Abstract
The current standard of care for prostate cancer includes hormone therapy, radiation therapy and radical prostatectomy, each with its own set of undesirable side effects. In this regard there is an unmet need to develop strategies that can prevent or delay the development of clinical prostate cancer. One potential area involves the use of natural compounds involving botanicals. Along these lines we have found that Nexrutine®, a dietary supplement derived from Phellodendron amurense bark extract, has prostate cancer prevention activity. The “extract” nature of this botanical, which constitutes a blend of several active protoberberine alkaloids, allows it to target several pathways deregulated in prostate cancer simultaneously. In this review, we will emphasize the prospective translational benefit of Nexrutine® as a chemopreventive agent for prostate cancer management. The potential of Nexrutine® was first identified and has subsequently been most exhaustively studied with reference to prostate cancer. Therefore the focus of this review is on the use of Nexrutine® in prostate cancer. In addition we have summarized the emerging evidence regarding the use of Nexrutine® in other tumor models to demonstrate the potential benefits of Nexrutine®.
Keywords: Botanicals, Chemoprevention, Herbal extracts, Natural products, Nexrutine® Prostate cancer
Introduction
Prostate cancer (PCA) is the second leading cause of cancer-related deaths in American men (1). Early localized disease has a 5-year survival rate of almost 100 percent, with a myriad of treatment approaches such as active surveillance, radiation therapy, hormone therapy and radical prostatectomy (1, 2). Unfortunately these strategies are associated with several undesirable side effects and are limited by progression to metastatic castration-resistant prostate cancer (CRPC) (3, 4). Notably, the FDA recently approved 6 new drugs, which improve overall survival or bone metastasis-free survival for CRPC patients (Table 1) (5). However, the survival benefit is limited with a suggested reactivation of the androgen receptor (AR) axis (6–8). Poor quality of life for patients undergoing these treatments underscores the need to find alternative strategies that can improve quality of life and or prevent the development of PCA in the first place.
Table 1.
Change in treatment landscape for CRPC in the past four years. Adapted from www.cancer.gov and Yin et al., IJMS (2012).
| Category | Drug Name | Approved |
|---|---|---|
| Androgen Receptor Antagonist | Enzalutamide | Aug, 2012 |
| Cyp17 Inhibitor | Abiraterone | Dec, 2012 |
| Microtubule inhibitor | Cabazitaxel | June, 2010 |
| Vaccine against PAP antigen | Sipuleucel-T | Aug, 2010 |
| RANKL Antibody | Denosumab | Sept, 2011 |
| Radium 223- Ca2+ mimetic | Alpharadin | May, 2013 |
A long latency is involved in the development of PCA including proliferative inflammatory atrophy (PIA), low and high-grade prostatic intraepithelial neoplasia (PIN) that finally culminates into clinically significant PCA (9). Recent advances in technologies that detect alterations (next gen sequencing, transcriptome sequencing) in various cancer causing pathways, have improved our understanding of the molecular mechanisms involved in PCA (10). This sets the stage to use the ‘long latent development period’ to test preventive agents using mechanism-based markers.
Prostate Cancer Chemoprevention
The emergence of the field of cancer chemoprevention reiterates the old proverbial saying, “Prevention is better than cure”. Sporn et al. originally defined chemoprevention as the application of natural, synthetic or biological modalities to prevent, contain or reverse the initiation of carcinogenesis or progression of localized cancer to metastatic disease (11). The recent addition of a ‘delay’ in initiation or progression has added an extra dimension to this definition (12). Chemoprevention can be generally classified into three categories depending on the stage of cancer when the intervention begins. Primary chemoprevention refers to the use of chemopreventive agent to healthy and high risk population; secondary chemoprevention is used to prevent or delay progression of premalignant lesions to invasive cancer, while tertiary chemoprevention targets tumor recurrence and metastasis for patients undergoing successful treatment of local disease (12, 13). The FDA approval of 10 drugs for cancer risk reduction including tamoxifen, raloxifene for breast cancer and HPV vaccines for cervical cancer signifies the rising surge of cancer prevention (14).
The untapped potential for PCA prevention led to large-scale clinical trials using 5α-reductase inhibitors. Prostate Cancer Prevention Trial (PCPT) and Reduction by Dutasteride of Prostate Cancer Events (REDUCE) were randomized placebo-controlled trials using the 5α-reductase inhibitor finasteride and dutasteride respectively (15, 16). The PCPT was a large-scale trial with 18,882 men, but the final analysis included only 9060 men due to early study termination and men declining the end of study biopsies (16). It is also important to note the higher rates of non-adherence (14.7 % vs. 10.8 %) and increased sexual functions in the finasteride group (16). A drawback of the study design was the lack of baseline determination of 5α-reductase levels, which may have affected the treatment outcome. Recently, an 18-years follow-up of the PCPT trial showed that use of finasteride for a period of about 7 years had no significant difference in overall survival compared to placebo, further questioning potential use of finasteride in the clinic (17). While the finasteride trial showed that it could prevent lower grade cancer, it also identified high-grade tumors (Gleason 8–10) in the treatment group (16). Subsequently it has been suggested that finasteride helps in the detection of these high-grade tumors (18, 19). However, it does not fulfill the premise on which cancer chemoprevention as discussed above is based.
Dietary supplements as prospective chemopreventives
Diet is a modifiable risk factor, which can impact the progression of indolent disease to clinically significant PCA (20, 21). Epidemiological studies have suggested that the incidence of PCA is much lower in Asian populations consuming phytonutrients-rich diet compared to their western counterparts (22). Further, increased cancer prevalence in Asian populations that have migrated to the west, underscores the importance of diet, lifestyle and environmental factors in increased risk of PCA (22–24). Interestingly, cancer incidence data from Surveillance, Epidemiology, End Results (SEER) registries showed that PCA was the most common malignancy in a majority of Asian American men in the United States (25). In this regard, dietary supplements such as lycopene, selenium, vitamins, soy isoflavones, green tea polyphenols, and silibinin are some of the phytoconstituents tested in various preclinical and clinical settings for their chemopreventive capabilities in PCA (20, 26–28). The Selenium and Vitamin E Cancer Prevention Trial (SELECT) which randomly assigned 35,533 men to selenium, Vitamin E, selenium and Vitamin E or placebo groups was concluded after the 7-year interim analysis because of lack of benefit in PCA risk reduction (29). Selenium was administered in the form of selenomethionine in the SELECT trial, although the smaller Nutritional Prevention of Cancer (NPC) trial which showed chemopreventive potential of selenium used selenized yeast containing methyl selenocysteine suggesting the value of choice of selenium that maybe beneficial (30). Further, the variability in the endogenous levels of selenium in the study population could have affected the outcome. An increase in PCA incidence was noted in the Vitamin E arm, which may be due to the higher dose of Vitamin E used in SELECT compared to earlier trials (29). Interestingly, two recently published follow-up studies of the SELECT and PCPT trials have suggested that circulating Vitamin D can prevent clinically relevant PCA (31, 32). However, this effect was limited only to the African-American population in the SELECT trial with other men showing increased PCA risk. As we learn these lessons there is a general need to design more effective chemoprevention trials with careful consideration given to study design, formulation, dosage and patient selection criteria.
Phytoceuticals (plant derived chemicals) are a rich source of number of FDA approved drugs. Strikingly, approximately 50% of FDA approved drugs for cancer are natural products or their derivatives, which includes the taxanes and vinca alkaloids widely used in current cancer therapy (33). Herbal extracts used in Traditional Chinese medicine (TCM) and Ayurvedic medicine are anecdotal for treatment of various pathological conditions including cancer (34, 35). Although these extracts are under explored they provide the starting advantage of having minimal systemic toxicity. In addition to potential use as chemopreventives they may also be beneficial in reducing the dose of current toxic treatments and delay therapeutic resistance.
Nexrutine®
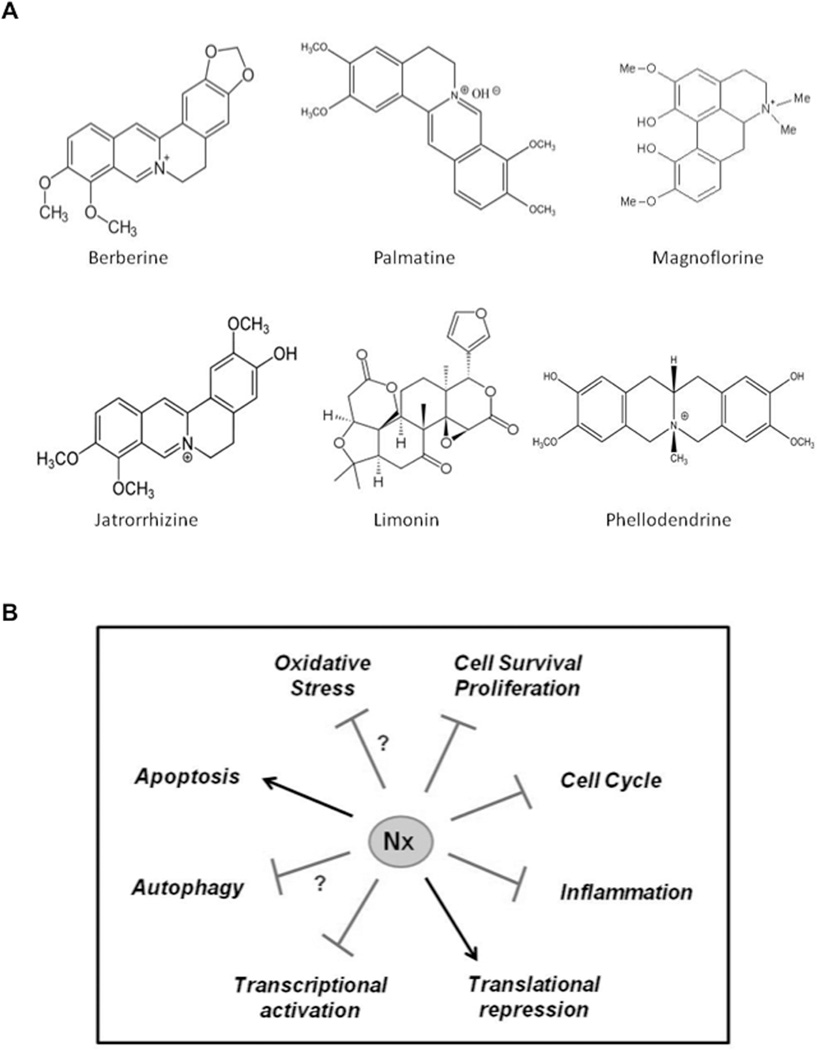
Nexrutine® is an inexpensive over the counter dietary supplement used to relieve joint pain. It is derived from Phellodendron amurense, more commonly known as the cork tree, which is native to Asia and belongs to family Rutaceae (36, 37). Isoquinoline alkaloids like berberine, palmatine, phellodendrine, jatrrorhizine and magnoflorine and the liminoid, limonin (Figure 1A) are considered as active components of this extract that exhibit biological activity (36, 38, 39). In traditional Chinese medicine, the bark extract is referred to as ‘Huang Bai’ and has been used for centuries to treat inflammatory conditions including psoriasis, gastroenteritis, abdominal pain, and diarrhea and also used as an anti-bacterial (36, 40). It has also been shown to be useful for symptoms of osteoarthritis and shows potential as a neuroprotective agent in Alzheimer’s disease (40, 41). Relora® (Next Pharmaceuticals, Salinas CA) is a proprietary formulation of Phellodendron amurense bark extract standardized to berberine and is believed to reduce stress and anxiety (42). Using activity-guided fractionation of Nexrutine®, we found that butanol fraction (F3) recapitulates the anti-proliferative and NF-κB inhibitory activity of the extract. Further, ultra-performance liquid-chromatography tandem mass spectrometry (UPLC–MS/MS) with multi reaction monitoring (MRM) analysis identified berberine and palmatine as the active constituents present in F3 (43).
Figure 1.
A) Major phytoconstituents of Phellodendron amurense bark extract.
B) Model depicting the molecular mechanism of action of Nx based on current data.
In vitro evaluation in PCA: One extract, many properties
Compared with other natural compounds, the use of Nexrutine® is relatively unexplored in the arena of chemoprevention. However, in the past decade the number of reports demonstrating anti-cancer properties of Nexrutine® has been on the rise with reports of its testing in melanoma, multiple myeloma, prostate, pancreatic, breast and nonmelanoma skin cancer (37, 43–50). Studies from our laboratory first discovered Nexrutine® has anti-proliferative properties against PCA cell lines irrespective of their androgen dependence status (37). Subsequent studies showed that Nexrutine® also inhibited invasion and anchorage independent growth of androgen independent PCA cell lines (37, 47). Mechanistic investigations revealed that Nexrutine® exerts its biological effects by modulating key cell-survival pathways such as PI3K/AKT and STAT3/NF-κB signaling leading to apoptosis (37, 43,44, 48, 50). Introduction of a constitutively-active form of AKT blocked the anti-proliferative effect of Nexrutine® in PCA cells, implying PI3K/AKT pathway as a target of Nexrutine® (37). Similarly, Nexrutine® decreased phosphorylation and DNA binding activity of CREB, a transcription factor downstream of PI3K/AKT signaling and elevated in high-Gleason grade human prostate tumors (37, 48). CREB transcriptionally regulates expression of a plethora of genes involved in various cellular processes including cell proliferation, survival, apoptosis, inflammation, invasion and metastasis (51). Nexrutine® was shown to inhibit NF-κB reporter and DNA binding activity in androgen independent PC-3 cells (43). Treatment with Nexrutine® reduced levels of Cyclin D1 (cell cycle), and COX-2 (inflammatory mediator) (44, 48). It is noteworthy to mention that, an integrated oncogenomic analysis of prostate tumors revealed that the PI3K/AKT pathway was deregulated in 42% of primary tumors and almost 100% of metastatic tumors (52). Alterations in PTEN, PIK3CA, PHLPP, and INPP4B genes are associated with poor prognosis and progression to metastatic. CRPC. Further, there is reciprocal cross-talk between PI3K and AR signaling; whereby inhibiting AR with anti-androgens causes increased PI3K/AKT signaling (53). Similarly, combined inhibition of AKT and AR delays progression to castrate resistant disease, underscoring the advantage of targeting the PI3K/AKT signaling in PCA (54). PI3K/AKT signaling mediated activation of NF-κB was shown to mediate PCA cell proliferation (55). Strikingly, nuclear expression of NF-κB is found to correlate with biochemical recurrence in PCA patients (56). Therefore, ability of Nexrutine® to modulate multiple critical deregulated signaling pathways will have enormous therapeutic benefit.
Preclinical Evaluation
Chemopreventive potential of Nexrutine® was evaluated in preclinical animal models (43, 44,46, 47, 50). Intervention with Nexrutine® not only prevented development of early-stage prostate tumors but also metastatic lesions in transgenic adenocarcinoma of mouse prostate (TRAMP) mice (47). Remarkably, intervention with Nexrutine® resulted in statistically significant increase in bone mineral density (BMD) in these animals (47). Bone is the most frequent site of metastasis in human PCA patients and is associated with increased pain and skeletal complications (57). Validation of these observations in large-scale studies will have significant impact to improve the quality of life for these patients. In addition, Nexrutine®-mediated in vivo effects were shown to be associated with decreased levels of Cyclin D1, AKT/CREB and NF-κB activation (43, 44).
From bench to bedside
Radiation therapy (RT) and radical prostatectomy are the common modes of treatment for localized PCA but are associated with several side effects with biochemical recurrence in the high and intermediate risk groups (58). Interestingly, Nexrutine® supplementation prior to RT inhibited progression of prostate tumors to poorly differentiated stage in TRAMP mice with no prominent toxicity (Hussain et al., unpublished data). Low dose radiation combined with Nexrutine® showed similar inhibition of surviving fraction as high dose radiation in androgen independent PC-3 cells (Hussain et al., unpublished data). Encouraged by the pre-clinical efficacy of Nexrutine®, Swanson et al. tested whether the supplementation of Nexrutine® would benefit PCA patients undergoing prostatectomy or RT. The 6–8 weeks period after diagnosis and before beginning treatment was effectively used to administer Nexrutine® to PCA patients with either Gleason score >6 or Prostate Specific Antigen (PSA) >10ng/ml. 9 patients receiving RT and 12 patients undergoing surgery were enrolled in this trial. Indeed, oral administration of Nexrutine® (500 mg tid) one to two months prior to radiation/surgery or with radiation decreased PSA in 81 % of patients with no signs of grade 3 toxicity (59). The trial also established the safety of Nexrutine®. This was the first clinical study, which tested the tolerance and efficacy of Nexrutine® in cancer patients. These studies have strengthened the potential use of Nexrutine® in combination with existing therapy to maximize clinical benefits for patients. Along these lines, given that reactivation of (AR) signaling is critical in the development of CRPC, studies to establish the potential of Nexrutine® alone and in combination with FDA approved androgen antagonists and androgen synthesis inhibitors are warranted (60). Given that androgen deprivation therapy (ADT) is an important treatment component of the armamentarium to treat patients with intermediate-to-high risk disease as well as to treat those men with recurrent PCA, use of non toxic alternatives will have substantial benefit in delaying the progression (2).
Antitumorigenic effects of Nexrutine® in other tumor models
Recent studies from our lab unraveled the benefits of Nexrutine® against pancreatic cancer (49, 50). Similarly, Nexrutine® decreased both NF-κB and STAT3 levels and transcriptional activity in pancreatic cancer cell lines (50). Disrupting the cross talk between NF-κB and STAT3, ensured inhibition of the feedback loop. Further, Nexrutine® was shown to inhibit NF-κB mediated transactivation of COX-2, which resulted in decreased expression of COX-2 (50). Inhibition of STAT3 reduced the elevated levels of ROS and autophagy (a survival mechanism) in pancreatic cancer cells (49). In a COX-2 over-expressing BK5–COX-2 preclinical model of pancreatic cancer, Nexrutine® intervention reduced fibrosis (50). Fibrosis or desmoplasia produced through tumor-stromal interactions impedes drug delivery leading to therapeutic resistance (61). Along these lines, Nexrutine®-mediated disruption of pancreatic desmoplasia makes it a particularly attractive adjuvant for conventional therapy.
Further Nexrutine® inhibited the survival of several melanoma cell lines by modulating their oxidative stress levels (Hambright et al., Oncotarget, in press). Supporting studies from other groups have demonstrated the chemopreventive potential in breast and skin cancer (45, 46). Interestingly, decreased cell viability and increased apoptosis in SkBr3 cells and induction of autophagy in MDA-MB231 ER negative breast cancer cells were reported (45). Nexrutine® also decreased COX-2 and PPARγ in breast cancer cells (45). Nexrutine® had negligible cytotoxic effect on primary murine keratinocytes (46). Using well established mouse two-stage carcinogenesis model, Kumar et al. showed reduced tumor incidence and associated decrease in the levels of COX-2 and NF-κB (46). In addition, Nexrutine® exhibited anti-tumorigenic activities in a multiple myeloma (MM) cells in vitro and in a preclinical model in vivo. Nexrutine® exposure reduced cell viability through apoptosis and inhibition of mTOR activation in murine 5TGM1 and human RPMI 8226 MM cells (62). Furthermore, Nexrutine® administration reduced overall tumor burden in a MM preclinical mouse model (62). That study also suggested potential for combining Nexrutine® with autophagy inhibitors for enhanced therapeutic benefit. Taken together, these preclinical observations suggest the potential utility of Nexrutine® as a secondary and/or tertiary chemopreventive agent for management of not only prostate but also other inflammation-associated malignancies.
Conclusions, future directions and challenges
In summary, Nexrutine® has been observed to have positive impact in inhibiting carcinogenesis pathways and biological processes involved not only in solid tumors but also in hematological malignancies (Figure 1B). These benefits have been reported to be potentially through both autophagy and apoptosis, perhaps in a cell type contextual manner as well as due to modulation of key inflammatory signaling pathways. It is well established that chronic inflammation and associated COX-2 overexpression is an early event in the pathogenesis of “inflammation-related” cancers (63). COX-2 is transcriptionally regulated by STAT3, NF-κB and CREB; therefore, it is possible that Nexrutine® suppresses COX-2 by down regulation of these transcription factors in tumor cells (64, 65). Suppression of this signaling not only inhibits tumor cell growth it could potentially sensitize cancer cells to conventional treatment. However, such concepts have not been tested. Furthermore, although epidemiological studies showed reduced cancer risk in people who regularly take NSAIDs, its long-term use has been associated with gastrointestinal or cardiovascular side effects (66, 67). In this scenario Nexrutine® could provide an alternative strategy for its anti-inflammatory use. Imbalance of pro- and anti-inflammatory cytokines and increased oxidative stress is associated with negative energy balance in which energy consumed (caloric intake) is greater than the energy expended (caloric expenditure) (68).This negative energy contributes to obesity, one of the risk factors for number of malignancies including PCA (69). Accordingly, given the ability of Nexrutine® to modulate inflammatory molecules, its preventative benefits in modulating obesity possibly as an exercise mimetic is an interesting hypothesis to test. In addition, the potential of Nexrutine® to alter adverse effects associated with cancer and cancer treatment including fatigue also need to be evaluated. However, further research involving long-term pharmacokinetic studies to establish the toxicity profile of Nexrutine® and biomarker-driven trials are warranted to determine the full beneficial impact of Nexrutine®.
Even though herbal extracts have shown promising results in preclinical models, the transition from ‘bench to bedside’ is hindered by their lack of bioavailability, difficulties in setting quality control parameters and more importantly the varied effect observed during different stages of disease or at different doses. In the case of Nexrutine® we used the solid support matrix in UPLC, which can cause adsorptive sample loss, thus limiting the fractionation of natural extracts containing diverse bioactive molecules. Hence, use of a high throughput counter current chromatography (CCC) fractionation method that excludes the solid support matrix can help elaborate the composition of Nexrutine® (70, 71). Quantitative composition activity relationship (QCAR), a newly identified approach based on algorithms to correlate the chemical composition of an extract to its biological activity could help in designing the quality control and standardization protocols for Nexrutine® thus alleviating some concerns of variation due to its natural origin (72, 73).
Acknowledgments
This work was supported in part by the funds from Veterans Affairs-Merit Award I01 BX 000766-01; National Cancer Institute 1R01 AT007448 (APK); and R01 CA 149516 (RG).
Footnotes
Compliance with Ethics Guidelines
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Conflict of Interest
Suleman S. Hussain, Darpan Patel, Rita Ghosh and Addanki P. Kumar declare that they have no conflict of interest.
References
Papers of particular interest, published recently, have been highlighted as:
• Of importance •• Of major importance
- 1.Siegel R, Ma J, Zou Z, Jemal A. Cancer statistics, 2014. CA: a cancer journal for clinicians. 2014;64:9–29. doi: 10.3322/caac.21208. [DOI] [PubMed] [Google Scholar]
- 2.Dominic T, Kimberley C. Advances in prostate cancer treatment. Nature Reviews Drug Discovery. 2013;12:823–824. doi: 10.1038/nrd4068. This review highlights the recently approved therapies and late-stage pipeline products for prostate cancer treatment. [DOI] [PubMed] [Google Scholar]
- 3.Kate DL, James WFC. Gastrointestinal Toxicity Following Radiotherapy for Prostate Cancer: A Ring of Fire. European Urology. 2011;60:917–919. doi: 10.1016/j.eururo.2011.07.003. [DOI] [PubMed] [Google Scholar]
- 4.Sanda MG, Dunn RL, Michalski J, Sandler HM, Northouse L, Hembroff L, et al. Quality of life and satisfaction with outcome among prostate-cancer survivors. The New England journal of medicine. 2008;358:1250–1261. doi: 10.1056/NEJMoa074311. [DOI] [PubMed] [Google Scholar]
- 5.Yin L, Hu Q, Hartmann RW. Recent progress in pharmaceutical therapies for castration-resistant prostate cancer. International journal of molecular sciences. 2012;14:13958–13958. doi: 10.3390/ijms140713958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kirby M, Hirst C, Crawford ED. Characterising the castration-resistant prostate cancer population: a systematic review. International Journal of Clinical Practice. 2011;65:1180–1192. doi: 10.1111/j.1742-1241.2011.02799.x. [DOI] [PubMed] [Google Scholar]
- 7.Korpal M, Korn J, Gao X, Rakiec D, Ruddy D, Doshi S, et al. An F876L Mutation in Androgen Receptor Confers Genetic and Phenotypic Resistance to MDV3100 (Enzalutamide) Cancer discovery. 2013;3:1030–1043. doi: 10.1158/2159-8290.CD-13-0142. F876L mutation in androgen receptor was identified to be responsible for antagonist to agonist shift of enzalutamide leading to progression to CRPC. [DOI] [PubMed] [Google Scholar]
- 8.Joseph J, Lu N, Qian J, Sensintaffar J, Shao G, Brigham D, et al. A Clinically Relevant Androgen Receptor Mutation Confers Resistance to Second-Generation Antiandrogens Enzalutamide and ARN-509. Cancer discovery. 2013;3:1020–1029. doi: 10.1158/2159-8290.CD-13-0226. F876L missense mutation in the AR was found to cause resistance to ARN-509 and could be detected in plasma of ARN-509-treated patients having progressive CRPC. [DOI] [PubMed] [Google Scholar]
- 9.Kelloff G, Lippman S, Dannenberg A, Sigman C, Pearce H, Reid B, et al. Progress in chemoprevention drug development: the promise of molecular biomarkers for prevention of intraepithelial neoplasia and cancer--a plan to move forward. Clinical cancer research: an official journal of the American Association for Cancer Research. 2006;12:3661–3697. doi: 10.1158/1078-0432.CCR-06-1104. [DOI] [PubMed] [Google Scholar]
- 10.Barbieri C, Bangma C, Bjartell A, Catto J, Culig Z, Grönberg H, et al. The Mutational Landscape of Prostate Cancer. European urology. 2013;64:567–576. doi: 10.1016/j.eururo.2013.05.029. This paper characterized the mutations in 50 heavily pretreated metastatic CRPC patients to elucidate resistance mechanisms. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sporn MB. Approaches to prevention of epithelial cancer during the preneoplastic period. Cancer research. 1976;36:2699–2702. [PubMed] [Google Scholar]
- 12.Steward W, Brown K. Cancer chemoprevention: a rapidly evolving field. British journal of cancer. 2013;109:1–7. doi: 10.1038/bjc.2013.280. This review outlines the current developments in chemoprevention and provides insights for effective design of chemoprevention clinical trials. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kelloff G, Johnson J, Crowell J, Boone C, DeGeorge J, Steele V, et al. Approaches to the development and marketing approval of drugs that prevent cancer. Cancer epidemiology, biomarkers & prevention: a publication of the American Association for Cancer Research, cosponsored by the American Society of Preventive Oncology. 1995;4:1–10. [PubMed] [Google Scholar]
- 14.Wu X, Patterson S, Hawk E. Chemoprevention--history and general principles. Best practice & research Clinical gastroenterology. 2011;25:445–459. doi: 10.1016/j.bpg.2011.10.012. [DOI] [PubMed] [Google Scholar]
- 15.Andriole G, Bostwick D, Brawley O, Gomella L, Marberger M, Montorsi F, et al. Effect of dutasteride on the risk of prostate cancer. The New England journal of medicine. 2010;362:1192–1202. doi: 10.1056/NEJMoa0908127. [DOI] [PubMed] [Google Scholar]
- 16.Thompson IM, Goodman PJ, Tangen CM, Lucia MS, Miller GJ, Ford LG, et al. The influence of finasteride on the development of prostate cancer. The New England journal of medicine. 2003;349:215–224. doi: 10.1056/NEJMoa030660. [DOI] [PubMed] [Google Scholar]
- 17.Thompson I, Goodman P, Tangen C, Parnes H, Minasian L, Godley P, et al. Long-term survival of participants in the prostate cancer prevention trial. The New England journal of medicine. 2013;369:603–610. doi: 10.1056/NEJMoa1215932. This was a 18-year follow-up of the prostate cancer prevention trial which showed no significant differnce in overall survival after administration of finasteride. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Thompson IM, Chi C, Ankerst DP, Goodman PJ, Tangen CM, Lippman SM, et al. Effect of finasteride on the sensitivity of PSA for detecting prostate cancer. Journal of the National Cancer Institute. 2006;98:1128–1133. doi: 10.1093/jnci/djj307. [DOI] [PubMed] [Google Scholar]
- 19.Thompson IM, Tangen CM, Goodman PJ, Lucia MS, Parnes HL, Lippman SM, et al. Finasteride improves the sensitivity of digital rectal examination for prostate cancer detection. The Journal of urology. 2007;177:1749–1752. doi: 10.1016/j.juro.2007.01.071. [DOI] [PubMed] [Google Scholar]
- 20.Venkateswaran V, Klotz L. Diet and prostate cancer: mechanisms of action and implications for chemoprevention. Nature reviews Urology. 2010;7:442–453. doi: 10.1038/nrurol.2010.102. [DOI] [PubMed] [Google Scholar]
- 21.Thapa D, Ghosh R. Antioxidants for prostate cancer chemoprevention: challenges and opportunities. Biochemical pharmacology. 2012;83:1319–1330. doi: 10.1016/j.bcp.2011.12.027. This review discussed the preclinical and clinical stuides of various antioxidants in prostate cancer chemoprevention and highlighted factors to be considered while designing chemoprevention trials. [DOI] [PubMed] [Google Scholar]
- 22.Tomomi K. East meets West: ethnic differences in prostate cancer epidemiology between East Asians and Caucasians. Chinese Journal of Cancer. 2012 doi: 10.5732/cjc.011.10324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kurahashi N, Sasazuki S, Iwasaki M, Inoue M, Tsugane S, Group JS. Green tea consumption and prostate cancer risk in Japanese men: a prospective study. American journal of epidemiology. 2008;167:71–77. doi: 10.1093/aje/kwm249. [DOI] [PubMed] [Google Scholar]
- 24.Miller BA, Chu KC, Hankey BF, Ries LA. Cancer incidence and mortality patterns among specific Asian and Pacific Islander populations in the U.S. Cancer causes & control: CCC. 2008;19:227–256. doi: 10.1007/s10552-007-9088-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gomez S, Noone A-M, Lichtensztajn D, Scoppa S, Gibson J, Liu L, et al. Cancer Incidence Trends Among Asian American Populations in the United States, 1990 to 2008. Journal of the National Cancer Institute. 2013;105(15):1096–1110. doi: 10.1093/jnci/djt157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chan J, Gann P, Giovannucci E. Role of diet in prostate cancer development and progression. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2005;23:8152–8160. doi: 10.1200/JCO.2005.03.1492. [DOI] [PubMed] [Google Scholar]
- 27.Hori S, Butler E, McLoughlin J. Prostate cancer and diet: food for thought? BJU international. 2011;107:1348–1359. doi: 10.1111/j.1464-410X.2010.09897.x. [DOI] [PubMed] [Google Scholar]
- 28.Gathirua-Mwangi W, Zhang J. Dietary factors and risk for advanced prostate cancer. European journal of cancer prevention : the official journal of the European Cancer Prevention Organisation (ECP) 2013;23(2):96–109. doi: 10.1097/CEJ.0b013e3283647394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lippman SM, Klein EA, Goodman PJ, Lucia MS, Thompson IM, Ford LG, et al. Effect of selenium and vitamin E on risk of prostate cancer and other cancers: the Selenium and Vitamin E Cancer Prevention Trial (SELECT) JAMA. 2009;301:39–51. doi: 10.1001/jama.2008.864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Marshall JR, Ip C, Romano K, Fetterly G, Fakih M, Jovanovic B, et al. Methyl selenocysteine: single-dose pharmacokinetics in men. Cancer prevention research (Philadelphia, Pa) 2011;4:1938–1944. doi: 10.1158/1940-6207.CAPR-10-0259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Schenk JM, Till CA, Tangen CM, Goodman PJ, Song X, Torkko KC, et al. Serum 25-Hydroxyvitamin D Concentrations and Risk of Prostate Cancer: Results from the Prostate Cancer Prevention Trial. Cancer Epidemiology Biomarkers & Prevention. 2014;23:1484–1493. doi: 10.1158/1055-9965.EPI-13-1340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kristal AR, Till C, Song X, Tangen CM, Goodman PJ, Neuhauser ML, et al. Plasma vitamin D and prostate cancer risk: results from the Selenium and Vitamin E Cancer Prevention Trial. Cancer epidemiology, biomarkers & prevention : a publication of the American Association for Cancer Research, cosponsored by the American Society of Preventive Oncology. 2014;23:1494–1504. doi: 10.1158/1055-9965.EPI-14-0115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Newman D, Cragg G. Natural products as sources of new drugs over the 30 years from 1981 to 2010. Journal of natural products. 2012;75:311–335. doi: 10.1021/np200906s. This review lays out detailed statistics of natural products which were approved in the past 30 years for cacner treatment. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Premalatha B, Rajgopal G. Cancer—an ayurvedic perspective. Pharmacological Research. 2005;51(1):19–30. doi: 10.1016/j.phrs.2004.04.010. [DOI] [PubMed] [Google Scholar]
- 35.Li X, Yang G, Li X, Zhang Y, Yang J, Chang J, et al. Traditional Chinese medicine in cancer care: a review of controlled clinical studies published in chinese. PloS one. 2012;8(4):e60338. doi: 10.1371/journal.pone.0060338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cuéllar M, Giner R, Recio M, Máñez S, Ríos J. Topical anti-inflammatory activity of some Asian medicinal plants used in dermatological disorders. Fitoterapia. 2001;72:221–229. doi: 10.1016/s0367-326x(00)00305-1. [DOI] [PubMed] [Google Scholar]
- 37.Garcia G, Nicole A, Bhaskaran S, Gupta A, Kyprianou N, Kumar A. Akt-and CREB-mediated prostate cancer cell proliferation inhibition by Nexrutine, a Phellodendron amurense extract. Neoplasia (New York, NY) 2006;8:523–533. doi: 10.1593/neo.05745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Akira I, Takayuki N, Hisao U. Indolopyridoquinazoline, furoquinoline and canthinone type alkaloids from Phellodendron amurense callus tissues. Phytochemistry. 1998:48. [Google Scholar]
- 39.Ryuk J, Zheng M, Lee M, Seo C, Li Y, Lee S, et al. Discrimination of Phellodendron amurense and P. chinense based on DNA analysis and the simultaneous analysis of alkaloids. Archives of pharmacal research. 2012;35:1045–1054. doi: 10.1007/s12272-012-0612-y. [DOI] [PubMed] [Google Scholar]
- 40.Xian Y-F, Lin Z-X, Ip S-P, Su Z-R, Chen J-N, Lai X-P. Comparison the neuropreotective effect of Cortex Phellodendri chinensis and Cortex Phellodendri amurensis against beta-amyloid-induced neurotoxicity in PC12 cells. Phytomedicine: international journal of phytotherapy and phytopharmacology. 2013;20:187–193. doi: 10.1016/j.phymed.2012.09.028. [DOI] [PubMed] [Google Scholar]
- 41.Oben J, Enonchong E, Kothari S, Chambliss W, Garrison R, Dolnick D. Phellodendron and Citrus extracts benefit joint health in osteoarthritis patients: a pilot, double-blind, placebo-controlled study. Nutrition journal. 2009;8:38. doi: 10.1186/1475-2891-8-38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Talbott S, Talbott J, Pugh M. Effect of Magnolia officinalis and Phellodendron amurense (Relora®) on cortisol and psychological mood state in moderately stressed subjects. Journal of the International Society of Sports Nutrition. 2013;10:37. doi: 10.1186/1550-2783-10-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Muralimanoharan S, Kunnumakkara A, Shylesh B, Kulkarni K, Haiyan X, Ming H, et al. Butanol fraction containing berberine or related compound from nexrutine inhibits NFkappaB signaling and induces apoptosis in prostate cancer cells. The Prostate. 2009;69:494–504. doi: 10.1002/pros.20899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kumar A, Bhaskaran S, Ganapathy M, Crosby K, Davis M, Kochunov P, et al. Akt/cAMP-responsive element binding protein/cyclin D1 network: a novel target for prostate cancer inhibition in transgenic adenocarcinoma of mouse prostate model mediated by Nexrutine, a Phellodendron amurense bark extract. Clinical cancer research : an official journal of the American Association for Cancer Research. 2007;13:2784–2794. doi: 10.1158/1078-0432.CCR-06-2974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Yan G, Lanza-Jacoby S, Wang C. Nexrutine inhibits survival and induces G1 cell cycle arrest, which is associated with apoptosis or autophagy depending on the breast cancer cell line. Nutrition and cancer. 2013;66:506–516. doi: 10.1080/01635581.2013.780627. [DOI] [PubMed] [Google Scholar]
- 46.Kumar R, Das M, Ansari KM. Nexrutine(R) inhibits tumorigenesis in mouse skin and induces apoptotic cell death in human squamous carcinoma A431 and human melanoma A375 cells. Carcinogenesis. 2012;33:1909–1918. doi: 10.1093/carcin/bgs219. [DOI] [PubMed] [Google Scholar]
- 47.Ghosh R, Graham H, Rivas P, Tan X, Crosby K, Bhaskaran S, et al. Phellodendron amurense bark extract prevents progression of prostate tumors in transgenic adenocarcinoma of mouse prostate: potential for prostate cancer management. Anticancer research. 2010;30:857–865. [PubMed] [Google Scholar]
- 48.Ghosh R, Garcia G, Crosby K, Inoue H, Thompson I, Troyer D, et al. Regulation of Cox-2 by cyclic AMP response element binding protein in prostate cancer: potential role for nexrutine. Neoplasia (New York, NY) 2007;9:893–899. doi: 10.1593/neo.07502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Gong J, Muñoz AR, Chan D, Ghosh R, Kumar AP. STAT3 down regulates LC3 to inhibit autophagy and pancreatic cancer cell growth. Oncotarget. 2014;5:2529–2541. doi: 10.18632/oncotarget.1810. This was the first study elucidating the effect of Nexrutine® on autophagy and ROS in pancreatic caner. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Gong J, Xie J, Bedolla R, Rivas P, Chakravarthy D, Freeman JW, et al. Combined targeting of STAT3/NF-κB/COX-2/EP4 for effective management of pancreatic cancer. Clinical cancer research : an official journal of the American Association for Cancer Research. 2014;20:1259–1273. doi: 10.1158/1078-0432.CCR-13-1664. The benefits of Nexrutine® for pancreatic caner treatment was established and it was shown that Nexrutine® can reduce fibrosis, which has important implication for therapeutic resistance. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Xiao X, Li BX, Mitton B, Ikeda A, Sakamoto KM. Targeting CREB for cancer therapy: friend or foe. Current cancer drug targets. 2010;10:384–391. doi: 10.2174/156800910791208535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Taylor BS, Schultz N, Hieronymus H, Gopalan A, Xiao Y, Carver BS, et al. Integrative genomic profiling of human prostate cancer. Cancer cell. 2010;18:11–22. doi: 10.1016/j.ccr.2010.05.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wang Y, Kreisberg JI, Ghosh PM. Cross-talk between the androgen receptor and the phosphatidylinositol 3-kinase/Akt pathway in prostate cancer. Current cancer drug targets. 2007;7:591–604. doi: 10.2174/156800907781662248. [DOI] [PubMed] [Google Scholar]
- 54.Thomas C, Lamoureux F, Crafter C, Davies BR, Beralidi E, Fazli L, et al. Synergistic targeting of PI3K/AKT-pathway and androgen-receptor axis significantly delays castration-resistant prostate cancer progression in vivo. Molecular cancer therapeutics. 2013;12(11):2342–2355. doi: 10.1158/1535-7163.MCT-13-0032. [DOI] [PubMed] [Google Scholar]
- 55.Sun H-ZZ, Yang T-WW, Zang W-JJ, Wu S-FF. Dehydroepiandrosterone-induced proliferation of prostatic epithelial cell is mediated by NFKB via PI3K/AKT signaling pathway. The Journal of endocrinology. 2010;204:311–318. doi: 10.1677/JOE-09-0270. [DOI] [PubMed] [Google Scholar]
- 56.Vincent F, Laurent L, Louis RB, Pierre K, Anne-Marie Mes M, Fred S. Nuclear Factor-κB Nuclear Localization Is Predictive of Biochemical Recurrence in Patients with Positive Margin Prostate Cancer. Clinical Cancer Research. 2004;10(24):8460–8464. doi: 10.1158/1078-0432.CCR-04-0764. [DOI] [PubMed] [Google Scholar]
- 57.Lee R, Saylor P, Smith M. Treatment and prevention of bone complications from prostate cancer. Bone. 2011;48:88–95. doi: 10.1016/j.bone.2010.05.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Alicikus ZA, Yamada Y, Zhang Z, Pei X, Hunt M, Kollmeier M, et al. Ten-year outcomes of high-dose, intensity-modulated radiotherapy for localized prostate cancer. Cancer. 2011;117:1429–1437. doi: 10.1002/cncr.25467. [DOI] [PubMed] [Google Scholar]
- 59.Swanson GP, Jones WE, Ha CS, Jenkins CA, Kumar AP, Basler J. Tolerance of Phellodendron amurense Bark Extract (Nexrutine®) in Patients with Human Prostate Cancer. Phytotherapy research: PTR. 2014 doi: 10.1002/ptr.5221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Karantanos T, Corn PG, Thompson TC. Prostate cancer progression after androgen deprivation therapy: mechanisms of castrate resistance and novel therapeutic approaches. Oncogene. 2013;32:5501–5511. doi: 10.1038/onc.2013.206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Erkan M, Hausmann S, Michalski CW, Fingerle AA, Dobritz M, Kleeff J, et al. The role of stroma in pancreatic cancer: diagnostic and therapeutic implications. Nature reviews Gastroenterology & hepatology. 2012;9:454–467. doi: 10.1038/nrgastro.2012.115. [DOI] [PubMed] [Google Scholar]
- 62.Oyajobi BO, Gupta A, McCluskey B, Mann M, Kumar AP. Antitumor effect of Nexrutine, a Phellodendron amurense bark extract in multiple myeloma. 102nd Annual Meeting of American Association for Cancer Research. 2011 Abstract 4216. [Google Scholar]
- 63.Alexander G, Helena JMS, Amy EM, Heather RR, Ann CW, Christos P, et al. The COX-2/PGE2 pathway: key roles in the hallmarks of cancer and adaptation to the tumour microenvironment. Carcinogenesis. 2009;30(3):377–386. doi: 10.1093/carcin/bgp014. [DOI] [PubMed] [Google Scholar]
- 64.Mayr B, Montminy M. Transcriptional regulation by the phosphorylation-dependent factor CREB. Nature reviews Molecular cell biology. 2001;2:599–609. doi: 10.1038/35085068. [DOI] [PubMed] [Google Scholar]
- 65.Xiongfei Z, Jingjing Z, Xiaomin Y, Xiao H. Several transcription factors regulate COX-2 gene expression in pancreatic β-cells. Molecular Biology Reports. 2007;34(3):199–206. doi: 10.1007/s11033-007-9085-3. [DOI] [PubMed] [Google Scholar]
- 66.Basler JW, Piazza GA. Nonsteroidal anti-inflammatory drugs and cyclooxygenase-2 selective inhibitors for prostate cancer chemoprevention. The Journal of urology. 2004;171(2 Pt 2):S59–S62. doi: 10.1097/01.ju.0000107839.06670.27. [DOI] [PubMed] [Google Scholar]
- 67.Harris RE. Cyclooxygenase-2 (cox-2) blockade in the chemoprevention of cancers of the colon, breast, prostate, and lung. Inflammopharmacology. 2009;17:55–67. doi: 10.1007/s10787-009-8049-8. [DOI] [PubMed] [Google Scholar]
- 68.Gilbert CA, Slingerland JM. Cytokines, obesity, and cancer: new insights on mechanisms linking obesity to cancer risk and progression. Annual review of medicine. 2012;64:45–57. doi: 10.1146/annurev-med-121211-091527. [DOI] [PubMed] [Google Scholar]
- 69.Eugenia EC, Rudolf K. Overweight, obesity and cancer: epidemiological evidence and proposed mechanisms. Nature Reviews Cancer. 2004;4:579–591. doi: 10.1038/nrc1408. [DOI] [PubMed] [Google Scholar]
- 70.Wu S, Yang L, Gao Y, Liu X, Liu F. Multi-channel counter-current chromatography for high-throughput fractionation of natural products for drug discovery. Journal of chromatography A. 2008;1180:99–107. doi: 10.1016/j.chroma.2007.12.024. [DOI] [PubMed] [Google Scholar]
- 71.Harvey A. Natural products in drug discovery. Drug discovery today. 2008;13:894–901. doi: 10.1016/j.drudis.2008.07.004. [DOI] [PubMed] [Google Scholar]
- 72.Cheng Y, Wang Y, Wang X. A causal relationship discovery-based approach to identifying active components of herbal medicine. Computational biology and chemistry. 2006;30(2):148–154. doi: 10.1016/j.compbiolchem.2005.11.003. [DOI] [PubMed] [Google Scholar]
- 73.Wang Y, Jin Y, Zhou C, Qu H, Cheng Y. Discovering active compounds from mixture of natural products by data mining approach. Medical & biological engineering & computing. 2008;46:605–611. doi: 10.1007/s11517-008-0323-1. [DOI] [PubMed] [Google Scholar]