Abstract
Cardiovascular disease is the primary cause of morbidity and mortality in diabetes, and endothelial dysfunction is commonly seen in these patients. Increased O-linked N-acetylglucosamine (O-GlcNAc) protein modification is one of the central pathogenic features of diabetes. Modification of proteins by O-GlcNAc (O-GlcNAcylation) is regulated by two key enzymes: β-N-acetylglucosaminidase [O-GlcNAcase (OGA)], which catalyzes the reduction of protein O-GlcNAcylation, and O-GlcNAc transferase (OGT), which induces O-GlcNAcylation. However, it is not known whether reducing O-GlcNAcylation can improve endothelial dysfunction in diabetes. To examine the effect of endothelium-specific OGA overexpression on protein O-GlcNAcylation and coronary endothelial function in diabetic mice, we generated tetracycline-inducible, endothelium-specific OGA transgenic mice, and induced OGA by doxycycline administration in streptozotocin-induced type 1 diabetic mice. OGA protein expression was significantly decreased in mouse coronary endothelial cells (MCECs) isolated from diabetic mice compared with control MCECs, whereas OGT protein level was markedly increased. The level of protein O-GlcNAcylation was increased in diabetic compared with control mice, and OGA overexpression significantly decreased the level of protein O-GlcNAcylation in MCECs from diabetic mice. Capillary density in the left ventricle and endothelium-dependent relaxation in coronary arteries were significantly decreased in diabetes, while OGA overexpression increased capillary density to the control level and restored endothelium-dependent relaxation without changing endothelium-independent relaxation. We found that connexin 40 could be the potential target of O-GlcNAcylation that regulates the endothelial functions in diabetes. These data suggest that OGA overexpression in endothelial cells improves endothelial function and may have a beneficial effect on coronary vascular complications in diabetes.
Keywords: diabetic vascular complications, endothelial cell dysfunction, vascular rarefaction, endothelium-dependent hyperpolarization, GCA
endothelial cells (ECs) regulate vascular tone and vascular formation under physiological conditions, and endothelial dysfunction plays an important role in the pathogenesis of vascular complications of diabetes (5). Attenuated coronary flow and vascular rarefaction cause heart failure due to a shortage of vascular supply relative to heart demand. Coronary ECs from diabetic mice were shown to be dysfunctional in the regulation of vascular tone and angiogenesis, as reported by us (22) and others (23). O-linked N-acetylglucosaminylation (O-GlcNAcylation) is a posttranslational modification, and it is closely controlled by enzymatic processes: the enzyme O-linked N-acetylglucosamine (O-GlcNAc) transferase (OGT) has been shown to catalyze the addition of a single N-acetylglucosamine to the serine and threonine residues of nuclear and cytoplasmic proteins (30, 31), while β-N-acetylglucosaminidase (OGA) can cleave this linkage and decrease protein O-GlcNAcylation (11). Hyperglycemia increases glucose flux through the hexosamine biosynthesis pathway and leads to a rise in cellular UDP-GlcNAc and O-GlcNAcylation of proteins. Several proteins prominent in vascular function are targets for O-GlcNAcylation, and increased O-GlcNAcylation interferes with endothelial function (1, 9, 10, 13, 16, 19–21, 29). The most extensively studied protein is endothelial nitric oxide (NO) synthase (eNOS); when eNOS is O-GlcNAcylated, it decreases NO production (1, 9, 16, 19). O-GlcNAcylation of Akt (a regulator of eNOS activity) attenuates endothelial migration and tube formation (21). VEGF-A expression levels are regulated by O-GlcNAcylation of Sp1 (6). However, the relationship between increased O-GlcNAcylation of proteins in diabetes and its influence on coronary endothelial dysfunction in vivo has not been explored. This study was designed to investigate whether hyperglycemia influences the elevation of protein O-GlcNAcylation and whether OGA overexpression is able to reverse coronary endothelial dysfunction in diabetes.1
MATERIALS AND METHODS
Biological materials and reagents.
Antibodies and reagents were purchased as follows: medium 199 (M199), antibiotics, and dispase II from Life Technologies (Grand Island, NY); anti-O-GlcNAc antibody (RL2) from Abcam (Cambridge, MA); anti-OGA from Sigma-Aldrich (St. Louis, MO); anti-OGT from Thermo Fisher Scientific (Waltham, MA); anti-eNOS, anti-connexin 40 (Cx40), and anti-actin from Santa Cruz Biotechnology (Santa Cruz, CA); anti-CD31 and endothelial cell growth supplement from BD Biosciences (San Jose, CA); streptozotocin from ALEXIS Biochemicals (San Diego, CA); collagenase II from Worthington Biochemical (Lakewood, NJ); and O-(2-acetamido-2-deoxy-d-glucopyranosylidene) amino N-phenylcarbamate (PugNAc) from Toronto Research Chemicals. All other chemicals were obtained from Sigma-Aldrich.
Animals.
This study was conducted in accordance with the guidelines established by the Animal Care and Use Committee at the University of Arizona. All animal use was in compliance with all current US government regulations concerning the care and use of laboratory animals. The laboratory personnel who conducted the experiments completed all training required for animal handling and were certified by the Institutional Animal Care and Use Committee. All surgery was performed under anesthesia with a mixture of ketamine (100 mg/kg ip) and xylazine (5 mg/kg ip), and all efforts were made to minimize animal suffering.
Generation of transgenic mice and OGA gene induction.
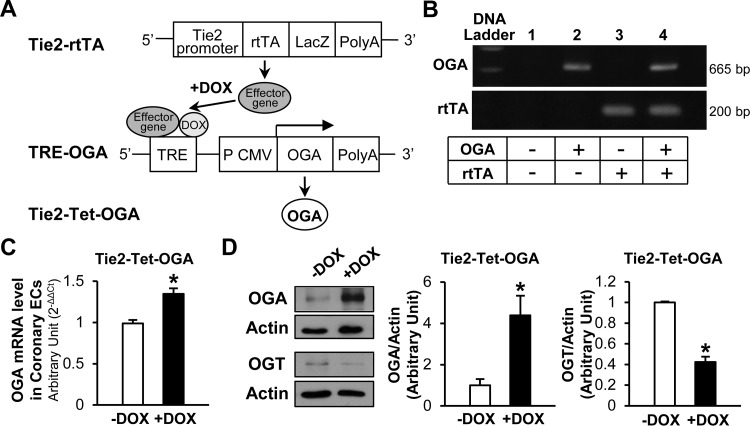
We constructed mice in which a tetracycline (Tet) response element (TRE) regulates transcription of a human OGA cDNA (TRE-OGA). These mice were crossed with transgenic mice in which an EC-specific Tie2 promoter drives expression of a reverse Tet-controlled transactivator (rtTA), termed Tie2-rtTA. Genotyping of transgenic mice was performed by PCR. Animals carrying both Tie2-rtTA and TRE-OGA transgenes, termed Tie2-Tet-OGA, were used for the experiments in Figs. 3, 4 and 5. Doxycycline (Dox) was administered in drinking water (0.2 mg/ml) for 4 wk to induce expression of OGA.
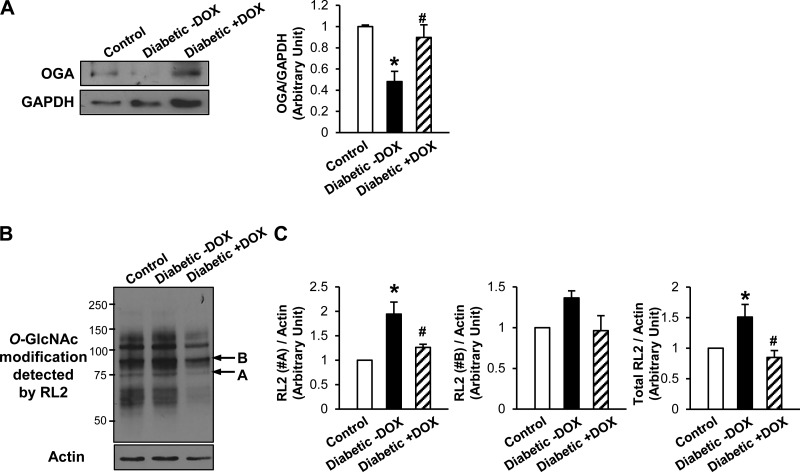
Fig. 3.
Overexpression of OGA significantly decreased protein O-GlcNAcylation in coronary ECs in Tie2-Tet-OGA diabetic mice. A: OGA protein expression levels in MCECs isolated from control, diabetic, and OGA-overexpressing diabetic mice. Values are means ± SE; n = 5 per group. *P < 0.05 vs. control; #P < 0.05 vs. diabetic −Dox. B: typical Western blot image of O-GlcNAcylation pattern detected by RL2 antibody. Arrows A and B indicate proteins that were O-GlcNAcylated in diabetes and whose O-GlcNAcylation levels were decreased by OGA overexpression. C: summarized data of the intensity of the bands with arrows (A and B) and total RL2 signals. Values are means ± SE; n = 6 control, 5 diabetic −Dox, and 6 diabetic +Dox. *P < 0.05 vs. control; #P < 0.05 vs. diabetic −Dox.
Fig. 4.
Overexpression of OGA increased capillary density in the left ventricle (LV) myocardium and restored endothelium-derived hyperpolarization (EDH)-induced relaxation in the coronary arteries in Tie2-Tet-OGA diabetic mice. A: representative capillary images in the LV of control, Dox-treated diabetic, or untreated diabetic heart. Scale bar = 10 μm. B: capillary density [number of capillaries per mm2 (NA/mm2)] in hearts from control, untreated diabetic, and Dox-treated diabetic mice. Values are means ± SE; n = 3 control, 3 untreated diabetic, and 7 Dox-treated diabetic mice. *P < 0.05 vs. control; #P < 0.05 vs. diabetic −Dox. C: endothelium-dependent relaxation by ACh. Coronary artery rings were contracted by treatment with PGF2α, and ACh was administered in a dose-dependent manner. Values are means ± SE; n = 4 per group. *P < 0.05 vs. control; #P <0.05 vs. diabetic −Dox. D: EDH-dependent relaxation induced by ACh in the presence of nitro-l-arginine methyl ester (l-NAME, an eNOS inhibitor, 100 μmol/l) and indomethacin (a cyclooxygenase inhibitor, 10 μmol/l). Values are means ± SE; n = 4 per group. *P < 0.05 vs. control. #P < 0.05 vs. diabetic −Dox. E: sodium nitruprusside (SNP)-induced relaxation (EC-independent). Values are means ± SE; n = 4 per group.
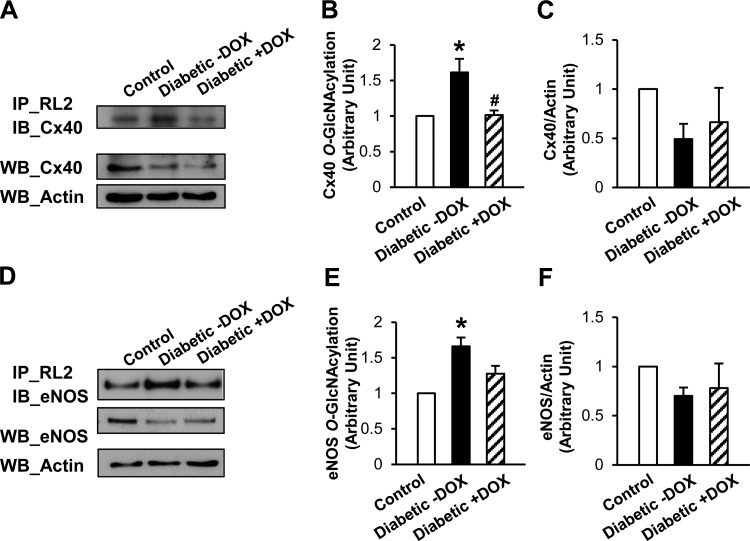
Fig. 5.
Effect of OGA overexpression on connexin 40 (Cx40) protein expression and O-GlcNAcylation in diabetic mice. A: immunoprecipitation (IP) with anti-RL2 antibody and immunoblotting (IB) with anti-Cx40 antibody (top) and Western blots (WB) of Cx40 and actin protein levels (bottom). B: O-GlcNAcylated Cx40 protein levels in MCECs isolated from control, untreated diabetic, and Dox-treated diabetic mice. Values are means ± SE; n = 3 per group. *P < 0.05 vs. control; #P < 0.05 vs. diabetic −Dox. C: Cx40 protein levels in MCECs isolated from control, untreated diabetic, and Dox-treated diabetic mice. Values are means ± SE; n = 4 per group. D: IP with anti-RL2 antibody and IB with anti-eNOS antibody (top) and Western blots of eNOS and actin protein levels (bottom). E: O-GlcNAcylated endothelial nitric oxide synthase (eNOS) protein levels in MCECs isolated from control, untreated diabetic, and Dox-treated diabetic mice. Values are means ± SE; n = 3 per group. *P < 0.05 vs. control. F: eNOS protein levels in MCECs isolated from control, untreated diabetic, and Dox-treated diabetic mice. Values are means ± SE; n = 4 per group.
Preparation of diabetic mice.
Six-week-old male mice were anesthetized and then injected with streptozotocin (133 mg/kg, dissolved in citrate buffer) through the tail vein. Dox treatment was started 6 wk after streptozotocin injection and continued for 4 wk (the duration of diabetes was 10 wk). Plasma glucose levels were 136.3 ± 5.1, 567.8 ± 12.2, and 581.3 ± 9.4 mg/dl for control mice, untreated diabetic mice, and Dox-treated diabetic mice, respectively. Data were obtained from 13–14 mice in each group.
Isolation of mouse coronary ECs.
Mouse coronary ECs (MCECs) were isolated using a method previously described (3, 22). Briefly, dissected heart tissues were minced and incubated with M199 containing 1 mg/ml collagenase II and 0.6 U/ml dispase II for 1 h at 37°C. The digested material was filtered through sterile 40-μm nylon mesh and washed in 2% fetal calf serum-M199. Cells were then incubated with Dynabeads (Invitrogen), which were prepared as follows: beads coated with sheep anti-rat IgG were incubated with purified rat anti-mouse CD31 monoclonal antibody (1 μg/ml) at 4°C overnight and then washed with PBS containing 0.1% BSA and 2 mmol/l EDTA. The cell suspension was incubated with beads for 1 h at 4°C, and then beads attached to ECs were captured by a Dynal magnet (Invitrogen).
Western blot analysis.
Samples were separated using a SDS-polyacrylamide gel and transferred to a nitrocellulose membrane. Blots were incubated with anti-RL2 (1:2,000 dilution), anti-OGA (1:1,000 dilution), anti-OGT (1:2,000 dilution), anti-Cx40 (1:2,000 dilution), anti-eNOS (1:2,000 dilution), or anti-actin (1:4,000 dilution) antibody and then with horseradish peroxidase-linked secondary antibody. The immunoblots were identified with SuperSignal West Pico chemiluminescent substrate (Thermo Fisher Scientific). Band intensity was calculated using ImageJ 1.48v (National Institutes of Health), and intensity data from focus proteins were normalized to actin controls and are expressed in arbitrary units.
Assay of OGA mRNA.
Ten nanograms of cDNA were used in each reaction for real-time PCR using the CFX384 Touch real-time PCR system (Bio-Rad Laboratories, Hercules, CA) (3). Threshold cycle (Ct) values of the target genes were normalized to the endogenous control gene (18S). The primer sequences are as follows: 5′-AAGCTTCTACCTGGAATTGA-3′ (forward) and 5′-GCATGAATGTTATCCCAGAT-3′ (reverse) for OGA and 5′-GTAACCCGTTGAACCCCATT-3′ (forward) and 5′-CCATCCAATCGGTAGTAGCG-3′ (reverse) for 18S. Differential expression in diabetic samples relative to control samples was calculated using the comparative Ct method.
Isometric tension measurement in coronary artery rings.
Isometric tension was measured to evaluate vascular function, as previously described (8, 22). The heart was isolated from the mouse and placed in modified Krebs-Henseleit solution (in mM: 118.0 NaCl, 4.7 KCl, 25.0 NaHCO3, 1.8 CaCl2, 1.2 NaH2PO4, 1.2 MgSO4, and 11.0 glucose) for dissection. Third-order small coronary arteries were used, and adherent connective tissues and cardiac myocytes were removed. Arteries were cut into 1- to 1.5-mm segments. Rings were mounted in a wire myograph over 20-μm wires, set at a resting tension of 0.1 g, and allowed to equilibrate for 45 min with intermittent washes every 15 min. After equilibration, each coronary artery ring was contracted by treatment with PGF2α. The degree of relaxation is shown as percentage of PGF2α-induced contraction.
Analysis of capillary density in left ventricular myocardium.
The heart was dissected, embedded in optimal cutting temperature compound (OCT, Sakura Finetek, Torrance, CA), frozen in 2-methylbutane precooled with liquid nitrogen, and kept at −80°C until they were sectioned (22). Sections (6 μm) were fixed in 4% formaldehyde for 5 min, blocked with 5% BSA for 30 min, and incubated with Bandeiraea simplicifolia lectin-FITC (BS-l) for 30 min. BS-l is used to probe the terminal β-galactosyl saccharides associated with ECs on the surface of arterioles and venules, as well as capillaries. Subepicardial regions of the left ventricle free wall on the section were photographed in sequence by a CCD camera connected to a fluorescence microscope with a ×20 objective lens. For every experimental condition, at least two sections from each sample were examined and at least eight microscopic fields were investigated. Capillary count was analyzed with ImageJ 1.47v. The average capillary numerical density (number of capillaries per mm2) was calculated for each heart.
Detection of O-GlcNAcylated eNOS and Cx40 proteins with immunoprecipitation.
MCECs (from 2–4 hearts/sample) were lysed, and 25 μg of proteins were incubated with ExactaCruz A immunoprecipitation (IP) matrix (Santa Cruz Biotechnology), which was prepared as follows: IP matrix (20 μl) was incubated with anti-RL2 (4 μg) at 4°C for 2 h and then washed with binding buffer. Lysate with IP matrix was incubated overnight at 4°C. The matrix-bound proteins were collected, and samples were processed for immunoblotting using anti-eNOS (1:1,000 dilution) or anti-Cx40 (1:1,000 dilution) antibody.
Dye-transfer assay.
Human coronary ECs were seeded in 12-well plates (105 cells/well). On the following day, the cells were infected with control adenovirus (Adv) or Cx40 Adv (200 plaque-forming units/cell) (22). After 24 h, the cells were washed and treated with high glucose (HG) for 48 h. For HG treatment, 30 mM glucose was added to the medium (final glucose concentration of 35 mM). In a control group of cells, equimolar mannitol was added to exclude the potential effect of changes in osmolality (normal glucose: 5 mM glucose). Some cells were treated with 100 μM PugNAc (an OGA inhibitor) during HG treatment. After treatment, cells were rinsed twice with Hanks' balanced salt solution. Lucifer yellow (0.5 mg/ml) was added to the well, and a scrape was made. Rhodamine-dextran (0.5 mg/ml) was added as negative loading control. The dye solution was left for 20 min in the well, and the cells were washed three times with Hanks' balanced salt solution and fixed with 3.7% formaldehyde for 10 min. Cells were mounted, and images of dye transfer were captured using a Nikon Ti-E fluorescence microscope. The distance of dye transfer from the location of the scrape to the farthest visual uptake of dye was measured using Image Pro-Plus 7.0 software (Media Cybernetics).
Statistical analysis.
Statistical analysis was performed using SigmaPlot 12.5 (Systat Software). Values are means ± SE. Responses were evaluated using one-way ANOVA with Dunn's multiple-range test. Statistical comparison between dose-response curves was made by two-way ANOVA, with Bonferroni's correction performed post hoc to correct for multiple comparisons. Differences were considered to be statistically significant when P < 0.05.
RESULTS
Decreased OGA protein levels, increased OGT protein levels, and elevated protein O-GlcNAc modification in coronary ECs isolated from diabetic mice.
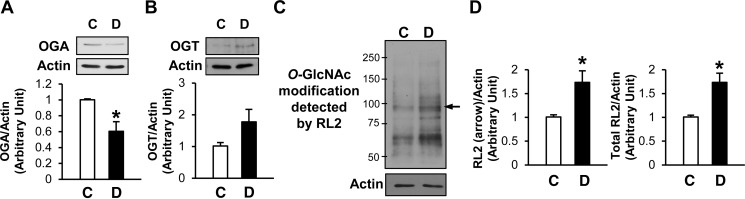
Diabetes was induced in 6-wk-old C57BL/6 mice (the background strain of our transgenic mice; Jackson Laboratory, Bar Harbor, ME). OGA protein levels were significantly decreased in diabetic compared with control mice (Fig. 1A), whereas OGT protein levels were markedly higher in diabetic than control MCECs (Fig. 1B). Figure 1, C and D, shows significantly higher protein O-GlcNAcylation in ECs isolated from diabetic than control mice, implying that decreased OGA protein expression and/or increased OGT protein expression may contribute to elevated protein O-GlcNAcylation in coronary ECs from diabetic mice.
Fig. 1.
Mouse coronary endothelial cells (MCECs) isolated from diabetic (D) mice exhibit decreased β-N-acetylglucosaminidase (OGA) protein levels and increased protein O-linked N-acetylglucosaminylation (O-GlcNAcylation) compared with endothelial cells (ECs) from control (C) mice. A: Western blots showing OGA and actin protein levels (top). Actin was used as a loading control. Bottom: OGA protein level normalized to actin. Values are means ± SE; n = 8 per group. *P < 0.05 vs. control. B: Western blots showing O-linked N-acetylglucosamine (O-GlcNAc) transferase (OGT) and actin protein levels (top). Bottom: OGT protein level normalized to actin. Values are means ± SE; n = 7 per group. C: increased protein O-GlcNAcylation was detected in diabetic ECs with the anti-O-GlcNAc antibody RL2. Arrow indicates a representative band of proteins showing increased O-GlcNAcylation. D: summarized data of the intensity of the band indicated by the arrow (left) and total RL2 signals (right). Values are means ± SE; n = 6 per group. *P < 0.05 vs. control.
Generation of Tie2-Tet-OGA mice.
Tie2-Tet-OGA transgenic mice were generated to test whether OGA overexpression attenuates hyperglycemia-induced protein O-GlcNAcylation in diabetic mice. Mice carrying the Tie2-rtTA were crossed with mice that have a TRE-OGA (Fig. 2, A and B). OGA mRNA (Fig. 2C) and protein (Fig. 2D) levels were significantly increased in Dox-treated compared with untreated Tie2-Tet-OGA mice. Interestingly, OGT protein expression level was significantly decreased in Tie2-Tet-OGA mice after Dox treatment. The following experiments in Figs. 3–5 were conducted using Tie2-Tet-OGA mice.
Fig. 2.
Generation of tetracycline (Tet)-inducible OGA transgenic (Tie2-Tet-OGA) mice. A: Tie2-rtTA mice were crossed with TRE-OGA mice to generate Tie2-Tet-OGA mice. rtTA, reverse Tet-controlled transactivator; TRE, Tet response element; CMV, cytomegalovirus promoter; Dox, doxycycline. B: genotyping of transgenic mice by PCR. Lane 4 carries both OGA and rtTA transgenes. C: Dox treatment in Tie2-Tet-OGA mice (lane 4) significantly increased OGA mRNA in coronary ECs. OGA mRNA expression level was determined by real-time PCR. Values are means ± SE; n = 6 per group. *P < 0.05 vs. untreated [without Dox (−Dox)]. D: Dox treatment in Tie2-Tet-OGA mice significantly increased OGA protein expression (n = 3 −Dox and 5 +Dox) but decreased OGT protein levels (n = 3 per group), as determined by Western blot analysis. Values are means ± SE. *P < 0.05 vs. −Dox.
Overexpression of OGA in ECs significantly decreases protein O-GlcNAcylation and restores coronary EC functions in diabetic mice.
Western blot data demonstrate that the decreased OGA protein levels in diabetic mice were significantly restored by Dox treatment (Fig. 3A). Furthermore, the protein O-GlcNAcylation level was successfully decreased in ECs from Dox-treated compared with untreated Tie2-Tet-OGA diabetic mice (Fig. 3, B and C). Capillary density was significantly lower in diabetic than control mice, while OGA transgene induction by Dox treatment in diabetic mice restored the capillary density to the level in the control mice (Fig. 4, A and B). In addition, we examined the effect of OGA overexpression on endothelium-dependent relaxation (EDR) and endothelium-derived hyperpolarization (EDH)-dependent relaxation by acetylcholine (ACh). EDR and EDH-dependent relaxation in coronary arteries was significantly attenuated in diabetic mice compared with control mice, and coronary vasodilatation by ACh was restored in diabetic mice when the OGA transgene was induced by Dox (Fig. 4, C and D). EC-independent relaxation determined by sodium nitroprusside did not change among the three groups (Fig. 4E).
Potential protein candidates that are O-GlcNAcylated in diabetic MCECs.
Cx40 and eNOS expression levels were lower in diabetic than control MCECs. However, OGA overexpression did not restore these proteins to levels in control MCECs [ratio to control: 0.49 ± 0.16 (untreated diabetic) and 0.66 ± 0.35 (Dox-treated diabetic) for Cx40 (Fig. 5C; P < 0.05); 0.70 ± 0.08 (untreated diabetic) and 0.78 ± 0.25 (Dox-treated diabetic) for eNOS (Fig. 5F; P < 0.05)]. Interestingly, Cx40 O-GlcNAcylation levels were significantly increased in diabetic mice, and OGA overexpression decreased the O-GlcNAcylation levels toward those in control MCECs (Fig. 5B). The levels of eNOS O-GlcNAcylation were also increased in diabetic mice, but the restoration of eNOS O-GlcNAcylation by OGA overexpression was not significant (Fig. 5E).
Inhibition of OGA attenuates Cx40-mediated gap junction communication.
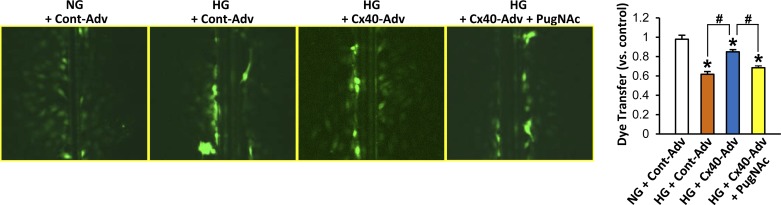
To examine whether O-GlcNAcylation of Cx40 would affect gap junction communication, we performed a dye-transfer experiment. Figure 6 demonstrates that HG treatment in ECs decreased the distance across which dye was transferred and that Cx40 overexpression in HG-treated ECs (22) significantly increased the distance. Furthermore, OGA inhibition by PugNAc treatment significantly decreased dye transfer in Cx40-overexpressing, HG-treated ECs, suggesting that O-GlcNAcylation of Cx40 contributes to attenuated gap junction communication in HG-treated ECs.
Fig. 6.
Effect of OGA inhibition on Cx40-induced gap junction communication. Left: Lucifer yellow dye transfer in ECs. Right: dye transfer (distance compared with control) in normal glucose (NG)-treated, control-adenovirus (Cont-Adv)-transduced ECs; high glucose (HG)-treated, control adenovirus-transduced ECs; HG-treated, Cx40 adenovirus-transduced ECs, and HG and PugNAc-treated, Cx40 adenovirus-transduced ECs. Values are means ± SE; n = 7 per group. *P < 0.05 vs. NG + Cont-Adv; #P < 0.05 vs. HG + Cx40-Adv.
DISCUSSION
Under physiological conditions, some proteins require O-GlcNAc binding for proper function (2, 4, 12). Under pathophysiological conditions, protein O-GlcNAcylation plays an adverse or a protective role, depending on the target proteins and the type of stress/disease (13, 14, 16, 25). There is increasing evidence that excess protein O-GlcNAcylation due to hyperglycemia is associated with EC dysfunction (1, 9, 10, 29). In Fig. 1, ECs from type 1 diabetic mice exhibit a significant increase in O-GlcNAcylation level, a decrease in OGA protein level, and an increase in OGT protein level. Using novel endothelium-specific OGA transgenic mice, we demonstrate here for the first time that decreasing protein O-GlcNAcylation in ECs from diabetic mice, through the overexpression of OGA in ECs in vivo, results in a significant improvement in coronary EC function. There are two major isoforms of OGA in mammals, full-length and short-length, and it has been reported that the full-length OGA is more important for the cleavage of O-GlcNAc from proteins (15, 17). Our mice overexpress the full-length OGA, and overexpression of OGA in type 1 diabetic mice significantly improved EDH-dependent vascular relaxation and capillary density, suggesting that overexpression of only the full-length OGA isoform would be sufficient to restore endothelial function.
Increased protein O-GlcNAcylation and endothelial dysfunction caused by protein O-GlcNAcylation in diabetes could be restored by decreasing OGT expression levels. Ubiquitous OGT knockout mice are embryonic lethal (28). Cardiac myocyte-specific OGT knockout mice rarely survive beyond 4 wk (33). These data indicate that OGT is regulating physiological function during development. To examine the effect of OGT downregulation on endothelial function, it is necessary to generate tissue-specific and/or conditional OGT knockout mice.
Capillary density is an indicator of oxygen transport efficiency and diffusion ability in the muscular tissue (26). Greater capillary density indicates a better capacity to transport oxygen because of the shorter diffusion distance to tissues. ECs play a critical role in new vascular formation, and the decrease in coronary EC function (e.g., migration and proliferation) in diabetes is attributed to a diminished regeneration of new capillary networks (22). Several reports indicate that HG treatment in ECs significantly increased O-GlcNAcylation of proteins that are known to regulate endothelial migration [e.g., Akt (21) and eNOS (7, 9)], but did not show direct evidence that decreased O-GlcNAcylation would restore endothelial physiological functions. In the present study we demonstrate that overexpression of OGA in ECs decreases protein O-GlcNAcylation in vivo and restores capillary density in the diabetic LV to normal levels (Fig. 4, A and B), suggesting that excessive protein O-GlcNAcylation resulting from chronic hyperglycemia dysregulates new vascular formation in coronary ECs, and the improvement of capillary density via OGA overexpression can be useful as a treatment for coronary vascular rarefaction in diabetes.
The endothelium relaxes vascular vessels by 1) producing and releasing vasodilators (e.g., NO, prostacyclin, and endothelium-derived hyperpolarizing factors) and 2) hyperpolarizing smooth muscle cells through gap junctions. We previously demonstrated that attenuated EDR plays an important role in the development of diabetes-associated vascular complications (22). In particular, EDH-dependent relaxation was significantly attenuated because of the lack of gap junction communication caused by decreased Cx40 protein levels in diabetic ECs. Lima et al. (18) reported that increased O-GlcNAcylation by PugNAc (an inhibitor of OGA) attenuated ACh-induced vascular relaxation in rat aorta and implied that the protein that regulates EDR might be affected by O-GlcNAcylation. Our data show that the decrease in EDR in diabetic coronary arteries is mainly due to decreased EDH-dependent relaxation and that OGA overexpression restores EDR by increasing EDH-dependent relaxation (Fig. 4, C and D), suggesting that EDH-related proteins are regulated by O-GlcNAcylation in diabetic mice. It is important to determine which protein or proteins are affected by O-GlcNAcylation in diabetes and regulate these effects. In a pilot study we tested several proteins, the levels of which were altered in diabetic MCECs in our previous reports (3, 8, 22, 27), for O-GlcNAc modification. We found that Cx40 and eNOS were decreased in diabetes but that these protein levels were not restored by OGA overexpression. Interestingly, O-GlcNAcylation of Cx40 and eNOS in diabetes was significantly increased compared with control levels, and Dox treatment in diabetic mice decreased Cx40 O-GlcNAcylation toward levels in control mice. It has been demonstrated that eNOS activity is regulated by O-GlcNAcylation through inhibition of phosphorylation sites in eNOS protein in HG-treated ECs (9), HG-treated gracilis muscle arterioles (1), and tissues in diabetes (24). eNOS activation is important not only for vascular relaxation, but also for cell migration and proliferation in vascular ECs. Decreased eNOS O-GlcNAcylation by OGA overexpression might, at least in part, play an important role in restoration of capillary density in diabetic mice. Connexins are main components of gap junctions, and ECs highly express Cx40, Cx37, and Cx43. Electrical signals (e.g., hyperpolarization) generated in ECs will be transferred to smooth muscle cells through the gap junctions, and a decrease in Cx40 protein level is one cause of endothelial dysfunction in diabetes (22). The study of Wang et al. (32) indicates that N-glycosylation regulates Cx43 function, but a relationship between O-GlcNAcylation and other connexin functions has not been reported. We demonstrate that attenuated gap junction communication resulting from HG treatment was significantly restored by Cx40 overexpression and that PugNAc treatment inhibited the beneficial effect of Cx40 overexpression in gap junction communication, suggesting that Cx40 function is negatively regulated by O-GlcNAcylation. In diabetes, not only decreased Cx40 protein levels (22), but also increased Cx40 O-GlcNAcylation, attenuates gap junction communication in MCECs and, subsequently, decreases EDH-induced coronary vascular relaxation.
In this study we demonstrate that OGA overexpression contributes to an improvement of coronary endothelium dysfunction in diabetic mice, suggesting that protein O-GlcNAcylation may represent a novel therapeutic target for the treatment of coronary vascular complications associated with diabetes.
GRANTS
This work was supported by National Heart, Lung, and Blood Institute Grant R01 HL-115578 (A. Makino) and Department of Veterans Affairs Grant 5I01BX001121-02 (W. H. Dillmann).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
A.M. developed the concept and designed the research; A.M., A.D., Y.H., K.D.Y., W.W., and H.W. performed the experiments; A.M., A.D., Y.H., K.D.Y., and W.W. analyzed the data; A.M., A.D., and Y.H. interpreted the results of the experiments; A.M. and Y.H. prepared the figures; A.M. and R.D. drafted the manuscript; A.M., K.D.Y., R.D., B.T.S., and W.H.D. edited and revised the manuscript; A.M., A.D., Y.H., K.D.Y., W.W., R.D., B.T.S., H.W., and W.H.D. approved the final version of the manuscript.
Footnotes
This article is the topic of an Editorial Focus by Tarik Issad (13a).
REFERENCES
- 1.Beleznai T, Bagi Z. Activation of hexosamine pathway impairs nitric oxide (NO)-dependent arteriolar dilations by increased protein O-GlcNAcylation. Vasc Pharmacol 56: 115–121, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Butkinaree C, Park K, Hart GW. O-linked β-N-acetylglucosamine (O-GlcNAc): extensive crosstalk with phosphorylation to regulate signaling and transcription in response to nutrients and stress. Biochim Biophys Acta 1800: 96–106, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cho YE, Basu A, Dai A, Heldak M, Makino A. Coronary endothelial dysfunction and mitochondrial reactive oxygen species in type 2 diabetic mice. Am J Physiol Cell Physiol 305: C1033–C1040, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cieniewski-Bernard C, Montel V, Stevens L, Bastide B. O-GlcNAcylation, an original modulator of contractile activity in striated muscle. J Muscle Res Cell Motil 30: 281–287, 2009. [DOI] [PubMed] [Google Scholar]
- 5.De Vriese AS, Verbeuren TJ, Van de Voorde J, Lameire NH, Vanhoutte PM. Endothelial dysfunction in diabetes. Br J Pharmacol 130: 963–974, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Donovan K, Alekseev O, Qi X, Cho W, Azizkhan-Clifford J. O-GlcNAc modification of transcription factor Sp1 mediates hyperglycemia-induced VEGF-A upregulation in retinal cells. Invest Ophthalmol Vis Sci 55: 7862–7873, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Du XL, Edelstein D, Dimmeler S, Ju Q, Sui C, Brownlee M. Hyperglycemia inhibits endothelial nitric oxide synthase activity by posttranslational modification at the Akt site. J Clin Invest 108: 1341–1348, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Estrada IA, Donthamsetty R, Debski P, Zhou MH, Zhang SL, Yuan JX, Han W, Makino A. STIM1 restores coronary endothelial function in type 1 diabetic mice. Circ Res 111: 1166–1175, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Federici M, Menghini R, Mauriello A, Hribal ML, Ferrelli F, Lauro D, Sbraccia P, Spagnoli LG, Sesti G, Lauro R. Insulin-dependent activation of endothelial nitric oxide synthase is impaired by O-linked glycosylation modification of signaling proteins in human coronary endothelial cells. Circulation 106: 466–472, 2002. [DOI] [PubMed] [Google Scholar]
- 10.Gurel Z, Sieg KM, Shallow KD, Sorenson CM, Sheibani N. Retinal O-linked N-acetylglucosamine protein modifications: implications for postnatal retinal vascularization and the pathogenesis of diabetic retinopathy. Mol Vis 19: 1047–1059, 2013. [PMC free article] [PubMed] [Google Scholar]
- 11.Hart GW, Housley MP, Slawson C. Cycling of O-linked β-N-acetylglucosamine on nucleocytoplasmic proteins. Nature 446: 1017–1022, 2007. [DOI] [PubMed] [Google Scholar]
- 12.Hart GW, Kreppel LK, Comer FI, Arnold CS, Snow DM, Ye Z, Cheng X, DellaManna D, Caine DS, Earles BJ, Akimoto Y, Cole RN, Hayes BK. O-GlcNAcylation of key nuclear and cytoskeletal proteins: reciprocity with O-phosphorylation and putative roles in protein multimerization. Glycobiology 6: 711–716, 1996. [DOI] [PubMed] [Google Scholar]
- 13.Hilgers RH, Xing D, Gong K, Chen YF, Chatham JC, Oparil S. Acute O-GlcNAcylation prevents inflammation-induced vascular dysfunction. Am J Physiol Heart Circ Physiol 303: H513–H522, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13a.Issad T. O-GlcNAcylation of connexin 40: a sweet connection between diabetes and endothelial cell dysfunction? Focus on “O-GlcNAcase overexpression reverses coronary endothelial cell dysfunction in type 1 diabetic mice.” Am J Physiol Cell Physiol (September 16, 2015). doi: 10.1152/ajpcell.00260.2015. [DOI] [PubMed] [Google Scholar]
- 14.Issad T, Masson E, Pagesy P. O-GlcNAc modification, insulin signaling and diabetic complications. Diabetes Metab 36: 423–435, 2010. [DOI] [PubMed] [Google Scholar]
- 15.Kim EJ, Kang DO, Love DC, Hanover JA. Enzymatic characterization of O-GlcNAcase isoforms using a fluorogenic GlcNAc substrate. Carbohydr Res 341: 971–982, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Laczy B, Hill BG, Wang K, Paterson AJ, White CR, Xing D, Chen YF, Darley-Usmar V, Oparil S, Chatham JC. Protein O-GlcNAcylation: a new signaling paradigm for the cardiovascular system. Am J Physiol Heart Circ Physiol 296: H13–H28, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Li J, Huang CL, Zhang LW, Lin L, Li ZH, Zhang FW, Wang P. Isoforms of human O-GlcNAcase show distinct catalytic efficiencies. Biochemistry (Mosc) 75: 938–943, 2010. [DOI] [PubMed] [Google Scholar]
- 18.Lima VV, Giachini FR, Carneiro FS, Carneiro ZN, Fortes ZB, Carvalho MH, Webb RC, Tostes RC. Increased vascular O-GlcNAcylation augments reactivity to constrictor stimuli. J Am Soc Hypertens 2: 410–417, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lima VV, Giachini FR, Choi H, Carneiro FS, Carneiro ZN, Fortes ZB, Carvalho MH, Webb RC, Tostes RC. Impaired vasodilator activity in deoxycorticosterone acetate-salt hypertension is associated with increased protein O-GlcNAcylation. Hypertension 53: 166–174, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Liu H, Yu S, Zhang H, Xu J. Identification of nitric oxide as an endogenous inhibitor of 26S proteasomes in vascular endothelial cells. PLos One 9: e98486, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Luo B, Soesanto Y, McClain DA. Protein modification by O-linked GlcNAc reduces angiogenesis by inhibiting Akt activity in endothelial cells. Arterioscler Thromb Vasc Biol 28: 651–657, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Makino A, Platoshyn O, Suarez J, Yuan JX, Dillmann WH. Downregulation of connexin40 is associated with coronary endothelial cell dysfunction in streptozotocin-induced diabetic mice. Am J Physiol Cell Physiol 295: C221–C230, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mather KJ. The vascular endothelium in diabetes—a therapeutic target? Rev Endocr Metab Disord 14: 87–99, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Musicki B, Kramer MF, Becker RE, Burnett AL. Inactivation of phosphorylated endothelial nitric oxide synthase (Ser-1177) by O-GlcNAc in diabetes-associated erectile dysfunction. Proc Natl Acad Sci USA 102: 11870–11875, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ngoh GA, Facundo HT, Zafir A, Jones SP. O-GlcNAc signaling in the cardiovascular system. Circ Res 107: 171–185, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rakusan K, Hoofd L, Turek Z. The effect of cell size and capillary spacing on myocardial oxygen supply. Adv Exp Med Biol 180: 463–475, 1984. [DOI] [PubMed] [Google Scholar]
- 27.Sasaki K, Donthamsetty R, Heldak M, Cho YE, Scott BT, Makino A. VDAC: old protein with new roles in diabetes. Am J Physiol Cell Physiol 303: C1055–C1060, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Shafi R, Iyer SP, Ellies LG, O'Donnell N, Marek KW, Chui D, Hart GW, Marth JD. The O-GlcNAc transferase gene resides on the X chromosome and is essential for embryonic stem cell viability and mouse ontogeny. Proc Natl Acad Sci USA 97: 5735–5739, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Shrikhande GV, Scali ST, da Silva CG, Damrauer SM, Csizmadia E, Putheti P, Matthey M, Arjoon R, Patel R, Siracuse JJ, Maccariello ER, Andersen ND, Monahan T, Peterson C, Essayagh S, Studer P, Guedes RP, Kocher O, Usheva A, Veves A, Kaczmarek E, Ferran C. O-glycosylation regulates ubiquitination and degradation of the anti-inflammatory protein A20 to accelerate atherosclerosis in diabetic ApoE-null mice. PLos One 5: e14240, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Spiro RG. Protein glycosylation: nature, distribution, enzymatic formation, and disease implications of glycopeptide bonds. Glycobiology 12: 43R–56R, 2002. [DOI] [PubMed] [Google Scholar]
- 31.Varki A, Cummings RD, Esko JD, Freeze HH, Stanley P, Bertozzi CR, Hart GW, Etzler ME. Essentials of Glycobiology (2nd ed). Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press, 2009. [PubMed] [Google Scholar]
- 32.Wang Y, Mehta PP, Rose B. Inhibition of glycosylation induces formation of open connexin-43 cell-to-cell channels and phosphorylation and Triton X-100 insolubility of connexin-43. J Biol Chem 270: 26581–26585, 1995. [DOI] [PubMed] [Google Scholar]
- 33.Watson LJ, Long BW, DeMartino AM, Brittian KR, Readnower RD, Brainard RE, Cummins TD, Annamalai L, Hill BG, Jones SP. Cardiomyocyte Ogt is essential for postnatal viability. Am J Physiol Heart Circ Physiol 306: H142–H153, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]