Abstract
Adriamycin (ADR) is a chemotherapeutic drug. Its toxicities may associate with mitochondriopathy. Selenium (Se) is a trace element for essential intracellular antioxidant enzymes. However, there is lack of data related to the effect of selenium on the liver tissue of ADR-induced mitochondrial dysfunction. The study was to investigate whether Se could restore mitochondrial dysfunction of liver-exposed ADR. Rats were divided into four groups as a control, ADR, Se, co-treated ADR with Se groups. The biochemical measurements of the liver were made in mitochondrial and cytosol. ATP level and mitochondria membrane potential (MMP) were measured. Total oxidant (TOS), total antioxidant (TAS) status were determined and oxidative stress index (OSI) was calculated by using TOS and TAS. ADR increased TOS in mitochondria and also oxidative stress in mitochondria. ADR sligtly decreased MMP, and ATP level. Partial recovery of MMP by Se was able to elevate the ATP production in cotreatment of ADR with Se. TOS in mitochondria and cytosol was diminished, as well as OSI. We concluded that selenium could potentially be used against oxidative stress induced by ADR in liver, resulting from the restoration of MMP and ATP production and prevention of mitochondrial damage in vivo.
Keywords: Adriamycin induced hepatotoxicity, Selenium, Mitochondrial ATP, Mitochondrial membrane potential, Oxidative stress index
Introduction
Cancer is still keeping in increasing due to a gradually increase in the population growth rate and ageing. It was reported that about 2 million people were diagnosed with cancer in 2012. Survival rate of childhood cancer patients was reported around 80 % today, while more than 50 % of childhood cancer survivors have been treated with anthracyclines (Tacar et al. 2013). Among the various chemotherapeutic drugs, adriamycin (ADR) is widely used an anthracycline drug due to its therapeutic efficacy on a wide spectrum of solid and childhood cancers (Ingawale et al. 2014; Manjanatha et al. 2014; Qian et al. 2011; Raskovic et al. 2011). However, this anti-cancer drug also produces significant toxic side effect by damaging heart (Raskovic et al. 2011), liver (Wang et al. 2014), and kidney (Taskin and Dursun 2012; Taskin et al. 2014). The possibilities of how to minimize the effect of ADR induced toxicities on noncancerous tissues like liver, heart, kidney has, therefore, become quite an important research goal (Alshabanah et al. 2010; Hanusova et al. 2013; Qian et al. 2011; Raskovic et al. 2011).
The liver is an organ responsible for the maintenance of metabolic function as well as detoxification of exogenous and endogenous chemicals like anticancer (ADR, cisplatin), xenobiotics, immuno-suppressant (cyclosporine) drugs etc. (Goldstein et al. 2013). The liver may, therefore, be a main target of cytotoxic effects of drugs. Drugs even at a therapeutic dose range may cause liver injury in susceptible individuals. The most frequent known cause of hepatic dysfunction is drug induced liver injury (Ingawale et al. 2014).
ADR seems to accumulate mostly in the liver, most likely due to the organ’s role in metabolism (Tacar et al. 2013). It undergoes single electron reduction through a metabolic activation caused by NADPH reductase or other flavin-containing enzymes in microsomes to form ADR semiquinone free radicals in the cell especially hepatocytes. In the presence of molecular oxygen, the ADR semiquinone rapidly reduces oxygen to superoxide to form superoxide anions. Thus, ADR depletes antioxidant enzymes status and its toxicity has been ascribed to its active metabolites (ADR semiquinone) (Ingawale et al. 2014). Depletion of antioxidant by ADR may not be so surprising because the liver plays an important role to detoxify endogenous and exogenous toxins and become thus more vulnerable to oxidative damage. ADR tends to diffuse from cytosol to mitochondria and accumulates there. Mitochondria are an organel responsible for many cellular process, e.g., producing ATP depented its membrane potential (Dursun and Taskin 2011; Taskin and Dursun 2012; Taskin et al. 2014). Reactive oxygen species are physiologically produced during the oxidative phosphorylation process in the mitochondria, so mitochondria also contain some antioxidant enzymes, including CAT, SOD etc to detoxify them. (Prahalathan et al. 2005). Function of mitochondria is to keep the balance among generating and detoxifying ROS. Otherwise, it may quickly perturb its function and lead to excessive mitochondrial fragmentation and trigger apoptosis (Pereira et al. 2012). Mitochondria in liver have an important role to detoxify drugs as well. So, liver mitochondria in cancer patients treated with ADR is much more under risk to develop a mitochondriopathy related to oxidative stress.
This study was designed to investigate the Se effect on liver oxidative stress induced via ADR for five reasons: Firstly, we found in our previous studies that Se has a great restoration effect on mitochondrial dysfunction induced by ADR in heart (Dursun and Taskin 2011) and kidney (Taskin and Dursun 2012) tissues. Secondly, ADR’s hepatotoxic effect is basically associated to oxidative stress. Thirdly, the liver’s antioxidant defence is a mainly Se dependent system (Chen et al. 1986). Fourthly, the efficacy of the antioxidants in the liver may change due to its metabolic tasks. So Se might support the tissue to protect tissues against the undesired effects of ADR (Wang and Kang 1999). Lastly, Se has been reported to increase ADR’s anticancer effect on different cell lines (Vadgama et al. 2000). Selenium (Se) is one of essential trace elements in the human body (Tan et al. 2009). Se in the form of selenocysteine is part of the active centre of several seleno-enzymes which have antioxidant function, e.g. the glutathione peroxidases (GPx), the deiodinases and the thioredoxine reductases (Bordoni et al. 2008). It has been found that selenium can prevent free radicals from damaging cells and tissues in vivo. It is well documented that any significant modification of Se level would lead to changes in the activity of the seleno-enzymes and have important consequences on the susceptibility of tissues to oxidative stress (Bordoni et al. 2008). Supplementation of Se may, therefore, attenuate the toxicity of ADR in liver tissue. The hypothesis in the present study was that if ADR hepatotoxicity is related to free radical formation and oxidative stress, selenium may protect against its mitochondrial dysfunction induced by oxidative stress in the liver. Therefore, the aim of this study was to investigate the potential hepatoprotective effects of selenium on liver mitochondria dysfunction in rats with ADR-induced oxidative stress.
Materials and methods
Animals
Sprague–Dawley rats were housed under hygienic and standard environmental conditions (24 ± 1 °C, humidity 60–70 %, 12 h light/dark cycle). The animals were allowed a standard diet and water ad libitum. Animals were randomly separated into four different treatment groups as a Control (CONT), ADR, Selenium (Se) and ADR + Se. Each group consists of seven animals. Animals of the ADR group (Adriamycin HCl, Adriblastina vial 10 mg, Pharmacia) were treated by four intraperitoneal injections of 16 mg/kg cumulative dose within 8 days, and the control group received the same volume of physiological saline. Se was daily injected with sodium selenite at 50 µg/kg/i.p. dose for 21 days (Fig. 1). The synergistic effect of Se in combination with ADR was investigated. All experimental protocols were approved by the Medical Faculty Ethics Committee on Animal Research at the Erciyes University.
Fig. 1.
Experimental design of groups. ADR was intraperitoneally given equally in four equal doses in the ADR and Se + ADR groups. Se was intraperitoneally injected for 21 days in the Se and Se + ADR groups. The CONT group has received only physiological serum. S physiological serum; Se selenium group
24 h after the last treatment of drugs, liver tissues were collected then kept at −80 °C until use. Eventually, the tissues were separated into cytosol and mitochondria by centrifugation and used for mitochondrial function measurements and biochemical assays.
Preparation of mitochondria and cytosol
The measurement of mitochondrial functions and mitochondria derived oxidative stress were performed in the mitochondria and cytosol parts from liver tissues as described before (Dursun and Taskin 2011; Taskin and Dursun 2012; Taskin et al. 2014). Liver tissue homogenization was firstly performed in icecold buffer. A including 250 mM sucrose, 2 mM EGTA, 5 mM Tris HCl homogenizer followed by centrifugation at 2,000 g for for 8 min at 4 °C. The supernatant was centrifuged at 12,000 g for 10 min at 4 °C as used cytosol sample. The pellet of mitochondria was suspended in ice–cold buffer B containing 140 mM potassium, 20 mM Tris HCl. The mitochondria and cytosols were kept at −80 °C until use.
Measurement of mitochondrial membrane potential in (MMP) mitochondria from liver
The mitochondrial-specific fluorescent cationic dye 5,5′,6,6′-tetrachloro-1,1′,3,3′- tetraethyl benzimidazolylcarbocyanine iodide (JC-1) was used for assessment of MMP (Cayman Chemical, Ann Arbor Mi, USA). JC-1 tends to accumulate based on mitochondrial membrane potential in the mitochondria. Mitochondria with a normal MMP aggregated JC-1 (red fluorescence) and in depolarized mitochondria, JC-1 formed monomers (green fluorescence). Mitochondria fractions were added to each well of microplate, pre incubated with 10 μM JC-1 for 20 min at 37 °C in the dark; then the mitochondria were analysed by a fluorescent plate reader (Biotek, Synergy HT). In healthy cells, JC-1 forms J-aggregates which displayed strong fluorescent intensity with excitation and emission wave length at 560 and 595 nm, respectively. In apoptotic or unhealthy cells, JC-1 existed as monomers which showed strong fluorescence intensity with excitation and emission wave length at 485 and 535 nm, respectively. The ratio of fluorescent intensity of J-aggregates to fluorescent intensity of monomers was used as an indicator of cell health.
Determination of ATP content in mitochondria from liver
The measurement of ATP level in mitochondria was measured by using a bioluminescent kit (Cambrex Bio Science, Rockland, USA). When ATP is the limiting reagent, the light emitted is proportional to the ATP present. ATP is consumed and light is emitted when firefly luciferase catalyzes the oxidation of luciferin. Briefly, a 10 μl aliquot of a mitochondria sample was mixed with 100 μl cell lysis reagent and incubated at room temperature for 10 min to extract ATP from cells. Following the addition of 100 μl ATP monitoring reagent, luminescence was measured using a luminometer (Biotek, Synergy HT). A standard curve was generated from known concentrations of ATP and used to calculate the concentration of ATP in each sample. Luminescence increased linearly with the negative log of the ATP concentration in the samples over the range of measured concentrations.
Biochemical studies
Measurement of total antioxidant status
Total antioxidant status (TAS) of the cytosol and mitochondria of the liver was measured using a Rel Assay kit (Rel Assay Diagnostics, Gaziantep, TURKEY). TAS was measured according to the manufacturer's protocol.
Measurement of total oxidant status
Total oxidant status (TOS) of the cytosol and mitochondria of the liver was measured using a Rel Assay Kit. TOS was measured according to the manufacturer's protocol. The results were expressed in μmol H2O2/L.
Calculation of oxidative stress index
The ratio of TOS and TAS was evaluated as the oxidative stress index (OSI). The ratio of TOS and TAS was evaluated as the oxidative stress index (OSI).
Statistical data analysis
Statistical analyses were conducted using Excel and SPSS version 16.0. All results were expressed as mean ± SEM. Multiple analyses of variance (ANOVA) was used for comparing all groups by using Turkey test. p < 0.05 was considered to be significant for all data.
Results
The effect of selenium on adriamycin changed in liver mitochondrial and cytosolic total oxidant status (TOS), total antioxidant (TAS) status and oxidative stress index (OSI)
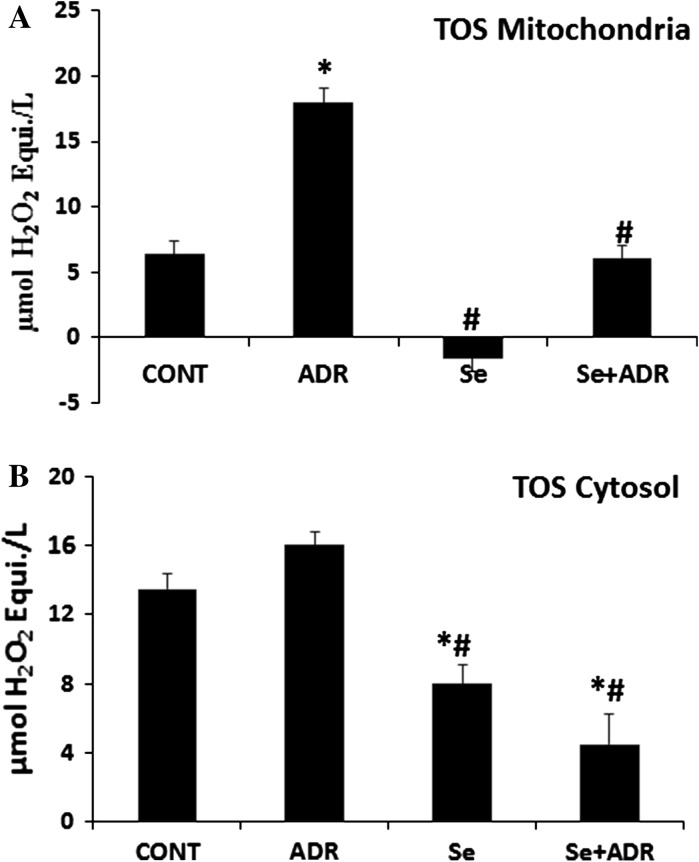
It has been well documented that ADR-induced toxicity is related to increased oxidative stress, as evidenced from increased levels of reactive oxygen species and decreased levels of antioxidants. TAS and TOS were measured in mitochondria, and cytosol from liver tissue. ADR significantly elevated mitochondrial and cytosolic TOS (p < 0.05; Fig. 2A, B). The elevation of TOS in mitochondria and cytosol could be attenuated by Se. In additional, the Se only group strongly attenuated TOS in mitochondria and cytosol (p < 0.05; Fig. 2A, B).
Fig. 2.
The effect of selenium on mitochondrial A and cytosolic B total oxidant status in rats with adriamycin-induced oxidative stress. TOS total oxidant status, Cont control group, ADR adriamycin group, Se selenium group, Se + ADR group selenium plus adriamycin group.*p < 0.05 versus Cont, #p < 0.05 versus ADR. All data were expressed as mean ± SEM
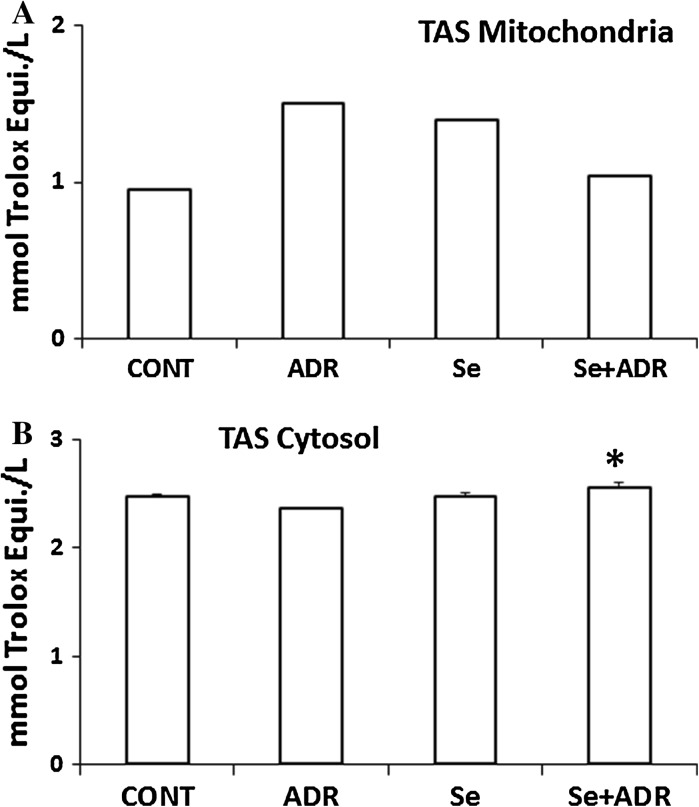
There was no significant change in the mitochondrial and cytosolic TAS by the ADR vs control group (Fig. 3A, B). But, TAS in the Se + ADR group had a significant elevation compared to the ADR only group in the cytosol (p < 0.05; Fig. 3B), since Se is known to play an essential role for being part of several antioxidant enzymes.
Fig. 3.
The effect of selenium on mitochondrial A and cytosolic B total antioxidant status in rats with adriamycin-induced oxidative stress. TOS total oxidant status, Cont control group, ADR adriamycin group, Se selenium group, Se + ADR group selenium plus adriamycin group. *p < 0.05 versus ADR. All data were expressed as mean ± SEM
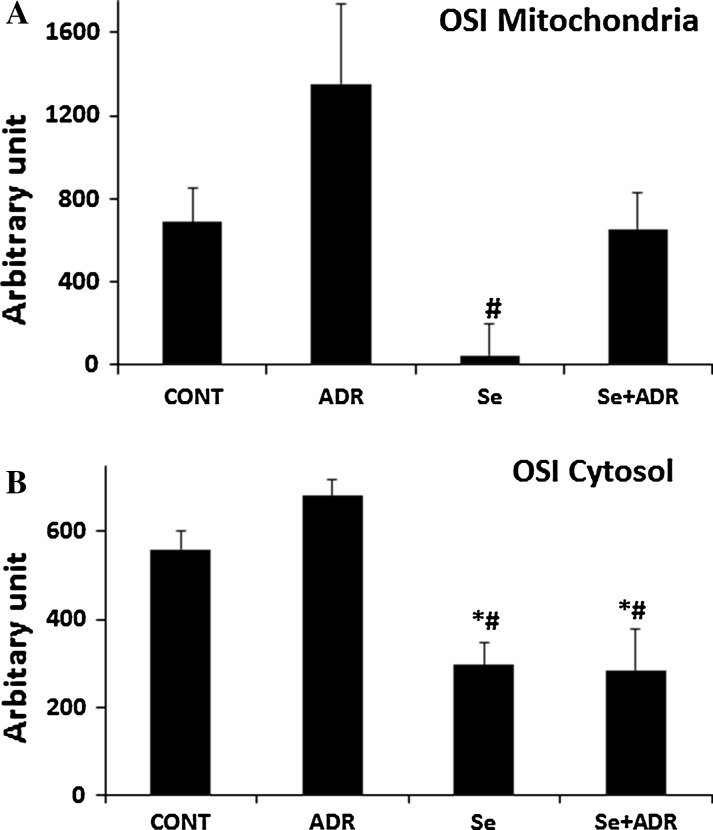
The level of oxidative stress was calculated by TOS to TAS ratio in the mitochondria and cytosol as well. OSI in mitochondria and cytosol was not significantly different in the ADR group compared to the control group (Fig. 4A, B). But, the Se only group showed a significant decline in the mitochondrial OSI vs the ADR group (p < 0.05, Fig. 4B). Furthermore, cytosolic OSI in both Se groups (Se and Se+ADR) was significantly decreased versus that of the ADR and the control groups (p < 0.05, Fig. 4B).
Fig. 4.
The effect of selenium on mitochondrial A and cytosolic B oxidative stress index in rats with adriamycin-induced oxidative stress. OSI oxidative stress index, Cont control group, ADR adriamycin group, Se selenium group, Se + ADR group selenium plus adriamycin group. *p < 0.05 versus Cont, #p < 0.05 versus ADR. All data were expressed as mean ± SEM
The effect of selenium on attenuation of mitochondrial function by adriamycin in liver
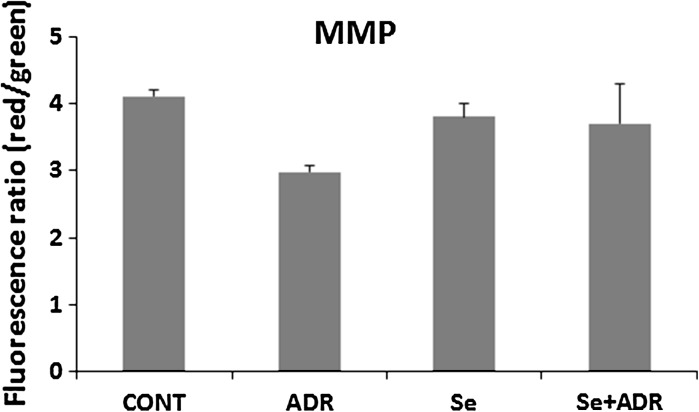
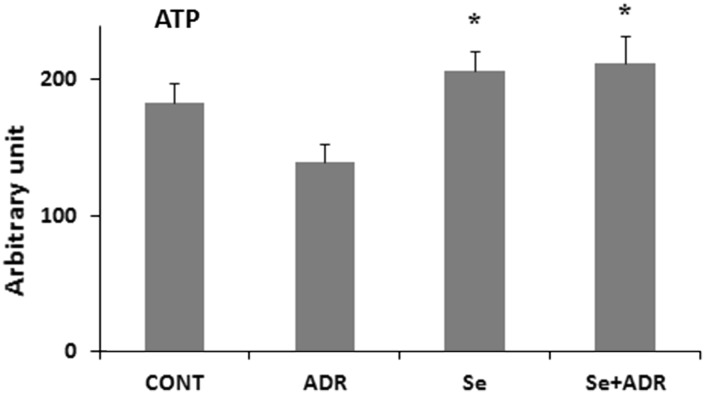
The mitochondrion is one of main target of ADR-toxicity at a subcellular level (Octavia et al. 2012). Restoration of mitochondrial dysfunction might be helped to attenuate of ADR-induced hepatic toxicity. So that next step was determination of mitochondrial function impairment by using MMP and ATP production. MMP was measured by using a specific mitochondrial cationic dye (JC-1). JC-1 is able to aggregate at mitochondrial depolarization, indicated by a decrease in the red/green fluorescence intensity ratio. But, JC-1 formed monomers in normal mitochondrial membrane potential as representing a increase in the red/green fluorescence intensity ratio. MMP was estimated via the ratio of red and green fluorescence to demonstrate ADR’s toxicity in mitochondria. ADR reduced weakly the dissipation of MMP, but this was not statistically significant (Fig. 5). In parallel, the weak effect of ADR on MMP was accompanied by a weak decrease in ATP production, but this was not statistically significant (Fig. 6). However, for both Se groups (Se and Se+ADR) a significant increase in the ATP level in the liver mitochondria (p < 0.05; Fig. 6) was observed. These data demonstrated that selenium strongly attenuated to ADR′s undesired effect on hepatic mitochondria.
Fig. 5.
The effect of selenium on mitochondrial membrane potential in rats with oxidative stress induced by adriamycin. MMP mitochondrial membrane potential, Cont control group, ADR adriamycin group, Se selenium group, Se + ADR group selenium plus adriamycin group. All data were expressed as mean ± SEM
Fig. 6.
The effect of selenium on ATP level in rats with oxidative stress induced by adriamycin. Cont control group, ADR adriamycin group, Se selenium group, Se + ADR group selenium plus adriamycin group.*p < 0.05 versus ADR. All data were expressed as mean ± SEM
Discussion
In this study, we evaluated the effect of selenium combined with ADR on mitochondrial function. At the end of the treatment period in the our present study, we observed that ADR caused the reduction of the mitochondrial funtion of the liver due to decline of MMP and ATP production based on elevation of OSI associated to increased TOS in the mitochondria. Surprisingly, only the Se group improved the mitochondrial functions even better than the control group by decreasing OSI based on decreasing TOS in mitochondria and cytosol of the liver tissue. Cotreatment of Se and ADR also led to enhancement of the mitochondrial functions of the liver by decreasing mitochondrial derived oxidative stress and an eventual increase in the ATP production.
Cancer cell mortality has been shown to correlate with dosage and exposure time of the drug (Dursun and Taskin 2011; Hanusova et al. 2013), but the systemic toxicity of ADR strongly limits its maximal therapeutic dose (Qian et al. 2011), whereas low dose ADR treatment may result in decline of therapeutic effect (Qian et al. 2011). Until now, ADR has been well documented to have toxic effects on mostly heart, liver, brain, kidney, and testis tissues (Wang et al. 2014), but its cardio and hepatotoxicities are mainly limiting factors in the cancer therapy using this agent (Alshabanah et al. 2010). Furthermore, the risk of impaired hepatic function in patients treated with ADR increases in dependence of its dose escalation (Cheng et al. 2011). Therefore, to find a candidate to overcome its toxicities on noncancerous tissues like liver has become quite an important research goal.
There is no doubt about hepatocellular damage and declined liver function in animals exposed to ADR (Alshabanah et al. 2010). To be found the mechanism of ADR caused liver toxicity is so important to overcome these adverse effect. Liver is vulnerable to the toxicity of ADR simply because of its higher metabolic function (Tacar et al. 2013), hepatocytes are very rich in mitochondria and have a high respiratory activity (Barogi et al. 2000). Additionally, mitochondrial ADR concentration is much higher than its cytosolic concentration (Dursun and Taskin 2011). ADR may transform to its semiquinone form by a mitochondria electron transport system (Chen et al. 2013) from mainly cytochrome-P450 reductase enzyme (Benchekroun et al. 1993) which is important for the metabolism of both endogenous (e.g. lipids, steroids) and exogenous (e.g. drugs, toxins) compounds (Goldstein et al. 2013). It was shown that the liver has higher cytochrome-P450 reductase level than does cardiac tissue (Myers et al. 1982). The semiquinone subsequently reacts with oxygen, iron, and hydrogen peroxide to produce ROS causing apoptosis and cell damage (Benchekroun et al. 1993; Chen et al. 2013). Therefore, oxidative stress induced in rat liver by ADR was found to be higher than in the controls in our current study. Redox cycling transformation occurs in cytoplasm, endoplasmatic reticulum, and especially in mitochondria, resulting in generating ROS (Dudka et al. 2012). So, we calculated oxidative stress index in both, cytosol and mitochondria, from liver tissue, and measured total oxidant, antioxidant status as well. As expected, our data are in agreement with other reports showing strong oxidative stress especially in the mitochondria. Besides different mechanism involved in the derivation of the oxidative stress in ADR, there is also evidence that ADR may depress natural antioxidant defense system. In light of the implication of oxidative stress in the pathogenic mechanism, a number of attempts have been made to assess the value of antioxidants in protecting against ADR liver toxicity (Popovic et al. 2007). One previous study has shown that the effect of adriamycin effect was mediated by a complex oxyradical cascade involving superoxide, hydroxylradical, and small amounts of iron (Hida et al. 1995).
We found in the study that ADR led to mitochondrial derived oxidative stress. Although ROS may freely diffuse through intracellular membranes, subcellular compartmentalization of glutathione, an essential intracellular antioxidant, may significantly modulate the harmful activity of ROS in subcellular compartments (Dudka et al. 2012). In a recent study, it was also shown that redox communication between peroxisomes and mitochondria may be important for ROS diffusion between subcellular compartments (Wang et al. 2013). The liver due to the ADR metabolism may produce an important amount of ROS, but at the same time the antioxidant defence is a few times higher than, for example, that in the heart (Revis and Marusic 1978). Cytosolic ROS might be detoxified by these higher antioxidant activity in liver. Therefore, the level of mitochondrial oxidative stress is thought to be probably more important than that of cytosol. According to this assumption, a moderately higher level of ROS for example in cytoplasm, does probably not destroy the cell membrane but mitochondria ROS overproduction may cause the dissipation of MMP resulting from reduced ATP synthesis and trigger liver injury.
It is accepted that ADR toxicity involving disruption of mitochondrial function is completely independent from its antitumor activity (Pereira et al. 2012). Both of ATP and MMP were used as a good indicator for mitochondrial functions in our current study. ADR-induced mitochondrial dysfunction in the tissues include inhibition of oxidative phosphorylation, decreased calcium-loading capacity and increased reactive oxygen species (ROS) production (Pereira et al. 2012). Hepatocytes are very rich in mitochondria and have a high respiratory activity (Barogi et al. 2000), ADR tend to accumulate in mitochondria (Dursun and Taskin 2011; Taskin and Dursun 2012). Therefore, ADR-mediated toxicity is responsible for decreasing levels of inorganic phosphate, both ADP and ATP as well as AMP which causes pathological conditions in hepatocytes. ATP is an essential fuel for all metabolic processes, and since ADR causes ATP to decrease, the ability of the cell to perform energy-dependent tasks is also decreased (Tacar et al. 2013). In other words, ADR gives rise to cause energy stress in cells. Maybe that is why the patients undergoing chemotherapy begin to weaken and feel constant muscular and mental fatigue (Tacar et al. 2013). In the present study, the reduced mitochondrial membrane potential in mitochondria of the liver was in good agreement with literature (Barogi et al. 2000). ADR treatment of rat hepatocytes was shown to induce a significant dose dependent decrease of MMP and ATPase activity (Barogi et al. 2000). Furthermore, the loss of mitochondrial membrane potential, resulting in the release of cytochrome c may induce the activation of caspase and lead to apoptotic cell death (Qian et al. 2011). These results are consistent with a previous report which reported that cellular organelles, such as ER, Golgi apparatus and cytoskeleton, mitochondria ccontributed to the early ADR effect (Qian et al. 2011). The knowledge about toxic effects of ADR on mitochondria is limited, but it may be related with inactivation of complex I and II in ADR-treated mitochondria (Ascensao et al. 2005; Pereira et al. 2012). In conclusion, our data confirm that mitochondrial dysfunction is one major cause of ADR-selective liver toxicity. One of ADR’s metabolite was reported to increase the MMP of liver mitochondria by inducing a Ca2+-dependent pathway. Moreover, this phenomenon was accompanied by a release of mitochondrial Ca2+, mitochondrial swelling, collapse of the membrane potential, and oxidation of mitochondrial pyridine nucleotides (Sokolove 1994).
It has also been reported that supplementation with antioxidants in ADR-induced myocardial and hepatic injury has a favourable effect on the tissues (Benedetti et al. 2012; Raskovic et al. 2011). Recent researches have revealed some potential therapeutic agents, such as Se (Benedetti et al. 2012; Dursun and Taskin 2011; Koukay et al. 1990; Taskin and Dursun 2012), vitamin E (Koukay et al. 1990), NAC (N-acetylcysteine) (Wang et al. 2014), α-tocopherol (Chautan et al. 1992; Myers et al. 1982), quercetin (Chen et al. 2013) and L-carnosine (Ozdogan et al. 2011) effective in limiting the extent of ADR-induced toxicities, all of which are based on the antioxidant properties of the candidate compound. We have already suggested the selenium to be a possible candidate to eliminate its mitochondrial toxicity in heart (Dursun and Taskin 2011) and kidney (Taskin and Dursun 2012) tissues. Until now, there was no report on the affect of selenium on mitochondrial dysfunction of the liver induced by adriamycin.
It was interestingly shown that rat and hamster liver have much higher levels of selenium-dependent glutathione peroxidase activity than the livers of sheep, pigs, chickens, humans and guinea pigs (Chen et al. 1986). So, probably selenium depletion might occur in the ADR-exposed liver, resulting from the generation of more antioxidant defence due to the toxicity of ADR in the tissue. To overcome this deficiency, selenium supplementation to decrease the toxicity of ADR in normal liver cells was intensively studied in the current study. So, we used a rat model to test our hypotheses that Se supplementation might achieve to elevate the total antioxidant status of the ADR-exposed liver, and Se administration might recover to liver mitochondrial dysfunction-exposed ADR in rats. Our results have provided some evidence supporting the hypotheses. Se produces its protective effects against ADR-induced liver damage at least partially by enhancing the antioxidation status of the liver. We found that Se could significantly attenuate the decrease in the TOS and OSI which may at least partially account for the TAS-produced attenuation by ADR in the ADR-treated livers. Because Se is an essential trace element which must be supplied by daily diet; its main role is that of an antioxidant in the GPx and thioredoxin reductase enzymes. GPx, the main intracellular antioxidant, and thioredoxin reductase have been reported to be critical antioxidant defences. A previous study has reported the elevation of ROS level and the decrease of total antioxidant after ADR treatment in plasma and liver as well (Bordoni et al. 2008). The oxidative damage-induced via adriamycin in the rat liver was counteracted by Se supplementation (Bordoni et al. 2008; Bulucu et al. 2009).The higher activity of liver GPx and TrxR observed in the Se-fed animals in the basal conditions partly explains the resistance to the ADR-induced oxidative damage and could reflect their higher Se intake (Benedetti et al. 2012). Another study has also shown that Se deficiency was caused by 90 % lower hepatic glutathione peroxidase activity (Fischer et al. 1992). According to our knowledge so far, there has been no data in the literature on the effect of selenium on MMP in liver exposed to ADR administration yet. Although it is not easy, at present, to explain the physiologic relevance or the underlying mechanism of the previous and present observations, these deserve attention in future studies. But our previous studies have suggested that Se decreased oxidative stress-induced in kidney (Taskin and Dursun 2012), and heart (Dursun and Taskin 2011) by preventing the pathological downstream events induced by oxidative stress, such as mitochondrial membrane potential. These data showed that ADR not only increased the free radical formation but also decreased its ability to detoxify reactive oxygen species. Not only was Se supplementation reported without any change of ADR’s antineoplastic effect, but it also enhanced its chemotherapeutic efficiency on different cell lines, including liver, breast, lung, and small intestinal tumor cells (Vadgama et al. 2000).
Conclusion
Taken together, our present study has indicated that Se could restore the functions of liver mitochondria after dysfunction induced by ADR in rats. The restoration of mitochondrial dysfunction via Se supplementation was related to decreased oxidative stress-induced injury by directly decreasing oxidative stress. As a result, selenium might be offered a great candidate for recovery of ADR-induced mitochondrial dysfunction in liver. Nevertheless, future studies will need to evaluate this beneficial effects in human studies and especially to prove this counteraction effect of selenium rich food in cancer patients treated with ADR.
Acknowledgments
The authors wish to tank to Assoc. Prof. Dr. Mukerrem Betul YERER AYCAN for helping to use a fluorescent plate reader, to Prof. Dr. Cem SUER and Prof. Dr. Yunus DURSUN to help analysis the data. This study was supported financially by the Research Foundation of Erciyes University.
Conflict of interest
The authors have no conflicts of interest to disclose.
References
- Alshabanah OA, Hafez MM, Al-Harbi MM, Hassan ZK, Al Rejaie SS, Asiri YA, Sayed-Ahmed MM (2010) Doxorubicin toxicity can be ameliorated during antioxidant L-carnitine supplementation. Oxid Med Cell Longev 3:428–433. doi:10.4161/oxim.3.6.14416 [DOI] [PMC free article] [PubMed]
- Ascensão A, Magalhães J, Soares JM, Ferreira R, Neuparth MJ, Marques F, Oliveira PJ, Duarte JA (2005) Moderate endurance training prevents doxorubicin induced in vivo mitochondriopathy and reduces the development of cardiac apoptosis. Am J Physiol Heart Circ Physiol 289:H722–731. doi:10.1152/ajpheart.01249.2004 [DOI] [PubMed]
- Barogi S, Baracca A, Cavazzoni M, Parenti Castelli G, Lenaz G (2000) Effect of the oxidative stress induced by adriamycin on rat hepatocyte bioenergetics during ageing. Mech ageing Dev 113:1–21. doi:10.1016/S0047-6374(99)00089-55 [DOI] [PubMed]
- Benchekroun MN, Pourquier P, Schott B, Robert J. Doxorubicin-induced lipid peroxidation and glutathione peroxidase activity in tumor cell lines selected for resistance to doxorubicin. Eur J Biochem. 1993;211:141–146. doi: 10.1111/j.1432-1033.1993.tb19880.x. [DOI] [PubMed] [Google Scholar]
- Benedetti S, Primiterra M, Tagliamonte MC, Carnevali A, Gianotti A, Bordoni A, Canestrari F. Counteraction of oxidative damage in the rat liver by an ancient grain (Kamut brand khorasan wheat) Nutrition. 2012;28:436–441. doi: 10.1016/j.nut.2011.08.006. [DOI] [PubMed] [Google Scholar]
- Bordoni A, Danesi F, Malaguti M, Di Nunzio M, Pasqui F, Maranesi M, Biagi PL. Dietary selenium for the counteraction of oxidative damage: fortified foods or supplements? Br J Nutr. 2008;99:191–197. doi: 10.1017/S0007114507793911. [DOI] [PubMed] [Google Scholar]
- Bulucu F, Ocal R, Karadurmus N, Sahin M, Kenar L, Aydin A, Oktenli C, Koc B, Inal V, Yamanel L, Yaman H (2009) Effects of N-acetylcysteine, deferoxamine and selenium on doxorubicin-induced hepatotoxicity. Biol Trace Elem Res 132:184–196. doi:10.1007/s12011-009-8377-y [DOI] [PubMed]
- Chautan M, Leonardi J, Calaf R, Lechene P, Grataroli R, Portugal H, Pauli AM, Lafont H, Nalbone G (1992) Heart and liver membrane phospholipid homeostasis during acute administration of various antitumoral drugs to the rat. Biochem Pharmacol 44:1139–1147. doi:10.1016/0006-2952(92)90378-V [DOI] [PubMed]
- Chen XS, Xue A, Morris VC, Ferrans VJ, Herman EH, el-Hage A, Levander OA. Effect of selenium deficiency on the chronic toxicity of adriamycin in rats. J Nutr. 1986;116:2453–2465. doi: 10.1093/jn/116.12.2453. [DOI] [PubMed] [Google Scholar]
- Chen JY, Hu RY, Chou HC. Quercetin-induced cardioprotection against doxorubicin cytotoxicity. J Biomed Sci. 2013;20:95. doi: 10.1186/1423-0127-20-95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng X, Gao F, Xiang J, Jiang X, Chen J, Zhang J (2011) Galactosylated alpha, beta-poly[(2-hydroxyethyl)-L-aspartamide]-bound doxorubicin: improved antitumor activity against hepatocellular carcinoma with reduced hepatotoxicity. Anticancer Drug 22:136–147. doi:10.1097/CAD.0b013e3283406e85 [DOI] [PubMed]
- Dudka J, Gieroba R, Korga A, Burdan F, Matysiak W, Jodlowska-Jedrych B, Mandziuk S, Korobowicz E, Murias M (2012) Different effects of resveratrol on dose-related doxorubicin-induced heart and liver toxicity. Evid Based Complement Alternat Med 2012:606183. doi:10.1155/2012/606183 [DOI] [PMC free article] [PubMed]
- Dursun N, Taskin E, Yerer Aycan MB, Sahin L. Selenium-mediated cardioprotection against adriamycin-induced mitochondrial damage . Drug Chem Toxicol. 2011;34:199–207. doi: 10.3109/01480545.2010.538693. [DOI] [PubMed] [Google Scholar]
- Fischer JG, Tackett RL, Howerth EW, Johnson MA. Copper and selenium deficiencies do not enhance the cardiotoxicity in rats due to chronic doxorubicin treatment. J Nutr. 1992;122:2128–2137. doi: 10.1093/jn/122.11.2128. [DOI] [PubMed] [Google Scholar]
- Goldstein I, Rivlin N, Shoshana OY, Ezra O, Madar S, Goldfinger N, Rotter V. Chemotherapeutic agents induce the expression and activity of their clearing enzyme CYP3A4 by activating p53. Carcinogenesis. 2013;34:190–198. doi: 10.1093/carcin/bgs318. [DOI] [PubMed] [Google Scholar]
- Hanusova V, Tomsik P, Kriesfalusyova L, Pakostova A, Bousova I, Skalova L. In vivo effect of oracin on doxorubicin reduction, biodistribution and efficacy in Ehrlich tumor bearing mice. Pharmacol Rep. 2013;65:445–452. doi: 10.1016/S1734-1140(13)71020-X. [DOI] [PubMed] [Google Scholar]
- Hida H, Coudray C, Calop J, Favier A. Effect of antioxidants on adriamycin-induced microsomal lipid peroxidation. Biol Trace Elem Res. 1995;47:111–116. doi: 10.1007/BF02790107. [DOI] [PubMed] [Google Scholar]
- Ingawale DK, Mandlik SK, Naik SR. Models of hepatotoxicity and the underlying cellular, biochemical and immunological mechanism(s): a critical discussion. Environ Toxicol Pharmacol. 2014;37:118–133. doi: 10.1016/j.etap.2013.08.015. [DOI] [PubMed] [Google Scholar]
- Koukay N, Mouhieddine S, Richard MJ, Arnaud J, de Leiris J, Favier A. Influence of selenium on lipid peroxidation and cardiac functions in chronically adriamycin-treated rats. Adv Exp Med Biol. 1990;264:353–359. doi: 10.1007/978-1-4684-5730-8_55. [DOI] [PubMed] [Google Scholar]
- Manjanatha MG, Bishop ME, Pearce MG, Kulkarni R, Lyn-Cook LE, Ding W. Genotoxicity of doxorubicin in F344 rats by combining the comet assay, flow-cytometric peripheral blood micronucleus test, and pathway-focused gene expression profiling. Environ Mol Mutagen. 2014;55:24–34. doi: 10.1002/em.21822. [DOI] [PubMed] [Google Scholar]
- Myers CE, Katki A, Travis E. Effect of tocopherol and selenium on defenses against reactive oxygen species and their effect on radiation sensitivity. Ann NY Acad Sci. 1982;393:419–425. doi: 10.1111/j.1749-6632.1982.tb31280.x. [DOI] [PubMed] [Google Scholar]
- Octavia Y, Tocchetti CG, Gabrielson KL, Janssens S, Crijns HJ, Moens AL. Doxorubicin-induced cardiomyopathy: from molecular mechanisms to therapeutic strategies. J Mol Cell Cardiol. 2012;52:1213–1225. doi: 10.1016/j.yjmcc.2012.03.006. [DOI] [PubMed] [Google Scholar]
- Ozdogan K, Taskin E, Dursun N. Protective effect of carnosine on adriamycin-induced oxidative heart damage in rats. Anadolu Kardiyol Derg. 2011;11:3–10. doi: 10.5152/akd.2011.003. [DOI] [PubMed] [Google Scholar]
- Pereira GC, Pereira SP, Pereira CV, Lumini JA, Magalhães J, Ascensão A, Santos MS, Moreno AJ, Oliveira PJ (2012) Mitochondrionopathy phenotype in doxorubicin-treated Wistar rats depends on treatment protocol and is cardiac-specific. PLoS One 7(6):e38867. doi:10.1371/journal.pone.0038867 [DOI] [PMC free article] [PubMed]
- Popovic M, Kolarovic J, Mikov M, Trivic S, Kaurinovic B. Anthracycline-based combined chemotherapy in the mouse model. Eur J Drug Metab Pharmacokinet. 2007;32:101–108. doi: 10.1007/BF03190998. [DOI] [PubMed] [Google Scholar]
- Prahalathan C, Selvakumar E, Varalakshmi P. Lipoic acid ameliorates adriamycin-induced testicular mitochondriopathy. Reprod Toxicol. 2005;20:111–116. doi: 10.1016/j.reprotox.2004.12.005. [DOI] [PubMed] [Google Scholar]
- Qian H, Yang Y, Wang X. Curcumin enhanced adriamycin-induced human liver-derived Hepatoma G2 cell death through activation of mitochondria-mediated apoptosis and autophagy. Eur J Pharm Sci. 2011;43:125–131. doi: 10.1016/j.ejps.2011.04.002. [DOI] [PubMed] [Google Scholar]
- Raskovic A, Stilinovic N, Kolarovic J, Vasovic V, Vukmirovic S, Mikov M. The protective effects of silymarin against doxorubicin-induced cardiotoxicity and hepatotoxicity in rats. Molecules. 2011;16:8601–8613. doi: 10.3390/molecules16108601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Revis NW, Marusic N (1978) Glutathione peroxidase activity and selenium concentration in the hearts of doxorubicin-treated rabbits. J Mol Cell Cardiol 10:945–951. doi:10.1016/0022-2828(78)90340-1 [DOI] [PubMed]
- Sokolove PM. Interactions of adriamycin aglycones with mitochondria may mediate adriamycin cardiotoxicity. Int J Biochem. 1994;26:1341–1350. doi: 10.1016/0020-711X(94)90176-7. [DOI] [PubMed] [Google Scholar]
- Tacar O, Sriamornsak P, Dass CR. Doxorubicin: an update on anticancer molecular action, toxicity and novel drug delivery systems. J Pharm Pharmacol. 2013;65:157–170. doi: 10.1111/j.2042-7158.2012.01567.x. [DOI] [PubMed] [Google Scholar]
- Tan L, Jia X, Jiang X, Zhang Y, Tang H, Yao S, Xie Q. In vitro study on the individual and synergistic cytotoxicity of adriamycin and selenium nanoparticles against Bel7402 cells with a quartz crystal microbalance. Biosens Bioelectron. 2009;24:2268–2272. doi: 10.1016/j.bios.2008.10.030. [DOI] [PubMed] [Google Scholar]
- Taskin E, Dursun N. The protection of selenium on adriamycin-induced mitochondrial damage in rat. Biol Trace Elem Res. 2012;147:165–171. doi: 10.1007/s12011-011-9273-9. [DOI] [PubMed] [Google Scholar]
- Taskin E, Ozdogan K, Kunduz KE, Dursun N (2014) The restoration of kidney mitochondria function by inhibition of angiotensin-II production in rats with acute adriamycin-induced nephrotoxicity. Ren Fail 36(4):606–612. doi:10.3109/0886022X.2014.882737 [DOI] [PubMed]
- Vadgama JV, Wu Y, Shen D, Hsia S, Block J. Effect of selenium in combination with adriamycin or taxol on several different cancer cells. Anticancer Res. 2000;20:1391–1414. [PubMed] [Google Scholar]
- Wang GW, Kang YJ. Inhibition of doxorubicin toxicity in cultured neonatal mouse cardiomyocytes with elevated metallothionein levels. J Pharmacol Exp Ther. 1999;288:938–944. [PubMed] [Google Scholar]
- Wang B, Van Veldhoven PP, Brees C, Rubio N, Nordgren M, Apanasets O, Kunze M, Baes M, Agostinis P, Fransen M (2013) Mitochondria are targets for peroxisome-derived oxidative stress in cultured mammalian cells. Free Radic Biol Med 65:882–894. doi:10.1016/j.freeradbiomed.2013.08.173 [DOI] [PubMed]
- Wang B, Ma Y, Kong X, Ding X, Gu H, Chu T, Ying W (2014) NAD administration decreases doxorubicin-induced liver damage of mice by enhancing antioxidation capacity and decreasing DNA damage. Chem Biol Interact. 212:65–71. doi:10.1016/j.cbi.2014.01.013 [DOI] [PubMed]