Abstract
Carbon tetrachloride (CCl4) is widely used to induce liver toxicity in in vitro/in vivo models. Lipid peroxidation (LPO) begins with toxicity and affects cell viability. Recently, the beneficial effects of melatonin and Vitamin D on cell proliferation in human normal and cancer cells were found. This study was planned to evaluate antioxidant and cytoprotective activity of melatonin and Vitamin D in CCl4 induced cytotoxicity in HepG2 and Hep3B hepatoma cell lines. Based on the cytotoxicity assay, melatonin and Vitamin D were evaluated for cytotoprotective potential against CCl4 induced toxicity in HepG2 and Hep3B liver cell lines by monitoring cell viability, LPO and glutathione (GSH) level. Different dosages of CCl4 (0.1, 0.2, 0.3 and 0.4 % v/v) were applied to HepG2 and Hep3B cells in order to determine the most toxic dosage of it in a time dependent manner. The same experiments were repeated with exogenously applied melatonin (MEL) and Vitamin D to groups treated with/without CCL4. Cell viability was determined with MTT measurements at the 2nd, 24th and 48th h. GSH content and Malondialdehyde levels were measured from the cell lysates. As a result, both melatonin and Vitamin D administration during CCl4 exposure protected liver cells from CCl4 induced cell damage. Increase in LPO and decrease in GSH were found in the CCl4 groups of both cells. Contrary to these results administration of MEL and Vitamin D on cells exhibited results similar to the control groups. Therefore, melatonin and Vitamin D might be a promising therapeutic agent in several toxic hepatic diseases.
Keywords: CCl4, Melatonin, Vitamin D, Cell proliferation, Liver cytotoxicity, HepG2, Hep3B
Introduction
Carbon tetrachloride (CCl4) is a clear liquid that evaporates very easily, and low background levels of CCl4 are found in air, water, and soil because of past and present releases (ATSDR 2005). The liver is especially sensitive to CCl4 since it contains a large amount of the enzymes that modify the form of the chemical. Some of the breakdown products may attack cell proteins, interfering with the functions of the liver cells. Products that attack cell membranes may result in the death of the cells (Thrall et al. 2000). In vivo and in vitro studies showed that inflammation and oxidative damage are the main mechanisms of CCl4 induced toxicity (Ding et al. 2005; Wang et al. 2005; Liu et al. 2006; Hong et al. 2009).
The antioxidant and hepatoprotective effects of melatonin have already been investigated (Carbajo-Pescador et al. 2009; Fan et al. 2010). It influences both the membrane and nucleus receptors of the cells (Martín-Renedo et al. 2008). HepG2 and Hep3B cell lines have been shown to retain parenchymal cell morphology (Knowles et al. 1980; Zannis et al. 1981). They can hydroxylate Vitamin D3 compounds (Masuda et al. 1996). One of the pharmacologic effects of 1.25(OH)2D3, the active form of Vitamin D is cell proliferative function (Eisman et al. 1989; Akhter et al. 2001). It has been used for disorders related to proliferation (Binderup and Bramm 1988; Kragballe 1992).
Taking into consideration the cited properties of melatonin and Vitamin D, it was hypothesized that both of these agents may accomplish the oxidative stress affecting the cell viability during CCl4-induced cytotoxicity. Hence, the present study focused on evaluating the hepatoprotective activity of melatonin and Vitamin D against carbon tetrachloride (CCl4) induced cytotoxicity in HepG2 and Hep3B cell lines.
Materials and methods
Cell lines, chemicals and materials
Human hepatoma cell line HepG2 and Hep3B cells were obtained from the ATCC (Manassas, VA, USA). Cells were cultured in Dulbecco’s Modified Eagle Medium (DMEM) (LONZA, Basel, Switzerland), supplemented with fetal calf serum (FCS), (LONZA), l-glutamine (LONZA), streptomycin (LONZA) and penicillin (LONZA). Carbon tetrachloride (CCl4), (Sigma Chemical Co., St. Louis, MO, USA) was dissolved in dimethyl sulfoxide (DMSO) (LONZA) in a serum-free DMEM (LONZA). Melatonin (Sigma) was dissolved in a volumetric mixture of 10,000 parts phosphate buffer saline (PBS) and 1 part Ethanol (96 % v/v). Melatonin and 1.25(OH)2D3 (DEVA, Istanbul, Turkey) were sterilized by 0.22 μm pore size cellulose acetate membrane filters, and added to cultures at the indicated time and concentrations. CCl4 toxicity was studied in the HepG2 and Hep3B cell lines. Cell counts were assessed by 3-[4.5-dimethylthiazol-2-yl]-2.5 diphenyltetrazolium bromide test (MTT, Sigma).
Cell culture and experimental protocol
The human hepatoma cell lines HepG2 and Hep3B were cultured in a DMEM medium supplemented with 10 % v/v FCS, 2 mmol/L l-glutamine, streptomycin (100 μg/ml) and penicillin (100 IU/ml) in a humidified atmosphere containing 5 % CO2 at 37 °C. The cells were passaged at a split ratio of 1:3 every 2–3 days. One day before the experiments, cells were seeded on 96-well microtitre plates (Sarstedt, Nümbrecht, Germany) at 2 × 105 cells/ml. The cells were treated with 0.1 ml each of 0.1, 0.2, 0.3 and 0.4 % (v/v) CCl4 dissolved in 0.25 % DMSO in a serum-free DMEM for optimizing the most toxic dosage of CCl4. According to the result of CCl4 dosage, another setup was designed. Cells were seeded again on 96-well microtitre plates at 2 × 105 cells/ml. The groups were arranged into eight groups for both cell lines; Control (DMEM), sham control (0.01 % PBS), melatonin (MEL) (10−8 M MEL dissolved in 0.01 % PBS) (Bonior et al. 2005), Vitamin D [2.5 × 10−6 M 1.25(OH)2D3] (Abramowitch et al. 2011), DMSO (0.25 %), CCl4 (dissolved in 0.25 % DMSO), CCl4 + MEL and CCl4 + Vitamin D. All experiments were repeated 3 times for both HepG2 and Hep3B cells.
Evaluation of cellular proliferation or death
MTT, a colorimetric assay based upon the ability of living cells to reduce MTT into formazan, was used for the evaluation of the effects of CCl4, melatonin and 1.25(OH)2 D3 on cell death or proliferation (at the 2nd, 24th, 48th h). Cell number % was calculated as the ratio of the cell number of the effected group vs control group (×100) at the pre-determined hour.
Determination of lipid peroxidation
Malondialdehyde (MDA), the end product of lipid peroxidation (LPO), was calculated using thio barbituric acid reactive substance (TBARS) assay with some modifications (Ohkawa et al. 1979). After 48 h of exposure, medium was aspirated; cells were trypsinized, suspended in 0.5 ml of PBS and sonicated for 10 s. To this 0.5 ml of TCA–TBA reagent was added and heated at 100 °C for 1 h. Then it was rapidly cooled in ice bath and centrifuged. The extent of LPO was quantified by computing the levels of MDA. The absorbance of color developed using 1,1,3,3-tetramethoxypropane as an external standard was calculated at 535 nm. The results were expressed as nmole MDA equivalent formed/mg protein at 37 °C.
Measurement of glutathione levels
Total glutathione level was calculated by DTNB-GSSG reductase recycling assay method (Buege and Aust 1978). After 48 h of exposure, cells were washed twice with the cooled PBS. 100 ml of 5 % (w/v) sulfosalicylic acid was added and the plate was left on ice for 10 min. Cell suspension was transferred to microtube and centrifuged at 13,000g at 4 °C for 5 min. For total GSH observation, 20 ml of supernatant and 80 ml of 1 mM EDTA in 0.1 M PBS (pH7.5) was added in each well of 96-well plate. Next, 100 ml of reaction mixture (0.15 mM 5,5′-dithio-bis-2-nitrobenzoic acid, 0.2 mM NADPH, 1U GSH reductase) was added. Absorbance of yellow product in the well was measured at a wave length of 405 nm using the microplate reader at 30 s intervals for 10 min. The total glutathione level was determined by the kinetic method from standard curve of reduced glutathione (GSH). The results were expressed as nmole GSH per mg of protein.
Statistical analysis
Results of the experiments were analyzed by Kruskal–Wallis followed by a multiple comparison test using SPSS 20.0. The Chi square test was implemented in relation of the categorical variable and the disparity between the groups. p < 0.05 was accepted as statistically significant. Results are given as mean ± SEM.
Results
Toxicity of CCl4
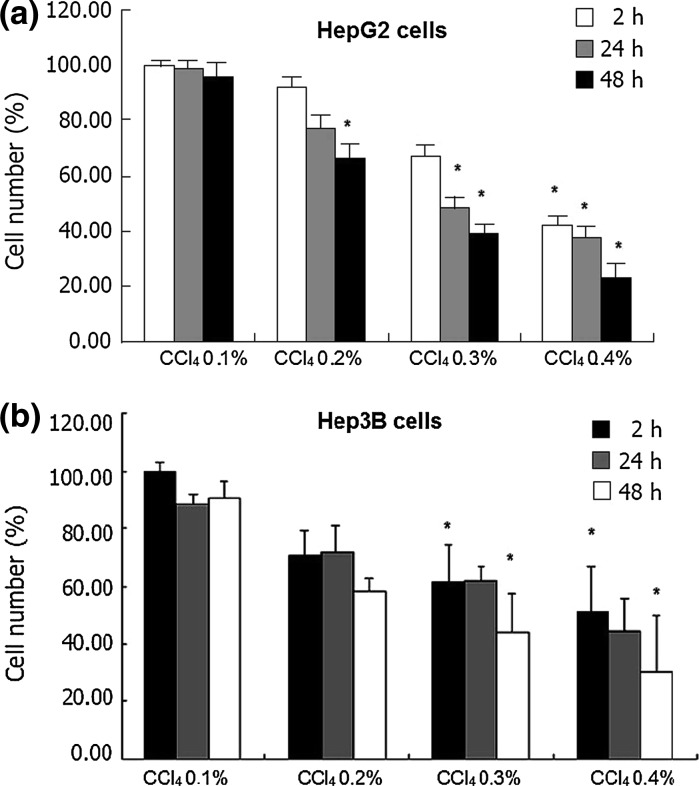
We designated the concentration-dependent cytotoxic effect of CCl4 on human cell lines, HepG2 and Hep3B as a function of time. CCl4 treatment diminished living cell number depending on the dosage. At the 0.1 % CCl4 dosage in HepG2 cells, a minimal cytotoxic (<4 %) effect was determined. As shown in Fig. 1a, cell exposure to 0.4 % CCl4 for up to 2 h only mildly affected cell viability as revealed by MTT measurements. However, compared to control group values calculated in untreated cells, it became apparent at the 24th and 48th h. Like HepG2 cells, Hep3B cells were affected from CCl4 exposure in a dose and time dependent manner (p < 0.05, Fig. 1b).
Fig. 1.
Cell death (%) was determined at the 2nd, 24th and 48th h by the MTT assay. CCl4 exposure induced prominent cell death in liver cell lines in a dose and time dependent manner. Data are from six independent experiments for each condition. a 0.4 % exposure of CCl4 affected HepG2 cell viability the most. b Reduction of Hep3B cell number was prominent at 0.4 % CCl4 dosage. Data are presented as mean ± SEM. *p < 0.05 versus control group
According to these data, 0.4 % CCl4 concentration with a high toxic impact was chosen for showing the effects of melatonin and 1.25(OH)2 D3 on cell viability of HepG2 and Hep3B cells.
Cell viability of HepG2 and Hep3B cells
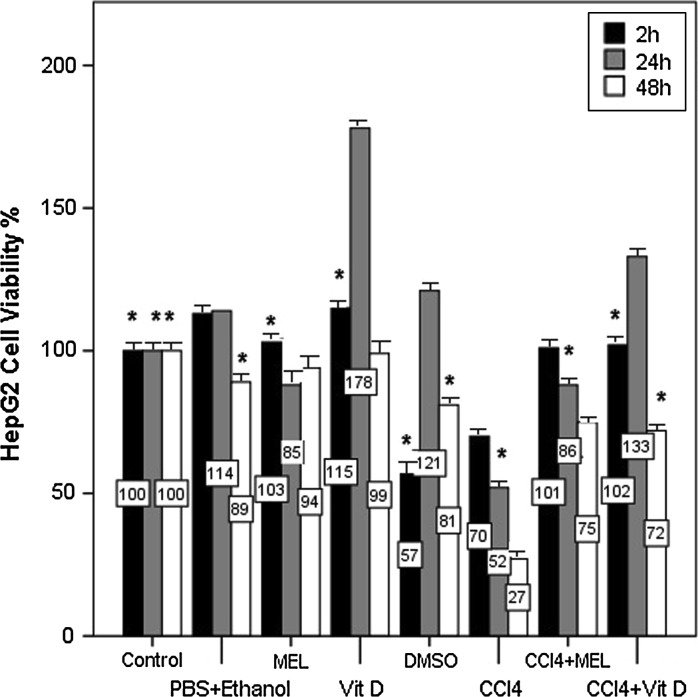
The cell viability ratio of the control group was accepted as 100 %. Other groups were compared to the control groups. Sham control and DMSO group were the solvents of MEL and CCl4 and these groups were planned to show if they had any effect on cell proliferation. As a result they did not exhibit remarkable alteration on cell number in HepG2 cells (p < 0.05, Fig. 2). The MEL group showed its best impact on cell number at the 2nd h. Compared to the MEL group, the Vitamin D group presented the highest potency on cell number (178 %) at the 24th h. CCl4 decreased the cell number in a time dependent manner and at the 48th h a few cells were left (27 %). In the MEL + CCl4 group the cell viability was protected in a time dependent manner. Even at the 48th h, the percentage of cell number was 75 %. Cell viability percentage decreased uniformly in the MEL + CCl4 group as time passed; while the Vitamin D + CCl4 group did not exhibit such a characteristic. The Vitamin D + CCl4 group showed its highest efficiency on cell proliferation at the 24th h similar to Vitamin D group (p < 0.05, Fig. 2).
Fig. 2.
Cell number (%) was determined by MTT assay following 2, 24, and 48 h of exposure of MEL and Vitamin D alone or with CCl4. The percentages of the cell number were shown on the bars of the graphic. Decrease in cell number as a result of CCl4 exposure in HepG2 cells was assessed. Supplementation of MEL and Vitamin D to CCl4 almost protected the cell viability in a time dependent manner. Increase in cell number of Vitamin D alone and with CCl4 groups compared to the control group at 24 h was prominent. Data are presented as mean ± SEM. *p < 0.05 versus control group
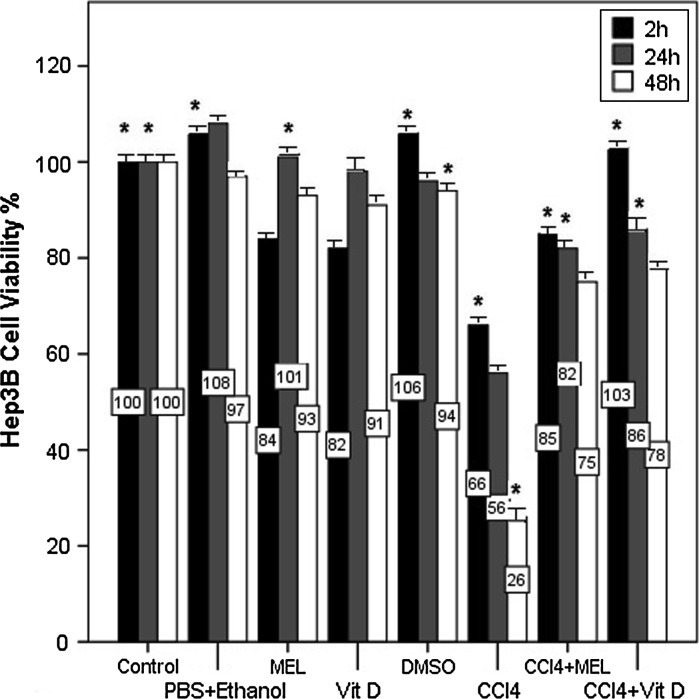
In Hep3B cells, sham control and DMSO groups exhibited a cell number ratio similar to the control group (p < 0.05, Fig. 3). The cell numbers of MEL and Vitamin D group were determined akin to the control group and the greatest impact of the cell number percentage of both groups were found at the 24th h. CCl4 exposure diminished cell viability of Hep3B cells in a time dependent manner and at the 48th h it was 26 %. Compared to the CCl4 group, the MEL + CCl4 group of Hep3B liver cells exhibited higher cell viability ratios. The same protection against CCl4 cytotoxicity was determined in the Vitamin D + CCl4 group. Even at the 48th h the cell number ratio of this group was found 78 % (p < 0.05, Fig. 3).
Fig. 3.
The cell number (%) of MEL and Vitamin D on CCl4 exposure in Hep3B liver cells by MTT assay on the 2nd, 24th and 48th h of exposure. The percentages of the cell number in MEL and Vitamin D treatment alone were similar to the control group. Hep3B cell number was diminished after CCl4 exposure. MEL and Vitamin D treatment with CCl4 increased cell proliferation in Hep3B liver cells in a time dependent manner. Data are presented as mean ± SEM. *p < 0.05 versus control group
Lipid peroxidation and antioxidant activity
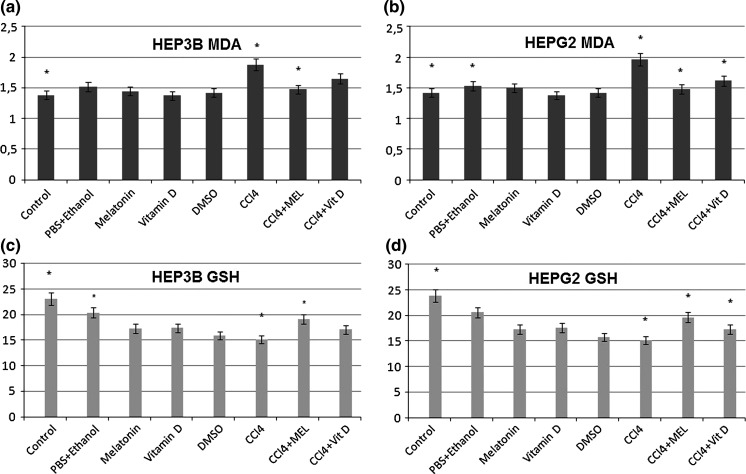
Between the results of the groups in both HepG2 and Hep3B cells the highest values for LPO were observed in the CCl4 groups (p < 0.05, Table 1). The increment in MDA was found 38 % in HepG2 cells and 32 % in Hep3B cells. In the MEL + CCl4 groups LPO was found lower than in the Vitamin D + CCl4 groups in both cell lines. Similarly, as a result of CCl4 exposure, a significant (p < 0.05) increase in LPO with concurrent, remarkable decrease in glutathione levels were noted compared to the control groups of both cells (p < 0.05, Table 2). The levels of GSH in the MEL and Vitamin D alone or with CCl4 group showed similarity to the control groups.
Table 1.
MDA levels in HepG2 and Hep3B cell lines
| Groups | HepG2 | Hep3B |
|---|---|---|
| 1. Control | 1.42 ± 0.19a | 1.39 ± 0.05a |
| 2. PBS + Ethanol | 1.53 ± 0.06a | 1.51 ± 0.04 |
| 3. MEL | 1.50 ± 0.23 | 1.44 ± 0.06 |
| 4. Vit D | 1.39 ± 0.10 | 1.37 ± 0.02 |
| 5. DMSO | 1.42 ± 0.04 | 1.42 ± 0.03 |
| 6. CCl4 | 1.96 ± 0.18a | 1.88 ± 0.20a |
| 7. CCl4 + MEL | 1.48 ± 0.08a | 1.47 ± 0.05a |
| 8. CCl4 + Vit D | 1.61 ± 0.14a | 1.51 ± 0.18 |
Data are expressed as means ± standard errors of the means (SEM)
a p < 0.05 versus CCl4
Table 2.
GSH activities in HepG2 and Hep3B cell lines
| Groups | HepG2 | Hep3B |
|---|---|---|
| 1. Control | 23.88 ± 2.82a | 23.09 ± 3.11a |
| 2. PBS + Ethanol | 20.57 ± 2.35 | 20.40 ± 1.37a |
| 3. MEL | 19.60 ± 0.41 | 19.11 ± 0.81 |
| 4. Vit D | 17.61 ± 2.71 | 17.36 ± 3.43 |
| 5. DMSO | 16.70 ± 2.01 | 17.01 ± 1.28 |
| 6. CCl4 | 15.09 ± 0.29a | 15.08 ± 0.45a |
| 7. CCl4 + MEL | 17.47 ± 1.19a | 17.30 ± 0.51a |
| 8. CCl4 + Vit D | 17.26 ± 0.14a | 17.07 ± 1.94 |
Data are expressed as means ± standard errors of the means (SEM)
a p < 0.05 versus CCl4
The chi-square test was applied to determine whether there is a significant association between the groups. Analysis of MDA values gave the following results: The relation between the control and CCl4 groups (p = 0.007), CCl4 and CCl4 + MEL groups (p = 0.021), in Hep3B cells (Fig. 4a) and the control and CCl4 groups (p = 0.028), PBS + Ethanol and CCl4 groups (p = 0.034), CCl4 and CCl4 + MEL groups (p = 0.004), CCl4 and CCl4 + Vitamin D groups (p = 0.047) in HepG2 cells (Fig. 4b) were found to be statistically significant. Analysis of the GSH values gave the following results: The relation between the control and CCl4 groups (p = 0.000), PBS + Ethanol and CCl4 groups (p = 0.002), CCl4 and CCl4 + MEL groups (p = 0.010) in Hep3B cells (Fig. 4c) and the control and CCl4 groups (p = 0.012), CCl4 and CCl4 + MEL groups (p = 0.034), CCl4 and CCl4 + Vit D groups (p = 0.018) in HepG2 cells (Fig. 4d) were found to be statistically significant.
Fig. 4.
The discrepancy between groups was evaluated. a MDA values of HEP3B cells are shown. b The variability of MDA content was determined in HepG2 cells. c GSH content of HEP3B cells are shown. d The date of GSH in HepG2 cells showed parallel results with the data of HEP3B cells. Data are presented as mean ± SEM. *p < 0.05 versus CCl4 value was accepted as statistically significant (versus CCL4)
Discussion
The HepG2 cell line is a preferential model for studying liver toxicity and the metabolism of xenobiotics because it has similar properties to normal human hepatocytes (Krithika et al. 2009). The Hep3B cell line has also been shown to display preserved parenchymal cell morphology (Knowles et al. 1980; Zannis et al. 1981). Therefore we preferred to study the cytoprotective effects of melatonin and 1.25(OH)2D3 on the cell viability of these cell lines.
The experimental hepatotoxicity induced by CCl4 starts with LPO and results in tissue damage (Marques et al. 2012). To see the true toxicity time, we tried different dosages of CCl4 and determined the highest toxic value of it. Similarly to our study, CCl4 exposure caused remarkable loss of cell viability (Harries et al. 2001; Krithika et al. 2012), increase in LPO (Krithika et al. 2009) and simultaneously attenuation of GSH (Krithika et al. 2012) in HepG2 cells.
It has been determined that the antioxidants reduce cytotoxicity (Kim et al. 2001; Martin et al. 2008). Melatonin is one of the widely used antioxidants for its hepatoprotective effects (Pan et al. 2006; Subramanian et al. 2007; Tahan et al. 2009). Low melatonin levels are connected with high cancer risk (Maestroni and Conti 1996) and the inhibition of cell proliferation depended on treatment time and dosage (Martín-Renedo et al. 2008; Carbajo-Pescador et al. 2009). It has been shown that in vitro, melatonin inhibits cell growth in prostate, breast and colon cancer cells, as well as in glioma, melanoma, and hepatoma cell lines (Cos et al. 2001; Martín et al. 2007; Carbajo-Pescador et al. 2009; Fan et al. 2010; Proietti et al. 2011). In breast cancer cells, it has been noted that 10−9 M melatonin raised the fraction of cells in G1 and reduced the number of cells in the S phase of the cell cycle after 5 days of incubation (Cos et al. 2001). When the same dosages of melatonin were given to prostate cancer cells, increases in cell viability in the G0/G1 phase and decrease in the cell population were found in the S phase after 7 days of incubation time (Marelli et al. 2000). As a result of high concentration of melatonin exposure to human melanoma cells led to the reduction of cell viability (Cabrera et al. 2010). Contrary to these results, after administration of melatonin at a low, physiological concentration over <5 days, remarkable increases in cell number were observed (Cid et al. 2012). Similar to our results, melatonin triggered the proliferation of neural stem cells (NSCs) from fetal mouse brain in a time and dose dependent manner (Moriya et al. 2007). These different studies with melatonin on cell proliferation, and the existing results point out that this neurohormone exerts in HepG2 a biphasic, non-linear impact, according to its concentration and the length of the treatment. Melatonin is also responsible for protecting the intracellular level of GSH; and shows antioxidant properties both in vitro (Vijayalaxmi et al. 1995; Gulcin et al. 2002) and in vivo (Vijayalaxmi et al. 1996; Kaya et al. 1999; El-Missiry et al. 2007) against LPO. Our results demonstrated congruity to this.
Vitamin D plays a significant role in cell proliferation, differentiation and apoptosis in many normal and malignant cells (DeLuca 2008; Plum and DeLuca 2009). Lutzow-Hollow and colleagues showed that administration of calcitriol to mouse skin induced proliferation (Lutzow-Holm et al. 1993). Proliferation of cultured keratinocytes is increased at low concentrations of 1.25-dihydroxyvitamin D (Bollag et al. 1995). HepG2 and Hep3B cell lines are convenient in vitro models for studying the effects of physiological factors on the 25-hydroxylation of Vitamin D3 (Tam et al. 1988). Accordingly, HepG2 and Hep3B cells were used in this study to investigate the effects of 1.25(OH)2D3 on CCl4 cytotoxicity. Especially through these data it is clear that, individually administered melatonin and Vitamin D revealed its highest protective effect on cell viability at the 24th h of incubation time in HepG2 cells. In contrast, there have been several studies about the anti-proliferative effects of Vitamin D in cancer cells. 10−8 mol/L 1.25(OH)2D3 inhibited the cell proliferation of HepG2 cells in 5 weeks of incubation time (Akhter et al. 2001). The treatment of human breast cancer cells (T 47D) with 10−9 to 10−8 M 1.25(OH)2D3 resulted in a dose- and time-dependent decline in cell numbers over 6 days (Eisman et al. 1989). Protective effects of Vitamin D from oxidative stress (Sezgin et al. 2013; Uberti et al. 2014) and increment in GSH content were observed (Sezgin et al. 2013; Jain and Micinski, 2013).
As a conclusion, the highest toxic degree of CCl4 on HepG2 and Hep3B cells was determined. Afterwards, melatonin and Vitamin D3 were given to the hepatic cell lines. It was observed that both of them protected the cells from oxidative stress. Therefore, the beneficial effects of exogenous melatonin and Vitamin D to diminish CCl4-induced damage would provide a new perspective for innovation in the treatment of CCl4 intoxications.
Acknowledgments
This study was supported by Ankara University Research Foundation, Ankara Turkey (Project Number: BAP12B4240012). We are indebted to Gulizar Aydogdu, Biotechnology Institute of Ankara University, for helping in the experiment protocol.
Contributor Information
Dilşad Özerkan, Phone: +903662801956, Email: dilsadokan@gmail.com.
Nesrin Özsoy, Phone: +903122126720/1067, Email: ozsoy@science.ankara.edu.tr.
Erkan Yılmaz, Phone: +90312 2225826/200, Email: eyilmaz@ankara.edu.tr.
References
- Abramovitch S, Dahan-Bachar L, Sharvit E, Weisman Y, Ben Tov A, Brazowski E, Reif S. Vitamin D inhibits proliferation and profibrotic marker expression in hepatic stellate cells and decreases thioacetamide-induced liver fibrosis in rats. Gut. 2011;60:1728–1737. doi: 10.1136/gut.2010.234666. [DOI] [PubMed] [Google Scholar]
- Agency for Toxic Substances and Disease Registry (ATSDR) Toxicological profile for carbon tetrachloride. Atlanta, GA: U.S. Department of Health and Human Services, Public Health Service; 2005. [PubMed] [Google Scholar]
- Akhter J, Lu Y, Finlay I, Pourgholami MH, Morris DL. 1α,25(OH)2D3 and its analogues, EB1089 and CB1093, profoundly inhibit the in vitro proliferation of the human hepatoblastoma cell line HepG2. ANZ J Surg. 2001;71:414–417. doi: 10.1046/j.1440-1622.2001.02147.x. [DOI] [PubMed] [Google Scholar]
- Binderup L, Bramm E. Effects of a novel vitamin D analogue MC 903 on cell proliferation and differentiation in vitro and on calcium metabolism in vivo. Biochem Pharmacol. 1988;37:889–895. doi: 10.1016/0006-2952(88)90177-3. [DOI] [PubMed] [Google Scholar]
- Bollag WB, Ducote J, Harmon CS. Biphasic effect of 1,25-dihyroxyvitamin D3 on primary mouse epidermal keratinocyte proliferation. J Cell Physiol. 1995;163:248–256. doi: 10.1002/jcp.1041630205. [DOI] [PubMed] [Google Scholar]
- Bonior J, Jaworek J, Konturek SJ, Pawlik WW. Increase of heat shock protein gene expression by melatonin in AR42J cells. J Physiol Pharmacol. 2005;56:471–481. [PubMed] [Google Scholar]
- Buege J, Aust S. Microsomal lipid peroxidation. Methods Enzymol. 1978;52:302–310. doi: 10.1016/S0076-6879(78)52032-6. [DOI] [PubMed] [Google Scholar]
- Cabrera J, Negrín G, Estévez F, Loro J, Reiter RJ, Quintana J. Melatonin decreases cell proliferation and induces melanogenesis in human melanoma SK-MEL-1 cells. J Pineal Res. 2010;49:45–54. doi: 10.1111/j.1600-079X.2010.00765.x. [DOI] [PubMed] [Google Scholar]
- Carbajo-Pescador S, Martín-Renedo J, García-Palomo A, Tuñón MJ, Mauriz JL, González-Gallego J. Changes in the expression of melatonin receptors induced by melatonin treatment in hepatocarcinoma HepG2 cells. J Pineal Res. 2009;47:330–338. doi: 10.1111/j.1600-079X.2009.00719.x. [DOI] [PubMed] [Google Scholar]
- Cid MA, Ubeda A, Hernández-Bule ML, Martínez MA, Trillo MÁ. Antagonistic effects of a 50 Hz magnetic field and melatonin in the proliferation and differentiation of hepatocarcinoma cells. Cell Physiol Biochem. 2012;30:1502–1516. doi: 10.1159/000343338. [DOI] [PubMed] [Google Scholar]
- Cos S, Garcia-Bolado A, Sánchez-Barceló EJ. Direct antiproliferative effects of melatonin on two metastatic cell sublines of mouse melanoma (B16BL6 and PG19) Melanoma Res. 2001;11:197–201. doi: 10.1097/00008390-200104000-00016. [DOI] [PubMed] [Google Scholar]
- DeLuca HF (2008) Evolution of our understanding of vitamin D. Nutr Rev 66 :573e8 [DOI] [PubMed]
- Ding J, Yu J, Wang C, Hu W, Li D, Luo Y, Luo H, Yu H. Ginkgo biloba extract alleviates liver fibrosis induced by CCl4 in rats. Liver Int. 2005;25:1224–1232. doi: 10.1111/j.1478-3231.2005.01169.x. [DOI] [PubMed] [Google Scholar]
- Eisman JA, Koga M, Sutherland RL, Barkla DH, Tutton PJM. 1,25-Dihydroxyvitamin D3 and the regulation of human cancer cell replication. Proc Soc Exp Biol Med. 1989;191:221–226. doi: 10.3181/00379727-191-42912. [DOI] [PubMed] [Google Scholar]
- El-Missiry MA, Fayed TA, El-Sawy MR, El-Sayed AA. Ameliorative effect of melatonin against gamma-irradiation-induced oxidative stress and tissue injury. Ecotoxicol Environ Saf. 2007;66:278–286. doi: 10.1016/j.ecoenv.2006.03.008. [DOI] [PubMed] [Google Scholar]
- Fan LL, Sun GP, Wei W, Wang ZG, Ge L, Fu WZ, Wang H. Melatonin and doxorubicin synergistically induce cell apoptosis in human hepatoma cell lines. World J Gastroenterol. 2010;16:1473–1481. doi: 10.3748/wjg.v16.i12.1473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gulcin I, Buyukokuroglu ME, Oktay M, Kufrevioglu OI. On the in vitro antioxidative properties of melatonin. J Pineal Res. 2002;33:167–171. doi: 10.1034/j.1600-079X.2002.20920.x. [DOI] [PubMed] [Google Scholar]
- Harries HM, Fletcher ST, Duggan CM, Baker VA. The use of genomic technology to investigate gene expression changes in cultured human liver cells. Toxicol In Vitro. 2001;15:399–405. doi: 10.1016/S0887-2333(01)00043-1. [DOI] [PubMed] [Google Scholar]
- Hong RT, Xu JM, Mei Q. Melatonin ameliorates experimental hepatic fibrosis induced by carbon tetrachloride in rats. World J Gastroenterol. 2009;15:1452–1458. doi: 10.3748/wjg.15.1452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jain SK, Micinski D (2013) Vitamin D upregulates glutamate cysteine ligase and glutathione reductase, and GSH formation, and decreases ROS and MCP-1 and IL-8 secretion in high-glucose exposed U937 monocytes. Biochem Biophys Res Commun19:437:7-11 [DOI] [PMC free article] [PubMed]
- Kaya H, Oral B, Ozguner F, Tahan V, Babar Y, Delibas N. The effect of melatonin application on lipid peroxidation during cyclophosphamide therapy in female rats. Zentralbl Gynakol. 1999;12:499–502. [PubMed] [Google Scholar]
- Kim SY, Kim RH, Huh TL, Park JW. Alpha-phenyl-N-t-butylnitrone protects oxidative damage to HepG2 cells. J Biochem Mol Biol. 2001;34:43–46. [Google Scholar]
- Knowles BB, Howe CC, Aden DP. Human hepatocellular carcinoma cell lines secrete the major plasma proteins and hepatitis B surface antigen. Science. 1980;209:497–499. doi: 10.1126/science.6248960. [DOI] [PubMed] [Google Scholar]
- Kragballe K. Vitamin D analogues in the treatment of psoriasis. J Cell Biochem. 1992;49:46–52. doi: 10.1002/jcb.240490109. [DOI] [PubMed] [Google Scholar]
- Krithika R, Mohankumarb R, Vermaa RJ, Shrivastavc PS, Mohamadd IM, Gunasekarand S, Narasimhanb S. Isolation, characterization and antioxidative effect of phyllanthin against CCl4-induced toxicity in HepG2 cell line. Chem-Biol Interact. 2009;181:351–358. doi: 10.1016/j.cbi.2009.06.014. [DOI] [PubMed] [Google Scholar]
- Krithika R, Verma RJ, Shrivastav PS. Antioxidative and cytoprotective effects of andrographolide against CCl4-induced hepatotoxicity in HepG2 cells. Hum Exp Toxicol. 2012;32:530–543. doi: 10.1177/0960327112459530. [DOI] [PubMed] [Google Scholar]
- Liu JY, Chen CC, Wang WH, Hsu JD, Yang MY, Wang CJ. The protective effects of Hibiscus sabdariffa extract on CCl4-induced liver fibrosis in rats. Food Chem Toxicol. 2006;44:336–343. doi: 10.1016/j.fct.2005.08.003. [DOI] [PubMed] [Google Scholar]
- Lutzow-Holm C, De Angelis P, Grosvik H, Clausen OP. 1,25-Dihyroxyvitamin D3 and the vitamin D analogue KH1060 induce hyperproliferation in normal mouse epidermis. A BrdUrd/DNA flow cytometric study. Exp Dermatol. 1993;2:113–120. doi: 10.1111/j.1600-0625.1993.tb00018.x. [DOI] [PubMed] [Google Scholar]
- Maestroni GJ, Conti A. Melatonin in human breast cancer tissue: association with nuclear grade and estrogen receptor status. Lab Invest. 1996;75:557–561. [PubMed] [Google Scholar]
- Marelli MM, Limonta P, Maggi R, Motta M, Marelli RM. Growth-inhibitory activity of melatonin on human androgen-independent DU 145 prostate cancer cells. Prostate. 2000;45:238–244. doi: 10.1002/1097-0045(20001101)45:3<238::AID-PROS6>3.0.CO;2-W. [DOI] [PubMed] [Google Scholar]
- Marques TG, Chaib E, da Fonseca JH, Lourenço AC, Silva FD, Ribeiro MA Jr, Galvão FH, D'Albuquerque LA (2012) Review of experimental models for inducing hepatic cirrhosis by bile duct ligation and carbon tetrachloride injection. Acta Cir Bras 27:589–594 [DOI] [PubMed]
- Martín V, Herrera F, García-Santos G, Antolín I, Rodriguez-Blanco J, Medina M, Rodriguez C. Involvement of protein kinase C in melatonin’s oncostatic effect in C6 glioma cells. J Pineal Res. 2007;43:239–244. doi: 10.1111/j.1600-079X.2007.00468.x. [DOI] [PubMed] [Google Scholar]
- Martín-Renedo J, Mauriz JL, Jorquera F, Ruiz-Andrés O, González P, González-Gallego J. Melatonin induces cell cycle arrest and apoptosis in hepatocarcinoma HepG2 cell line. J Pineal Res. 2008;45:532–540. doi: 10.1111/j.1600-079X.2008.00641.x. [DOI] [PubMed] [Google Scholar]
- Masuda S, Byford V, Kremer R. In vitro metabolism of the vitamin D analog, 22-oxacalcitriol, using culture osteosarcoma, hepatoma and keratinocyte cell lines. J Biol Chem. 1996;271:8700–8718. doi: 10.1074/jbc.271.15.8700. [DOI] [PubMed] [Google Scholar]
- Moriya T, Horie N, Mitome M, Shinohara K. Melatonin influences the proliferative and differentiative activity of neural stem cells. J Pineal Res. 2007;42:411–418. doi: 10.1111/j.1600-079X.2007.00435.x. [DOI] [PubMed] [Google Scholar]
- Martin MA, Ramos S, Mateos R, Granado Serrano AB, Izquierdo-Pulido M, Bravo L, Goya L. Protection of human HepG2 cells against oxidative stress by cocoa phenolic extract. J Agric Food Chem. 2008;56:7765–7772. doi: 10.1021/jf801744r. [DOI] [PubMed] [Google Scholar]
- Ohkawa H, Ohishi N, Yagi K. Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Analytic Biochem. 1979;95:351–358. doi: 10.1016/0003-2697(79)90738-3. [DOI] [PubMed] [Google Scholar]
- Pan M, Song YL, Xu JM, Gan HZ. Melatonin ameliorates nonalcoholic fatty liver induced by high-fat diet in rats. J Pineal Res. 2006;41:79–84. doi: 10.1111/j.1600-079X.2006.00346.x. [DOI] [PubMed] [Google Scholar]
- Plum LA, DeLuca HF. The functional metabolism and molecular biology of vitamin D action. In: Holick MF, editor. Vitamin D: Physiology, Molecular Biology and Clinical Applications. 2. Totowa, NJ: The Humana Press; 2009. [Google Scholar]
- Proietti S, Cucina A, D’Anselmi F, Dinicola S, Pasqualato A, Lisi E, Bizzarri M. Melatonin and vitamin D3 synergistically down-regulate Akt and MDM2 leading to TGFβ-1-dependent growth inhibition of breast cancer cells. J Pineal Res. 2011;50:150–158. doi: 10.1111/j.1600-079X.2010.00824.x. [DOI] [PubMed] [Google Scholar]
- Sezgin G, Oztürk G, Güney S, Sinanoğlu O, Tunçdemir M. Protective effect of melatonin and 1,25-dihydroxyvitamin D3 on renal ischemia-reperfusion injury in rats. Ren Fail. 2013;35:374–379. doi: 10.3109/0886022X.2012.760409. [DOI] [PubMed] [Google Scholar]
- Subramanian P, Mirunalini S, Pandi-Perumal SR, Trakht I, Cardinali DP. Melatonin treatment improves the antioxidant status and decreases lipid content in brain and liver of rats. Eur J Pharmacol. 2007;571:116–119. doi: 10.1016/j.ejphar.2007.06.011. [DOI] [PubMed] [Google Scholar]
- Tahan V, Atug O, Akin H, Eren F, Tahan G, Tarcin O, Uzun H, Ozdogan O, Tarcin O, Imeryuz N, Ozguner F, Celikel C, Avsar E, Tozun N. Melatonin ameliorates methionine- and choline-deficient diet-induced nonalcoholic steatohepatitis in rats. J Pineal Res. 2009;46:401–407. doi: 10.1111/j.1600-079X.2009.00676.x. [DOI] [PubMed] [Google Scholar]
- Tam SP, Strugnell S, Deeley RG, Jones G. 25-Hydroxylation of vitamin D3 in the human hepatoma cell lines Hep G2 and Hep3B. J Lipid Res. 1988;29:1637–1642. [PubMed] [Google Scholar]
- Thrall KD, Vucelick ME, Gies RA, Benson JM. Comparative metabolism of carbon tetrachloride in rats, mice, and hamsters using gas uptake and PBPK modeling. J Toxicol Environ Health A. 2000;60:531–548. doi: 10.1080/00984100050082085. [DOI] [PubMed] [Google Scholar]
- Uberti F, Lattuada D, Morsanuto V, Nava U, Bolis G, Vacca G, Squarzanti DF, Cisari C, Molinari C (2014) Vitamin D protects human endothelial cells from oxidative stress through the autophagic and survival pathways. J Clin Endocrinol Metab 99:1367–1374 [DOI] [PubMed]
- Vijayalaxmi, Reiter RJ, Meltz ML. Melatonin protects human blood lymphocytes from radiation induced chromosome damage. Mutat Res. 1995;34:623–631. doi: 10.1016/0165-7992(95)90065-9. [DOI] [PubMed] [Google Scholar]
- Vijayalaxmi, Reiter RJ, Herman TS, Meltz ML. Melatonin and radioprotection from genetic damage: in vivo/in vitro studies with human volunteers. Mutat Res. 1996;37:221–228. doi: 10.1016/S0165-1218(96)90110-X. [DOI] [PubMed] [Google Scholar]
- Wang H, Wei W, Wang NP, Gui SY, Wu L, Sun WY, Xu SY. Melatonin ameliorates carbon tetrachloride-induced hepatic fibrogenesis in rats via inhibition of oxidative stress. Life Sci. 2005;77:1902–1915. doi: 10.1016/j.lfs.2005.04.013. [DOI] [PubMed] [Google Scholar]
- Zannis VI, Breslow JL, SanGiacomo TR, Aden DP, Knowles BB. Characterization of the major apolipoproteins secreted by two human hepatoma cell lines. Biochemistry. 1981;20:7089–7096. doi: 10.1021/bi00528a006. [DOI] [PubMed] [Google Scholar]