Abstract
The tumor microenvironment is a complex system, playing an important role in tumor development and progression. Besides cellular stromal components, extracellular matrix fibers, cytokines, and other metabolic mediators are also involved. In this review we outline the potential role of hypoxia, a major feature of most solid tumors, within the tumor microenvironment and how it contributes to immune resistance and immune suppression/tolerance and can be detrimental to antitumor effector cell functions. We also outline how hypoxic stress influences immunosuppressive pathways involving macrophages, myeloid-derived suppressor cells, T regulatory cells, and immune checkpoints and how it may confer tumor resistance. Finally, we discuss how microenvironmental hypoxia poses both obstacles and opportunities for new therapeutic immune interventions.
Keywords: hypoxia, hypoxia-inducible factor, tumor microenvironment, myeloid cells, lymphoid cells, immune suppression, cancer stem cells, programmed death-ligand 1, epithelial-mesenchymal transition, circulating tumor cells, autophagy and antitumor immune response
it has become increasingly apparent that malignant cells exist in a complex cellular and extracellular microenvironment that plays key roles in the initiation and maintenance of the malignant phenotype. Among the microenvironmental factors that play a dominant role in neoplasia, hypoxia is believed to be one of the most relevant in the neoplastic response of tumor cells. It is widely appreciated that the majority of malignancies create a hostile hypoxic microenvironment that can hamper cell-mediated immunity and dampen the efficacy of the immune response. Poorly vascularized and hypoxic zones inside solid tumors contribute to immune tolerance of tumor cells by impeding the homing of immunocompetent cells into tumors and inhibiting their antitumor efficacy. Several regulatory mechanisms can occur concurrently within the hypoxic tumor microenvironment, resulting in multiple redundant levels of immune cell plasticity and suppression, tumor plasticity, and functional heterogeneity. Hypoxic stress clearly plays a crucial role in tumor promotion and immune escape by controlling angiogenesis and favoring immune suppression and tumor resistance. Like other therapeutic attempts, immunotherapy is heavily hampered by the morphologically aberrant tumor microvasculature, preventing migration of immune effector cells into established tumors. Many strategies are emerging for changing the immunosuppressive nature of the tumor to a microenvironment able to support antitumor immunity. The identification of ways to induce a permissive and less hostile tumor microenvironment to avoid tumor resistance and immune suppression is of major interest and pertinence.
Hypoxia as an Integral Component of the Tumor Microenvironment
All life on Earth depends on O2, and all physiological and pathological processes of a living cell rely principally on O2 (103). Hypoxia is characterized by lack of O2, and hypoxic tissues are inadequately oxygenated (107). A physiologically hypoxic microenvironment has been observed in a variety of normal tissues, including retina, kidney medulla, skin epidermis, and hypoxic niches within the bone marrow (103). Both normal and tumor cells adapt to a hypoxic microenvironment by regulating the hypoxia-inducible factor (HIF) family of transcription factors. HIFs are dimeric proteins composed of an O2-sensitive α-subunit (HIF-1α, HIF-2α, or HIF-3α) and a β-subunit (HIF-1β/aryl hydrocarbon receptor nuclear translocator). HIF-1α and HIF-2α are regulated by O2-dependent von Hippel-Lindau (VHL) tumor suppressor-mediated degradation (103). HIF-1α and HIF-2α are structurally similar in DNA binding and dimerization domains but differ in their transactivation domains. Consequently, they share overlapping target genes, but each also regulates a set of unique targets. The genes induced by hypoxia-dependent HIF-1α and HIF-2α play important roles in regulating different aspects of tumor biology, such as angiogenesis, cell survival, chemo- and radioresistance, proliferation, invasion and metastasis, pH regulation and metabolism, resistance to the immune system, and maintenance of cancer stem cells (CSCs) (104).
Hypoxic Stress and the Immune System
Hypoxia reeducates myeloid cells toward an immunosuppressive phenotype.
Increasing evidence demonstrates that tumor hypoxia impacts the antitumor immune response by promoting local immune suppression and inhibiting immune killing functions. Hypoxic zones in solid tumors are infiltrated by a large number of immunosuppressive cells, such as myeloid-derived suppressor cells, tumor-associated macrophages, and T-regulatory (Treg) cells (83). These cells are among the most widely studied immunosuppressive cells within the tumor microenvironment, and the role of tumor hypoxia in their recruitment and immunosuppressive functions is becoming evident (37).
tumor-associated macrophages.
Macrophages constitute a principal component of the immune infiltrate in solid tumors (22). Within the solid tumor microenvironment, macrophages differentiate into tumor-associated macrophages (TAMs) with expression of markers such as CD206 (67). Tumor-derived cytokines, such as IL-4 and IL-10, are able to convert TAMs to polarized type 2, or M2, macrophages with more immunosuppressive activities, resulting in tumor progression (67). TAMs have been found to be preferentially located in tumor hypoxic areas, where they accumulate HIF-1 and HIF-2 (13, 56). The relative contribution of HIF-1 and HIF-2 to the regulation of gene expression in TAMs is not completely clear. Besides studies reporting a role of HIF-1 and HIF-2 in the promotion of macrophage angiogenic properties (117, 118), HIF-1α was also reported to be crucial for macrophage-mediated inhibition of T cells in hypoxic conditions (28). In hypoxic areas of tumors, TAMs also upregulate the expression of matrix metalloproteinase-7 protein in hypoxic areas of tumors (11). Matrix metalloproteinase-7 is known to cleave the Fas ligand from neighboring cells, making tumor cells less responsive to lysis by natural killer (NK) and T cells (32).
myeloid-derived suppressor cells.
Myeloid-derived suppressor cells (MDSCs) directly promote immune tolerance (37). In tumor-bearing hosts, tumor-derived factors, such as VEGF, granulocyte-macrophage colony-stimulating factor, and prostaglandins, favor the accumulation of MDSCs in tumoral tissues and secondary lymphoid organs (34). In these sites, MDSCs induce T cell anergy, restrain the effector phase of the CD8+ T cell, and can promote antigen-specific Treg cell proliferation (34, 106). HIF-1α has been directly shown to regulate the function and differentiation of MDSCs within the hypoxic tumor microenvironment. Tumor-derived MDSCs are more immunosuppressive than splenic MDSCs mostly because of the induction of HIF-1α-dependent increased arginase activity and nitric oxide production (22). Very recently, our group showed that tumoral MDSC expression of programmed death-ligand 1 (PD-L1) upregulated under hypoxia increased MDSC-mediated T cell tolerance (80). We first found a differentially higher expression of PD-L1 on tumor-infiltrating MDSCs than splenic MDSCs (80), and hypoxia dramatically and significantly increased the percentage of PD-L1-positive MDSCs isolated from the spleen in different tumor-bearing mice. We and others (10) further provided evidence that HIF-1α is a major regulator of PD-L1 mRNA and protein expression and that HIF-1α regulates the expression of PD-L1 by binding directly to a hypoxia response element in the PD-L1 proximal promoter. The immunosuppressive function of MDSCs enhanced under hypoxia was abrogated following PD-L1 blockade, and hypoxia-upregulated IL-6 and IL-10 in MDSCs was significantly attenuated after PD-L1 blockade (79, 80). These data seem to indicate a more general mechanism, as blockade of tumor microenvironment-induced PD-L1 on dendritic cells (DCs) (24) has been shown to decrease DC-mediated immune suppression, improve T cell function, and decrease tumor progression.
Cross talk between MDSCs and macrophages, suggesting that MDSCs downregulate IL-12 production by macrophages and increase their own production of IL-10 in response to signals from macrophages, has also been reported. This interaction between MDSCs and macrophages polarizes classically activated (M1) macrophages toward a type 2 and immunosuppressive phenotype and accentuates M2 macrophages (113).
dendritic cells.
DCs are dominant antigen-presenting cells that are specialized for the activation of resting T cells and the initiation and regulation of antitumor immune responses (8). The effect of hypoxia on the survival, differentiation, and maturation of DCs and their impact on antitumor immune responses have been well investigated. Indeed, DCs are also diverted by hypoxia from their highly specialized antigen-presenting and T cell-activating functions.
Under hypoxia, DCs exhibit decreased expression of several differentiation and maturation markers (CD1a, CD40, CD80, CD83, CD86, and myosin heavy chain class II molecules) in response to lipopolysaccharide. Similarly, hypoxia inhibits the stimulatory capacity of DCs for activating T cell functions. On the contrary, the production of proinflammatory cytokines, such as TNF and IL-1, as well as the inflammatory C-C chemokine receptor (CCR) type 5 (CCR5), was strongly upregulated under hypoxia in DCs (65).
Moreover, several studies have provided evidence that the maturation and function of DCs are influenced by several hypoxia-modulated factors, such as VEGF and IL-10, present in the tissue microenvironment. The production of VEGF by human tumors inhibits the functional maturation of DCs and, thereby, promotes immune escape of tumor cells (36). Recombinant VEGF administered to tumor-free mice resulted in repressed DC development associated with accumulation of Gr-1+ immature MDSCs that inhibit T cell functions (35), illustrating the proper immunosuppressive functions of VEGF. Tumor-associated DCs, in response to tumor-derived VEGF, increase the expression of PD-L1, which is a negative regulator of T cell function (24). Moreover, anti-VEGF therapy was associated with a decrease in immature myeloid cells and DCs in patients (85).
IL-10 has been shown to prevent the differentiation of monocytes to DCs while promoting their maturation to macrophages (4). Mammalian and viral IL-10 enhances CCR5 but downregulates CCR7 expression by DCs, thus impacting chemotactic responses and in vivo homing ability (109).
Hypoxia Interferes With T Lymphocyte Function
Hypoxia regulates cytotoxic T lymphocyte cytotoxic activity.
O2-independent inducers of HIF resulting in HIF stabilization have been identified in T cells. In this regard, T cell receptor (TCR)-mediated stabilization of HIF-1 has been reported following antibody-mediated engagement of TCR/CD3 via the phosphoinositide 3-kinase/mammalian target of rapamycin pathway, leading to increased HIF-1α protein synthesis (76). TCR-activated T cells also increased HIF-1α mRNA synthesis by mechanisms involving protein kinase C and Ca2+/calcineurin (61). Independently of TCR stimulation, HIF-1α mRNA is augmented in T cells in the presence of transforming growth factor (TGF)-β and/or IL-6 by a mechanism involving STAT3 (25).
In physiological conditions, CD8+ T cells from lymphoid organs (spleen and lymph nodes) were found to bind pimonidazole, indicating a hypoxic state within these organs (84). Moreover, CD8+ (and CD4+) T cells were found in hypoxic adipose tissues of obese mice (92). This suggests that CD8+ T cells could be found in hypoxic tissue zones. In a tumoral context, CD8+ T cells have been found in hypoxic tumors (87), but their distribution inside the tumors, i.e., whether CD8+ T cells are inside or outside the intratumoral hypoxic zones, has not been elucidated.
The effects of hypoxic stress on the killing functions of CD8+ T cells have been analyzed by several groups. Caldwell et al. (12) revealed that, starting from mouse splenocytes, hypoxic stress delayed CD8+ T cell development but potentiated the lytic capacities of developed CD8+ T cells. More recently, deletion of Vhl in CD8+ T cells, which resulted in constitutive expression of HIF-1 and HIF-2, delayed CD8+ T cell differentiation into effector cells but increased their cytotoxic functions, which correlated with increased expression of granzyme B (27). These increased effector capacities were dependent on HIF-1 and HIF-2 and resulted in a better ability to inhibit tumor growth in mice. HIF-1 was also shown to control the expression of granzyme D, E, and F genes (33), but whether HIFs directly regulate the expression of granzyme genes was not documented. HIF-1 was also shown to regulate perforin expression in an indirect manner (33). These results illustrate the in vitro effects of hypoxic stress on CD8+ T cell activity and suggest that hypoxic stress increases lytic functions of CD8+ T cells and decreases their proliferative and differentiating capacities.
In mice challenged with tumors, intratumoral hypoxia increased expression of the co-stimulatory receptor CD137 at the surface of tumor-infiltrating CD8+ T cells in a HIF-1-dependent manner. The ligation of CD137 by agonist antibodies increased CD8+ T cell activity on the basis of increased production of IFN-γ and TNF-α by CD137+CD8+ T cells in vitro and decreased tumor growth in vivo (87). However, the beneficial effects of CD137 upregulation on tumor progression were found to be tumor-specific, since spontaneous breast carcinoma was resistant to anti-CD137 immunotherapy. Moreover, antigenic stimulation of T cells was necessary for optimal upregulation of CD137 by hypoxia, implying that, in tumors with a loss of antigen expression, the hypoxia-induced upregulation of CD137 may be impaired. Therefore, CD8+ T cells facing hypoxic conditions do not lose their cytolytic properties and even seem to be more lytic due to their upregulation of cytotoxic proteins, TCR, and adhesion molecules.
On the other hand, the effect of hypoxia on cytokine production by CD8+ T cells is less well documented. In vitro cultured hypoxic CD8+ T cells secreted less IFN-γ and less IL-2 (12). IFN-γ production was not altered in in vitro-activated CD8+ T cells with constitutive HIF-1 (33). Vhl-deficient CD8+ T cells isolated from mice expressed more IFN-γ and TNF (27). This diversity in culture conditions and in the activating signal (hypoxia, antigenic stimulation, or VHL tumor suppressor deletion) may have led to different impacts on cytokine production by CD8+ T cells.
Hypoxia potentiates Treg cell immunosuppressive function.
The effects of hypoxia on CD4+ T cells are better described. Under hypoxic stress and in the presence of TGF-β, CD4+ T cells upregulate Foxp3 through direct binding of HIF-1 to the Foxp3 promoter region, inducing Treg cell formation (18). On the another hand, Foxp3-restricted VHL tumor suppressor deletion in Treg cells, which resulted in constitutive HIF-1 stabilization, skewed Treg cells toward a T helper type 1 (Th1)-like phenotype (55). These Treg cells exhibited a massive IFN-γ production by direct binding of HIF-1 to the IFN-γ promoter and a negligible increase in IL-17 production. As suggested by Lee et al. (55), the discrepancy between these findings and the previous study may reside in the fact that Clambey et al. (18) analyzed naïve CD4+ T cells, whereas they used differentiated Treg cells. This HIF-1-mediated IFN-γ production by Treg cells suggests that, inside tumors, IFN-γ production may be high in HIF-1-positive Treg cells, but tumoral Treg cells have been described to be rather immunosuppressive and a source of anti-inflammatory cytokines. Further studies on the consequences of Foxp3-restricted VHL deletion in the tumor microenvironment are needed. DCs that have constitutive HIF-1 signaling following SIRT1 deletion showed increased IL-12 and decreased TGF-β1 production and induced CD4+ differentiation toward Th1-like T cells (59). Again, these experiments must be performed in a tumoral context to gain insights into the consequences of HIF-1 stabilization in DCs in CD4+ T cell differentiation.
Tumor hypoxia also attracts Treg cells inside the tumor bed by impacting the cytokine profile inside the microenvironment. Facciabene et al. (31) recently reported that hypoxic stress increases the expression and secretion of CCL28 by ovarian tumor cells. CCL28 acts as a chemoattractant for Treg cells, which have well-documented immunosuppressive functions on CD8+ T cells. We have also provided evidence that hypoxic stress, by inducing the pluripotency factor NANOG in tumor cells, activates the expression and secretion of the immunosuppressive TGF-β1 by tumor cells by a mechanism involving at least direct binding of NANOG to the TGF-β1 promoter. Targeting NANOG in B16-F10 melanoma cells decreases TGF-β1 and reverses the intratumoral immune cell infiltrate by increasing the number of CD8+ T cells and decreasing the number of macrophages and Treg cells (44). These findings connect stem cell-associated factors with inhibition of the immune response in the hypoxic tumor environment.
Effect of Hypoxic Stress on Tumor Target Plasticity
Tumor cell heterogeneity: CSCs.
Tumor growth is dependent on the presence of CSCs, a subpopulation with stem cell-like properties, within the tumor (93). CSCs are in an undifferentiated state, undergo self-renewal, and, when implanted in immunodeficient mice, are able to develop tumors and reestablish the bulk tumoral heterogeneity (88). Also, because CSCs are able to resist conventional antitumor therapies (115), they are a probable cause of tumor recurrence after treatment. Therefore, their eradication in the tumor is a therapeutic challenge that justifies a better understanding of their emergence and persistence in the tumoral tissue. In this regard, hypoxia and HIFs have been described to induce tumor cell dedifferentiation toward an immature phenotype and, similarly, to maintain tumor cells with stem cell properties (51). Several reports show the role of hypoxia and HIFs in promoting a stem cell-like phenotype through the expression of pluripotency factors such as OCT4, SOX2, and NANOG, required for maintenance of self-renewal in stem cells or activation of the Notch-signaling pathway, which regulates self-renewal and differentiation (51). Jogi et al. (48) showed that culturing neuroblastoma cells under hypoxia led to an increase in genes expressed in neural crest progenitors and a decrease in neuronal marker genes. McCord et al. (70) reported that glioblastoma neurospheres under hypoxia show an increased proportion of CD133+ stem cell-like cells and induction of embryonic markers, such as OCT4 and SOX2. This was associated with a selective increase in HIF-2α. Hypoxia was reported by Chen et al. (15) to activate Notch signaling in lung adenocarcinomas, which was essential, since use of a Notch-signaling inhibitor under hypoxia induced cell death. However, the Notch pathway can also promote cell differentiation in keratinocytes and certain neural stem cells (75, 119). This ability of hypoxia to increase the stem cell-like subset inside a tumor cell population reflects the plasticity of the CSC compartment and the role of microenvironmental stimuli in shaping this particular subset. Some of the effects of hypoxia on tumor cell differentiation are directly mediated by the HIFs. Li et al. (58) reported that targeting HIF-1α and HIF-2α in CD133+ glioma stem cells decreased their survival and their tumorigenic and angiogenic potentials. They also reported a preferential expression of HIF-2α in CD133+ glioma stem cells, whereas HIF-1α was present in both stem and nonstem tumor cells, and their stabilization required more severe hypoxia. Another study using human neuroblastoma cells also found a selective expression of HIF-2α in an immature cell subset, with induction of differentiation when targeting HIF-2α (91). Overexpression of HIF-2α in nonglioma stem cells was sufficient to induce a stem cell-like phenotype (sphere-forming ability and larger tumors after mouse engraftment) (45). HIF-2 was also shown to directly activate the expression of SOX2 (74). At a clinical level, HIF-2α expression in patients correlated with poorer prognosis. These findings support a preferential targeting of HIF-2α for selective eradication of CSCs without adverse effects on normal progenitor cells. HIF-1α is not outdone, since a recent study by Wang et al. (116) using human acute lymphocytic leukemia showed a selective activation of HIF-1α in leukemia stem cells under normoxic conditions compared with the bulk of leukemia blasts. This HIF activity is due to a selective VHL deficiency in the leukemia stem cell subset, and blocking HIF-1α activity enabled the elimination of leukemia stem cells without an effect on the normal hematopoietic stem cells. HIF-1α is required for induction of the breast cancer stem cell phenotype in response to hypoxia (120). We and others have identified HIF-1 as the inducer of NANOG expression under hypoxic stress in non-small cell lung carcinoma (68) and in B16-F10 melanoma cells, and NANOG contributed to the acquisition of stem cell-like features under hypoxic stress (43, 44). These studies and others describe the effects of hypoxia in converting differentiated cancer cells to stem cell-like cancer cells via the expression of embryonic factors or the induction of stem cell properties. The tumoral expression of transcription factors associated with stemness may also lead to tumor target resistance to cytotoxic T lymphocyte (CTL)-mediated lysis. Our group has identified hypoxia-induced NANOG as a critical molecule involved in resistance to CTL-mediated lysis in a HIF-1-dependent manner and by a mechanism involving STAT3. Indeed, hypoxia-induced NANOG was found to be implicated in the phosphorylation of STAT3 under hypoxic stress and, thereby, in its translocation to the nucleus (43). Hypoxia-induced NANOG was also found to directly regulate TGF-β1 expression by binding to TGF-β1 promoter in B16 melanoma cells, which is involved in tumor infiltration by immunosuppressive cells (43, 44). Similarly, constitutive expression of NANOG in cervical cancer cells also mediates resistance to lysis by CTL by a mechanism involving Akt (77).
Tumor cell plasticity: epithelial-mesenchymal transition and consequences on antitumor immunity.
Epithelial-mesenchymal transition (EMT) is a developmental transdifferentiation process in which polarized epithelial cells lose their epithelial traits while gaining mesenchymal traits (111). This process can be reactivated during cancer progression, providing certain cancer cells with increased capacity to migrate, invade, and resist cell death. EMT can be partial, with cells conserving many salient features of epithelial cells, and transient, with cells transitioning from an epithelial to a mesenchymal state and then reverting to an epithelial state, either partially or fully (mesenchymal-epithelial transition). This illustrates the innate plasticity of cells undergoing EMT or mesenchymal-epithelial transition. Cells experiencing EMT can also reactivate stem cell-associated self-renewal programs, which suppose some degrees of plasticity (66). Several reports suggest that hypoxia promotes EMT in various cancer types, including prostate, breast, renal, pancreatic, myeloma, lung, ovarian, and squamous carcinoma (6, 46, 62, 64, 97, 100, 108). Experimental studies have revealed that the stabilization of HIF-1α by hypoxia can directly or indirectly stimulate the expression of several E-box-binding transcription factors known to regulate EMT, including TWIST1, ZEB2, and SNAIL (64, 90, 121, 124), while discrepancies among models suggest context-dependent events. Additionally, under some circumstances, hypoxia may sustain major CSC and EMT-regulatory pathways, such as TGF-β, nuclear factor-κB, and Notch signaling pathways (16, 69, 96, 122, 123). Recently, a new concept, that EMT can modulate the antitumor immune response, has emerged. Transduction of Snail in B16 melanoma cells resulted in inhibition of the CTL lysis activity toward the cancer cells concomitantly with inhibition of DC maturation and expansion of a population of Treg cell-like CD4+Foxp3+ (54). Exploiting the human mammary carcinoma model MCF7, we found that MCF7 cells that experienced EMT after 1) introduction of SNAIL, 2) prolonged exposure to TNF-α, or 3) modulation of the WISP2-TGF-β-KLF4 axis, presented with resistance to CTL-mediated lysis (1–3). The acquired mesenchymal phenotypes were associated with incipient stem cell-like and autophagic states, which we found to be mainly responsible for promoting reduced susceptibility to CTL-mediated lysis. Ricciardi et al. (94) recently reported that EMT following exposure to inflammatory cytokines (i.e., TGF-β, IFN-γ, and TNF-α) can promote a multitude of immune-modulatory effects, including inhibition of lymphocyte proliferation (NK, T, and B cells), expansion of regulatory and T and B cells, and stimulation of NK or T cell apoptosis. These effects were variable among the tested models, which suppose a high degree of complexity when these mechanisms are considered in the clinical setting. It is also noteworthy that previous studies performed in models of human keratinocytes and colorectal cancer cells have found that EMT can render cancer cells more susceptible to NK cell-mediated lysis in a manner that appears to be dependent on EMT-mediated induction of NK group 2 ligand D [i.e., myosin heavy chain class I chain-related molecule A (MICA)/myosin heavy chain class I chain-related molecule and ULBP1-3] (60). In another significant breakthrough, Chen and colleagues (14) showed in lung cancer models that downregulation of microRNA 200s and overexpression of ZEB1 induced upregulation of PD-L1 expression in a microRNA 200-dependent fashion, resulting in exhaustion of intratumoral CD8+ T lymphocytes and a reduced number of tumor-infiltrating lymphocytes, the latter correlated with the development of lung metastases derived from subcutaneous engrafted tumor cells (14).
Hypoxia and circulating tumor cells.
Circulating tumor cells (CTCs) invade and colonize potential distant organs after reaching the lymphatic or hematogenous circulation through intravasation. CTCs generally represent a small proportion of cancer cells that have escaped from the primary tumor site. Nevertheless, CTCs have been detected in the peripheral blood of cancer patients and provide an index of disease severity and aggressiveness in patients with distant metastasis (26). EMT can occur at the origin of a CTC and may be involved in their capacity to migrate into blood vessels. Several studies have demonstrated the upregulation of EMT markers in CTCs, supporting this hypothesis (112). During EMT, CTCs are generated from the primary tumor and, subsequently, invade and colonize distant organs (41). Hypoxia is involved directly and indirectly in the induction of EMT and, consequently, in higher rates of metastases (49). Although the relationship between CTCs and tumor hypoxia has not been fully investigated, some preliminary reports demonstrated an impact of hypoxic tumor microenvironment on modulation and determination of the metastatic ability of CTCs. In their investigation of HIF-1α and VEGF expression in CTCs of metastatic breast cancer patients, Kallergi and colleagues (50) showed that CTCs from these patients express VEGF mRNA and protein. Moreover, they demonstrated that VEGF receptor (VEGFR) type 2 (VEGFR2) is also expressed on the plasma membrane of CTCs, suggesting an autocrine loop involving VEGF and VEGFR2. More interestingly, double- and triple-staining experiments on CTCs showed that VEGF was coexpressed with HIF-1α and VEGFR2. Hence, VEGF production was probably regulated by HIF-1α and/or phosphorylated focal adhesion kinase, as suggested by the significant correlation between the expression of these molecules (50). Ameri et al. (5), using the human triple-negative breast cancer cell line MDA-MB-231, generated orthotopic xenografts in mice that produced CTCs (30) and resulted in lung metastases. These xenografts were found to be profoundly hypoxic and produced CTCs that were captured and cultured. These CTCs demonstrated an altered response to hypoxia compared with the parental MDA-MB-231 cells from which they were derived and also exhibited increased aggressiveness in vivo. In addition to expressing the HIFs at higher levels, CTCs also demonstrated an altered regulation of HIF target genes. CTCs did induce BNIP3, CAIX, and GLUT1, but the induction of these factors was much lower than in parental MDA-MB-231 cells (5). The impact of hypoxia on CTC number, CTC phenotype, and CSC-like properties of CTCs has not been examined. Further investigations are needed to better understand the relationship between hypoxia, CTCs, epithelial and mesenchymal marker levels, EMT process, tumor progression, and treatment response.
Hypoxic Stress Impairs Tumor Target Susceptibility to Effector-Mediated Lysis Through Different Mechanisms
Hypoxia-induced HIF-1α and phosphorylated STAT3 confer resistance to tumor targets against effector cell-mediated killing.
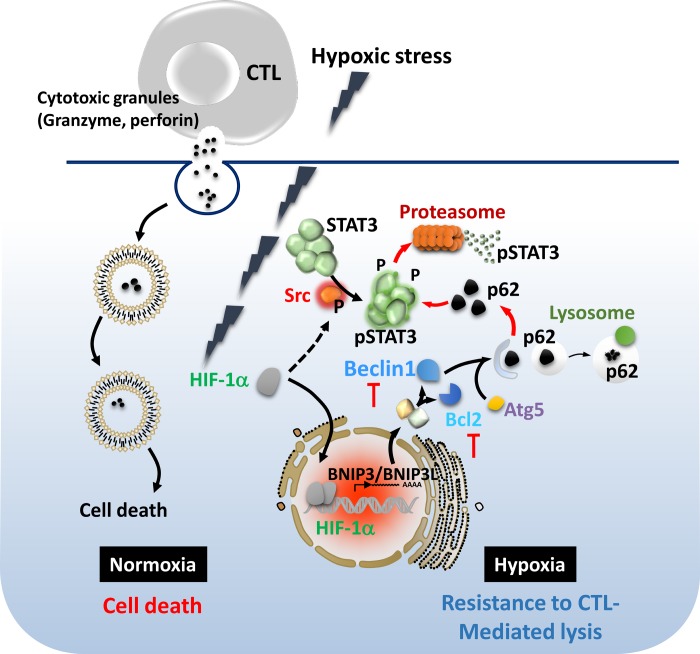
Accumulating experimental and clinical evidence indicates that multiple mechanisms suppressing the antitumor immune functions are induced in the tumor microenvironment. More attention has been focused on the mechanisms by which hypoxic stress within the tumor microenvironment alters tumor susceptibility to immune cell attack. We demonstrated that exposure of tumor cells to hypoxia inhibits specific CTL-mediated lysis by a mechanism involving nuclear translocation of HIF-1α, phosphorylation of STAT3, and VEGF secretion by tumor cells (78). Silencing of STAT3 resulted in HIF-1α inhibition and a significant restoration of target cell susceptibility to CTL-induced killing under hypoxic conditions by a mechanism involving, at least in part, downregulation of Akt phosphorylation. This study highlights the functional link of the concomitant hypoxic induction of phosphorylated STAT3 and HIF-1α to the alteration of tumor susceptibility to CTL-mediated killing (Fig. 1).
Fig. 1.
Hypoxic stress impairs tumor cell susceptibility to cytotoxic T lymphocyte (CTL)-mediated lysis through induction of hypoxia-inducible factor (HIF)-1α, phosphorylated STAT3 (pSTAT3), and autophagy. Under hypoxia, tumor cells stabilize HIF-1α and activate pSTAT3, which renders them resistant to CTL-mediated lysis. Similarly, hypoxia-induced autophagy is responsible for the acquisition of resistance to CTL-mediated killing by selective degradation of pSTAT3 under autophagy inhibitions under hypoxia (78, 82).
Moreover, it has been reported that hypoxia induces tumor cell resistance to immune-mediated lysis via a HIF-1α-dependent pathway linked to increased expression of the metalloproteinase ADAM10. Indeed, ADAM10 is required for the hypoxia-induced shedding of MICA, a ligand that triggers the cytolytic action of immune effectors, from the surface of tumor cells. Such findings show a mechanistic link between hypoxia-induced accumulation of HIF-1α, increased expression of ADAM10, and decreased surface MICA levels, leading to tumor cell resistance to lysis mediated by innate immune effectors (9).
Hypoxia-induced autophagy plays a major role in the resistance of tumors to immune cell-mediated killing.
Recent data have shown that hypoxia-induced autophagy is an important regulator of the innate and adaptive antitumor immunity mediated by NK cells and CTLs, respectively. The first evidence for such a role of autophagy was provided by our group; we demonstrated that hypoxic lung carcinoma cells can evade CTL-mediated lysis through autophagy induction. The inhibition of autophagy restored tumor cell sensitivity to CTL-mediated lysis. This was correlated with a decrease in hypoxia-dependent induction of phosphorylated STAT3. Using a mouse melanoma model, we further showed that the combination of the autophagy inhibitor hydroxychloroquine and a tyrosinase-related protein-2 peptide-based vaccination strategy resulted in a significant decrease in tumor growth in mice compared with control and with each treatment alone. These results strongly demonstrate that in vivo inhibition of autophagy improves the antitumor effect of a tyrosinase-related protein-2-based vaccine (81, 82) (Fig. 1).
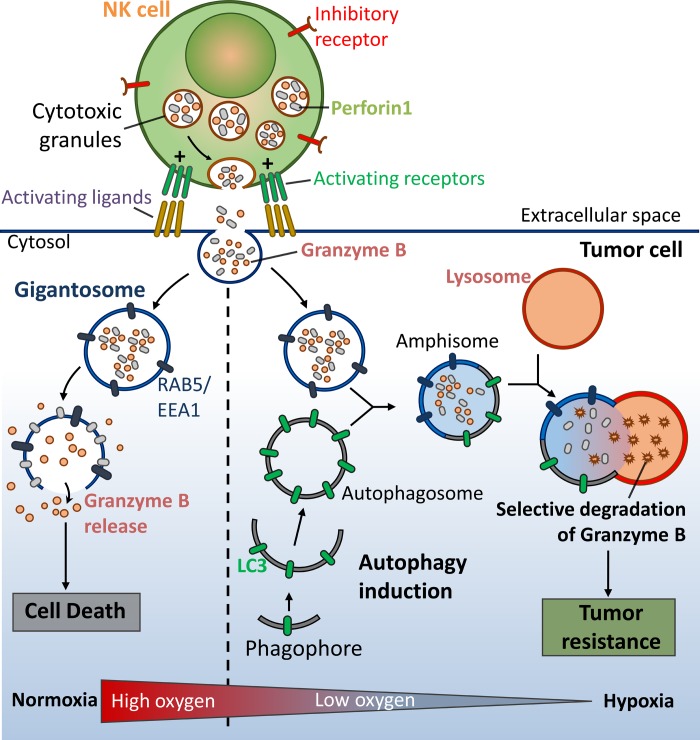
The role of autophagy activation has been extended to the NK cell-mediated antitumor immune response. We recently described the first evidence that tumor cells can escape NK cell-mediated immune surveillance by activating autophagy under hypoxia (7, 114). At the mechanistic level, we showed that granzyme B is selectively degraded upon autophagy activation in hypoxic cells, thereby inhibiting NK cell-mediated target cell apoptosis (Fig. 2). In light of these in vitro observations, we showed a significant decrease in tumor volume in autophagy-defective tumors, presumably as a consequence of potentiation of tumor cell killing by NK cells.
Fig. 2.
Hypoxia-induced autophagy in cancer cells acts as an intrinsic resistance mechanism to natural killer (NK) cell-mediated killing. After recognition by NK cells, the cytolytic effectors perforin 1 and granzyme B enter the target cells through endocytosis and traffic to enlarged endosomes, called “gigantosomes.” In normoxic cells, perforin forms pores in the gigantosome membrane, allowing granzyme B release and initiation of cell death. In hypoxic cells, excessive autophagy leads to fusion of gigantosomes with autophagosomes and the subsequent formation of amphisomes, which contain granzyme B and perforin 1. Fusion of amphisomes with lysosomes triggers selective degradation of granzyme B, making hypoxic tumor cells less sensitive to NK cell-mediated killing (114).
More recently, the role of autophagy in regulating the NK cell-mediated immune response was investigated in a clear cell renal cell carcinoma cell model displaying a mutation in the VHL gene and resistance to NK cell-mediated killing. Using VHL-mutated (786-O) and VHL-corrected (WT-7) renal carcinoma cells, we showed that inositol 1,4,5-triphosphate receptor type 1 (ITPR1) was overexpressed in 786-O compared with WT-7 cells. Interestingly, targeting ITPR1 in 786-O cells was sufficient to dramatically restore NK cell-mediated lysis of these cells. The accumulation of ITPR1 in 786-O cells was associated with their ability to activate autophagy by a signal derived from NK cells, as targeting ITPR1 in 786-O cells abrogates the ability of NK cells to activate autophagy (72). Taken together, these results suggest that inhibition of ITPR1/autophagy in tumors improves their elimination by NK cells in vivo (71, 73).
Targeting Hypoxia to Improve Current Cutting-Edge Immunotherapy Approaches
Immunotherapeutic strategies aimed at triggering or enhancing antitumour immunity are disappointing because of diverse tumor escape mechanisms from immunosurveillance (17, 29). It seems obvious that more could be achieved by combining therapies that tackle malignancies from multiple angles, with the tumor microenvironment conditioned to support a powerful effector arm generated by immunotherapy.
Tumor immunotherapy in the clinic has not taken into account the hypoxic microenvironment and its impact on the therapeutic outcome. A number of anticancer drugs have been shown to inhibit HIFs (99, 101, 104). Clearly, given the central role of hypoxia in the regulation of tumor progression and immune suppression, it is conceivable that its targeting might be considered in new combined cancer therapies.
In this regard, it is tempting to speculate that targeting tumor hypoxia will decrease immunosuppressive cell numbers, inhibit their function, increase effector T cells, improve vaccine efficacy, and, in general, improve antitumoral immunotherapy. Novel immunotherapy approaches, along with targeting tumor hypoxia by using HIF-1α inhibitors, will eventually be beneficial for improving the antitumor immune response in various cancer patients. Whether the suppression of hypoxia may be a promising strategy that is selective for facilitating immunotherapeutic efficacy in cancer patients is under investigation.
Compensation of Hypoxia and Potential of Vessel Normalization for Immunotherapy
Regulation of stroma reactivity by tumor angiogenic activity.
It is widely admitted that the endothelial response to tumor hypoxia signaling is the angiogenic switch (42) involving HIF stabilization and transcription (102), along with production of VEGFs, angiopoietin 2, IL-8, and other factors (38). As antiangiogenic treatments have shown that destruction of angiogenesis leads to deep hypoxia and induces tumor resistance (86), efforts are focusing on rendering the tumor vessels functional to help treat the tumor by anticancer drugs for their increased availability, by radiotherapy for the higher Po2, and by immunotherapy to synergistically boost the immune response. By normalizing vessels (38, 47), the challenge is to alleviate hypoxia to counteract most deleterious effects related to tumor microenvironment (105).
Normalization and alleviation of hypoxia are reciprocal results.
Persistent normalization is an alternative to antiangiogenesis strategies (98) due to the profound effect on the microenvironmental components, namely, the immune cells.
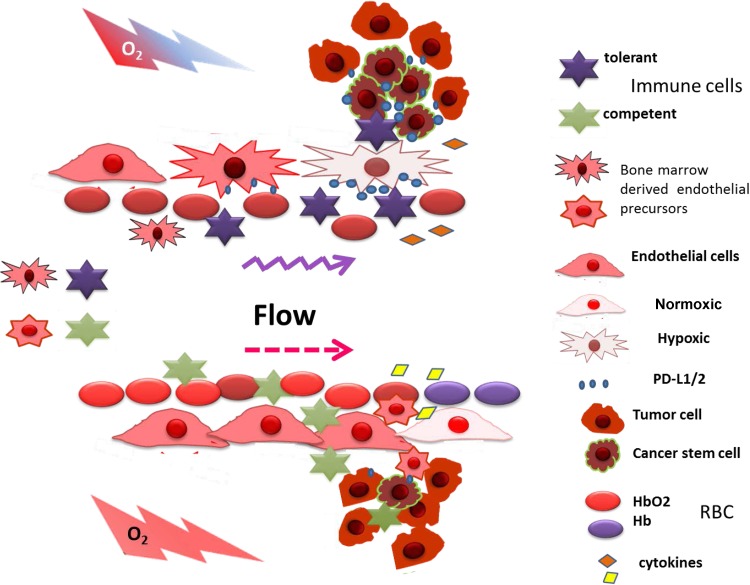
Normalization approaches directed to the VEGF/VEGFR blockade have given promising data in the perspective of tumor immunotherapy by increasing CTL entry into the tumor mass. Normalization was found to direct macrophage polarization to the M1 phenotype and, inducing a Th1 immune cytokine response by IFN-γ and IL-12, allows CTL infiltration and correlates with a reduced Treg cell recruitment (Fig. 3) (53). CD4+CD25+Foxp3+ Treg cells are recruited by CCL-22 (23) and CCL-28 induced by tumor hypoxia (31, 110). CTL and NK cell entry and activity (73a, 109a) are regulated by the immune checkpoint proteins CTLA4 and PD1/PD-L1/PD-L2. PD-L1 and PD-L2 are also expressed on the endothelial cell surface (95) and appear to restrict extravasation of T cells into the tumor (39). Hence, PD-L1 regulation by endothelial phosphatase and tensin homolog activity (89) is a potential target for normalization-improved antitumor immunotherapy (52).
Fig. 3.
Influence of hypoxia on tumor microenvironment-mediated immune cell recruitment. Under hypoxia, the tumor microenvironment recruits tolerance-inducing immune cells: T regulatory cells and M2 tumor-associated macrophages. When normalized, tumor vessels alleviate hypoxia and change the cell recruitment signals (cytokines) to attract immunocompetent programmed death-ligand 1 (PD-L1)-positive cells, such as NK cells and CTLs. Hypoxia compensation reduces expression of immune checkpoint ligands (PD-L1 and PD-L2) on tumor cells, immune suppressor cells, and endothelial cells, allowing the immune cells to extravasate the normalized vessel wall, meet tumor cells, and exert their cytotoxic action.
In addition, the closed cross talk between vessel normalization/hypoxia alleviation and immune response was confirmed by Treg cell depletion, which restored functional vessels (57). A vessel normalization strategy based on VEGF/VEGFR2 (adapted to be active only under hypoxia) leads to hypoxia alleviation and tumor reduction (19–21). Very recently, chloroquine was also reported to induce endothelial cell Notch1-mediated tumor vessel normalization (63). The allosteric effector of hemoglobin, inositol trispyrophosphate, was also found to normalize vessels and to increase Po2 by improving O2 delivery at hypoxic sites (40, 52).
Indeed, efforts are focused on the identification of pertinent ways to remodel the tumor microenvironment through vascular normalization to avoid tumor resistance and immune suppression. This could offer innovative therapeutic approaches allowing the development of new combinatorial antitumor-targeting therapeutic treatments based on the combination of a stable normalization-inducing strategy with peptide vaccination and immune checkpoint molecule inactivation.
Concluding Remarks
The role of the microenvironment during the progression of carcinogenesis is now realized to be of critical importance for an enhanced understanding of fundamental cancer biology, as well as exploitation of this source of relatively new knowledge for improved immune intervention. Approaches such as those using antibodies, adoptive cell transfer, or chimeric antigen receptor are opening new treatment opportunities for cancer immunotherapies. However, most immunotherapeutic approaches on their own are of limited value against the majority of malignancies, as solid tumors create a hostile hypoxic microenvironment that can hamper cell-mediated immunity and dampen the efficacy of the immune response. It is well documented that intratumoral hypoxia can cause genetic changes that promote a microenvironment that selects cells of a more aggressive phenotype. In addition, several regulatory mechanisms can occur concurrently within the hypoxic tumor microenvironment, resulting in multiple redundant levels of immune cell plasticity and suppression, tumor plasticity, and functional heterogeneity. In this regard, several elements of the hypoxia-response pathway are good candidates for therapeutic targeting. Exploitation of hypoxia-signaling pathways for clinical application, in particular in solid tumors, is challenging. It will be of major interest to take into account the manipulation of hypoxic stress in future integrative and innovative cancer immunotherapy approaches. Targeting the hypoxic tumor microenvironment to awaken or reawaken immune cells or to redirect it from a protumor to an antitumor state is very pertinent. In this context, remodeling of the tumor microenvironment through vascular normalization to avoid tumor resistance and immune suppression could also offer innovative therapeutic approaches allowing the development of new combinatorial antitumor-targeting therapeutic strategies.
GRANTS
This work was supported by a grant from Equipe Labellisée par la Ligue Contre le Cancer.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
M.Z.N., C.K., and B.J. prepared the figures; M.Z.N., M.H., Y.M., S.T., C.K., B.J., and S.C. drafted the manuscript; M.Z.N., M.H., S.T., C.K., B.J., and S.C. edited and revised the manuscript; M.Z.N., M.H., S.T., C.K., B.J., and S.C. approved the final version of the manuscript.
REFERENCES
- 1.Akalay I, Janji B, Hasmim M, Noman MZ, Andre F, De Cremoux P, Bertheau P, Badoual C, Vielh P, Larsen AK, Sabbah M, Tan TZ, Keira JH, Hung NT, Thiery JP, Mami-Chouaib F, Chouaib S. Epithelial-to-mesenchymal transition and autophagy induction in breast carcinoma promote escape from T-cell-mediated lysis. Cancer Res 73: 2418–2427, 2013. [DOI] [PubMed] [Google Scholar]
- 2.Akalay I, Janji B, Hasmim M, Noman MZ, Thiery JP, Mami-Chouaib F, Chouaib S. EMT impairs breast carcinoma cell susceptibility to CTL-mediated lysis through autophagy induction. Autophagy 9: 1104–1106, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Akalay I, Tan TZ, Kumar P, Janji B, Mami-Chouaib F, Charpy C, Vielh P, Larsen AK, Thiery JP, Sabbah M, Chouaib S. Targeting WNT1-inducible signaling pathway protein 2 alters human breast cancer cell susceptibility to specific lysis through regulation of KLF-4 and miR-7 expression. Oncogene 34: 2261–2271, 2015. [DOI] [PubMed] [Google Scholar]
- 4.Allavena P, Piemonti L, Longoni D, Bernasconi S, Stoppacciaro A, Ruco L, Mantovani A. IL-10 prevents the differentiation of monocytes to dendritic cells but promotes their maturation to macrophages. Eur J Immunol 28: 359–369, 1998. [DOI] [PubMed] [Google Scholar]
- 5.Ameri K, Luong R, Zhang H, Powell AA, Montgomery KD, Espinosa I, Bouley DM, Harris AL, Jeffrey SS. Circulating tumour cells demonstrate an altered response to hypoxia and an aggressive phenotype. Br J Cancer 102: 561–569, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Azab AK, Hu J, Quang P, Azab F, Pitsillides C, Awwad R, Thompson B, Maiso P, Sun JD, Hart CP, Roccaro AM, Sacco A, Ngo HT, Lin CP, Kung AL, Carrasco RD, Vanderkerken K, Ghobrial IM. Hypoxia promotes dissemination of multiple myeloma through acquisition of epithelial to mesenchymal transition-like features. Blood 119: 5782–5794, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Baginska J, Viry E, Berchem G, Poli A, Noman MZ, van Moer K, Medves S, Zimmer J, Oudin A, Niclou SP, Bleackley RC, Goping IS, Chouaib S, Janji B. Granzyme B degradation by autophagy decreases tumor cell susceptibility to natural killer-mediated lysis under hypoxia. Proc Natl Acad Sci USA 110: 17450–17455, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Banchereau J, Steinman RM. Dendritic cells and the control of immunity. Nature 392: 245–252, 1998. [DOI] [PubMed] [Google Scholar]
- 9.Barsoum IB, Hamilton TK, Li X, Cotechini T, Miles EA, Siemens DR, Graham CH. Hypoxia induces escape from innate immunity in cancer cells via increased expression of ADAM10: role of nitric oxide. Cancer Res 71: 7433–7441, 2011. [DOI] [PubMed] [Google Scholar]
- 10.Barsoum IB, Smallwood CA, Siemens DR, Graham CH. A mechanism of hypoxia-mediated escape from adaptive immunity in cancer cells. Cancer Res 74: 665–674, 2014. [DOI] [PubMed] [Google Scholar]
- 11.Burke B, Giannoudis A, Corke KP, Gill D, Wells M, Ziegler-Heitbrock L, Lewis CE. Hypoxia-induced gene expression in human macrophages: implications for ischemic tissues and hypoxia-regulated gene therapy. Am J Pathol 163: 1233–1243, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Caldwell CC, Kojima H, Lukashev D, Armstrong J, Farber M, Apasov SG, Sitkovsky MV. Differential effects of physiologically relevant hypoxic conditions on T lymphocyte development and effector functions. J Immunol 167: 6140–6149, 2001. [DOI] [PubMed] [Google Scholar]
- 13.Chaturvedi P, Gilkes DM, Takano N, Semenza GL. Hypoxia-inducible factor-dependent signaling between triple-negative breast cancer cells and mesenchymal stem cells promotes macrophage recruitment. Proc Natl Acad Sci USA 111: E2120–E2129, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chen L, Gibbons DL, Goswami S, Cortez MA, Ahn YH, Byers LA, Zhang X, Yi X, Dwyer D, Lin W, Diao L, Wang J, Roybal JD, Patel M, Ungewiss C, Peng D, Antonia S, Mediavilla-Varela M, Robertson G, Jones S, Suraokar M, Welsh JW, Erez B, Wistuba II, Wang S, Ullrich SE, Heymach JV, Kurie JM, Qin FX. Metastasis is regulated via microRNA-200/ZEB1 axis control of tumour cell PD-L1 expression and intratumoral immunosuppression. Nat Commun 5: 5241, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chen Y, De Marco MA, Graziani I, Gazdar AF, Strack PR, Miele L, Bocchetta M. Oxygen concentration determines the biological effects of NOTCH-1 signaling in adenocarcinoma of the lung. Cancer Res 67: 7954–7959, 2007. [DOI] [PubMed] [Google Scholar]
- 16.Cheng ZX, Wang DW, Liu T, Liu WX, Xia WB, Xu J, Zhang YH, Qu YK, Guo LQ, Ding L, Hou J, Zhong ZH. Effects of the HIF-1α and NF-κB loop on epithelial-mesenchymal transition and chemoresistance induced by hypoxia in pancreatic cancer cells. Oncol Rep 31: 1891–1898, 2014. [DOI] [PubMed] [Google Scholar]
- 17.Chouaib S, El Hage F, Benlalam H, Mami-Chouaib F. [Immunotherapy of cancer: promise and reality]. Med Sci 22: 755–759, 2006. [DOI] [PubMed] [Google Scholar]
- 18.Clambey ET, McNamee EN, Westrich JA, Glover LE, Campbell EL, Jedlicka P, de Zoeten EF, Cambier JC, Stenmark KR, Colgan SP, Eltzschig HK. Hypoxia-inducible factor-1-dependent induction of FoxP3 drives regulatory T-cell abundance and function during inflammatory hypoxia of the mucosa. Proc Natl Acad Sci USA 109: E2784–E2793, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Collet G, Grillon C, Nadim M, Kieda C. Trojan horse at cellular level for tumor gene therapies. Gene 525: 208–216, 2013. [DOI] [PubMed] [Google Scholar]
- 20.Collet G, Lamerant-Fayel N, Tertil M, El Hafny-Rahbi B, Stepniewski J, Guichard A, Foucault-Collet A, Klimkiewicz K, Petoud S, Matejuk A, Grillon C, Jozkowicz A, Dulak J, Kieda C. Hypoxia-regulated overexpression of soluble VEGFR2 controls angiogenesis and inhibits tumor growth. Mol Cancer Ther 13: 165–178, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Collet G, Skrzypek K, Grillon C, Matejuk A, El Hafni-Rahbi B, Lamerant-Fayel N, and Kieda C. Hypoxia control to normalize pathologic angiogenesis: potential role for endothelial precursor cells and miRNAs regulation. Vasc Pharmacol 56: 252–261, 2012. [DOI] [PubMed] [Google Scholar]
- 22.Corzo CA, Condamine T, Lu L, Cotter MJ, Youn JI, Cheng P, Cho HI, Celis E, Quiceno DG, Padhya T, McCaffrey TV, McCaffrey JC, Gabrilovich DI. HIF-1α regulates function and differentiation of myeloid-derived suppressor cells in the tumor microenvironment. J Exp Med 207: 2439–2453, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Curiel TJ, Coukos G, Zou L, Alvarez X, Cheng P, Mottram P, Evdemon-Hogan M, Conejo-Garcia JR, Zhang L, Burow M, Zhu Y, Wei S, Kryczek I, Daniel B, Gordon A, Myers L, Lackner A, Disis ML, Knutson KL, Chen L, Zou W. Specific recruitment of regulatory T cells in ovarian carcinoma fosters immune privilege and predicts reduced survival. Nat Med 10: 942–949, 2004. [DOI] [PubMed] [Google Scholar]
- 24.Curiel TJ, Wei S, Dong H, Alvarez X, Cheng P, Mottram P, Krzysiek R, Knutson KL, Daniel B, Zimmermann MC, David O, Burow M, Gordon A, Dhurandhar N, Myers L, Berggren R, Hemminki A, Alvarez RD, Emilie D, Curiel DT, Chen L, Zou W. Blockade of B7-H1 improves myeloid dendritic cell-mediated antitumor immunity. Nat Med 9: 562–567, 2003. [DOI] [PubMed] [Google Scholar]
- 25.Dang EV, Barbi J, Yang HY, Jinasena D, Yu H, Zheng Y, Bordman Z, Fu J, Kim Y, Yen HR, Luo W, Zeller K, Shimoda L, Topalian SL, Semenza GL, Dang CV, Pardoll DM, Pan F. Control of TH17/Treg balance by hypoxia-inducible factor 1. Cell 146: 772–784, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dawood S, Broglio K, Valero V, Reuben J, Handy B, Islam R, Jackson S, Hortobagyi GN, Fritsche H, Cristofanilli M. Circulating tumor cells in metastatic breast cancer: from prognostic stratification to modification of the staging system? Cancer 113: 2422–2430, 2008. [DOI] [PubMed] [Google Scholar]
- 27.Doedens AL, Phan AT, Stradner MH, Fujimoto JK, Nguyen JV, Yang E, Johnson RS, Goldrath AW. Hypoxia-inducible factors enhance the effector responses of CD8+ T cells to persistent antigen. Nat Immunol 14: 1173–1182, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Doedens AL, Stockmann C, Rubinstein MP, Liao D, Zhang N, DeNardo DG, Coussens LM, Karin M, Goldrath AW, Johnson RS. Macrophage expression of hypoxia-inducible factor-1α suppresses T-cell function and promotes tumor progression. Cancer Res 70: 7465–7475, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dunn GP, Old LJ, Schreiber RD. The immunobiology of cancer immunosurveillance and immunoediting. Immunity 21: 137–148, 2004. [DOI] [PubMed] [Google Scholar]
- 30.Eliane JP, Repollet M, Luker KE, Brown M, Rae JM, Dontu G, Schott AF, Wicha M, Doyle GV, Hayes DF, Luker GD. Monitoring serial changes in circulating human breast cancer cells in murine xenograft models. Cancer Res 68: 5529–5532, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Facciabene A, Peng X, Hagemann IS, Balint K, Barchetti A, Wang LP, Gimotty PA, Gilks CB, Lal P, Zhang L, Coukos G. Tumour hypoxia promotes tolerance and angiogenesis via CCL28 and Treg cells. Nature 475: 226–230, 2011. [DOI] [PubMed] [Google Scholar]
- 32.Fingleton B, Vargo-Gogola T, Crawford HC, Matrisian LM. Matrilysin (MMP-7) expression selects for cells with reduced sensitivity to apoptosis. Neoplasia 3: 459–468, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Finlay DK, Rosenzweig E, Sinclair LV, Feijoo-Carnero C, Hukelmann JL, Rolf J, Panteleyev AA, Okkenhaug K, Cantrell DA. PDK1 regulation of mTOR and hypoxia-inducible factor 1 integrate metabolism and migration of CD8+ T cells. J Exp Med 209: 2441–2453, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gabrilovich D. Mechanisms and functional significance of tumour-induced dendritic-cell defects. Nat Rev 4: 941–952, 2004. [DOI] [PubMed] [Google Scholar]
- 35.Gabrilovich D, Ishida T, Oyama T, Ran S, Kravtsov V, Nadaf S, Carbone DP. Vascular endothelial growth factor inhibits the development of dendritic cells and dramatically affects the differentiation of multiple hematopoietic lineages in vivo. Blood 92: 4150–4166, 1998. [PubMed] [Google Scholar]
- 36.Gabrilovich DI, Chen HL, Girgis KR, Cunningham HT, Meny GM, Nadaf S, Kavanaugh D, Carbone DP. Production of vascular endothelial growth factor by human tumors inhibits the functional maturation of dendritic cells. Nat Med 2: 1096–1103, 1996. [DOI] [PubMed] [Google Scholar]
- 37.Gabrilovich DI, Nagaraj S. Myeloid-derived suppressor cells as regulators of the immune system. Nat Rev 9: 162–174, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Goel S, Duda DG, Xu L, Munn LL, Boucher Y, Fukumura D, Jain RK. Normalization of the vasculature for treatment of cancer and other diseases. Physiol Rev 91: 1071–1121, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Grabie N, Gotsman I, DaCosta R, Pang H, Stavrakis G, Butte MJ, Keir ME, Freeman GJ, Sharpe AH, Lichtman AH. Endothelial programmed death-ligand 1 (PD-L1) regulates CD8+ T-cell mediated injury in the heart. Circulation 116: 2062–2071, 2007. [DOI] [PubMed] [Google Scholar]
- 40.Grillon C, Matejuk A, Nadim M, Lamerant-Fayel N, Kieda C. News on microenvironmental physioxia to revisit skin cell targeting approaches. Exp Dermatol 21: 723–728, 2012. [DOI] [PubMed] [Google Scholar]
- 41.Gupta GP, Massague J. Cancer metastasis: building a framework. Cell 127: 679–695, 2006. [DOI] [PubMed] [Google Scholar]
- 42.Hashimoto T, Shibasaki F. Hypoxia-inducible factor as an angiogenic master switch. Front Pediatr 3: 33, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hasmim M, Noman MZ, Lauriol J, Benlalam H, Mallavialle A, Rosselli F, Mami-Chouaib F, Alcaide-Loridan C, Chouaib S. Hypoxia-dependent inhibition of tumor cell susceptibility to CTL-mediated lysis involves NANOG induction in target cells. J Immunol 187: 4031–4039, 2011. [DOI] [PubMed] [Google Scholar]
- 44.Hasmim M, Noman MZ, Messai Y, Bordereaux D, Gros G, Baud V, Chouaib S. Cutting edge: hypoxia-induced Nanog favors the intratumoral infiltration of regulatory T cells and macrophages via direct regulation of TGF-β1. J Immunol 191: 5802–5806, 2013. [DOI] [PubMed] [Google Scholar]
- 45.Heddleston JM, Li Z, McLendon RE, Hjelmeland AB, Rich JN. The hypoxic microenvironment maintains glioblastoma stem cells and promotes reprogramming towards a cancer stem cell phenotype. Cell Cycle 8: 3274–3284, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Huang J, Yao X, Zhang J, Dong B, Chen Q, Xue W, Liu D, Huang Y. Hypoxia-induced downregulation of miR-30c promotes epithelial-mesenchymal transition in human renal cell carcinoma. Cancer Sci 104: 1609–1617, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Jain RK. Antiangiogenesis strategies revisited: from starving tumors to alleviating hypoxia. Cancer Cell 26: 605–622, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Jogi A, Ora I, Nilsson H, Lindeheim A, Makino Y, Poellinger L, Axelson H, Pahlman S. Hypoxia alters gene expression in human neuroblastoma cells toward an immature and neural crest-like phenotype. Proc Natl Acad Sci USA 99: 7021–7026, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Jung HY, Fattet L, Yang J. Molecular pathways: linking tumor microenvironment to epithelial-mesenchymal transition in metastasis. Clin Cancer Res 21: 962–968, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kallergi G, Markomanolaki H, Giannoukaraki V, Papadaki MA, Strati A, Lianidou ES, Georgoulias V, Mavroudis D, Agelaki S. Hypoxia-inducible factor-1α and vascular endothelial growth factor expression in circulating tumor cells of breast cancer patients. Breast Cancer Res 11: R84, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Keith B, Simon MC. Hypoxia-inducible factors, stem cells, and cancer. Cell 129: 465–472, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kieda C, El Hafny-Rahbi B, Collet G, Lamerant-Fayel N, Grillon C, Guichard A, Dulak J, Jozkowicz A, Kotlinowski J, Fylaktakidou KC, Vidal A, Auzeloux P, Miot-Noirault E, Beloeil JC, Lehn JM, and Nicolau C. Stable tumor vessel normalization with Po2 increase and endothelial PTEN activation by inositol trispyrophosphate brings novel tumor treatment. J Mol Med 91: 883–899, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Klug F, Prakash H, Huber PE, Seibel T, Bender N, Halama N, Pfirschke C, Voss RH, Timke C, Umansky L, Klapproth K, Schakel K, Garbi N, Jager D, Weitz J, Schmitz-Winnenthal H, Hammerling GJ, Beckhove P. Low-dose irradiation programs macrophage differentiation to an iNOS+/M1 phenotype that orchestrates effective T cell immunotherapy. Cancer Cell 24: 589–602, 2013. [DOI] [PubMed] [Google Scholar]
- 54.Kudo-Saito C, Shirako H, Takeuchi T, Kawakami Y. Cancer metastasis is accelerated through immunosuppression during Snail-induced EMT of cancer cells. Cancer Cell 15: 195–206, 2009. [DOI] [PubMed] [Google Scholar]
- 55.Lee JH, Elly C, Park Y, Liu YC. E3 ubiquitin ligase VHL regulates hypoxia-inducible factor-1α to maintain regulatory T cell stability and suppressive capacity. Immunity 42: 1062–1074, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Lewis JS, Landers RJ, Underwood JC, Harris AL, Lewis CE. Expression of vascular endothelial growth factor by macrophages is up-regulated in poorly vascularized areas of breast carcinomas. J Pathol 192: 150–158, 2000. [DOI] [PubMed] [Google Scholar]
- 57.Li X, Kostareli E, Suffner J, Garbi N, Hammerling GJ. Efficient Treg depletion induces T-cell infiltration and rejection of large tumors. Eur J Immunol 40: 3325–3335, 2010. [DOI] [PubMed] [Google Scholar]
- 58.Li Z, Bao S, Wu Q, Wang H, Eyler C, Sathornsumetee S, Shi Q, Cao Y, Lathia J, McLendon RE, Hjelmeland AB, Rich JN. Hypoxia-inducible factors regulate tumorigenic capacity of glioma stem cells. Cancer Cell 15: 501–513, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Liu G, Bi Y, Xue L, Zhang Y, Yang H, Chen X, Lu Y, Zhang Z, Liu H, Wang X, Wang R, Chu Y, Yang R. Dendritic cell SIRT1-HIF1α axis programs the differentiation of CD4+ T cells through IL-12 and TGF-β1. Proc Natl Acad Sci USA 112: E957–E965, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Lopez-Soto A, Huergo-Zapico L, Galvan JA, Rodrigo L, de Herreros AG, Astudillo A, Gonzalez S. Epithelial-mesenchymal transition induces an antitumor immune response mediated by NKG2D receptor. J Immunol 190: 4408–4419, 2013. [DOI] [PubMed] [Google Scholar]
- 61.Lukashev D, Caldwell C, Ohta A, Chen P, Sitkovsky M. Differential regulation of two alternatively spliced isoforms of hypoxia-inducible factor-1α in activated T lymphocytes. J Biol Chem 276: 48754–48763, 2001. [DOI] [PubMed] [Google Scholar]
- 62.Lundgren K, Nordenskjold B, Landberg G. Hypoxia, Snail and incomplete epithelial-mesenchymal transition in breast cancer. Br J Cancer 101: 1769–1781, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Maes H, Kuchnio A, Peric A, Moens S, Nys K, De Bock K, Quaegebeur A, Schoors S, Georgiadou M, Wouters J, Vinckier S, Vankelecom H, Garmyn M, Vion AC, Radtke F, Boulanger C, Gerhardt H, Dejana E, Dewerchin M, Ghesquiere B, Annaert W, Agostinis P, Carmeliet P. Tumor vessel normalization by chloroquine independent of autophagy. Cancer Cell 26: 190–206, 2014. [DOI] [PubMed] [Google Scholar]
- 64.Mak P, Leav I, Pursell B, Bae D, Yang X, Taglienti CA, Gouvin LM, Sharma VM, Mercurio AM. ERβ impedes prostate cancer EMT by destabilizing HIF-1α and inhibiting VEGF-mediated Snail nuclear localization: implications for Gleason grading. Cancer Cell 17: 319–332, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Mancino A, Schioppa T, Larghi P, Pasqualini F, Nebuloni M, Chen IH, Sozzani S, Austyn JM, Mantovani A, Sica A. Divergent effects of hypoxia on dendritic cell functions. Blood 112: 3723–3734, 2008. [DOI] [PubMed] [Google Scholar]
- 66.Mani SA, Guo W, Liao MJ, Eaton EN, Ayyanan A, Zhou AY, Brooks M, Reinhard F, Zhang CC, Shipitsin M, Campbell LL, Polyak K, Brisken C, Yang J, Weinberg RA. The epithelial-mesenchymal transition generates cells with properties of stem cells. Cell 133: 704–715, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Mantovani A, Sozzani S, Locati M, Allavena P, Sica A. Macrophage polarization: tumor-associated macrophages as a paradigm for polarized M2 mononuclear phagocytes. Trends Immunol 23: 549–555, 2002. [DOI] [PubMed] [Google Scholar]
- 68.Mathieu J, Zhang Z, Zhou W, Wang AJ, Heddleston JM, Pinna CM, Hubaud A, Stadler B, Choi M, Bar M, Tewari M, Liu A, Vessella R, Rostomily R, Born D, Horwitz M, Ware C, Blau CA, Cleary MA, Rich JN, Ruohola-Baker H. HIF induces human embryonic stem cell markers in cancer cells. Cancer Res 71: 4640–4652, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Matsuoka J, Yashiro M, Doi Y, Fuyuhiro Y, Kato Y, Shinto O, Noda S, Kashiwagi S, Aomatsu N, Hirakawa T, Hasegawa T, Shimizu K, Shimizu T, Miwa A, Yamada N, Sawada T, Hirakawa K. Hypoxia stimulates the EMT of gastric cancer cells through autocrine TGFβ signaling. PLos One 8: e62310, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.McCord AM, Jamal M, Shankavaram UT, Lang FF, Camphausen K, Tofilon PJ. Physiologic oxygen concentration enhances the stem-like properties of CD133+ human glioblastoma cells in vitro. Mol Cancer Res 7: 489–497, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Messai Y, Noman MZ, Hasmim M, Escudier B, Chouaib S. HIF-2α/ITPR1 axis: a new saboteur of NK-mediated lysis. Oncoimmunology 4: e985951, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Messai Y, Noman MZ, Hasmim M, Janji B, Tittarelli A, Boutet M, Baud V, Viry E, Billot K, Nanbakhsh A, Ben Safta T, Richon C, Ferlicot S, Donnadieu E, Couve S, Gardie B, Orlanducci F, Albiges L, Thiery J, Olive D, Escudier B, Chouaib S. ITPR1 protects renal cancer cells against natural killer cells by inducing autophagy. Cancer Res 74: 6820–6832, 2014. [DOI] [PubMed] [Google Scholar]
- 73.Messai Y, Noman MZ, Janji B, Hasmim M, Escudier B, Chouaib S. The autophagy sensor ITPR1 protects renal carcinoma cells from NK-mediated killing. Autophagy 25: 0, 2015. [DOI] [PubMed] [Google Scholar]
- 73a.Mondini M, Nizard M, Tran T, Mauge L, Loi M, Clemenson C, Dugue D, Mouroun P, Louvel E, Adam J, Badoual C, Helley C, Dransart E, Johannes L, Vozenin MC, Perfettini JL, Tartour E, Deutsch E. Synergy of radiotherapy and a cancer vaccine for the treatment of HPV-associated head and neck cancer. Mol Cancer Ther 14: 1336–1345, 2015. [DOI] [PubMed] [Google Scholar]
- 74.Moreno-Manzano V, Rodriguez-Jimenez FJ, Acena-Bonilla JL, Fustero-Lardies S, Erceg S, Dopazo J, Montaner D, Stojkovic M, Sanchez-Puelles JM. FM19G11, a new hypoxia-inducible factor (HIF) modulator, affects stem cell differentiation status. J Biol Chem 285: 1333–1342, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Morrison SJ, Perez SE, Qiao Z, Verdi JM, Hicks C, Weinmaster G, Anderson DJ. Transient Notch activation initiates an irreversible switch from neurogenesis to gliogenesis by neural crest stem cells. Cell 101: 499–510, 2000. [DOI] [PubMed] [Google Scholar]
- 76.Nakamura H, Makino Y, Okamoto K, Poellinger L, Ohnuma K, Morimoto C, Tanaka H. TCR engagement increases hypoxia-inducible factor-1α protein synthesis via rapamycin-sensitive pathway under hypoxic conditions in human peripheral T cells. J Immunol 174: 7592–7599, 2005. [DOI] [PubMed] [Google Scholar]
- 77.Noh KH, Kim BW, Song KH, Cho H, Lee YH, Kim JH, Chung JY, Hewitt SM, Seong SY, Mao CP, Wu TC, Kim TW. Nanog signaling in cancer promotes stem-like phenotype and immune evasion. J Clin Invest 122: 4077–4093, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Noman MZ, Buart S, Van Pelt J, Richon C, Hasmim M, Leleu N, Suchorska WM, Jalil A, Lecluse Y, El Hage F, Giuliani M, Pichon C, Azzarone B, Mazure N, Romero P, Mami-Chouaib F, Chouaib S. The cooperative induction of hypoxia-inducible factor-1α and STAT3 during hypoxia induced an impairment of tumor susceptibility to CTL-mediated cell lysis. J Immunol 182: 3510–3521, 2009. [DOI] [PubMed] [Google Scholar]
- 79.Noman MZ, Chouaib S. Targeting hypoxia at the forefront of anticancer immune responses. Oncoimmunology 3: e954463, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Noman MZ, Desantis G, Janji B, Hasmim M, Karray S, Dessen P, Bronte V, Chouaib S. PD-L1 is a novel direct target of HIF-1α, and its blockade under hypoxia enhanced MDSC-mediated T cell activation. J Exp Med 211: 781–790, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Noman MZ, Janji B, Berchem G, Mami-Chouaib F, Chouaib S. Hypoxia-induced autophagy: a new player in cancer immunotherapy? Autophagy 8: 704–706, 2012. [DOI] [PubMed] [Google Scholar]
- 82.Noman MZ, Janji B, Kaminska B, Van Moer K, Pierson S, Przanowski P, Buart S, Berchem G, Romero P, Mami-Chouaib F, Chouaib S. Blocking hypoxia-induced autophagy in tumors restores cytotoxic T-cell activity and promotes regression. Cancer Res 71: 5976–5986, 2011. [DOI] [PubMed] [Google Scholar]
- 83.Noman MZ, Messai Y, Carre T, Akalay I, Meron M, Janji B, Hasmim M, Chouaib S. Microenvironmental hypoxia orchestrating the cell stroma cross talk, tumor progression and antitumor response. Crit Rev Immunol 31: 357–377, 2011. [DOI] [PubMed] [Google Scholar]
- 84.Ohta A, Diwanji R, Kini R, Subramanian M, Sitkovsky M. In vivo T cell activation in lymphoid tissues is inhibited in the oxygen-poor microenvironment. Front Immunol 2: 27, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Osada T, Chong G, Tansik R, Hong T, Spector N, Kumar R, Hurwitz HI, Dev I, Nixon AB, Lyerly HK, Clay T, Morse MA. The effect of anti-VEGF therapy on immature myeloid cell and dendritic cells in cancer patients. Cancer Immunol Immunother 57: 1115–1124, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Paez-Ribes M, Allen E, Hudock J, Takeda T, Okuyama H, Vinals F, Inoue M, Bergers G, Hanahan D, Casanovas O. Antiangiogenic therapy elicits malignant progression of tumors to increased local invasion and distant metastasis. Cancer Cell 15: 220–231, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Palazon A, Martinez-Forero I, Teijeira A, Morales-Kastresana A, Alfaro C, Sanmamed MF, Perez-Gracia JL, Penuelas I, Hervas-Stubbs S, Rouzaut A, de Landazuri MO, Jure-Kunkel M, Aragones J, Melero I. The HIF-1α hypoxia response in tumor-infiltrating T lymphocytes induces functional CD137 (4-1BB) for immunotherapy. Cancer Discovery 2: 608–623, 2012. [DOI] [PubMed] [Google Scholar]
- 88.Pardal R, Clarke MF, Morrison SJ. Applying the principles of stem-cell biology to cancer. Nat Rev Cancer 3: 895–902, 2003. [DOI] [PubMed] [Google Scholar]
- 89.Parsa AT, Waldron JS, Panner A, Crane CA, Parney IF, Barry JJ, Cachola KE, Murray JC, Tihan T, Jensen MC, Mischel PS, Stokoe D, Pieper RO. Loss of tumor suppressor PTEN function increases B7-H1 expression and immunoresistance in glioma. Nat Med 13: 84–88, 2007. [DOI] [PubMed] [Google Scholar]
- 90.Peinado H, Del Carmen Iglesias-de la Cruz M, Olmeda D, Csiszar K, Fong KS, Vega S, Nieto MA, Cano A, Portillo F. A molecular role for lysyl oxidase-like 2 enzyme in snail regulation and tumor progression. EMBO J 24: 3446–3458, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Pietras A, Gisselsson D, Ora I, Noguera R, Beckman S, Navarro S, Pahlman S. High levels of HIF-2α highlight an immature neural crest-like neuroblastoma cell cohort located in a perivascular niche. J Pathol 214: 482–488, 2008. [DOI] [PubMed] [Google Scholar]
- 92.Rausch ME, Weisberg S, Vardhana P, Tortoriello DV. Obesity in C57BL/6J mice is characterized by adipose tissue hypoxia and cytotoxic T-cell infiltration. Int J Obes (Lond) 32: 451–463, 2008. [DOI] [PubMed] [Google Scholar]
- 93.Reya T, Morrison SJ, Clarke MF, Weissman IL. Stem cells, cancer, and cancer stem cells. Nature 414: 105–111, 2001. [DOI] [PubMed] [Google Scholar]
- 94.Ricciardi M, Zanotto M, Malpeli G, Bassi G, Perbellini O, Chilosi M, Bifari F, Krampera M. Epithelial-to-mesenchymal transition (EMT) induced by inflammatory priming elicits mesenchymal stromal cell-like immune-modulatory properties in cancer cells. Br J Cancer 112: 1067–1075, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Rodig N, Ryan T, Allen JA, Pang H, Grabie N, Chernova T, Greenfield EA, Liang SC, Sharpe AH, Lichtman AH, Freeman GJ. Endothelial expression of PD-L1 and PD-L2 down-regulates CD8+ T cell activation and cytolysis. Eur J Immunol 33: 3117–3126, 2003. [DOI] [PubMed] [Google Scholar]
- 96.Sahlgren C, Gustafsson MV, Jin S, Poellinger L, Lendahl U. Notch signaling mediates hypoxia-induced tumor cell migration and invasion. Proc Natl Acad Sci USA 105: 6392–6397, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Salnikov AV, Liu L, Platen M, Gladkich J, Salnikova O, Ryschich E, Mattern J, Moldenhauer G, Werner J, Schemmer P, Buchler MW, Herr I. Hypoxia induces EMT in low and highly aggressive pancreatic tumor cells but only cells with cancer stem cell characteristics acquire pronounced migratory potential. PLos One 7: e46391, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Sato Y. Persistent vascular normalization as an alternative goal of anti-angiogenic cancer therapy. Cancer Sci 102: 1253–1256, 2011. [DOI] [PubMed] [Google Scholar]
- 99.Scholz CC, Taylor CT. Targeting the HIF pathway in inflammation and immunity. Curr Opin Pharmacol 13: 646–653, 2013. [DOI] [PubMed] [Google Scholar]
- 100.Scortegagna M, Martin RJ, Kladney RD, Neumann RG, Arbeit JM. Hypoxia-inducible factor-1α suppresses squamous carcinogenic progression and epithelial-mesenchymal transition. Cancer Res 69: 2638–2646, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Semenza GL. HIF-1 inhibitors for cancer therapy: from gene expression to drug discovery. Curr Pharm Design 15: 3839–3843, 2009. [DOI] [PubMed] [Google Scholar]
- 102.Semenza GL. Hydroxylation of HIF-1: oxygen sensing at the molecular level. Physiology (Bethesda) 19: 176–182, 2004. [DOI] [PubMed] [Google Scholar]
- 103.Semenza GL. Hypoxia-inducible factors in physiology and medicine. Cell 148: 399–408, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Semenza GL. Hypoxia-inducible factors: mediators of cancer progression and targets for cancer therapy. Trends Pharmacol Sci 33: 207–214, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Semenza GL. Vasculogenesis, angiogenesis, and arteriogenesis: mechanisms of blood vessel formation and remodeling. J Cell Biochem 102: 840–847, 2007. [DOI] [PubMed] [Google Scholar]
- 106.Serafini P, Mgebroff S, Noonan K, Borrello I. Myeloid-derived suppressor cells promote cross-tolerance in B-cell lymphoma by expanding regulatory T cells. Cancer Res 68: 5439–5449, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Shay JE, Celeste Simon M. Hypoxia-inducible factors: crosstalk between inflammation and metabolism. Semin Cell Dev Biol 23: 389–394, 2012. [DOI] [PubMed] [Google Scholar]
- 108.Sun L, Li H, Chen J, Iwasaki Y, Kubota T, Matsuoka M, Shen A, Chen Q, Xu Y. PIASy mediates hypoxia-induced SIRT1 transcriptional repression and epithelial-to-mesenchymal transition in ovarian cancer cells. J Cell Sci 126: 3939–3947, 2013. [DOI] [PubMed] [Google Scholar]
- 109.Takayama T, Morelli AE, Onai N, Hirao M, Matsushima K, Tahara H, Thomson AW. Mammalian and viral IL-10 enhance C-C chemokine receptor 5 but down-regulate C-C chemokine receptor 7 expression by myeloid dendritic cells: impact on chemotactic responses and in vivo homing ability. J Immunol 166: 7136–7143, 2001. [DOI] [PubMed] [Google Scholar]
- 109a.Tartour E, Pere H, Maillere B, Terme M, Merillon N, Taieb J, Sandoval F, Quintin-Colonna F, Lacerda K, Karadimou A, Badoual C, Tedgui A, Fridman WH, Oudard S. Angiogenesis and immunity: a bidirectional link potentially relevant for the monitoring of antiangiogenic therapy and the development of novel therapeutic combination with immunotherapy. Cancer Metastasis Rev 30: 83–95, 2011. [DOI] [PubMed] [Google Scholar]
- 110.Terme M, Pernot S, Marcheteau E, Sandoval F, Benhamouda N, Colussi O, Dubreuil O, Carpentier AF, Tartour E, Taieb J. VEGFA-VEGFR pathway blockade inhibits tumor-induced regulatory T-cell proliferation in colorectal cancer. Cancer Res 73: 539–549, 2013. [DOI] [PubMed] [Google Scholar]
- 111.Thiery JP, Acloque H, Huang RY, Nieto MA. Epithelial-mesenchymal transitions in development and disease. Cell 139: 871–890, 2009. [DOI] [PubMed] [Google Scholar]
- 112.Tinhofer I, Saki M, Niehr F, Keilholz U, Budach V. Cancer stem cell characteristics of circulating tumor cells. Int J Radiat Biol 90: 622–627, 2014. [DOI] [PubMed] [Google Scholar]
- 113.Trinchieri G. Interleukin-12 and the regulation of innate resistance and adaptive immunity. Nat Rev 3: 133–146, 2003. [DOI] [PubMed] [Google Scholar]
- 114.Viry E, Baginska J, Berchem G, Noman MZ, Medves S, Chouaib S, Janji B. Autophagic degradation of GZMB/granzyme B: a new mechanism of hypoxic tumor cell escape from natural killer cell-mediated lysis. Autophagy 10: 173–175, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Visvader JE, Lindeman GJ. Cancer stem cells in solid tumours: accumulating evidence and unresolved questions. Nat Rev Cancer 8: 755–768, 2008. [DOI] [PubMed] [Google Scholar]
- 116.Wang Y, Liu Y, Malek SN, Zheng P. Targeting HIF1α eliminates cancer stem cells in hematological malignancies. Cell Stem Cell 8: 399–411, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Werno C, Menrad H, Weigert A, Dehne N, Goerdt S, Schledzewski K, Kzhyshkowska J, Brune B. Knockout of HIF-1α in tumor-associated macrophages enhances M2 polarization and attenuates their pro-angiogenic responses. Carcinogenesis 31: 1863–1872, 2010. [DOI] [PubMed] [Google Scholar]
- 118.White JR, Harris RA, Lee SR, Craigon MH, Binley K, Price T, Beard GL, Mundy CR, Naylor S. Genetic amplification of the transcriptional response to hypoxia as a novel means of identifying regulators of angiogenesis. Genomics 83: 1–8, 2004. [DOI] [PubMed] [Google Scholar]
- 119.Wilson A, Radtke F. Multiple functions of Notch signaling in self-renewing organs and cancer. FEBS Lett 580: 2860–2868, 2006. [DOI] [PubMed] [Google Scholar]
- 120.Xiang L, Gilkes DM, Hu H, Takano N, Luo W, Lu H, Bullen JW, Samanta D, Liang H, Semenza GL. Hypoxia-inducible factor 1 mediates TAZ expression and nuclear localization to induce the breast cancer stem cell phenotype. Oncotarget 5: 12509–12527, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Yang MH, Wu MZ, Chiou SH, Chen PM, Chang SY, Liu CJ, Teng SC, Wu KJ. Direct regulation of TWIST by HIF-1α promotes metastasis. Nat Cell Biol 10: 295–305, 2008. [DOI] [PubMed] [Google Scholar]
- 122.Yu LX, Zhou L, Li M, Li ZW, Wang DS, Zhang SG. The Notch1/cyclooxygenase-2/Snail/E-cadherin pathway is associated with hypoxia-induced hepatocellular carcinoma cell invasion and migration. Oncol Rep 29: 362–370, 2013. [DOI] [PubMed] [Google Scholar]
- 123.Zhang L, Huang G, Li X, Zhang Y, Jiang Y, Shen J, Liu J, Wang Q, Zhu J, Feng X, Dong J, Qian C. Hypoxia induces epithelial-mesenchymal transition via activation of SNAIL by hypoxia-inducible factor-1α in hepatocellular carcinoma. BMC Cancer 13: 108, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Zhang W, Shi X, Peng Y, Wu M, Zhang P, Xie R, Wu Y, Yan Q, Liu S, Wang J. HIF-1α promotes epithelial-mesenchymal transition and metastasis through direct regulation of ZEB1 in colorectal cancer. PLos One 10: e0129603, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]