Abstract
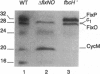
We report the discovery of a Bradyrhizobium japonicum gene cluster (fixNOQP) in which mutations resulted in defective soybean root-nodule bacteroid development and symbiotic nitrogen fixation. The predicted, DNA-derived protein sequences suggested that FixN is a heme b and copper-binding oxidase subunit, FixO a monoheme cytochrome c, FixQ a polypeptide of 54 amino acids, and FixP a diheme cytochrome c and that they are all membrane-bound. The isolation and analysis of membrane proteins from B. japonicum wild-type and mutant cells revealed two c-type cytochromes of 28 and 32 kDa as the likely products of the fixO and fixP genes and showed that both were synthesized only under oxygen-limited growth conditions. Furthermore, fixN insertion and fixNO deletion mutants grown microaerobically or anaerobically (with nitrate) exhibited a strong decrease in whole-cell oxidase activity as compared with the wild type. The data suggest that the fixNOQP gene products are induced at low oxygen concentrations and constitute a member of the bacterial heme/copper cytochrome oxidase superfamily. The described features are compatible with the postulate that this oxidase complex is specifically required to support bacterial respiration in endosymbiosis.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anthamatten D., Hennecke H. The regulatory status of the fixL- and fixJ-like genes in Bradyrhizobium japonicum may be different from that in Rhizobium meliloti. Mol Gen Genet. 1991 Jan;225(1):38–48. doi: 10.1007/BF00282640. [DOI] [PubMed] [Google Scholar]
- Anthamatten D., Scherb B., Hennecke H. Characterization of a fixLJ-regulated Bradyrhizobium japonicum gene sharing similarity with the Escherichia coli fnr and Rhizobium meliloti fixK genes. J Bacteriol. 1992 Apr;174(7):2111–2120. doi: 10.1128/jb.174.7.2111-2120.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Appleby C. A., James P., Hennecke H. Characterization of three soluble c-type cytochromes isolated from soybean root nodule bacteroids of Bradyrhizobium japonicum strain CC705. FEMS Microbiol Lett. 1991 Oct 1;67(2):137–144. doi: 10.1016/0378-1097(91)90344-a. [DOI] [PubMed] [Google Scholar]
- Batut J., Daveran-Mingot M. L., David M., Jacobs J., Garnerone A. M., Kahn D. fixK, a gene homologous with fnr and crp from Escherichia coli, regulates nitrogen fixation genes both positively and negatively in Rhizobium meliloti. EMBO J. 1989 Apr;8(4):1279–1286. doi: 10.1002/j.1460-2075.1989.tb03502.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bott M., Bolliger M., Hennecke H. Genetic analysis of the cytochrome c-aa3 branch of the Bradyrhizobium japonicum respiratory chain. Mol Microbiol. 1990 Dec;4(12):2147–2157. doi: 10.1111/j.1365-2958.1990.tb00576.x. [DOI] [PubMed] [Google Scholar]
- Bott M., Preisig O., Hennecke H. Genes for a second terminal oxidase in Bradyrhizobium japonicum. Arch Microbiol. 1992;158(5):335–343. doi: 10.1007/BF00245362. [DOI] [PubMed] [Google Scholar]
- Bott M., Ritz D., Hennecke H. The Bradyrhizobium japonicum cycM gene encodes a membrane-anchored homolog of mitochondrial cytochrome c. J Bacteriol. 1991 Nov;173(21):6766–6772. doi: 10.1128/jb.173.21.6766-6772.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chepuri V., Lemieux L., Au D. C., Gennis R. B. The sequence of the cyo operon indicates substantial structural similarities between the cytochrome o ubiquinol oxidase of Escherichia coli and the aa3-type family of cytochrome c oxidases. J Biol Chem. 1990 Jul 5;265(19):11185–11192. [PubMed] [Google Scholar]
- Daniel R. M., Appleby C. A. Anaerobic-nitrate, symbiotic and aerobic growth of Rhizobium japonicum: effects on cytochrome P 450 , other haemoproteins, nitrate and nitrite reductases. Biochim Biophys Acta. 1972 Sep 20;275(3):347–354. doi: 10.1016/0005-2728(72)90215-0. [DOI] [PubMed] [Google Scholar]
- Esposti M. D. Prediction and comparison of the haem-binding sites in membrane haemoproteins. Biochim Biophys Acta. 1989 Dec 7;977(3):249–265. doi: 10.1016/s0005-2728(89)80079-9. [DOI] [PubMed] [Google Scholar]
- Gabel C., Maier R. J. Nucleotide sequence of the coxA gene encoding subunit I of cytochrome aa3 of Bradyrhizobium japonicum. Nucleic Acids Res. 1990 Oct 25;18(20):6143–6143. doi: 10.1093/nar/18.20.6143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gennis R. B. Site-directed mutagenesis studies on subunit I of the aa3-type cytochrome c oxidase of Rhodobacter sphaeroides: a brief review of progress to date. Biochim Biophys Acta. 1992 Jul 17;1101(2):184–187. [PubMed] [Google Scholar]
- Kahn D., David M., Domergue O., Daveran M. L., Ghai J., Hirsch P. R., Batut J. Rhizobium meliloti fixGHI sequence predicts involvement of a specific cation pump in symbiotic nitrogen fixation. J Bacteriol. 1989 Feb;171(2):929–939. doi: 10.1128/jb.171.2.929-939.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keister D. L., Marsh S. S. Hemoproteins of Bradyrhizobium japonicum Cultured Cells and Bacteroids. Appl Environ Microbiol. 1990 Sep;56(9):2736–2741. doi: 10.1128/aem.56.9.2736-2741.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsushita K., Shinagawa E., Adachi O., Ameyama M. o-Type cytochrome oxidase in the membrane of aerobically grown Pseudomonas aeruginosa. FEBS Lett. 1982 Mar 22;139(2):255–258. doi: 10.1016/0014-5793(82)80864-8. [DOI] [PubMed] [Google Scholar]
- Minagawa J., Mogi T., Gennis R. B., Anraku Y. Identification of heme and copper ligands in subunit I of the cytochrome bo complex in Escherichia coli. J Biol Chem. 1992 Jan 25;267(3):2096–2104. [PubMed] [Google Scholar]
- O'Brian M. R., Maier R. J. Molecular aspects of the energetics of nitrogen fixation in Rhizobium-legume symbioses. Biochim Biophys Acta. 1989 May 30;974(3):229–246. doi: 10.1016/s0005-2728(89)80239-7. [DOI] [PubMed] [Google Scholar]
- Raitio M., Jalli T., Saraste M. Isolation and analysis of the genes for cytochrome c oxidase in Paracoccus denitrificans. EMBO J. 1987 Sep;6(9):2825–2833. doi: 10.1002/j.1460-2075.1987.tb02579.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramseier T. M., Göttfert M. Codon usage and G + C content in Bradyrhizobium japonicum genes are not uniform. Arch Microbiol. 1991;156(4):270–276. doi: 10.1007/BF00262997. [DOI] [PubMed] [Google Scholar]
- Regensburger B., Hennecke H. RNA polymerase from Rhizobium japonicum. Arch Microbiol. 1983 Aug;135(2):103–109. doi: 10.1007/BF00408017. [DOI] [PubMed] [Google Scholar]
- Rossbach S., Loferer H., Acuña G., Appleby C. A., Hennecke H. Cloning, sequencing and mutational analysis of the cytochrome c552 gene (cycB) from Bradyrhizobium japonicum strain 110. FEMS Microbiol Lett. 1991 Oct 1;67(2):145–152. doi: 10.1016/0378-1097(91)90345-b. [DOI] [PubMed] [Google Scholar]
- Saraste M. Structural features of cytochrome oxidase. Q Rev Biophys. 1990 Nov;23(4):331–366. doi: 10.1017/s0033583500005588. [DOI] [PubMed] [Google Scholar]
- Thöny-Meyer L., James P., Hennecke H. From one gene to two proteins: the biogenesis of cytochromes b and c1 in Bradyrhizobium japonicum. Proc Natl Acad Sci U S A. 1991 Jun 1;88(11):5001–5005. doi: 10.1073/pnas.88.11.5001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thöny-Meyer L., Stax D., Hennecke H. An unusual gene cluster for the cytochrome bc1 complex in Bradyrhizobium japonicum and its requirement for effective root nodule symbiosis. Cell. 1989 May 19;57(4):683–697. doi: 10.1016/0092-8674(89)90137-2. [DOI] [PubMed] [Google Scholar]
- Tinoco I., Jr, Borer P. N., Dengler B., Levin M. D., Uhlenbeck O. C., Crothers D. M., Bralla J. Improved estimation of secondary structure in ribonucleic acids. Nat New Biol. 1973 Nov 14;246(150):40–41. doi: 10.1038/newbio246040a0. [DOI] [PubMed] [Google Scholar]
- Tully R. E., Sadowsky M. J., Keister D. L. Characterization of cytochromes c550 and c555 from Bradyrhizobium japonicum: cloning, mutagenesis, and sequencing of the c555 gene (cycC). J Bacteriol. 1991 Dec;173(24):7887–7895. doi: 10.1128/jb.173.24.7887-7895.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]