Abstract
While all forms of glaucoma are characterized by a specific pattern of retinal ganglion cell death, they are clinically divided into several distinct subclasses, including normal tension glaucoma, primary open angle glaucoma, congenital glaucoma, and secondary glaucoma. For each type of glaucoma there are likely numerous molecular pathways that control susceptibility to the disease. Given this complexity, a single animal model will never precisely model all aspects of all the different types of human glaucoma. Therefore, multiple animal models have been utilized to study glaucoma but more are needed. Because of the powerful genetic tools available to use in the laboratory mouse, it has proven to be a highly useful mammalian system for studying the pathophysiology of human disease. The similarity between human and mouse eyes coupled with the ability to use a combination of advanced cell biological and genetic tools in mice have led to a large increase in the number of studies using mice to model specific glaucoma phenotypes. Over the last decade, numerous new mouse models and genetic tools have emerged, providing important insight into the cell biology and genetics of glaucoma. In this review, we describe available mouse genetic models that can be used to study glaucoma-relevant disease/pathobiology. Furthermore, we discuss how these models have been used to gain insights into ocular hypertension (a major risk factor for glaucoma) and glaucomatous retinal ganglion cell death. Finally, the potential for developing new mouse models and using advanced genetic tools and resources for studying glaucoma are discussed.
Keywords: neuroinflammation, IOP, axonal degeneration, trabecular meshwork, mouse genetics, genomics, neurodegeneration, DBA/2J
1. Introduction
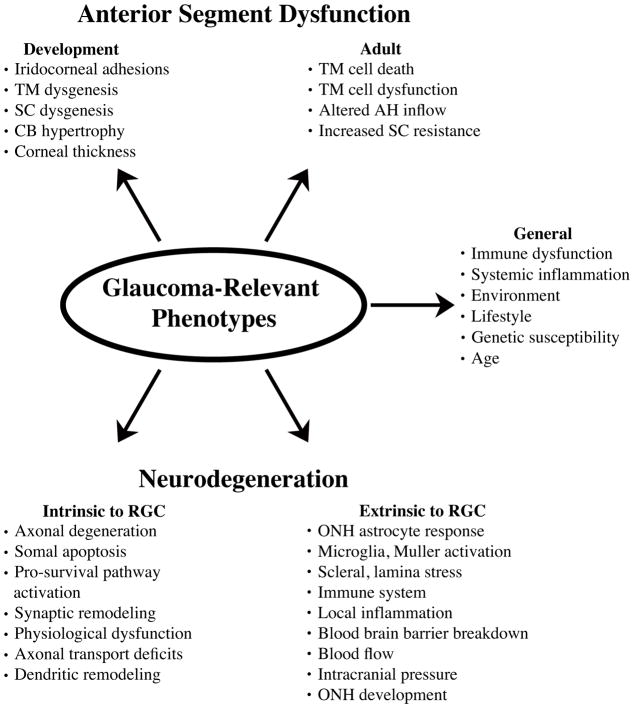
Glaucoma is defined and unified by a distinct pattern of retinal ganglion cell (RGC) death. However, glaucoma is a heterogeneous disorder (often termed ‘glaucomas’) with RGCs likely insulted in multiple ways. Even within a patient, different RGCs may experience different stresses that trigger or contribute to cell loss (Casson et al., 2012). Age, elevated intraocular pressure (IOP; or ocular hypertension), and family history are major risk factors for glaucoma. IOP regulation is in itself complex and in most cases where IOP is a factor in glaucoma, it is not known why it becomes pathologically elevated. Given this complexity, multiple models of glaucoma are required to fully understand the disease. A single animal model can never precisely model all aspects of all human glaucomas, and modeling specific aspects of the cell biology and/or the genetics of human glaucoma requires careful consideration. The choice of the animal model should be made based on the precise glaucoma-relevant phenotype(s) being assessed. With this perspective, mice are a powerful tool to unlock the genetic causes, susceptibility factors, and physiological pathways underlying human glaucoma. The similarity between human and mouse eyes, coupled with the ability to use a combination of advanced cell biological and genetic tools in mice, make the mouse an excellent system for unraveling the molecular pathways that control glaucoma pathophysiology (see Figure 1 and 2 for overview).
Figure 1. Glaucoma-relevant phenotypes that can be modeled and studied in mice.
To study the cell biology of glaucoma, it is helpful to breakdown the disease into the individual events and/or phenotypes that can contribute to the disease. TM, trabecular meshwork; SC, Schlemm’s canal; CB, ciliary body; ONH, optic nerve head; RGC, retinal ganglion cell.
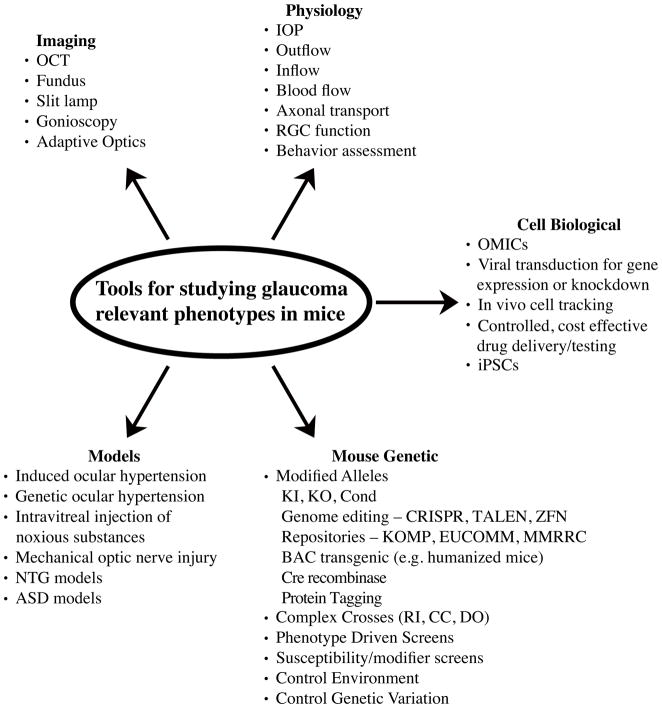
Figure 2. Tools available to study ocular disease in mice.
There are many tools available to researchers using mice to study glaucoma-relevant phenotypes including: various imaging technologies, both advanced technologies which can only be used in model organisms and standard clinical imaging tools; physiological measurements to assess both anterior and posterior function; advanced cell biological analysis that can be used to probe the molecular mechanisms of glaucoma; mouse genetics, which can be used to discover new genes and molecular pathways underlying glaucoma and to critically test predicted pathways involved in glaucomatous pathophysiology; and numerous mouse models with glaucoma-relevant phenotypes that can be used to gain insight into human glaucoma. OCT, optical coherence tomography; IOP, intraocular pressure; RGC, retinal ganglion cell OMICs, genomics, proteomics and metabolomics; iPSCs, induced pluripotent stem cells; NTG, normal tension glaucoma; ASD, anterior segment dysgenesis; KI, knock in; KO, knock out; Cond, conditional allele; CRISPR, clustered regularly interspaced short palindromic repeats; TALEN, transcription activator-like effector nucleases; ZFN, xinc-finger nucleases; KOMP, knock out mouse project; EUCOMM, European Conditional Mouse Mutagenesis Program; MMRRC, Mutant Mouse Regional Resource Center; RI, recombinant inbred; CC, collaborative cross; DO, diversity outbred cross.
Several genes that cause glaucoma-relevant phenotypes have recently been identified in mice. Moreover, mouse models with mutations in known human glaucoma genes have been used to study the genetic underpinnings of glaucoma pathobiology. These models provide insight into (i) the cell biology underlying specific phenotypes of glaucoma, (ii) the physiology of glaucoma-relevant tissues, (iii) the pathophysiological response of cells subjected to glaucoma-relevant insults, and (iv) the molecular genetics of glaucoma-relevant phenotypes and endophenotypes. As there are many in depth reviews about mouse models of glaucoma (e.g. Libby et al., 2005b; Lindsey and Weinreb, 2005; Morrison et al., 2011) and about how mouse genetics can be used to study physiology and pathophysiology (Ermann and Glimcher, 2012; Justice et al., 2011; Schofield et al., 2012; van der Weyden et al., 2011), in this review we discuss the strengths and the utility of using genetic mouse models of glaucoma to gain insights into the cell biology and genetics underlying glaucoma-relevant phenotypes (Section 2), key aspects of anterior segment disease (Section 3) and retinal ganglion cell death (Section 4) that have been elucidated through the use of genetic models. Furthermore, we highlight ways that the mouse can be used to address unanswered questions about glaucoma and how it can be used in the future to help unravel the complexities of human glaucoma.
2. Strengths and utility of mice to model glaucoma-relevant phenotypes
In order to study a disease-relevant phenotype, it is ideal, though not always possible, to have characteristics of the phenotype carefully defined in both humans and the animal model. This is especially critical when studying a heterogeneous disease like glaucoma in which multiple distinct pathological insults are observed in several ocular tissues. Mouse models have in the past both improved our understanding of human disease and have proved to be an invaluable tool for discovering therapeutic targets. Overall, current knowledge of the physiology of the anterior segment, retina, and optic nerve in humans and mice strongly support the utility of the mouse to model key phenotypes associated with glaucoma. However, as our understanding of the key molecular pathogenic events in human glaucoma continues to grow, animal models will need to be refined to include specific aspects of human glaucoma as new information becomes available.
2.1. Glaucoma relevance of the mouse anterior segment
Over the last decade, many studies have found that mouse models can be used to examine glaucoma-relevant aspects of anterior segment physiology. Mouse and human both have similar cyclical IOP patterns (Aihara et al., 2003; Li and Liu, 2008; Liu et al., 2003; Mosaed et al., 2005; Savinova et al., 2001). Also, similar mutations lead to anterior segment disease in both mice and humans (see below). Most importantly, in terms of IOP regulation, key physiological parameters are similar between mice and humans, including: similar mechanisms regulating aqueous humor production and outflow; similar response to IOP lowering drugs; and no detectable washout rate in either species (e.g. Aihara et al., 2003; Boussommier-Calleja et al., 2012; Lei et al., 2011; Lindsey and Weinreb, 2002). Thus, physiological measurements show that the mouse is a good model system for studying glaucoma-relevant anterior segment physiology and pathophysiology. The mouse does however, have a proportionately larger lens than humans and correspondingly, the shape of the anterior chamber is different in mice (Schmucker and Schaeffel, 2004). With respect to glaucoma, consequences of this variation have not been studied in depth, but would likely change the presentation of clinical features associated with secondary forms of glaucoma, including iris-lens rubbing (pigment dispersion syndrome) and lenticular accumulations of exfoliative material (exfoliation syndrome). Overall, the basic physiology and anatomy of glaucoma relevant structures in the mouse and human anterior segment are very similar.
2.2. Glaucoma relevance of the mouse retina and optic nerve head
The utility of the mouse as a model for studying ocular hypertension-induced RGC degeneration has been questioned in the past primarily because of differences in the morphology and structure of the optic nerve head. The mouse optic nerve head does not have collagen beams and instead contains pores created by optic nerve head astrocytes (this has been termed a glial lamina; Howell et al., 2007). Because the optic nerve head is a key site in ocular hypertensive glaucoma, it is reasonable to assume that differences in optic nerve head morphology may limit the utility of the mouse in modeling ocular hypertension-induced optic neuropathy. However, over the last decade, findings from several groups have validated the usefulness of the mouse as a model for ocular hypertension-induced RGC death. Mouse models exhibit many hallmarks thought to characterize human glaucoma, including segmented axon loss, a defined insult to RGC axons at the optic nerve head, early loss of axonal transport, ocular hypertension-induced abnormalities in RGC physiology, specific loss of RGCs, and reduced glaucomatous damage by interventions that lower IOP (Buckingham et al., 2008; Filippopoulos et al., 2006; Howell et al., 2007; Jakobs et al., 2005; Nagaraju et al., 2007; Schlamp et al., 2006; Schuettauf et al., 2002; Wong and Brown, 2013). Furthermore, many molecular pathways shown to be important in human glaucoma through gene expression studies have also been reported in mouse models of ocular hypertension-induced RGC death. Thus, ocular hypertensive mouse models recapitulate key aspects of human glaucoma.
3. Glaucoma-relevant anterior segment disease
Over the last several decades, technical advances have greatly increased the usefulness of the mouse for studying glaucoma-relevant anterior segment physiology. Intraocular pressure and aqueous outflow can now be reliably measured in the mouse (Aihara et al., 2003; Avila et al., 2001; Boussommier-Calleja and Overby, 2013; Cohan and Bohr, 2001; John et al., 1997; Lei et al., 2011; Wang et al., 2005). Also, many imaging technologies used to assess human anterior segment pathology are used in mice, including OCT imaging (Li et al., 2014; Nair et al., 2011), and gonioscopy (Smith et al., 2002; Trantow et al., 2009). Advanced mouse genetic and cell biological tools are also being employed to study anterior segment biology. For example, several groups recently used genetically altered mice (conditional alleles and reporter alleles) to study Schlemm’s canal development (Aspelund et al., 2014; Kizhatil et al., 2014; Park et al., 2014; Thomson et al., 2014). Mouse strains also differ in IOP level, outflow rate, susceptibility to steroid-induced ocular hypertension, and susceptibility to ocular hypertension due to other anterior segment diseases. Standard mouse genetic tools can be used to identify the genes responsible for the differences between mouse strains regarding these important glaucoma-relevant phenotypes (Anderson et al., 2006; Boussommier-Calleja and Overby, 2013; John et al., 1997; Savinova et al., 2001; Whitlock et al., 2010). Identifying these genes will likely provide fundamental insight into IOP regulation, opening up new areas of investigation and models for studying how IOP becomes dysregulated. Below, we describe currently available mouse genetic models that are being used by many groups to understand anterior segment development and disease.
3.1 Ocular Hypertensive Mice
3.1.1 DBA/2J mice
DBA/2J mice develop a pigment dispersing iris disease that leads to elevated IOP and RGC loss (Chang et al., 1999; John et al., 1998; Libby et al., 2005a). Mutations in two genes, Gpnmb and Tyrp1 (the mutant alleles are GpnmbR150X and Tyrp1b respectively) cause an iris disease that has two main components: iris pigment dispersion and iris stromal atrophy. The iris pigment dispersion phenotype resembles pigment dispersion syndrome (PDS) in humans (Anderson et al., 2002). The iris disease and subsequent IOP elevation can be mitigated by reconstituting the bone marrow of DBA/2J mice with bone marrow from DBA/2J-Gpnmb+ mice (Anderson et al., 2008; Mo et al., 2003). Since bone marrow contains progenitor cells of the immune system, it has been suggested that the GpnmbR150X bone marrow-derived cell lineages of standard DBA/2J mice contribute to an abnormal inflammatory response resulting in pigment dispersion and subsequent elevation in IOP (Anderson et al., 2008; Mo et al., 2003). Adaptive immune components (T and B cell mediated) have been ruled out in the propagation of the iris disease (Anderson et al., 2008) and it is likely driven by innate immune responses (Nair et al., 2014). Together, these data highlight the need to further study how ocular immune processes and melanosome function can contribute to glaucoma-relevant anterior segment disease.
DBA/2J mice are also an important resource to study the genetics of ocular hypertension. C57BL/6J mice that are homozygous for the GpnmbR150X and Tyrp1b mutations develop a DBA/2J-like iris disease with severe dispersion of iris pigment (Anderson et al., 2006). However, these mice are much less likely to develop ocular hypertension. These results indicate that DBA/2J mice have a greater susceptibility to IOP elevation than C57BL/6J mice. Thus, genetic factors could be key mediators for how the iridocorneal angle responds to dispersed pigment in pigmentary glaucoma and other ocular hypertensive diseases. The differential response of C57BL/6J and DBA/2J mice to pigment dispersion is similar to the differing responses of people with PDS, where only some individuals with PDS develop ocular hypertension (Siddiqui et al., 2003). The discovery of molecules or genetic pathways that segregate between DBA/2J and C57BL/6J may hold clues about human glaucoma pathophysiology and the genetic susceptibility factors that cause/modulate glaucoma in heterogeneous populations.
3.1.2 Myocilin mutant mice
Mutations in the myocilin gene (MYOC) cause ocular hypertension and glaucoma (Menaa et al., 2011; Stone et al., 1997). In humans and mice, MYOC is expressed in numerous ocular tissues, most notably in trabecular meshwork (TM) cells (for a review see; Resch and Fautsch, 2009). In cultured human TM cells, MYOC is induced by glucocorticoids, oxidative stress, cell stress signaling pathways, and potentially other unidentified factors in the aqueous humor (Fautsch et al., 2005; Polansky et al., 1997; Tamm et al., 1999). TM cells normally secrete MYOC, however mutations in MYOC can prevent secretion of mutant and wild type MYOC (Jacobson et al., 2001). These data and other studies raised important questions about whether myocilin had a normal function in IOP regulation and how its level and/or location of expression contribute to ocular hypertension. In a series of experiments, several groups have used mouse genetics to gain a better understanding of the normal and pathological functions of myocilin.
The first generation of genetic models manipulated levels of the mouse Myoc gene. Null mutations in mice do not result in ocular hypertension, RGC loss, or other gross ocular abnormalities (Kim et al., 2001). There is a similar absence of glaucoma-related phenotypes observed when Myoc is overexpressed in the mouse at levels comparable to the elevated MYOC expression seen in humans with steroid-induced IOP elevation (Gould et al., 2004a). These results helped explain genetic studies of glaucoma in human patients; in humans, a likely null mutation has been identified that is not associated with glaucoma (Pang et al., 2002). Conversely, >90% of glaucoma-related mutations are in a well-conserved functional domain of MYOC, the olfactamedin domain, and likely affect MYOC structure (Resch and Fautsch, 2009). The results gained from genetic models indicated that MYOC does not have a significant function in normal IOP regulation and that mutant MYOC proteins with abnormal function (gain of function) were likely causing ocular hypertension.
To determine how mutant MYOC leads to glaucoma, several groups began testing disease-specific alleles in mice. Gould et al. (Gould et al., 2006) and Senatorov et al. (Senatorov et al., 2006) focused on the Y437H allele, a mutation that causes a severe form of juvenile open angle glaucoma. Results varied, but generally, expression of mouse Myoc with a mutation orthologous to Y437H affected secretion of both mutant and wild type Myoc without causing either substantially elevated IOP or RGC loss in young or aged mice across multiple mouse strains. Expression of human MYOC and MYOCY437H in the mouse lens also did not cause IOP elevation or RGC loss (Zillig et al., 2005). An important finding was made when Shepard et al. used an adenovirus to express human MYOCY437H in the iridocorneal angle and saw those eyes develop high IOP (Shepard et al., 2007). Human myocilin contains a targeting signal for peroxisomes that does not exist in mouse myocilin (Shepard et al., 2007). This domain appears to be critical for mutant myocilin-induced toxicity in trabecular meshwork cells. Subsequently, three groups have reported IOP elevation using different strategies for expressing human MYOCY437H in the mouse eye (McDowell et al., 2012; Zhou et al., 2008; Zode et al., 2011). Most recently, transgenic mice were generated that express MYOCY437H under control of a CMV promoter in the iridocorneal angle and sclera (Zode et al., 2011). Tg-MYOCY437H mice show reduced secretion of MYOC into the aqueous humor, elevated IOP after 3 months of age and RGC loss after 4 months of age. Other MYOC mutations are suggested to have alternative pathogenic mechanisms (McKay et al., 2013). Expressing these other human disease-associated alleles in mice (humanized mice) will likely provide an important method by which to distinguish the pathogenic mechanism and test whether therapies need to be designed based on specific variants. As an excellent example of this, Zode and colleagues showed that treating mice with a drug that reduces ER stress significantly lessened IOP elevation in the Tg-MYOCY437H (Zode et al., 2011). Overall, humanized mice represent a valuable to tool to study the genetics, function, and treatment of myocilin disease variants in ocular hypertension and glaucoma.
3.1.3 Other mouse genetic models for studying IOP
Mouse anterior segment physiology is similar to humans and there is now a full range of tools to interrogate ocular fluid dynamics in the mouse (discussed above). As a result, there are a growing number of reports using both forward and reverse genetic approaches to study anterior segment physiology and pathophysiology in mice. Reverse genetic approaches, specifically altering the genome, are being used to test specific hypotheses about a molecule’s role in IOP regulation (Chatterjee et al., 2014; Haddadin et al., 2009; Haddadin et al., 2012; Luo et al., 2014; Zhang et al., 2009). While these mutants generally have only a small increase or decrease in IOP, they do provide valuable information about the molecules and pathways contributing to IOP homeostasis. Furthermore, this approach is likely to become commonplace as more and more mouse mutants with targeted alterations in specific genes are made available. A growing number of mutant mouse alleles are being generated by individual investigators and large consortiums (Schofield et al., 2012; van der Weyden et al., 2011). Liu and colleagues have made several valuable Cre lines that use the Myocilin promoter to control gene expression (Liu et al., 2011). Furthermore, it is important to develop Cre lines specific for each cell type in the anterior segment that contributes to IOP regulation and to make these Cre lines inducible, so that they can be driven at specific developmental and adult time points.
Forward genetic approaches in the mouse, identifying genes based on a specific phenotype, have generated new mouse models as well. One example is the generation of new mutants from an N-ethyl-N-nitrosourea (ENU) based mutagenesis screen that was designed to find mouse mutants with glaucoma-relevant anterior segment pathology. This approach was used to generate a mutant with cardinal features of human angle closure glaucoma (ACG) (Nair et al., 2011). In ACG, the iris is pushed against the trabecular meshwork (TM), thus obstructing aqueous humor outflow and subsequently leading to elevated IOP (Quigley, 2009). Multiple anatomical and physiological factors are thought to participate in the pathogenesis of ACG. Similar to patients with ACG, the mutant mice have features that predispose them to angle closure and high IOP, including reduced ocular axial length, a relatively large lens, and a shallow angle (Lowe, 1970; Nair et al., 2011; Quigley, 2009). In these mice, the causal mutation was identified in the serine protease, Prss56 (Nair et al., 2011). Importantly, mutations in PRSS56 contribute to posterior microphthalmos or nanophthalmos in humans, a condition with severe reduction in ocular axial length (Gal et al., 2011; Nair et al., 2011; Orr et al., 2011). As a result of this severe anatomical predisposition, some individuals go on to develop ACG (Gal et al., 2011; Nair et al., 2011; Orr et al., 2011). In addition, rare variants of PRSS56 have been reported to be associated with primary angle closure glaucoma (PACG; Jiang et al., 2013). Future studies utilizing the mutant Prss56 mouse model will provide a better understanding of ACG relevant phenotypes at a mechanistic level. Another mutant strain from an ENU-based mutagenesis screen was found to develop cataracts and high IOP as a result of a mutation in Tdrd7, a component of RNA granules (Lachke et al., 2011). Interestingly, despite developing elevated IOP, Tdrd7 mutants do not exhibit any gross morphological abnormalities in the ocular drainage tissues and their angles are primarily open. It was hypothesized that IOP elevation in Tdrd7 mutant mice develops as a consequence of abnormalities in the protective stress response in the drainage tissues, in particular, oxidative stress. The Tdrd7 mutants provide a model to study the role of RNA granules in drainage tissue-specific stress responses.
3.2 Developmental glaucoma models
Primary Congenital Glaucoma (PCG) is a less common, though severe subtype of glaucoma resulting from abnormal development of the anterior segment. Accordingly, PCG falls under the category of Anterior Segment Dysgenesis (ASD), a group of diseases characterized by the improper development of anterior ocular tissues. Aberrant morphogenesis of glaucoma-relevant anterior segment tissues—e.g. trabecular meshwork and Schlemm’s canal—often leads to elevated IOP and ultimately, RGC death and vision loss (Figure 1). The cellular and molecular mechanisms underlying development of the anterior segment remain largely undefined. For instance, for the majority of cell types in the iridocorneal angle, including the trabecular meshwork, we do not yet understand the factors required for cell determination and commitment. Similarly, for many of the genes that cause ASD, we do not know when and where these genes function in normal development, or how mutations in them lead to ASD. Mice are ideally suited for asking basic developmental biological questions and assessing how mutations affect tissue morphogenesis. Due to the availability of many ASD-relevant mutants, the ability to genetically manipulate the genome in a developmentally controlled manner, and the numerous cell biological tools available in the mouse to study development, mice have been used extensively over the last several decades to study the molecular genetics of ASD (recently reviewed in depth; Ito and Walter, 2014; Reis and Semina, 2011).
Fundamental insight into the pathogenicity of human disease variants in ASD has been achieved through a combination of human genetics and the use of mouse models (Gould and John, 2002; Gould et al., 2004b; Liu and Allingham, 2011). Mice and humans with orthologous mutations develop similar phenotypes in many cases. Cloning of ASD mutations in mice has led to identifying ASD genes in humans (Kuo et al., 2012; McKeone et al., 2011). Additionally, many phenotypes characteristic of ASD—ciliary body and trabecular meshwork hypoplasia, peripheral iridocorneal adhesion, buphthalmos, and elevated IOP—have been found in both spontaneous and genetically modified mouse mutants (Chang et al., 2001; Gould et al., 2007; Kroeber et al., 2010; Libby et al., 2003; Mao et al., 2011; Sarode et al., 2014; Smith et al., 2000; Sowden, 2007; Weng et al., 2008; Zhou et al., 2013). Mutations in transcription factors (PITX2, FOXC1, FOXF2, LMX1B, PAX6) are responsible for numerous cases of ASD and PCG (Gould et al., 2004b) and mouse mutants have been used to study the cell biology of these genes in the eye. Recent work with mice carrying mutations in various cell-cell signaling components is beginning to give us insight into the inductive events that drive anterior segment development. For instance, BMP4, a secreted molecule belonging to the TGFβ superfamily, is believed to be necessary for proper formation of both the ciliary body and the iridocorneal angle (Chang et al., 2001; Zhou et al., 2013). Additionally, mutations in several genes influencing the extracellular matrix (eg. CollagenIVa1, CollagenXVIIIa1, and Peroxidasin) have been shown to lead to ASD-relevant phenotypes in mice (Gould et al., 2007; Mao et al., 2011; Yan et al., 2014; Ylikarppa et al., 2003). Recently, mouse studies demonstrated that Schlemm’s canal develops from existing blood vessels by a newly discovered process of vascular development with features of vascuologeneiss, angiogeneisis and lymphangiogenesis (Aspelund et al., 2014; Kizhatil et al., 2014; Park et al., 2014; Thomson et al., 2014). Various vascular receptors are expressed during this process including KDR (VEGFR2) and TEK (TIE2), with KDR function being essential for normal development of Schlemm’s canal (Kizhatil et al., 2014). These receptors and their downstream proteins are excellent candidates to contribute to PCG and other developmental glaucomas (Kizhatil et al., 2014; Thomson et al., 2014). Together, these results implicate many different developmental events and signaling pathways in the morphogenesis of anterior segment structures. On an even more basic level, these studies and others have identified factors involved in patterning the mammalian eye as well as the general time line of events in anterior segment morphogenesis (Cvekl and Tamm, 2004; Heavner and Pevny, 2012; Ito and Walter, 2014; Napier and Kidson, 2007; Smith et al., 2001).
It is important to note that having any form of ASD puts children at a 50% increased risk for developing glaucoma later in life (Sowden, 2007), however it is unknown precisely why this occurs. It is also possible that clinically undetectable abnormalities in the anterior segment in early childhood could significantly contribute to IOP elevation later in life. Furthermore, clinical severity of ASD does not appear to correlate with the development of glaucoma, suggesting that clinically undetectable changes are critical for the development of ocular hypertension (Ito and Walter, 2014). ASD appears to be highly susceptible to genetic modifiers in both humans and mice (Gould et al., 2007; Gould et al., 2004b; Ito and Walter, 2014; Libby et al., 2003). The ability to perform precise histological analysis and measure glaucoma-relevant physiological parameters (e.g. IOP and outflow measurements) make the mouse ideally suited to study how ASD leads to ocular hypertension and to identify the morphological and genetic factors controlling this progression.
4. Glaucoma-relevant RGC death in mice
Standard genetic tools, such as genetically modified mice, have been used extensively to study glaucoma-relevant RGC loss. Also, other methods have been used in mice to test various hypotheses of glaucoma-relevant RGC death and the effectiveness of particular treatments at stopping RGC loss. Such methods include using viruses to deliver or knockdown genes specifically in RGCs and using microbeads or stem cells to deliver therapeutic substances (reviewed in Harvey et al., 2009; Lebrun-Julien and Di Polo, 2008). Acute injuries in mice, such as mechanical optic nerve crush or intravitreally injecting substances hypothesized to injure RGCs in glaucoma have provided important cell biological information about RGC death; though, it is likely that these acute perturbations do not model key aspects of glaucomatous neurodegeneration. Genetic models offer important advantages over other model systems for studying glaucoma-relevant RGC death as they often show a greater variability and slower progression of IOP elevation (more akin to many human glaucomas), in addition to providing the ability to study the mutation in the context of aging and environmental stressors. Fortunately, there are an increasing number of genetic mouse models being used to gain insight into how RGCs die in both ocular hypertensive and normotensive glaucoma.
4.1 Ocular Hypertensive Mice
4.1.1 DBA/2J mice
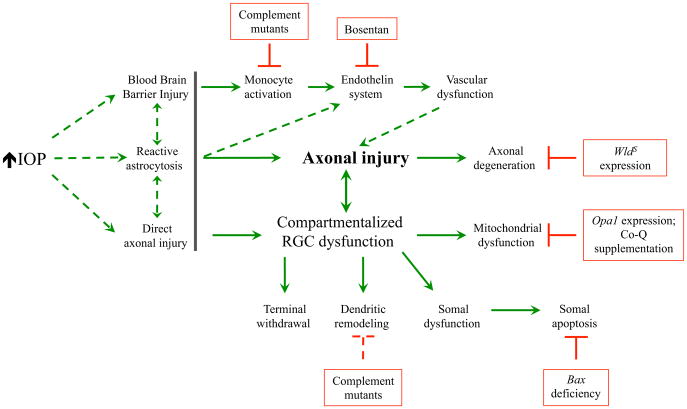
In DBA/2J eyes, ocular hypertension is age-related and asynchronous. IOP increases in the majority of eyes from 8 to 12 months of age (Libby et al., 2005a). By 10 months of age, approximately half of DBA/2J eyes have significant optic nerve damage and by 12 months, the majority of eyes have severe glaucomatous damage (Libby et al., 2005a). Reducing IOP in DBA/2J mice pharmaceutically, genetically, or through surgical therapy prevents significant RGC damage (Anderson et al., 2006; Matsubara et al., 2006; Schuettauf et al., 2002; Wong and Brown, 2012, 2013). Thus, RGC loss in DBA/2J mice is dependent upon ocular hypertension. DBA/2J mice have been used to study many cell biological questions relevant to ocular hypertension-induced optic nerve degeneration and RGC death. Below, we describe a few examples where DBA/2J mice were used in combination with mouse genetic tools or pharmaceutical interventions to study glaucoma-relevant RGC death and optic nerve injury (see Figure 3 for examples of genetic or pharmaceutical manipulation that have been used in DBA/2J mice to dissect the pathological processes involved in ocular hypertension-induced RGC death).
Figure 3. Ocular hypertension leads to numerous events in distinct subcompartments of RGCs that contribute to RGC loss in DBA/2J mice.
DBA/2J mice have been used extensively to study the pathogenesis of ocular hypertension-induced RGC death. These studies have led to the idea that different cellular compartments of RGCs respond to ocular hypertension and that targeting these events can lessen RGC loss. This diagram assumes a critical early injury occurs to RGCs axons (axonal injury) in the optic nerve head. However, it is unclear (dashed lines) whether the initial driving event is an insult directly to the axons or through extrinsic means (the gray line is used to separate these potential early events to the later important event of axonal injury). Red boxes represent either pharmaceutical or genetic interventions that blocked a specific event in the degeneration cascade and have lessened RGC loss in DBA/2J mice.
There is ample evidence that RGCs die by apoptosis in human glaucoma and in various mouse models of glaucoma (Guo et al., 2005; Nickells, 1996, 1999; Nickells et al., 2008; Quigley et al., 1995; Whitmore et al., 2005). In DBA/2J mice, several components of the Bcl2 family (major regulators of apoptotic death) are expressed in RGCs during the window of RGC death (Harder et al., 2012; Libby et al., 2005c). Deficiency in the proapoptotic Bcl2 family member Bax, prevented RGC somal loss in DBA/2J mice but did not prevent axonal degeneration (Libby et al., 2005c). Interestingly, DBA/2J mice expressing a mutant form of FasL had an earlier onset and more severe loss of RGCs. These data suggest that modulating FasL signaling may reduce RGC apoptosis in glaucoma and indicate that the extrinsic apoptotic pathway is capable of activating ocular hypertension-induced RGC death (Gregory et al., 2011). During the window of RGC death in DBA/2J glaucoma, several pathological insults have been observed including axonal injury at the glial lamina, structural and functional alterations in RGCs, mitochondrial dysfunction, glial dysfunction, neuroinflammation and vascular dysfunction. Along with dissociating the insults that accompany RGC degeneration from the insults that trigger RGC degeneration, determining how these cell intrinsic and cell extrinsic events finally converge on BAX activation leading to the execution of RGC death is an area of ongoing investigation.
Anderson, Hendricks, Quigley, and others suggested that axonal injury is an important early event in human glaucoma and in primate models of glaucoma (Anderson and Hendrickson, 1974; Anderson and Hendrickson, 1977; Quigley and Anderson, 1976; Quigley and Addicks, 1980, 1981). In DBA/2J mice, an early site of axonal injury is at the glial lamina (Buckingham et al., 2008; Howell et al., 2007; Jakobs et al., 2005; Schlamp et al., 2006). Consistent with RGC axons being insulted at the glial lamina, in Bax deficient DBA/2J mice, RGC axons survived to the optic nerve head while the axon behind the glial lamina degenerated. Further support for a key role of axonal injury in DBA/2J mice was provided by the finding showing DBA/2J mice that express the WldS allele, a naturally occurring mutation that slows axonal degeneration (Fang et al., 2005; Lyon et al., 1993; Mack et al., 2001; Perry et al., 1992), were significantly protected from both axonal and somal degeneration compared to wild type DBA/2J mice (Howell et al., 2007). Thus, axonal injury response and axonal degeneration appear to be key pathological insults for ocular hypertension-induced RGC death in DBA/2J mice. It will be important to identify the genes that control these events, as they could be therapeutic targets for human glaucoma.
The work in DBA/2J mice complements studies in human glaucoma and in other animal models, showing that early changes occur in multiple compartments of an RGC after an ocular hypertensive insult (reviewed in Almasieh et al., 2012; Morquette and Di Polo, 2008; Whitmore et al., 2005; Yu et al., 2013). Along with axonal stress/injury, numerous studies have also shown early changes in other RGC compartments in response to ocular hypertension, including changes in the soma and dendrites. Crish and colleagues (Crish et al., 2010) showed failure of axonal transport begins in the distal axon and progresses toward the soma in DBA/2J mice. Early RGC dendritic remodeling has also been described in retinas of DBA/2J mice (Stevens et al., 2007; Williams et al., 2013). In a series of papers, Ju, Weinreb and colleagues established the potential importance of mitochondria physiology in regulating RGC loss in DBA/2J mice (Ju et al., 2009; Ju et al., 2010; Ju et al., 2008; Lee et al., 2014). Abnormalities in mitochondrial dynamics correlated with ocular hypertension-induced RGC loss. Importantly, genetically or pharmacologically protecting mitochondrial function also lessened glaucomatous damage in DBA/2J mice. Determining whether the molecular pathways responsible for these structural and functional alterations in distinct RGC compartments interact with each other, and defining which changes are critical for RGC death should be a priority of future research.
Neuroinflammation has been implicated both in human glaucoma (Baltmr et al., 2010; Cheung et al., 2008; Soto and Howell, 2014) and in RGC loss in DBA/2J mice (Anderson et al., 2008; Danesh-Meyer, 2011; Fan et al., 2010; Howell et al., 2011; Howell et al., 2012; Nickells et al., 2012; Zhou et al., 2005). The complement system, a component of the innate immune system, is a key part of the neuroinflammatory response and complement activation has been reported in human glaucoma and in animal models of glaucoma (Brennan et al., 2012; Fan et al., 2010; Harvey and Durant, 2014; Howell et al., 2011; Kuehn et al., 2006; Orsini et al., 2014; Ren and Danias, 2010; Stasi et al., 2006; Stevens et al., 2007; Tezel et al., 2010). DBA/2J mice are naturally deficient in complement component C5 (a key protein in the formation of the membrane attack complex), and C5 sufficient DBA/2J show more RGC loss than standard DBA/2J mice, suggesting C5 plays a damaging role after an ocular hypertensive injury. Similarly, C1qa mutant DBA/2J mice have less RGC loss, suggesting that the initiating C1 complex of the classical arm of the complement cascade is damaging in glaucoma (Howell et al., 2011; Howell et al., 2014; Howell et al., 2013). Interestingly, C5 seems much less important than C1, as C1qa deficiency is much more protective than C5 deficiency, suggesting that substantial damaging effects of C1 are independent of C5 and completion of the classical arm of the complement cascade. It is unclear how decreasing complement activation protects RGCs, though complement has been implicated in various pathological processes thought to be involved in ocular hypertension-induced RGC death in DBA/2J mice, including synaptic remodeling and monocyte recruitment (Figure 3; Howell et al., 2011; Howell et al., 2013; Howell et al., 2012; Stevens et al., 2007).
Neuroinflammation is often linked closely with vascular compromise and changes in vascular tone have been reported in glaucoma (Gerber et al., 2014). Further implicating the vasculature in glaucoma is the suggestion that blood pressure may play a role in glaucoma (Chauhan, 2008; Flammer et al., 2013; Prasanna et al., 2005; Prasanna et al., 2002; Prasanna et al., 2011; Shoshani et al., 2012). The endothelin system, which controls a variety of processes including blood pressure and astrocyte activation has recently been implicated in neurodegenerative conditions (Jo et al., 2014). Components of the endothelin system are upregulated in human and animal models of glaucoma (Howell et al., 2011; Krishnamoorthy et al., 2008; Prasanna et al., 2005; Prasanna et al., 2002; Rattner and Nathans, 2005). Intravitreally injected EDN1 or END2 peptide kills RGCs in rodents (Howell et al., 2011; Krishnamoorthy et al., 2008; Rattner and Nathans, 2005), especially in the presence of elevated IOP (Howell et al., 2012). Treating DBA/2J mice with endothelin receptor antagonists both slowed and lessened the severity of RGC axonal damage, suggesting that endothelin signaling has a significant role in ocular hypertension-induced RGC death (Howell et al., 2011; Howell et al., 2014). Interestingly, axonal loss in the DBA/2J mouse has been associated with monocyte entry into the optic nerve head (Howell et al., 2012), further implicating neuroinflammatory responses. An important future direction will be to mechanistically explore the roles of neuroinflammation and vascular dysfunction in glaucoma. However, this work is confounded by the fact that components of these systems are activated in both aging, a major risk factor for glaucoma, and multiple cell types, including astrocytes, RGCs, microglia and cells associated with the vasculature. Here, the mouse can play a major role in determining the beneficial and/or damaging roles of specific genes and cell types in aging and glaucoma using conditional alleles whereby genes are ablated in particular cell types. Importantly, as anti-inflammatory therapies are already commonplace, the inhibition of glaucomatous damage using anti-inflammatory approaches poses a tangible therapeutic option.
Glia are involved in many different processes that can contribute to neurodegeneration, including neuroinflammation (Maragakis and Rothstein, 2006; Mrak and Griffin, 2005; Verkhratsky et al., 2014). Microglia, Müller glia and optic nerve head astrocytes have been hypothesized to play critical roles in glaucomatous neurodegeneration (Chong and Martin, 2015; Seitz et al., 2013). Both retinal and optic nerve head glia have been implicated in ocular hypertensive RGC death in DBA/2J mice. Astrocytes at the optic nerve head have been shown to undergo morphological changes prior to RGC death (Lye-Barthel et al., 2013). Also, gene expression studies have suggested that molecular changes in astrocytes could be involved in the pathogenesis of RGC death in DBA/2J mice (Howell et al., 2011; Howell et al., 2014; Nguyen et al., 2011). Microglial activation has been correlated with RGC death (Bosco et al., 2015) and reducing microglial activation has been suggested to increase RGC viability in DBA/2J mice (Bosco et al., 2008; Bosco et al., 2015; Inman and Horner, 2007). It will be important to functionally test specific aspects of glial activation in DBA/2J mice to precisely define the role of glia in the disease process.
In summary, multiple pathways, both intrinsic and extrinsic to RGCs, have been suggested to contribute to ocular hypertension-induced RGC death in DBA/2J mice (Figure 3). However, the initial molecular changes that trigger neuronal injury remain unclear. Highlighting the complexity of glaucoma, numerous cell biological events are suggested as key players in the disease, including axonal injury, inflammation, glial activation, growth factor deficiency, mitochondrial dysfunction, and synapse withdrawal. Thus, there is still much to be learned about RGC loss in DBA/2J mice. It will be important to continue to critically test molecules and pathways hypothesized to be important through functional tests, including assessing their roles in specific cell types and determining critical molecular events. Careful functional testing of any specific pathway in multiple models (DBA/2J as well as others) will identify potential targets for therapies to prevent glaucomatous neurodegeneration.
4.1.2 Other Models for Studying Ocular Hypertension-Induced RGC death
Along with genetic models, there are inducible models of IOP elevation that are being used to study ocular hypertension-induced RGC death, including, microbead injections (Morgan Chapter REF, this issue), episcleral vein and aqueous outflow sclerosis (Morrison et al., 2015, this issue), acute IOP elevation (Crowston et al., 2015, this issue), glucocorticoid-induced IOP elevation (Overby and Clark, 2015, this issue), and viral gene transfer to generate TM cell dysfunction and resulting IOP elevation (Pang et al., 2015, this issue). For instance, the microbead model has been used to test the importance of a variety of molecular pathways in ocular hypertension induced RGC death (Hu et al., 2012; Huang et al., 2011; Ward et al., 2014) and to investigate susceptibility factors involved in response to IOP elevation (Cone et al., 2010; Steinhart et al., 2012). The large number of models being used and developed will allow researchers to validate results in multiple diverse models, species, and strains within species.
4.2 Normal tension glaucoma models
Normal tension glaucoma (NTG) is a subset of primary open angle glaucoma that is characterized by RGC loss and optic neuropathy in the absence of detecting elevated IOP. NTG is an interesting type of glaucoma because it suggests that factors intrinsic to retina and/or optic nerve are a primary site of pathophysiology. Below we highlight three different mouse models of NTG, where mice have been used to gain insight into mechanisms that lead to RGC loss.
4.2.1 Optineurin (OPTN)
The OPTN E50K mutation was the first mutation identified in familial normal tension glaucoma (Rezaie et al., 2002). Transgenic mice overexpressing the optineurin E50K mutation (E50Ktg ) were made to investigate the biology of this specific mutation (Chi et al., 2010a). E50Ktg mice did not have elevated IOP but did have loss of RGCs (~25% loss by 16 months of age). A caveat of the E50Ktg model is the significant thinning of the ONL and INL and loss of other retinal neurons, including amacrine cells, bipolar cells, and photoreceptors (Chi et al., 2010a), cell loss that is not generally observed in glaucoma patients. The high expression levels of E50K OPTN in the E50Ktg mice were hypothesized to result in non-specific loss of other retinal neurons through a toxic gain of function mechanism. To test this hypothesis a BAC transgenic mouse expressing human E50K OPTN (BAC-hOPTNE50K) close to physiological levels was made (Tseng et al., 2015). Interestingly, in these mice there was age-related loss of RGCs without apparent loss of other retinal cell types. Thus, BAC-hOPTNE50K mice are a valuable resource for gaining insight into the molecular mechanisms of RGC death in NTG. In the mouse eye, OPTN is expressed in RGCs, as well as in the cornea, trabecular meshwork, ciliary epithelium and iris (Kroeber et al., 2006) and it remains unclear why mutations in OPTN lead to RGC loss. Optineurin functions in several physiological processes, most prominently in secretory vesicle transport (Bond et al., 2011) and autophagy (Wild et al., 2011). Determining which, if any, of these processes is critical for RGC death in NTG is an important future direction.
4.2.2 WD-repeat-containing protein 36 (WDR36)
Several studies have reported an association between WDR36 and both NTG and POAG. Interestingly, these studies have shown that WDR36 can be causative for glaucoma and be a susceptibility factor for developing glaucoma (Allingham et al., 2009; Hauser et al., 2006; Monemi et al., 2005). WDR36 is expressed throughout the eye, including in tissues important in glaucoma pathogenesis (sclera, iris, ciliary muscle, ciliary body, retina, and optic nerve ; Monemi et al., 2005). We have included WDR36 in the NTG section because of the data generated from mice (discussed below); however, we realize that it could potentially be included in the ocular hypertension discussion or in a section focusing on general susceptibility factors. Several mouse mutant alleles have been generated to study the role of WDR36 in ocular biology and disease. Unfortunately, mice homozygous for a null allele of Wdr36 (Wdr36−/− mice) die preimplantation (Gallenberger et al., 2011). Wdr36+/− mice do not have RGC loss at 12 months, nor do RGCs in these mice show increased susceptibility to death when exposed to an excitotoxic or ocular hypertensive insult (Gallenberger et al., 2014). There have been 3 mouse alleles generated that have been designed to either create an orthologous mutation to that found in human glaucoma patients or to disrupt molecular function in a region of the gene with an orthologous mutation (Chi et al., 2010b). Transgenic mice overexpressing one of these alleles, Wdr36 (Del605-607), had a dramatic reduction in peripheral retinal thickness and loss of RGCs as well as amacrine cells and bipolar cells at 16 months of age (Chi et al., 2010b). Wdr36 (Del605-607) mutant mice do not have elevated IOP and the lens, cornea, and anterior segment of these mice appear to be histologically normal. In the future, it will be important to make a conditional allele of Wdr36 so that it can be deleted selectively in adult trabecular meshwork, RGCs, or optic nerve head astrocytes to allow assessment of the tissue-specific function of Wdr36 in the pathogenesis of glaucoma. Also, it will be important to assess the effects of mutant mouse alleles that have orthologous mutations to those found in human glaucoma patients knocked into the mouse Wdr36 locus.
4.2.3 Glutamate Transporters
To our knowledge, there are no hereditary studies that implicate alleles involved in glutamate regulation in human NTG or in any other form of glaucoma. However, elevated levels of extracellular glutamate cause RGC death and mice deficient in the glutamate transporters, GLAST or EAAC1, exhibit RGC degeneration with varying time courses. GLAST deficient mice had a progressive attrition of RGCs with as much as a 50% loss by 8 months (Harada et al., 2007). These mice were also suggested to have other hallmarks of glaucomatous neurodegeneration including optic nerve cupping and degenerating axons in the optic nerve. EAAC1 deficient mice had RGC degeneration as early as 8 weeks of age, but also have thinning of the inner retina (Harada et al., 2007). At 8 weeks of age, there is a 20% reduction in the number of cells in the ganglion cell layer in EAAC1 deficient mice. Both GLAST and EAAC1 deficient mice have normal IOP and normal iridocorneal angle morphology, suggesting IOP does not directly cause RGC death in these mice (Harada et al., 2007). Both glutamate toxicity and oxidative stress have been hypothesized to contribute to RGC loss in GLAST and EAAC1 deficient mice (Kimura et al., 2015; Namekata et al., 2013; Semba et al., 2014). Glutamate receptor antagonists lessened RGC death at early time points in GLAST deficient mice, indicating that glutamate neurotoxicity contributes to RGC degeneration in these mice (Harada et al., 2007; Namekata et al., 2013). Similarly, attenuating oxidative stress was shown to reduce RGC loss in GLAST knockout mice (Kimura et al., 2015). Collectively, the data from animal models of glutamate transporter loss show that both RGC intrinsic as well as extrinsic mechanisms contribute to RGC degeneration. It will be important in the future to determine if this system is involved in RGC loss in NTG in humans.
4.2.4 Summary of NTG Models
Thus far, mouse models of NTG have revealed a diversity of physiological processes that when disrupted in the retina can lead to RGC degeneration independent of IOP elevation. An important caveat with some of the NTG mouse models is that they appear to have loss of other retinal neurons, which is generally thought to be inconsistent with glaucomatous neurodegeneration. Furthermore, there are other genetic causes of NTG that remain to be modeled. For instance, copy number variants of TBK1 cause NTG (Awadalla et al., 2015; Ritch et al., 2014). The normal physiology and pathophysiology of altered TBK1 expression remain to be determined. Using induced pluripotent stem cells from patients with TBK1 copy number variations, evidence is emerging that autophagy is enhanced in these cells (Tucker et al., 2014). Mice are routinely used for studying the effects of copy number variation and can easily be made to express different levels of specific human or mouse alleles (Le et al., 2003; Smithies and Kim, 1994; van der Weyden and Bradley, 2006). Modeling TBK1 expression level variants should provide a helpful addition to the toolbox for studying NTG, especially if the pathophysiology involves an aging component or requires the interaction between different cell types. As new genes are found to cause NTG, regardless of the nature of the mutation, mouse mutants will be important tools for determining the pathophysiological mechanisms resulting from the mutations.
5 The future of genetic models of glaucoma
The genomics revolution over the last twenty years is beginning to bear fruit for glaucoma research and a steady stream of genes and genetic loci are being discovered that are linked or associated with glaucoma. Recent large-scale genome wide association studies (GWAS; Hysi et al., 2014; Springelkamp et al., 2014) have identified genes/loci associated with glaucoma-relevant phenotypes. However, in some cases, it is not clear how a gene or a specific allelic variant impacts the disease. For instance, while CDKN2B-AS1, an anti-sense transcript that is transcribed on the opposite strand to CDKN2A and CDKN2B, is strongly associated with glaucoma, the function of CDKN2B-AS1 remains unclear (Burdon et al., 2012; Burdon et al., 2011; Ng et al., 2014). In a second example, copy number variations in TBK1 are associated with glaucoma, and although the function of the gene is better understood (Helgason et al., 2013; Minegishi et al., 2013; Tucker et al., 2014; Weidberg and Elazar, 2011), the reason for why copy number variations that include TBK1 impact glaucoma progression is less clear.
A major limitation of GWAS is that it generally implicates genetic loci and not candidate variants. Plus, GWAS analysis cannot find evolutionarily recent or low frequency genetic variants (Manolio et al., 2009). To address this, studies are now beginning to take advantage of next generation sequencing (NGS) of human samples, using targeted exome and whole genome sequencing approaches. However, progress with these approaches will often not be straightforward and complementary animal and human studies will often be necessary. This is due to both the complex nature of glaucoma, where variations in multiple genes contribute to the disease, and to difficulty ascribing causality to any specific variants among the large numbers of variants detected by sequencing. As with GWAS, NGS studies will likely need to involve large numbers of samples coordinated in a consortium-like manner that has proved successful for GWAS (e.g. the Neighbor and Neighborhood studies Burdon et al., 2011; Lu et al., 2013; Springelkamp et al., 2014; Wiggs et al., 2013; Wiggs et al., 2012). The discovery that naturally existing nucleases, first zinc fingers (Urnov et al., 2010) then TALENS (Joung and Sander, 2013) and now CRISPRs (Sander and Joung, 2014) can be used to alter single bases or a few bases (‘genome editing’), means that genetically engineered mice can be created in months rather than years. Further advances that include incorporating larger stretches of DNA into the mouse genome will enable insertion of human gene sequences into the mouse genome and the generation of conditional alleles and new Cre driver lines to study the temporal and spatial function(s) of genes. Thus, new genomic technologies will allow us to readily assess putative causal variants identified in human genetic studies in mice. An important goal should be to incorporate genetic variants implicated in human glaucoma into mouse models, improving utility for preclinical and translational studies. These experiments can complement genetic manipulation strategies in human tissues/cells, including the ability to assess variants in specific cell types derived using induced pluripotent stem cells (IPSCs; Ding et al., 2014; Tucker et al., 2014).
The mouse can play other key roles in the development of improved therapies for glaucoma. For instance, human genetic and genomic studies are difficult because of the obvious limitations of working with human subjects and samples. Therefore, the mouse can continue to inform us about novel genes and pathways that impact glaucoma. These experiments can include hypothesis driven approaches where the importance of individual genes can be assessed. Alternatively, unbiased approaches should continue to be pursued where genes are mutated at random (for instance, by ethylnitrosourea mutagenesis). This approach has already identified novel genes for glaucoma research (e.g. Cross et al., 2014; Lachke et al., 2011; Nair et al., 2011; Van Agtmael et al., 2005). Given the genetic complexity of glaucoma, sensitized approaches are also being pursued where mutagenesis results in mice carrying genetic mutations that increase susceptibility for glaucoma-relevant phenotypes but do not directly cause glaucoma. Unbiased genetic approaches may be greatly aided by incorporating the latest generation of inbred and outbred mouse crosses, (collaborative cross and diversity outbred cross respectively; Chesler et al., 2008;
Churchill et al., 2004; Churchill et al., 2012). These are currently the most powerful strains for identifying genetic variations that impact complex disease, and are ideal for mapping glaucoma-relevant modifier genes. Diversity outbred mice are also ideal for assessing efficacy and pharmacokinetics in the preclinical space where potential new drugs can be tested in genetically unique mice under controlled environmental conditions. Finally, as the number of mouse strains with unique mutations and engineered manipulations relevant to glaucoma continue to grow, so will the need for efficient sharing of them amongst investigators and institutions. Several commercial and non-profit repositories for sharing mice exist (see Figure 2 for some examples) and it will be critical that our community remains dedicated to using them fully.
Mice can also be used to probe some of the outstanding questions about complex physiological interactions that may be important in glaucoma. For instance, it has been suggested that both blood pressure (He et al., 2014; Moore et al., 2008) and intracranial pressure (Fleischman and Allingham, 2013) help mediate glaucoma susceptibility. It is possible to manipulate these traits in mice along with IOP to determine the importance of these parameters in RGC susceptibility to IOP elevation (Nusbaum et al., 2015; Takahashi and Smithies, 2004; Verouti et al., 2015). Also, recent work is starting to examine endophenotypes of glaucoma, such as corneal thickness and optic disc morphology in mice (Harder et al., 2012; Lively et al., 2010a; Lively et al., 2010b; Prasov et al., 2012). These studies will open up avenues of research examining how these phenotypes affect the pathophysiology of glaucoma. Overall, mice have provided important cell biological insight into glaucoma-relevant phenotypes. In the future, continually integrating information gained from human glaucoma studies into mouse models will further enhance the power of mice for studying glaucoma pathophysiology.
Highlights.
Mice can be used to model specific glaucoma-relevant phenotypes.
Mice are a valuable tool to determine the molecular mechanisms underlying glaucoma phenotypes.
Mice are a valuable tool to probe the cell biology underlying findings from human genetic studies.
Mice can be used to find new genes and molecular pathways that contribute to human glaucoma.
Acknowledgments
This work was supported by The Glaucoma Foundation (RTL, GRH), The Glaucoma Research Foundation (RTL, GRH), EY018606 (RTL), EY021525 (GRH), EY011721 (SWMJ), EY022891 (KSN), EY017673 (MGA), T32 EY007125 (RLR), Research to Prevent Blindness unrestricted grant to the Department of Ophthalmology at the University of Rochester. S.W.M. John is an Investigator of the Howard Hughes Medical Institute.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aihara M, Lindsey JD, Weinreb RN. Aqueous humor dynamics in mice. Invest Ophthalmol Vis Sci. 2003;44:5168–5173. doi: 10.1167/iovs.03-0504. [DOI] [PubMed] [Google Scholar]
- Allingham RR, Liu Y, Rhee DJ. The genetics of primary open-angle glaucoma: a review. Exp Eye Res. 2009;88:837–844. doi: 10.1016/j.exer.2008.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Almasieh M, Wilson AM, Morquette B, Cueva Vargas JL, Di Polo A. The molecular basis of retinal ganglion cell death in glaucoma. Prog Retin Eye Res. 2012;31:152–181. doi: 10.1016/j.preteyeres.2011.11.002. [DOI] [PubMed] [Google Scholar]
- Anderson DR, Hendrickson A. Effect of intraocular pressure on rapid axoplasmic transport in monkey optic nerve. Invest Ophthalmol. 1974;13:771–783. [PubMed] [Google Scholar]
- Anderson DR, Hendrickson AE. Failure of increased intracranial pressure to affect rapid axonal transport at the optic nerve head. Invest Ophthalmol Vis Sci. 1977;16:423–426. [PubMed] [Google Scholar]
- Anderson MG, Libby RT, Mao M, Cosma IM, Wilson LA, Smith RS, John SW. Genetic context determines susceptibility to intraocular pressure elevation in a mouse pigmentary glaucoma. BMC Biol. 2006;4:20. doi: 10.1186/1741-7007-4-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson MG, Nair KS, Amonoo LA, Mehalow A, Trantow CM, Masli S, John SW. GpnmbR150X allele must be present in bone marrow derived cells to mediate DBA/2J glaucoma. BMC Genet. 2008;9:30. doi: 10.1186/1471-2156-9-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson MG, Smith RS, Hawes NL, Zabaleta A, Chang B, Wiggs JL, John SW. Mutations in genes encoding melanosomal proteins cause pigmentary glaucoma in DBA/2J mice. Nat Genet. 2002;30:81–85. doi: 10.1038/ng794. [DOI] [PubMed] [Google Scholar]
- Aspelund A, Tammela T, Antila S, Nurmi H, Leppanen VM, Zarkada G, Stanczuk L, Francois M, Makinen T, Saharinen P, Immonen I, Alitalo K. The Schlemm’s canal is a VEGF-C/VEGFR-3-responsive lymphatic-like vessel. J Clin Invest. 2014;124:3975–3986. doi: 10.1172/JCI75395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avila MY, Carre DA, Stone RA, Civan MM. Reliable measurement of mouse intraocular pressure by a servo-null micropipette system. Invest Ophthalmol Vis Sci. 2001;42:1841–1846. [PubMed] [Google Scholar]
- Awadalla MS, Fingert JH, Roos BE, Chen S, Holmes R, Graham SL, Chehade M, Galanopolous A, Ridge B, Souzeau E, Zhou T, Siggs OM, Hewitt AW, Mackey DA, Burdon KP, Craig JE. Copy Number Variations of TBK1 in Australian Patients With Primary Open-Angle Glaucoma. Am J Ophthalmol. 2015;159:124–130. e121. doi: 10.1016/j.ajo.2014.09.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baltmr A, Duggan J, Nizari S, Salt TE, Cordeiro MF. Neuroprotection in glaucoma - Is there a future role? Exp Eye Res. 2010;91:554–566. doi: 10.1016/j.exer.2010.08.009. [DOI] [PubMed] [Google Scholar]
- Bond LM, Peden AA, Kendrick-Jones J, Sellers JR, Buss F. Myosin VI and its binding partner optineurin are involved in secretory vesicle fusion at the plasma membrane. Mol Biol Cell. 2011;22:54–65. doi: 10.1091/mbc.E10-06-0553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bosco A, Inman DM, Steele MR, Wu G, Soto I, Marsh-Armstrong N, Hubbard WC, Calkins DJ, Horner PJ, Vetter ML. Reduced retina microglial activation and improved optic nerve integrity with minocycline treatment in the DBA/2J mouse model of glaucoma. Invest Ophthalmol Vis Sci. 2008;49:1437–1446. doi: 10.1167/iovs.07-1337. [DOI] [PubMed] [Google Scholar]
- Bosco A, Romero CO, Breen KT, Chagovetz AA, Steele MR, Ambati BK, Vetter ML. Neurodegeneration severity is anticipated by early microglia alterations monitored in vivo in a mouse model of chronic glaucoma. Dis Model Mech. 2015 doi: 10.1242/dmm.018788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boussommier-Calleja A, Bertrand J, Woodward DF, Ethier CR, Stamer WD, Overby DR. Pharmacologic manipulation of conventional outflow facility in ex vivo mouse eyes. Invest Ophthalmol Vis Sci. 2012;53:5838–5845. doi: 10.1167/iovs.12-9923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boussommier-Calleja A, Overby DR. The influence of genetic background on conventional outflow facility in mice. Invest Ophthalmol Vis Sci. 2013;54:8251–8258. doi: 10.1167/iovs.13-13025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brennan FH, Anderson AJ, Taylor SM, Woodruff TM, Ruitenberg MJ. Complement activation in the injured central nervous system: another dual-edged sword? J Neuroinflammation. 2012;9:137. doi: 10.1186/1742-2094-9-137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckingham BP, Inman DM, Lambert W, Oglesby E, Calkins DJ, Steele MR, Vetter ML, Marsh-Armstrong N, Horner PJ. Progressive ganglion cell degeneration precedes neuronal loss in a mouse model of glaucoma. J Neurosci. 2008;28:2735–2744. doi: 10.1523/JNEUROSCI.4443-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burdon KP, Crawford A, Casson RJ, Hewitt AW, Landers J, Danoy P, Mackey DA, Mitchell P, Healey PR, Craig JE. Glaucoma risk alleles at CDKN2B-AS1 are associated with lower intraocular pressure, normal-tension glaucoma, and advanced glaucoma. Ophthalmology. 2012;119:1539–1545. doi: 10.1016/j.ophtha.2012.02.004. [DOI] [PubMed] [Google Scholar]
- Burdon KP, Macgregor S, Hewitt AW, Sharma S, Chidlow G, Mills RA, Danoy P, Casson R, Viswanathan AC, Liu JZ, Landers J, Henders AK, Wood J, Souzeau E, Crawford A, Leo P, Wang JJ, Rochtchina E, Nyholt DR, Martin NG, Montgomery GW, Mitchell P, Brown MA, Mackey DA, Craig JE. Genome-wide association study identifies susceptibility loci for open angle glaucoma at TMCO1 and CDKN2B-AS1. Nat Genet. 2011;43:574–578. doi: 10.1038/ng.824. [DOI] [PubMed] [Google Scholar]
- Casson RJ, Chidlow G, Ebneter A, Wood JP, Crowston J, Goldberg I. Translational neuroprotection research in glaucoma: a review of definitions and principles. Clin Experiment Ophthalmol. 2012;40:350–357. doi: 10.1111/j.1442-9071.2011.02563.x. [DOI] [PubMed] [Google Scholar]
- Chang B, Smith RS, Hawes NL, Anderson MG, Zabaleta A, Savinova O, Roderick TH, Heckenlively JR, Davisson MT, John SW. Interacting loci cause severe iris atrophy and glaucoma in DBA/2J mice. Nat Genet. 1999;21:405–409. doi: 10.1038/7741. [DOI] [PubMed] [Google Scholar]
- Chang B, Smith RS, Peters M, Savinova OV, Hawes NL, Zabaleta A, Nusinowitz S, Martin JE, Davisson ML, Cepko CL, Hogan BL, John SW. Haploinsufficient Bmp4 ocular phenotypes include anterior segment dysgenesis with elevated intraocular pressure. BMC Genet. 2001;2:18. doi: 10.1186/1471-2156-2-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chatterjee A, Villarreal G, Jr, Oh DJ, Kang MH, Rhee DJ. AMP-activated protein kinase regulates intraocular pressure, extracellular matrix, and cytoskeleton in trabecular meshwork. Invest Ophthalmol Vis Sci. 2014;55:3127–3139. doi: 10.1167/iovs.13-12755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chauhan BC. Endothelin and its potential role in glaucoma. Can J Ophthalmol. 2008;43:356–360. doi: 10.3129/i08-060. [DOI] [PubMed] [Google Scholar]
- Chesler EJ, Miller DR, Branstetter LR, Galloway LD, Jackson BL, Philip VM, Voy BH, Culiat CT, Threadgill DW, Williams RW, Churchill GA, Johnson DK, Manly KF. The Collaborative Cross at Oak Ridge National Laboratory: developing a powerful resource for systems genetics. Mamm Genome. 2008;19:382–389. doi: 10.1007/s00335-008-9135-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheung W, Guo L, Cordeiro MF. Neuroprotection in glaucoma: drug-based approaches. Optom Vis Sci. 2008;85:406–416. doi: 10.1097/OPX.0b013e31817841e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chi ZL, Akahori M, Obazawa M, Minami M, Noda T, Nakaya N, Tomarev S, Kawase K, Yamamoto T, Noda S, Sasaoka M, Shimazaki A, Takada Y, Iwata T. Overexpression of optineurin E50K disrupts Rab8 interaction and leads to a progressive retinal degeneration in mice. Hum Mol Genet. 2010a;19:2606–2615. doi: 10.1093/hmg/ddq146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chi ZL, Yasumoto F, Sergeev Y, Minami M, Obazawa M, Kimura I, Takada Y, Iwata T. Mutant WDR36 directly affects axon growth of retinal ganglion cells leading to progressive retinal degeneration in mice. Hum Mol Genet. 2010b;19:3806–3815. doi: 10.1093/hmg/ddq299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chong RS, Martin KR. Glial cell interactions and glaucoma. Curr Opin Ophthalmol. 2015;26:73–77. doi: 10.1097/ICU.0000000000000125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Churchill GA, Airey DC, Allayee H, Angel JM, Attie AD, Beatty J, Beavis WD, Belknap JK, Bennett B, Berrettini W, Bleich A, Bogue M, Broman KW, Buck KJ, Buckler E, Burmeister M, Chesler EJ, Cheverud JM, Clapcote S, Cook MN, Cox RD, Crabbe JC, Crusio WE, Darvasi A, Deschepper CF, Doerge RW, Farber CR, Forejt J, Gaile D, Garlow SJ, Geiger H, Gershenfeld H, Gordon T, Gu J, Gu W, de Haan G, Hayes NL, Heller C, Himmelbauer H, Hitzemann R, Hunter K, Hsu HC, Iraqi FA, Ivandic B, Jacob HJ, Jansen RC, Jepsen KJ, Johnson DK, Johnson TE, Kempermann G, Kendziorski C, Kotb M, Kooy RF, Llamas B, Lammert F, Lassalle JM, Lowenstein PR, Lu L, Lusis A, Manly KF, Marcucio R, Matthews D, Medrano JF, Miller DR, Mittleman G, Mock BA, Mogil JS, Montagutelli X, Morahan G, Morris DG, Mott R, Nadeau JH, Nagase H, Nowakowski RS, O’Hara BF, Osadchuk AV, Page GP, Paigen B, Paigen K, Palmer AA, Pan HJ, Peltonen-Palotie L, Peirce J, Pomp D, Pravenec M, Prows DR, Qi Z, Reeves RH, Roder J, Rosen GD, Schadt EE, Schalkwyk LC, Seltzer Z, Shimomura K, Shou S, Sillanpaa MJ, Siracusa LD, Snoeck HW, Spearow JL, Svenson K, Tarantino LM, Threadgill D, Toth LA, Valdar W, de Villena FP, Warden C, Whatley S, Williams RW, Wiltshire T, Yi N, Zhang D, Zhang M, Zou F Complex Trait C. The Collaborative Cross, a community resource for the genetic analysis of complex traits. Nat Genet. 2004;36:1133–1137. doi: 10.1038/ng1104-1133. [DOI] [PubMed] [Google Scholar]
- Churchill GA, Gatti DM, Munger SC, Svenson KL. The Diversity Outbred mouse population. Mamm Genome. 2012;23:713–718. doi: 10.1007/s00335-012-9414-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohan BE, Bohr DF. Measurement of intraocular pressure in awake mice. Invest Ophthalmol Vis Sci. 2001;42:2560–2562. [PubMed] [Google Scholar]
- Cone FE, Gelman SE, Son JL, Pease ME, Quigley HA. Differential susceptibility to experimental glaucoma among 3 mouse strains using bead and viscoelastic injection. Exp Eye Res. 2010;91:415–424. doi: 10.1016/j.exer.2010.06.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crish SD, Sappington RM, Inman DM, Horner PJ, Calkins DJ. Distal axonopathy with structural persistence in glaucomatous neurodegeneration. Proc Natl Acad Sci U S A. 2010;107:5196–5201. doi: 10.1073/pnas.0913141107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cross SH, Macalinao DG, McKie L, Rose L, Kearney AL, Rainger J, Thaung C, Keighren M, Jadeja S, West K, Kneeland SC, Smith RS, Howell GR, Young F, Robertson M, van T’ Hof R, John SW, Jackson IJ. A dominant-negative mutation of mouse Lmx1b causes glaucoma and is semi-lethal via LBD1-mediated dimerisation. PLoS Genet. 2014;10:e1004359. doi: 10.1371/journal.pgen.1004359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crowston JG, Kong YX, Trounce IA, Dang TM, Fahy ET, Bui BV, Morrison JC, Chrysostomou V. An acute intraocular pressure challenge to assess retinal ganglion cell injury and recovery in the mouse. Exp Eye Res. 2015 doi: 10.1016/j.exer.2015.03.006. [DOI] [PubMed] [Google Scholar]
- Cvekl A, Tamm ER. Anterior eye development and ocular mesenchyme: new insights from mouse models and human diseases. Bioessays. 2004;26:374–386. doi: 10.1002/bies.20009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danesh-Meyer HV. Neuroprotection in glaucoma: recent and future directions. Curr Opin Ophthalmol. 2011;22:78–86. doi: 10.1097/ICU.0b013e32834372ec. [DOI] [PubMed] [Google Scholar]
- Ding QJ, Zhu W, Cook AC, Anfinson KR, Tucker BA, Kuehn MH. Induction of trabecular meshwork cells from induced pluripotent stem cells. Invest Ophthalmol Vis Sci. 2014;55:7065–7072. doi: 10.1167/iovs.14-14800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ermann J, Glimcher LH. After GWAS: mice to the rescue? Curr Opin Immunol. 2012;24:564–570. doi: 10.1016/j.coi.2012.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan W, Li X, Wang W, Mo JS, Kaplan H, Cooper NG. Early Involvement of Immune/Inflammatory Response Genes in Retinal Degeneration in DBA/2J Mice. Ophthalmol Eye Dis. 2010;1:23–41. doi: 10.4137/oed.s2854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fang C, Bernardes-Silva M, Coleman MP, Perry VH. The cellular distribution of the Wld s chimeric protein and its constituent proteins in the CNS. Neuroscience. 2005;135:1107–1118. doi: 10.1016/j.neuroscience.2005.06.078. [DOI] [PubMed] [Google Scholar]
- Fautsch MP, Howell KG, Vrabel AM, Charlesworth MC, Muddiman DC, Johnson DH. Primary trabecular meshwork cells incubated in human aqueous humor differ from cells incubated in serum supplements. Invest Ophthalmol Vis Sci. 2005;46:2848–2856. doi: 10.1167/iovs.05-0101. [DOI] [PubMed] [Google Scholar]
- Filippopoulos T, Danias J, Chen B, Podos SM, Mittag TW. Topographic and morphologic analyses of retinal ganglion cell loss in old DBA/2NNia mice. Invest Ophthalmol Vis Sci. 2006;47:1968–1974. doi: 10.1167/iovs.05-0955. [DOI] [PubMed] [Google Scholar]
- Flammer J, Konieczka K, Flammer AJ. The primary vascular dysregulation syndrome: implications for eye diseases. EPMA J. 2013;4:14. doi: 10.1186/1878-5085-4-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleischman D, Allingham RR. The role of cerebrospinal fluid pressure in glaucoma and other ophthalmic diseases: A review. Saudi J Ophthalmol. 2013;27:97–106. doi: 10.1016/j.sjopt.2013.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gal A, Rau I, El Matri L, Kreienkamp HJ, Fehr S, Baklouti K, Chouchane I, Li Y, Rehbein M, Fuchs J, Fledelius HC, Vilhelmsen K, Schorderet DF, Munier FL, Ostergaard E, Thompson DA, Rosenberg T. Autosomal-recessive posterior microphthalmos is caused by mutations in PRSS56, a gene encoding a trypsin-like serine protease. Am J Hum Genet. 2011;88:382–390. doi: 10.1016/j.ajhg.2011.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallenberger M, Kroeber M, Marz L, Koch M, Fuchshofer R, Braunger BM, Iwata T, Tamm ER. Heterozygote Wdr36-deficient mice do not develop glaucoma. Exp Eye Res. 2014;128:83–91. doi: 10.1016/j.exer.2014.09.008. [DOI] [PubMed] [Google Scholar]
- Gallenberger M, Meinel DM, Kroeber M, Wegner M, Milkereit P, Bosl MR, Tamm ER. Lack of WDR36 leads to preimplantation embryonic lethality in mice and delays the formation of small subunit ribosomal RNA in human cells in vitro. Hum Mol Genet. 2011;20:422–435. doi: 10.1093/hmg/ddq478. [DOI] [PubMed] [Google Scholar]
- Gerber AL, Harris A, Siesky B, Lee E, Schaab TJ, Huck A, Amireskandari A. Vascular Dysfunction in Diabetes and Glaucoma: A Complex Relationship Reviewed. J Glaucoma. 2014 doi: 10.1097/IJG.0000000000000137. [DOI] [PubMed] [Google Scholar]
- Gould DB, John SW. Anterior segment dysgenesis and the developmental glaucomas are complex traits. Hum Mol Genet. 2002;11:1185–1193. doi: 10.1093/hmg/11.10.1185. [DOI] [PubMed] [Google Scholar]
- Gould DB, Marchant JK, Savinova OV, Smith RS, John SW. Col4a1 mutation causes endoplasmic reticulum stress and genetically modifiable ocular dysgenesis. Hum Mol Genet. 2007;16:798–807. doi: 10.1093/hmg/ddm024. [DOI] [PubMed] [Google Scholar]
- Gould DB, Miceli-Libby L, Savinova OV, Torrado M, Tomarev SI, Smith RS, John SW. Genetically increasing Myoc expression supports a necessary pathologic role of abnormal proteins in glaucoma. Mol Cell Biol. 2004a;24:9019–9025. doi: 10.1128/MCB.24.20.9019-9025.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gould DB, Reedy M, Wilson LA, Smith RS, Johnson RL, John SW. Mutant myocilin nonsecretion in vivo is not sufficient to cause glaucoma. Mol Cell Biol. 2006;26:8427–8436. doi: 10.1128/MCB.01127-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gould DB, Smith RS, John SW. Anterior segment development relevant to glaucoma. Int J Dev Biol. 2004b;48:1015–1029. doi: 10.1387/ijdb.041865dg. [DOI] [PubMed] [Google Scholar]
- Gregory MS, Hackett CG, Abernathy EF, Lee KS, Saff RR, Hohlbaum AM, Moody KS, Hobson MW, Jones A, Kolovou P, Karray S, Giani A, John SW, Chen DF, Marshak-Rothstein A, Ksander BR. Opposing roles for membrane bound and soluble Fas ligand in glaucoma-associated retinal ganglion cell death. PLoS One. 2011;6:e17659. doi: 10.1371/journal.pone.0017659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo L, Moss SE, Alexander RA, Ali RR, Fitzke FW, Cordeiro MF. Retinal ganglion cell apoptosis in glaucoma is related to intraocular pressure and IOP-induced effects on extracellular matrix. Invest Ophthalmol Vis Sci. 2005;46:175–182. doi: 10.1167/iovs.04-0832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haddadin RI, Oh DJ, Kang MH, Filippopoulos T, Gupta M, Hart L, Sage EH, Rhee DJ. SPARC-null mice exhibit lower intraocular pressures. Invest Ophthalmol Vis Sci. 2009;50:3771–3777. doi: 10.1167/iovs.08-2489. [DOI] [PubMed] [Google Scholar]
- Haddadin RI, Oh DJ, Kang MH, Villarreal G, Jr, Kang JH, Jin R, Gong H, Rhee DJ. Thrombospondin-1 (TSP1)-null and TSP2-null mice exhibit lower intraocular pressures. Invest Ophthalmol Vis Sci. 2012;53:6708–6717. doi: 10.1167/iovs.11-9013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harada T, Harada C, Nakamura K, Quah HM, Okumura A, Namekata K, Saeki T, Aihara M, Yoshida H, Mitani A, Tanaka K. The potential role of glutamate transporters in the pathogenesis of normal tension glaucoma. J Clin Invest. 2007;117:1763–1770. doi: 10.1172/JCI30178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harder JM, Fernandes KA, Libby RT. The Bcl-2 family member BIM has multiple glaucoma-relevant functions in DBA/2J mice. Sci Rep. 2012;2:530. doi: 10.1038/srep00530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harvey AR, Hellstrom M, Rodger J. Gene therapy and transplantation in the retinofugal pathway. Prog Brain Res. 2009;175:151–161. doi: 10.1016/S0079-6123(09)17510-6. [DOI] [PubMed] [Google Scholar]
- Harvey H, Durant S. The role of glial cells and the complement system in retinal diseases and Alzheimer’s disease: common neural degeneration mechanisms. Exp Brain Res. 2014;232:3363–3377. doi: 10.1007/s00221-014-4078-7. [DOI] [PubMed] [Google Scholar]
- Hauser MA, Allingham RR, Linkroum K, Wang J, LaRocque-Abramson K, Figueiredo D, Santiago-Turla C, del Bono EA, Haines JL, Pericak-Vance MA, Wiggs JL. Distribution of WDR36 DNA sequence variants in patients with primary open-angle glaucoma. Invest Ophthalmol Vis Sci. 2006;47:2542–2546. doi: 10.1167/iovs.05-1476. [DOI] [PubMed] [Google Scholar]
- He Z, Vingrys AJ, Armitage JA, Nguyen CT, Bui BV. Chronic Hypertension Increases Susceptibility to Acute IOP Challenge in Rats. Invest Ophthalmol Vis Sci. 2014;55:7888–7895. doi: 10.1167/iovs.14-15207. [DOI] [PubMed] [Google Scholar]
- Heavner W, Pevny L. Eye development and retinogenesis. Cold Spring Harb Perspect Biol. 2012:4. doi: 10.1101/cshperspect.a008391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helgason E, Phung QT, Dueber EC. Recent insights into the complexity of Tank-binding kinase 1 signaling networks: the emerging role of cellular localization in the activation and substrate specificity of TBK1. FEBS Lett. 2013;587:1230–1237. doi: 10.1016/j.febslet.2013.01.059. [DOI] [PubMed] [Google Scholar]
- Howell GR, Libby RT, Jakobs TC, Smith RS, Phalan FC, Barter JW, Barbay JM, Marchant JK, Mahesh N, Porciatti V, Whitmore AV, Masland RH, John SW. Axons of retinal ganglion cells are insulted in the optic nerve early in DBA/2J glaucoma. J Cell Biol. 2007;179:1523–1537. doi: 10.1083/jcb.200706181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howell GR, Macalinao DG, Sousa GL, Walden M, Soto I, Kneeland SC, Barbay JM, King BL, Marchant JK, Hibbs M, Stevens B, Barres BA, Clark AF, Libby RT, John SW. Molecular clustering identifies complement and endothelin induction as early events in a mouse model of glaucoma. J Clin Invest. 2011;121:1429–1444. doi: 10.1172/JCI44646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howell GR, MacNicoll KH, Braine CE, Soto I, Macalinao DG, Sousa GL, John SW. Combinatorial targeting of early pathways profoundly inhibits neurodegeneration in a mouse model of glaucoma. Neurobiol Dis. 2014;71:44–52. doi: 10.1016/j.nbd.2014.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howell GR, Soto I, Ryan M, Graham LC, Smith RS, John SW. Deficiency of complement component 5 ameliorates glaucoma in DBA/2J mice. J Neuroinflammation. 2013;10:76. doi: 10.1186/1742-2094-10-76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howell GR, Soto I, Zhu X, Ryan M, Macalinao DG, Sousa GL, Caddle LB, MacNicoll KH, Barbay JM, Porciatti V, Anderson MG, Smith RS, Clark AF, Libby RT, John SW. Radiation treatment inhibits monocyte entry into the optic nerve head and prevents neuronal damage in a mouse model of glaucoma. J Clin Invest. 2012;122:1246–1261. doi: 10.1172/JCI61135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu Y, Park KK, Yang L, Wei X, Yang Q, Cho KS, Thielen P, Lee AH, Cartoni R, Glimcher LH, Chen DF, He Z. Differential effects of unfolded protein response pathways on axon injury-induced death of retinal ganglion cells. Neuron. 2012;73:445–452. doi: 10.1016/j.neuron.2011.11.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang W, Xing W, Ryskamp DA, Punzo C, Krizaj D. Localization and phenotype-specific expression of ryanodine calcium release channels in C57BL6 and DBA/2J mouse strains. Exp Eye Res. 2011;93:700–709. doi: 10.1016/j.exer.2011.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hysi PG, Cheng CY, Springelkamp H, Macgregor S, Bailey JN, Wojciechowski R, Vitart V, Nag A, Hewitt AW, Hohn R, Venturini C, Mirshahi A, Ramdas WD, Thorleifsson G, Vithana E, Khor CC, Stefansson AB, Liao J, Haines JL, Amin N, Wang YX, Wild PS, Ozel AB, Li JZ, Fleck BW, Zeller T, Staffieri SE, Teo YY, Cuellar-Partida G, Luo X, Allingham RR, Richards JE, Senft A, Karssen LC, Zheng Y, Bellenguez C, Xu L, Iglesias AI, Wilson JF, Kang JH, van Leeuwen EM, Jonsson V, Thorsteinsdottir U, Despriet DD, Ennis S, Moroi SE, Martin NG, Jansonius NM, Yazar S, Tai ES, Amouyel P, Kirwan J, van Koolwijk LM, Hauser MA, Jonasson F, Leo P, Loomis SJ, Fogarty R, Rivadeneira F, Kearns L, Lackner KJ, de Jong PT, Simpson CL, Pennell CE, Oostra BA, Uitterlinden AG, Saw SM, Lotery AJ, Bailey-Wilson JE, Hofman A, Vingerling JR, Maubaret C, Pfeiffer N, Wolfs RC, Lemij HG, Young TL, Pasquale LR, Delcourt C, Spector TD, Klaver CC, Small KS, Burdon KP, Stefansson K, Wong TY, Group BG, Consortium N, Viswanathan A, Mackey DA, Craig JE, Wiggs JL, van Duijn CM, Hammond CJ, Aung T Wellcome Trust Case Control C. Genome-wide analysis of multi-ancestry cohorts identifies new loci influencing intraocular pressure and susceptibility to glaucoma. Nat Genet. 2014;46:1126–1130. doi: 10.1038/ng.3087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inman DM, Horner PJ. Reactive nonproliferative gliosis predominates in a chronic mouse model of glaucoma. Glia. 2007;55:942–953. doi: 10.1002/glia.20516. [DOI] [PubMed] [Google Scholar]
- Ito YA, Walter MA. Genomics and anterior segment dysgenesis: a review. Clin Experiment Ophthalmol. 2014;42:13–24. doi: 10.1111/ceo.12152. [DOI] [PubMed] [Google Scholar]
- Jacobson N, Andrews M, Shepard AR, Nishimura D, Searby C, Fingert JH, Hageman G, Mullins R, Davidson BL, Kwon YH, Alward WL, Stone EM, Clark AF, Sheffield VC. Non-secretion of mutant proteins of the glaucoma gene myocilin in cultured trabecular meshwork cells and in aqueous humor. Hum Mol Genet. 2001;10:117–125. doi: 10.1093/hmg/10.2.117. [DOI] [PubMed] [Google Scholar]
- Jakobs TC, Libby RT, Ben Y, John SW, Masland RH. Retinal ganglion cell degeneration is topological but not cell type specific in DBA/2J mice. J Cell Biol. 2005;171:313–325. doi: 10.1083/jcb.200506099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang D, Yang Z, Li S, Xiao X, Jia X, Wang P, Guo X, Liu X, Zhang Q. Evaluation of PRSS56 in Chinese subjects with high hyperopia or primary angle-closure glaucoma. Mol Vis. 2013;19:2217–2226. [PMC free article] [PubMed] [Google Scholar]
- Jo WK, Law AC, Chung SK. The neglected co-star in the dementia drama: the putative roles of astrocytes in the pathogeneses of major neurocognitive disorders. Mol Psychiatry. 2014;19:159–167. doi: 10.1038/mp.2013.171. [DOI] [PubMed] [Google Scholar]
- John SW, Hagaman JR, MacTaggart TE, Peng L, Smithes O. Intraocular pressure in inbred mouse strains. Invest Ophthalmol Vis Sci. 1997;38:249–253. [PubMed] [Google Scholar]
- John SW, Smith RS, Savinova OV, Hawes NL, Chang B, Turnbull D, Davisson M, Roderick TH, Heckenlively JR. Essential iris atrophy, pigment dispersion, and glaucoma in DBA/2J mice. Invest Ophthalmol Vis Sci. 1998;39:951–962. [PubMed] [Google Scholar]
- Joung JK, Sander JD. TALENs: a widely applicable technology for targeted genome editing. Nat Rev Mol Cell Biol. 2013;14:49–55. doi: 10.1038/nrm3486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ju WK, Kim KY, Angert M, Duong-Polk KX, Lindsey JD, Ellisman MH, Weinreb RN. Memantine blocks mitochondrial OPA1 and cytochrome c release and subsequent apoptotic cell death in glaucomatous retina. Invest Ophthalmol Vis Sci. 2009;50:707–716. doi: 10.1167/iovs.08-2499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ju WK, Kim KY, Duong-Polk KX, Lindsey JD, Ellisman MH, Weinreb RN. Increased optic atrophy type 1 expression protects retinal ganglion cells in a mouse model of glaucoma. Mol Vis. 2010;16:1331–1342. [PMC free article] [PubMed] [Google Scholar]
- Ju WK, Kim KY, Lindsey JD, Angert M, Duong-Polk KX, Scott RT, Kim JJ, Kukhmazov I, Ellisman MH, Perkins GA, Weinreb RN. Intraocular pressure elevation induces mitochondrial fission and triggers OPA1 release in glaucomatous optic nerve. Invest Ophthalmol Vis Sci. 2008;49:4903–4911. doi: 10.1167/iovs.07-1661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Justice MJ, Siracusa LD, Stewart AF. Technical approaches for mouse models of human disease. Dis Model Mech. 2011;4:305–310. doi: 10.1242/dmm.000901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim BS, Savinova OV, Reedy MV, Martin J, Lun Y, Gan L, Smith RS, Tomarev SI, John SW, Johnson RL. Targeted Disruption of the Myocilin Gene (Myoc) Suggests that Human Glaucoma-Causing Mutations Are Gain of Function. Mol Cell Biol. 2001;21:7707–7713. doi: 10.1128/MCB.21.22.7707-7713.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimura A, Guo X, Noro T, Harada C, Tanaka K, Namekata K, Harada T. Valproic acid prevents retinal degeneration in a murine model of normal tension glaucoma. Neurosci Lett. 2015;588:108–113. doi: 10.1016/j.neulet.2014.12.054. [DOI] [PubMed] [Google Scholar]
- Kizhatil K, Ryan M, Marchant JK, Henrich S, John SW. Schlemm’s canal is a unique vessel with a combination of blood vascular and lymphatic phenotypes that forms by a novel developmental process. PLoS Biol. 2014;12:e1001912. doi: 10.1371/journal.pbio.1001912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krishnamoorthy RR, Rao VR, Dauphin R, Prasanna G, Johnson C, Yorio T. Role of the ETB receptor in retinal ganglion cell death in glaucoma. Can J Physiol Pharmacol. 2008;86:380–393. doi: 10.1139/Y08-040. [DOI] [PubMed] [Google Scholar]
- Kroeber M, Davis N, Holzmann S, Kritzenberger M, Shelah-Goraly M, Ofri R, Ashery-Padan R, Tamm ER. Reduced expression of Pax6 in lens and cornea of mutant mice leads to failure of chamber angle development and juvenile glaucoma. Hum Mol Genet. 2010;19:3332–3342. doi: 10.1093/hmg/ddq237. [DOI] [PubMed] [Google Scholar]
- Kroeber M, Ohlmann A, Russell P, Tamm ER. Transgenic studies on the role of optineurin in the mouse eye. Exp Eye Res. 2006;82:1075–1085. doi: 10.1016/j.exer.2005.11.004. [DOI] [PubMed] [Google Scholar]
- Kuehn MH, Kim CY, Ostojic J, Bellin M, Alward WL, Stone EM, Sakaguchi DS, Grozdanic SD, Kwon YH. Retinal synthesis and deposition of complement components induced by ocular hypertension. Exp Eye Res. 2006;83:620–628. doi: 10.1016/j.exer.2006.03.002. [DOI] [PubMed] [Google Scholar]
- Kuo DS, Labelle-Dumais C, Gould DB. COL4A1 and COL4A2 mutations and disease: insights into pathogenic mechanisms and potential therapeutic targets. Hum Mol Genet. 2012;21:R97–110. doi: 10.1093/hmg/dds346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lachke SA, Alkuraya FS, Kneeland SC, Ohn T, Aboukhalil A, Howell GR, Saadi I, Cavallesco R, Yue Y, Tsai AC, Nair KS, Cosma MI, Smith RS, Hodges E, Alfadhli SM, Al-Hajeri A, Shamseldin HE, Behbehani A, Hannon GJ, Bulyk ML, Drack AV, Anderson PJ, John SW, Maas RL. Mutations in the RNA granule component TDRD7 cause cataract and glaucoma. Science. 2011;331:1571–1576. doi: 10.1126/science.1195970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le TH, Kim HS, Allen AM, Spurney RF, Smithies O, Coffman TM. Physiological impact of increased expression of the AT1 angiotensin receptor. Hypertension. 2003;42:507–514. doi: 10.1161/01.HYP.0000092000.07559.57. [DOI] [PubMed] [Google Scholar]
- Lebrun-Julien F, Di Polo A. Molecular and cell-based approaches for neuroprotection in glaucoma. Optom Vis Sci. 2008;85:417–424. doi: 10.1097/OPX.0b013e31817841f7. [DOI] [PubMed] [Google Scholar]
- Lee D, Shim MS, Kim KY, Noh YH, Kim H, Kim SY, Weinreb RN, Ju WK. Coenzyme Q10 inhibits glutamate excitotoxicity and oxidative stress-mediated mitochondrial alteration in a mouse model of glaucoma. Invest Ophthalmol Vis Sci. 2014;55:993–1005. doi: 10.1167/iovs.13-12564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lei Y, Overby DR, Boussommier-Calleja A, Stamer WD, Ethier CR. Outflow physiology of the mouse eye: pressure dependence and washout. Invest Ophthalmol Vis Sci. 2011;52:1865–1871. doi: 10.1167/iovs.10-6019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li G, Farsiu S, Qiu J, Dixon A, Song C, McKinnon SJ, Yuan F, Gonzalez P, Stamer WD. Disease progression in iridocorneal angle tissues of BMP2-induced ocular hypertensive mice with optical coherence tomography. Mol Vis. 2014;20:1695–1709. [PMC free article] [PubMed] [Google Scholar]
- Li R, Liu JH. Telemetric monitoring of 24 h intraocular pressure in conscious and freely moving C57BL/6J and CBA/CaJ mice. Mol Vis. 2008;14:745–749. [PMC free article] [PubMed] [Google Scholar]
- Libby RT, Anderson MG, Pang IH, Robinson ZH, Savinova OV, Cosma IM, Snow A, Wilson LA, Smith RS, Clark AF, John SW. Inherited glaucoma in DBA/2J mice: pertinent disease features for studying the neurodegeneration. Vis Neurosci. 2005a;22:637–648. doi: 10.1017/S0952523805225130. [DOI] [PubMed] [Google Scholar]
- Libby RT, Gould DB, Anderson MG, John SW. Complex genetics of glaucoma susceptibility. Annu Rev Genomics Hum Genet. 2005b;6:15–44. doi: 10.1146/annurev.genom.6.080604.162209. [DOI] [PubMed] [Google Scholar]
- Libby RT, Li Y, Savinova OV, Barter J, Smith RS, Nickells RW, John SW. Susceptibility to neurodegeneration in a glaucoma is modified by Bax gene dosage. PLoS Genet. 2005c;1:17–26. doi: 10.1371/journal.pgen.0010004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Libby RT, Smith RS, Savinova OV, Zabaleta A, Martin JE, Gonzalez FJ, John SW. Modification of ocular defects in mouse developmental glaucoma models by tyrosinase. Science. 2003;299:1578–1581. doi: 10.1126/science.1080095. [DOI] [PubMed] [Google Scholar]
- Lindsey JD, Weinreb RN. Identification of the mouse uveoscleral outflow pathway using fluorescent dextran. Invest Ophthalmol Vis Sci. 2002;43:2201–2205. [PubMed] [Google Scholar]
- Lindsey JD, Weinreb RN. Elevated intraocular pressure and transgenic applications in the mouse. J Glaucoma. 2005;14:318–320. doi: 10.1097/01.ijg.0000169411.09258.f6. [DOI] [PubMed] [Google Scholar]
- Liu JH, Bouligny RP, Kripke DF, Weinreb RN. Nocturnal elevation of intraocular pressure is detectable in the sitting position. Invest Ophthalmol Vis Sci. 2003;44:4439–4442. doi: 10.1167/iovs.03-0349. [DOI] [PubMed] [Google Scholar]
- Liu P, Fu X, Johnson RL. Efficient in vivo doxycycline and cre recombinase-mediated inducible transgene activation in the murine trabecular meshwork. Invest Ophthalmol Vis Sci. 2011;52:969–974. doi: 10.1167/iovs.09-5052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y, Allingham RR. Molecular genetics in glaucoma. Exp Eye Res. 2011;93:331–339. doi: 10.1016/j.exer.2011.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lively GD, Jiang B, Hedberg-Buenz A, Chang B, Petersen GE, Wang K, Kuehn MH, Anderson MG. Genetic dependence of central corneal thickness among inbred strains of mice. Invest Ophthalmol Vis Sci. 2010a;51:160–171. doi: 10.1167/iovs.09-3429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lively GD, Koehn D, Hedberg-Buenz A, Wang K, Anderson MG. Quantitative trait loci associated with murine central corneal thickness. Physiol Genomics. 2010b;42:281–286. doi: 10.1152/physiolgenomics.00140.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowe RF. Aetiology of the anatomical basis for primary angle-closure glaucoma. Biometrical comparisons between normal eyes and eyes with primary angle-closure glaucoma. Br J Ophthalmol. 1970;54:161–169. doi: 10.1136/bjo.54.3.161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu Y, Vitart V, Burdon KP, Khor CC, Bykhovskaya Y, Mirshahi A, Hewitt AW, Koehn D, Hysi PG, Ramdas WD, Zeller T, Vithana EN, Cornes BK, Tay WT, Tai ES, Cheng CY, Liu J, Foo JN, Saw SM, Thorleifsson G, Stefansson K, Dimasi DP, Mills RA, Mountain J, Ang W, Hoehn R, Verhoeven VJ, Grus F, Wolfs R, Castagne R, Lackner KJ, Springelkamp H, Yang J, Jonasson F, Leung DY, Chen LJ, Tham CC, Rudan I, Vatavuk Z, Hayward C, Gibson J, Cree AJ, MacLeod A, Ennis S, Polasek O, Campbell H, Wilson JF, Viswanathan AC, Fleck B, Li X, Siscovick D, Taylor KD, Rotter JI, Yazar S, Ulmer M, Li J, Yaspan BL, Ozel AB, Richards JE, Moroi SE, Haines JL, Kang JH, Pasquale LR, Allingham RR, Ashley-Koch A, Consortium N, Mitchell P, Wang JJ, Wright AF, Pennell C, Spector TD, Young TL, Klaver CC, Martin NG, Montgomery GW, Anderson MG, Aung T, Willoughby CE, Wiggs JL, Pang CP, Thorsteinsdottir U, Lotery AJ, Hammond CJ, van Duijn CM, Hauser MA, Rabinowitz YS, Pfeiffer N, Mackey DA, Craig JE, Macgregor S, Wong TY. Genome-wide association analyses identify multiple loci associated with central corneal thickness and keratoconus. Nat Genet. 2013;45:155–163. doi: 10.1038/ng.2506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo N, Conwell MD, Chen X, Kettenhofen CI, Westlake CJ, Cantor LB, Wells CD, Weinreb RN, Corson TW, Spandau DF, Joos KM, Iomini C, Obukhov AG, Sun Y. Primary cilia signaling mediates intraocular pressure sensation. Proc Natl Acad Sci U S A. 2014;111:12871–12876. doi: 10.1073/pnas.1323292111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lye-Barthel M, Sun D, Jakobs TC. Morphology of astrocytes in a glaucomatous optic nerve. Invest Ophthalmol Vis Sci. 2013;54:909–917. doi: 10.1167/iovs.12-10109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyon MF, Ogunkolade BW, Brown MC, Atherton DJ, Perry VH. A gene affecting Wallerian nerve degeneration maps distally on mouse chromosome 4. Proc Natl Acad Sci U S A. 1993;90:9717–9720. doi: 10.1073/pnas.90.20.9717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mack TG, Reiner M, Beirowski B, Mi W, Emanuelli M, Wagner D, Thomson D, Gillingwater T, Court F, Conforti L, Fernando FS, Tarlton A, Andressen C, Addicks K, Magni G, Ribchester RR, Perry VH, Coleman MP. Wallerian degeneration of injured axons and synapses is delayed by a Ube4b/Nmnat chimeric gene. Nat Neurosci. 2001;4:1199–1206. doi: 10.1038/nn770. [DOI] [PubMed] [Google Scholar]
- Manolio TA, Collins FS, Cox NJ, Goldstein DB, Hindorff LA, Hunter DJ, McCarthy MI, Ramos EM, Cardon LR, Chakravarti A, Cho JH, Guttmacher AE, Kong A, Kruglyak L, Mardis E, Rotimi CN, Slatkin M, Valle D, Whittemore AS, Boehnke M, Clark AG, Eichler EE, Gibson G, Haines JL, Mackay TF, McCarroll SA, Visscher PM. Finding the missing heritability of complex diseases. Nature. 2009;461:747–753. doi: 10.1038/nature08494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mao M, Hedberg-Buenz A, Koehn D, John SW, Anderson MG. Anterior segment dysgenesis and early-onset glaucoma in nee mice with mutation of Sh3pxd2b. Invest Ophthalmol Vis Sci. 2011;52:2679–2688. doi: 10.1167/iovs.10-5993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maragakis NJ, Rothstein JD. Mechanisms of Disease: astrocytes in neurodegenerative disease. Nat Clin Pract Neurol. 2006;2:679–689. doi: 10.1038/ncpneuro0355. [DOI] [PubMed] [Google Scholar]
- Matsubara A, Nakazawa T, Husain D, Iliaki E, Connolly E, Michaud NA, Gragoudas ES, Miller JW. Investigating the effect of ciliary body photodynamic therapy in a glaucoma mouse model. Invest Ophthalmol Vis Sci. 2006;47:2498–2507. doi: 10.1167/iovs.05-0959. [DOI] [PubMed] [Google Scholar]
- McDowell CM, Luan T, Zhang Z, Putliwala T, Wordinger RJ, Millar JC, John SW, Pang IH, Clark AF. Mutant human myocilin induces strain specific differences in ocular hypertension and optic nerve damage in mice. Exp Eye Res. 2012;100:65–72. doi: 10.1016/j.exer.2012.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKay BS, Congrove NR, Johnson AA, Dismuke WM, Bowen TJ, Stamer WD. A role for myocilin in receptor-mediated endocytosis. PLoS One. 2013;8:e82301. doi: 10.1371/journal.pone.0082301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKeone R, Vieira H, Gregory-Evans K, Gregory-Evans CY, Denny P. Foxf2: a novel locus for anterior segment dysgenesis adjacent to the Foxc1 gene. PLoS One. 2011;6:e25489. doi: 10.1371/journal.pone.0025489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menaa F, Braghini CA, Vasconcellos JP, Menaa B, Costa VP, Figueiredo ES, Melo MB. Keeping an eye on myocilin: a complex molecule associated with primary open-angle glaucoma susceptibility. Molecules. 2011;16:5402–5421. doi: 10.3390/molecules16075402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minegishi Y, Iejima D, Kobayashi H, Chi ZL, Kawase K, Yamamoto T, Seki T, Yuasa S, Fukuda K, Iwata T. Enhanced optineurin E50K-TBK1 interaction evokes protein insolubility and initiates familial primary open-angle glaucoma. Hum Mol Genet. 2013;22:3559–3567. doi: 10.1093/hmg/ddt210. [DOI] [PubMed] [Google Scholar]
- Mo JS, Anderson MG, Gregory M, Smith RS, Savinova OV, Serreze DV, Ksander BR, Streilein JW, John SW. By altering ocular immune privilege, bone marrow-derived cells pathogenically contribute to DBA/2J pigmentary glaucoma. J Exp Med. 2003;197:1335–1344. doi: 10.1084/jem.20022041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monemi S, Spaeth G, DaSilva A, Popinchalk S, Ilitchev E, Liebmann J, Ritch R, Heon E, Crick RP, Child A, Sarfarazi M. Identification of a novel adult-onset primary open-angle glaucoma (POAG) gene on 5q22.1. Hum Mol Genet. 2005;14:725–733. doi: 10.1093/hmg/ddi068. [DOI] [PubMed] [Google Scholar]
- Moore D, Harris A, Wudunn D, Kheradiya N, Siesky B. Dysfunctional regulation of ocular blood flow: A risk factor for glaucoma? Clin Ophthalmol. 2008;2:849–861. doi: 10.2147/opth.s2774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morquette JB, Di Polo A. Dendritic and synaptic protection: is it enough to save the retinal ganglion cell body and axon? J Neuroophthalmol. 2008;28:144–154. doi: 10.1097/WNO.0b013e318177edf0. [DOI] [PubMed] [Google Scholar]
- Morrison JC, Cepurna WO, Johnson EC. Modeling glaucoma in rats by sclerosing aqueous outflow pathways to elevate intraocular pressure. Exp Eye Res. 2015 doi: 10.1016/j.exer.2015.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrison JC, Cepurna Ying Guo WO, Johnson EC. Pathophysiology of human glaucomatous optic nerve damage: insights from rodent models of glaucoma. Exp Eye Res. 2011;93:156–164. doi: 10.1016/j.exer.2010.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mosaed S, Liu JH, Weinreb RN. Correlation between office and peak nocturnal intraocular pressures in healthy subjects and glaucoma patients. Am J Ophthalmol. 2005;139:320–324. doi: 10.1016/j.ajo.2004.09.062. [DOI] [PubMed] [Google Scholar]
- Mrak RE, Griffin WS. Glia and their cytokines in progression of neurodegeneration. Neurobiol Aging. 2005;26:349–354. doi: 10.1016/j.neurobiolaging.2004.05.010. [DOI] [PubMed] [Google Scholar]
- Nagaraju M, Saleh M, Porciatti V. IOP-dependent retinal ganglion cell dysfunction in glaucomatous DBA/2J mice. Invest Ophthalmol Vis Sci. 2007;48:4573–4579. doi: 10.1167/iovs.07-0582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nair KS, Barbay J, Smith RS, Masli S, John SW. Determining immune components necessary for progression of pigment dispersing disease to glaucoma in DBA/2J mice. BMC Genet. 2014;15:42. doi: 10.1186/1471-2156-15-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nair KS, Hmani-Aifa M, Ali Z, Kearney AL, Ben Salem S, Macalinao DG, Cosma IM, Bouassida W, Hakim B, Benzina Z, Soto I, Soderkvist P, Howell GR, Smith RS, Ayadi H, John SW. Alteration of the serine protease PRSS56 causes angle-closure glaucoma in mice and posterior microphthalmia in humans and mice. Nat Genet. 2011;43:579–584. doi: 10.1038/ng.813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Namekata K, Kimura A, Kawamura K, Guo X, Harada C, Tanaka K, Harada T. Dock3 attenuates neural cell death due to NMDA neurotoxicity and oxidative stress in a mouse model of normal tension glaucoma. Cell Death Differ. 2013;20:1250–1256. doi: 10.1038/cdd.2013.91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Napier HR, Kidson SH. Molecular events in early development of the ciliary body: a question of folding. Exp Eye Res. 2007;84:615–625. doi: 10.1016/j.exer.2006.07.012. [DOI] [PubMed] [Google Scholar]
- Ng SK, Casson RJ, Burdon KP, Craig JE. Chromosome 9p21 primary open-angle glaucoma susceptibility locus: a review. Clin Experiment Ophthalmol. 2014;42:25–32. doi: 10.1111/ceo.12234. [DOI] [PubMed] [Google Scholar]
- Nguyen JV, Soto I, Kim KY, Bushong EA, Oglesby E, Valiente-Soriano FJ, Yang Z, Davis CH, Bedont JL, Son JL, Wei JO, Buchman VL, Zack DJ, Vidal-Sanz M, Ellisman MH, Marsh-Armstrong N. Myelination transition zone astrocytes are constitutively phagocytic and have synuclein dependent reactivity in glaucoma. Proc Natl Acad Sci U S A. 2011;108:1176–1181. doi: 10.1073/pnas.1013965108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nickells RW. Retinal ganglion cell death in glaucoma: the how, the why, and the maybe. J Glaucoma. 1996;5:345–356. [PubMed] [Google Scholar]
- Nickells RW. Apoptosis of retinal ganglion cells in glaucoma: an update of the molecular pathways involved in cell death. Surv Ophthalmol. 1999;43(Suppl 1):S151–161. doi: 10.1016/s0039-6257(99)00029-6. [DOI] [PubMed] [Google Scholar]
- Nickells RW, Howell GR, Soto I, John SW. Under pressure: cellular and molecular responses during glaucoma, a common neurodegeneration with axonopathy. Annu Rev Neurosci. 2012;35:153–179. doi: 10.1146/annurev.neuro.051508.135728. [DOI] [PubMed] [Google Scholar]
- Nickells RW, Semaan SJ, Schlamp CL. Involvement of the Bcl2 gene family in the signaling and control of retinal ganglion cell death. Prog Brain Res. 2008;173:423–435. doi: 10.1016/S0079-6123(08)01129-1. [DOI] [PubMed] [Google Scholar]
- Nusbaum DM, Wu SM, Frankfort BJ. Elevated intracranial pressure causes optic nerve and retinal ganglion cell degeneration in mice. Exp Eye Res. 2015;136:38–44. doi: 10.1016/j.exer.2015.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orr A, Dube MP, Zenteno JC, Jiang H, Asselin G, Evans SC, Caqueret A, Lakosha H, Letourneau L, Marcadier J, Matsuoka M, Macgillivray C, Nightingale M, Papillon-Cavanagh S, Perry S, Provost S, Ludman M, Guernsey DL, Samuels ME. Mutations in a novel serine protease PRSS56 in families with nanophthalmos. Mol Vis. 2011;17:1850–1861. [PMC free article] [PubMed] [Google Scholar]
- Orsini F, De Blasio D, Zangari R, Zanier ER, De Simoni MG. Versatility of the complement system in neuroinflammation, neurodegeneration and brain homeostasis. Front Cell Neurosci. 2014;8:380. doi: 10.3389/fncel.2014.00380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Overby DR, Clark AF. Animal models of glucocorticoid-induced glaucoma. Exp Eye Res. 2015 doi: 10.1016/j.exer.2015.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pang CP, Leung YF, Fan B, Baum L, Tong WC, Lee WS, Chua JK, Fan DS, Liu Y, Lam DS. TIGR/MYOC gene sequence alterations in individuals with and without primary open-angle glaucoma. Invest Ophthalmol Vis Sci. 2002;43:3231–3235. [PubMed] [Google Scholar]
- Pang IH, Millar JC, Clark AF. Elevation of intraocular pressure in rodents using viral vectors targeting the trabecular meshwork. Exp Eye Res. 2015 doi: 10.1016/j.exer.2015.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park DY, Lee J, Park I, Choi D, Lee S, Song S, Hwang Y, Hong KY, Nakaoka Y, Makinen T, Kim P, Alitalo K, Hong YK, Koh GY. Lymphatic regulator PROX1 determines Schlemm’s canal integrity and identity. J Clin Invest. 2014;124:3960–3974. doi: 10.1172/JCI75392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perry VH, Brown MC, Tsao JW. The Effectiveness of the Gene Which Slows the Rate of Wallerian Degeneration in C57BL/Ola Mice Declines With Age. Eur J Neurosci. 1992;4:1000–1002. doi: 10.1111/j.1460-9568.1992.tb00126.x. [DOI] [PubMed] [Google Scholar]
- Polansky JR, Fauss DJ, Chen P, Chen H, Lutjen-Drecoll E, Johnson D, Kurtz RM, Ma ZD, Bloom E, Nguyen TD. Cellular pharmacology and molecular biology of the trabecular meshwork inducible glucocorticoid response gene product. Ophthalmologica. 1997;211:126–139. doi: 10.1159/000310780. [DOI] [PubMed] [Google Scholar]
- Prasanna G, Hulet C, Desai D, Krishnamoorthy RR, Narayan S, Brun AM, Suburo AM, Yorio T. Effect of elevated intraocular pressure on endothelin-1 in a rat model of glaucoma. Pharmacol Res. 2005;51:41–50. doi: 10.1016/j.phrs.2004.04.006. [DOI] [PubMed] [Google Scholar]
- Prasanna G, Krishnamoorthy R, Clark AF, Wordinger RJ, Yorio T. Human optic nerve head astrocytes as a target for endothelin-1. Invest Ophthalmol Vis Sci. 2002;43:2704–2713. [PubMed] [Google Scholar]
- Prasanna G, Krishnamoorthy R, Yorio T. Endothelin, astrocytes and glaucoma. Exp Eye Res. 2011;93:170–177. doi: 10.1016/j.exer.2010.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prasov L, Nagy M, Rudolph DD, Glaser T. Math5 (Atoh7) gene dosage limits retinal ganglion cell genesis. Neuroreport. 2012;23:631–634. doi: 10.1097/WNR.0b013e328355f260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quigley H, Anderson DR. The dynamics and location of axonal transport blockade by acute intraocular pressure elevation in primate optic nerve. Invest Ophthalmol. 1976;15:606–616. [PubMed] [Google Scholar]
- Quigley HA. Angle-closure glaucoma-simpler answers to complex mechanisms: LXVI Edward Jackson Memorial Lecture. Am J Ophthalmol. 2009;148:657–669. e651. doi: 10.1016/j.ajo.2009.08.009. [DOI] [PubMed] [Google Scholar]
- Quigley HA, Addicks EM. Chronic experimental glaucoma in primates. II Effect of extended intraocular pressure elevation on optic nerve head and axonal transport. Invest Ophthalmol Vis Sci. 1980;19:137–152. [PubMed] [Google Scholar]
- Quigley HA, Addicks EM. Regional differences in the structure of the lamina cribrosa and their relation to glaucomatous optic nerve damage. Arch Ophthalmol. 1981;99:137–143. doi: 10.1001/archopht.1981.03930010139020. [DOI] [PubMed] [Google Scholar]
- Quigley HA, Nickells RW, Kerrigan LA, Pease ME, Thibault DJ, Zack DJ. Retinal ganglion cell death in experimental glaucoma and after axotomy occurs by apoptosis. Invest Ophthalmol Vis Sci. 1995;36:774–786. [PubMed] [Google Scholar]
- Rattner A, Nathans J. The genomic response to retinal disease and injury: evidence for endothelin signaling from photoreceptors to glia. J Neurosci. 2005;25:4540–4549. doi: 10.1523/JNEUROSCI.0492-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reis LM, Semina EV. Genetics of anterior segment dysgenesis disorders. Curr Opin Ophthalmol. 2011;22:314–324. doi: 10.1097/ICU.0b013e328349412b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ren L, Danias J. A role for complement in glaucoma? Adv Exp Med Biol. 2010;703:95–104. doi: 10.1007/978-1-4419-5635-4_7. [DOI] [PubMed] [Google Scholar]
- Resch ZT, Fautsch MP. Glaucoma-associated myocilin: a better understanding but much more to learn. Exp Eye Res. 2009;88:704–712. doi: 10.1016/j.exer.2008.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rezaie T, Child A, Hitchings R, Brice G, Miller L, Coca-Prados M, Heon E, Krupin T, Ritch R, Kreutzer D, Crick RP, Sarfarazi M. Adult-onset primary open-angle glaucoma caused by mutations in optineurin. Science. 2002;295:1077–1079. doi: 10.1126/science.1066901. [DOI] [PubMed] [Google Scholar]
- Ritch R, Darbro B, Menon G, Khanna CL, Solivan-Timpe F, Roos BR, Sarfarzi M, Kawase K, Yamamoto T, Robin AL, Lotery AJ, Fingert JH. TBK1 gene duplication and normal-tension glaucoma. JAMA Ophthalmol. 2014;132:544–548. doi: 10.1001/jamaophthalmol.2014.104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sander JD, Joung JK. CRISPR-Cas systems for editing, regulating and targeting genomes. Nat Biotechnol. 2014;32:347–355. doi: 10.1038/nbt.2842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarode B, Nowell CS, Ihm J, Kostic C, Arsenijevic Y, Moulin AP, Schorderet DF, Beermann F, Radtke F. Notch signaling in the pigmented epithelium of the anterior eye segment promotes ciliary body development at the expense of iris formation. Pigment Cell Melanoma Res. 2014;27:580–589. doi: 10.1111/pcmr.12236. [DOI] [PubMed] [Google Scholar]
- Savinova OV, Sugiyama F, Martin JE, Tomarev SI, Paigen BJ, Smith RS, John SW. Intraocular pressure in genetically distinct mice: an update and strain survey. BMC Genet. 2001;2:12. doi: 10.1186/1471-2156-2-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlamp CL, Li Y, Dietz JA, Janssen KT, Nickells RW. Progressive ganglion cell loss and optic nerve degeneration in DBA/2J mice is variable and asymmetric. BMC Neurosci. 2006;7:66. doi: 10.1186/1471-2202-7-66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmucker C, Schaeffel F. A paraxial schematic eye model for the growing C57BL/6 mouse. Vision Res. 2004;44:1857–1867. doi: 10.1016/j.visres.2004.03.011. [DOI] [PubMed] [Google Scholar]
- Schofield PN, Hoehndorf R, Gkoutos GV. Mouse genetic and phenotypic resources for human genetics. Hum Mutat. 2012;33:826–836. doi: 10.1002/humu.22077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuettauf F, Quinto K, Naskar R, Zurakowski D. Effects of anti-glaucoma medications on ganglion cell survival: the DBA/2J mouse model. Vision Res. 2002;42:2333–2337. doi: 10.1016/s0042-6989(02)00188-8. [DOI] [PubMed] [Google Scholar]
- Seitz R, Ohlmann A, Tamm ER. The role of Muller glia and microglia in glaucoma. Cell Tissue Res. 2013;353:339–345. doi: 10.1007/s00441-013-1666-y. [DOI] [PubMed] [Google Scholar]
- Semba K, Namekata K, Guo X, Harada C, Harada T, Mitamura Y. Renin-angiotensin system regulates neurodegeneration in a mouse model of normal tension glaucoma. Cell Death Dis. 2014;5:e1333. doi: 10.1038/cddis.2014.296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Senatorov V, Malyukova I, Fariss R, Wawrousek EF, Swaminathan S, Sharan SK, Tomarev S. Expression of mutated mouse myocilin induces open-angle glaucoma in transgenic mice. J Neurosci. 2006;26:11903–11914. doi: 10.1523/JNEUROSCI.3020-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shepard AR, Jacobson N, Millar JC, Pang IH, Steely HT, Searby CC, Sheffield VC, Stone EM, Clark AF. Glaucoma-causing myocilin mutants require the Peroxisomal targeting signal-1 receptor (PTS1R) to elevate intraocular pressure. Hum Mol Genet. 2007;16:609–617. doi: 10.1093/hmg/ddm001. [DOI] [PubMed] [Google Scholar]
- Shoshani YZ, Harris A, Shoja MM, Rusia D, Siesky B, Arieli Y, Wirostko B. Endothelin and its suspected role in the pathogenesis and possible treatment of glaucoma. Curr Eye Res. 2012;37:1–11. doi: 10.3109/02713683.2011.622849. [DOI] [PubMed] [Google Scholar]
- Siddiqui Y, Ten Hulzen RD, Cameron JD, Hodge DO, Johnson DH. What is the risk of developing pigmentary glaucoma from pigment dispersion syndrome? Am J Ophthalmol. 2003;135:794–799. doi: 10.1016/s0002-9394(02)02289-4. [DOI] [PubMed] [Google Scholar]
- Smith RS, Korb D, John SW. A goniolens for clinical monitoring of the mouse iridocorneal angle and optic nerve. Mol Vis. 2002;8:26–31. [PubMed] [Google Scholar]
- Smith RS, Zabaleta A, Kume T, Savinova OV, Kidson SH, Martin JE, Nishimura DY, Alward WL, Hogan BL, John SW. Haploinsufficiency of the transcription factors FOXC1 and FOXC2 results in aberrant ocular development. Hum Mol Genet. 2000;9:1021–1032. doi: 10.1093/hmg/9.7.1021. [DOI] [PubMed] [Google Scholar]
- Smith RS, Zabaleta A, Savinova OV, John SW. The mouse anterior chamber angle and trabecular meshwork develop without cell death. BMC Dev Biol. 2001;1:3. doi: 10.1186/1471-213X-1-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smithies O, Kim HS. Targeted gene duplication and disruption for analyzing quantitative genetic traits in mice. Proc Natl Acad Sci U S A. 1994;91:3612–3615. doi: 10.1073/pnas.91.9.3612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soto I, Howell GR. The complex role of neuroinflammation in glaucoma. Cold Spring Harb Perspect Med. 2014:4. doi: 10.1101/cshperspect.a017269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sowden JC. Molecular and developmental mechanisms of anterior segment dysgenesis. Eye (Lond) 2007;21:1310–1318. doi: 10.1038/sj.eye.6702852. [DOI] [PubMed] [Google Scholar]
- Springelkamp H, Hohn R, Mishra A, Hysi PG, Khor CC, Loomis SJ, Bailey JN, Gibson J, Thorleifsson G, Janssen SF, Luo X, Ramdas WD, Vithana E, Nongpiur ME, Montgomery GW, Xu L, Mountain JE, Gharahkhani P, Lu Y, Amin N, Karssen LC, Sim KS, van Leeuwen EM, Iglesias AI, Verhoeven VJ, Hauser MA, Loon SC, Despriet DD, Nag A, Venturini C, Sanfilippo PG, Schillert A, Kang JH, Landers J, Jonasson F, Cree AJ, van Koolwijk LM, Rivadeneira F, Souzeau E, Jonsson V, Menon G, Weinreb RN, de Jong PT, Oostra BA, Uitterlinden AG, Hofman A, Ennis S, Thorsteinsdottir U, Burdon KP, Consortium N, Spector TD, Mirshahi A, Saw SM, Vingerling JR, Teo YY, Haines JL, Wolfs RC, Lemij HG, Tai ES, Jansonius NM, Jonas JB, Cheng CY, Aung T, Viswanathan AC, Klaver CC, Craig JE, Macgregor S, Mackey DA, Lotery AJ, Stefansson K, Bergen AA, Young TL, Wiggs JL, Pfeiffer N, Wong TY, Pasquale LR, Hewitt AW, van Duijn CM, Hammond CJ Blue Mountains Eye Study Gg, Wellcome Trust Case Control C. Meta-analysis of genome-wide association studies identifies novel loci that influence cupping and the glaucomatous process. Nat Commun. 2014;5:4883. doi: 10.1038/ncomms5883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stasi K, Nagel D, Yang X, Wang RF, Ren L, Podos SM, Mittag T, Danias J. Complement component 1Q (C1Q) upregulation in retina of murine, primate, and human glaucomatous eyes. Invest Ophthalmol Vis Sci. 2006;47:1024–1029. doi: 10.1167/iovs.05-0830. [DOI] [PubMed] [Google Scholar]
- Steinhart MR, Cone FE, Nguyen C, Nguyen TD, Pease ME, Puk O, Graw J, Oglesby EN, Quigley HA. Mice with an induced mutation in collagen 8A2 develop larger eyes and are resistant to retinal ganglion cell damage in an experimental glaucoma model. Mol Vis. 2012;18:1093–1106. [PMC free article] [PubMed] [Google Scholar]
- Stevens B, Allen NJ, Vazquez LE, Howell GR, Christopherson KS, Nouri N, Micheva KD, Mehalow AK, Huberman AD, Stafford B, Sher A, Litke AM, Lambris JD, Smith SJ, John SW, Barres BA. The classical complement cascade mediates CNS synapse elimination. Cell. 2007;131:1164–1178. doi: 10.1016/j.cell.2007.10.036. [DOI] [PubMed] [Google Scholar]
- Stone EM, Fingert JH, Alward WL, Nguyen TD, Polansky JR, Sunden SL, Nishimura D, Clark AF, Nystuen A, Nichols BE, Mackey DA, Ritch R, Kalenak JW, Craven ER, Sheffield VC. Identification of a gene that causes primary open angle glaucoma. Science. 1997;275:668–670. doi: 10.1126/science.275.5300.668. [DOI] [PubMed] [Google Scholar]
- Takahashi N, Smithies O. Human genetics, animal models and computer simulations for studying hypertension. Trends Genet. 2004;20:136–145. doi: 10.1016/j.tig.2004.01.004. [DOI] [PubMed] [Google Scholar]
- Tamm ER, Russell P, Epstein DL, Johnson DH, Piatigorsky J. Modulation of myocilin/TIGR expression in human trabecular meshwork. Invest Ophthalmol Vis Sci. 1999;40:2577–2582. [PubMed] [Google Scholar]
- Tezel G, Yang X, Luo C, Kain AD, Powell DW, Kuehn MH, Kaplan HJ. Oxidative stress and the regulation of complement activation in human glaucoma. Invest Ophthalmol Vis Sci. 2010;51:5071–5082. doi: 10.1167/iovs.10-5289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomson BR, Heinen S, Jeansson M, Ghosh AK, Fatima A, Sung HK, Onay T, Chen H, Yamaguchi S, Economides AN, Flenniken A, Gale NW, Hong YK, Fawzi A, Liu X, Kume T, Quaggin SE. A lymphatic defect causes ocular hypertension and glaucoma in mice. J Clin Invest. 2014;124:4320–4324. doi: 10.1172/JCI77162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trantow CM, Mao M, Petersen GE, Alward EM, Alward WL, Fingert JH, Anderson MG. Lyst mutation in mice recapitulates iris defects of human exfoliation syndrome. Invest Ophthalmol Vis Sci. 2009;50:1205–1214. doi: 10.1167/iovs.08-2791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tseng HC, Riday TT, McKee C, Braine CE, Bomze H, Barak I, Marean-Reardon C, John SW, Philpot BD, Ehlers MD. Visual impairment in an optineurin mouse model of primary open-angle glaucoma. Neurobiol Aging. 2015;36:2201–2212. doi: 10.1016/j.neurobiolaging.2015.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tucker BA, Solivan-Timpe F, Roos BR, Anfinson KR, Robin AL, Wiley LA, Mullins RF, Fingert JH. Duplication of TBK1 Stimulates Autophagy in iPSC-derived Retinal Cells from a Patient with Normal Tension Glaucoma. J Stem Cell Res Ther. 2014;3:161. doi: 10.4172/2157-7633.1000161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urnov FD, Rebar EJ, Holmes MC, Zhang HS, Gregory PD. Genome editing with engineered zinc finger nucleases. Nat Rev Genet. 2010;11:636–646. doi: 10.1038/nrg2842. [DOI] [PubMed] [Google Scholar]
- Van Agtmael T, Schlotzer-Schrehardt U, McKie L, Brownstein DG, Lee AW, Cross SH, Sado Y, Mullins JJ, Poschl E, Jackson IJ. Dominant mutations of Col4a1 result in basement membrane defects which lead to anterior segment dysgenesis and glomerulopathy. Hum Mol Genet. 2005;14:3161–3168. doi: 10.1093/hmg/ddi348. [DOI] [PubMed] [Google Scholar]
- van der Weyden L, Bradley A. Mouse chromosome engineering for modeling human disease. Annu Rev Genomics Hum Genet. 2006;7:247–276. doi: 10.1146/annurev.genom.7.080505.115741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Weyden L, White JK, Adams DJ, Logan DW. The mouse genetics toolkit: revealing function and mechanism. Genome Biol. 2011;12:224. doi: 10.1186/gb-2011-12-6-224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verkhratsky A, Parpura V, Pekna M, Pekny M, Sofroniew M. Glia in the pathogenesis of neurodegenerative diseases. Biochem Soc Trans. 2014;42:1291–1301. doi: 10.1042/BST20140107. [DOI] [PubMed] [Google Scholar]
- Verouti SN, Boscardin E, Hummler E, Frateschi S. Regulation of blood pressure and renal function by NCC and ENaC: lessons from genetically engineered mice. Curr Opin Pharmacol. 2015;21:60–72. doi: 10.1016/j.coph.2014.12.012. [DOI] [PubMed] [Google Scholar]
- Wang WH, Millar JC, Pang IH, Wax MB, Clark AF. Noninvasive measurement of rodent intraocular pressure with a rebound tonometer. Invest Ophthalmol Vis Sci. 2005;46:4617–4621. doi: 10.1167/iovs.05-0781. [DOI] [PubMed] [Google Scholar]
- Ward NJ, Ho KW, Lambert WS, Weitlauf C, Calkins DJ. Absence of transient receptor potential vanilloid-1 accelerates stress-induced axonopathy in the optic projection. J Neurosci. 2014;34:3161–3170. doi: 10.1523/JNEUROSCI.4089-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weidberg H, Elazar Z. TBK1 mediates crosstalk between the innate immune response and autophagy. Sci Signal. 2011;4:pe39. doi: 10.1126/scisignal.2002355. [DOI] [PubMed] [Google Scholar]
- Weng J, Luo J, Cheng X, Jin C, Zhou X, Qu J, Tu L, Ai D, Li D, Wang J, Martin JF, Amendt BA, Liu M. Deletion of G protein-coupled receptor 48 leads to ocular anterior segment dysgenesis (ASD) through down-regulation of Pitx2. Proc Natl Acad Sci U S A. 2008;105:6081–6086. doi: 10.1073/pnas.0708257105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitlock NA, McKnight B, Corcoran KN, Rodriguez LA, Rice DS. Increased intraocular pressure in mice treated with dexamethasone. Invest Ophthalmol Vis Sci. 2010;51:6496–6503. doi: 10.1167/iovs.10-5430. [DOI] [PubMed] [Google Scholar]
- Whitmore AV, Libby RT, John SW. Glaucoma: thinking in new ways-a role for autonomous axonal self-destruction and other compartmentalised processes? Prog Retin Eye Res. 2005;24:639–662. doi: 10.1016/j.preteyeres.2005.04.004. [DOI] [PubMed] [Google Scholar]
- Wiggs JL, Hauser MA, Abdrabou W, Allingham RR, Budenz DL, Delbono E, Friedman DS, Kang JH, Gaasterland D, Gaasterland T, Lee RK, Lichter PR, Loomis S, Liu Y, McCarty C, Medeiros FA, Moroi SE, Olson LM, Realini A, Richards JE, Rozsa FW, Schuman JS, Singh K, Stein JD, Vollrath D, Weinreb RN, Wollstein G, Yaspan BL, Yoneyama S, Zack D, Zhang K, Pericak-Vance M, Pasquale LR, Haines JL. The NEIGHBOR consortium primary open-angle glaucoma genome-wide association study: rationale, study design, and clinical variables. J Glaucoma. 2013;22:517–525. doi: 10.1097/IJG.0b013e31824d4fd8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiggs JL, Yaspan BL, Hauser MA, Kang JH, Allingham RR, Olson LM, Abdrabou W, Fan BJ, Wang DY, Brodeur W, Budenz DL, Caprioli J, Crenshaw A, Crooks K, Delbono E, Doheny KF, Friedman DS, Gaasterland D, Gaasterland T, Laurie C, Lee RK, Lichter PR, Loomis S, Liu Y, Medeiros FA, McCarty C, Mirel D, Moroi SE, Musch DC, Realini A, Rozsa FW, Schuman JS, Scott K, Singh K, Stein JD, Trager EH, Vanveldhuisen P, Vollrath D, Wollstein G, Yoneyama S, Zhang K, Weinreb RN, Ernst J, Kellis M, Masuda T, Zack D, Richards JE, Pericak-Vance M, Pasquale LR, Haines JL. Common variants at 9p21 and 8q22 are associated with increased susceptibility to optic nerve degeneration in glaucoma. PLoS Genet. 2012;8:e1002654. doi: 10.1371/journal.pgen.1002654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wild P, Farhan H, McEwan DG, Wagner S, Rogov VV, Brady NR, Richter B, Korac J, Waidmann O, Choudhary C, Dotsch V, Bumann D, Dikic I. Phosphorylation of the autophagy receptor optineurin restricts Salmonella growth. Science. 2011;333:228–233. doi: 10.1126/science.1205405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams PA, Howell GR, Barbay JM, Braine CE, Sousa GL, John SW, Morgan JE. Retinal ganglion cell dendritic atrophy in DBA/2J glaucoma. PLoS One. 2013;8:e72282. doi: 10.1371/journal.pone.0072282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong AA, Brown RE. A neurobehavioral analysis of the prevention of visual impairment in the DBA/2J mouse model of glaucoma. Invest Ophthalmol Vis Sci. 2012;53:5956–5966. doi: 10.1167/iovs.12-10020. [DOI] [PubMed] [Google Scholar]
- Wong AA, Brown RE. Prevention of vision loss protects against age-related impairment in learning and memory performance in DBA/2J mice. Front Aging Neurosci. 2013;5:52. doi: 10.3389/fnagi.2013.00052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan X, Sabrautzki S, Horsch M, Fuchs H, Gailus-Durner V, Beckers J, Hrabe de Angelis M, Graw J. Peroxidasin is essential for eye development in the mouse. Hum Mol Genet. 2014;23:5597–5614. doi: 10.1093/hmg/ddu274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ylikarppa R, Eklund L, Sormunen R, Kontiola AI, Utriainen A, Maatta M, Fukai N, Olsen BR, Pihlajaniemi T. Lack of type XVIII collagen results in anterior ocular defects. FASEB J. 2003;17:2257–2259. doi: 10.1096/fj.02-1001fje. [DOI] [PubMed] [Google Scholar]
- Yu DY, Cringle SJ, Balaratnasingam C, Morgan WH, Yu PK, Su EN. Retinal ganglion cells: Energetics, compartmentation, axonal transport, cytoskeletons and vulnerability. Prog Retin Eye Res. 2013;36:217–246. doi: 10.1016/j.preteyeres.2013.07.001. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Davidson BR, Stamer WD, Barton JK, Marmorstein LY, Marmorstein AD. Enhanced inflow and outflow rates despite lower IOP in bestrophin-2-deficient mice. Invest Ophthalmol Vis Sci. 2009;50:765–770. doi: 10.1167/iovs.08-2501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou X, Li F, Kong L, Tomita H, Li C, Cao W. Involvement of inflammation, degradation, and apoptosis in a mouse model of glaucoma. J Biol Chem. 2005;280:31240–31248. doi: 10.1074/jbc.M502641200. [DOI] [PubMed] [Google Scholar]
- Zhou Y, Grinchuk O, Tomarev SI. Transgenic mice expressing the Tyr437His mutant of human myocilin protein develop glaucoma. Invest Ophthalmol Vis Sci. 2008;49:1932–1939. doi: 10.1167/iovs.07-1339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Y, Tanzie C, Yan Z, Chen S, Duncan M, Gaudenz K, Li H, Seidel C, Lewis B, Moran A, Libby RT, Kiernan AE, Xie T. Notch2 regulates BMP signaling and epithelial morphogenesis in the ciliary body of the mouse eye. Proc Natl Acad Sci U S A. 2013;110:8966–8971. doi: 10.1073/pnas.1218145110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zillig M, Wurm A, Grehn FJ, Russell P, Tamm ER. Overexpression and properties of wild-type and Tyr437His mutated myocilin in the eyes of transgenic mice. Invest Ophthalmol Vis Sci. 2005;46:223–234. doi: 10.1167/iovs.04-0988. [DOI] [PubMed] [Google Scholar]
- Zode GS, Kuehn MH, Nishimura DY, Searby CC, Mohan K, Grozdanic SD, Bugge K, Anderson MG, Clark AF, Stone EM, Sheffield VC. Reduction of ER stress via a chemical chaperone prevents disease phenotypes in a mouse model of primary open angle glaucoma. J Clin Invest. 2011;121:3542–3553. doi: 10.1172/JCI58183. [DOI] [PMC free article] [PubMed] [Google Scholar]