doi: 10.1016/j.bbi.2012.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Maples KR, Ma F, Zhang YK. Comparison of the radical trapping ability of PBN, S-PPBN and NXY-059. Free Radic Res. 2001;34:417–426. doi: 10.1080/10715760100300351. [DOI] [PubMed] [Google Scholar]
- 32.Croitoru MD, Ibolya F, Pop MC, Dergez T, Mitroi B, Dogaru MT, Tokes B. Nitrones are able to release nitric oxide in aqueous environment under hydroxyl free radical attack. Nitric Oxide. 2011;25:309–315. doi: 10.1016/j.niox.2011.05.007. [DOI] [PubMed] [Google Scholar]
- 33.Han M, He QP, Yong G, Siesjo BK, Li PA. NXY-059, a nitrone with free radical trapping properties inhibits release of cytochrome c after focal cerebral ischemia. Cell Mol Biol (Noisy-le-grand) 2003;49:1249–1252. [PubMed] [Google Scholar]
- 34.Savitz SI, Fisher M. NXY-059 for the treatment of stroke. N Engl J Med. 2007;357:2198. doi: 10.1056/NEJMc072612. author reply 2198–2199. [DOI] [PubMed] [Google Scholar]
- 35.Tirilazad mesylate in acute ischemic stroke: A systematic review. Tirilazad International Steering Committee. Stroke. 2000;31:2257–2265. doi: 10.1161/01.str.31.9.2257. [DOI] [PubMed] [Google Scholar]
- 36.Zhao R, Masayasu H, Holmgren A. Ebselen: a substrate for human thioredoxin reductase strongly stimulating its hydroperoxide reductase activity and a superfast thioredoxin oxidant. Proc Natl Acad Sci U S A. 2002;99:8579–8584. doi: 10.1073/pnas.122061399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ahmed S. Green tea polyphenol epigallocatechin 3-gallate in arthritis: progress and promise. Arthritis Res Ther. 2010;12:208. doi: 10.1186/ar2982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bjelakovic G, Nikolova D, Gluud LL, Simonetti RG, Gluud C. Mortality in randomized trials of antioxidant supplements for primary and secondary prevention: systematic review and meta-analysis. JAMA. 2007;297:842–857. doi: 10.1001/jama.297.8.842. [DOI] [PubMed] [Google Scholar]
- 39.Kato N, Yanaka K, Hyodo K, Homma K, Nagase S, Nose T. Stable nitroxide Tempol ameliorates brain injury by inhibiting lipid peroxidation in a rat model of transient focal cerebral ischemia. Brain Res. 2003;979:188–193. doi: 10.1016/s0006-8993(03)02918-4. [DOI] [PubMed] [Google Scholar]
- 40.Kutala VK, et al. Reactivity of superoxide anion radical with a perchlorotriphenylmethyl (trityl) radical. J Phys Chem B. 2008;112:158–167. doi: 10.1021/jp076656x. [DOI] [PubMed] [Google Scholar]
- 41.Soule BP, Hyodo F, Matsumoto K, Simone NL, Cook JA, Krishna MC, Mitchell JB. The chemistry and biology of nitroxide compounds. Free Radic Biol Med. 2007;42:1632–1650. doi: 10.1016/j.freeradbiomed.2007.02.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gariboldi MB, Lucchi S, Caserini C, Supino R, Oliva C, Monti E. Antiproliferative effect of the piperidine nitroxide TEMPOL on neoplastic and nonneoplastic mammalian cell lines. Free Radic Biol Med. 1998;24:913–923. doi: 10.1016/s0891-5849(97)00372-9. [DOI] [PubMed] [Google Scholar]
- 43.Chen A, et al. The Role of p21 in Apoptosis, Proliferation, Cell Cycle Arrest, and Antioxidant Activity in UVB-Irradiated Human HaCaT Keratinocytes. Med Sci Monit Basic Res. 2015;21:86–95. doi: 10.12659/MSMBR.893608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Rizzi C, Samouilov A, Kutala VK, Parinandi NL, Zweier JL, Kuppusamy P. Application of a trityl-based radical probe for measuring superoxide. Free Radic Biol Med. 2003;35:1608–1618. doi: 10.1016/j.freeradbiomed.2003.09.014. [DOI] [PubMed] [Google Scholar]
- 45.Dikalov SI, Kirilyuk IA, Voinov M, Grigor’ev IA. EPR detection of cellular and mitochondrial superoxide using cyclic hydroxylamines. Free Radic Res. 2011;45:417–430. doi: 10.3109/10715762.2010.540242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Nash KM, Rockenbauer A, Villamena FA. Reactive nitrogen species reactivities with nitrones: theoretical and experimental studies. Chem Res Toxicol. 2012;25:1581–1597. doi: 10.1021/tx200526y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kim SU, Liu Y, Nash KM, Zweier JL, Rockenbauer A, Villamena FA. Fast reactivity of a cyclic nitrone-calix[4]pyrrole conjugate with superoxide radical anion: theoretical and experimental studies. J Am Chem Soc. 2010;132:17157–17173. doi: 10.1021/ja105198c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Han Y, Tuccio B, Lauricella R, Villamena FA. Improved spin trapping properties by beta-cyclodextrin-cyclic nitrone conjugate. J Org Chem. 2008;73:7108–7117. doi: 10.1021/jo8007176. [DOI] [PubMed] [Google Scholar]
- 49.Ahmed S, Rahman A, Hasnain A, Goldberg VM, Haqqi TM. Phenyl N-tert-butylnitrone down-regulates interleukin-1 beta-stimulated matrix metalloproteinase-13 gene expression in human chondrocytes: suppression of c-Jun NH2-terminal kinase, p38-mitogen-activated protein kinase and activating protein-1. J Pharmacol Exp Ther. 2003;305:981–988. doi: 10.1124/jpet.102.048611. [DOI] [PubMed] [Google Scholar]
- 50.Khan N, et al. Spin traps: in vitro toxicity and stability of radical adducts. Free Radic Biol Med. 2003;34:1473–1481. doi: 10.1016/s0891-5849(03)00182-5. [DOI] [PubMed] [Google Scholar]
- 51.Franci G, Falanga A, Galdiero S, Palomba L, Rai M, Morelli G, Galdiero M. Silver Nanoparticles as Potential Antibacterial Agents. Molecules. 2015;20:8856–8874. doi: 10.3390/molecules20058856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Etheridge ML, Campbell SA, Erdman AG, Haynes CL, Wolf SM, McCullough J. The big picture on nanomedicine: the state of investigational and approved nanomedicine products. Nanomedicine. 2013;9:1–14. doi: 10.1016/j.nano.2012.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Li M, Al-Jamal KT, Kostarelos K, Reineke J. Physiologically based pharmacokinetic modeling of nanoparticles. ACS Nano. 2010;4:6303–6317. doi: 10.1021/nn1018818. [DOI] [PubMed] [Google Scholar]
- 54.Reddy MK, Labhasetwar V. Nanoparticle-mediated delivery of superoxide dismutase to the brain: an effective strategy to reduce ischemia-reperfusion injury. FASEB J. 2009;23:1384–1395. doi: 10.1096/fj.08-116947. [DOI] [PubMed] [Google Scholar]
- 55.Chen YP, et al. A new strategy for intracellular delivery of enzyme using mesoporous silica nanoparticles: superoxide dismutase. J Am Chem Soc. 2013;135:1516–1523. doi: 10.1021/ja3105208. [DOI] [PubMed] [Google Scholar]
- 56.Kajita M, Hikosaka K, Iitsuka M, Kanayama A, Toshima N, Miyamoto Y. Platinum nanoparticle is a useful scavenger of superoxide anion and hydrogen peroxide. Free Radic Res. 2007;41:615–626. doi: 10.1080/10715760601169679. [DOI] [PubMed] [Google Scholar]
- 57.Watanabe A, Kajita M, Kim J, Kanayama A, Takahashi K, Mashino T, Miyamoto Y. In vitro free radical scavenging activity of platinum nanoparticles. Nanotechnology. 2009;20:455105. doi: 10.1088/0957-4484/20/45/455105. [DOI] [PubMed] [Google Scholar]
- 58.Kim J, Shirasawa T, Miyamoto Y. The effect of TAT conjugated platinum nanoparticles on lifespan in a nematode Caenorhabditis elegans model. Biomaterials. 2010;31:5849–5854. doi: 10.1016/j.biomaterials.2010.03.077. [DOI] [PubMed] [Google Scholar]
- 59.Korsvik C, Patil S, Seal S, Self WT. Superoxide dismutase mimetic properties exhibited by vacancy engineered ceria nanoparticles. Chem Commun (Camb) 2007:1056–1058. doi: 10.1039/b615134e. [DOI] [PubMed] [Google Scholar]
- 60.Heckert EG, Karakoti AS, Seal S, Self WT. The role of cerium redox state in the SOD mimetic activity of nanoceria. Biomaterials. 2008;29:2705–2709. doi: 10.1016/j.biomaterials.2008.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Pirmohamed T, et al. Nanoceria exhibit redox state-dependent catalase mimetic activity. Chem Commun (Camb) 2010;46:2736–2738. doi: 10.1039/b922024k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Hirst SM, Karakoti AS, Tyler RD, Sriranganathan N, Seal S, Reilly CM. Anti-inflammatory properties of cerium oxide nanoparticles. Small. 2009;5:2848–2856. doi: 10.1002/smll.200901048. [DOI] [PubMed] [Google Scholar]
- 63.Dowding JM, Seal S, Self WT. Cerium oxide nanoparticles accelerate the decay of peroxynitrite (ONOO) Drug Deliv Transl Res. 2013;3:375–379. doi: 10.1007/s13346-013-0136-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Oberdorster G, Oberdorster E, Oberdorster J. Nanotoxicology: an emerging discipline evolving from studies of ultrafine particles. Environ Health Perspect. 2005;113:823–839. doi: 10.1289/ehp.7339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Celardo I, Traversa E, Ghibelli L. Cerium oxide nanoparticles: a promise for applications in therapy. J Exp Ther Oncol. 2011;9:47–51. [PubMed] [Google Scholar]
- 66.Lee D, et al. H2O2-responsive molecularly engineered polymer nanoparticles as ischemia/reperfusion-targeted nanotherapeutic agents. Sci Rep. 2013;3:2233. doi: 10.1038/srep02233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Wu SL, Chen JC, Li CC, Lo HY, Ho TY, Hsiang CY. Vanillin improves and prevents trinitrobenzene sulfonic acid-induced colitis in mice. J Pharmacol Exp Ther. 2009;330:370–376. doi: 10.1124/jpet.109.152835. [DOI] [PubMed] [Google Scholar]
- 68.Pieczenik SR, Neustadt J. Mitochondrial dysfunction and molecular pathways of disease. Exp Mol Pathol. 2007;83:84–92. doi: 10.1016/j.yexmp.2006.09.008. [DOI] [PubMed] [Google Scholar]
- 69.Marrache S, Dhar S. Engineering of blended nanoparticle platform for delivery of mitochondria-acting therapeutics. Proc Natl Acad Sci U S A. 2012;109:16288–16293. doi: 10.1073/pnas.1210096109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Marushima A, et al. Newly synthesized radical-containing nanoparticles enhance neuroprotection after cerebral ischemia-reperfusion injury. Neurosurgery. 2011;68:1418–1425. doi: 10.1227/NEU.0b013e31820c02d9. discussion 1425–1416. [DOI] [PubMed] [Google Scholar]
- 71.Bond JM, Chacon E, Herman B, Lemasters JJ. Intracellular pH and Ca2+ homeostasis in the pH paradox of reperfusion injury to neonatal rat cardiac myocytes. Am J Physiol. 1993;265:C129–137. doi: 10.1152/ajpcell.1993.265.1.C129. [DOI] [PubMed] [Google Scholar]
- 72.Chonpathompikunlert P, Yoshitomi T, Vong LB, Imaizumi N, Ozaki Y, Nagasaki Y. Recovery of Cognitive Dysfunction via Orally Administered Redox-Polymer Nanotherapeutics in SAMP8 Mice. PLoS One. 2015;10:e0126013. doi: 10.1371/journal.pone.0126013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Vong LB, Tomita T, Yoshitomi T, Matsui H, Nagasaki Y. An orally administered redox nanoparticle that accumulates in the colonic mucosa and reduces colitis in mice. Gastroenterology. 2012;143:1027–1036. e1023. doi: 10.1053/j.gastro.2012.06.043. [DOI] [PubMed] [Google Scholar]
- 74.Fuchs S, Pane-Farre J, Kohler C, Hecker M, Engelmann S. Anaerobic gene expression in Staphylococcus aureus. J Bacteriol. 2007;189:4275–4289. doi: 10.1128/JB.00081-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Radovic-Moreno AF, Lu TK, Puscasu VA, Yoon CJ, Langer R, Farokhzad OC. Surface charge-switching polymeric nanoparticles for bacterial cell wall-targeted delivery of antibiotics. ACS Nano. 2012;6:4279–4287. doi: 10.1021/nn3008383. [DOI] [PMC free article] [PubMed] [Google Scholar]


