Abstract
Patients with metabolically healthy obesity (MHO) do not present the cluster of metabolic abnormalities that define the metabolic syndrome (MetS). Whether MHO is associated with lower impairment of vasoreactivity than the MetS is unknown. For this purpose, forearm blood flow (FBF) responses were measured by strain-gauge plethysmography during the intra-arterial infusion of acetylcholine (ACh), sodium nitroprusside (SNP), and/or the selective endothelin type A (ETA) receptor blocker BQ-123 in 119 obese individuals with MHO (n = 34) or with the MetS (n = 85) and in healthy lean controls (n = 56). ACh and SNP caused a significant vasodilation in both obese and lean participants (all P < 0.001). However, the response to both agents was significantly lower in the obese than in the control group (both P < 0.001). Among the obese participants, the reactivity to ACh was higher in MHO than in MetS patients, whereas the responsiveness to SNP was equally impaired in both groups (P = 0.45). Infusion of BQ-123 significantly increased FBF in obese patients (P < 0001), but not in the lean participants; hence, FBF following ETA receptor blockade was higher in both obese groups than in controls (both P < 0.001). FBF response to BQ-123 was significantly higher in patients with the MetS than in those with MHO (P = 0.007). In conclusion, patients with MHO have abnormal vascular reactivity, although their endothelial dysfunction is less pronounced than in patients with the MetS. These findings indicate that obesity is associated with vascular damage independent of those metabolic abnormalities underlying the MetS.
Keywords: obesity, endothelium, endothelin-1, metabolic syndrome, vasodilation
obesity, particularly in the presence of abdominal fat accumulation, is linked to the occurrence of insulin resistance, impaired glucose metabolism, atherogenic lipid profile, and elevated blood pressure (20). The clustering of these abnormalities forms the basis for the diagnosis of the metabolic syndrome (MetS) (3) and contributes to the doubling of the relative risk for cardiovascular events and the fivefold increment in the risk of developing type 2 diabetes observed in patients with this condition (16). Interestingly, a proportion of obese individuals does not present with the metabolic disturbances underlying the MetS (33), and therefore, these individuals are considered to have a metabolically healthy obesity (MHO). Although some observational data appear to indicate that obesity in the absence of the MetS is not associated with increased risk of cardiovascular disease (18), other studies have cast doubts on this evidence (5, 12).
We and others have demonstrated that patents with the MetS have impaired endothelial vasodilator responses (26, 29) and enhanced ET-1 mediated vasoconstrictor tone (30). This state of endothelial dysfunction is a pivotal player in the pathogenesis of atherosclerosis (14) and is an independent predictor of cardiovascular events (17). Interestingly, Weil et al. (32) have reported an elevated endothelin (ET)-1-mediated vasoconstriction in overweight and obese adults independent of other cardiovascular risk factors. Given the absence of significant metabolic abnormalities, however, it is reasonable to speculate that individuals with MHO may have less marked impairment of endothelial function. Thus, the current investigation was designed to compare vasodilator reactivity and ET-1-mediated vasoconstrictor tone between patients with the MHO and those with the MetS.
METHODS
Study participants.
The study population included adult obese patients [body mass index (BMI) ≥30 kg/m2] with a diagnosis of MetS or MHO and age-matched lean healthy controls (BMI <27 kg/m2) who participated in studies conducted in our laboratory to investigate endothelium-dependent and -independent dilation and/or endothelin type A (ETA)-dependent vasoconstrictor reactivity. The MetS was defined according to the International Diabetes Federation (IDF) criteria (2), whereas MHO was defined as the presence of obesity in the absence of any of the metabolic abnormalities characteristic of the MetS (i.e., raised fasting plasma glucose, elevated triglycerides, and reduced HDL cholesterol). None of the study participants was taking any medication, including aspirin or vitamin supplements, at the time of vascular function assessment. In patients with the MetS taking antihypertensive, oral hypoglycemic, and/or lipid-lowering drugs, treatment was discontinued 1 wk prior to the vascular study. During this time, blood pressure and/or plasma glucose levels were repeatedly measured, and when needed, treatments were restarted with the exclusion of the patient from the study. None of the participants was treated with insulin, none was a smoker, and all participants were asked to refrain from drinking alcohol and beverages containing caffeine for ≥24 h before the study. None of the participants was engaged in programs of regular physical activity, and the levels of physical activity were comparable between the three groups. The study protocols were approved by the local institutional review boards, and all participants gave written informed consent before their participation in the studies.
Protocols.
All studies were performed in the morning in a quiet room with a temperature of ≈22°C. Each study consisted of infusion of drugs into the brachial artery and measurement of the response of the forearm vasculature by means of strain-gauge venous occlusion plethysmography. Drugs were prepared by the local pharmaceutical service, following specific procedures to ensure accurate bioavailability and sterility of the solutions. A 20-gauge Teflon catheter (Arrow, Limeric, PA) was inserted into the brachial artery of the nondominant arm (left arm in most cases) for drug infusion. The extended arm was positioned slightly above the level of the right atrium, and a mercury-filled strain gauge was placed around the widest part of the forearm. The strain gauge was connected to a plethysmograph (model EC-6; Hokanson, Bellevue, WA) calibrated to measure the percent change in volume and connected to a personal computer through an analog-to-digital converter. For each measurement, a cuff placed around the upper arm was inflated to 40 mmHg with a rapid cuff inflator (model E-10; Hokanson) to occlude venous outflow from the extremity. A wrist cuff was inflated to suprasystolic pressures 1 min before each measurement to exclude the hand circulation. Flow measurements were recorded for ∼7 s every 15 s; seven readings were obtained for each mean value. Blood pressure was recorded with the use of a standard mercury manometer. Throughout all of the studies, volumes infused were matched by administration of variable amounts of saline.
Assessment of endothelium-dependent and -independent vascular reactivity and of ET-1-mediated vasoconstriction.
Endothelial vasodilator function was tested as described previously in detail (8). Briefly, forearm blood flow (FBF) was measured by strain-gauge plethysmography at baseline and after intra-arterial infusion of increasing doses of the endothelium-dependent vasodilator acetylcholine (ACh; Clinalfa) (infusion rates: 7.5, 15, and 30 μg/min) and of the endothelium-independent vasodilator sodium nitroprusside (SNP; Malesci) (infusion rates: 0.8, 1.6, and 3.2 μg/min). Drug sequence was randomized to avoid bias related to the order of infusion. To assess ET-1-dependent vasoconstrictor tone, BQ-123 (Bachem, Bubendorf, Switzerland), a selective blocker of ETA receptors, was infused at 10 nmol/min (10 nmol/ml solution), a dose that effectively counteracts the vasoconstrictor effect of ET-1 infusion in the human forearm (7).
Statistical analyses.
Group comparisons were performed by unpaired t-test, one- and two-way ANOVA, as appropriate. Within-group comparisons were performed by two-way ANOVA for repeated measurements. Univariate analyses of associations were assessed by use of standard linear regression analysis. Multivariate analyses of associations were assessed using the backward stepwise regression technique. Multivariate regression models for the dependent variables ACh (highest dose) and SNP (highest dose) or response to BQ-123 (last 30 min of infusion) included diagnosis (absence of obesity, MHO, MetS) as well as other relevant covariates of interest (BMI, mean arterial pressure, plasma glucose, total cholesterol, triglycerides, and HDL cholesterol) as main effects. All calculated probability values are two-tailed, and a P value of <0.05 was considered statistically significant. All group data are reported as means ± SE.
RESULTS
A total of 119 obese patients (85 with the MetS and 34 with MHO) and 56 lean controls participated in some or all of the various protocols of this study.
The baseline anthropometric and biochemical characteristics of the participants are reported in Table 1. All obese patients had a waist circumference above the IDF criterion (≥94 cm in men and ≥80 cm in women); blood pressure (BP) was above the IDF criterion (systolic BP ≥135 mmHg or diastolic BP ≥85 mmHg) in 12 of 34 patients (35%) with MHO and 44 of 85 patients (52%) with the MetS. Among patients with the MetS, fasting plasma glucose levels were above the IDF criterion (≥100 mg/dl) in 36 of 85 patients (42%); 30 of 85 patients (35%) had type 2 diabetes based on the American Diabetes Association classification (1). Any combination of plasma triglycerides and HDL cholesterol levels above or below the IDF criteria, respectively, was present in 71 of 85 patients (84%) with the MetS. Within the MetS group, three components of the syndrome were present in 57 patients (67%), four components were present in 23 patients (27%), and all five of the components were present in five patients (6%).
Table 1.
Clinical characteristics of the study population
| Lean Controls (n = 56) | Metabolically Healthy Obesity (n = 34) | Metabolic Syndrome (n = 85) | P Value | |
|---|---|---|---|---|
| Sex (males/females) | 30/26 | 16/18 | 42/43 | |
| Age, yr | 43 ± 1 | 44 ± 2 | 46 ± 2 | 0.09 |
| BMI, kg/m2 | 23 ± 1 | 35 ± 1* | 36 ± 1* | <0.001 |
| Waist, cm | 111 ± 3 | 113 ± 2 | 0.37 | |
| MAP, mmHg | 84 ± 1 | 94 ± 2* | 98 ± 2* | <0.001 |
| Glucose, mg/dl | 89 ± 2 | 93 ± 2 | 128 ± 6¶ | <0.001 |
| Total cholesterol, mg/dl | 171 ± 4 | 167 ± 5 | 192 ± 4¶ | <0.001 |
| HDL cholesterol, mg/dl | 49 ± 2 | 51 ± 2 | 42 ± 1¶ | <0.001 |
| Triglycerides, mg/dl | 90 ± 5 | 88 ± 6 | 172 ± 10¶ | <0.001 |
| Insulin, μU/ml | 18 ± 3 | 18 ± 2 | 0.81 |
Data are expressed as means ± SE.
BMI, body mass index; MAP, mean arterial pressure.
Comparisons were performed by 1-way analysis of variance, followed by Holm-Sidak test for pairwise multiple comparisons and unpaired t-test, as appropriate.
P < 0.01 vs. lean controls;
P > 0.01 vs. both lean controls and healthy obesity.
Both MHO and MetS patients had higher BMI and BP compared with lean controls, without significant differences between them (both P > 0.05). Fasting plasma glucose, total cholesterol, and triglyceride levels were higher in patients with the MetS than in the other two groups, whereas no significant difference in these parameters was observed between patients with MHO and lean controls (all P > 0.05). HDL plasma levels were lower in patients with the MetS than in the other two groups, without significant difference between patients with MHO and lean subjects (P > 0.05). Insulin plasma levels were not different in the two obese groups.
Throughout the studies, mean arterial pressure and heart rate did not change significantly after infusion of any of the drugs used, thus indicating that the drug effects were limited to the infused forearm and did not extend to the systemic circulation.
Endothelium-dependent and -independent vascular reactivity.
Vascular responses to ACh and SNP were assessed in 81 obese patients (58 with the MetS and 23 with MHO) and 25 lean controls. Infusion of graded doses of ACh resulted in a progressive vasodilator response in both obese and lean participants (P < 0.001). However, forearm vascular reactivity to ACh was significantly lower in obese patients than in lean controls (Fig. 1, left). Similarly, FBF responses to SNP were lower in obese patients than in lean controls (Fig. 1, right).
Fig. 1.

Plots showing forearm blood flow responses to intra-arterial infusion of escalating doses of acetylcholine (left) and sodium nitroprusside (right) in lean subjects and obese patients. P values refer to the comparisons of vascular responses by 2-way analysis of variance for repeated measurements. All values are means ± SE.
When the reactivity to ACh was compared between the two obese subgroups, patients with MHO had greater vasodilator reactivity than those with the MetS (Fig. 2, left). The response to ACh of MHO patients (14.3 ± 1.3 ml·min−1·dl−1 at the highest dose), however, remained significantly lower than that observed in lean subjects (22.5 ± 1.8 ml·min−1·dl−1, P < 0001). In contrast with the ACh results, endothelium-independent responsiveness to SNP did not differ between the two obese subgroups (Fig. 2, right).
Fig. 2.
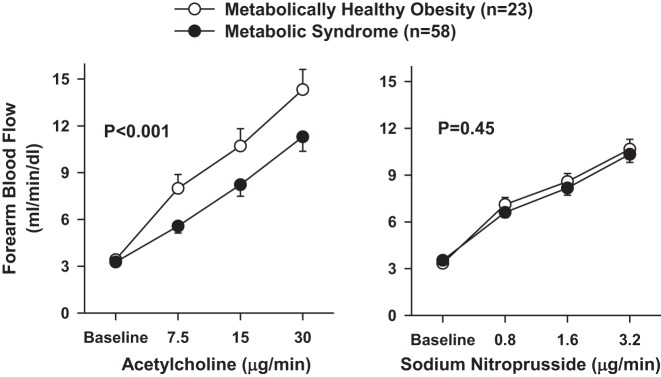
Plots showing forearm blood flow responses to intra-arterial infusion of escalating doses of acetylcholine (left) and sodium nitroprusside (right) in patients with metabolically healthy obesity and the metabolic syndrome. P values refer to the comparisons of vascular responses by 2-way analysis of variance for repeated measurements. All values are means ± SE.
Vascular responsiveness to ETA receptor blockade.
Forearm vascular reactivity to selective ETA antagonism was measured in 70 obese patients (49 with the MetS and 21 with MHO) and 31 lean controls. Infusion of BQ-123 did not result in significant FBF changes in lean subjects (9 ± 4% after 60 min, P = 0.45), whereas it induced a significant vasodilator response in obese patients (33 ± 4% after 60 min, P < 0001). Thus, the percent increase in FBF from baseline following ETA receptor blockade was higher in obese patients than in lean participants (Fig. 3).
Fig. 3.
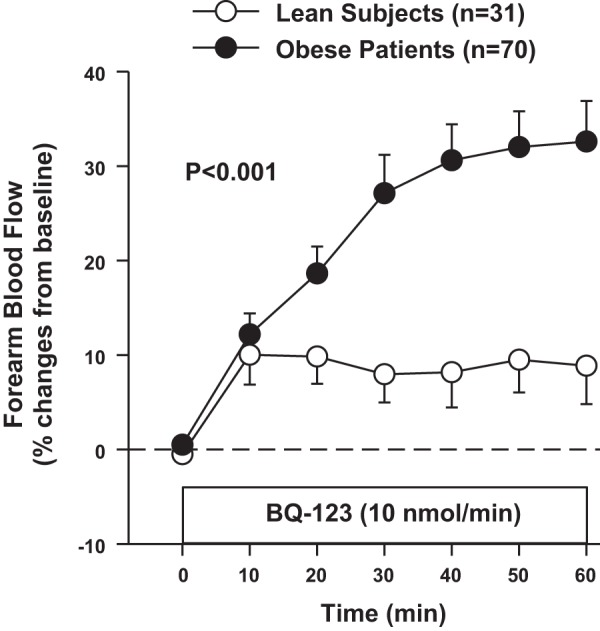
Plot showing forearm blood flow responses to intra-arterial infusion of the selective endothelin type A (ETA) blocker BQ-123 in lean subjects and obese patients. P value refers to the comparisons of vascular responses to BQ-123 by 2-way analysis of variance for repeated measurements. All values are means ± SE.
When the responses to BQ-123 were compared between the two obese groups, the FBF increases from baseline were significantly higher in patients with the MetS than in those with MHO (Fig. 4). In patients with MHO, however, the vasodilator effect of BQ-123 (28 ± 6% at 60 min) was higher than in lean controls (P < 0001), thereby indicating already enhanced ETA-dependent vasoconstriction in patients with MHO.
Fig. 4.
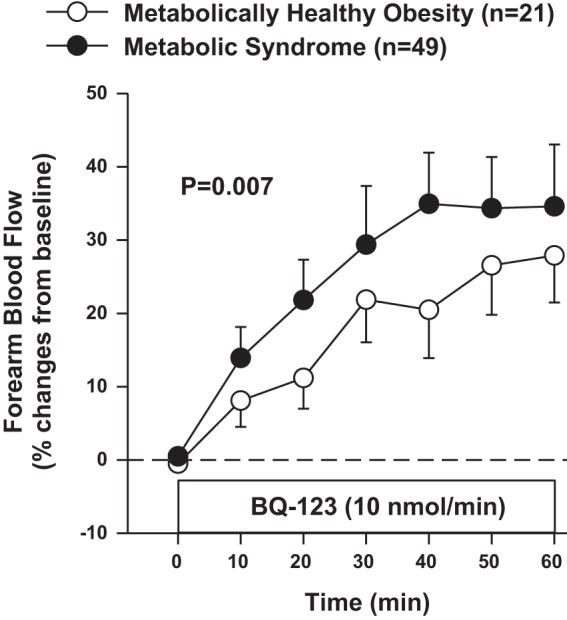
Plot showing forearm blood flow responses to intra-arterial infusion of the selective ETA blocker BQ-123 in patients with metabolically healthy obesity and the metabolic syndrome. P value refers to the comparisons of vascular responses to BQ-123 by 2-way analysis of variance for repeated measurements. All values are means ± SE.
Predictors of vascular responses to ACh, SNP, or BQ-123.
Linear regression analyses showed that, in the whole population, the vasodilator response to ACh had a strong negative association with BMI, plasma glucose, and diagnosis (all P < 0.001) and a weaker negative association with total cholesterol (P = 0.03) and triglycerides (P = 0.02). In the backward stepwise analysis, diagnosis (P < 0.001), plasma glucose (P = 0.01), and BMI (P = 0.04) remained independent predictors of the response to ACh (r = 0.56, r2 = 0.31, P < 0.001). In the obese patients, fasting plasma glucose (P = 0.02) and diagnosis (P = 0.02) were again significant predictors of the response to ACh in the multivariate analysis (r = 0.35, r2 = 0.12, P = 0.01).
Differently from the Ach results, BMI was the only variable showing a linear association with the vasodilator response to SNP in both the whole population (r = 0.48, P = 0.008) and the obese group (r = 0.34, P = 0.03).
In the whole population, the vasodilator response to BQ-123 was linearly associated with diagnosis (P < 0.001), BMI (P < 0.001), mean arterial pressure (P < 0.001), and fasting plasma glucose (P = 0.003). In the backward stepwise analysis, mean arterial pressure (P < 0.001) and fasting plasma glucose (P = 0.02) remained the only significant predictors of the vasodilator response to ETA receptor blockade (r = 0.58, r2 = 0.34, P < 0.001). In the obese patients, mean arterial pressure (P < 0.001) and fasting plasma glucose (P = 0.02) persisted as significant predictors of vascular reactivity to BQ-123 in the multivariate analysis (r = 0.52, r2 = 0.27, P = 0.002).
DISCUSSION
The main novel findings of this investigation are that, compared with lean controls, patients with MHO have impaired endothelium-dependent and -independent vasodilation as well as increased ETA-dependent vasoconstrictor tone. In patients with MHO, however, the vasodilation induced by ACh is higher and the response to ETA antagonism by BQ-123 lower than in patients with the MetS; the vasodilator effect of SNP, conversely, is equally impaired in the two obese subgroups. Taken together, these results suggest that MHO is not an innocuous condition but is associated with vascular function abnormalities, although they are milder that those observed in patients with the MetS.
The results of the current study expand and refine previous observations from our group and other groups of an abnormal endothelium-dependent vascular reactivity in overweight and obese individuals (32) and in patients with the MetS (26, 29) (22). In particular, we now demonstrate that the impairment in endothelial vasodilator function is present even in patients with MHO, suggesting that obesity per se is associated with endothelial dysfunction and that the presence of the MetS exerts additive detrimental effects on the vascular endothelium. The concept that MHO is not a benign condition is in line with a series of recent observations showing higher cardiovascular and metabolic risk in patients with MHO. Thus, Arnlöv et al. (5) have reported that overweight and obese men without the MetS have increased risk for cardiovascular events during more than 30 years of followup. More recently, Chang et al. (12) demonstrated that men and women with MHO have increased coronary artery calcifications compared with metabolically healthy, normal-weight individuals. These findings are at odds with older reports suggesting that overweight and obese individuals without associated metabolic abnormalities do not exhibit higher rates of cardiovascular disease and mortality (4, 25) but strengthen the results of a systematic review and meta-analysis evaluating mortality and cardiovascular events in individuals classified according to BMI categories and metabolic status, which has shown that obese persons are at risk for adverse long-term outcomes even in the absence of metabolic abnormalities (19). Similarly, a meta-analysis of prospective cohort studies has shown that the risk of incident type 2 diabetes in patients with MHO is intermediate between that of normal-weight adults and patients with the MetS (6). Taken together, these results suggest that, although increased adiposity may increase the likelihood of unwanted consequences per se, coexistence of metabolic abnormalities carries an additional risk of endothelial damage. This concept is supported by the results of our backward regression analysis, in which diagnosis and plasma glucose, together with BMI, remained independent predictors of endothelium-dependent vasodilator responsiveness in the whole study population. In this regard, it is important to emphasize that among the different metabolic abnormalities characteristic of the MetS, hyperglycemia, but not lipid changes, was independently associated with endothelial dysfunction. Because we used IDF criteria to define the MetS, it is possible that inclusion in our study of some patients with already overt T2D might have contributed to this outcome. Several mechanisms might explain the higher degree of endothelial dysfunction driven by obesity-associated metabolic abnormalities, in particular the activation of those inflammatory pathways (21), leading to increased production of a number of cytokines, including tumor necrosis factor-α, interleukin-6, and monocyte chemoattractant protein-1 (13).
Another important finding of our investigation is that, compared with lean controls, MHO and MetS participants had similarly reduced responses to endothelium-independent, NO-mediated stimulation with SNP. This finding is in contrast with the data from Weil et al. (32), who reported no differences in the magnitude of vasodilation to SNP between overweight and obese patients and normal-weight controls. The reasons for this discrepancy are probably related to more severe degrees of obesity and insulin resistance present in our obese cohort compared with the overweight and obese populations decribed by Weil et al. (32). Our results, however, are consistent with those reported recently by Walther et al. (31), who observed that patients with the MetS, with or without T2D, have generalized vascular dysfunction, including impaired vascular smooth muscle response to exogenous nitric oxide, in both the brachial artery and the microvasculature. Our previous evidence of generalized impairment of insulin-mediated sensitization to vasodilator stimuli in patients with the MetS (23) supports the notion that obesity-associated vascular dysfunction extends beyond the endothelium to involve smooth muscle cells in the media (9, 15). Vascular insulin resistance, likely related to perivascular adipose tissue changes occurring in obesity (10), is the potential mechanism underlying this abnormality. This view is supported by the results of our regression analysis showing that BMI was the only significant correlate of the vasodilator response to SNP. However, because our study was performed in the intact human circulation in vivo, we could not gain more direct insights in this regard.
In line with prior work from our laboratory and others (30, 32), in the current study the obese participants as a group showed significant vasodilator response during BQ-123 infusion, hence confirming the concept of obesity-related enhanced ETA-dependent vasoconstrictor tone. Coherently with the results observed with the endothelium-dependent response to ACh, vasodilation during ETA receptor blockade was less marked in MHO participants than in MetS patients, thereby suggesting that obesity and its metabolic complications exert independent and additive detrimental effects on the vascular ET-1 system. Therefore, because previous investigations have shown that vasoconstriction during exogenous ET-1 infusion is blunted in overweight and obese adults (32) and in overweight type 2 diabetics (10), the likely mechanism of the increased vascular ET-1 activity in obesity relates to higher bioavailability of the peptide within the vessel wall. Among the numerous stimuli known to promote ET-1 production in endothelial cells, insulin is likely a leading player in hyperinsulinemic states like obesity and MetS (11). However, additional mechanisms involved in blood pressure regulation, such as hyperactivation of the sympathetic system and of the renin-angiotensin axis (27, 28), are probably at play, as suggested by the results of our multivariate regression analysis, showing that high blood pressure is the strongest predictor of the response to BQ-123.
One possible limitation of the present study is the fact that the criteria for defining MHO were selected arbitrarily among several other possibilities to identify the metabolic health status (24); a similar reasoning applies to the definition of the MetS, and all of these arbitrary choices might have influenced the outcomes. Another potential limit of this study relates to the absence of a group of lean, metabolically unhealthy subjects whose presence might have allowed us to better ascertain the role of increased adiposity vs. metabolic abnormalities in the pathophysiology of vascular dysfunction. Even with these limitations, however, this study clearly demonstrates that obesity is associated with vascular dysfunction even in the absence of metabolic abnormalities. Therefore, this concept might have potential clinical implications, helping to define preventive strategies for vascular protection in these patients.
GRANTS
This work was partially supported by Fondi d'Ateneo grants from Catholic University and from an Italian Ministry of Health (Ministero della Salute) grant (RF-2010-2313809) to C. Cardillo.
DISCLOSURES
The authors declare that they have no potential conflicts of interest, financial or otherwise, to disclose.
AUTHOR CONTRIBUTIONS
F.S., N.M., V.R., and C.C. performed experiments; F.S., U.C., and V.R. prepared figures; F.S., M.I., and U.C. drafted manuscript; F.S., M.I., U.C., N.M., V.R., M.T., N.D.D., and C.C. approved final version of manuscript; M.I., U.C., N.M., V.R., M.T., N.D.D., and C.C. edited and revised manuscript; U.C., M.T., and C.C. analyzed data; M.T. and C.C. interpreted results of experiments; N.D.D. and C.C. conception and design of research.
REFERENCES
- 1.American Diabetes Association. Diagnosis and classification of diabetes mellitus. Diabetes Care 37, Suppl 1: S81–S90, 2014. [DOI] [PubMed] [Google Scholar]
- 2.Alberti G, Zimmet P, Shaw J, Grundy SM. IDF Metabolic Syndrome Consensus Definition. Brussels, Belgium: IDF Communications, 2006. [Google Scholar]
- 3.Alberti KG, Zimmet P, Shaw J; IDF Epidemiology Task Force Consensus Group. The metabolic syndrome—a new worldwide definition. Lancet 366: 1059–1062, 2005. [DOI] [PubMed] [Google Scholar]
- 4.Andres R. Effect of obesity on total mortality. Int J Obes 4: 381–386, 1980. [PubMed] [Google Scholar]
- 5.Arnlöv J, Ingelsson E, Sundström J, Lind L. Impact of body mass index and the metabolic syndrome on the risk of cardiovascular disease and death in middle-aged men. Circulation 121: 230–236, 2010. [DOI] [PubMed] [Google Scholar]
- 6.Bell JA, Kivimaki M, Hamer M. Metabolically healthy obesity and risk of incident type 2 diabetes: a meta-analysis of prospective cohort studies. Obes Rev 15: 504–515, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bohm F, Ahlborg G, Pernow J. Endothelin-1 inhibits endothelium-dependent vasodilatation in the human forearm: reversal by ETA receptor blockade in patients with atherosclerosis. Clin Sci 102: 321–327, 2002. [PubMed] [Google Scholar]
- 8.Campia U, Matuskey LA, Panza JA. Peroxisome proliferator-activated receptor-gamma activation with pioglitazone improves endothelium-dependent dilation in nondiabetic patients with major cardiovascular risk factors. Circulation 113: 867–875, 2006. [DOI] [PubMed] [Google Scholar]
- 9.Campia U, Tesauro M, Cardillo C. Human obesity and endothelium-dependent responsiveness. Br J Pharmacol 165: 561–573, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Campia U, Tesauro M, Di Daniele N, Cardillo C. The vascular endothelin system in obesity and type 2 diabetes: pathophysiology and therapeutic implications. Life Sci 118: 149–155, 2014. [DOI] [PubMed] [Google Scholar]
- 11.Cardillo C, Campia U, Bryant MB, Panza JA. Increased activity of endogenous endothelin in patients with type II diabetes mellitus. Circulation 106: 1783–1787, 2002. [DOI] [PubMed] [Google Scholar]
- 12.Chang Y, Kim BK, Yun KE, Cho J, Zhang Y, Rampal S, Zhao D, Jung HS, Choi Y, Ahn J, Lima JA, Shin H, Guallar E, Ryu S. Metabolically-healthy obesity and coronary artery calcification. J Am Coll Cardiol 63: 2679–2686, 2014. [DOI] [PubMed] [Google Scholar]
- 13.Daniele G, Guardado Mendoza R, Winnier D, Fiorentino TV, Pengou Z, Cornell J, Andreozzi F, Jenkinson C, Cersosimo E, Federici M, Tripathy D, Folli F. The inflammatory status score including IL-6, TNF-α, osteopontin, fractalkine, MCP-1 and adiponectin underlies whole-body insulin resistance and hyperglycemia in type 2 diabetes mellitus. Acta Diabetol 51: 123–131, 2014. [DOI] [PubMed] [Google Scholar]
- 14.Davignon J, Ganz P. Role of endothelial dysfunction in atherosclerosis. Circulation 109: III27–III32, 2004. [DOI] [PubMed] [Google Scholar]
- 15.Grassi G, Seravalle G, Scopelliti F, Dell'Oro R, Fattori L, Quarti-Trevano F, Brambilla G, Schiffrin EL, Mancia G. Structural and functional alterations of subcutaneous small resistance arteries in severe human obesity. Obesity (Silver Spring) 18: 92–98, 2010. [DOI] [PubMed] [Google Scholar]
- 16.Grundy SM, Cleeman JI, Daniels SR, Donato KA, Eckel RH, Franklin BA, Gordon DJ, Krauss RM, Savage PJ, Smith SC Jr, Spertus JA, Fernando C. Diagnosis and management of the metabolic syndrome: an American Heart Association/National Heart, Lung, and Blood Institute scientific statement: Executive Summary. Crit Pathw Cardiol 4: 198–203, 2005. [DOI] [PubMed] [Google Scholar]
- 17.Halcox JP, Schenke WH, Zalos G, Mincemoyer R, Prasad A, Waclawiw MA, Nour KR, Quyyumi AA. Prognostic value of coronary vascular endothelial dysfunction. Circulation 106: 653–658, 2002. [DOI] [PubMed] [Google Scholar]
- 18.Hamer M, Stamatakis E. Metabolically healthy obesity and risk of all-cause and cardiovascular disease mortality. J Clin Endocrinol Metab 97: 2482–2488, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kramer CK, Zinman B, Retnakaran R. Are metabolically healthy overweight and obesity benign conditions?: A systematic review and meta-analysis. Ann Intern Med 159: 758–769, 2013. [DOI] [PubMed] [Google Scholar]
- 20.Lemieux I, Pascot A, Couillard C, Lamarche B, Tchernof A, Alméras N, Bergeron J, Gaudet D, Tremblay G, Prud'homme D, Nadeau A, Després JP. Hypertriglyceridemic waist: A marker of the atherogenic metabolic triad (hyperinsulinemia; hyperapolipoprotein B; small, dense LDL) in men? Circulation 102: 179–184, 2000. [DOI] [PubMed] [Google Scholar]
- 21.Monroy A, Kamath S, Chavez AO, Centonze VE, Veerasamy M, Barrentine A, Wewer JJ, Coletta DK, Jenkinson C, Jhingan RM, Smokler D, Reyna S, Musi N, Khokka R, Federici M, Tripathy D, DeFronzo RA, Folli F. Impaired regulation of the TNF-alpha converting enzyme/tissue inhibitor of metalloproteinase 3 proteolytic system in skeletal muscle of obese type 2 diabetic patients: a new mechanism of insulin resistance in humans. Diabetologia 52: 2169–2181, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Perticone F, Ceravolo R, Candigliota M, Ventura G, Iacopino S, Sinopoli F, Mattioli PL. Obesity and body fat distribution induce endothelial dysfunction by oxidative stress: protective effect of vitamin C. Diabetes 50: 159–165, 2001. [DOI] [PubMed] [Google Scholar]
- 23.Schinzari F, Tesauro M, Rovella V, Galli A, Mores N, Porzio O, Lauro D, Cardillo C. Generalized impairment of vasodilator reactivity during hyperinsulinemia in patients with obesity-related metabolic syndrome. Am J Physiol Endocrinol Metab 299: E947–E952, 2010. [DOI] [PubMed] [Google Scholar]
- 24.Seo MH, Rhee EJ. Metabolic and cardiovascular implications of a metabolically healthy obesity phenotype. Endocrinol Metab (Seoul) 29: 427–434, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sims EA. Are there persons who are obese, but metabolically healthy? Metabolism 50: 1499–1504, 2001. [DOI] [PubMed] [Google Scholar]
- 26.Steinberg HO, Chaker H, Leaming R, Johnson A, Brechtel G, Baron AD. Obesity/insulin resistance is associated with endothelial dysfunction. Implications for the syndrome of insulin resistance. J Clin Invest 97: 2601–2610, 1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Stiefel P, Vallejo-Vaz AJ, García Morillo S, Villar J. Role of the Renin-Angiotensin system and aldosterone on cardiometabolic syndrome. Int J Hypertens 2011: 685238, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tentolouris N, Liatis S, Katsilambros N. Sympathetic system activity in obesity and metabolic syndrome. Ann NY Acad Sci 1083: 129–152, 2006. [DOI] [PubMed] [Google Scholar]
- 29.Tesauro M, Schinzari F, Iantorno M, Rizza S, Melina D, Lauro D, Cardillo C. Ghrelin improves endothelial function in patients with metabolic syndrome. Circulation 112: 2986–2992, 2005. [DOI] [PubMed] [Google Scholar]
- 30.Tesauro M, Schinzari F, Rovella V, Di Daniele N, Lauro D, Mores N, Veneziani A, Cardillo C. Ghrelin restores the endothelin 1/nitric oxide balance in patients with obesity-related metabolic syndrome. Hypertension 54: 995–1000, 2009. [DOI] [PubMed] [Google Scholar]
- 31.Walther G, Obert P, Dutheil F, Chapier R, Lesourd B, Naughton G, Courteix D, Vinet A. Metabolic syndrome individuals with and without type 2 diabetes mellitus present generalized vascular dysfunction: cross-sectional study. Arterioscler Thromb Vasc Biol 35: 1022–1029, 2015. [DOI] [PubMed] [Google Scholar]
- 32.Weil BR, Westby CM, Van Guilder GP, Greiner JJ, Stauffer BL, DeSouza CA. Enhanced endothelin-1 system activity with overweight and obesity. Am J Physiol Heart Circ Physiol 301: H689–H695, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wildman RP, Muntner P, Reynolds K, McGinn AP, Rajpathak S, Wylie-Rosett J, Sowers MR. The obese without cardiometabolic risk factor clustering and the normal weight with cardiometabolic risk factor clustering: prevalence and correlates of 2 phenotypes among the US population (NHANES 1999–2004). Arch Intern Med 168: 1617–1624, 2008. [DOI] [PubMed] [Google Scholar]


