Abstract
Hypertonicity increases urea transport, as well as the phosphorylation and membrane accumulation of UT-A1, the transporter responsible for urea permeability in the inner medullary collect duct (IMCD). Hypertonicity stimulates urea transport through PKC-mediated phosphorylation. To determine whether PKC phosphorylates UT-A1, eight potential PKC phosphorylation sites were individually replaced with alanine and subsequently transfected into LLC-PK1 cells. Of the single mutants, only ablation of the S494 site dampened induction of total UT-A1 phosphorylation by the PKC activator phorbol dibutyrate (PDBu). This result was confirmed using a newly generated antibody that specifically detected phosphorylation of UT-A1 at S494. Hypertonicity increased UT-A1 phosphorylation at S494. In contrast, activators of cAMP pathways (PKA and Epac) did not increase UT-A1 phosphorylation at S494. Activation of both PKC and PKA pathways increased plasma membrane accumulation of UT-A1, although activation of PKC alone did not do so. However, ablating the PKC site S494 decreased UT-A1 abundance in the plasma membrane. This suggests that the cAMP pathway promotes UT-A1 trafficking to the apical membrane where the PKC pathway can phosphorylate the transporter, resulting in increased UT-A1 retention at the apical membrane. In summary, activation of PKC increases the phosphorylation of UT-A1 at a specific residue, S494. Although there is no cross talk with the cAMP-signaling pathway, phosphorylation of S494 through PKC may enhance vasopressin-stimulated urea permeability by retaining UT-A1 in the plasma membrane.
Keywords: vasopressin, urea transport, cAMP, UT-A1, PKC
transport of urea across the inner medullary collecting duct (IMCD) is vital to establishing the corticomedullary osmolality gradient that is necessary for producing a concentrated urine (19, 31). Once thought to be passive diffusion, several lines of evidence conclude that urea reabsorption is mediated by facilitated transport through urea transporters in the renal medulla (5, 27, 30). Exclusively located in the IMCD, the urea transporter UT-A1 plays a physiologically significant role in urine concentration as evidenced by the polyuria observed in the UT-A1/UT-A3 knockout mouse (6–8).
Regulation of UT-A1 activity by posttranslational modifications has only recently become a topic of interest and has particularly focused on vasopressin-mediated regulation of UT-A1 through stimulation of the cAMP pathway. Upon binding to the basolateral V2-vasopressin receptor, vasopressin increases cellular cAMP levels (15, 22). By triggering downstream targets of cAMP, vasopressin stimulates the phosphorylation and apical plasma membrane accumulation of UT-A1 through two cAMP-dependent signaling pathways: protein kinase A (PKA) and Epac (exchange protein activated by cAMP) (3, 23, 32, 35). PKA phosphorylates UT-A1 at two serines, S486 and S499 (3), and possibly at a third serine, S84 (13). UT-A1 phosphorylation at either S486 or S499 is required for an increase in UT-A1 apical plasma membrane accumulation and urea transport (3, 10, 18). Epac also phosphorylates UT-A1 (32), but the amino acid that is phosphorylated is unknown, except that it is not the PKA sites S486 or S499 (10).
Hypertonicity also increases UT-A1 phosphorylation, apical plasma membrane accumulation, and urea transport in the IMCD (14, 20, 28, 33, 34). Interestingly, the effect of hypertonicity is independent of vasopressin and is also synergistic with vasopressin (34). Recent studies show that hypertonicity stimulates UT-A1 through a protein kinase C (PKC) signaling pathway (20, 33, 34). The hypertonicity-stimulated increase in urea permeability is diminished by two PKC blockers, chelerythrine and rottlerin, and a direct PKC activator, phorbol dibutyrate (PDBu), further increases urea permeability above the vasopressin-stimulated level (34). All of these findings suggest that hypertonicity independently regulates UT-A1 and urea permeability in the IMCD through a PKC signaling pathway; however, if UT-A1 is directly phosphorylated by PKC, the amino acid(s) that this occurs at is unknown.
UT-A1 has eight putative PKC phosphorylation sites that have high probability of being the substrate(s) for the kinase. The goal of our investigation is to determine if one or more of these sites is a substrate for PKC and, if so, elucidate if PKC-mediated phosphorylation of UT-A1 works in concert with other known posttranslational modifications of the transporter. This investigation revealed that UT-A1 is phosphorylated through PKC at one site, S494. Further studies revealed that this site is phosphorylated in response to hypertonicity but is not targeted through cross talk with a cAMP-stimulated pathway, and phosphorylation of UT-A1 through PKC at S494 may be important for UT-A1 membrane localization.
METHODS
Animals.
All animal protocols were approved by the Emory University Institutional Animal Care and Use Committee. Male Sprague-Dawley rats (Charles River Laboratories, Wilmington, MA), weighing 100–150 g, received free access to water and standard rat chow (Test Diet 5001; Purina) containing 23% protein.
Kidneys were removed, and the inner medulla was collected and cut into small pieces. Inner medullary pieces were treated with either vehicle, a phorbol ester [phorbol dibutyrate (PDBu); 2 μM], a PKC inhibitor (chelerythrine, 10 μM), an Epac activator (Sp-8-pCPT-2′-O-methyl-cAMPS; 75 μM), or the adenylyl cyclase stimulator forskolin (10 μM) for 30 min in DMEM medium for 30 min at 37°C in a CO2 incubator. For hypertonic treatment of inner medullary tissue, osmolality was raised by adding sucrose to the incubation medium.
Site-directed mutagenesis.
Meta-prediction of PKC phosphorylation sites within rat UT-A1 was performed with the phosphorylation site-predicting program NetPhosK (1). Of the potential phosphorylation sites identified that were consistent with the consensus sequence for substrate recognition by PKC (17), we targeted the following eight residues: S23, S79, T447, S494, T545, T549, S554, and S910. Each site was mutated from rat UT-A1 with the QuickChange site-directed mutagenesis kit (Agilent Technologies, Santa Clara, CA) according to the manufacturer's instructions. The serine (S) or threonine (T) residue was substituted with an alanine (A) residue with the appropriately designed oligonucleotide resulting in the following mutated UT-A1 transporters: S23A, S79A, T447A, S494A, T545A, T549A, S554A, and S910A. All mutant constructs were verified by nucleotide sequence analysis (Macrogen, Rockville, MD).
Cell culture and transfections.
LLC-PK1 cells were maintained in DMEM containing 10% FBS, 5% l-glutamine, and 5% penicillin-streptomycin at 37°C and gassed with 5% CO2-95% air. Cells were grown on six-well plates. At 60% confluence, cells were transfected with UT-A1 (wild type), S23A, S79A, T447A, S494A, T545A, T549A, S554A, S910A, or vehicle, using Effectene (Qiagen, Hilden, Germany) according to the manufacturer's instructions. Maximal protein expression occurred after 48 h.
Generation of mouse IMCD3 cell lines stably expressing UT-A1.
Mouse (m)IMCD3 cells that stably expressed wild-type UT-A1 were generated as previously described (18). Briefly, mIMCD3 cells were transfected with pFRT/lacZeo using Lipofectamine 2000 (Invitrogen) and grown for 10 days in 100 μg/ml Zeocin to select for cells stably expressing the FRT recombination site. The resulting mIMCD3-FRT cell line was then cotransfected with pOG44 and with pcDNA5-FRT-UT-A1 for homologous recombination with the cell's FRT site. Cells that underwent the recombination and incorporation were selected for by hygromycin resistance (400 μg/ml hygromycin). After clonal selection, the stable line was maintained in equal mixtures of DMEM and Ham's F-12 medium, supplemented with 10% fetal bovine serum, 100 U/ml penicillin, and 100 μg/ml streptomycin at 37°C in a humidified atmosphere of 95% air-5% CO2.
Metabolic labeling with 32P-orthophosphate.
Metabolic labeling with 32P-orthophosphate was performed with transfected LLC-PK1 cells, mIMCD3-UT-A1 cells, and inner medullary rat tissue as previously published (2, 3, 18). Briefly, confluent cells or inner medulla pieces were incubated in phosphate-free DMEM containing 0.15 mCi/ml of 32P-orthophosphate. After the 3-h labeling period, cells or tissues were incubated for a further 30 min with either vehicle or the designated treatment(s). Lysates were prepared and UT-A1 was immunoprecipitated. Precipitated proteins were separated by SDS-PAGE, and radiolabeled UT-A1 was determined by autoradiography.
Generation and characterization of new phospho-specific antibody to S494.
To acquire the 494 phospho-antibody, we commissioned PhosphoSolutions (Aurora, CO) to assist in the generation of the antibody. We designed an immunizing peptide prepared against our site of interest: HIEWSS(P)IRRRSK[C]-NH2. With the use of this phospho-peptide, a rabbit was immunized and serum was collected 8 wk later. To isolate the 494 phospho-specific antibody, an IgG fraction was collected from the serum and applied to a phospho-peptide affinity column. This column bound both the 494 phospho-specific antibody and a pan-specific antibody. These two antibodies were eluted from the phosphopeptide affinity column and then applied to a dephosphopeptide affinity column. This column bound the pan-specific antibody allowing the 494 phospho-specific antibody to be collected in the flow through. The 494 phospho-specific antibody was first characterized at PhosphoSolutions by ELISA.
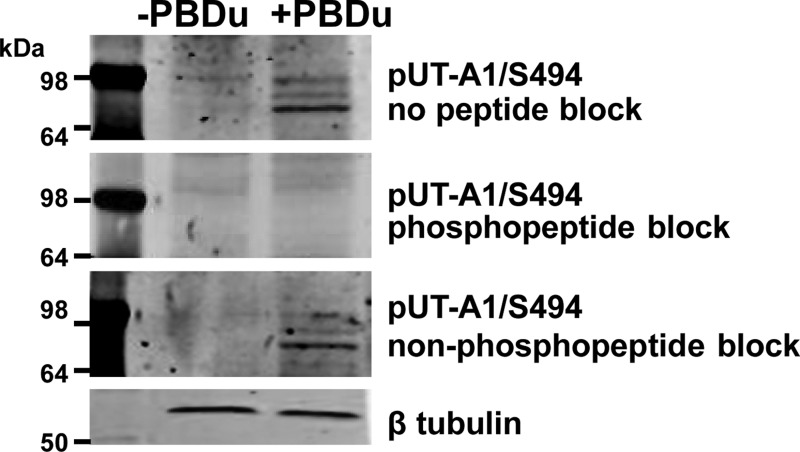
To demonstrate the specificity of the 494 phospho-specific antibody, we performed a peptide competition experiment with two control peptides; one with the sequence that recognizes the phosphorylated region by the antibody and a second corresponding nonphosphorylated peptide (both provided by PhosphoSolutions). Rat inner medullary tissue was incubated with either vehicle or PDBu (2 μM) for 30 min in DMEM at 37°C. Tissues were then homogenized, separated by SDS-PAGE, and transferred to a membrane. Blots were blocked as normal and incubated overnight at 4°C with the following: 1) 494 phospho-specific antibody with no added peptide; 2) 494 phospho-specific antibody that was preadsorbed with 1 μg/ml phosphopeptide; and 3) 494 phospho-specific antibody that was preadsorbed with 1 μg/ml nonphosphopeptide. As shown in Fig. 1, these studies demonstrated the specificity of the UT-A1 494 phospho-specific antibody.
Fig. 1.
Characterization of a newly generated phospho-specific UT-A1 antibody for residue S494. Rat inner medullary collecting duct tissues were incubated in the absence (−) or presence (+) of phorbol dibutyrate (PDBu; 2 μM) for 30 min at 37°C. Tissues were homogenized, separated by SDS-PAGE, and transferred to membranes. Blots were then probed with phospho-specific antibody (pUT-A1/S494) that was not preadsorbed with a peptide (1st row), pUT-A1/S494 preadsorbed with the phosphorylated peptide (2nd row) or pUT-A1/S494 preadsorbed with the nonphosphorylated peptide (3rf row). Equal loading was confirmed with β-tubulin (4th row).
Western blot analysis.
Proteins (20 μg/lane) were size-separated by SDS-PAGE on 10% gels and then electroblotted to polyvinylidene difluoride membranes (Immobilon; Millipore, Bedford, MA). After being blocked with 5% nonfat dry milk for 1 h, blots were incubated with primary antibody rocking overnight at 4°C. Primary antibodies used in these studies included the following: 1) a polyclonal antibody to the COOH terminal of UT-A1 to assess total UT-A1 at 1:1,000 (21); 2) a phospho-specific antibody to UT-A1 phosphorylated at S486 at 1:250 (18); 3) a phospho-specific antibody to UT-A1 phosphorylated at S499 at 1:250 (10); and 4) the new phospho-specific antibody to UT-A1 phosphorylated at S494 that was preadsorbed with 1 μg/ml nonphosphopeptide at 1:50. Blots were washed three times in Tris-buffered saline with 0.5% Tween 20 and then incubated for 2 h with Alexa Fluor 680-linked anti-rabbit (1:2,000; Molecular Probes, Eugene, OR). Bound secondary antibody was visualized using infrared detection with the LI-COR Odyssey protein analysis system (Lincoln, NE), and densitometry of the desired band was collected. To ensure equal loading and to quantify densitometric scanning, membranes were blocked and reprobed with either chicken anti-tubulin antibody (1:10,000; Abcam, Cambridge, MA) or rabbit anti-GAPDH (1:7,000; Abcam) as indicated. Membranes were then incubated with IRDye 800 conjugated anti-chicken secondary antibody (1:6,000; Rockland Immunochemicals, Limerick, PA) or Alexa Fluor 680-linked anti-rabbit and imaged via the LI-COR Odyssey system. Proteins were identified by known molecular mass (kDa) as indicated by a variety of prestained molecular mass ladders separated on the same gel. All densitometries were collected using ImageJ software (National Institutes of Health, Bethesda, MD) and used to normalize the Western blot data.
Biotinylation of plasma cell membranes.
UT-A1 and UT-A1ΔS494A transfected LLC-PK1 cells as well as mIMCD3-UT-A1 cells were biotinylated as previously described (3, 18). PDBu (2 μM) was added for 30 min at 37°C, and then samples were washed free of excess solution twice with PBS and three times with biotinylation buffer without biotin (in mM: 215 NaCl, 4 KCl, 1.2 MgSO4, 2 CaCl2, 5.5 glucose, 10 triethanolamine, and 2.5 Na2HPO4). PDBu was added back during the incubation with biotinylation buffer containing 3 mg/ml biotinamidohexanoic acid 3-sulfo-N-hydroxysuccinimide ester (Sigma-Aldrich, St. Louis, MO) for 60 min at 4°C. Cells were then washed free of unattached biotin by three washes with biotin quenching buffer (in mM: 0.1 CaCl2, 1 MgCl2, and 260 glycine in PBS), with the last wash incubated for 20 min at 4°C. Next, samples were washed three times with lysis buffer without detergent and the cells were solubilized for 1 h in lysis buffer containing 1% NP-40 (mM: 150 NaCl, 5 EDTA, and 50 Tris). After centrifugation (14,000 g, 10 min, 4°C) to remove insoluble particulates, streptavidin beads were added to the supernatant fractions and allowed to absorb biotinylated proteins overnight at 4°C. After being washed with high-salt and no-salt buffers, Laemmli SDS-PAGE sample buffer was added directly to the pellets, samples were boiled for 1 min, and the pool of biotinylated proteins was analyzed by Western blot.
Immunofluorescence.
After grown to confluence on Transwell supports (Corning, Manassas, VA), mIMCD3-UT-A1 cells or mIMCD3 cells transiently transfected with UT-A1 or the mutant S494A construct were treated with either 1) vehicle; 2) forskolin (10 μM); and PDBu (2 μM); or 3) PDBu alone (2 μM) for 30 min at 37°C. Following treatment, cells were rinsed twice in PBS and fixed in 1% formaldehyde/150 mM NaCl/20 mM HEPES, pH 7.8, at 20°C for 30 min. Fixation solution was washed twice with PBS and staining buffer (0.1% Triton X-100/100 mM NaCl/20 mM HEPES, pH 7.8) was added for 1 h. After being rinsed in staining buffer, the cells were incubated with UT-A1 primary antibody (1:200) for 3 h. After being rinsed in staining buffer three times, the cells were incubated with the secondary antibody, Alexa Fluor 488-linked goat anti-rabbit IgG (Invitrogen, Carlsbad, CA) for 1 h. After two washes in staining buffer, cells were incubated with Alexa Fluor 555 Phalloidin (Invitrogen) to selectivity label F-actin. Transwell filters were excised and mounted on glass slides using ProLong Gold antifade with DAPI (Invitrogen). Confocal microscopy was performed with a Zeiss LSM 510 META ZEN confocal microscope and LSM ZEN imaging software. Micrographs were acquired at a magnification of ×63, and acquisition settings were optimized and then matched for each pair of samples that were stained with the same antibody.
Statistics.
Data are presented as means ± SE; comparisons were made with either an unpaired t-test with or a one-way ANOVA with Tukey's honestly significant difference test where P < 0.05 was considered significant.
RESULTS
UT-A1 is phosphorylated at S494 following PKC activation.
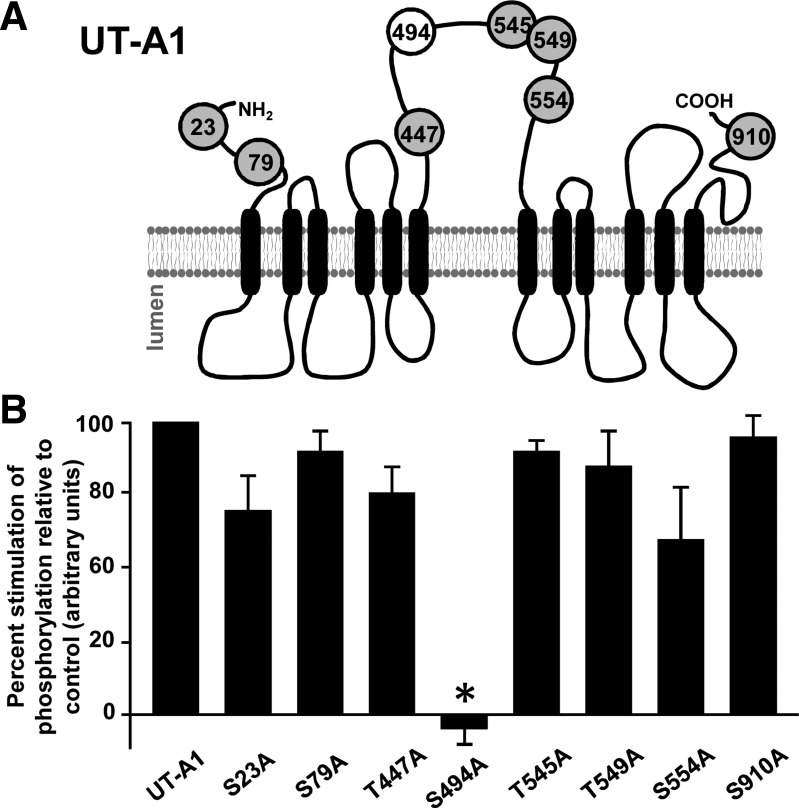
Performing an in silico prediction of PKC phosphorylation sites in the rat UT-A1 amino acid sequence revealed a number or possible candidate targets for the kinase including the following: S23, S79, T447, S494, T545, T549, S554, and S910. Interestingly, all of these sites are located in the cytosolic portion of the transporter and a majority are found in the large intracellular loop that is unique to UT-A1 (Fig. 2A). Each individual residue of interest was converted to an alanine using site-directed mutagenesis. Constructs were transiently transfected into LLC-PK1 cells to measure UT-A1 phosphorylation in response to the activation of PKC activity by PDBu. With the exception of one construct, S494A, PKC-mediated phosphorylation of UT-A1 was enhanced (Fig. 2B).
Fig. 2.
Identification of UT-A1 PKC phosphorylation sites. A: after identifying viable targets, we performed site directed mutagenesis to ablate potential PKC-substrate sites to alanine, a residue that will not be phosphorylated by the kinases and that does not alter UT-A1 protein structure. B: each single mutation was transiently transfected into LLC-PK1 cells and grown to confluence within 48 h. Cells were then metabolically labeled in [32P]orthophosphate (0.15 mCi/ml) before addition of either vehicle or the phorbol ester PDBu (2 μM) for 30 min at 37°C. UT-A1 was immunoprecipitated from lysates and subjected to Western blot analysis to confirm total protein abundance and autoradiography to measure total phosphorylation of UT-A1. Data are shown as the percentage of PDBu-mediated phosphorylation relative to control (average phosphorylation:total wild-type UT-A1). Data are presented as means ± SE and by ANOVA where *P < 0.05 was significant; n = 3.
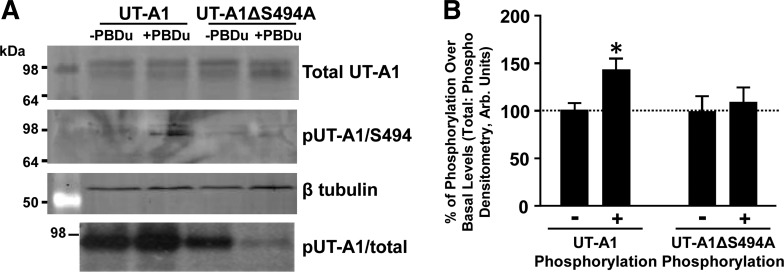
To confirm this result, we generated an antibody that specifically detected phosphorylation of UT-A1 at S494 (Fig. 3). PDBu-treated mIMCD3 cells transfected with a rat UT-A1 construct demonstrated an increase of total phosphorylation and phosphorylation at S494 (Fig. 3). This response was not observed in the mutated construct UT-A1ΔS494A.
Fig. 3.
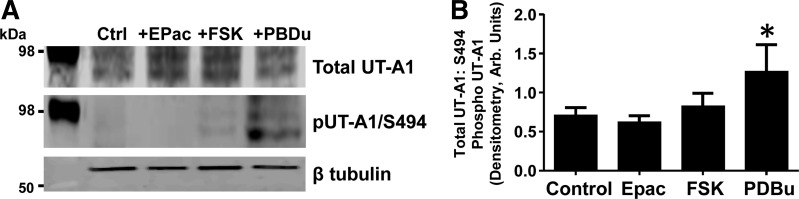
Confirmation of S494 as the PKC phosphorylation site with a phospho-specific antibody. A: either a control UT-A1 construct or UT-A1 containing the altered S494 residue (UT-A1ΔS494A) was transiently transfected into LLC-PK1 cells. We measured total protein by Western blot (A) and total phosphorylation in response to PDBu (2 μM; 30 min at 37°C) by autoradiography (B). We commissioned the generation of a phospho-specific antibody that was affinity purified to recognize S494 as described in detail in methods. Western blot analysis with the new phospho-specific antibody to UT-A1 phosphorylated at S494 that was 1st preadsorbed with nonphosphopeptide (pUT-A1/S494) proved that the antibody specifically recognized PDBu stimulation of phosphorylation of UT-A1 at S494 (A, 2nd row). Equal loading was confirmed with β-tubulin (A, 3rd row). Shown are representative images. B: after total UT-A1 abundance was normalized, a ratio of total UT-A1 to either total phospho-UT-A1 or S494 phospho-UT-A1 in response to (−) or (+) PDBu was determined using arbitrary units. Data are shown as means ± SE where *P < 0.05 was significant; n = 5.
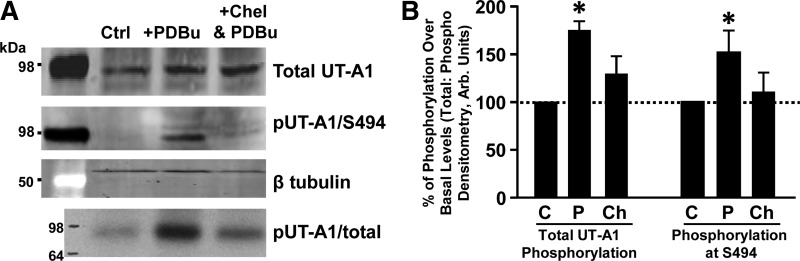
We also confirmed that phosphorylation of UT-A1 at S494 is increased by PKC activation in inner medullary tissue. Ex vivo treatment with PDBu increased both total UT-A1 phosphorylation and phosphorylation at S494 in rat inner medulla (Fig. 4). In tissues pretreated with the global PKC inhibitor chelerythrine, PDBu stimulation blunted total UT-A1 phosphorylation and prevented PKC-mediated phosphorylation at the S494 site (Fig. 4). Collectively, these results demonstrate that PKC increases phosphorylation of UT-A1, primarily at the S494 site.
Fig. 4.
Phosphorylation of UT-A1 at S494 is dependent on active PKC. Rat inner medullary tissue was metabolically labeled in [32P]orthophosphate (0.15 mCi/ml) before incubation with either vehicle (Ctrl), PDBu (2 μM), or chelerythrine (10 μM; Chel) followed by PDBu (2 μM) in DMEM medium for 30 min at 37°C. Tissues were lysed and subjected to Western blot analysis. A: blots from a representative experiment probed with UT-A1 (1st row) or pUT-A1/S494 preadsorbed with nonphosphopeptide (2nd row). Autoradiography was used to measure total phosphorylation of UT-A1 (4th row) and equal loading was confirmed with β-tubulin (3rd row). B: after total UT-A1 abundance was normalized, a ratio of total UT-A1 to either total phospho-UT-A1 or S494 phospho-UT-A1 was determined using arbitrary units for each treatment group (C, vehicle; P, PDB; Ch, chelerythrine followed by PDBu). Data are shown as means ± SE where *P < 0.05 was significant; n = 4.
Cyclic AMP pathways do not affect phosphorylation of UT-A1 at S494.
To examine if elevated cAMP levels stimulated UT-A1 phosphorylation at S494, we first treated mIMCD3-UT-A1 cells with the adenylyl cyclase stimulator forskolin. Elevation of cAMP triggers downstream targets including PKA and Epac. Treatment with forskolin failed to increase phosphorylation of UT-A1 at S494 (Fig. 5). We also specifically activated Epac with Sp-8-pCPT-2′-O-Me-cAMPS and saw no change in S494 phosphorylation (Fig. 5). These observations suggest that cAMP-stimulated pathways known to regulate UT-A1 function do not require phosphorylation of S494.
Fig. 5.
Phosphorylation of UT-A1 at S494 is independent of the cAMP pathway. Stably transfected mIMCD3-UT-A1 cells were treated with either 1) vehicle; 2) forskolin (FSK; 10 μM); 3) an activator of the Epac pathway (Sp-8-pCPT-2′-o-methyl-cAMPS; 75 μM); or 4) PDBu (2 μM) for 30 min at 37°C. Cells were lysed and subjected to Western blot analysis. A: blots from a representative experiment probed with UT-A1 (1st row) or pUT-A1/S494 preadsorbed with nonphosphopeptide (2nd row). Equal loading was confirmed with β-tubulin (3rd row). B: after total UT-A1 abundance was normalized, a ratio of total UT-A1 to S494 phospho-UT-A1 was determined using arbitrary units. Data shown as means ± SE where *P < 0.05 was significant; n = 5.
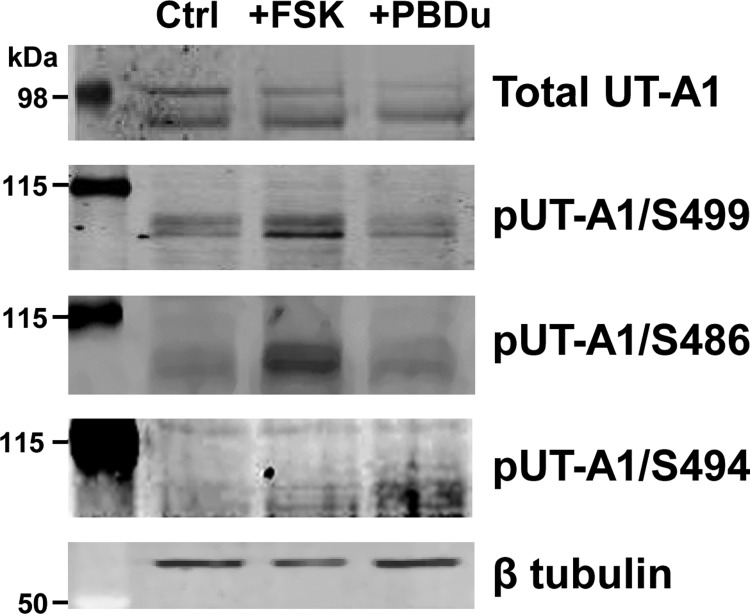
UT-A1 has two PKA sites, S486 and S499, located in the intracellular loop region of UT-A1 (3, 13) in close proximity to the PKC site S494. Because several proteins have multiple phosphorylation sites that can have distinct or opposing effects on protein regulation, we examined if posttranslational modification of UT-A1 at S486 or S499 was altered by PKC activation. Elevation of cAMP levels following forskolin treatment of mIMCD3-UT-A1 cells significantly increased UT-A1 phosphorylation at both S486 and S499 but not at the S494 residue (Fig. 6). Activating PKC activity with PDBu treatment failed to increase phosphorylation at either PKA site; however, phosphorylation at S494 was higher (Fig. 6). From these observations, both PKA- and PKC-mediated phosphorylation of UT-A1 appear to occur at distinctive sites.
Fig. 6.
Activation of PKC does not increase phosphorylation of UT-A1 at S486 and S499. Rat inner medullary tissue was treated either vehicle (Ctrl), forskolin (10 μM), or PDBu (2 μM) in DMEM medium for 30 min at 37°C. Tissues were lysed and subjected to Western blot analysis. Blots shown are from a representative experiment probed with the following antibodies: UT-A1, pUT-A1/S499, pUT-A1/S486, and pUT-A1/S494 preadsorbed with nonphosphopeptide. Two molecular mass ladders were used as shown and equal loading was confirmed with β-tubulin (5th row); n = 3.
Hypertonicity increases phosphorylation of UT-A1 at the PKC site S494.
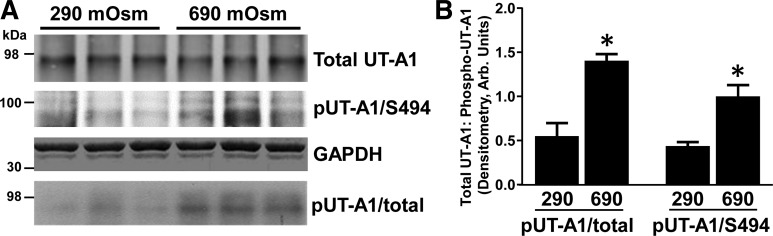
We have previously shown that PKC mediates a hypertonicity-stimulated increase in total UT-A1 phosphorylation (20) but were unable to determine if this event was due to direct phosphorylation of the transporter. We subjected rat inner medullary tissue to hypertonic conditions (690 mosmol/kgH2O). Hypertonicity predictably elevated total UT-A1 phosphorylation and also increased phosphorylation of UT-A1 at S494 (Fig. 7). These findings imply that the increase in UT-A1 phosphorylation under hypertonic conditions mainly occurs at the PKC-sensitive S494 site.
Fig. 7.
Total and S494 UT-A1 phosphorylation are elevated in response to hypertonicity in rat inner medulla. Metabolically labeled ([32P]orthophosphate) rat inner medullary tissue was incubated in either 290 mosmol/kgH2O buffer or 600 mosmol/kgH2O buffer for 30 min at 37°C. Osmolality was increased by the appropriate addition of sucrose and confirmed with an osmometer. Following treatment, lysates were subjected to immunoprecipitation for UT-A1 and analyzed by Western blot and autoradiography. A: autoradiogram (4th row) and Western blot of total UT-A1 (1st row) and pUT-A1/S494 (2nd row) are shown from 3 representative samples. Each lane represents the 2 inner medulla collected from 1 rat. Two molecular weight ladders were used as shown and equal loading was confirmed with GAPDH (3rd row). B: bar graph demonstrates the ratio of total phosphorylated UT-A1 to total UT-A1 in response to hypertonicity as well as the ratio of S494 phosphorylated UT-A1 to total UT-A1 from all collected samples (2 separate cohorts of animals; 3 per cohort). Data are presented as means ± SE where *P < 0.05 was significant; n = 6.
UT-A1 membrane accumulation is not dependent on PKC.
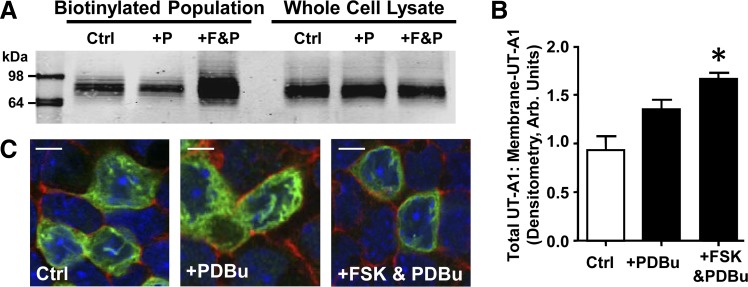
We previously observed that cAMP-mediated phosphorylation of UT-A1 is important to membrane insertion of the transporter (3). To examine the role of PKC in accumulation at the apical membrane, we biotinylated mIMCD3-UT-A1 cells following stimulation of PKC with the global PKC activator PDBu (Fig. 8A). We found that there was not a significant increase in UT-A1 abundance at the plasma membrane when PKC activity was elevated (Fig. 8B). Examination of UT-A1 localization in the cell following PDBu treatment showed that transporter expression remained diffuse throughout the cell, similar to untreated mIMCD3-UT-A1 cells (Fig. 8C). Pharmacological activation of both PKA and PKC does increase the amount of UT-A1 associated with the plasma membrane (Fig. 8B), and cellular location was strongly associated with the membrane (Fig. 8C). Despite our findings demonstrating a distinction between PKA- and PKC-mediated modifications of the transporter, UT-A1 cellular location appears to require an active cAMP pathway.
Fig. 8.
Stimulation of PKC alone is not sufficient to increase UT-A1 membrane accumulation. A: stably transfected mIMCD3-UT-A1 cells were treated with either 1) vehicle (Ctrl); 2) PDBu (2 μM; +P); or 3) forskolin (10 μM) and PDBu (2 μM) (+F&P) for 30 min at 37°C and then biotinylated to reveal membrane-associated UT-A1. A: biotinylated protein population and total lysate were subjected to Western blot analysis probed for UT-A1. Shown are representative samples. B: graph of the ratio of membrane UT-A1 to total UT-A1 from all samples presented as means ± SE where *P < 0.05 was significant; n = 4. C: representative images from mIMCD3-UT-A1 cells grown to confluence on permeable supports and treated with either 1) vehicle (Ctrl); 2) PDBu (2 μM); or 3) forskolin (10 μM) and PDBu (2 μM) for 30 min at 37°C. Cells were then fixed and UT-A1 cellular localization was determined by immunofluorescence. UT-A1: green; phalliodin: red; nucleus: blue. Scale bar = 10 mm; n = 3.
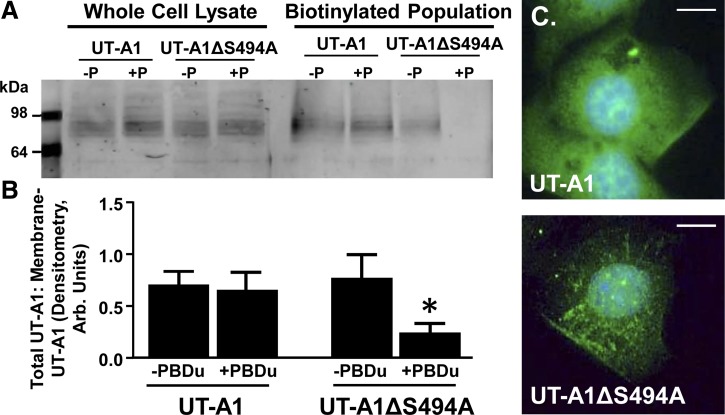
Although PKC may not mediate trafficking to the membrane, further studies suggest that S494 may play a role in UT-A1 localization to the membrane. LLC-PK1 cells were transiently transfected with either UT-A1 or UT-A1ΔS494A and stimulated with vehicle or PDBu before biotinylation. Investigation of the collected membrane pools reveals that although PDBu does not alter UT-A1 association with the plasma membrane, ablating the PKC substrate site S494 decreases UT-A1 abundance in the membrane (Fig. 9B).
Fig. 9.
UT-A1 requires S494 for membrane retention following PKC stimulation. A: Either a control UT-A1 construct or UT-A1 containing the altered S494 residue (UT-A1ΔS494A) was transiently transfected into LLC-PK1 cells. After 48 h cells were treated with either vehicle (−P) or PDBu (2 μM; +P) for 30 min at 37°C and then biotinylated. The biotinylated protein population and total lysate were subjected to Western blot analysis probed with anti-UT-A1. Shown are representative samples. B: graph of the ratio of membrane UT-A1 to total UT-A1 from all samples presented as means ± SE where *P < 0.05 was significant; n = 3. C: either UT-A1 or UT-A1ΔS494A was transiently transfected into mIMCD3 cells. After 24 h, PDBu (2 μM) was added for 30 min at 37°C. Cells were fixed and stained for the cellular location of UT-A1 (green) relative to the nucleus (DAPI; blue). Shown are representative images at maximum intensity. Scale bar = 10 mm; n = 3.
Intracellular localization was examined in mIMCD3 cells transiently transfected with either UT-A1 or UT-A1ΔS494A and grown on collagen-coated Transwell membranes. Following the addition of PDBu, UT-A1 expression was diffuse with some intense fluorescence associated with the plasma membrane (Fig. 9C, top). UT-A1ΔS494A, however, was found in an internal and punctated staining pattern, reflective of endosomes and lysosomes containing internalized proteins (Fig. 9C, bottom). The observations suggest that ablating S494 may lead to a trafficking defect that prevents UT-A1ΔS494A from accumulating at the membrane for a measurable period of time.
DISCUSSION
Mounting evidence has proven that hypertonicity increases urea permeability through a PKC-mediated signaling pathway (34). Furthermore, UT-A1 phosphorylation by PKC is required for elevated urea permeability in response to hypertonicity (20, 33); however, direct phosphorylation of UT-A1 by PKC was unknown. Our study is the first to identify that activation of PKC phosphorylates UT-A1 at residue S494. Discovery of a PKC-sensitive site provides additional findings that support previous reports as discussed below.
In addition to PKC phosphorylation, PKA phosphorylates UT-A1 at two sites, S486 and S499 (3). Previous work demonstrated that vasopressin stimulates phosphorylation at S486 and S499; however, treatment of rat inner medullary tissue with PDBu alone did not stimulate phosphorylation at S486 or S499. Similarly, we found that elevating cellular cAMP in mIMCD3-UT-A1 cells with forskolin failed to stimulate phosphorylation at S494. These findings add to our understanding of the synergistic effects of vasopressin and hypertonicity on urea transport. Not only do these two urea transport stimulators work through different kinases, the different kinases phosphorylate different serines in UT-A1. It is intriguing that the two PKA sites S486 and S499 and the PKC site S494 are all located in the intracellular loop region of UT-A1. This region is not present in other urea transporters and hence is unique to UT-A1 (18) (19). The three serines are also located in close proximity to one another raising the possibility that phosphorylation of one serine could induce a conformational change in UT-A1 that could have an effect on the other sites. However, future studies will be needed to evaluate this hypothesis.
Previous work showed that maximal increases in urea transport require stimulation of both cAMP and PKC-signaling pathways (33, 34). This suggests that regulation of UT-A1 function requires some mechanism connecting the cAMP and PKC signaling pathways. Several studies show that Epac mediates cAMP-cellular responses by activating PKC (4, 12, 25). This is particularly intriguing considering that the specific activation of Epac increases urea transport and UT-A1 phosphorylation (32). Despite these findings, we discovered that PKC-meditated phosphorylation of UT-A1 at S494 is not due to Epac stimulation. Therefore, Epac is not the key mediator of the cross talk between the cAMP and PKC signaling pathways in the inner medulla that regulates urea permeability.
The concept of a cAMP-independent mechanism to stimulate urea permeability certainly supports the need for an independent PKC-mediated pathway in the inner medulla. In contrast to the stimulation of PKC with PDBu (34) or angiotensin II (16) that does not show any stimulation of urea permeability in the absence of vasopressin, the hypertonicity-mediated increase in urea permeability is dependent on PKC activation, even without vasopressin (33, 34). Our finding that hypertonicity independently phosphorylates UT-A1 at the identified PKC-sensitive site S494 reaffirms that the increased urea permeability due to osmotic stress is likely a result of PKC-mediated UT-A1 phosphorylation. Furthermore, activation of PKC by hypertonicity without stimulating the cAMP pathway proves that PKC phosphorylation of S494 is independent of vasopressin-mediated signaling pathways.
We discovered that activation of the PKC pathway alone does not increase UT-A1 accumulation at the plasma membrane. These findings support previous studies by our group that demonstrate that direct activation of PKC by the phorbol ester PDBu fails to stimulate urea permeability (34). Interestingly, that same study found that urea permeability was increased when IMCDs were treated with vasopressin and PDBu simultaneously, an effect that was blocked by the PKC inhibitor chelerythrine (34). Although the role of PKC in activating UT-A1 is fairly clear, the exact manner in which this occurs is not completely understood. Frequently membrane trafficking is a major regulatory pathway for transporter function and is often governed by the phosphorylation of specific residues on the transporter protein in question. PKC-induced transporter trafficking is commonly linked with controlling recycling of the transporter protein from and/or to the plasma membrane. The importance of direct phosphorylation of UT-A1 by PKC for membrane retention is apparent following our finding herein that UT-A1 lacking S494 is actually reduced from the plasma membrane in response to PKC activation. This finding indicates that although phosphorylation of S494 is not involved in UT-A1 membrane insertion, PKC phosphorylation of this site is important for UT-A1 retention at the plasma membrane. A recent study revealed that ablation of a single PKC substrate residue in the osmoprotective, renal betaine-GABA transporter 1 (BGT1) decreased transporter retention in the plasma membrane (29). If the phosphorylation of UT-A1 at S494 by PKC acts similarly to BGT1, this could explain why stimulation of both the cAMP pathway and PKC lead to a synergistic effect on UT-A1 activity. In other words, the cAMP pathway promotes trafficking to the apical membrane where the activated PKC pathway phosphorylates the transporter resulting in increased UT-A1 retention at the apical membrane and thus elevated urea permeability.
Endocytosis is an effective mechanism to regulate cellular signaling events by controlling the number of receptors and transporters at the plasma membrane by controlling internalization (26). Furthermore, endocytosis can also regulate cellular function by localizing signaling complexes through the spatial restriction of signaling activities or by transporting signaling proteins to cellular locations unreachable by diffusion (11). It is possible that regulation of UT-A1 may exploit this system, possibly requiring dynamic cycling between phosphorylated and nonphosphorylated states to effectively traffic to the cell surface. We found that in the absence of the S494 site UT-A1 was predominately internalized into punctate structures suggesting that phosphorylation of this site may be important in the vesicular trafficking of the transporter. A key step in the fusion of recycling vesicles to the cell membrane is the formation of a stable N-ethylmaleimide-sensitive factor attachment protein receptor (SNARE) complex. Interestingly, PKC-mediated phosphorylation of SNAP23, an important regulator of transport vesicle docking and fusion that directly associates with UT-A1 (24), is critical in regulating proper vesicle fusion (9).
In summary, we have identified that stimulation of PKC increases phosphorylation of UT-A1 at a specific residue, S494. Although there is no cross talk with the cAMP-signaling pathway, phosphorylation of S494 thru PKC may enhance vasopressin-stimulated urea permeability by retaining UT-A1 in the plasma membrane. Discovery of the PKC-sensitive phosphorylation site in the UT-A1 protein provides a new aspect of posttranslational modification of the transporter that will need to be considered in future investigations.
GRANTS
This work was supported by National Institute of Diabetes and Digestive and Kidney Diseases Grants R01-DK-89828 (to J. M. Sands), T32-DK-07656 (to P. Cipriani), P01-DK-61521-S1 (to R. J. Ordas), K01-DK-082733 (to M. A. Blount), and K01-DK-082733-S1 (to M. A. Blount) and an American Physiological Society Undergraduate Summer Research Fellowship (to S. K. Redd).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: M.A.B., J.D.K., and J.M.S. conception and design of research; M.A.B., P.C., S.K.R., R.J.O., L.N.B., D.L.G., and C.A.H. performed experiments; M.A.B., P.C., S.K.R., R.J.O., L.N.B., D.L.G., and C.A.H. analyzed data; M.A.B., P.C., S.K.R., R.J.O., L.N.B., D.L.G., and C.A.H. interpreted results of experiments; M.A.B., P.C., S.K.R., R.J.O., L.N.B., D.L.G., and C.A.H. prepared figures; M.A.B. and J.M.S. drafted manuscript; M.A.B., P.C., S.K.R., R.J.O., L.N.B., D.L.G., C.A.H., J.D.K., and J.M.S. edited and revised manuscript; M.A.B., P.C., S.K.R., R.J.O., L.N.B., D.L.G., C.A.H., J.D.K., and J.M.S. approved final version of manuscript.
ACKNOWLEDGMENTS
We are grateful to Amy Archuleta of PhosphoSolutions for technical expertise and suggestions as well as graciously supplying peptides.
REFERENCES
- 1.Blom N, Sicheritz-Ponten T, Gupta R, Gammeltoft S, Brunak S. Prediction of post-translational glycosylation and phosphorylation of proteins from the amino acid sequence. Proteomics 4: 1633–1649, 2004. [DOI] [PubMed] [Google Scholar]
- 2.Blount MA, Klein JD, Martin CF, Tchapyjnikov D, Sands JM. Forskolin stimulates phosphorylation and membrane accumulation of UT-A3. Am J Physiol Renal Physiol 293: F1308–F1313, 2007. [DOI] [PubMed] [Google Scholar]
- 3.Blount MA, Mistry AC, Frohlich O, Price SR, Chen G, Sands JM, Klein JD. Phosphorylation of UT-A1 urea transporter at serines 486 and 499 is important for vasopressin-regulated activity and membrane accumulation. Am J Physiol Renal Physiol 295: F295–F299, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Borland G, Bird RJ, Palmer TM, Yarwood SJ. Activation of protein kinase C alpha by EPAC1 is required for the ERK- and C/EBPbeta-dependent induction of the SOCS-3 gene by cyclic AMP in COS1 cells. J Biol Chem 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fenton RA. Urea transporters and renal function: lessons from knockout mice. Curr Opin Nephrol Hypertens 17: 513–518, 2008. [DOI] [PubMed] [Google Scholar]
- 6.Fenton RA, Chou CL, Stewart GS, Smith CP, Knepper MA. Urinary concentrating defect in mice with selective deletion of phloretin-sensitive urea transporters in the renal collecting duct. Proc Natl Acad Sci USA 101: 7469–7474, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fenton RA, Flynn A, Shodeinde A, Smith CP, Schnermann J, Knepper MA. Renal phenotype of UT-A urea transporter knockout mice. J Am Soc Nephrol 16: 1583–1592, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fenton RA, Knepper MA. Urea and renal function in the 21st century: insights from knockout mice. J Am Soc Nephrol 18: 679–688, 2007. [DOI] [PubMed] [Google Scholar]
- 9.Hepp R, Puri N, Hohenstein AC, Crawford GL, Whiteheart SW, Roche PA. Phosphorylation of SNAP-23 regulates exocytosis from mast cells. J Biol Chem 280: 6610–6620, 2005. [DOI] [PubMed] [Google Scholar]
- 10.Hoban CA, Black LN, Ordas RJ, Gumina DL, Pulous FE, Sim JH, Sands JM, Blount MA. Vasopressin regulation of multisite phosphorylation of UT-A1 in the inner medullary collecting duct. Am J Physiol Renal Physiol 308: F49–F55, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Howe CL. Modeling the signaling endosome hypothesis: why a drive to the nucleus is better than a (random) walk. Theor Biol Med Model 2: 43, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hucho TB, Dina OA, Levine JD. Epac mediates a cAMP-to-PKC signaling in inflammatory pain: an isolectin B4(+) neuron-specific mechanism. J Neurosci 25: 6119–6126, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hwang S, Gunaratne R, Rinschen MM, Yu MJ, Pisitkun T, Hoffert JD, Fenton RA, Knepper MA, Chou CL. Vasopressin increases phosphorylation of Ser84 and Ser486 in Slc14a2 collecting duct urea transporters. Am J Physiol Renal Physiol 299: F559–F567, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jung JY, Kwon HM, Kim J. Regulation of urea transporters by tonicity-responsive enhancer binding protein. Electrolyte Blood Press 5: 28–33, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Juul KV, Bichet DG, Nielsen S, Norgaard JP. The physiological and pathophysiological functions of renal and extrarenal vasopressin V2 receptors. Am J Physiol Renal Physiol 306: F931–F940, 2014. [DOI] [PubMed] [Google Scholar]
- 16.Kato A, Klein JD, Zhang C, Sands JM. Angiotensin II increases vasopressin-stimulated facilitated urea permeability in rat terminal IMCDs. Am J Physiol Renal Physiol 279: F835–F840, 2000. [DOI] [PubMed] [Google Scholar]
- 17.Kennelly PJ, Krebs EG. Consensus sequences as substrate specificity determinants for protein kinases and protein phosphatases. J Biol Chem 266: 15555–15558, 1991. [PubMed] [Google Scholar]
- 18.Klein JD, Blount MA, Frohlich O, Denson CE, Tan X, Sim JH, Martin CF, Sands JM. Phosphorylation of UT-A1 on serine 486 correlates with membrane accumulation and urea transport activity in both rat IMCDs and cultured cells. Am J Physiol Renal Physiol 298: F935–F940, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Klein JD, Blount MA, Sands JM. Urea transport in the kidney. In: Comprehensive Physiology. New York: John Wiley & Sons, 2011. [DOI] [PubMed] [Google Scholar]
- 20.Klein JD, Martin CF, Kent KJ, Sands JM. Protein kinase C-alpha mediates hypertonicity-stimulated increase in urea transporter phosphorylation in the inner medullary collecting duct. Am J Physiol Renal Physiol 302: F1098–F1103, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Klein JD, Price SR, Bailey JL, Jacobs JD, Sands JM. Glucocorticoids mediate a decrease in AVP-regulated urea transporter in diabetic rat inner medulla. Am J Physiol Renal Physiol 273: F949–F953, 1997. [DOI] [PubMed] [Google Scholar]
- 22.Knepper MA, Nielsen S, Chou CL, DiGiovanni SR. Mechanism of vasopressin action in the renal collecting duct. Semin Nephrol 14: 302–321, 1994. [PubMed] [Google Scholar]
- 23.Knepper MA, Star RA. The vasopressin-regulated urea transporter in renal inner medullary collecting duct. Am J Physiol Renal Fluid Electrolyte Physiol 259: F393–F401, 1990. [DOI] [PubMed] [Google Scholar]
- 24.Mistry AC, Mallick R, Frohlich O, Klein JD, Rehm A, Chen G, Sands JM. The UT-A1 urea transporter interacts with snapin, a SNARE-associated protein. J Biol Chem 282: 30097–30106, 2007. [DOI] [PubMed] [Google Scholar]
- 25.Oestreich EA, Malik S, Goonasekera SA, Blaxall BC, Kelley GG, Dirksen RT, Smrcka AV. Epac and phospholipase Cepsilon regulate Ca2+ release in the heart by activation of protein kinase Cepsilon and calcium-calmodulin kinase II. J Biol Chem 284: 1514–1522, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Palfy M, Remenyi A, Korcsmaros T. Endosomal crosstalk: meeting points for signaling pathways. Trends Cell Biol 22: 447–456, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pannabecker TL. Comparative physiology and architecture associated with the mammalian urine concentrating mechanism: role of inner medullary water and urea transport pathways in the rodent medulla. Am J Physiol Regul Integr Comp Physiol 304: R488–R503, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sands JM, Schrader DC. An independent effect of osmolality on urea transport in rat terminal inner medullary collecting ducts. J Clin Invest 88: 137–142, 1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Schweikhard ES, Kempson SA, Ziegler C, Burckhardt BC. Mutation of a single threonine in the cytoplasmic NH2 terminus disrupts trafficking of the renal betaine-GABA transporter 1 during hypertonic stress. Am J Physiol Renal Physiol 307: F107–F115, 2014. [DOI] [PubMed] [Google Scholar]
- 30.Shayakul C, Clemencon B, Hediger MA. The urea transporter family (SLC14): physiological, pathological and structural aspects. Mol Aspects Med 34: 313–322, 2013. [DOI] [PubMed] [Google Scholar]
- 31.Stockand JD. Vasopressin regulation of renal sodium excretion. Kidney Int 78: 849–856, 2010. [DOI] [PubMed] [Google Scholar]
- 32.Wang Y, Klein JD, Blount MA, Martin CF, Kent KJ, Pech V, Wall SM, Sands JM. Epac regulates UT-A1 to increase urea transport in inner medullary collecting ducts. J Am Soc Nephrol 20: 2018–2024, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wang Y, Klein JD, Froehlich O, Sands JM. Role of protein kinase C-alpha in hypertonicity-stimulated urea permeability in mouse inner medullary collecting ducts. Am J Physiol Renal Physiol 304: F233–F238, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wang Y, Klein JD, Liedtke CM, Sands JM. Protein kinase C regulates urea permeability in the rat inner medullary collecting duct. Am J Physiol Renal Physiol 299: F1401–F1406, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhang C, Sands JM, Klein JD. Vasopressin rapidly increases phosphorylation of UT-A1 urea transporter in rat IMCDs through PKA. Am J Physiol Renal Physiol 282: F85–F90, 2002. [DOI] [PubMed] [Google Scholar]