Abstract
Cyclophosphamide (CYC) and doxorubicin (DOX) are among the most effective and widely used anticancer chemotherapeutic drugs. Potential chemopreventive and chemotherapeutic functions have recently been attributed to flavonoids. We hypothesized that Quercetin (QR) would protect against the toxic effects of chemotherapeutic agents applied prior to pregnancy. Rats were treated with the chemotherapeutic drugs CYC (27 mg/kg) and DOX (1.8 mg/kg) applied in a single intraperitoneal dose once every 3 weeks for 10 weeks. QR was administered at a dose of 10 mg/kg/day by oral gavage. 48 h following the experimental chemotherapy exposure, female rats were transferred to cages containing male rat for mating. Fetal brain tissues were removed from fetuses extracted by cesarean section on the 20th day of gestation for evaluation of antioxidant parameters. A significant increase in superoxide dismutase and malondialdehyde activity was observed in CYC and DOX treatment groups relative to the control group (p < 0.05). Similarly, carnitine acylcarnitine translocase and Glutathione activity was significantly reduced in the CYC and DOX groups relative to the control group (p < 0.05). Our results indicate that the use of chemotherapeutic drugs before pregnancy can result in oxidative damage to fetal brain tissue. Therefore, women who have been exposed to chemotherapy and may become pregnant should be treated with antioxidant compounds such as QR to reduce the risk of damage to fetal brain tissues.
Keywords: Chemotherapy, Cyclophosphamide, Doxorubicin, Fetal development, Quercetin
Introduction
Chemotherapy is the widely accepted standard of care for most forms of cancer. Cancers of the breast, cervix, and ovary are commonly treated with a variety of chemotherapeutic agents (Meistrich 2009).
Cyclophosphamide (CYC) and doxorubicin (DOX) are among the most effective and widely used anticancer chemotherapy drugs (Agarwal et al. 2003). The anthracycline antibiotic DOX represents the single most effective chemotherapeutic agent for the treatment of breast cancer, bone and soft tissue sarcomas, and malignant lymphomas (Bayne and Sohal 2002; Taskin and Dursun 2014a). DOX-induced toxicity stimulates the translocation of calreticulin to the cell surface in tumor cells, enhancing uptake and clearance by dendritic cells (Bines et al. 1996). Both in vitro and in vivo data support the hypothesis that a combination of the antioxidant Quercetin (QR) and DOX produces beneficial, synergistic effects in the treatment of breast cancer and leukemia (Black et al. 2002; Bolzan et al. 1997; Ceballos et al. 1992) However, exposure of pregnant women to chemotherapy results in congenital malformations in as many as 10–20 % of cases (Cevik et al. 2013).
CYC is a potent alkylating agent (Howell and Shalet 2005a). Alkylating agents are particularly gonadotoxic, producing adducts and cross-links in DNA that may result in prolonged azoospermia (Vaisheva et al. 2007). At moderate doses, recovery of normospermic conditions may occur within 1–3 years; however, increased exposure can result in extended or permanent azoospermia (Claeson et al. 2000; Cheng et al. 2002; Doganay et al. 2006; Ciftci et al. 2013; Delbes et al. 2010). Premature menopause and infertility may result from acute exposure to chemotherapy regimens including anthracycline and CYC (Du et al. 2010a; Estany et al. 2007). DOX exposure is known to increase oocyte apoptosis in cell culture based on experiments (Estany et al. 2007).
Potential chemopreventive and chemotherapeutic functions have recently been attributed to flavonoids (Bolzan et al. 1997; Fadillioğlu et al. 2002). QR (QT, 3,3′,4′,5,7-pentahydroxyflavone) is one type of naturally-occurring flavonoid. Several recent studies have demonstrated the antimicrobial, antiviral, antioxidative, and anti-inflammatory properties of QR, suggesting broad benefits to human health (Gilgun et al. 2001). The flavonoid QR is known to enhance cellular antioxidant potential through the Nrf2 pathway (Haliwel and Gutterridge 1992; Gutteridge 1995). A variety of evidence from animal tirals suggests that the antioxidant properties of QR reduce oxidative damage to the brain, heart, and other tissues during ischemic reperfusion injury and exposure to compounds that induce oxidative stress (Gutteridge 1995; Bayne and Sohal 2002).
In the present study, we evaluated the hypothesis that QR would protect against the toxic effects of chemotherapeutic agents applied prior to pregnancy.
Materials and methods
All experimental procedures were reviewed and approved (2009/32) by the local ethics committee of the Medical Sciences Experimental Search and Application Center (MSESAC) at the Inonu University.
Experimental treatments
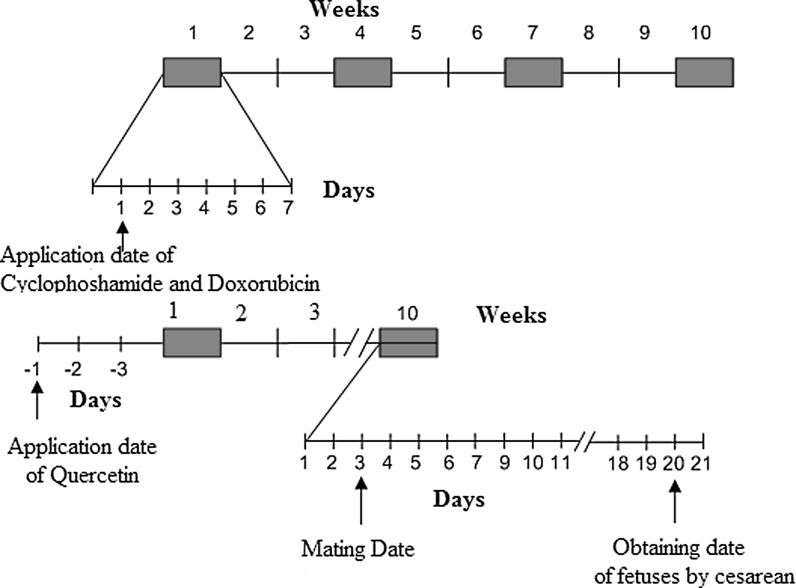
The chemotherapeutic agents CYC (Molekula, Shaftesbury, Dorset, UK) and DOX (Sigma-Aldrich, Oakville, ON, Canada) were used for experimental induction of chemotherapeutic oxidative injury. The doses calculated for treatment of experimental animals were equivalent to typical clinical dosages. CYC was administered four times at three week intervals at the total dose 27 mg/kg. DOX was administered four times at three week intervals at the total dose 1.8 mg/kg. (Fig. 1) (Agarwal et al. 2003). The doses of the drugs administered to rats were analogous to human doses after adjustment for the differences in surface area to weight ratio.
Fig. 1.
Cyclophosphamide, Doxorubicin, and Quersetin treatment regimen
QR (Quercetin dihydrate, 97%, Alfa Aesar GmbH, Karlsruhe, Germany, CAS: 6151-25-3) was suspended in corn oil and administered at a dose of 10 mg/kg/day by oral gavage throughout the course of the study (Howell and Shalet 2005b). Control groups have received only corn oil by gavage.
Animals
A total of 53 female Wistar rats weighing approximately 250 g each were used in this study. At 2 days post-chemotherapy exposure, female rats were introduced to cages containing a male rat for the purpose of breeding. Four females were housed with a single male in a wire mesh cage and pregnancy was determined by the presence or absence of a vaginal plug. The day of plug release was considered gestation day 1. Male rats were removed from the cage following confirmation of pregnancy. Food and water were given without restriction. The animals were subjected to a schedule of 12 h of light and 12 h of darkness (lights on at 06:00), and an ambient temperature of 22 ± 2 °C was maintained at all times.
Experimental groups
The experimental rats were divided into six groups: control (CONT), QR, CYC, DOX, CYC + QR, and DOX + QR. On the 20th day of gestation, fetuses were removed by cesarian section under combination of ketamine (Ketalar® 50mg/ml Pfizer, Berlin, Germany) and xylazine (Rompun® 2%, 20 mg•ml−1, Bayer, Berlin, Germany) anaesthesia (Ketamine/Xylazine, 90/10 mg/kg body weight, i.p. injection). Subsequently, fetal brain tissues were extracted for the measurement of antioxidant parameters including malondialdehyde antioxidant (MDA), glutathione (GSH), superoxide dismutase (SOD), and carnitine acylcarnitine translocase (CAT).
Biochemical analysis
Fetal brain tissue samples were homogenized in ice-cold 0.1 M Tris–HCl buffer (pH 7.5) containing protease inhibitor, phenylmethylsulfonyl fluoride, 1 mM using a tissue homogenizer (IKA, Staufen, Germany, Ultra-Turrax T 25 basic) at 16,000 rpm for 2 min at +4 °C. These homogenates were subsequently used for measurement of biochemical analysis.
MDA and GSH
Thiobarbituric acid reactive substances including MDA were measured by the addition of thiobarbituric acid to tissue homogenates and the measurement of light absorbance at 535 and 520 nm in a spectrophotometer as previously described (Mihara and Uchiyama 1978). The results were reported as nmol/g wet tissue. Fetal brain homogenate GSH concentrations were measured using the reduced glutathione assay according to the spectrophotometric Ellman’s method (Ellman 1959). Again, results were reported as nmol/g wet tissue.
SOD assay
SOD activity was measured as the total reduction of nitroblue tetrazolium by the superoxide anion produced by xanthine and xanthine oxidase (Jolitha et al. 2006). One unit of SOD activity was defined as the quantity of protein inhibiting the rate of NBT reduction by 50 %, with the results reported as units per milligram protein. The total protein content of the brain tissue homogenate samples was determined by the method of Lowry et al. (Lenton et al.1999).
Determination of CAT activity
CAT activity was measured using Aebi’s method (Aebi 1974), the determination of the rate constant k (dimension: s-1, k) of H2O2 (initial concentration 10 mM) as indicated by absorbance at 240 nm in a spectrophotometer (Casado et al. 2001). Activity was reported as k (constant rate).
Statistical analysis
All continuously variable data were expressed as mean ± SEM. The results were analyzed using SPSS 15.0 statistical software. One-way analysis of variance (ANOVA) and the post hoc Tukey’s HSD test were used to evaluate the differences in biochemical parameters between multiple groups. For statistical significance a p value of less than 0.05 was accepted.
Results
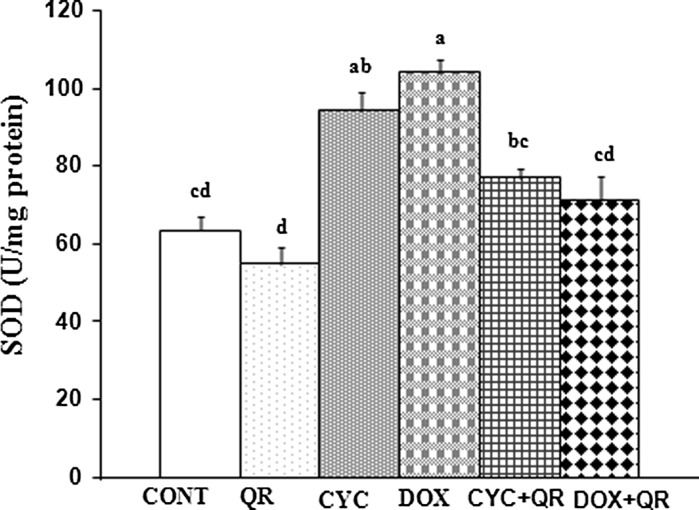
The results of the biochemical analysis of antioxidant parameters in fetal brain tissue were provided. A significant increase in SOD activity was observed in the CYC and DOX groups relative to the control group, while SOD activity was significantly decreased in the DOX + QR treatment group compared to the group exposed to DOX alone (p < 0.05) (Fig. 2).
Fig. 2.
Bar graph indicating SOD parameters in fetal brain tissue. abcd superscript symbols within the same column indicate statistically significant differences (p < 0.05). N = 5 rats per group
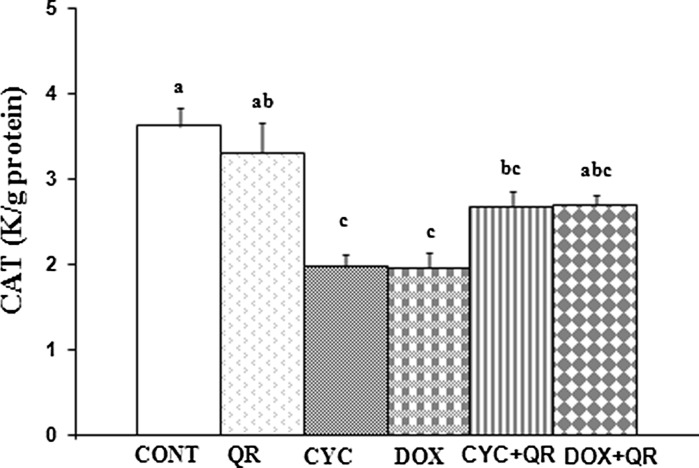
A significant decrease in CAT activity was observed in the CYC and DOX groups relative to the control group (p < 0.05). CAT activity was increased in the CYC + QR group relative to the group treated with CYC alone, however this difference did not reach the level of statistical significance (p > 0.05). Similarly, differences in CAT activity in the DOX + QR group relative to the DOX treatment group were not statistically significant neither (p < 0.05) (Fig. 3).
Fig. 3.
Bar graph indicating CAT parameters in fetal brain tissue. abc superscript symbols within the same column indicate statistically significant differences (p < 0.05). N = 5 rats per group
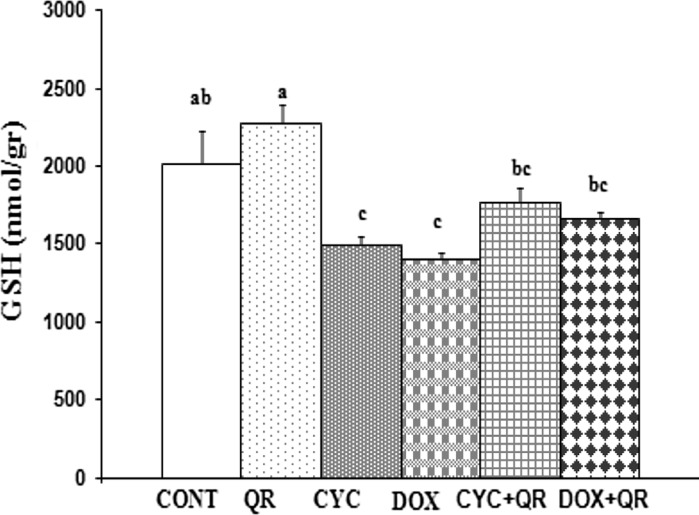
A significant decrease in GSH levels was observed in the CYC and DOX treatment groups relative to the control group (p < 0.05), however an increase in GSH in the CYC + QR and DOX + QR treatment groups did not reach the level of statistical significance (p > 0.05) (Fig. 4).
Fig. 4.
Bar graph indicating GSH parameters in fetal brain tissue. abc superscript symbols within the same column indicate statistically significant differences (p < 0.05). N = 5 rats per group
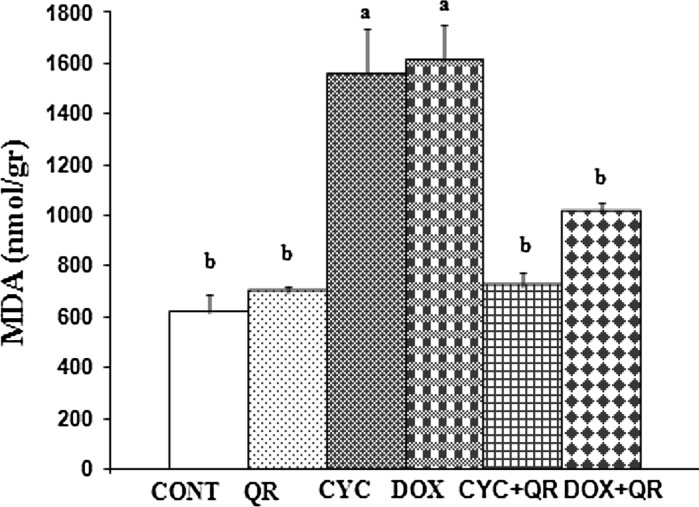
Similar to the other antioxidant parameters measured, a statistically significant increase in MDA levels was observed in the CYC and DOX groups relative to the control group (p < 0.05), although in this case MDA was also significantly decreased in the CYC + QR treatment group relative to the group treated with CYC alone (p < 0.05). However, there was no statistically significant difference in MDA levels in the DOX + QR treatment group relative to the group treated with DOX alone (p < 0.05) (Fig. 5).
Fig. 5.
Bar graph indicating MDA parameters in fetal brain tissue. ab superscript symbols within the same column indicate statistically significant differences (p < 0.05). N = 5 rats per group
Discussion
Oxidative stress occurs as a result of insufficient antioxidant protection, elevated free radical production, or a combination of the two processes (Lowry et al. 1951; Leyder et al. 2011). The central nervous system, possessing relatively weak endogenous and exogenous antioxidant capabilities, is particularly sensitive to oxidative stress induced by the presence of free radicals (Matouk et al. 2013). In the present study, we examined markers of oxidative stress and evaluated antioxidant capacity in fetal brain tissues following exposure of the mother to chemotherapeutic agents known to induce oxidative stress.
The catalysis of superoxide to hydrogen peroxide by SOD (Meistrich 2009) results in the inhibition of lipid peroxidation, a protective function in the presence of oxygen free radicals (Mihara and Uchiyama 1978). SOD activity is elevated in tissues with high oxygen demands. CAT is an antioxidant enzyme that is itself highly sensitive to oxidative damage, resulting in loss of antioxidant activity. CAT enzymatic activity has been demonstrated to decrease following oxidative damage (Mihara and Uchiyama 1978; Niestroy et al. 2011). In the present study, fetal brain tissues were obtained from mothers exposed to the chemotherapeutic drugs CYC and DOX just prior to pregnancy. Our results demonstrate that while fetal brain SOD activity was significantly increased by CYC or DOX exposure, CAT activity was simultaneously decreased in the same tissues. This is consistent with previous reports regarding the mechanisms contributing to oxidative damage of fetal brain tissues (Mihara and Uchiyama 1978; Niestroy et al. 2011). Recent studies have implicated oxidative stress in the pathogenesis of a variety of widely prevalent human diseases (Niestroy et al. 2011). Neurodegenerative disease (Obeid et al. 2013), cardiovascular disease (Pryzant et al. 1993), cancer (Ray et al. 2000), and infertility (Senturker et al. 2002) have all been associated with increased oxidative stress. The present study indicates that chemotherapy agents significantly increase SOD activity and that QR co-treatment with the drugs significantly ameliorates the effects of chemotherapy-induced oxidative damage. Previous studies support the therapeutic effect of QR in pathologies involving oxidative stress (Gilgun et al. 2001; Bayne and Sohal 2002).
Increased availability of GSH, the reduced form of glutathione, enhances the detoxification of free radicals. The neutralization of free radicals within the cytoplasm results in GSH depletion (Sestili et al. 1998). A significant decrease in the non-enzymatic anti-oxidant GSH was observed in fetal brain tissues exposed to chemotherapeutic agents CYC and DOX relative to the control group. The depletion of GSH in the chemotherapy-exposed tissue is a clear indicator of significant oxidative damage in the fetal brain tissue. A recent study reported that elevated oxidative stress give rise to attenuate mitochondrial functions resulting from decreased mitochondrial membrane potential and ATP production (Taskin et al. 2014b; Taskin and Dursun 2014a). Attenuation of mitochondrial function probably worsened the fetal brain damage.
Numerous reports suggest that MDA expression is enhanced by lipid peroxidation resulting from oxidative stress (Sun et al. 1988; Singh et al.2003; Shen et al. 2008; Staedler et al. 2011), while SOD activity is known to suppress lipid peroxidation (Vaisheva et al. 2007). Despite elevated SOD activity in the CYC- and DOX-exposed fetal brain tissues, increased MDA expression is a clear indicator of ongoing lipid peroxidation.
The antioxidant capacity of QR is quite strong relative to other known flavonoids (Vanhees et al. 2013). Previous studies support the notion that QR acts as a free radical scavenger and is capable of suppressing lipid peroxidation (Bines et al. 1996; Walle et al. 2007; Delbes et al. 2010; Vanhees et al. 2013). The present data demonstrate that CYC and DOX exposure increases MDA levels and SOD activity in fetal brain tissue while it decreased CAT enzymatic activity indicating oxidative damage, consistent with previous studies (Wessels et al. 2011). However, the presence of QR resulted in enhancement of the antioxidant capacity of the fetal tissues and reduction of free radical induced oxidative damage, a result supported by previous work (Tanir et al. 2005).
In conclusion, our study data suggest that the therapeutic use of CYC or DOX in pregnancy should be strongly discouraged due to the risk oxidative damage to fetal brain tissue. Metabolites resulting from CYC and DOX exposure, described in this paper, accumulate in fetal brain tissues as a result of oxidative damage. In such cases where CYC and DOX must be used, QR may be a suitable anti-oxidant capable of reducing the risk of damage to fetal neurological development. Our data demonstrate that exposure to chemotherapeutic drugs prior to pregnancy can result in significant oxidative damage to fetal brain tissues. Therefore, women who have been exposed to chemotherapy and may become pregnant should be treated with antioxidant compounds such as QR to reduce the risk of damage to fetal brain tissues.
Acknowledgments
This research was supported by Inonu University Research Fund (INU-BAP 2010/58).
References
- Aebi H, Catalase BH (1974) Methods of enzymatic analysis. Academic Press, New York and London, pp 673–677
- Agarwal A, Saleh RA, Bedaiwy MA. Role of reactive oxygen species in the pathophysiology of human reproduction. Fertil Steril. 2003;79:829–843. doi: 10.1016/S0015-0282(02)04948-8. [DOI] [PubMed] [Google Scholar]
- Bayne AV, Sohal RS. Effects of superoxide dismutase/catalase mimetics on life span and oxidative stress resistance in the housefly, musca domestica. Free Radic Biol Med. 2002;32:1229–1234. doi: 10.1016/S0891-5849(02)00849-3. [DOI] [PubMed] [Google Scholar]
- Bines J, Oleske DM, Cobleigh MA. Ovarian function in premenopausal women treated with adjuvant chemotherapy for breast cancer. J Clin Oncol. 1996;14:1718–1729. doi: 10.1200/JCO.1996.14.5.1718. [DOI] [PubMed] [Google Scholar]
- Black PH, Garbutt LD. Stress, inflammation and cardiovascular disease. J Psychosom Res. 2002;52:1–23. doi: 10.1016/S0022-3999(01)00302-6. [DOI] [PubMed] [Google Scholar]
- Bolzan AD, Bianchi MS, Bianchi NO. Superoxide dismutase, catalase and glutathione peroxidase activities in human blood: influence of sex age and cigarette smoking. Clin Biochem. 1997;30:449–454. doi: 10.1016/S0009-9120(97)00047-7. [DOI] [PubMed] [Google Scholar]
- Casado A, de La Torre R, Lopéz-Fernández E (2001) Antioxidant enzyme levels in red blood cells from cataract patients. Gerontol 47:186–188 [DOI] [PubMed]
- Ceballos PI, Trivier JM, Nicole A. Age- correlated modification of copper: zinc superoxide dismutase and glutationic related enzyme activities in human erythrocytes. Clin Chem. 1992;38:66. [PubMed] [Google Scholar]
- Cevik O, Cadırcı S, Sener TE, Tinay I, Akbal C, Tavuk HH, Cetinel S, Kıran D, Sener G. Quercetin treatment against ischemia/reperfusion injury in rat corpus cavernosum tissue: a role on apoptosis and oxidative stress. Free Radic Res. 2013;47:683–691. doi: 10.3109/10715762.2013.814912. [DOI] [PubMed] [Google Scholar]
- Cheng FC, Jen JF, Tsai TH. Hydroxyl radical in living systems and its separation methods. J Chromatogr B Analyt Technol Biomed Life. 2002;781:481–496. doi: 10.1016/S1570-0232(02)00620-7. [DOI] [PubMed] [Google Scholar]
- Ciftci O, Vardi N, Ozdemir I. Effects of quercetin and chrysin on 2,3,7,8-tetrachlorodibenzo-p-dioxin induced hepatotoxicity in rats. Environ Toxicol. 2013;28:146–154. doi: 10.1002/tox.20707. [DOI] [PubMed] [Google Scholar]
- Claeson K, Aberg F, Karlberg B. Free malondialdehyde determination in rat brain tissue by capillary zone electrophoresis: evaluation of two protein removal procedures. J Chromatogr B Biomed Sci Appl. 2000;31:87–92. doi: 10.1016/S0378-4347(00)00030-X. [DOI] [PubMed] [Google Scholar]
- Delbes G, Vaisheva F, Luu T, Marcon L, Hales BF, Robaire B. Reversibility of the effects of the chemotherapeutic regimen for non-Hodgkin lymphoma, cyclophosphamide, doxorubicin, vincristine, and prednisone, on the male rat reproductive system and progeny outcome. Reprod Toxicol. 2010;29:332–338. doi: 10.1016/j.reprotox.2010.01.007. [DOI] [PubMed] [Google Scholar]
- Doganay S, Borozan M, Iraz M, Ciqremis Y. The effect of resveratrol in experimental cataract model formed by sodium selenite. Curr Eye Res. 2006;31:147–153. doi: 10.1080/02713680500514685. [DOI] [PubMed] [Google Scholar]
- Du G, Lin H, Yang Y, Zhang S, Wu X, Wang M, Ji L, Lu L, Yu L, Han G. Dietary quercetin combining intratumoral doxorubicin injection synergistically induces rejection of established breast cancer in mice. Int Immunopharmacol. 2010;10:819–826. doi: 10.1016/j.intimp.2010.04.018. [DOI] [PubMed] [Google Scholar]
- Ellman GL (1959) Tissue sulfhydryl groups. Arch Biochem Biophys 82:70–77 [DOI] [PubMed]
- Estany S, Palacio JR, Barnadas R, Sabes M, Iborra A, Mart´ınez P. Antioxidant activity of N-acetylcysteine, flavonoids and α-tocopherol on endometrial cells in culture. J Reprod Immunol. 2007;75:1–10. doi: 10.1016/j.jri.2007.01.007. [DOI] [PubMed] [Google Scholar]
- Fadillioğlu E, Erdoğan H, Polat A, Emre H (2002) Renal antioxidant status in rats with hypertension ınduced by N sup omega nitro-l-arginine methyl ester. Kidney Blood Pres Res 25:211–216. doi:10.1159/000066341 [DOI] [PubMed]
- Gilgun Y, Melaned E, Offen D. Oxidative stress induced neurodegenerative diseases: the need for antioxidants that penetrate the blood brain barrier. Neuropharmacology. 2001;40:959–975. doi: 10.1016/S0028-3908(01)00019-3. [DOI] [PubMed] [Google Scholar]
- Gutteridge JM. Lipid peroxidation and antioxidants as biomarkers of tissue damage. Clin Chem. 1995;41:1819–1828. [PubMed] [Google Scholar]
- Haliwel B, Gutterridge JM, Cross CE. Free radicals antioxidants and human disease: where are we now? J Lab Clin Med. 1992;119:598–620. [PubMed] [Google Scholar]
- Howell SJ, Shalet SM. Effect of cancer therapy on pituitary–testicular axis. Int J Androl. 2002;25:269–276. doi: 10.1046/j.1365-2605.2002.00365.x. [DOI] [PubMed] [Google Scholar]
- Howell SJ, Shalet SM. Spermatogenesis after cancer treatment: damage and recovery. J Natl Cancer Inst Monogr. 2005;34:12–17. doi: 10.1093/jncimonographs/lgi003. [DOI] [PubMed] [Google Scholar]
- Ilhan N, Halifeoglu I, Ozercan HI, Ilhan N. Tissue malondialdehyde andadenosine triphosphatase level after experimental liver ischaemia-reperfusion damage. Cell Biochem Funct. 2001;19:207–212. doi: 10.1002/cbf.912. [DOI] [PubMed] [Google Scholar]
- Jolitha AB, Subramanyam MV, Devi SA (2006) Modification by vitamin E and exercise of oxidative stress in regions of aging rat brain: studies on superoxide dismutase isoenzymes and protein oxidation status. Exp Gerontol 41:753–763 [DOI] [PubMed]
- Lenton KJ, Therriault H, Fulop T, Payette H, Wagner JR. Glutathione and ascorbate are negatively correlated with oxidative DNA damage in human lymphocytes. Carcinogenesis. 1999;20:607–613. doi: 10.1093/carcin/20.4.607. [DOI] [PubMed] [Google Scholar]
- Leyder M, Laubach M, Breugelmans M, Keymolen K, Greve JD, Foulon W. Specific congenital malformations after exposure to cyclophosphamide, epirubicin and 5-fluorouracil during the first trimester of pregnancy. Gynecol Obstet Invest. 2011;71:141–144. doi: 10.1159/000317264. [DOI] [PubMed] [Google Scholar]
- Lowry O, Rosenbraugh N, Farr L, Rondell R. Protein measurement with the folin-phenol reagent. J Biol Chem. 1951;183:265–275. [PubMed] [Google Scholar]
- Matouk AI, Taye A, Heeba GH, El-Moselhy MA. Quercetin augments the protective effect of losartan against chronic doxorubicin cardiotoxicity in rats. Environ Toxicol Pharmacol. 2013;36:443–450. doi: 10.1016/j.etap.2013.05.006. [DOI] [PubMed] [Google Scholar]
- Meistrich ML. Male gonadal toxicity. Pediatr Blood Cancer. 2009;53:261–266. doi: 10.1002/pbc.22004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mihara M, Uchiyama M. Determination of malonaldehyde precursor in tissues by thiobarbituric acid test. Anal Biochem. 1978;86:271–278. doi: 10.1016/0003-2697(78)90342-1. [DOI] [PubMed] [Google Scholar]
- Niestroy J, Barbara A, Herbst K, van Rode S, Liempt M, Roos PH. Single and concerted effects of benzo [a] pyrene and flavonoids on the AhR and Nrf2-pathway in the human colon carcinoma cell line Caco-2. Toxicol InVitro. 2011;25:671–683. doi: 10.1016/j.tiv.2011.01.008. [DOI] [PubMed] [Google Scholar]
- Obeid M, Tesniere A, Ghiringhelli F, Fimia GM, Apetoh L, Perfettini JL. Calreticulin exposure dictates the immunogenicity of cancer cell death. Nat Med. 2013;13:54–61. doi: 10.1038/nm1523. [DOI] [PubMed] [Google Scholar]
- Pryzant RM, Meistrich ML, Wilson E, Brown B, Mclaghlin P. Long-term reduction in sperm count after chemotherapy with and without radiation therapy for non-Hodgkin’s lymphomas. J Clin Oncol. 1993;11:239–247. doi: 10.1200/JCO.1993.11.2.239. [DOI] [PubMed] [Google Scholar]
- Ray G, Batra S, Shukla NK, Deo S, Raina V, Ashok S, Husain SA. Lipid peroxidation, free radical production and antioxidant status in breast cancer. Breast Cancer Res Treat. 2000;59:163–170. doi: 10.1023/A:1006357330486. [DOI] [PubMed] [Google Scholar]
- Senturker S, Tschirret-GuthR MJ, Levine R, Shacter E. Induction of apoptosis by chemotherapeutic drugs without generation of reactive oxygen species. Arch Biochem Biophys. 2002;397:262–272. doi: 10.1006/abbi.2001.2681. [DOI] [PubMed] [Google Scholar]
- Sestili P, Guidarelli A, Dacha M, Cantoni O. Quercetin prevents DNA single strand breakage and cytotoxicity caused by tert-butylhydroperoxide: free radical scavenging versus iron chelating mechanism. Free Radic Biol Med. 1998;25:196–200. doi: 10.1016/S0891-5849(98)00040-9. [DOI] [PubMed] [Google Scholar]
- Shen J, Zhang W, Wu J, Zhu Y. The synergistic reversal effect of multidrug resistance by quercetin and hyperthermia in doxorubicin-resistant human myelogenous leukemia cells. Int J Hyperthermia. 2008;24:151–159. doi: 10.1080/02656730701843109. [DOI] [PubMed] [Google Scholar]
- Singh A, Naidu PS, Kulkarni SK. Reversal of aging and chronic ethanol-induced cognitive dysfunction by quercetin a bioflavonoid. Free Radic Res. 2003;37:1245–1252. doi: 10.1080/10715760310001616014. [DOI] [PubMed] [Google Scholar]
- Staedler D, Idrizi E, Kenzaoui BH, Juillerat-Jeanneret L. Drug combinations with quercetin: doxorubicin plus quercetin in human breast cancer cells. Cancer Chemother Pharmacol. 2011;68:1161–1172. doi: 10.1007/s00280-011-1596-x. [DOI] [PubMed] [Google Scholar]
- Sun Y, Oberly LW, Li Y. A simple method for clinical assay of SOD. Clin Chem. 1988;34:479–500. [PubMed] [Google Scholar]
- Tanir MH, Sener T, Inal M, Akyuz F, Uzuner K, Sivri E. Effect of quercetin and glutathione on the level of superoxide dismutase, catalase, malonyldialdehyde, blood pressure and neonatal outcome in a rat model of pre-eclampsia induced by NG-nitro-l-arginine-methyl ester. Eur J of Obstet Gynecol Reprod Biol. 2005;118:190–195. doi: 10.1016/j.ejogrb.2004.04.033. [DOI] [PubMed] [Google Scholar]
- Taskin E, Dursun N. Recovery of adriamycın induced mitochondrıal dysfunctıon in liver by selenium. Cytotechnology. 2014 doi: 10.1007/s10616-014-9736-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taskin E, Ozdogan K, Kunduz Kindap E, Dursun N. The restoration of kidney mitochondria function by inhibition of angiotensin-II production in rats with acute adriamycin-induced nephrotoxicity. Ren Fail. 2014;36:606–612. doi: 10.3109/0886022X.2014.882737. [DOI] [PubMed] [Google Scholar]
- Vaisheva F, Delbes G, Hales BF, Robaire B. Effects of the chemotherapeutic agents for non-hodgkin lymphoma, cyclophosphamide, doxorubicin, vincristine, and prednisone (CHOP), on the male rat reproductive system and progeny outcome. J Androl. 2007;28:578–587. doi: 10.2164/jandrol.106.002428. [DOI] [PubMed] [Google Scholar]
- Vanhees K, Schooten FJ, Khosrovani SB, Helden SV, Munnia A, Peluso M, Briede JJ, Haenen GR, Godschalk RW. Intrauterine exposure to flavonoids modifies antioxidant status at adulthood and decreases oxidative stress-induced DNA damage. Free Radic Biol Med. 2013;57:154–161. doi: 10.1016/j.freeradbiomed.2012.12.021. [DOI] [PubMed] [Google Scholar]
- Walle T, Ta N, Kawamori T, Wen X, Tsuji PA, Walle UK. Cancer chemopreventive properties of orally bioavailable flavonoids—methylated versus unmethylated flavones. Biochem Pharmacol. 2007;73:1288–1296. doi: 10.1016/j.bcp.2006.12.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wessels AM, Flockhart DA, Carpenter JS, Radovich M, Li L, Miller KD, Sledge GW, Storniolo AM, Otte JL, Lemler SM, Schneider BP. Cytochrome P450 polymorphisms and their relationship with premature ovarian failure in premenopausal women with breast cancer receiving doxorubicin and cyclophosphamide. Breast J . 2011;17:536–538. doi: 10.1111/j.1524-4741.2011.01144.x. [DOI] [PubMed] [Google Scholar]