Abstract
The aim of the study was to obtain the highest number of multipotent adipose-derived mesenchymal stem cells (ADMSCs) by using culture conditions which favour cell expansion without loss of mesenchymal stem cells (MSC)-like properties. Based on the assumption that stem cells reside in niches characterized by hypoxic condition, we investigated if the low oxygen tension may improve the proliferation and stemness of ADMSCs. Intact adipose tissue was resected from eight subjects, and the stromal vascular fraction was obtained by using type II collagenase. The heterogeneity of cellular lineages was confirmed by immunophenotypic analysis that showed the presence of leukocytes (CD45+), endothelial cells (CD34+), and pericytes (CD140+). The immunophenotype of confluent ADMSCs was similar to that of bone marrow-derived MSCs, except for the expression of CD34, which was variable (donor-dependent) and inversely correlated to the CD36 expression. ADMSCs showed a high clonal efficiency (94.5 ± 1 %) and were able to generate osteoblastic, chondrocytic and adipocytic lineages. ADMSCs were cultured under normoxic (21 % O2) and hypoxic (1 % O2) conditions, and we found that hypoxia significantly favoured ADMSC proliferation and preserved the expression of stemness genes, i.e. Nanog and Sox2. Since hypoxia reflects the microenvironment in which ADMSCs must proliferate and differentiate, the culture in hypoxic condition allows to better understand the biology of these cells and their regenerative potential. Low oxygen concentrations promote cell proliferation and stemness, thus enriching the pool of cells potentially able to differentiate into multi-lineages, and extending the possibility of a long-term expansion.
Keywords: Adipose-derived MSC, Intact adipose tissue, Hypoxia, Stemness, Proliferation, Regenerative medicine
Introduction
Mesenchymal stem cells (MSC) possess a self-renewal capacity and the potential to differentiate into many cell types including chondrocytes, osteoblasts adipocytes, myoblasts, and fibroblasts (Bruder et al. 1998). Over the past 10–20 years, adult MSC have been widely used for a plurality of applications, including studies on the pathophysiology of bone diseases, cell and gene therapies, and tissue engineering for bone regeneration (Arvidson et al. 2011; Barrilleaux et al. 2006; Mansilla et al. 2007; Salem and Thiemermann 2010; Granchi et al. 2010; Ciapetti et al. 2012; Scotti et al. 2013; Lecourt et al. 2012). For a long time, bone marrow aspiration has been considered the most reliable source of MSC, even though the low yield of stem cells may be a limitation in both pre-clinical and clinical applications.
Recently, MSCs derived from adipose tissue (ADMSCs) have received increasing interest for tissue engineering applications owing to their relative ease of harvest, abundance, and multi-lineage differentiation potential (Schäffler and Büchler 2007). In cosmetic surgery, the fat that is removed as intact tissue or aspirated by liposuction is usually discarded, but it represents an abundant and accessible source of multipotent stromal cells (Zuk et al. 2001). There are relevant differences between MSCs resident in the bone marrow stroma and ADMSCs. For instance, the first ones constitute about 0.001–0.01 % of the total marrow nucleated cells, whereas the amount of ADMSCs isolated from an equivalent amount of adipose tissue is approximately 500-fold greater (Kern et al. 2006; Strioga et al. 2012). Therefore, ADMSCs could be more suitable for clinical applications or research purposes when a large amount of cells is required. Despite their enormous potential, there are still several steps that need to be resolved before they may be used widely in clinical practice, especially in the field of bone regeneration (Schroeder et al. 2011). Presently, about two hundred clinical studies concerning the use of ADMSCs as therapeutic intervention are registered in ClinicalTrial.gov (http://clinicaltrials.gov), but only eight trials are related to orthopaedic conditions and in none of these the use of ADMSCs is intended for repairing bone defects (Table 1). An essential requirement for the effectiveness of regenerative strategies is the good yield of stem cells, which is mainly depending on source and processing method. In addition, it is desirable that cells retain over time the capacity to proliferate and maintain their multipotency (Pawitan 2011; Huang et al. 2009).
Table 1.
List of clinical trials related to orthopaedics and registered on ClinicalTrial.gov until May 2013
| Condition | Id number | Title |
|---|---|---|
| Articular cartilage lesion of the femoral condyle | NCT01399749 | Autologous mesenchymal stem cells vs. chondrocytes for the repair of chondral knee defects |
| Degenerative arthritis | NCT01300598 | Autologous adipose tissue derived mesenchymal stem cells transplantation in patient with degenerative arthritis |
| Lumbar intervertebral disc degeneration | NCT01643681 | Autologous adipose tissue derived mesenchymal stem cells transplantation in patient with lumbar intervertebral disc degeneration |
| Osteoarthritis | NCT01585857 | ADIPOA—clinical study |
| NCT01739504 | Autologous adipose-derived stromal cells delivered intra-articularly in patients with osteoarthritis | |
| NCT01809769 | Autologous adipose tissue derived mesenchymal progenitor cells therapy for patients with knee osteoarthritis | |
| Osteoporotic fractures | NCT01532076 | Effectiveness of adipose tissue derived mesenchymal stem cells as osteogenic component in composite grafts |
| Rheumatoid arthritis aggravated | NCT01663116 | Cx611-0101, eADMSCs Intravenous administration to refractory rheumatoid arthritis patients |
Several methods have been employed to isolate and cultivate ADMSCs (Stocchero and Stocchero 2011; Bourin et al. 2013). Most of the protocols involve enzymatic digestion of the sample, but there is a large variety in the kind and concentration of the enzyme used, time and conditions of incubation for the tissue digestion. Moreover, some authors purified the vascular fraction on a gradient density, while others use the total stromal vascular fraction (Bunnell et al. 2008). The development of a standardized and reproducible method for isolating and culturing ADMSCs is a basic requirement to validate their use in experimental protocols, since the methodological differences may influence the properties of isolated cells and influence the functional results.
The main goal of this study was to identify the best method to obtain a high number of multipotent ADMSCs, by using culture condition that allowed cell expansion without loss of MSC-like properties. As a source we used intact adipose tissue resected from the abdomen of morbidly obese subjects. We compared two different methods for isolating ADMSCs and assessed the cell yield. Moreover, we analyzed the immunophenotype of the cultured ADMSCs and the stem properties, including cell cloning capacity and multipotency. Based on the assumption that stem cells reside in niches characterized by hypoxic condition (Mohyeldin et al. 2010), we investigated if the low oxygen tension may improve the proliferation and stemness of ADMSCs in early stages of the culture.
Materials and methods
Cell isolation
ADMSCs were isolated from intact adipose tissues of 8 subjects (four women; mean age 42.2 years; four men; mean age: 39.6 years) undergoing abdominal plastic surgery following a remarkable weight loss (BMI < 30) (Fraccalvieri et al. 2007). Informed consent was obtained from the enrolled patients, all of whom agreed that discarded biological samples could be used for research purposes. A code number was assigned to each sample in order to assure subject anonymity. All samples were processed within 24 h.
Adipose tissue was washed with PBS, minced into small pieces, and treated by mechanical or enzymatic method. Through the mechanic method the sample were agitated at least for one minute, three times, using a vortex equipment and then the samples were incubated at 37 °C for 15 min. Enzymatic digestion was conducted by 0.075 % type II collagenase (Gibco, Invitrogen, Monza, Italy) and incubated under continuous shaking conditions for 60 min at 37 °C. Mature adipocytes and connective tissues were separated from pellets by centrifugation (400 g for 10 min). The pellets were resuspended and filtered with a 100-mm mesh (Sigma-Aldrich, Milan, Italy).
Cell culture
Freshly isolated stromal vascular fraction (SVF), was resuspended in Alpha-MEM (Sigma-Aldrich, Milan, Italy) supplemented with 10 % FBS (Lonza, Basel, Switzerland), 100 U/mL penicillin (Lonza), 0.1 mg/mL streptomycin (Lonza). Aliquots of cells were stored frozen in liquid nitrogen to be re-used in following experiments. The cells were seeded at a density of 100,000 or 500,000 cell/cm2 and maintained at 37 °C in 5 % CO2/21 % O2 in humidified atmosphere. The culture medium was discarded and replaced with fresh medium every 3–4 days. All experiments were performed with 2nd passage cells. To create the hypoxic conditions the cells were incubated in the Hypoxia Incubator Chamber (Stem Cell Technology, Vancuover, Canada). Briefly, the cultures were enclosed in the chamber and flushed with a mixture of gasses (95 % N2 and 5 % CO2). The final oxygen tension was 1–3 %, measured by oximeter Oxybaby M+ (Witt Technology, Solza, Italy).
Flow cytometry
Immunophenotyping of ADMSCs was performed using monoclonal antibodies conjugated with fluorescin isothiocynate (FITC), or R-Phycoerythrin (RD1), or RD1-Cyanin 5.1 (PC5) (Instrumentation Laboratory, Milan, Italy). The description of the tested antigens is shown in Table 2.
Table 2.
Percentage (mean value ± SEM) of cells expressing markers used for immunophenotypic characterization of ADMSC
| Cluster differentiation or Antigen | Description | SVF | 1st confluence of adherent cells (ADMSC) |
|---|---|---|---|
| CD34 | Hemopoietic progenitor cell antigen 1 (HPCA1) | 46 ± 4 | 55 ± 22 |
| CD36 | Thrombospondin receptor, collagen receptor (type I, IV, V) | 29 ± 5 | 34 ± 15 |
| CD45 | Leukocyte common antigen; protein tyrosine phosphatase receptor type C | 30 ± 3 | 5 ± 1 |
| CD49e | α5 integrin; fibronectin receptor | 22 ± 6 | 97 ± 1 |
| CD90 | Thymus cell antigen 1 (Thy-1) | 59 ± 7 | 97 ± 2 |
| CD105 | Endoglin; binds TGFβ1 and TGFβ3, activin A, BMP7, BMP2 but requires coexpression of the respective ligand binding kinase receptor | 5 ± 2 | 94 ± 2 |
| CD117 | c-Kit; stem cell factor receptor (SCFR) | 6 ± 1 | 8 ± 3 |
| CD140b | Platelet-derived growth factor receptor-β (PDGFRβ) | 26 ± 9 | 78 ± 8 |
| STRO-1 | Unidentified antigen; STRO-1 +ve cells are able to differentiate into multiple mesenchymal lineages | 9 ± 2 | 4 ± 1 |
Monoclonal antibodies and cells (105/test) were incubated for 20 min at 4 °C, and the portion of positive cells was evaluated on 10,000 events by using a flow cytometer EPICS XL-MCL (Beckman Coulter, Fullerton, CA, USA).
Fibroblastoid colony-forming unit (CFU-F) evaluation
Briefly, following trypsinization of adherent cells at the first confluence, 50 cells/cm2 were plated in 6 well plates, and cultured with complete medium. After 14 days, duplicate wells were stained with crystal violet dye (Sigma) (25 % crystal violet dye in 20 % methanol for 10 min at room temperature). An aggregate with more than 50 cells was visually scored as a colony and counted at optical microscope. The experiment was performed in duplicate and repeated twice.
Multipotency assays
For osteogenic differentiation, 100,000 cells/well were seeded in 6 well plate in duplicate, and cultured until subconfluency. Then, the culture medium was replaced with the differentiation medium consisting of α-MEM, 10 % FBS, supplemented with 50 μg/mL l-ascorbic acid 2-phosphate (Sigma), and 10−8 M dexamethasone (Sigma). At cell confluence, the medium was supplemented with 10 mM β-glycerophosphate (Sigma) in order to induce the deposition of mineralized matrix. After 21 days, cells were fixed in 3.7 % paraformaldehyde for 20 min and stained with 1 % Alizarin Red (pH 4.2) (Sigma) for one hour at room temperature.
Chondrogenic differentiation was induced in pellet culture. Briefly, 200,000 cells were resuspended in complete High Glucose D-MEM (Euroclone, Milan, Italy), supplemented with insulin (6.25 μg/mL) (Roche, Applied Science, Monza, Italy), l-ascorbic acid 2-phosphate (50 μM) and TGFβ3 (10 ng/mL) (Prospec, Rehovot, Israel). After 21 days the pellet was fixed in 10 % buffered formalin, embedded in paraffin, sectioned at 5 μm, deparaffinized, hydrated, and finally stained with Alcian Blue (pH 2.5) (Sigma) for 30 min at room temperature.
For adipogenic differentiation, 100,000 cells/well were seeded on 6 well plate, cultured until subconfluency, and then the culture medium was replaced with the differentiation medium consisting of complete medium supplemented with dexamethasone (0.5 μM), indomethacin (50 μM) (Sigma) and isobutylmethylxanthine (0.5 mM) (Sigma). To detect lipid accumulation, cultures were fixed in 3.7 % paraformaldehyde for 20 min and stained with 0.3 % Oil Red O in isopropanol for one hour at room temperature.
Proliferation assay
Cells were seeded at 15,000/well, and cell proliferation was evaluated after 0, 24, 72, 96, and 144 h. Cell counting was performed on harvested cells using erythrosin B (Sigma) dye exclusion assay.
Gene expression analysis
The gene expression was analyzed after seven days of culture, by quantifying the transcripts of genes useful to monitor the ADMSCs stemness (Table 3). RNA was extracted with NucleoSpin RNA II (Macherey–nagel, Düren, Germany) and the retrotranscription was performed with MuLV Reverse Transcriptase (Applied Biosystems, Foster City, Ca, USA). Real-Time Polymerization Chain Reaction (Real-time PCR) was performed by amplifying 1 μg of cDNA using the Light Cycler instrument and the Universal Probe Library system (Roche). Probes and primers were selected using the web-based assay design software (ProbeFinder: http://www.roche-applied-science.com). β-actin was used as housekeeping gene to normalize the expression of the genes of interest. The results were expressed as a ratio between gene of interest and β-actin reference gene.
Table 3.
List of primers and probes selected to analyse the expression of genes related to the cell stemness
| Gene | ID number | Primer sequence (5′–3′) | Probe |
|---|---|---|---|
| β-actin | NM_001101.2 |
Sense
ccaaccgcgagaagatga
Antisense agggctgtcctgaataagca |
#64 |
| Nanog | NM_024865.2 |
Sense
atgcctcacacggagactgt
Antisense agggctgtcctgaataagca |
#69 |
| Sox-2 | NM_003106.3 |
Sense
gggaatggaccttgtatag
Antisense gcaaagctcctaccgtacca |
#65 |
| Oct-4 | NM_002701.4 |
Sense
cttcgcaagccctcatttc
Antisense gagaagggaaatccgaag |
#60 |
Statistical analysis
The statistical analysis was performed by StatView5.01 software (SAS Institute Inc., Cary, NC, USA). Quantitative results were expressed as arithmetic mean plus or minus the standard error of the mean (SEM). Mann–Whitney test was performed as unpaired comparison for two independent variables. All p values <0.05 were considered as statistically significant.
Results
Isolation of ADMSCs
The enzymatic and the mechanic method of isolation were compared in terms of cell yield. The enzymatic digestion with collagenase type II 0.075 % resulted to be the best method of MSCs isolation from adipose tissue. The total number of cells isolated from tissue treated by enzyme was about 1.5 fold higher than mechanic treatment (data not shown). Moreover, after five days of culture, ADMSCs isolated using collagenase reached a confluence of 70–80 %, while by mechanic treatment only a few number of cells were able to adhere to plastic (Fig. 1). In order to find the best seeding density after cell isolation, two different amounts of cell, 100,000 and 500,000 cells/cm2, were assessed. Cells seeded at low cell density reached a 40–50 % confluence, while cells seeded at 500,000 cells/cm2 reached an 80–90 % confluence within seven days. Based on the above results, the following experiments were performed employing the isolation method that allowed the best yield, i.e., enzymatic digestion of the adipose tissue and a seeding density of 500,000 cells/cm2.
Fig. 1.
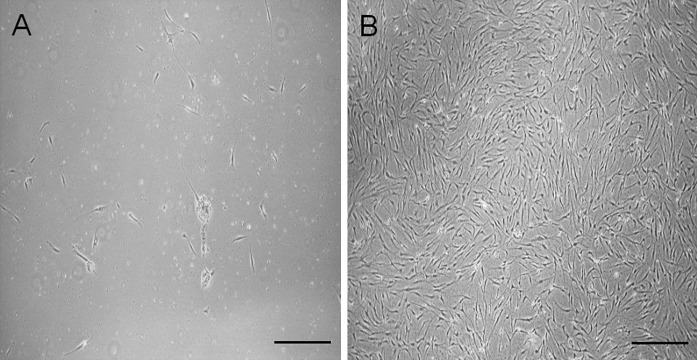
The images represent the 7-day growth of cells treated with mechanic A or enzymatic B method. Bars represent 50 μm
ADMSCs characterization
Immunophenotype
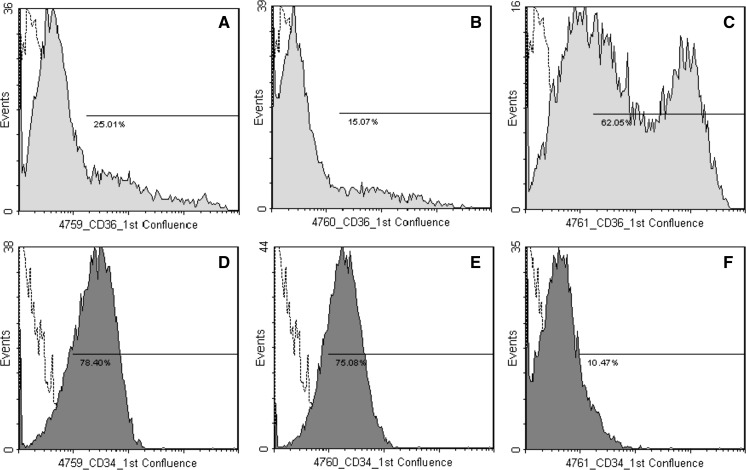
The immunophenotype was characterized before the seeding of the SVF (stromal vascular fraction) and at the first confluence of ADMSCs (Table 2). We found heterogeneous population of cells in SVF, since in the same pool of cells hematopoietic and mesenchymal antigens coexisted. In SVF the mean percentage of CD105, CD117 and STRO1 positive cells was lower than 10 %. The expression of CD117 and STRO-1 continued to be low at the first confluence of the cells, CD45 disappeared, while CD49e, CD90, CD105 were highly expressed (>90 %). The mean percentage of CD140b positive ADMSCs was 78 ± 8 %, thus testifying that “Platelet-derived growth factor receptor-β” antigen was not uniformly expressed by the whole population. Even though the cultures were obtained from three different individuals, the immunophenotype of confluent cells was quite homogenous, except for CD34 and CD36 that showed a degree of variability (SEM ≥ 15 %). As shown in Fig. 2, the expression of CD34 and CD36 was donor-dependent, and the two antigens appeared to be inversely related.
Fig. 2.
Flow cytometric analysis of 1st ADMSCs confluence. The images show the inter-individual differences in CD36 (A–C) and CD34 expression (D–F) in three different subjects (4759, 4760, 4761). Histogram plots represent the percentage of positive cells in the whole population of ADMSCs versus isotype controls (open histograms). 10,000 events were scored
Cloning capacity and multipotency
The CFU-F evaluation was performed after 10 days of culture. ADMSCs were able to form a high number of colonies of at least 50 cells (47.2 ± 3) with a clonal efficiency equal to 94.5 ± 1 %. An important marker of stemness is the ability of ADMSCs to differentiate in vitro in a different cellular lineage. We tested the ability of ADMSCs to differentiate in osteogenic, condrogenic and adipogenic lineage. After 21 days of culture with specific inductors, cell differentiation was assessed using different staining (Fig. 3). The osteogenic differentiation medium induced cells to form multiple individual clusters with cells growing in several layers, producing a mineralized extracellular matrix positively stained with Alizarin Red (Fig. 3a). Cultured cell pellets maintained integrity and showed intense Alcian blue staining, indicating a high content of sulfated proteoglycans (Fig. 3b). Cells treated with adipogenic medium contained single lipid droplets stained by Oil Red O (Fig. 3c).
Fig. 3.

In vitro differentiation of ADMSCs into osteoblasts (A), chondocytes (B), and adipocytes (C). Bars represent 20 μm
Effect of hypoxia on proliferation and stemness of ADMSCs
Cell proliferation
We evaluated if the hypoxic condition could reduce the time required to reach the cellular confluence. As shown in Fig. 4, we found that the adhesion of freshly isolated cells was hampered by low oxygen tension, while cells maintained in standard conditions of culture behaved as expected.
Fig. 4.

The images represent the adhesion of ADMSCs after five days of culture in hypoxic (A) and normoxic (B) conditions. Bars represent 50 μm
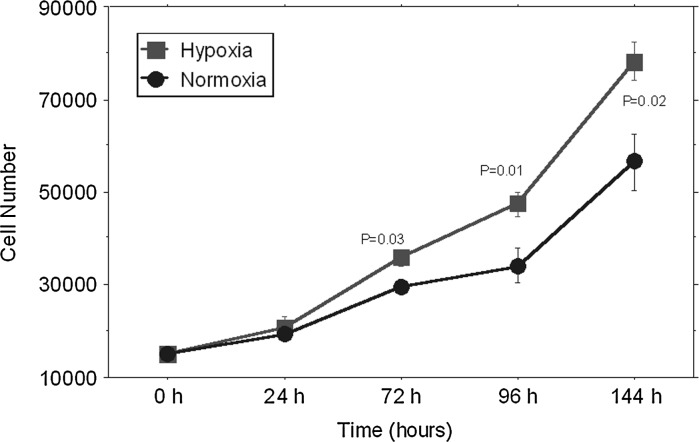
Therefore ADMSCs were cultured in normal oxygen tension until the first confluence. Then, the proliferation of ADMSCs was analyzed under normoxic and hypoxic conditions at five endpoints (0, 24, 72, 96 and 144 h). We found that the low oxygen tension favoured the proliferation rate, and significant differences between the two culture conditions were observed at 72, 96 and 144 h (Fig. 5).
Fig. 5.
ADMSCs were cultured for the indicated times in monolayer culture under hypoxic (filled square) or normoxic (filled circle) conditions. Results are expressed as mean ± SEM
Expression of stemness genes
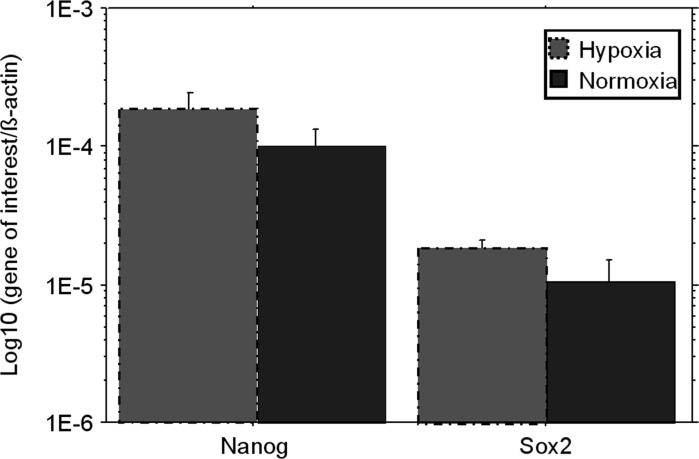
The gene expression of Oct-4, Nanog and Sox2 was analyzed on ADMSCs cultured under normoxic and hypoxic conditions for 7 days. Oct-4 was undetectable in all cultures, while the expression of Nanog and Sox2 increased under low O2 tension, although the differences were not statistically significant (Fig. 6).
Fig. 6.
Expression of genes related to ADMSCs stemness. The quantitative analysis of transcripts was performed on first confluent ADMSCs, in three separate experiments. Bars express the mean ± SEM of the ratios between “gene of interest” and “β-actin” (Log10 scale)
Multipotency
The effect of hypoxia on the multipotent ability of the ADMSCs was evaluated after 21 days of cell culture with the differentiating agents in hypoxic or normoxic condition. Figure 7 shows that hypoxia did not alter the trilineage differentiation capacity of the induced cells.
Fig. 7.
In vitro differentiation of ADMSCs into osteoblasts (A, D), chondocytes (B, E), and adipocytes (C, F) after cultivation in hypoxic (A–C) or normoxic (D–F) condition. Bars represent 20 μm
Discussion
In the field of regenerative medicine, ADMSCs represent a plentiful source of adult multipotent stem cells that provide wide possibilities for clinical applications. This is due to their abundant quantity in the body, their ability to self-renew with high proliferative capacity, and their versatility to differentiate into several cell types (Zuk et al. 2002). However, the use of ADMSCs in orthopedics, especially in bone regenerative medicine, faces still some obstacles, including the lack of standardization of manipulation methods to maximize the therapeutic potential of these cells, especially when they are used for tissue engineering applications (Otte et al. 2013). The aim of the study was to identify the optimal method to obtain the highest number of multipotent ADMSCs by using culture condition that allow cell expansion without loss of MSC-like properties. For this purpose, our work was focused into three main points, namely (1) the adipose tissue source, (2) the isolation technique of the ADMSCs, and (3) the culture method that favoured the maintenance of self-renewal ability, as essential requirements to enhance cell yield and to ensure long-term expansion.
Based on literature data, we have chosen to obtain ADMSCs from intact adipose tissue, thus obtaining abundant amounts of material to store and recover over time. Some authors demonstrated that sliced fat tissue obtained through lipectomy generated a much higher CFU number when compared to fat samples obtained through liposuction (Stocchero and Stocchero 2011; Schreml et al. 2009). The relative paucity of ADMSCs seems to depend on the distribution of multipotent stem cells, which are mainly located around large vessels and thus remain in the donors when the fat is aspirated. Actually, Eto et al. (2009) showed that aspirated adipose tissue was lacking in large vasculature structures, presented a high number of small lipid droplets released from damaged adipocytes, and an ADMSCs yield approximately one-half than in intact tissue.
Secondly, we faced the choice of manipulation techniques to obtain the SVF. To date, most laboratories use enzymatic digestion or mechanical disruption to process the adipose tissue. Inevitably, the methodological differences lead to different characteristics of isolated cells (Bourin et al. 2013; Baptista et al. 2009; Zachar et al. 2011; Gimble et al. 2011). In our hands, the best results were obtained using the enzymatic method that is also the most widely employed. This is in contrast to Baptista et al., who found a higher yield of adherent cells after mechanic treatment (Baptista et al. 2009). The main reason for this discrepancy could be the different source of adipose tissue, since they used lipoaspiration. Since the aspirated tissue is already disrupted, it is reasonable to assume that the ADMSCs fraction is more available than in intact tissue, and the mechanic treatment is sufficient to obtain an acceptable result. On the contrary, the enzymatic treatment is required to enhance the release of the multipotent cells that are trapped in the stroma of the intact tissue, and allows to maximize the yield that, as above described, is considerably higher than that obtained with lipoaspiration.
Since there are not clear indications about the right cell density for cell seeding, we compared two concentrations of cells to obtain a monolayer in 5–7 days. We found that the 80–90 % confluence was obtained with the seeding of 500,000 cells. Since the whole SVF includes a plurality of cell types, it is not surprising that a so high number of cells should be used. The heterogeneity of cellular lineages contained in SVF was confirmed by immunophenotypic analysis that showed the presence of circulating leukocytes (CD45+), endothelial cells (CD34+), and pericytes (CD140+). Our findings agree with literature data that show as endothelial, haematopoietic and pericytic lineages represent 10–20 %, 25–45 % and 3–5 %, respectively, of the total nucleated cells of SVF (Bourin et al. 2013).
The immunophenotype of adherent ADMSCs was similar to that of bone marrow-derived MSCs. CD45 cells disappeared at the first confluence, while the distribution of CD49e, CD90, and CD105 was more homogenous. Differently from bone marrow-derived MSCs, CD34+ was detectable in confluent ADMSCs, even though its expression was highly variable. This result was consistent with the finding of other authors who described the donor-dependent variability of CD34 expression in ADMSCs (Baer et al. 2013). CD34 is reckoned as a hematopoietic stem cells-associated surface marker and the functional role in ADMSCs has not been discovered. However, its expression decreases in later passages, and Suga et al. suggested that the lost of CD34 may reflect the differentiation or commitment of ADMSCs (Suga et al. 2009). They showed that CD34+ cells are more proliferative and have a higher ability to form colonies, while CD34− cells have a greater ability to differentiate into adipogenic and osteogenic lineages. As described for CD34, we observed donor-specific differences in CD36 expression, which resulted to be inversely correlated to the CD34 expression. Actually, other authors used a five-color flow-cytometry and demonstrated that the co-expression of CD34 and CD36 was lacking in cultured ADMSCs, whilst both CD34+CD36− and CD34−CD36+ subpopulations were positive for other MSCs markers (Baer et al. 2013).
The CFU-F assay is recommended to define the number of progenitor cells and to predict the performance of any cell therapy product (Bourin et al. 2013). In our hands, ADMSCs showed a clonal efficiency higher than that reported by others, thus testifying the high proliferative ability of multipotent cells obtained from intact tissue (Stocchero and Stocchero 2011). The multipotency of ADMSCs was further confirmed, since when cultured with proper inducers they were able to generate osteoblastic, chondrocytic and adipocytic lineages.
The third aim of this study was to test culture conditions that favoured the maintenance of self-renewal ability and stemness of ADMSCs. This is an important requirement that needs to be addressed for the development of effective regenerative strategies. In this study, we hypothesized to favour the maintenance of ADMSCs stemness by using unconventional culture conditions reflecting the physiological microenvironment of the stem cell. Normally, cell cultures are maintained under 21 % oxygen tension, corresponding to that of inspired air. Nevertheless, air enters the lungs and travels in the blood throughout the body, so that the oxygen partial pressure (pO2) progressively decreases. Even though MSCs are located close to vascular structures, the physiological oxygen tension of MSC niche ranges between 2 and 8 % (Mohyeldin et al. 2010). These findings suggests that oxygen tension may act as a regulator for the maintenance of MSCs in an undifferentiated state, and let to hypothesize that proliferation and stem cell quiescence may be regulated by gradients of oxygen tension. We found that in the early phases of SVF culture, hypoxia strongly inhibited cell adhesion, but favoured cell proliferation and stemness maintenance if ADMSCs were grown in normoxic condition until the first confluence. This is in contrast with findings of Weijers et al. who described a similar cell adhesion of SVF in 21 and 1 % O2 tension (Weijers et al. 2011). The main reason for the different results between the two studies could be the selection process of their isolation method, which consisted of steps aimed to remove contaminant cells, namely cell filtration and separation by density gradient. In our culture conditions, the effect of hypoxia on the other cells present in the whole SVF could affect the adhesion and growth of ADMSCs subpopulation. However, it should be noted that the mechanisms of cell adhesion are intimately related to the processes of tissue morphogenesis, since signals generated locally by adhesion junctions can interact with classic signal transduction pathways to control cell differentiation (Gumbiner 1996). Different methods have been developed to support the proliferation of bone marrow derived MSC in a nonadherence status (Isern et al. 2013; Baksh et al. 2003; Leonardi et al. 2009). So, MSCs tend to form mesenspheres that maintain their ability to differentiate into mesenchymal lineages, but display a relatively undifferentiated phenotype and increased self-renewal capacity. In this context, the “nonadherence” induced by hypoxia could be synonymous of “undifferentiated” status, and this is not necessarily an undesiderable effect. At present, no study has clarified the relationship among hypoxia, nonadherence, and stemness on primary cultures of adipose-derived MSC, and also our experimental plan is not able to confirm the above hypotheses. However, we can assert that using our isolation protocol is necessary to maintain cells in normoxic conditions until the first confluence to allow a rapid cell proliferation.
The oxygen concentration influences the expression of hypoxia-inducible factors (HIFs), and it has been demonstrated that HIFs regulate the expression of Oct-4, Sox2 and Nanog, the transcription factors essential for the maintenance of pluripotency (Covello et al. 2006; Forristal et al. 2010). Their function is to repress genes that promote differentiation, even if the role of Oct-4 is most relevant in the embryonic cells rather than in adult MSCs (Pierantozzi et al. 2011). In fact, Liedtke et al. demonstrated that the expression of Oct-4 in adult stem cells is essentially missing, and false-positive results can be generated by the trascription of pseudo-genes (Liedtke et al. 2008). These data are supported by several studies which demonstrated that the tissue-specific ablation of Oct-4 did not compromise their proliferative and regenerative capacity (Berg and Goodell 2007). According to these findings, we found that Oct-4 levels were always below the detection limit, while hypoxia favoured the expression of stemness markers, namely Nanog and Sox2, even if not significantly.
Taken together, our results agree with the assumption that hypoxia plays a crucial role in maintaining the stemness of the MSC. However, most of the data regarding the effect of different oxygen tension on proliferation and stemness of MSC have been conducted on bone marrow-derived cells (Mohyeldin et al. 2010; Kim et al. 2013; D’Ippolito et al. 2006; Fehrer et al. 2007; Grayson et al. 2007), while few studies have been performed on ADMSCs and the results are conflicting. Valorani et al. showed that ADMSCs derived from lipoaspirate exhibit a higher growth rate in hypoxic conditions (Valorani et al. 2012). Conversely, Wang et al. reported that hypoxia inhibits proliferation of cells obtained from intact adipose tissue and cultured in alginate beads, thus mimicking a microenvironment favouring the chondrogenic differentiation (Wang et al. 2005). Here, we confirmed that hypoxia enhances ADMSCs proliferation and maintains the multipotency status, allowing the differentiation in specific lineages in the presence of proper factors.
In summary, ADMSCs culture in hypoxic condition seems to be more favourable from different points of view. On the one hand, the hypoxia reflects the microenvironment in which stem cells must proliferate and differentiate. In regenerative medicine applications, it is expected that ADMSCs populate the lesion site where they find low oxygen levels, mainly due to the lack of blood vessels. Therefore, in vitro methods that mimic the pathophysiology of damaged tissue allow to better understand the ADMSCs biology and their regenerative potential.
In addition, the low concentrations of oxygen promote cell proliferation and stemness, thus enriching the pool of cells potentially able to differentiate into multi-lineages, and extending the possibility of a long-term expansion. The above mentioned characteristics can be extremely beneficial in regenerative medicine applications, since they offer the possibility of modulating the proliferative and differentiative status by simply changing the culture conditions. Finally, our findings represent the rationale for future scientific and clinical investigations, aimed to demonstrate if these methods may really improve the expectations of the regenerative approach.
Acknowledgments
This study was supported by grants from the Italian Ministry of the Health RF-EMR-2008-1207087 (Exploring innovative strategies to enhance bone regeneration based on novel mesenchymal stromal/stem cells.)
References
- Arvidson K, Abdallah BM, Applegate LA, Baldini N, Cenni E, Gomez-Barrena E, Granchi D, Kassem M, Konttinen YT, Mustafa K, Pioletti DP, Sillat T, Finne-Wistrand A. Bone regeneration and stem cells. J Cell Mol Med. 2011;15:718–746. doi: 10.1111/j.1582-4934.2010.01224.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baer PC, Kuçi S, Krause M, Kuçi Z, Zielen S, Geiger H, Bader P, Schubert R. Comprehensive phenotypic characterization of human adipose-derived stromal/stem cells and their subsets by a high throughput technology. Stem Cells Dev. 2013;22:330–339. doi: 10.1089/scd.2012.0346. [DOI] [PubMed] [Google Scholar]
- Baksh D, Davies JE, Zandstra PW. Adult human bone marrow-derived mesenchymal progenitor cells are capable of adhesion-independent survival and expansion. Exp Hematol. 2003;31:723–732. doi: 10.1016/S0301-472X(03)00106-1. [DOI] [PubMed] [Google Scholar]
- Baptista LS, do Amaral RJ, Carias RB, Aniceto M, Claudio-da-Silva C, Borojevic R. An alternative method for the isolation of mesenchymal stromal cells derived from lipoaspirate samples. Cytotherapy. 2009;11:706–715. doi: 10.3109/14653240902981144. [DOI] [PubMed] [Google Scholar]
- Barrilleaux B, Phinney DG, Prockop DJ, O’Connor KC. Review: ex vivo engineering of living tissues with adult stem cells. Tissue Eng. 2006;12:3007–3019. doi: 10.1089/ten.2006.12.3007. [DOI] [PubMed] [Google Scholar]
- Berg JS, Goodell MA. An argument against a role for Oct4 in somatic stem cells. Cell Stem Cell. 2007;1:359–360. doi: 10.1016/j.stem.2007.09.007. [DOI] [PubMed] [Google Scholar]
- Bourin P, Bunnell BA, Casteilla L, Dominici M, Katz AJ, March KL, Redl H, Rubin JP, Yoshimura K, Gimble JM. Stromal cells from the adipose tissue-derived stromal vascular fraction and culture expanded adipose tissue-derived stromal/stem cells: a joint statement of the International Federation for Adipose Therapeutics and Science (IFATS) and the International Society for Cellular Therapy (ISCT) Cytotherapy. 2013;15:641–648. doi: 10.1016/j.jcyt.2013.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruder SP, Jaiswal N, Ricalton NS, Mosca JD, Kraus KH, Kadiyala S. Mesenchymal stem cells in osteobiology and applied bone regeneration. Clin Orthop Relat Res. 1998;355:S247–S256. doi: 10.1097/00003086-199810001-00025. [DOI] [PubMed] [Google Scholar]
- Bunnell BA, Flaat M, Gagliardi C, Patel B, Ripoll C. Adipose-derived stem cells: isolation, expansion and differentiation. Methods. 2008;45:115–120. doi: 10.1016/j.ymeth.2008.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciapetti G, Granchi D, Baldini N. The combined use of mesenchymal stromal cells and scaffolds for bone repair. Curr Pharm Des. 2012;18:1796–1820. doi: 10.2174/138161212799859648. [DOI] [PubMed] [Google Scholar]
- Covello KL, Kehler J, Yu H, Gordan JD, Arsham AM, Hu CJ, Labosky PA, Simon MC, Keith B. HIF-2alpha regulates Oct-4: effects of hypoxia on stem cell function, embryonic development, and tumor growth. Genes Dev. 2006;20:557–570. doi: 10.1101/gad.1399906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D’Ippolito G, Diabira S, Howard GA, Roos BA, Schiller PC. Low oxygen tension inhibits osteogenic differentiation and enhances stemness of human MIAMI cells. Bone. 2006;39:513–522. doi: 10.1016/j.bone.2006.02.061. [DOI] [PubMed] [Google Scholar]
- Eto H, Suga H, Matsumoto D, Inoue K, Aoi N, Kato H, Araki J, Yoshimura K. Characterization of structure and cellular components of aspirated and excised adipose tissue. Plast Reconstr Surg. 2009;124:1087–1097. doi: 10.1097/PRS.0b013e3181b5a3f1. [DOI] [PubMed] [Google Scholar]
- Fehrer C, Brunauer R, Laschober G, Unterluggauer H, Reitinger S, Kloss F, Gülly C, Gassner R, Lepperdinger G. Reduced oxygen tension attenuates differentiation capacity of human mesenchymal stem cells and prolongs their lifespan. Aging Cell. 2007;6:745–757. doi: 10.1111/j.1474-9726.2007.00336.x. [DOI] [PubMed] [Google Scholar]
- Forristal CE, Wright KL, Hanley NA, Oreffo RO, Houghton FD. Hypoxia inducible factors regulate pluripotency and proliferation in human embryonic stem cells cultured at reduced oxygen tensions. Reproduction. 2010;139:85–97. doi: 10.1530/REP-09-0300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fraccalvieri M, Datta G, Bogetti P, Verna G, Pedrale R, Bocchiotti MA, Boriani F, Obbialero FD, Kefalas N, Bruschi S. Abdominoplasty after weight loss in morbidly obese patients: a 4-year clinical experience. Obes Surg. 2007;17:1319–1324. doi: 10.1007/s11695-007-9235-7. [DOI] [PubMed] [Google Scholar]
- Gimble JM, Bunnell BA, Chiu ES, Guilak F. Concise review: adipose-derived stromal vascular fraction cells and stem cells: let’s not get lost in translation. Stem Cells. 2011;29:749–754. doi: 10.1002/stem.629. [DOI] [PubMed] [Google Scholar]
- Granchi D, Ochoa G, Leonardi E, Devescovi V, Baglìo SR, Osaba L, Baldini N, Ciapetti G. Gene expression patterns related to osteogenic differentiation of bone marrow-derived mesenchymal stem cells during ex vivo expansion. Tissue Eng Part C Methods. 2010;16:511–524. doi: 10.1089/ten.tec.2009.0405. [DOI] [PubMed] [Google Scholar]
- Grayson WL, Zhao F, Bunnell B, Ma T. Hypoxia enhances proliferation and tissue formation of human mesenchymal stem cells. Biochem Biophys Res Commun. 2007;358:948–953. doi: 10.1016/j.bbrc.2007.05.054. [DOI] [PubMed] [Google Scholar]
- Gumbiner BM. Cell adhesion: the molecular basis of tissue architecture and morphogenesis. Cell. 1996;84:345–357. doi: 10.1016/S0092-8674(00)81279-9. [DOI] [PubMed] [Google Scholar]
- http://clinicaltrials.gov (date last accessed 03 June 2013)
- Huang GTJ, Gronthos S, Shi S. Mesenchymal stem cells derived from dental tissues vs. those from other sources. Their biology and role in regenerative medicine. J Dent Res. 2009;88:792–806. doi: 10.1177/0022034509340867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isern J, Martín-Antonio B, Ghazanfari R, Martín AM, López JA, Del Toro R, Sánchez-Aguilera A, Arranz L, Martín-Pérez D, Suárez-Lledó M, Marín P, Van Pel M, Fibbe WE, Vázquez J, Scheding S, Urbano-Ispizúa A, Méndez-Ferrer S. Self-renewing human bone marrow mesenspheres promote hematopoietic stem cell expansion. Cell Rep. 2013;3:1714–1724. doi: 10.1016/j.celrep.2013.03.041. [DOI] [PubMed] [Google Scholar]
- Kern S, Eichler H, Stoeve J, Klüter H, Bieback K. Comparative analysis of mesenchymal stem cells from bone marrow, umbilical cord blood, or adipose tissue. Stem Cells. 2006;24:1294–1301. doi: 10.1634/stemcells.2005-0342. [DOI] [PubMed] [Google Scholar]
- Kim JH, Park SG, Song SY, Kim JK, Sung JH. Reactive oxygen species-responsive miR-210 regulates proliferation and migration of adipose-derived stem cells via PTPN2. Cell Death Dis. 2013 doi: 10.1038/cddis.2013.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lecourt S, Vanneaux V, Cras A, Freida D, Heraoui D, Herbi L, Caillaud C, Chomienne C, Marolleau JP, Belmatoug N, Larghero J. Bone marrow microenvironment in an in vitro model of Gaucher disease: consequences of glucocerebrosidase deficiency. Stem Cells Dev. 2012;21:239–248. doi: 10.1089/scd.2011.0365. [DOI] [PubMed] [Google Scholar]
- Leonardi E, Ciapetti G, Baglìo SR, Devescovi V, Baldini N, Granchi D. Osteogenic properties of late adherent subpopulations of human bone marrow stromal cells. Histochem Cell Biol. 2009;132:547–557. doi: 10.1007/s00418-009-0633-x. [DOI] [PubMed] [Google Scholar]
- Liedtke S, Stephan M, Kögler G. Oct4 expression revisited: potential pitfalls for data misinterpretation in stem cell research. Biol Chem. 2008;389:845–850. doi: 10.1515/BC.2008.098. [DOI] [PubMed] [Google Scholar]
- Mansilla E, Drago H, Sturla F, Bossi S, Salas E, Marín GH, Ibar R, Soratti C. Matrix superhighways configurations: new concepts for complex organ regeneration. Transpl Proc. 2007;39:2431–2433. doi: 10.1016/j.transproceed.2007.06.070. [DOI] [PubMed] [Google Scholar]
- Mohyeldin A, Garzón-Muvdi T, Quiñones-Hinojosa A. Oxygen in stem cell biology: a critical component of the stem cell niche. Cell Stem Cell. 2010;7:150–161. doi: 10.1016/j.stem.2010.07.007. [DOI] [PubMed] [Google Scholar]
- Otte A, Bucan V, Reimers K, Hass R. Mesenchymal stem cells maintain long-term in vitro stemness during explant culture. Tissue Eng Part C Methods. 2013;19:937–948. doi: 10.1089/ten.tec.2013.0007. [DOI] [PubMed] [Google Scholar]
- Pawitan JA. Future research in adipose stem cell engineering. In: Illouz YG, Sterodimas A, editors. Adipose Stem Cells and Regenerative Medicine. Berlin: Springer; 2011. pp. 257–272. [Google Scholar]
- Pierantozzi E, Gava B, Manini I, Roviello F, Marotta G, Chiavarelli M, Sorrentino V. Pluripotency regulators in human mesenchymal stem cells: expression of NANOG but not of OCT-4 and SOX-2. Stem Cells Dev. 2011;20:915–923. doi: 10.1089/scd.2010.0353. [DOI] [PubMed] [Google Scholar]
- Salem HK, Thiemermann C. Mesenchymal stromal cells: current understanding and clinical status. Stem Cells. 2010;28:585–596. doi: 10.1002/stem.269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schäffler A, Büchler C. Concise review: adipose tissue-derived stromal cells—basic and clinical implications for novel cell-based therapies. Stem Cells. 2007;25:818–827. doi: 10.1634/stemcells.2006-0589. [DOI] [PubMed] [Google Scholar]
- Schreml S, Babilas P, Fruth S, Orsó E, Schmitz G, Mueller MB, Nerlich M, Prantl L. Harvesting human adipose tissue-derived adult stem cells: resection versus liposuction. Cytotherapy. 2009;11:947–957. doi: 10.3109/14653240903204322. [DOI] [PubMed] [Google Scholar]
- Schroeder JE, Beyth S, Liebergall M. Orthopedic use of adipose-derived stem cells. In: Illouz YG, Sterodimas A, editors. Adipose Stem Cells and Regenerative Medicine. Berlin: Springer; 2011. pp. 181–191. [Google Scholar]
- Scotti C, Piccinini E, Takizawa H, Todorov A, Bourgine P, Papadimitropoulos A, Barbero A, Manz MG, Martin I. Engineering of a functional bone organ through endochondral ossification. Proc Natl Acad Sci USA. 2013;110:3997–4002. doi: 10.1073/pnas.1220108110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stocchero IN, Stocchero GF. Isolation of stem cells from human adipose tissue: technique, problems and pearls. In: Illouz YG, Sterodimas A, editors. Adipose Stem Cells and Regenerative Medicine. Berlin: Springer; 2011. pp. 13–18. [Google Scholar]
- Strioga M, Viswanathan S, Darinskas A, Slaby O, Michalek J. Same or not the same? Comparison of adipose tissue-derived versus bone marrow-derived mesenchymal stem and stromal cells. Stem Cells Dev. 2012;21:2724–2752. doi: 10.1089/scd.2011.0722. [DOI] [PubMed] [Google Scholar]
- Suga H, Matsumoto D, Eto H, Inoue K, Aoi N, Kato H, Araki J, Yoshimura K. Functional implications of CD34 expression in human adipose-derived stem/progenitor cells. Stem Cells Dev. 2009;18:1201–1210. doi: 10.1089/scd.2009.0003. [DOI] [PubMed] [Google Scholar]
- Valorani MG, Montelatici E, Germani A, Biddle A, D’Alessandro D, Strollo R, Patrizi MP, Lazzari L, Nye E, Otto WR, Pozzilli P, Alison MR. Pre-culturing human adipose tissue mesenchymal stem cells under hypoxia increases their adipogenic and osteogenic differentiation potentials. Cell Prolif. 2012;45:225–238. doi: 10.1111/j.1365-2184.2012.00817.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang DW, Fermor B, Gimble JM, Awad HA, Guilak F. Influence of oxygen on the proliferation and metabolism of adipose derived adult stem cells. J Cell Physiol. 2005;204:184–191. doi: 10.1002/jcp.20324. [DOI] [PubMed] [Google Scholar]
- Weijers EM, Van Den Broek LJ, Waaijman T, Van Hinsbergh VW, Gibbs S, Koolwijk P. The influence of hypoxia and fibrinogen variants on the expansion and differentiation of adipose tissue-derived mesenchymal stem cells. Tissue Eng Part A. 2011;17:2675–2685. doi: 10.1089/ten.tea.2010.0661. [DOI] [PubMed] [Google Scholar]
- Zachar V, Rasmussen JG, Fink T. Isolation and growth of adipose tissue-derived stem cells. Methods Mol Biol. 2011;698:37–49. doi: 10.1007/978-1-60761-999-4_4. [DOI] [PubMed] [Google Scholar]
- Zuk PA, Zhu M, Mizuno H, Huang J, Futrell JW, Katz AJ, Benhaim P, Lorenz HP, Hedrick MH. Multi-lineage cells from human adipose tissue: implications for cell-based therapies. Tissue Eng. 2001;7:211–228. doi: 10.1089/107632701300062859. [DOI] [PubMed] [Google Scholar]
- Zuk PA, Zhu M, Ashjian P, De Ugarte DA, Huang JI, Mizuno H, Alfonso ZC, Fraser JK, Benhaim P, Hedrick MH. Human adipose tissue is a source of multipotent stem cells. Mol Biol Cell. 2002;13:4279–4295. doi: 10.1091/mbc.E02-02-0105. [DOI] [PMC free article] [PubMed] [Google Scholar]