Abstract
This study evaluates the cytotoxic and genotoxic potential of alloxydim sodium using micronucleus (MN) assay, in human peripheral lymphocytes. MN assay was used to investigate the genotoxic effects of alloxydim sodium in human peripheral lymphocytes treated with 250, 500, 750, 1,000 µg/ml concentrations of alloxydim sodium for 24 and 48 h. Solvent, negative and positive controls were also used in the experiments in parallel. The obtained results were evaluated in statistical analyses by using Dunnett-t test (two sided) and p < 0.05 was accepted as significant. Alloxydim sodium significantly increased the MN formation compared with the negative control, at both 750 and 1,000 µg/ml concentrations and treatment periods. We also evaluated the nuclear division index (NDI) for cytotoxicity of this pesticide in the experiment, and finally observed a significant decrease of the NDI values at all concentrations of alloxydim sodium and at both treatment periods.
Keywords: Alloxydim sodium, Genotoxicity, Micronucleus assay, MN frequency, Nuclear division index
Introduction
Pesticides are chemicals designed to eliminate the pests, such as weeds, insects, rodents and fungi. They also improve the quantity and quality of yield in agriculture without causing much damage to non-target species (Zahm and Blair 1993). Although the benefits of conventional pesticides have been immense, humans and other living organisms are often exposed to them in the environment (WHO 1990). Widespread usage of synthetic pesticides for agricultural purpose has an adverse effect on living organisms and can lead to several hematological and neurological complications in individuals (Broughton et al. 1990; Vojdani et al. 1992; Wells and Nerland 1991).
Pesticides can be characterized on the basis of their function as herbicidal, insecticidal, fungicidal, etc. and also on the basis of chemical nature, i.e. organophosphates, organochlorides, s-triazines and pyrethroids (Jamil et al. 2004; Whitney et al. 1995; Aksoy 1989). Herbicides which are used intensively in agriculture, are designed for the control of weeds (Bolognesi 2003). They are the most widely applied agrochemicals and their application is the most accepted and effective method for plant protection from weeds (Nikoloff et al. 2012a).
Alloxydim sodium is one of the most intensively used cyclohexene oxime herbicides in agriculture for the control graminaceous weeds in a wide range of broad-leaf crops. It is absorbed from roots and leaves of grasses, acts on active meristematic tissues and gives rise to necrotic death of the plants (Veeresakeran and Catchpole 2006; Iwataki and Hirono 1979).
Alloxydim sodium shows its toxic effect especially to aquatic organisms. LC50 values for Cyprinus carpio, Oncorhynchus mykiss and Poecilia reticulata species were found to be 191, 174, 394 μg/l after 48 h application, respectively (Svobodova et al. 1986). Although it is known that this herbicide is easily degraded under different conditions, there is little information in the literature on the fate of this compound in the environment (Sandín-España et al. 2005).
Genotoxicity and cytotoxicity studies are frequently used to test numerous agrochemical compounds with different test systems (Ergene et al. 2007; Lin and Garry 2000; Rakitsky et al. 2000; Soloneski et al. 2007, 2008; Soloneski and Larramendy 2010; Zeljezic et al. 2006). These test systems are valuable and very well-known tools for the early and sensitive detection or estimation of genotoxic potential of chemicals. In addition, in vitro and in vivo methods focusing on test compounds provide more informative evidence about the genotoxic effects of specific pesticides (Santovito et al. 2012). The use of in vitro cell cultures for genotoxic and cytotoxic evaluation is rather economic and they are highly sensitive methods for the early detection of chemical exposure and toxicity (DiPaolo et al. 1981). Among them, one of the most used system for clastogenic and/or aneugenic screening is the micronucleus assay in human peripheral blood lymphocytes (Ali et al. 2011; González et al. 2011; Nikoloff et al. 2012b; Soloneski et al. 2008; Vera-Candioti et al. 2013).
The micronucleus (MN) assay is widely used and a good indicator of evaluation for pesticide genotoxicity estimation. Micronuclei are whole or partial chromosomes that have not been incorporated into the daughter nucleus following mitosis due to the chromosome breaking (clastogenic process) or mitotic spindle dysfunction (aneugenic process) (Fenech 2000; 2007). Micronuclei are indirect indicators of numerical and structural chromosomal aberrations (Albertini et al. 2000).
The aim of this study was to determine if alloxydim sodium induces genotoxic damage in cultured human lymphocytes by using the in vitro micronucleus assay.
Materials and methods
Chemicals
The test substance alloxydim sodium was obtained from Fluka (Buchs, Switzerland; CAS No. 55635-13-7, molecular weight: 345.37 g/mol, purity of 97.2 %) and dissolved in DMSO (CAS No. 67-68-5). Mitomycin-C, cytochalasin B (CAS No. 14930-96-2) chromosome medium B (Biochrom, Cambridge, UK; cat. No. F5023) and Giemsa were purchased from Sigma-Aldrich (St. Louis, MO, USA). The other chemicals were obtained from Merck (Darmstadt, Germany) and Riedel-de Haën (Buchs, Switzerland). The chemical structure of alloxydim sodium is shown in Fig. 1.
Fig. 1.
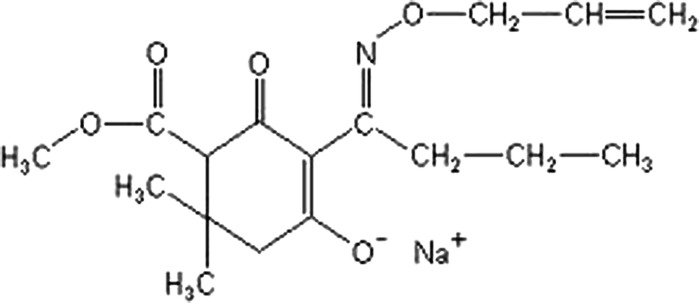
The chemical structure of alloxydim sodium
Lymphocyte cultures
Whole blood samples were obtained by venipuncture in heparinized tubes for genotoxicity testing. Peripheral venous blood was collected from four healthy donors (non-smokers, non-drinkers, not under drug therapy, and with no recent history of exposure to mutagens and aged 22–30 years) under sterile conditions. Informed consent was obtained from all donors and the study was carried out according to the local ethics committee.
Micronucleus assay
MN technique was carried out according to the method described by Fenech (2000) with some modification. The blood samples obtained from 4 healthy donors were added to 2.5 ml Chromosome Medium B (containing MEM Joklik with non-essential aminoacids, fetal bovine serum, heparin, penicillin G-sodium salt, streptomycin sulphate, phytohaemagglutinin L, ascorbic acid, glutathione-reduced) and incubated at 37 °C for 68 h. Cytochalasin B (final concentration 6 µg/ml) was added into the medium to arrest cytokinesis 44 h from the initiation. Mitomycin-C (MMC, 0.20 µg/ml) was used as positive control, and a negative control (untreated cultures) was also used in parallel. Different concentrations of alloxydim sodium (250, 500, 750, 1,000 µg/ml) were added 24 and 48 h after the incubation. These doses were determined based on the highest doses causing a reduction in the mitotic index of more than 50 % according to Sivikova and Dianovsky (2000). At the end of the incubation period, the cells treated with hypotonic solution (0.4 % KCl). Cells were re-centrifuged and fixed once with fixative (methanol:glacial acetic acid, 0.9 % NaCl 5:1:6) for 20 min. Fixation was repeated twice with methanol:glacial acetic acid (5:1). Microscope slides were prepared in duplicate by dropping cell samples, air-drying, and staining with 5 % Giemsa solution at pH 6.8 for 14 min. They were finally washed in distilled water, and dried at room temperature.
Slide evaluation
Micronuclei were scored from 2,000 binucleated cells per donor with well-preserved cytoplasm (totally 8,000 binucleated cells per concentrations). Criteria for scoring binucleated cells and MN were applied according to Fenech (2000). Cell proliferation was evaluated, using the nuclear division index (NDI), which indicates the average number of cell cycles. Totally 2,000 viable cells were scored to evaluate the percentage of cells with 1, 2, 3 and 4 nuclei. NDI was calculated using the formula: [(1 × M1) + (2 × M2) + (3 × M3) + (4 × M4)]/N; where M1–M4 represent the number of cells with one to four nuclei and N is the total number of intact cells scored (Fenech 2000). For the statistical analysis of the results, differences between treated samples and controls were tested with the Dunnett-t test (two sided).
Results
The results of the MN assay are summarized in Table 1. To evaluate the effects of alloxydim sodium on human peripheral lymphocytes, the MN frequency was investigated at different concentrations (250, 500, 750, 1,000 µg/ml) and different treatment periods (24 and 48 h) of the herbicide. In the MN assay, micronuclei were scored in 2,000 bi-nucleated lymphocytes with well-preserved cytoplasm per donor (total 8,000 bi-nucleated cells per concentration), for evaluation of MN according to Fenech (2000). A total of 2,000 lymphocytes per donor were scored to assess the percentage of cells with 1–4 nuclei. Figure 2a, b shows a binucleated cell with one and two micronuclei.
Table 1.
The frequency of micronucleus and nuclear division index in cultured human lymphocytes treated with alloxydim sodium
| Test substance | Treatment time (h) | Concentration (µg/ml) | Micronucleated binuclear cells (%) ± SD | Nuclear division index ± SD |
|---|---|---|---|---|
| Solvent control (−) Control (DMSO) MMC Alloxydim sodium |
24 | – | 0.18 ± 0.01 | 1.56 ± 0.04 |
| 0.16 ± 0.21 | 1.58 ± 0.08 | |||
| 0.20 | 11.28 ± 2.44* | 1.15 ± 0.04* | ||
| 250 | 0.25 ± 0.10 | 1.44 ± 0.04* | ||
| 500 | 0.28 ± 0.05 | 1.42 ± 0.05* | ||
| 750 | 0.45 ± 0.24* | 1.40 ± 0.04* | ||
| 1,000 | 0.68 ± 0.26* | 1.36 ± 0.04* | ||
| Solvent control (−) Control (DMSO) MMC Alloxydim sodium |
48 | – | 0.23 ± 0.02 | 1.49 ± 0.05 |
| 0.20 ± 0.02 | 1.56 ± 0.04 | |||
| 0.20 | 12.02 ± 4.55* | 1.18 ± 0.06* | ||
| 250 | 0.26 ± 0.05 | 1.42 ± 0.05* | ||
| 500 | 0.30 ± 0.01 | 1.40 ± 0.05* | ||
| 750 | 0.52 ± 0.01* | 1.38 ± 0.06* | ||
| 1,000 | 0.72 ± 0.14* | 1.34 ± 0.04* |
* The mean difference is significant at the 0.05 level Dunnett t test (2-sided)
SD standard deviation, MMC mitomycin-C
Fig. 2.
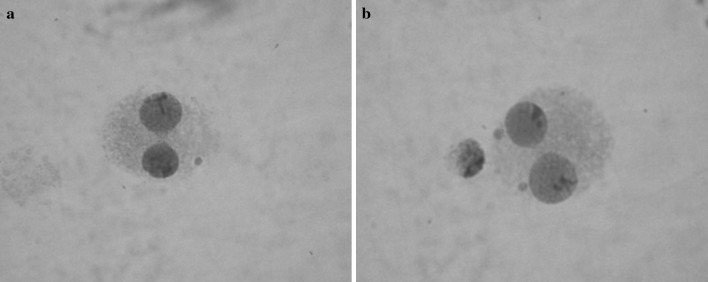
A binucleated cell with one and two micronuclei
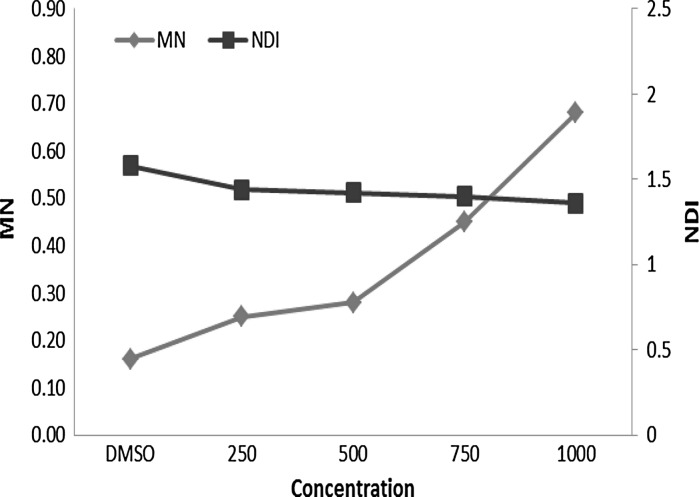
According to the results, the solvent control groups did not display any difference with the negative controls confirming that, at this low concentration (1 %), DMSO has no biological effects. Alloxydim sodium significantly (p < 0.05) increased the MN formation compared to the negative control, at concentrations of 750 and 1,000 µg/ml for both treatment periods. MMC was used as a positive control in this experiment and it showed a significantly increased MN formation compared with the negative and solvent controls for both treatment periods. We also evaluated the NDI for cytotoxicity of this pesticide in the experiment. Finally, we observed a significant decrease of the NDI values at all concentrations of alloxydim sodium and both treatment periods. MN formation and NDI values are shown graphically in Figs. 3, 4.
Fig. 3.
Micronucleus frequency (MN) and nuclear division index (NDI) of alloxydim sodium for 24 h treatment
Fig. 4.
Micronucleus frequency (MN) and nuclear division index (NDI) with alloxydim sodium for 48 h treatment
Discussion
A number of references reported on the possible genotoxic and cytotoxic effects of pesticides with different models. Therefore, we have much information about these methods but their clastogenic and aneugenic effects should be investigated.
Among the genotoxic methods, MN assay is often used to investigate the genotoxicity of chemicals due to its sensitivity and reliability as a marker of cytogenetic damage (Fenech 1993; Bolognesi 2003). MN assay in peripheral lymphocytes can be a useful biomarker for evaluating the early biologic effects of exposure to pesticides. It may arise from a whole lagging chromosome (aneugenic event leading to chromosome loss) or an acentric chromosome fragment detaching from a chromosome after breakage (clastogenic event) which do not integrate in the daughter nuclei (Fenech and Morley 1985; Tucker and Preston 1996).
In this study, the MN assay was chosen to investigate the potential risk for chromosome damage in cultured human lymphocytes exposed to different concentrations of alloxydim sodium (250, 500, 750, 1,000 µg/ml) for 24 and 48 h. Although alloxydim sodium is one of the most widely used herbicides in agriculture, for the control graminaceous weeds in a wide range of broad-leaf crops, the available information on the genotoxic potential of these herbicide is insufficient.
Alloxydim sodium is one of the postemergence systemic herbicides and belongs to the cyclohexanedione family of herbicides and an inhibitor of Acetyl CoA carboxylase (ACCase). This enzyme is the key enzyme in the fatty acid biosynthesis that catalyses the carboxylation of acetyl-CoA to malonyl-CoA which is necessary for the biosynthesis of fatty acids and secondary metabolites (Nikolskaya et al. 1999; Radwan 2012). Banas et al. (1993, 2000) have reported that ACCase inhibitor herbicides such as Haloxyfop and Alloxydim have a mode of action related to the overproduction of free radicals and oxidative stress. The application of herbicides can increase the superoxide levels and cause reactive oxygen species (ROS) accumulation which accompanied by lipid peroxidation (Feierabend and Winkelhüsener 1982). Accumulation of ROS can not only cause lipid peroxidation but also induce membrane damage and cause serious defects in the physiological metabolism of plants leading to cell death and stimulated plant senescence (Guo et al. 2006; Ogweno et al. 2009).
Our results showed that alloxydim sodium induced significant MN formation in cells treated with concentrations of 750 and 1,000 µg/ml for both treatment periods (24h, 48h). MN frequency also increased in the two other concentrations (250 and 500 µg/ml), however, the results were not found statistically significant. Besides, a significant reduction of the NDI value was observed in all cultures treated with alloxydim sodium for all exposure time. These results indicate that this herbicide can inhibit ACCase and increase MN frequency.
The cyclohexanedione family of herbicides has been studied by previously investigators with different test systems except the micronucleus assay and different results were obtained. Clethodim is also one of the postemergence systemic herbicides and belongs to the cyclohexanedione family of herbicides (Prostko et al. 2001; Clewis et al. 2002). Radwan (2012) applied this herbicide in maize and Clethodim application to maize leaves caused leaftip yellowing, browning or drying in some parts of the leaf. These injuries were probably due to enhanced production of ROS and/or accumulation of H2O2 and oxidative stress leading to death of some leaf parts. In a different study Sethoxydim which is an herbicide belonging to same group was investigated with the Salmonella assay and was not found mutagenic in this method (Claxton et al. 2004). In addition, Alloxydim was investigated with a standard microtest procedure based on the decrease of light emission by the marine bacterium Vibrio fischeri. The results indicated that the toxicity of the photoproducts was higher than the toxicity of the parent compound (Sandín-España et al. 2013).
Although there was no study with micronucleus assay in cyclohexanedione herbicides, MN test system was used by many investigators for other pesticide groups and they reported increases in MN formation (Zeljezic and Garaj-Vrhovac 2004; Yüzbasioglu et al. 2006; Ergene et al. 2007; Ali et al. 2008; Revankar and Shyama 2009). These results support our findings. However, some investigators reported that different pesticides did not lead to MN formation (Surrales et al. 1995; Gollapudi et al. 1995; Villarini et al. 1998). These results contradict our findings. On the other hand, Alloxydim sodium has cytotoxic effect due to decreasing the NDI and similar results have been reported by Ila et al. (2008) and İstifli and Topaktaş (2013).
The emergence of so many different results can be a result of contact time with the pesticides, the dose of the substance, or the way of how it is metabolized – directly or indirectly. Organophosphates, pyrethroids, organochlorines, and carbamates have been reported to be genotoxic, generating free radicals that react with cell membranes. The accumulation of these radicals can cause oxidative stress, depending on the antioxidant capacity of individuals exposed to these pesticides (Hérnandez et al. 2005; Salvador et al. 2008). Binucleated cells were also found to be more frequent in the group exposed to pesticides. They might be indicative of the failure of cytokinesis due to aneuploidy (Bonassi et al. 2011). The genotoxicity of the investigated compounds could depend on the chemical structure, biological activity, having rings in the structure and the positions of the binding location etc. (Kutlu et al. 2011). Moreover, it might be related to differences in lifestyle, climate and environmental conditions, and the pesticides are used at different quantities, at different durations of exposure and individual eating habits (Omenn 1991). Therefore, it can be explained, why some studies find an increase of genetic damage whilst in others the results are negative. Due to these reasons, genotoxic evaluation using different test systems are useful and necessary to determinate genotoxic effects of chemicals. By assessing genotoxic modifications in individuals, those who are at risk to develop diseases such as cancer may be identified and greater attention may be recommended.
In conclusion, alloxydim sodium was found to be cytotoxic and genotoxic due to decreasing of NDI and increasing of MN frequency. Micronucleus assay is highly sensitive in the detection of hazards arising from pesticides; further investigations are needed to determine the toxicity of these pesticides with in vivo and in vitro test systems.
References
- Aksoy M. Hematoxicity and carcinogenicity of benzene. Environ Health Perspect. 1989;82:193–197. doi: 10.1289/ehp.8982193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albertini RJ, Anderson D, Douglas GR, Hagmar L, Hemminki K, Merlo F, Natarajan AT, Norppa H, Shuker DEG, Tice R, Waters MD, Aitio A. IPCS guidelines for the monitoring of genotoxic effects of carcinogens in humans. Mutat Res. 2000;463:111–172. doi: 10.1016/S1383-5742(00)00049-1. [DOI] [PubMed] [Google Scholar]
- Ali D, Nagpure NS, Kumar S, Kumar R, Kushwaha B. Genotoxicity assessment of acute exposure of chlorpyrifos to freshwater fish Channa punctatus (Bloch) using micronucleus assay and alkaline single-cell gel electrophoresis. Chemosphere. 2008;71:1823–1831. doi: 10.1016/j.chemosphere.2008.02.007. [DOI] [PubMed] [Google Scholar]
- Ali R, Mittelstaedt RA, Shaddock JG, Ding W, Bhalli JA, Khan QM, Heflich RH. Comparative analysis of micronuclei and DNA damage induced by ochratoxin a in two mammalian cell lines. Mutat Res. 2011;723:58–64. doi: 10.1016/j.mrgentox.2011.04.002. [DOI] [PubMed] [Google Scholar]
- Banas A, Johansson I, Stenlid G, Stymne S. Free radical scavengers and inhibitors of lipoxygenases as antagonists the herbicides haloxyfop and alloxydim. Swed J Agric Res. 1993;23:67. [Google Scholar]
- Banas A, Johansson I, Stenlid G, Stymne S. Selective increase in acyl hydrolase activity by graminicides in wheat. Biochem Soc Trans. 2000;28:777. doi: 10.1042/bst0280777. [DOI] [PubMed] [Google Scholar]
- Bolognesi C. Genotoxicity of pesticides: a review of human biomonitoring studies. Mutat Res. 2003;543:251–272. doi: 10.1016/S1383-5742(03)00015-2. [DOI] [PubMed] [Google Scholar]
- Bonassi S, Coskun E, Ceppi M, Lando C, Bolognesi C, Burgaz S, Holland N, Kirsh-Volders M, Knasmueller S, Zeiger E, Carnesoltas D, Cavallo D, daSilva J, deAndrade VM, Demircigil GC, Odio Domínguez A, Donmez-Altuntas H, Gattas G, Giri A, Giri S, Gómez-Meda B, Gómez-Arroyo S, Hadjidekova V, Haveric A, Kamboj M, Kurteshi K, Martino-Roth MG, Montero Montoya R, Nersesyan A, Pastor-Benito S, Salvadori DMF, Shaposhnikova A, Stopper H, Thomas P, Torres-Bugarín O, Yadav AS, Zú˜niga González G, Fenech M. The human micronucleus project on exfoliated buccal cells [HUMN(XL)]: the role of life-style, host factors, occupational exposures, health status, and assay protocol. Mutat Res. 2011;728:88–97. doi: 10.1016/j.mrrev.2011.06.005. [DOI] [PubMed] [Google Scholar]
- Broughton A, Thrasher JD, Madison R. Chronic health effects and immunological alterations associated with exposure to pesticides. Comment Toxicol. 1990;4:59–71. [Google Scholar]
- Claxton LD, Matthews PP, Warren SH. The genotoxicity of ambient outdoor air, a review: Salmonella mutagenicity. Mutat Res. 2004;567:347–399. doi: 10.1016/j.mrrev.2004.08.002. [DOI] [PubMed] [Google Scholar]
- Clewis SB, Askew SD, Wilcut JW. Economic assessment of diclosulam and flumioxazion in strip- and conventional-tillage peanut. Weed Sci. 2002;50:378–385. doi: 10.1614/0043-1745(2002)050[0378:EAODAF]2.0.CO;2. [DOI] [Google Scholar]
- DiPaolo JA, DeMarinis AJ, Evans CH, Doniger J. Regulation of expression and promoted stages of irradiation carcinogenesis in Syrian hamster embryo cells. Cancer Lett. 1981;14:243–249. doi: 10.1016/0304-3835(81)90150-6. [DOI] [PubMed] [Google Scholar]
- Ergene S, Celik A, Cavaş T, Kaya F. Genotoxic biomonitoring study of population residing in pesticide contaminated regions in Goksu Delta: micronucleus, chromosomal aberrations and sister chromatid exchanges. Environ Int. 2007;33:877–885. doi: 10.1016/j.envint.2007.04.003. [DOI] [PubMed] [Google Scholar]
- Feierabend J, Winkelhüsener T. Nature of photooxidative events in leaves treated with chlorosis-inducing herbicides. Plant Physiol. 1982;70:1277–1282. doi: 10.1104/pp.70.5.1277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fenech M. The cytokinesis-block micronucleus technique and its application to genotoxicity studies in human populations. Environ Health Perspect. 1993;101:101–107. doi: 10.1289/ehp.93101s3101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fenech M. The in vitro micronucleus technique. Mutat Res. 2000;455:81–95. doi: 10.1016/S0027-5107(00)00065-8. [DOI] [PubMed] [Google Scholar]
- Fenech M. Cytokinesis-block micronucleus cytoma assay. Nat Protoc. 2007;2:1084–1104. doi: 10.1038/nprot.2007.77. [DOI] [PubMed] [Google Scholar]
- Fenech M, Morley A (1985) Measurement of micronuclei in lymphocytes’. Mutat Res 147:29–36 [DOI] [PubMed]
- Gollapudi BB, Mendrala AL, Linscombe VA. Evaluation of the genetic toxicity of the organophosphate insecticide Chlorpyrifos. Mutat Res Genet Toxicol. 1995;342:25–36. doi: 10.1016/0165-1218(95)90087-X. [DOI] [PubMed] [Google Scholar]
- González NV, Nikoloff N, Soloneski S, Larramendy ML. A combination of the cytokinesis-block micronucleus cytome assay and centromeric identification for evaluation of the genotoxicity of dicamba. Toxicol Lett. 2011;207:204–212. doi: 10.1016/j.toxlet.2011.09.013. [DOI] [PubMed] [Google Scholar]
- Guo YP, Zhou HF, Zhang LC. Photosynthetic characteristics and protective mechanisms against photooxidation during high temperature stress in two citrus species. Sci Hortic. 2006;108:260–267. doi: 10.1016/j.scienta.2006.01.029. [DOI] [Google Scholar]
- Hérnandez AF, López O, Rodrigo L, Gil F, Pena G, Serrano JL, Parrón T, Alvarez JC, Lorante JÁ, Pla A. Changes in erythrocyte enzymes in humans long-term exposed to pesticides influence of several markers of individual susceptibility. Toxicol Lett. 2005;159:13–21. doi: 10.1016/j.toxlet.2005.04.008. [DOI] [PubMed] [Google Scholar]
- Ila HB, Topaktaş M, Rencüzoğulları E, Kayraldız A, Dönbak L, Dağlıoğlu YK. Genotoxic potential of cyfluthrin. Mutat Res. 2008;656:49–54. doi: 10.1016/j.mrgentox.2008.07.005. [DOI] [PubMed] [Google Scholar]
- Istifli ES, Topaktaş M. Genotoxicity of pemetrexed in human peripheral blood lymphocytes. Cytotechnology. 2013;65:621–628. doi: 10.1007/s10616-012-9516-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwataki I, Hirono Y. The chemical structure and herbicidal activity of alloxydim-sodium and related compounds. In: Geissbuhler H, editor. Advances in pesticide sciences, Part 2. Oxford: Pergarnorn Press; 1979. pp. 235–243. [Google Scholar]
- Jamil K, Shaik AP, Mahboob M, Krishna D. Effect of organophosphorus and organochlorine pesticides (monochrotophos, chlorpyriphos, dimethoate, and endosulfan) on human lymphocytes in vitro. Drug Chem Toxicol. 2004;27:133–144. doi: 10.1081/DCT-120030725. [DOI] [PubMed] [Google Scholar]
- Kutlu M, Öztaş E, Kılıç GA, Işıkdağ İ, Özkay Y (2011) An Investigation of mutagenic activities of some 9-substituted phenanthrene derivatives with ames/salmonella/microsome test. AUJST–C Life Sci Biotechnol 1:83–94
- Lin N, Garry VF. In vitro studies of cellular and molecular developmental toxicity of adjuvants, herbicides, and fungicides commonly used in Red River Valley Minnesota. J Toxicol Environ Health. 2000;60:423–439. doi: 10.1080/00984100050033494. [DOI] [PubMed] [Google Scholar]
- Nikoloff N, Soloneski S, Larramendy ML (2012a) Genotoxic and cytotoxic evaluation of the herbicide flurochloridone on Chinese hamster ovary (CHO-K1) cells. Toxicol In Vitro 26:157–163 [DOI] [PubMed]
- Nikoloff N, Larramendy ML, Soloneski S (2012b) Comparative evaluation in vitro of the herbicide flurochloridone by cytokinesis-block micronucleus cytome and comet assays. Environ Toxicol 29:884–892 [DOI] [PubMed]
- Nikolskaya T, Zagnitko O, Tevzadze G, Haselkorn R, Gornicki P (1999) Herbicide sensitivity determinant of wheat plastid acetyl-CoA carboxylase is located in a 400-amino acid fragment of the carboxyltransferase domain. Proc Natl Acad Sci USA 96:14647–14651 [DOI] [PMC free article] [PubMed]
- Ogweno JO, Song XS, Hu WH, Shi K, Zhou YH, Yu JQ. Detached leaves of tomato differ in their photosynthetic physiological response to moderate high and low temperature stress. Sci Hortic. 2009;123:17–22. doi: 10.1016/j.scienta.2009.07.011. [DOI] [Google Scholar]
- Omenn GS. Future research directions in cancer ecogenetics. Mutat Res. 1991;247:283–291. doi: 10.1016/0027-5107(91)90023-H. [DOI] [PubMed] [Google Scholar]
- Prostko EP, Johnson WC, Mullinix BG. Annual grass control with preplant incorporated and preemergence applications of ethalfluralin and pendimethalin in peanut (Arachis hypogaea) Weed Technol. 2001;15:36–41. doi: 10.1614/0890-037X(2001)015[0036:AGCWPI]2.0.CO;2. [DOI] [Google Scholar]
- Radwan DEM. Salicylic acid induced alleviation of oxidative stress caused by clethodim in maize (Zea mays L.) leaves. Pestic Biochem Physiol. 2012;102:182–188. doi: 10.1016/j.pestbp.2012.01.002. [DOI] [Google Scholar]
- Rakitsky VN, Koblyakov VA, Turusov VS. Nongenotoxic (epigenetic) carcinogens: pesticides as an example: a critical review. Teratog Carcinog Mutagen. 2000;20:229–240. doi: 10.1002/1520-6866(2000)20:4<229::AID-TCM5>3.0.CO;2-M. [DOI] [PubMed] [Google Scholar]
- Revankar PR, Shyama SK. Genotoxic effects of monocrotophos, an organophosphorous pesticide, on an estuarine bivalve, Meretrix ovum. Food Chem Toxicol. 2009;47:1618–1623. doi: 10.1016/j.fct.2009.04.010. [DOI] [PubMed] [Google Scholar]
- Salvador M, Bordin DL, Andreazza AC, daSilva J, Henriques J, Erdtmann B. Determination of oxidative stress markers and serum cholinesterase among pesticide sprayers in southern Brazil. Toxicol Environ Chem. 2008;4:809–814. doi: 10.1080/02772240701742656. [DOI] [Google Scholar]
- Sandín-España P, Santín I, Magrans JO, Alonso-Prados JL, García-Baudín JM. Degradation of alloxydim in chlorinated water. Agron Sustain Dev. 2005;25:331–334. doi: 10.1051/agro:2005007. [DOI] [Google Scholar]
- Sandín-España P, Sevilla-Morán B, Calvo L, Mateo-Miranda M, Alonso-Prados JL. Photochemical behavior of alloxydim herbicide in environmental waters. Structural elucidation and toxicity of degradation products. Microchem J. 2013;106:212–219. doi: 10.1016/j.microc.2012.07.003. [DOI] [Google Scholar]
- Santovito A, Cervella P, Delpero M. Micronucleus frequency in human lymphocytes after exposure to diphenylamine in vitro. Mutat Res. 2012;747:135–137. doi: 10.1016/j.mrgentox.2012.05.003. [DOI] [PubMed] [Google Scholar]
- Sivikova K, Dianovsky J. Cytogenetic effect of technical glyphosate on cultivated bovine peripheral lymphocytes. Int J Hyg Environ Health. 2000;209:15–20. doi: 10.1016/j.ijheh.2005.07.005. [DOI] [PubMed] [Google Scholar]
- Soloneski S, Larramendy M. Sister chromatid exchanges and chromosomal aberrations in Chinese hamster ovary (CHO-K1) cells treated with insecticide pirimicarb. J Hazard Mater. 2010;174:410–415. doi: 10.1016/j.jhazmat.2009.09.068. [DOI] [PubMed] [Google Scholar]
- Soloneski S, Gonzalez NV, Reigosa MA, Larramendy ML. Herbicide 2,4dichlorophenoxyacetic acid (2,4-D) -induced cytogenetic damage in human lymphocytes in vitro in presence of erythrocytes. Cell Biol Int. 2007;31:1316–1322. doi: 10.1016/j.cellbi.2007.05.003. [DOI] [PubMed] [Google Scholar]
- Soloneski S, Reigosa MA, Molinari G, González NV, Larramendy ML. Genotoxic and cytotoxic effects of carbofuran and furadan on Chinese hamster ovary (CHOK1) cells. Mutat Res. 2008;656:68–73. doi: 10.1016/j.mrgentox.2008.07.007. [DOI] [PubMed] [Google Scholar]
- Surrales J, Xamena N, Creus A, Catalfin J, Norppa H, Marcos R. Induction of micronuclei by five pyrethroid insecticides in whole-blood and isolated human lymphocyte cultures. Mutat Res. 1995;341:169–184. doi: 10.1016/0165-1218(95)90007-1. [DOI] [PubMed] [Google Scholar]
- Svobodova Z, Machova J, Faina R, Kocova A, Kunce P. Acute toxicity of selected pesticides to aquatic organisms. Bull VURH Vodnany. 1986;1:8–20. [Google Scholar]
- Tucker JD, Preston JR. Chromosome aberrations, micronuclei, aneuploidy, sister chromatid exchanges, and cancer risk assessment. Mutat Res. 1996;365:147–159. doi: 10.1016/S0165-1110(96)90018-4. [DOI] [PubMed] [Google Scholar]
- Veeresakeran P, Catchpole AH. Studies on the selectivity of alloxydim-sodium in plants. Pest Manag Sci. 2006;13:452–462. doi: 10.1002/ps.2780130416. [DOI] [Google Scholar]
- Vera-Candioti J, Soloneski S, Larramendy ML. Evaluation of the genotoxic and cytotoxic effects of glyphosate-based herbicides in the ten spotted livebearer fish Cnesterodon decemmaculatus (Jenyns, 1842) Ecotoxicol Envriron Saf. 2013;89:166–173. doi: 10.1016/j.ecoenv.2012.11.028. [DOI] [PubMed] [Google Scholar]
- Villarini M, Moratti M, Paquini R, Scassellati-Sforzolini G, Fatigoni C, Marcarelli M, Monarca S, Rodriguez AV. In vitro genotoxic effects of the insecticide deltamethrin in human peripheral blood leukocytes: DNA damage (comet assay) in relation to the induction of sister-chromatid exchanges and micronuclei. Toxicol. 1998;130:129–139. doi: 10.1016/S0300-483X(98)00097-3. [DOI] [PubMed] [Google Scholar]
- Vojdani A, Ghoneum M, Brautbar N. Immune alteration associated with exposure to toxic chemicals. Toxicol Ind Health. 1992;8:239–254. [PubMed] [Google Scholar]
- Wells MS, Nerland DE. Hematotoxicity and concentration-dependent conjugation of phenol in mice following inhalation exposure to Benzene. Toxicol Lett. 1991;56:159–166. doi: 10.1016/0378-4274(91)90102-C. [DOI] [PubMed] [Google Scholar]
- Whitney KD, Seidler FJ, Slotkin TA. Developmental neurotoxicity of chlorpyrifos: cellular mechanisms. Toxicol Appl Pharmacol. 1995;134:53–62. doi: 10.1006/taap.1995.1168. [DOI] [PubMed] [Google Scholar]
- WHO . Public health impacts of pesticides used in agriculture (WHO in collaboration with the United Nations Environment Programme. Geneva): World Health Organization; 1990. [Google Scholar]
- Yüzbaşıoğlu D, Çelik M, Yılmaz S, Ünal F, Aksoy H (2006) Clastogenicity of the fungicide Afugan in cultured human lymphocytes. Mutat Res 53–59 [DOI] [PubMed]
- Zahm SH, Blair A. Cancer among migrant and seasonal farmworkers: an epidemiologic review and research agenda. Am J Indust Med. 1993;24:753–766. doi: 10.1002/ajim.4700240612. [DOI] [PubMed] [Google Scholar]
- Zeljezic D, Garaj-Vrhovac V. Chromosomal aberrations, micronuclei and nuclear buds induced in human lymphocytes by 2,4-dichlorophenoxyacetic acid pesticide formulation. Toxicol. 2004;200:39–47. doi: 10.1016/j.tox.2004.03.002. [DOI] [PubMed] [Google Scholar]
- Zeljezic D, Garaj-Vrhovac V, Perkovic P. Evaluation of DNA damage induced by atrazine and atrazine-based herbicide in human lymphocytes in vitro using a comet and DNA diffusion assay. Toxicol In Vitro. 2006;20:923–935. doi: 10.1016/j.tiv.2006.01.017. [DOI] [PubMed] [Google Scholar]