Abstract
Low oxygen concentrations or hypoxia is a trait common to inflamed tissues. Therefore it is not surprising that pathways of hypoxic stress response, largely governed by hypoxia-inducible factors (HIF), are highly relevant to the proper function of immune cells. HIF expression and stabilization in immune cells can be triggered not only by hypoxia, but also by a variety of stimuli and pathological stresses associated with leukocyte activation and inflammation. In addition to its role as a sensor of oxygen scarcity, HIF is also a major regulator of immune cell metabolic function. Rapid progress is being made in elucidating the roles played by HIF in diverse aspects of both innate and adaptive immunity. Here we discuss a number of breakthroughs that have shed light on how HIF expression and activity impact the differentiation and function of diverse T cell populations. The insights gained from these findings may serve as the foundation for future therapies aimed at fine-tuning the immune response.
Keywords: hypoxia-inducible factor, metabolism, immune activation, inflammation, T cells
hypoxia-inducible factor (HIF) is expressed across a remarkable range of species (in fact, all metazoan species analyzed to date), and it controls the cell's metabolic response to low oxygen status (33). HIF is a heterodimeric transcription factor composed of a constitutively expressed HIF-1β subunit [also known as the aryl hydrocarbon receptor nuclear translocator (ARNT)] and an α-subunit (either HIF-1α or HIF-2α) that is tightly regulated at the protein level. While HIF-1 and HIF-2 are similar in their regulation, as well as the target genes they regulate in response to hypoxia, they are not identical in either respect. A third molecule, HIF-3α, has also been described as a negative feedback mechanism for inhibiting the activity of HIF-1α (48). Both HIF-1α and HIF-1β are members of the bHLH-PAS superfamily of proteins known to contain both basic-helix-loop-helix (bHLH) and PER-ARNT-SIM (PAS) domains, which are involved in DNA binding and heteromerization, respectively (26).
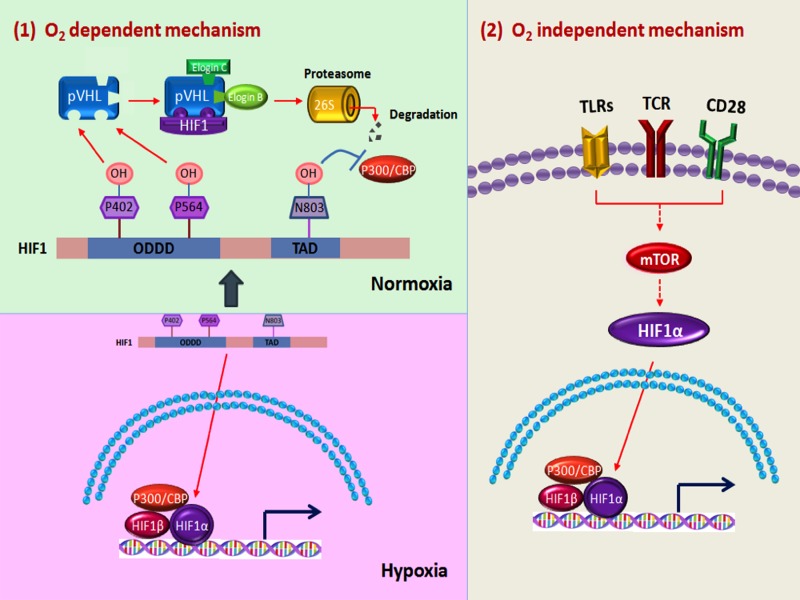
Under normoxic conditions, HIF-1 expression is tightly regulated in an oxygen-dependent manner (56). HIF-1 is rapidly downregulated at the protein level through the ubiquitin-proteasome pathway (Fig. 1). This process begins when HIF-1α protein is hydroxylated at prolines 402 and 564 by certain members of a family of prolyl hydroxylase domain proteins (PHD1, 2, and 3), which require oxygen for their function. These modifications permit the recruitment of the von Hippel-Lindau (VHL) tumor suppressor, ubiquitin E3 ligase complex (including elongin B/C, RBX1, and cullin2). Subsequent polyubiquitination of HIF-1α by the VHL complex marks the molecule for degradation by way of the 26S proteasome (48).
Fig. 1.
Regulation of hypoxia-inducible factor-1 (HIF-1) expression and function by multiple inputs. Across diverse cell types, HIF-1 is regulated at the protein level by oxygen-dependent mechanisms (panel 1). HIF subunits are readily transcribed. However, in the presence of oxygen, proline residues of the HIF-1α subunit are hydroxylated by prolyl hydroxylase domain (PHD) enzymes (which are inactive under hypoxic conditions). Modified HIF-1 molecules then interact with the von Hippel-Lindau (VHL)/E3 ligase complex, become polyubiquitinated, and are then degraded by the 26S proteasome. Additionally, hydroxylation of residue N803 can disrupt interaction with transcription cofactors. In immune cells, and particularly T cells, HIF-1 expression can be transcriptionally upregulated even in the presence of oxygen in response to stimuli triggering Toll-like receptor (TLR), T cell receptor (TCR), costimulation signaling pathways leading to mammalian target of rapamycin (mTOR) pathway activation (panel 2).
In contrast, under low-oxygen conditions, inactive PHDs do not modify HIF-1α subunits, which do not interact with VHL, and are resultantly spared from degradation. Upon translocation to the nucleus, HIF subunit dimerization occurs. The assembled HIF complex can then interact with cofactors such as p300 to bind the promoters of HIF-target genes at defined five-nucleotide sequences termed hypoxia response elements (HREs, 5′-[A/G]CGTG-3′). Among the many genes regulated by HIF are those critical for the response to hypoxia including metabolism, angiogenesis, and apoptosis, to name a few (48, 49).
Hypoxia is a hallmark trait of inflamed tissues (along with acidosis, hypoglycemia, and an abundance of free oxygen radicals). Inflammation-associated injury to the microvasculature can compromise regional supplies of oxygen as does ravenous consumption by invading microbes and activated leukocytes accumulating at inflammatory loci. Resultant oxygen levels can reach as low as 0.5–3% by volume (compared with ∼11% for normoxic tissues and ∼20% for standard in vitro culture conditions) (8, 40). Solid tumors are also notorious for harboring niches of extreme oxygen deprivation. The rapid growth of malignant cells that outpaces the aberrant and disorganized neovascularization seen in tumors accounts for both the chronic and cycling hypoxia typical of tumor microenvironments (15). Therefore it is not surprising that adaptation to oxygen scarcity, and particularly the action of HIF-1, is highly pertinent to the function of cells participating in the immune responses to both infectious and cancerous threats. Additionally, a number of other stimuli of considerable immune relevance can upregulate HIF-1 expression, mostly by enhancing transcription of the Hif1a gene. These factors include cytokines, Toll-like receptor ligands as well as triggers of T cell receptor (TCR) and mammalian target of rapamycin (mTOR) signaling (5, 39, 53).
Here we will review what is known about the role played by HIF-1 across diverse T cell subsets with functions ranging from the inflammatory to the immunosuppressive. As will be discussed, these roles will involve layers of transcriptional, posttranscriptional, and protein-level regulation. Particularly we will focus on how these regulatory mechanisms influence the processes of T cell differentiation, cell survival as well as phenotypic stability and function. In reviewing the many and diverse roles played by HIF-1 in T cell biology we aim to highlight the therapeutic potential of HIF-1 targeting strategies for the correction of immune dysregulation underlying both autoimmune/inflammatory diseases and ineffectual anti-tumor immunity.
Effector CD4+ T Cells
Upon activation, naïve CD4+ T cells are capable of acquiring remarkably specialized effector functions in response to lineage-driving cytokines in the microenvironment. Signaling events downstream of the cytokine-cytokine receptor interaction activate the expression of “master regulator” transcription factors responsible for establishing and enforcing specific gene expression programs. These, in turn, underlie similarly unique T helper lineage-defining functions (64).
T helper (Th) 1 cells, for instance, produce interferon-γ (IFN-γ) under the control of the transcription factor T-bet and drive cell-mediated immunity to protect against intracellular viral, bacterial, and parasitic infection. The Th1 response is also beneficial for anti-tumor immunity. Th2 cells produce interleukin-4 (IL-4) and are critical for controlling extracellular parasites such as helminthes. In these cells, GATA3 is a critical regulator of Th2-associated gene expression. Th17 cell differentiation is driven by cytokines utilizing the STAT3 signaling pathway (e.g., IL-6) and activating the transcriptional regulator RORγt. These cells are responsible for expelling extracellular bacteria and fungi through secretion of IL-17a, IL-17f, and IL-22. However, Th17 cells, with their considerable inflammatory potential, are perhaps better known for their contribution to autoimmune disease and immune pathology (64).
Naïve T cells can also acquire the ability to suppress the activation of other immune cells including the aforementioned effector subsets. By upregulating the transcription factor Foxp3 in response to signaling triggered by the cytokines transforming growth factor-β (TGF-β) and IL-2 in conjunction with moderate TCR activation, naïve T cells can differentiate into induced (i)Tregs or peripheral (p)Tregs (27).
Interestingly, the notoriously proinflammatory Th17 lineage and these immune-suppressing iTregs, while functionally opposite, share common elements in their differentiation pathways. Specifically, the cytokine TGF-β, for instance, is required for both Th17 and iTreg generation (32). High concentrations of TGF-β (among other factors) can sustain Foxp3 expression and commitment to an iTreg fate. The presence of STAT3-activating cytokines (such as IL-6, IL-21, and IL-23), in contrast, promotes the upregulation of the Th17-promoting transcription factor RORγt and the characteristic cytokine IL-17 (4, 32). Illustrating the interwoven developmental pathways of Th17 and iTreg cells, Foxp3 expression has been noted in the early stages of both subsets. Since Foxp3 is known to actively suppress RORγt-driven expression of Th17-associated genes (63), timely downregulation of Foxp3 activity from cells poised at the crossroads of these divergent lineages is likely necessary for optimal commitment to the Th17 fate.
STAT3-dependent signaling has been reported to stabilize HIF-1 expression in non-T cells (29, 62). We therefore suspected such a pathway might be operative in developing Th17 cells, influencing the relative balance between Th17 and Treg generating processes. In our studies, we found that during Th17 differentiation, HIF-1 is indeed induced in naïve CD4+ T cells, even in the presence of oxygen in a STAT3-dependent manner. Suggesting that HIF-1 is important for Th17 differentiation, HIF-1α-deficient naïve CD4+ T cells (from CD4cre+HIF-1αflox/flox mice) display stunted upregulation of Th17 genes, including those encoding RORγt and IL-17, compared with wild-type cells. Further investigation would reveal that HIF-1 promotes expression of several Th17-linked genes through the activation of RORγt expression and function. Furthermore, we found that subjecting differentiating CD4+ T cells to periodic hypoxia (which boosts HIF-1 levels) also enhances Th17 commitment (12).
Interestingly, in the absence of HIF-1, T cells show reciprocal upregulation of Foxp3 protein but not its transcript (12), suggesting a HIF-1-dependent mechanism for the rapid downregulation of Foxp3 protein at the crossroads of Th17 and iTreg differentiation. Reflecting an impaired commitment to the Th17 fate in favor of an iTreg one, T cell-specific HIF-1-deficient mice were protected from the more severe disease seen in wild-type mice in the experimental autoimmune encephalomyelitis model of multiple sclerosis. The influence of HIF-1 on the reciprocal differentiation of Th17 and iTregs was independently observed by Shi et al. (51). In their study, HIF-1's part in shaping the nature of the T cell response was tied to the molecule's role as a regulation of glycolytic metabolism (51).
In addition to cytokines, metabolic factors and processes can impact T cell differentiation. These metabolic factors can indeed mean the difference between T cell activation or T cell anergy and immune tolerance (41, 60). Furthermore, a considerable body of literature identifies specific metabolic sensors, enzymes, and byproducts as particular regulators of the balance between Th17 and iTreg lineages (1).
Naïve T cells are quiescent and have relatively modest biosynthetic and energetic demands that are met by glucose oxidation in the tricarboxylic acid or TCA cycle and through the oxidation of lipids. These processes maintain cellular homeostasis (41). T cell activation, however, drastically increases the metabolic demands on T cells. An increase in cell size and proliferation rate coupled with the need for energy to fuel the synthesis of macromolecules, intracellular mediators, and effector gene products (i.e., cytokines) all require a metabolic reprogramming of T cells upon activation (59, 60).
To meet the demands of their activated lifestyle, T cells downregulate the pathways characteristic of resting cells in favor of aerobic glycolysis and glutamine catabolism. This is triggered by the signaling cascades downstream of the T cell receptor (TCR), costimulatory molecules and cytokines, which involve MAPK/ERK, PI3kinase (PI3K)/Akt, mTOR, and NF-κB pathways (59). These events result in the induction of the transcription factor Myc as well as HIF-1α. These induce a number of genes important for glycolysis and glutaminolysis. To differentiate from uncommitted naïve CD4+ precursors into specialized effectors (Th1, Th2, Th17, etc.), proper upregulation of glucose metabolism is an absolute requisite. An inability to do so inhibits T effector cell differentiation both in vitro and in vivo (19). Glycolytic inadequacies or forced utilization of fatty acid metabolism instead results in either T cell anergy or the shunting of potential effector T cells to the iTreg lineage (14, 38).
In T cells, as in cells of the tumor, HIF-1 drives expression of a number of genes necessary for the shift to a glycolysis-dominated metabolism (50, 55). Reflecting this, in the study by Shi et al. (51), disrupting HIF-1 in T cells results in stunted expression of several glycolysis genes including those encoding Glut1, a glucose transporter; hexokinase 2; glucose-6-phosphate isomerase; enolase 1; pyruvate kinase muscle; and lactate dehydrogenase alongside the Th17-associated factors IL-23R, IL-21, IL-22, and IL-17. Supporting an important role for HIF-1 in the metabolic reprogramming necessary for Th17 differentiation, blocking glycolysis with the inhibitory glucose analog 2-deoxyglucose (2-DG) recapitulated the results of genetic HIF-1 ablation in CD4+ T cells, as did the mTOR inhibiting rapamycin. These results suggest that HIF-1 is an important player, along with mTOR and PI3K/Akt, in a metabolic reprogramming pathway shaping the nature of the T cell response (51).
Another study by Gomez-Rodriguez and colleagues (21) more recently expounded on the role of HIF-1 and mTOR in setting the balance between Th17 and iTreg balance. This study concluded that the Tec family kinase known as IL-2-inducible T cell kinase (Itk), an important participant in TCR signaling cascades, drives Th17 differentiation from naïve precursors while suppressing reciprocal iTreg commitment. Naïve CD4+ T cells from Itk-deficient mice cultured under Th17-skewing conditions show reduced upregulation of IL-17, and instead they induce Foxp3 expression. Additionally, Foxp3 levels were also elevated in Itk knockout T cells under iTreg-skewing conditions (21). Having previously shown Itk to promote Th17 differentiation (20), these authors further characterized this altered balance between pro- and anti-inflammatory T cell subsets. They found that in the absence of Itk, differentiating naïve T cells display defective mTOR/Akt activation. Importantly, expression of metabolic factors downstream of mTOR, including HIF-1α and the glucose transporter Glut1, was lacking without Itk (21), further linking HIF-1 expression to a metabolic profile conducive to Th17 generation.
These results suggest that elevated HIF-1 levels, stabilized by either cytokine or TCR signaling or hypoxic conditions, should promote a strong and enduring Th17 response. A couple of reports, while supporting the role of HIF-1 as driver of Th17 commitment, indicate that the relationship between Th17 programming and hypoxia is considerably more nuanced.
A study by Ikejiri et al. (25) suggested that the duration of hypoxic stress can have significant consequences for the enhancement of Th17 differentiation by HIF-1. These authors found that Th17 skewing could indeed be boosted by short-term, HIF-1-inducing, hypoxic culture. Importantly, however, optimal Th17 commitment was seen only when cells were primed with a brief period of hypoxia followed by reoxygenation. This boost in Th17 skewing, which was not observed for other Th cell lineages, was found to be HIF-1-dependent as it was not seen in T cells lacking HIF-1 expression (25).
More recently, Wang et al. (57) confirmed that not only does short-term hypoxic priming enhance IL-17 upregulation by naïve CD4+ T cells, they also showed that such treatment actually results in more robust HIF-1 levels than prolonged hypoxic culture. Correspondingly, in their study, the upregulation of IL-17 under prolonged hypoxia was less robust than after transient hypoxia/reoxygenation. These observations were explained by the discovery of a hypoxia/HIF-1-dependent upregulation of a microRNA (miR210) capable of targeting HIF-1's own transcript (57). Short-term hypoxic priming apparently avoids this built-in mechanism for negative feedback control. In this study, knocking out miR210 resulted in even higher levels of HIF-1 and HIF-1-dependent Th17 differentiation following hypoxic priming compared with that seen in wild-type T cells (57).
In addition to this role as a primer of the Th17 response, HIF-1 has also been implicated as a factor sustaining inflammatory Th17 cells and their function. Kryczek et al. (30) found that long-lived and highly plastic human Th17 cells can be recovered from numerous types of diseased tissues. In their study they found that Th17 cells express heightened levels of HIF-1 message compared with other T cell subsets, in agreement with the aforementioned mouse studies (12, 51). Suspecting that the ability of Th17 cells to survive in various inflamed tissues was mediated by HIF-1, the authors tested the effects of HIF-1 inhibition (echinomycin treatment) on the in vivo persistence of these cells. Supporting a role for HIF-1 in promoting the longevity of Th17 cells, inhibiting the molecule promoted the in vivo apoptosis of Th17 cells, an effect attributed to HIF-1's control of Notch signaling and antiapoptotic gene expression (30).
These findings not only paint HIF-1 as a promoter of the Th17 lineage, but they also strongly suggest that the regulation of this molecule and its contribution to CD4+ T cell differentiation are tightly regulated and potentially dynamic.
Regulatory T Cells
The activation, proliferation, and functions of effector T cell subsets are subject to attenuation by the suppressive mechanisms of regulatory T cells (Tregs). These cells moderate the intensity of immune responses, thereby preventing collateral tissue damage, and they also prevent autoimmunity by inhibiting self-antigen directed responses (2, 47, 64). For some time, Tregs have been divided into two subpopulations. Peripheral regulatory T cells (pTregs) arise in extrathymic tissue niches from naive T cells under the control of TGF-β and are propagated by IL-2 (9, 27). Thymic derived (t)Tregs, on the other hand, emerge from their namesake tissue of origin genetically and epigenetically programmed for a suppressive phenotype. Both subsets play important, but perhaps nonoverlapping roles in maintaining immune homeostasis (28). They also share a heavy reliance on the transcription factor Foxp3 for their characteristic Treg-gene expression profile typified by repression of effector genes and upregulation of those needed for immune suppression (27, 47).
While HIF-1 drives commitment of naïve CD4+ T cells to a Th17 fate, it does so apparently at the expense of reciprocal induction of Foxp3+ Tregs. We and others have found that T cells lacking HIF-1 preferentially upregulate Foxp3 protein under Th17-inducing conditions, in vitro (12, 51). Interestingly, in our study, this effect of disrupting HIF-1 function occurred at the protein level as the Foxp3 message was not markedly altered between wild-type and HIF-1-deficient CD4+ T cells (12).
We would go on to demonstrate that HIF-1 and Foxp3 physically interact, and that step-wise increases in ectopic HIF-1 expression levels cause a dose-dependent loss of Foxp3 protein in cell lines. Importantly, Foxp3 protein was observed to be both ubiquitinated and reduced in developing iTregs exposed to hypoxic culture conditions. This downregulation could be prevented by inhibiting the proteasome (MG132 treatment), suggesting that, like HIF-1 itself, Foxp3 is subject to posttranslational regulation via the ubiquitin-proteasome pathway. Supporting this notion, cells expressing mutant HIF-1 molecules rendered insensitive to oxygen-dependent modification and degradation failed to display Foxp3 downregulation in our experiments. Also, knocking down components of the HIF-1 degradation machinery also stabilized Foxp3 levels. These findings suggest that HIF-1 mediates the degradation of Foxp3 through the same pathway responsible for its own oxygen-dependent regulation (Fig. 1) (12). Whether or not HIF-1 and Foxp3 can be co-degraded in complex together remains to be elucidated, as do the precise molecular events involved in this process.
Interestingly, others have reported that hypoxia and HIF-1 can positively contribute to Foxp3 expression (3, 11) primarily by enhancing transcription of the Foxp3 gene under certain conditions. This activation of Foxp3 transcription has been shown to have consequences for established Treg function under inflammatory conditions (11). These different roles for HIF-1 suggest that it is possible that transcriptional and protein-level pathways controlling Foxp3 expression may be poised in opposition. What's more, uncharacterized factors may determine the winner of such a “tug-of-war.” While HIF-1-deficient T cells, in our hands, upregulate Foxp3 message on pace with their wild-type counterparts during in vitro T cell differentiation (12), under different conditions (i.e., in vivo inflammation, extreme hypoxia, etc.), HIF-1-driven transcription at Treg-critical loci such as Ctla4 and Foxp3 (11) may be necessary for optimal Treg functional stability. In support of this notion, HIF-1-deficient Tregs were found to be less effective than their wild-type counterparts at suppressing adoptive transfer-induced colitis (11, 24). In contrast, however, Itk-deficient iTregs, which have reduced HIF-1α levels, appear more functional in vitro and in vivo than their wild-type counterparts. How much of this improved performance occurs because of, or in spite of, low HIF-1 levels is unclear as Itk−/− Tregs are also hypersensitive to IL-2 signaling (21).
These results suggest that HIF-1 blockade may favor the generation of new Foxp3-expressing CD4+ T cells at the expense of potentially inflammatory Th17, but it may hinder the function or phenotypic stability of established Tregs in some situations. Adding to the complex picture of HIF-1's role in Tregs, a pair of recent studies explored the consequences of an overly robust HIF-1 pool.
Hsiao et al. (24) recently found that mice with Treg-restricted deletion of Deltex (DTX1)—a promoter of HIF-1 protein turnover—predictably display high levels of HIF-1 expression. These elevated HIF-1 levels did not remarkably change the baseline Foxp3 levels, expression of Treg-associated molecules and coregulatory factors, or the in vitro suppressive function of Tregs in this study. DTX1-deficient Tregs, however, were shown to be less effective suppressors than their wild-type counterparts in vivo (using models of airway inflammation and colitis). This was linked to unstable expression of Foxp3 which was observed in parallel with bolstered HIF-1 levels in the absence of DTX1. In this study, DTX expression could prevent a hypoxia-induced reduction of the Foxp3 protein pool in T cells. Importantly, the authors showed that simultaneous HIF-1 and DTX1 deficiencies in Tregs restored their in vivo Foxp3 expression level, which largely rescued the ability of DTX1-deficient Tregs to function in vivo (24).
In another recent study, Lee et al. (31) also shed light on the consequences of excessive HIF-1 stabilization in Tregs. This study involved conditional, Foxp3-driven knockout of VHL. As this E3 ligase complex mediates the degradation of HIF, it was not surprising that Foxp3+ cells from these mice display elevated levels of HIF-1 compared with wild-type Tregs. Notably, however, the authors found that stabilized HIF expression in the absence of VHL undermined the suppressive phenotype of Tregs as evidenced by both the spontaneous inflammation and early mortality seen in Foxp3Cre+/VHLfl/fl mice. Furthermore, these mice showed signs of high baseline levels of T cell activation, and Tregs isolated from these mice had reduced Foxp3 expression, with tissues most expected to be hypoxic witnessing the most pronounced downregulation (31). In line with an inability to maintain immune homeostasis, VHL-deficient Tregs also do not control disease in the adoptive transfer colitis model (31).
The authors attributed the phenotypic instability of VHL-deficient Tregs to their heightened HIF-1 levels, which were linked to the acquisition of an abhorrent, Th1 effector-like phenotype. Additionally, high HIF-1 expression in the absence of VHL was observed to promote a metabolic lifestyle incompatible with that typically seen in Tregs, which the authors credit with enhancing the breakdown of Treg phenotype (31).
These studies of Hsiao et al. (24) and Lee et al. (31) suggest that very high levels HIF-1 expression or activity can negatively impact Treg phenotype. Taken with the observation that established Tregs can be less effective in vivo without HIF-1, one comes away with the notion that to some degree, HIF-1 can stabilize Tregs, but “too much a good thing” can have the opposite effect. These findings have substantial implications for the study of Treg behavior in diverse microenvironments, and they suggest that the relationship between HIF-1 levels and Treg stability may be complex.
Recently, Mascanfroni et al. (36) demonstrated a complex role for HIF-1 in the programming of a distinct regulatory T cell subset, the Tr1 cells. These cells, which do not express Foxp3, are characterized by their ready production of the anti-inflammatory cytokine IL-10. Like their Foxp3+ counterparts, Tr1 are important in the maintenance of immune homeostasis and are capable of limiting inflammatory damage in a number of disease models including those for neuroinflammation and inflammatory bowel disease (43, 45, 46).
In this study, hypoxia inhibited Tr1 generation. Specifically, HIF-1 upregulation, brought about by extracellular ATP buildup (a hallmark of hypoxia), negatively impacted the differentiation of Tr1 cells. This antagonism of Tr1 generation by high HIF-1 levels was found to stem from the negative effects of HIF-1 on both the protein pool and activity of a key player in the Tr1 differentiation program, the aryl hydrocarbon receptor (AHR). This was linked to the competition between HIF-1α and AHR for their common binding partner HIF-1β/ARNT. The latter of these requires ARNT to activate expression of key Tr1-associated genes like Il10, Il21, and Entpd1 (36). Interestingly, HIF-1 was also found to play a positive role in the early differentiation of Tr1 by promoting an aerobic glycolysis-dominated metabolic profile necessary for the Tr1 phenotype. These findings suggest that HIF-1 plays a duplicitous role in the biology of this unique Treg subset.
Taken together, these studies present a complicated picture of HIF-1's role in the biology of Tregs. A number of variables may determine the ultimate part played. For instance, the mechanisms governing the generation of Tregs may be distinct from those needed to maintain the Treg phenotype “under fire.” Also, in light of HIF-1's augmentation of Th17 differentiation being apparently highly sensitive to the timing and duration of hypoxia (25, 57), it is tempting to speculate that its impact on Tregs is similarly dependent on the duration or degree of HIF-1 stabilization. Studies of mice with stabilized HIF-1 levels seem aligned with this notion. Future work will certainly shed light on this topic.
CD8+ T Cells
Hypoxia and HIF-1 also play important, and somewhat complex, roles in the CD8+ T cell compartment. Hypoxia has been reported to alter specific aspects of CD8+ T cell biology. While Fas ligand- and perforin-mediated killing by CD8+ cytotoxic T lymphocytes (CTL) is not altered by low oxygen concentrations, at physiologically relevant hypoxic conditions (2.5%), the frequency of CD8+ T cells appears adversely affected. Intriguingly, while fewer in number, these CD8+ T cells exposed to hypoxia display heightened cytolytic potential. These findings suggest that while hypoxia does not interfere with the killing function in CD8+ T cells, it does promote the differentiation of a highly potent subset of CTL (7). Involvement of HIF-1 in CD8+ T cell differentiation has also been reported by several groups.
Just as appears to be the case with activated CD4+ T cells, the involvement of HIF-1 in the differentiation and function of CD8+ T cell populations involves the molecule's part in shifting the gears of cellular metabolism in addition to its control over the hypoxic response.
Resting CD8+ T cells, like their CD4+ counterparts, are fueled by fatty acid oxidation while quiescent. However, following activation, these cells adopt a glycolysis-dominated metabolism needed to support their proliferation and differentiation into effector and memory subsets (18, 19, 44, 54). The triggering of TCR signaling and costimulation through CD28 induces expression of Myc and expression of Myc-dependent genes that are critical for the initial activation and expansion of T cells. Another wave of TCR-triggered genes including AP4, IRF4, and HIF-1 is thought to continue the cellular commitment to glycolysis by facilitating the upregulation of enzymes involved in glycolysis and glutaminolysis (10). Effector CD8+ T cells are characteristically short-lived and their numbers contract with the waning of the immune response. Memory CD8+ T cells, on the other hand, persist (61) and can respond to antigenic re-challenge with rapid kinetics (37). Among the effector and memory CD8+ T cell subsets are those with defining killing capacity, the CTLs.
CTLs are crucial mediators of cell-mediated immunity. Activation of these cells can trigger the killing of infected or altered (malignant) host cells mediated by the production and release of perforin and granzymes. CTLs are also capable of driving proinflammatory immune responses by producing cytokines including TNF-α and IFN-γ (23).
Finlay et al. (17) identified HIF-1 as a major facilitator of the metabolic shift in newly activated CD8+ T cells (17). These authors found that, upon encountering their cognate antigen in the presence of IL-2, CD8+ T cells show enhanced mTOR complex 1 (mTORC1) activity, which leads to robust levels of HIF-1α and -β subunits, and isolated CTLs also showed high expression of HIF-1 as well. Upregulation of HIF-1 by mTORC1 in these activated CD8+ T cells was found to promote glucose uptake and glycolysis through the upregulation of numerous glycolytic enzymes and the glucose transporter Glut1. Assessment of the gene expression changes associated with HIF-1 deficiency (achieved by deletion of the HIF-1β subunit) confirmed the importance of this molecule as a glycolysis-promoting factor. They also revealed a downregulation of certain granzymes and perforin—important mediators of CTL function—in the absence of HIF-1 (17). Importantly, these authors also found that HIF-1-inducing hypoxic culture conditions could enhance the expression of metabolic and effector molecules (perforin and Glut1) in CTLs (17), clearly linking HIF-1 to the metabolic lifestyle of activated, effector CD8+ T cells. These results are in accord with earlier findings reported by Caldwell et al. (7), which found that hypoxia favors the development of highly cytotoxic CD8+ T cells.
In another study, IRF4, a transcription factor important for sustaining the activation and function of effector CD8+ T cells, was found to support commitment to the glycolytic lifestyle. IRF4 was found to bind a number of genes important for CD8+ T cell differentiation and function as well as those encoding metabolic regulators including HIF-1α. Furthermore, IRF4−/−-derived CD8+ T cells expressed lower levels of HIF-1α and a number of glycolysis participants. Correspondingly, these T cells display reduced rates of glycolysis compared with their wild-type counterparts, and they also fail to maintain clonal expansion after activation (35). These results are in line with the notion that HIF-1 is important for meeting the metabolic needs of effector CD8+ T cells.
Antigen-mediated activation of CTLs precipitates their cytotoxic and proinflammatory potential. Persistent antigen exposure, much like one would expect in the case of chronic infection or cancer, on the other hand, has been shown to dampen the intensity of CTL responses (61). Using a model for chronic viral infection, lymphocytic choriomeningitis virus (LCMV), Doedens et al. (16) investigated the importance of hypoxia inducible factors in such a scenario.
In this study, ablation of the VHL complex, which mediates the degradation of HIFs in the presence of oxygen, enhanced levels of HIF-1 and HIF-2 in activated CD8+ T cells. Interestingly, peripheral T cell-restricted VHL deficiency (accomplished by crossing mice carrying a floxed VHL gene to a Lck promoter-driven Cre transgenic mouse) also resulted in high mortality rates in mice upon persistent viral challenge (16).
The authors of this study would go on to show that this enhanced mortality stemmed from immunopathologies resulting from a highly inflammatory, exhaustion-resistant CD8+ T cell phenotype that arose in the midst of HIF overabundance. These T cells lacked KLRG1, a marker of cells likely to terminally differentiate into short-lived effector CTLs. Furthermore, analysis of the genes expressed by CD8+ T cells in both the presence and absence of VHL revealed that VHL deficiency and reciprocally high levels of HIF-1 activity results in a bolstered, sustained effector phenotype marked by elevated expression of genes related to glycolysis, CTL effector function, and T cell activation. Meanwhile, transcription factors responsible for CD8+ differentiation (i.e., Tcf7, Eomes, T-bet) were downregulated in the presence of elevated HIF-1 levels. Furthermore, in this study, stabilization of HIF-1 levels by hypoxia could recapitulate the effects of VHL knockout. Specifically, hypoxic culture resulted in a HIF-1-dependent enhancement of granzyme B, activation-induced surface markers (i.e., LAG3, CTLA-4) while transcription factors involved in T cell differentiation (i.e., T-bet and TCF-1) were downregulated by hypoxia as they were in T cells lacking VHL (16).
HIF-1 also plays a role in memory CD8+ T cells, which persist beyond the immune response's contraction phase, outlasting their terminally differentiated effector counterparts. Like naïve T cells, these cells are quiescent. However, they can traffic to diverse tissues and respond to antigenic re-challenge with accelerated kinetics comparable to cells of the innate immune system. This functional shift is accompanied by an “immediate early” metabolic transition towards a reliance on aerobic glycolysis.
Gubser et al. (22) found that effector memory (EM) CD8+ T cell subsets activated by CD3 and CD28 cross-linking antibodies dramatically upregulate glycolytic metabolism, to an extent greater than their naïve T cell counterparts. This surge requires costimulation as well as an adequate glucose supply. The authors of this study would go on to demonstrate a dependence on PI3K/Akt (and mTORC2, but not mTORC1) signaling for this metabolic shift. This rapid response was linked to a remodeling of chromatin at the IFNG promoter favoring activation of gene expression. Inhibition of glycolysis by 2-DG treatment interfered with these epigenetic events and reduced expression of the cytokine (22). While it was not clear from this study whether HIF-1 plays an indispensable role in the implementation or maintenance of this metabolic shift, the importance of the molecule for this form of metabolism certainly suggests that HIF-1 contributes the awakening of memory CD8+ T cell subsets.
These results suggest that stabilized HIF-1 expression, resulting either from hypoxia or other means, should optimize the killing and potential of the CD8+ T cell compartment. Interestingly, however, Sukumar et al. (52) found that while chemical inhibition of glycolysis (and HIF-1 expression) led to reduced commitment to a short-lived effector CD8+ T cell fate, these conditions led instead to an enhanced, memory-like population (identifiable as KLRG1low/CD62Lhigh). In addition, artificially enforcing glycolytic metabolism in CD8+ T cells could restrict the generation of memory cells in this study (52). These results are consistent with those reported by Pollizzi et al. (42), which showed that commitment to a highly glycolysis-driven metabolic program favored short-lived effector memory cells while negatively affecting memory populations. Interestingly, these memory CD8+ accumulating upon glycolysis-blockade and HIF-1 downregulation were actually more effective at combating tumor growth than control cells more inclined to become effectors (52). These results suggest that while HIF-1 may be key for establishing a glycolysis-driven metabolism upon CD8++ T cell activation, it is likely that the process and the molecule play an unexpectedly negative role in the formation of highly function memory CD8+ T cell populations.
In line with these findings, our own unpublished results suggest that knocking out HIF-1 in T cells has a considerable effect on both the memory phenotype and inflammatory potential of CD8+ T cells. We found that implanted B16 melanomas grow much less aggressively in mice lacking HIF-1 in the T cell compartment with evidence of an improved anti-tumor immune response compared with wild-type controls despite an apparently competent Foxp3+Treg population. Interestingly, CD8+ T cells from these conditional knockout mice display surface and transcriptional characteristics of memory CD8+ T cells and have enhanced capacities for killing and IFN-γ production (our unpublished results). These findings are consistent with the notion that HIF-1 suppresses the acquisition of a highly cytotoxic and proinflammatory CD8+ T cell population.
Also supporting this notion, treatment of tumor-bearing mice with HIF-1 inhibitors slows tumor progression and recapitulates the effects of genetic HIF-1 ablation. Suspecting that HIF-1 inhibition could have the counterproductive outcome of elevating Foxp3+ Treg frequencies (at the expense of the Th17 cell pool), coupling HIF-1 inhibition with periodic Treg depletion leads to an even more dramatic anti-tumor effect (our unpublished results). Taken together, these findings point to distinct roles for HIF-1 in different CD8+ T cell subsets.
Summary and Discussion
In this review we have highlighted the distinct roles played by HIF-1 across diverse T cell subsets. It is clear that during CD4+ T cell differentiation, HIF-1 can be an important driver of potentially inflammatory, IL-17-producing effector cells. Since HIF-1 deficiency favors a reciprocal commitment to Foxp3 upregulation in differentiating naïve T cells, the metabolic sensor is likely among the factors determining the balance between the distinct Treg and Th17 cell lineages. This appears to be the result of HIF-1's activation of pro-Th17 transcriptional program, its protein-level antagonism of Foxp3, and/or its critical role promoting a glycolytic life style in T cells. In regulatory T cell populations, HIF-1's role appears decidedly more complex with both beneficial and negative effects attributed to its stabilization and activation. These disparate outcomes of HIF-1 activity in Tregs may well depend on a number of factors including the level, duration, and timing of HIF-1 expression. Additionally, in CD8+ T cells, HIF-1 seems to have very subset-specific roles—facilitating a glycolysis-dominated metabolism necessary for effector cell function—while also negatively affecting the generation of a surprisingly inflammatory and tumoricidal memory CD8 T cell subset.
These findings have considerable implications for the study of T cell behavior in a number of microenvironments and under a variety of conditions both homeostatic and pathological. Importantly, they also are relevant to the development of novel immunotherapies.
Since inflammatory and autoimmune diseases are often associated with relative deficiencies in either Treg presence or function (13, 34) and sometimes a reciprocal abundance of proinflammatory T cell subsets (such as the Th17 cells), there is much interest in finding ways to therapeutically correct such imbalances. Targeting HIF-1 may prove an effective means to this end given the reduction in Th17 cell differentiation and the increase in potentially suppressive Foxp3-expressing cell frequency seen upon HIF-1 knockout. These encouraging results must nevertheless be weighed with possible trade-offs in Treg stability or function that might occur in the accumulating Treg population.
In the cancer setting, chemically targeting HIF-1 appears to be a highly viable strategy with multiple potential benefits arising from the suppression of several tumor-promoting processes. An additional result of HIF-1 inhibition during cancer may be the suppressed production of IL-17, a cytokine that contributes to tumor progression (58). Undermining the Th17 differentiation pathway along with multiple pro-tumor processes by chemically targeting general HIF-1 function is a tempting therapeutic strategy. However, studies using mice with HIF-1-deficient T cells sound a note of caution when considering HIF-1 inhibition as monotherapy cancer treatment.
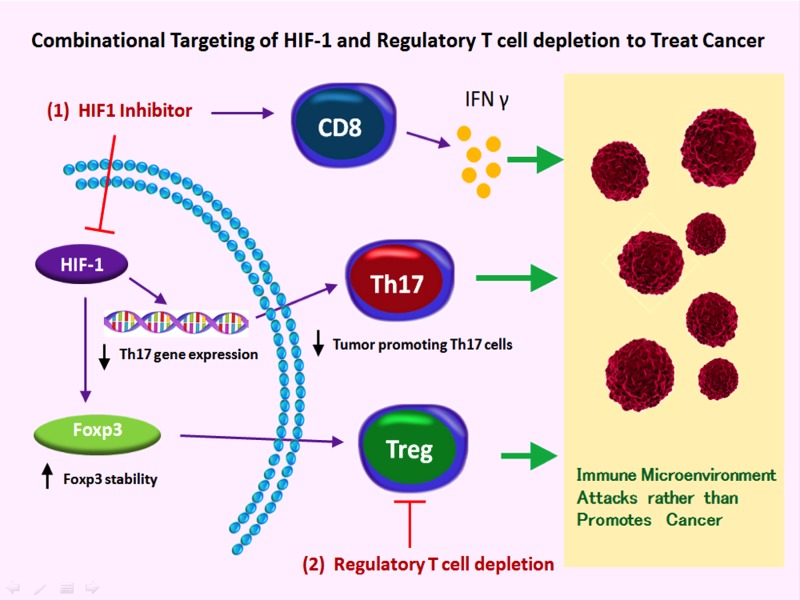
While HIF-1 inhibitors can interfere with the tumor-promoting processes of angiogenesis and cancer cell metabolism while negating the pro-tumor effects of IL-17. They may, as suggested by the previous work of our group and others, also elevate the frequency of immune suppressive Treg cells. In cancer, these cells tend to be enriched, either locally in developing tumors or systemically at advanced stages, and the immune-suppressing nature of these cells hampers the anti-tumor response permitting tumor growth (6). Sabotaging Tregs or blocking their function has been explored as an immunotherapeutic measure to enhance the anti-tumor immune response and increase the effectiveness of anti-cancer therapies including tumor vaccines. Therefore the anti-tumor efficacy of HIF-1 inhibition should be evaluated in combination with additional agents aimed at counteracting potential suppressor cell accumulation such as the drugs used to deplete Treg cells. Such a combinational approach should, in theory, simultaneously neutralize two tumor-promoting T cell populations (Fig. 2).
Fig. 2.
A combinational approach for enhancing the anti-tumor immune response through HIF-1-targeting approaches. Inhibition of HIF-1 activity or expression (1) undermines expression of Th17-associated genes including the tumor-abetting IL-17. Disrupting HIF-1 also enhances the frequency of a highly proinflammatory subset of CD8+ T cells capable of driving more effective anti-tumor immune responses. While HIF-1 inhibition is expected to elevate frequencies of potentially suppressive Foxp3+ T cells as well, simultaneous depletion or inhibition of Tregs (2) is likely to either prevent this counterproductive potentiality or enhance anti-tumor immunity further.
Another possible benefit of targeting HIF-1 during cancer presents itself in the finding that HIF-1- and glycolytic-deficiencies are associated with the rise of a CD8+ T cell population capable of directing a much more efficient anti-tumor immune response (Fig. 2). In all, these many roles for HIF-1 in the heterogeneous biology of T cells make this molecule an intriguing candidate for immunotherapies aimed at fighting immune dysregulation in very different pathological settings.
GRANTS
Funding support comes from grants from the Melanoma Research Alliance, the National Institutes of Health (RO1AI099300 and RO1AI089830), “Kelly's Dream” Foundation, the Janey Fund, and the Seraph Foundation, and gifts from Bill and Betty Topecer and Dorothy Needle. F. Pan is a Stewart Trust Scholar. J. Barbi is supported by a Crohn's and Colitis Foundation of America Research Fellowship.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
J.-H.T. prepared figures; J.-H.T., J.B., and F.P. approved final version of manuscript; J.B. drafted manuscript; J.B. and F.P. edited and revised manuscript.
REFERENCES
- 1.Barbi J, Pardoll D, Pan F. Metabolic control of the Treg/Th17 axis. Immunol Rev 252: 52–77, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Barnes MJ, Krebs P, Harris N, Eidenschenk C, Gonzalez-Quintial R, Arnold CN, Crozat K, Sovath S, Moresco EM, Theofilopoulos AN, Beutler B, Hoebe K. Commitment to the regulatory T cell lineage requires CARMA1 in the thymus but not in the periphery. PLoS Biol 7: e51, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ben-Shoshan J, Maysel-Auslender S, Mor A, Keren G, George J. Hypoxia controls CD4+CD25+ regulatory T-cell homeostasis via hypoxia-inducible factor-1alpha. Eur J Immunol 38: 2412–2418, 2008. [DOI] [PubMed] [Google Scholar]
- 4.Bettelli E, Carrier Y, Gao W, Korn T, Strom TB, Oukka M, Weiner HL, Kuchroo VK. Reciprocal developmental pathways for the generation of pathogenic effector TH17 and regulatory T cells. Nature 441: 235–238, 2006. [DOI] [PubMed] [Google Scholar]
- 5.Blouin CC, Page EL, Soucy GM, Richard DE. Hypoxic gene activation by lipopolysaccharide in macrophages: implication of hypoxia-inducible factor 1alpha. Blood 103: 1124–1130, 2004. [DOI] [PubMed] [Google Scholar]
- 6.Byrne WL, Mills KH, Lederer JA, O'Sullivan GC. Targeting regulatory T cells in cancer. Cancer Res 71: 6915–6920, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Caldwell CC, Kojima H, Lukashev D, Armstrong J, Farber M, Apasov SG, Sitkovsky MV. Differential effects of physiologically relevant hypoxic conditions on T lymphocyte development and effector functions. J Immunol 167: 6140–6149, 2001. [DOI] [PubMed] [Google Scholar]
- 8.Carreau A, El Hafny-Rahbi B, Matejuk A, Grillon C, Kieda C. Why is the partial oxygen pressure of human tissues a crucial parameter? Small molecules and hypoxia. J Cell Mol Med 15: 1239–1253, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chen Q, Kim YC, Laurence A, Punkosdy GA, Shevach EM. IL-2 controls the stability of Foxp3 expression in TGF-beta-induced Foxp3+ T cells in vivo. J Immunol 186: 6329–6337, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chisolm DA, Weinmann AS. TCR-signaling events in cellular metabolism and specialization. Front Immunol 6: 292, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Clambey ET, McNamee EN, Westrich JA, Glover LE, Campbell EL, Jedlicka P, de Zoeten EF, Cambier JC, Stenmark KR, Colgan SP, Eltzschig HK. Hypoxia-inducible factor-1 alpha-dependent induction of FoxP3 drives regulatory T-cell abundance and function during inflammatory hypoxia of the mucosa. Proc Natl Acad Sci USA 109: E2784–E2793, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dang EV, Barbi J, Yang HY, Jinasena D, Yu H, Zheng Y, Bordman Z, Fu J, Kim Y, Yen HR, Luo W, Zeller K, Shimoda L, Topalian SL, Semenza GL, Dang CV, Pardoll DM, Pan F. Control of T(H)17/T(reg) balance by hypoxia-inducible factor 1. Cell 146: 772–784, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dejaco C, Duftner C, Grubeck-Loebenstein B, Schirmer M. Imbalance of regulatory T cells in human autoimmune diseases. Immunology 117: 289–300, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Delgoffe GM, Pollizzi KN, Waickman AT, Heikamp E, Meyers DJ, Horton MR, Xiao B, Worley PF, Powell JD. The kinase mTOR regulates the differentiation of helper T cells through the selective activation of signaling by mTORC1 and mTORC2. Nat Immunol 12: 295–303, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dewhirst MW, Cao Y, Moeller B. Cycling hypoxia and free radicals regulate angiogenesis and radiotherapy response. Nat Rev Cancer 8: 425–437, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Doedens AL, Phan AT, Stradner MH, Fujimoto JK, Nguyen JV, Yang E, Johnson RS, Goldrath AW. Hypoxia-inducible factors enhance the effector responses of CD8(+) T cells to persistent antigen. Nat Immunol 14: 1173–1182, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Finlay DK, Rosenzweig E, Sinclair LV, Feijoo-Carnero C, Hukelmann JL, Rolf J, Panteleyev AA, Okkenhaug K, Cantrell DA. PDK1 regulation of mTOR and hypoxia-inducible factor 1 integrate metabolism and migration of CD8+ T cells. J Exp Med 209: 2441–2453, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Frauwirth KA, Thompson CB. Regulation of T lymphocyte metabolism. J Immunol 172: 4661–4665, 2004. [DOI] [PubMed] [Google Scholar]
- 19.Gerriets VA, Rathmell JC. Metabolic pathways in T cell fate and function. Trends Immunol 33: 168–173, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gomez-Rodriguez J, Sahu N, Handon R, Davidson TS, Anderson SM, Kirby MR, August A, Schwartzberg PL. Differential expression of interleukin-17A and -17F is coupled to T cell receptor signaling via inducible T cell kinase. Immunity 31: 587–597, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gomez-Rodriguez J, Wohlfert EA, Handon R, Meylan F, Wu JZ, Anderson SM, Kirby MR, Belkaid Y, Schwartzberg PL. Itk-mediated integration of T cell receptor and cytokine signaling regulates the balance between Th17 and regulatory T cells. J Exp Med 211: 529–543, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gubser PM, Bantug GR, Razik L, Fischer M, Dimeloe S, Hoenger G, Durovic B, Jauch A, Hess C. Rapid effector function of memory CD8+ T cells requires an immediate-early glycolytic switch. Nat Immunol 14: 1064–1072, 2013. [DOI] [PubMed] [Google Scholar]
- 23.Harty JT, Tvinnereim AR, White DW. CD8+ T cell effector mechanisms in resistance to infection. Annu Rev Immunol 18: 275–308, 2000. [DOI] [PubMed] [Google Scholar]
- 24.Hsiao HW, Hsu TS, Liu WH, Hsieh WC, Chou TF, Wu YJ, Jiang ST, Lai MZ. Deltex1 antagonizes HIF-1alpha and sustains the stability of regulatory T cells in vivo. Nat Commun 6: 6353, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ikejiri A, Nagai S, Goda N, Kurebayashi Y, Osada-Oka M, Takubo K, Suda T, Koyasu S. Dynamic regulation of Th17 differentiation by oxygen concentrations. Int Immunol 24: 137–146, 2012. [DOI] [PubMed] [Google Scholar]
- 26.Jiang BH, Rue E, Wang GL, Roe R, Semenza GL. Dimerization, DNA binding, and transactivation properties of hypoxia-inducible factor 1. J Biol Chem 271: 17771–17778, 1996. [DOI] [PubMed] [Google Scholar]
- 27.Josefowicz SZ, Lu LF, Rudensky AY. Regulatory T cells: mechanisms of differentiation and function. Annu Rev Immunol 30: 531–564, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Josefowicz SZ, Niec RE, Kim HY, Treuting P, Chinen T, Zheng Y, Umetsu DT, Rudensky AY. Extrathymically generated regulatory T cells control mucosal TH2 inflammation. Nature 482: 395–399, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jung JE, Lee HG, Cho IH, Chung DH, Yoon SH, Yang YM, Lee JW, Choi S, Park JW, Ye SK, Chung MH. STAT3 is a potential modulator of HIF-1-mediated VEGF expression in human renal carcinoma cells. FASEB J 19: 1296–1298, 2005. [DOI] [PubMed] [Google Scholar]
- 30.Kryczek I, Zhao E, Liu Y, Wang Y, Vatan L, Szeliga W, Moyer J, Klimczak A, Lange A, Zou W. Human TH17 cells are long-lived effector memory cells. Sci Transl Med 3: 104ra100, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lee JH, Elly C, Park Y, Liu YC. E3 ubiquitin ligase VHL regulates hypoxia-inducible factor-1alpha to maintain regulatory T cell stability and suppressive capacity. Immunity 42: 1062–1074, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lee YK, Mukasa R, Hatton RD, Weaver CT. Developmental plasticity of Th17 and Treg cells. Curr Opin Immunol 21: 274–280, 2009. [DOI] [PubMed] [Google Scholar]
- 33.Loenarz C, Coleman ML, Boleininger A, Schierwater B, Holland PW, Ratcliffe PJ, Schofield CJ. The hypoxia-inducible transcription factor pathway regulates oxygen sensing in the simplest animal, Trichoplax adhaerens. EMBO Rep 12: 63–70, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Long SA, Buckner JH. CD4+FOXP3+ T regulatory cells in human autoimmunity: more than a numbers game. J Immunol 187: 2061–2066, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Man K, Miasari M, Shi W, Xin A, Henstridge DC, Preston S, Pellegrini M, Belz GT, Smyth GK, Febbraio MA, Nutt SL, Kallies A. The transcription factor IRF4 is essential for TCR affinity-mediated metabolic programming and clonal expansion of T cells. Nat Immunol 14: 1155–1165, 2013. [DOI] [PubMed] [Google Scholar]
- 36.Mascanfroni ID, Takenaka MC, Yeste A, Patel B, Wu Y, Kenison JE, Siddiqui S, Basso AS, Otterbein LE, Pardoll DM, Pan F, Priel A, Clish CB, Robson SC, Quintana FJ. Metabolic control of type 1 regulatory T cell differentiation by AHR and HIF1-alpha. Nat Med 21: 638–646, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Masopust D, Picker LJ. Hidden memories: frontline memory T cells and early pathogen interception. J Immunol 188: 5811–5817, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Michalek RD, Gerriets VA, Jacobs SR, Macintyre AN, MacIver NJ, Mason EF, Sullivan SA, Nichols AG, Rathmell JC. Cutting edge: distinct glycolytic and lipid oxidative metabolic programs are essential for effector and regulatory CD4+ T cell subsets. J Immunol 186: 3299–3303, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Nakamura H, Makino Y, Okamoto K, Poellinger L, Ohnuma K, Morimoto C, Tanaka H. TCR engagement increases hypoxia-inducible factor-1 alpha protein synthesis via rapamycin-sensitive pathway under hypoxic conditions in human peripheral T cells. J Immunol 174: 7592–7599, 2005. [DOI] [PubMed] [Google Scholar]
- 40.Nizet V, Johnson RS. Interdependence of hypoxic and innate immune responses. Nat Rev Immunol 9: 609–617, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Pearce EL. Metabolism in T cell activation and differentiation. Curr Opin Immunol 22: 314–320, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Pollizzi KN, Patel CH, Sun IH, Oh MH, Waickman AT, Wen J, Delgoffe GM, Powell JD. mTORC1 and mTORC2 selectively regulate CD8(+) T cell differentiation. J Clin Invest 125: 2090–2108, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Pot C, Apetoh L, Kuchroo VK. Type 1 regulatory T cells (Tr1) in autoimmunity. Semin Immunol 23: 202–208, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Powell JD, Pollizzi K. Fueling memories. Immunity 36: 3–5, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Roncarolo MG, Gregori S, Bacchetta R, Battaglia M. Tr1 cells and the counter-regulation of immunity: natural mechanisms and therapeutic applications. Curr Top Microbiol Immunol 380: 39–68, 2014. [DOI] [PubMed] [Google Scholar]
- 46.Roncarolo MG, Gregori S, Battaglia M, Bacchetta R, Fleischhauer K, Levings MK. Interleukin-10-secreting type 1 regulatory T cells in rodents and humans. Immunol Rev 212: 28–50, 2006. [DOI] [PubMed] [Google Scholar]
- 47.Sakaguchi S, Yamaguchi T, Nomura T, Ono M. Regulatory T cells and immune tolerance. Cell 133: 775–787, 2008. [DOI] [PubMed] [Google Scholar]
- 48.Semenza GL. Defining the role of hypoxia-inducible factor 1 in cancer biology and therapeutics. Oncogene 29: 625–634, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Semenza GL. HIF-1 and human disease: one highly involved factor. Genes Dev 14: 1983–1991, 2000. [PubMed] [Google Scholar]
- 50.Semenza GL. HIF-1 mediates metabolic responses to intratumoral hypoxia and oncogenic mutations. J Clin Invest 123: 3664–3671, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Shi LZ, Wang R, Huang G, Vogel P, Neale G, Green DR, Chi H. HIF1alpha-dependent glycolytic pathway orchestrates a metabolic checkpoint for the differentiation of TH17 and Treg cells. J Exp Med 208: 1367–1376, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Sukumar M, Liu J, Ji Y, Subramanian M, Crompton JG, Yu Z, Roychoudhuri R, Palmer DC, Muranski P, Karoly ED, Mohney RP, Klebanoff CA, Lal A, Finkel T, Restifo NP, Gattinoni L. Inhibiting glycolytic metabolism enhances CD8+ T cell memory and antitumor function. J Clin Invest 123: 4479–4488, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Takeda N, O'Dea EL, Doedens A, Kim JW, Weidemann A, Stockmann C, Asagiri M, Simon MC, Hoffmann A, Johnson RS. Differential activation and antagonistic function of HIF-α isoforms in macrophages are essential for NO homeostasis. Genes Dev 24: 491–501, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.van der Windt GJ, Pearce EL. Metabolic switching and fuel choice during T-cell differentiation and memory development. Immunol Rev 249: 27–42, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Waickman AT, Powell JD. mTOR, metabolism, and the regulation of T-cell differentiation and function. Immunol Rev 249: 43–58, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wang GL, Jiang BH, Rue EA, Semenza GL. Hypoxia-inducible factor 1 is a basic-helix-loop-helix-PAS heterodimer regulated by cellular O2 tension. Proc Natl Acad Sci USA 92: 5510–5514, 1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Wang H, Flach H, Onizawa M, Wei L, McManus MT, Weiss A. Negative regulation of Hif1a expression and TH17 differentiation by the hypoxia-regulated microRNA miR-210. Nat Immunol 15: 393–401, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Wang L, Yi T, Zhang W, Pardoll DM, Yu H. IL-17 enhances tumor development in carcinogen-induced skin cancer. Cancer Res 70: 10112–10120, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Wang R, Green DR. The immune diet: meeting the metabolic demands of lymphocyte activation. F1000 Biol Rep 4: 9, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Wang R, Green DR. Metabolic reprogramming and metabolic dependency in T cells. Immunol Rev 249: 14–26, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Williams MA, Bevan MJ. Effector and memory CTL differentiation. Annu Rev Immunol 25: 171–192, 2007. [DOI] [PubMed] [Google Scholar]
- 62.Xu Q, Briggs J, Park S, Niu G, Kortylewski M, Zhang S, Gritsko T, Turkson J, Kay H, Semenza GL, Cheng JQ, Jove R, Yu H. Targeting Stat3 blocks both HIF-1 and VEGF expression induced by multiple oncogenic growth signaling pathways. Oncogene 24: 5552–5560, 2005. [DOI] [PubMed] [Google Scholar]
- 63.Zhou L, Lopes JE, Chong MM, Ivanov II, Min R, Victora GD, Shen Y, Du J, Rubtsov YP, Rudensky AY, Ziegler SF, Littman DR. TGF-beta-induced Foxp3 inhibits T(H)17 cell differentiation by antagonizing RORgammat function. Nature 453: 236–240, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Zhu J, Yamane H, Paul WE. Differentiation of effector CD4 T cell populations (*). Annu Rev Immunol 28: 445–489, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]