Abstract
Classifying primary progressive aphasia (PPA) into variants that may predict the underlying pathology is important. However, some PPA patients cannot be classified. A 78-year-old woman had unclassifiable PPA characterized by anomia, dysarthria and apraxia of speech without agrammatism. MRI revealed left mesial temporal atrophy and 18-flourodeoxy-glucose PET showed left anterior temporal and posterior frontal (premotor) hypometabolism. Autopsy revealed a mixed tauopathy (argyrophilic grain disease) and TAR-DNA-binding-protein-43 proteinopathy. Dual pathologies may explain the difficulty classifying some PPA patients and recognizing this will be important as new imaging techniques (particularly tau-PET) are introduced and patients begin enrollment in clinical trials targeting the underlying proteinopathy.
Keywords: frontotemporal lobar degeneration, primary progressive aphasia, argyrophilic grain disease, tau, TDP 43
Primary progressive aphasia (PPA) is a neurodegenerative disease in which language disturbance predominates at presentation and is classified into three variants: 1) progressive nonfluent/agrammatic variant (agPPA); 2) logopenic variant (lvPPA); and 3) semantic variant (svPPA).(Gorno-Tempini et al., 2011) Apraxia of speech (AOS) is a motor speech disorder associated with difficulties in planning and programming the movements associated with speech production. It is common in agPPA, but not in other PPA variants.(Gorno-Tempini et al., 2011) Clinicopathological studies suggest that agPPA is most often associated with tau, lvPPA with tau and amyloid and svPPA with TAR-DNA-binding protein of 43 kDa (TDP-43) pathology.(Gorno-Tempini et al., 2011) It has been recognized that many PPA patients do not fit into any of these clinical variants,(Wicklund et al., 2014) but the reason is uncertain. Herein we report an autopsied case of PPA that was difficult to classify in life but explained by dual pathology.
METHODS
IMAGING ANALYSIS
A three-dimensional MRI magnetization-prepared rapid gradient-echo sequence was acquired at 3 Tesla. Atrophy patterns were assessed using Differential-STAND (Differential Diagnosis Based on Structural Abnormality in Neurodegeneration). This system measures grey matter volumes in 91 regions of interest, adjusted for age and head size, which are then compared to an elderly population without cognitive or language difficulties generating Z scores for each region of interest.(Vemuri et al., 2011) An 18F-Fluorodeoxyglucose-positron-emission-tomography (FDG-PET) was also acquired. Individual patterns of hypometabolism were assessed using the clinical tool of 3D stereotactic surface projections (SSP).(Minoshima, Frey, Koeppe, Foster, & Kuhl, 1995) The activity in each subject’s FDG-PET data set was normalized to the pons and compared with an age-segmented normative database, yielding a 3D SSP z-score image. The image produced by this analysis produces a metabolic map using the z-scores as calculated for each surface pixel. The software package used to perform these analyses was CortexID (GE Healthcare).
PATHOLOGICAL ANALYSIS
For the autopsy in this case, sections of the neocortex (x7), hippocampus (x2), basal forebrain, basal ganglia, thalamus, midbrain, pons, medulla, cervical spinal cord and cerebellum (x2) were examined with Hematoxylin and Eosin (H&E) staining. Sections of the neocortex (x5), hippocampus, basal forebrain, basal ganglia and cerebellum were studied with thioflavin-S fluorescent microscopy. Sections of basal forebrain (with amygdala and basal ganglia) were studied with α-synuclein immunohistochemistry (NACP, 1:3,000). Sections of hippocampus and cerebellum were screened for inclusions with p62/sequestosome (guinea pig polyclonal; 1;2500; Progen Biotechnik GmbH, Heidelberg, Germany). Sections of cortex, hippocampus, basal ganglia, thalamus, midbrain, pons, medulla and cerebellum were studied with immunohistochemistry for phospho-tau (CP13,1:100; Peter Davies, Albert Einstein College of Medicine, Bronx, NY). Sections of frontal cortex, hippocampus, basal forebrain, midbrain and medulla were studied with immunohistochemistry for TDP-43 (rabbit polyclonal: 1:3,000; ProteinTech Group, Chicago, IL).
CASE REPORT
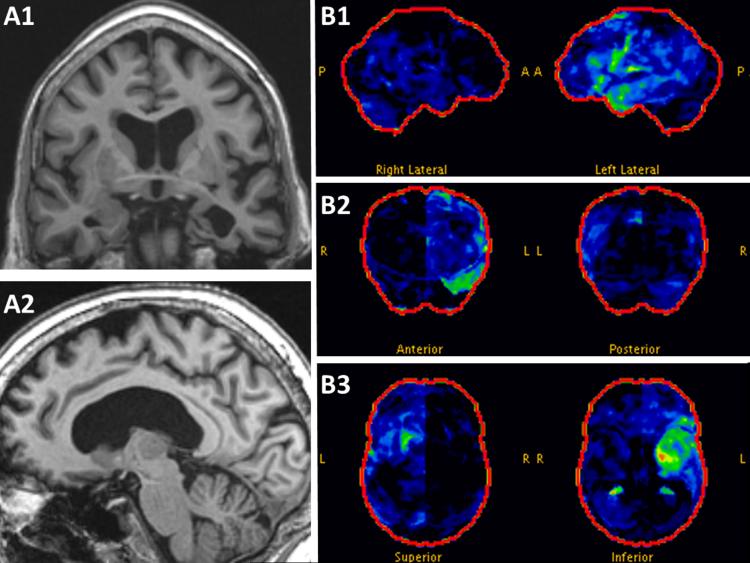
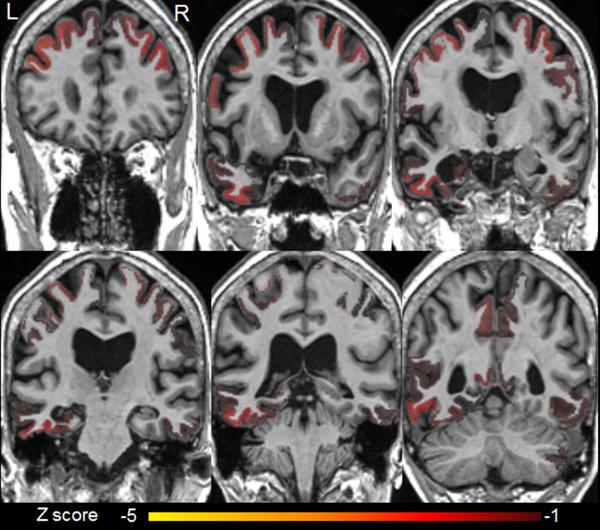
A 78-year-old right-handed woman had an insidious onset of difficulties pronouncing words accompanied by dysphagia that progressed over 5 months. There was no pertinent family history. Examination revealed mild AOS with inconsistent articulatory errors characterized by substitutions and distorted substitutions and slowed speech rate (Video). She also had a mild upper motor neuron dysarthria, mild right central face and tongue weakness. The Western Aphasia Battery(Kertesz, 2007) revealed only anomia. She scored10/15 (below expected for age) on the 15 item Boston Naming Test.(Lansing, Ivnik, Cullum, & Randolph, 1999) Lexical fluency was mildly impaired and single word receptive vocabulary, measured by the Peabody Picture Vocabulary Test,(Dunn & Dunn, 2006) was low average. There was no agrammatism. She scored 10/10 on the Northwestern Anagram test.(Weintraub et al., 2009) Head MRI revealed left amygdala, hippocampal, and anterior temporal lobe atrophy (Figure 1A) and 18-F-Flouro-deoxy-glucose-positron-emission-tomography (FDG-PET) scan showed left anterior medial temporal and posterior inferior frontal hypometabolism (Figure 1B). The differential diagnosis based on structural abnormality in neurodegeneration (differential-STAND) maps (figure 2) showed atrophy in the following regions: bilateral mid and superior frontal regions, bilateral precentral regions with left hemisphere being more severely involved, bilateral precuneus, and postcentral regions. The left hippocampus, fusiform, mid temporal regions also showed atrophy. An amyloid PET (Pittsburgh Compound B) scan was negative. Electromyography was normal. Genetic screening of progranulin, C9ORF72 and tau genes was negative. Her apolipoprotein genotype was 3/3. Our PPA working group was unable to classify her PPA as clearly fitting one of the three recognized PPA variants. She progressed rapidly and died fourteen months after onset.
Figure 1. MRI and FDG-PET imaging.
Magnetization-prepared rapid acquisition with gradient-echo (MP-RAGE) images show left mesial temporal atrophy on coronal images (A1) and medial pre-central atrophy on sagittal images (A2). FDG-PET measuring glucose metabolism (green = mild hypometabolism; yellow=moderate hypometabolism; red=severe hypometabolism) shows mostly mild hypometabolism in the left anterior temporal lobe and left inferior frontal region (B1) which is also evident on coronal (B2) and axial images (B3). Abbreviations: A, anterior; L, left; P, posterior; R, right.
Figure 2.
MRI atrophy patterns using differential diagnosis based on structural abnormality in neurodegeneration (differential-STAND).
There is atrophy in bilateral mid and superior frontal regions, bilateral precentral regions (worse on the left), bilateral precuneus, and postcentral regions. The left hippocampus, fusiform and mid temporal regions also showed significant atrophy.
Abbreviations (z score refers to the number of standard deviations): L, left; R, right (note for this analysis the normal MRI left-right orientation is reversed; the left brain is on the left of the figure and the right brain is on the right of the figure for each panel).
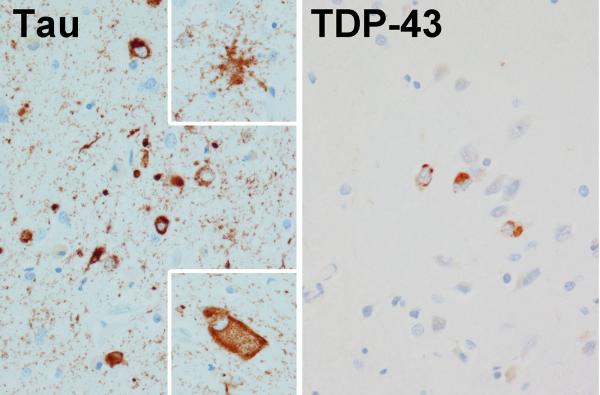
Autopsy revealed mixed tauopathy (argyrophilic grain disease [AGD]) and TDP-43 Type B proteinopathy (Figure 3). The whole brain weight was 1040 grams. The argyrophilic grain disease (with many grains, pre-tangles and coiled bodies) was largely limited to hippocampus, amygdala and nucleus accumbens (Figure 3). In the amygdala there were also many tau-positive ramified astrocytes and a few ballooned neurons. Argyrophilic grains were not found in the remainder of the temporal lobe or in the frontal lobe. Neurofibrillary tangles were most dense in the Ca2/CA3 region (3-18/40x field) and a few (0-1/ 40x field) were noted in the entorhinal cortex, CA1, subiculum and superior temporal cortex. A few dystrophic neurites were observed in the amygdala only. The Braak neurofibrillary tangle stage was Stage III. TDP-43 immunoreactive neuronal cytoplasmic inclusions were moderate in amygdala and entorhinal, occipitotemporal and inferior temporal cortices, and sparse in peri-Rolandic cortices, dentate fascia, CA1 and subiculum of the hippocampus and basal nucleus of Meynert (Figure 3). TDP-43 was not found in the Betz cells, midfrontal cortex, basal ganglia, hypoglossal nucleus, or the spinal cord. There were no senile plaques (Thal A-beta stage 0).(Thal, Rub, Orantes, & Braak, 2002) No Lewy bodies were detected within the brainstem nuclei (including the substantia nigra) or amygdala. No inclusions typical of c9FTD were detected. The hippocampus had mild focal neuronal loss and gliosis in the anterior subiculum, but no neuronal loss in endplate, CA2/3 or CA1.
Figure 3.
Figure 3 demonstrates two separate pathological processes in our patient with unclassified PPA.
On the left are tau immunoreactive coiled bodies and grains consistent with argyrophilic grains disease. On the right are TDP-43 immunoreactive neuronal cytoplasmic inclusions consistent with frontotemporal lobar degeneration with TDP; Type B pathology.
DISCUSSION
This case highlights the difficulties in classifying some PPA cases. The AOS best fit agPPA but naming of nouns is usually preserved in early agPPA and grammar was normal.(Gorno-Tempini et al., 2011) The AOS argued against svPPA and lvPPA while anomia is a feature of both svPPA and lvPPA. In retrospect, we could have predicted the underlying dual pathologies. AOS strongly predicts underlying tau pathology and one tauopathy strongly associated with focal anterior medial temporal atrophy and hypometabolism in the absence of amyloid deposition is AGD.(Josephs et al., 2008) The dysphagia, upper motor neuron dysarthria and tongue weakness could suggest motor neuron disease which is strongly associated with TDP type B pathology.(Josephs, Stroh, Dugger, & Dickson, 2009). However, an EMG was normal and neither TDP-43 deposits nor neuronal loss were found in the hypoglossal nucleus or spinal cord making it difficult to explain the dysphagia and dysarthria in this case.
AGD is characterized neuropathologically by spindle shaped grains and pretangles (composed of hyperphosphorylated 4-repeat [4R] Tau) in brain limbic regions.(Ferrer, Santpere, & van Leeuwen, 2008) AGD may occur in normals, coexist with other neurodegenerative pathologies or exist in isolation. In contrast to other reports which suggested that the nucleus accumbens is usually affected by tau in the last stage of AGD,(Saito et al., 2004) in our case the hippocampus, amygdala and nucleus accumbens were involved while the remainder of the frontal and temporal cortices were spared. A single svPPA case with isolated AGD pathology has been described.(Deramecourt et al., 2010) TDP-43 co-existed with AGD in 60% of cases in one series, but no patients in that report had PPA.(Fujishiro et al., 2009) This is the first case, to our knowledge, of PPA with mixed AGD and TDP-Type B pathology. Dual pathology has been described previously in a patient with agPPA in which autopsy revealed Pick’s disease and Alzheimer’s disease pathology.(Caso et al., 2013) Imaging of tau using PET is currently being validated to predict pathology in PPA and other dementias and help select patients for clinical trials of treatments targeting the underlying proteinopathy. However, in validating the use of tau-PET in PPA, some patients with anterior medial temporal atrophy and negative amyloid scan were unexpectedly tau positive.(Dickerson et al., 2014) AGD, like in our case, is one potential explanation for this finding.
Supplementary Material
Footnotes
Financial Disclosure Statements
Dr. Flanagan, has no disclosures.
Dr Duffy is funded by the NIH (NIDCD).
Dr Whitwell is funded by the NIH (NICD) and the Alzheimer’s Association
Dr Vemuri has no disclosures
Dr Dickson is funded by the NIH
Dr. Josephs is funded by the NIH
Consent
The patient consented to having her speech video-taped.
REFERENCES
- Caso F, Gesierich B, Henry M, Sidhu M, LaMarre A, Babiak M, Gorno-Tempini ML. Nonfluent/agrammatic PPA with in-vivo cortical amyloidosis and Pick's disease pathology. Behavioural neurology. 2013;26(1-2):95–106. doi: 10.3233/BEN-2012-120255. doi: 10.3233/BEN-2012-120255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deramecourt V, Lebert F, Debachy B, Mackowiak-Cordoliani MA, Bombois S, Kerdraon O, Pasquier F. Prediction of pathology in primary progressive language and speech disorders. Neurology. 2010;74(1):42–49. doi: 10.1212/WNL.0b013e3181c7198e. doi: 10.1212/WNL.0b013e3181c7198e. [DOI] [PubMed] [Google Scholar]
- Dickerson B, Domoto-Reilly K, Hochberg D, Brickhouse M, Stepanovic M, Johnson K. Imaging Tau Pathology In Vivo in FTLD: Initial Experience With 18F T807 PET. Neurology. 2014;82 (No. 10), Supplement S8.007. [Google Scholar]
- Dunn L, Dunn D. Peabody Picture Vocabulary Test. Pearson Canada Assessment Inc; Toronto: 2006. [Google Scholar]
- Ferrer I, Santpere G, van Leeuwen FW. Argyrophilic grain disease. Brain : a journal of neurology. 2008;131:1416–1432. doi: 10.1093/brain/awm305. Pt 6. doi: 10.1093/brain/awm305. [DOI] [PubMed] [Google Scholar]
- Fujishiro H, Uchikado H, Arai T, Hasegawa M, Akiyama H, Yokota O, Hirayasu Y. Accumulation of phosphorylated TDP-43 in brains of patients with argyrophilic grain disease. Acta neuropathologica. 2009;117(2):151–158. doi: 10.1007/s00401-008-0463-2. doi: 10.1007/s00401-008-0463-2. [DOI] [PubMed] [Google Scholar]
- Gorno-Tempini ML, Hillis AE, Weintraub S, Kertesz A, Mendez M, Cappa SF, Grossman M. Classification of primary progressive aphasia and its variants. Neurology. 2011;76(11):1006–1014. doi: 10.1212/WNL.0b013e31821103e6. doi: 10.1212/WNL.0b013e31821103e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Josephs KA, Stroh A, Dugger B, Dickson DW. Evaluation of subcortical pathology and clinical correlations in FTLD-U subtypes. Acta neuropathologica. 2009;118(3):349–358. doi: 10.1007/s00401-009-0547-7. doi: 10.1007/s00401-009-0547-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Josephs KA, Whitwell JL, Parisi JE, Knopman DS, Boeve BF, Geda YE, Dickson DW. Argyrophilic grains: a distinct disease or an additive pathology? Neurobiology of aging. 2008;29(4):566–573. doi: 10.1016/j.neurobiolaging.2006.10.032. doi: 10.1016/j.neurobiolaging.2006.10.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kertesz A. Western Aphasia Battery (Revised) PsychCorp; San Antonio: 2007. [Google Scholar]
- Lansing AE, Ivnik RJ, Cullum CM, Randolph C. An empirically derived short form of the Boston naming test. Archives of clinical neuropsychology : the official journal of the National Academy of Neuropsychologists. 1999;14(6):481–487. [PubMed] [Google Scholar]
- Minoshima S, Frey KA, Koeppe RA, Foster NL, Kuhl DE. A diagnostic approach in Alzheimer's disease using three-dimensional stereotactic surface projections of fluorine-18-FDG PET. Journal of nuclear medicine : official publication, Society of Nuclear Medicine. 1995;36(7):1238–1248. [PubMed] [Google Scholar]
- Saito Y, Ruberu NN, Sawabe M, Arai T, Tanaka N, Kakuta Y, Murayama S. Staging of argyrophilic grains: an age-associated tauopathy. Journal of neuropathology and experimental neurology. 2004;63(9):911–918. doi: 10.1093/jnen/63.9.911. [DOI] [PubMed] [Google Scholar]
- Thal DR, Rub U, Orantes M, Braak H. Phases of A beta-deposition in the human brain and its relevance for the development of AD. Neurology. 2002;58(12):1791–1800. doi: 10.1212/wnl.58.12.1791. [DOI] [PubMed] [Google Scholar]
- Vemuri P, Simon G, Kantarci K, Whitwell JL, Senjem ML, Przybelski SA, Jack CR., Jr. Antemortem differential diagnosis of dementia pathology using structural MRI: Differential-STAND. NeuroImage. 2011;55(2):522–531. doi: 10.1016/j.neuroimage.2010.12.073. doi: 10.1016/j.neuroimage.2010.12.073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weintraub S, Mesulam MM, Wieneke C, Rademaker A, Rogalski EJ, Thompson CK. The northwestern anagram test: measuring sentence production in primary progressive aphasia. American journal of Alzheimer's disease and other dementias. 2009;24(5):408–416. doi: 10.1177/1533317509343104. doi: 10.1177/1533317509343104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wicklund MR, Duffy JR, Strand EA, Machulda MM, Whitwell JL, Josephs KA. Quantitative application of the primary progressive aphasia consensus criteria. Neurology. 2014;82(13):1119–1126. doi: 10.1212/WNL.0000000000000261. doi: 10.1212/WNL.0000000000000261. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.