Abstract
Impairment of vasodilator action of insulin is associated with endothelial dysfunction and insulin resistance. Activation of Toll-like receptor 4 (TLR4) induces proinflammatory response and endoplasmic reticulum (ER) stress. Saturated fatty acids (SFA) activate TLR4, which induces ER stress and endothelial dysfunction. Therefore, we determined whether TLR4-mediated ER stress is an obligatory step mediating SFA-induced endothelial dysfunction. Palmitate stimulated proinflammatory responses and ER stress, and this was suppressed by knockdown of TLR4 in primary human aortic endothelial cells (HAEC). Next, we examined the role of TLR4 in vasodilatory responses in intact vessels isolated from wild-type (WT, C57BL/6) and TLR4-KO mice after feeding high-fat (HFD) or normal chow diet (NCD) for 12 wk. Arterioles isolated from HFD WT mice exhibited impaired insulin-stimulated vasodilation compared with arterioles isolated from NCD WT mice. Deficiency of TLR4 was protective from HFD-induced impairment of insulin-stimulated vasodilation. There were no differences in acetylcholine (Ach)- or sodium nitroprusside (SNP)-stimulated vasodilation between the two groups. Furthermore, we examined whether ER stress is involved in SFA-induced impairment of vasodilator actions of insulin. Infusion of palmitate showed the impairment of vasodilatory response to insulin, which was ameliorated by coinfusion with tauroursodeoxycholic acid (TUDCA), an ER stress suppressor. Taken together, the results suggest that TLR4-induced ER stress may be an obligatory step mediating the SFA-mediated endothelial dysfunction.
Keywords: endothelial dysfunction, TLR4, ER stress, insulin, nitric oxide
one important role of vascular endothelium is to regulate vascular tone by balancing the production of vasodilators and vasoconstrictors such as nitric oxide (NO), endothelial-derived hyperpolarizing factors, and endothelin-1 (ET-1) (6, 18, 27). The relationship between endothelial dysfunction and insulin resistance (27) underlies clustering of cardiovascular and metabolic diseases including hypertension, atherosclerosis, diabetes, and obesity (42). Endothelial dysfunction caused by reduced bioavailability of NO plays an important role in the pathogenesis of cardiometabolic syndrome (47, 55, 57). One of the vascular actions of insulin increases blood flow that facilitates delivery of nutrients and hormones (25, 56, 65, 66). Vasodilator actions of insulin contribute to glucose uptake in skeletal muscle (2, 30, 37). Endothelial production of NO in response to insulin stimulation is mediated by signaling pathways involving the insulin receptor (IR)/IRS-1/PI 3-kinase/PDK-1/Akt. Akt directly phosphorylates endothelial NO synthase (eNOS) at Ser1177 (40, 41, 65). Phosphorylation of eNOS at Ser1177 stimulates enzymatic activity, resulting in increased production of NO (9, 16). Insulin-stimulated vasodilation is diminished in insulin-resistant animals and humans, which promotes various cardiovascular diseases including hypertension, atherosclerosis, and coronary heart disease (42, 57, 62).
Palmitate (PA) is the most abundant saturated fatty acid in human bloodstream (7, 58). Elevated circulating fatty acids in human subjects with obesity and diabetes contribute to impaired vascular function, which is associated with metabolic and cardiovascular disorders (1, 7, 58). Obesity-induced inflammatory responses are mediated, in part, by Toll-like receptors (TLRs) that are activated by saturated fatty acids (SFA) (12, 14, 24, 29, 32, 53). SFA-stimulated TLR4 (33) increases expression of proinflammatory cytokines, including TNFα, IL-1β, and IL-6, as well as adhesion molecules, including E-selectin and intercellular adhesion molecule (ICAM) (24, 44, 60). Furthermore, TLR4-deficient mice are protected from various cardiovascular diseases including septic cardiomyopathy, ischemia/reperfusion injury, heart failure, toxic cardiomyopathy, and cardiac hypertrophy (15). Increased expression of TLR4 is observed in atherosclerotic plaques (11, 38, 39). Moreover, TLR4-deficient mice are protected against obesity-induced insulin resistance compared with wild-type mice (44, 52, 53). Thus, TLR4 may be a key link between obesity and insulin resistance diseases including diabetes and its cardiovascular complications.
Endoplasimc reticulum (ER) stress has been implicated as a cause of inflammation and insulin resistance, diabetes, and cardiovascular diseases (17, 28, 46). Despite numerous studies regarding TLR4-mediated proinflammatory responses and insulin resistance, it is unknown whether TLR4-induced ER stress is a mechanism by which SFA impair the vasodilator actions of insulin. In the present study, we investigated whether TLR4-induced ER stress is an obligatory step mediating the SFA-induced proinflammatory responses and the impairment of vasodilator actions of insulin.
MATERIALS AND METHODS
Materials
Anti-eIFα, p-eIFα, anti-p-IκBα, anti-p-Akt, and anti-Akt antibodies were obtained from Cell Signaling (Beverly, MA). Anti-IRS-1 antibody was from Millipore (Billerica, MA), anti-p-JNK was obtained from Invitrogen (Carlsbad, CA), and anti-phosphotyrosine, anti-p-p65 NF-κB, and anti-JNK antibodies were obtained from Santa Cruz Biotechnology (Santa Cruz, CA). dsiRNA for human TLR4 and nontargeted scrambled siRNA and primers for the RT-PCR were purchased from Integrated DNA Technologies (Coralville, IA).
Animals
All animal procedures were performed in accordance with the Animal Use and Care Committee guidelines at The University of Alabama at Birmingham. Wild-type mice (C57BL/6J) were purchased from Jackson Laboratory (Bar Harbor, ME). TLR4-KO mice weare a kind gift from Drs. Shizuo Akira and Osamu Takeuchi (19). All animals were maintained in a temperature-controlled facility with a 12:12-h light-dark cycle. At 6 wk of age, mice were fed either chow (7917 Harlan Diet, 11% calories from fat) or high-fat diet (Test Diet 5SPQ, 54% calories from fat, Richmond, IN) for 10 wk. Mice were fasted overnight and anesthetized with pentabarbitol sodium (50 mg/kg) before euthanization.
Cell Culture and Transfection
Endothelial cell culture.
Bovine aortic endothelial cells (BAECs) were maintained in F-12K medium containing 5% fetal bovine serum (FBS), endothelial cell growth supplement (BD Biosciences 30 mg/l), heparin sulfate (50 μg/ml), penicillin (100 U/ml), and streptomycin (100 μg/ml). HAECs (Lonza, Walkersville, MD) in primary culture were grown in F-12K medium containing EGM-2 single-quote supplements (Lonza). All experiments were conducted on HAECs and BAECs before their sixth passage. HAECs were transiently transfected with 100 nM siRNA duplex oligonucleotides (nontargeted scrambled or TLR4 DsiRNA) using Lipofectamine 2000 (Invitrogen, Carlsbad, CA) according to the manufacturer's instructions. siRNA sequences are described in Table 1. Two days after transfection, cells were serum starved for 2 h and then treated with BSA or palmitate as indicated in the figure legends. BAECs were transfected with Lipofectamine with Plus as per the manufacturer's instructions. Cells were transiently transfected with TLR4-DN or pcDNA3.1 (vector only) as described previously (33, 34). Cells were incubated with transfection complex for 4 h in OPTI-MEM medium and then replaced with regular medium containing growth factors and serum for 48 h.
Table 1.
Primers for RT-PCR
| Genes | |
| hGRP78 | F, CATCACGCCGTCCTATGTCG |
| R, CGTCAAAGACCTGTTCTG | |
| hXBP-1 s | F, GGTCTGCTGAGTCCGCAGCAGC |
| R, GGGCTTGGAATATGTGG | |
| hCHOP | F, GGAGAACCAGAAACGGAAAC |
| R, TCTCCTTCATGCCTGCTTT | |
| hIL-1β | F, GGGCTCAAGGAAAAGAATC |
| R, TTCTGCTTGAGAGGTGCTGA | |
| hMCP-1 | F, CCCCAGTCACCTGCTGTTAT |
| R, TGGAATCCTGAACCCACTTC | |
| hTNFα | F, CAGAGGGCCTGTACCTCATC |
| R, GGAAGACCCCTCCCAGATAG | |
| hE-Selectin | F, GGTTTGGTGAGGTGGCT |
| R, TGATCTTTCCCG GAACTG | |
| hIL-6 | F, AGTGCGTCTTTGCTGCTTTCAC |
| R, TGACAAACAAATTCGGTACATCCT | |
| hTLR2 | F, CCAGCAGGAACATCTGCTAT |
| R, CTTGCA CCACTCACTCTTCA | |
| hTLR4 | F, GTCCTCAGTGTGCTTGTAGT |
| R, GGTGATGTTGGCAGCAATG | |
| β-Actin | F, CTGGCACCCAGCACAATGAAG |
| R, TAGAAGCATTGCGGTGGACG | |
| DsiRNA | |
| Scrambled | rArUrArCrGrCrGrUrArUrUrArUrArCrGrCrGrArUrUrArArCrGrArC |
| rCrGrUrUrArArUrCrGrCrGrUrArUrArArUrArCrGrCrGrUAT | |
| hTLR4 | rUrCrUrArArArUrArCrUrUrUrArGrGrCrUrGrGrUrUrGrUrCrCrCrA |
| rGrGrArCrArArCrCrArGrCrCrUrArArArGrUrArUrUrUAGA | |
| bTLR4 | rUrGrGrCrArArArUrUrCrUrGrUrArGrUrUrCrUrUrGrCrUrCrCrUrU |
| rGrGrArGrCrArArGrArArCrUrArCrArGrArArUrUrUrGrCCA | |
F, forward; R, reverse.
Stable transfection.
TLR4-WT and MD2 were described previously (33, 34). HEK-293 cells were transfected with TLR4-WT and MD2 and treated with geneticine 418 (50 μg/ml) for 3 wk. Growing colonies were selected.
Preparation of Palmitate
Preparation of palmitate was carried out as described by Mott et al. (63). Briefly, 10.5% BSA (Fitzgerald, MA) was dissolved in 25 mM HEPES-DMEM, and filtered (0.22 μM, Millipore). Sodium palmitate was heated to be dissolved in water and rapidly added to warmed BSA solution. Then, this BSA-conjugated palmitate was added to reach the proper concentration of pamitate. We used endotoxin-free reagents and tested all the reagents we used, including BSA, palmitate, media, and reagent diluents. We checked endotoxin levels of all the reagents we used in this study with a Chromogenic Endotoxin Quantitation assay kit (Pierce). Lower than 25 pg/ml is undetectable.
Measurement of NO Production
Production of NO was assessed using the NO-specific fluorescent dye 4,5-diaminofluorescein diacetate (DAF-2 DA; EMD Biosciences, Gibbstown, NJ), as described previously (1). Briefly, endothelial cells were grown to 95% confluence in 24-well black plates (Denville, Metuchen, NJ) and serum starved as indicated in the figure legends. Then, the cells were treated with BSA or palmitate. Cells were then loaded with DAF-2 DA (final concentration, 1 μM) for 10 min at 37°C, rinsed 3 times with F-12K, and kept in the dark. Cells were then treated without or with insulin, as indicated in the figure legends. After stimulation, cells were fixed in 2% paraformaldehyde for 5 min at 4°C. Fixed cells were visualized with a Zeiss inverted epifluorescence microscope (Axio Observer A1) using appropriate filters for a peak excitation wavelength of 480 nm and a peak emission wavelength of 510 nm. Images were captured and analyzed by using AV Rel 4.7 software with multichannel modules.
Functional Assessment for Isolated Mesentery Arterioles
Mice were anesthetized with an intraperitoneal injection of pentobarbital sodium (50 mg/kg). Mesenteric arterioles were excised from the animals and placed in a cooled (4°C) chamber containing dissection buffer [145 mM NaCl, 4.7 mM KCl, 2 mM CaCl, 1.2 mM MgSO4, 1 mM NaH2PO4, 5 mM glucose, 3 mol/l,3-(N-morpholino) propanesulfonic acid (MOPS) buffer, 2 mM pyruvate, 0.02 mM EDTA, and 1% BSA, pH 7.4]. The isolated arterioles were then cannulated with glass micropipettes with 10-0 monofilament suture and mounted in a custom-designed tissue chamber (Living System Instrumentation, Burlington, VT). The arterioles were pressurized to 45 mmHg intraluminally with the same buffer without flow and superfused with buffer without albumin. The vessel preparations were positioned on the stage of an inverted microscope. The vessel segments were gradually warmed to 37°C during a 30-min equilibration period. After baseline diameter was established, arterioles were exposed to phenylephrine (1 μmol/l) until a maximal contraction was achieved (5 min). The vessels were subsequently stimulated with various stimulators (10−11 to ∼10−5 mol/l, 3 min per concentration), including insulin, Ach, or SNP. The dilator responses to insulin were observed and recorded. Measurement of vessel diameter (in μm) was performed with an electronic video caliper (Living Systems Instumentation, St. Albans, VT) and recorded by using the software Lab Chart (AD instruments, Colorado, Springs, CO). In some experiments, palmitate (200 μM) was intraluminally infused into vessels with and without TUDCA (500 μM) and incubated for 4 h. Then, vessels were incubated with the indicated vasodilators in different concentrations as described above.
The data are expressed as means ± SE. The vasodilator responses to insulin were calculated as percent relaxation from the phenylephrine constriction according to the equation: %relaxation = (IDtreat − IDPE) × 100/(IDw/oCa2+ − IDPE),
where IDtreat is the inner diameter obtained when the vessel was treated with insulin, IDPE is the diameter obtained when the vessel was constricted with penylephrine, and IDw/oCa2+ is the maximal passive diameter observed when the vessel was fully dilated in buffer containing 2 mM EGTA and 100 μM adenosine without Ca2+.
Preparation of Cell Lysates and Immunoblotting
Before lysis, cells were briefly washed with ice-cold PBS. Cells were then scraped in lysis buffer containing 50 mM Tris (pH 7.2), 125 mM NaCl, 1% Triton X-100, 0.5% NP-40, 1 mM EDTA, 1 mM Na3VO4, 20 mM NaF, 1 mM Na pyrophosphate, and complete protease inhibitor cocktail (Roche Applied Science). Cell debris were pelleted by centrifugation of samples at 17,000 g for 10 min at 4°C. Supernatants were then boiled with Laemmle sample buffer for 5 min, and proteins were resolved by 10% SDS-PAGE, transferred to nitrocellulose membranes, and immunoblotted with specific antibodies using standard methods as described in the figures. Immunoblots were visualized with quantified by Image analyzer (Vision Works LS) and UVP.
RT-PCR
The cells were treated as described in the figure legends. Total RNA was prepared using TRIzol (Invitrogen) according to the manufacturer's instructions. One microgram of total RNA was used for cDNA synthesis using an Omniscript RT Kit (Qiagen, Valencia, CA). Then, the cDNA was subjected to semiquantitative PCR analysis using Hot Star Taq Master Mix kit (Qiagen). PCR product was visualized with fluorescent dye (Envirosafe DNA/RNA Stain; Helixx Technologies, Ontario, Canada), and the image was analyzed and quantified by Image Analyzer (Vision Works LS) and UVP. The primers for each gene are described in Table 1.
Statistical Analysis
Values are presented as means ± SE. Western blots were analyzed by one-way ANOVA. For the comparisons of vasodilatory responses, repeated-measures two-way ANOVA was used.
RESULTS
Palmitate Stimulates Proinflammatory Responses by a TLR4-Dependent Mechanism
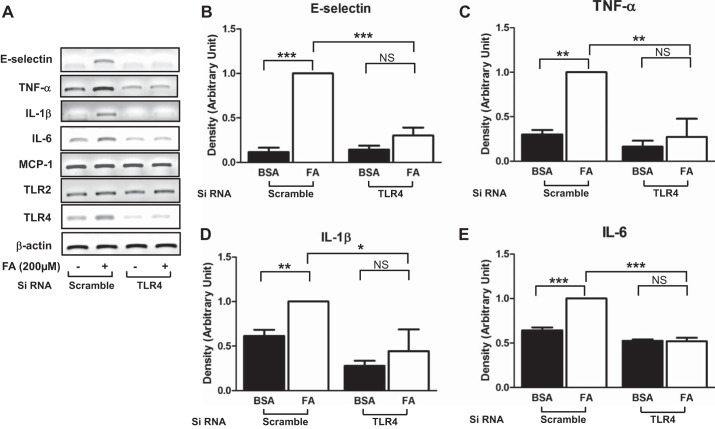
To determine whether palmitate stimulates inflammatory responses through TLR4 in vascular endothelial cells, cells were transiently transfected with siRNA for TLR4 or scrambled, and treated HAEC with BSA or palmitate. Next, we examined the expressions of proinflammatory genes. Transfection of siRNA for TLR4 reduced the expression of TLR4, but the expression of TLR2 was not altered. Treatment with palmitate increased the expression of proinflammatory genes including E-selectin, TNFα, IL-1β, and IL-6, but not MCP-1, and knockdown of TLR4 abolished the palmitate-induced inflammatory responses (Fig. 1).
Fig. 1.
Palmitate stimulates proinflammatory responses through a Toll-like receptor 4 (TLR4)-dependent mechanism. Human aortic endothelial cells (HAECs) were transfected with siRNA for TLR4 or scrambled and then incubated for 48 h. Cells were treated with palmitate (PA, 200 μM, 6 h), and total RNA was isolated. RNA was subjected to RT-PCR analysis with indicated specific primers. Treatment with palmitate stimulated proinflammatory responses, and knockdown of TLR4 blunted palmitate-stimulated upregulation of proinflammatory gene expressions. Quantification of 3 independent experiments is shown in bar graphs (means ± SE). ***P < 0.001, **P < 0.01, *P < 0.05: samples are statistically different.
Palmitate Stimulates Proinflammatory Signaling Molecules Through a TLR4-Dependent Mechanism
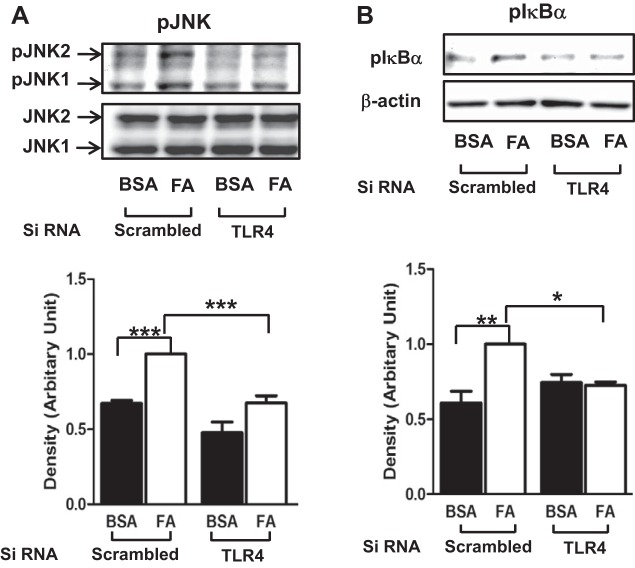
Next, we examined whether palmitate stimulates proinflammatory signaling molecules. Phosphorylations of JNK and IκBα were evaluated by immunoblottings. Treatment with palmitate stimulated phosphorylation of JNK and IκBα, whereas knockdown of TLR4 blunted the effects of palmitate to stimulate phosphorylation of JNK and p-IκBα. (Fig. 2).
Fig. 2.
Palmitate stimulates proinflammatory signaling molecules through a TLR4-dependent mechanism. HAECs were transfected with siRNA for TLR4 or scrambled and then incubated for 48 h. Cells were treated with palmitate (PA, 200 μM, 30 min), and cell lysate was isolated. Cell lysate was subjected to Western blotting with specific antibodies. Treatment with palmitate stimulated phosphoprylation of JNK and IkBα, which was blunted by knockdown of TLR4. Quantification of 3 independent experiments is shown in bar graphs (means ± SE). ***P < 0.001, **P < 0.01, *P < 0.05: samples are statistically different.
Palmitate Stimulates ER Stress Through a TLR4-Dependent Mechanism
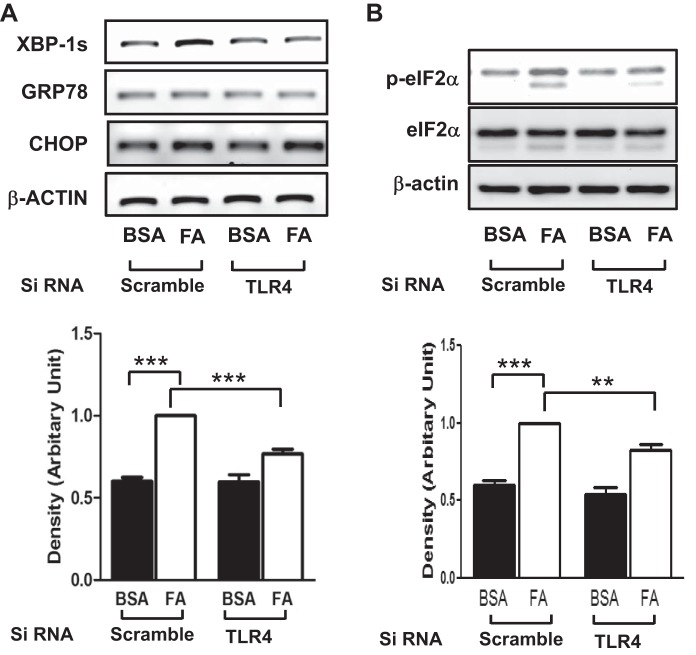
Metabolic stress causes ER stress that leads to inflammatory responses (20). We examined whether TLR4 contributes to palmitate-induced ER stress. Treatment with palmitate stimulated splicing of XBP-1 in cells transfected with scrambled siRNA, whereas splicing of XBP-1 was not increased in TLR4 knockdown endothelial cells (Fig. 3A). By contrast, the expressions of other ER stress markers, including GRP78 and CHOP, were not altered by treatment with palmitate (Fig. 3A). As a cellular protective mechanism, translation of proteins is inhibited when cells undergo ER stress. Phosphorylation of eIF2α is one of the markers for ER stress. Treatment with palmitate increased phosphorylation of eIF2α, and knockdown of TLR4 blunted the palmitate-induced phosphorylation of eIF2α (Fig. 3B).
Fig. 3.
Palmitate stimulates endoplasmic reticulum (ER) stress through a TLR4-dependent mechanism. HAECs were transfected with siRNA for TLR4 or scrambled and then incubated for 48 h. Cells were treated with palmitate (PA, 200 μM, 6 h), and total RNA (A) or cell lysate (B) was isolated. RNA was subjected to RT-PCR, and cell lysate was subjected to Western blotting. Treatment with palmitate stimulated splicing of XBP-1 and phosphorylation of eIF2α; knockdown of TLR4 blunted palmitate-stimulated XBP-1 splicing and phosphorylation of eIF2α. ***P < 0.001, **P < 0.01: samples are statistically different.
Activation of TLR4 Impairs Insulin Signaling
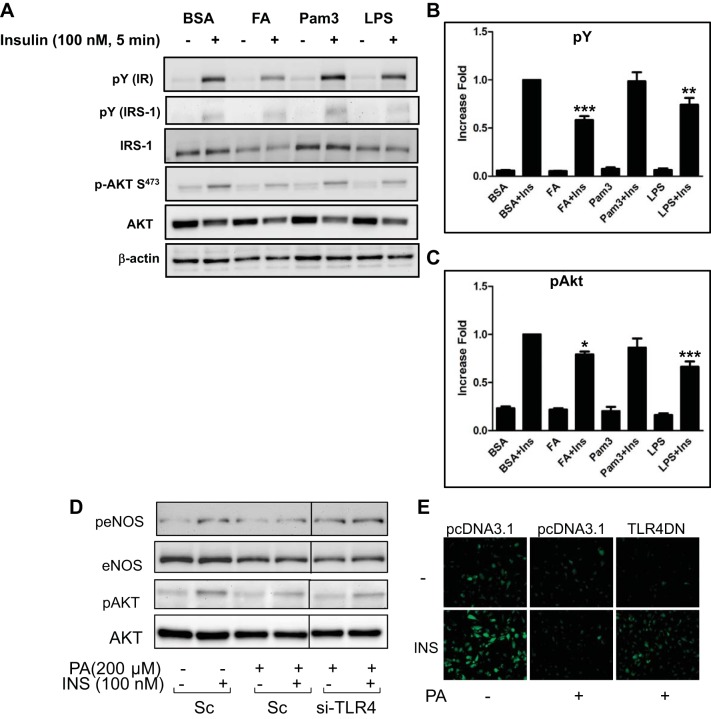
There have been studies suggesting that stimulation of TLR4 is a cause of insulin resistance (49, 50, 53, 60). To confirm that SFA-induced insulin resistance is mediated through TLR4 signaling pathways, we stably overexpressed TLR4 with MD2 in HEK-293 cells, which do not express TLR4. TLR4-overexpressing HEK-293 cells were treated with palmitate, Pam3CSk4, or LPS, and then the cells were stimulated without or with insulin. Treatment with palmitate or LPS, but not Pam3Csk4, inhibited insulin-stimulated tyrosine phosphorylation of insulin receptor (IR) and insulin receptor substrate-1 (IRS-1) (Fig. 4A). Moreover, insulin-stimulated phosphorylation of Akt was reduced by treatment with palmitate or LPS but not by Pam3Csk4. This suggests that stimulation of TLR4 impairs insulin-signaling pathways. To confirm the inhibitory action of TLR4 on insulin signaling in primary endothelial cells, HAECs were transfected with siRNA for scrambled or TLR4. Then insulin-stimulated phosphorylation of eNOS and Akt was examined. Treatment with palmitate inhibited insulin-stimulated phosphorylation of eNOS and Akt, which was restored by knockdown of TLR4 (Fig. 4D). To examine the role of TLR4 in impairment of insulin-stimulated NO production, bovine aortic endothelial cells (BAEC) were transfected with vector only or dominant negative DNA construct of TLR4 (TLR4-DN) and then examined for insulin-stimulated NO production using the fluorescent NO-specific dye DAF2-DA, as we previously reported (22, 26). Insulin-stimulated NO production was inhibited by treatment with palmitate. Overexpression of TLR4-DN partially restored the insulin-stimulated NO production in the presence of palmitate (Fig. 4E).
Fig. 4.
Activation of TLR4 impairs insulin signaling. A–C: HEK-293 cells were stably transfected with TLR4 and MD2 as described in material and methods. Cells were serum starved with medium containing 0.1% horse serum for 2 h and then treated with palmitate (PA, 100 μM), Pam3Csk4 (Pam3, 1 μg/ml), or LPS (1 μg/ml) for 24 h, followed by treatment with insulin (100 nM, 5 min). Cell lysates were collected and subjected to Western blot analysis. Blots were incubated with indicated antibodies. Representative blots are from 3 independent experiments. Tyrosine phosphorylation of insulin receptor (IR) and IRS-1 was detected by blotting a whole lysate with anti-phosphotyrosine antibody. A: phosphorylation of Akt was detected using anti-pAkt. B: tyrosine phosphorylation was normalized to β-actin. C: p-Akt is normalized to total Akt. Quantification of 3 independent experiments is shown in bar graphs (means ± SE). ***P < 0.001, **P < 0.01, *P < 0.05: samples are statistically different vs. insulin-treated samples. D: bovine aortic endothelial cells (BAECs) were trasfected with scrambled or siRNA for TLR4. Two days later, cells were treated with BSA or palmitate (200 μM, 4 h). Cell lysates were collected and subjected to immunoblotting. Representative blots are from 3 independent experiments. Demarcated lines are due to the noncontiguous lanes but are from a single gel. E: BAECs were transiently transfected with vector (pcDNA 3.1) or dominant negative construct of TLR4 as described in material and methods. After cells were incubated for 48 h, they were serum starved for 2 h and then were loaded with the NO-specific fluorescent dye DAF2-DA. Cells were pretreated with BSA or palmitate (200 μML, 4 h) and then treated without or with insulin (100 nM) for 20 min. After insulin treatment, cells were fixed with 2% paraformaldehyde and visualized with an epifluorescent microscope as described in material and methods. Emission of green fluorescence is indicative of NO production. Experiments shown are representative of those independently repeated ≥3 times.
TLR4 Knockout Mice Are Protected from HFD-Induced Endothelial Dysfunction
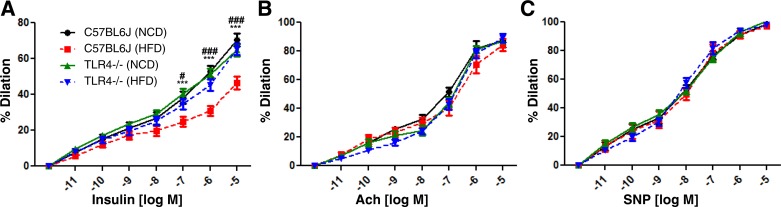
Vascular action of insulin is associated with glucose disposal in skeletal muscle, which suggests the coordinating relationship between insulin action and endothelial function (3, 8, 27, 30, 61). Next, we examined the vasodilatory responsiveness of vessels isolated from WT (C57BL/6J) or TLR4-KO mice. We fed mice normal chow diet (NCD) or high-fat diet (HFD) for 10 wk. Mesentery arterioles were isolated from the mice and examined for the vasodilatory responses to various stimuli, including insulin, Ach, or SNP. Dilation of the vessels isolated from WT-HFD mice was impaired in response to insulin compared with those isolated from WT-NCD mice. In contrast, the vessels isolated from TLR4-KO-HFD mice similarly dilated in response to insulin as those isolated from TLR4-KO-NCD or WT-NCD mice (Fig. 5A). Interestingly, there was no difference in vasodilatory responses to Ach (Fig. 5B) or SNP (Fig. 5C) between groups. These results suggest that TLR4 mediates the HFD-induced impairment of vasodilator actions of insulin specifically.
Fig. 5.
TLR4 knockout (KO) mice are protected from high-fat diet (HFD)-induced vasodilator action of insulin. C57BL/6 mice or TLR4-KO mice were fed normal chow diet (NCD; 10% calorie from fat) or HFD (60% calorie from fat) for 10 wk. Mesentery arterioles were isolated and subjected to videomicroscopic analysis for vasodilation in response to various stimulators, including insulin (A), acetylcholine (Ach; B), and sodium nitroprusside (SNP; C). Vessels isolated from WT HFD mice (C57BL/6J) were impaired to dilate in response to insulin vs. those isolated from WT NCD mice. By contrast, arterioles isolated from both NCD and HFD TLR4-KO mice similarly dilated in response to insulin vs. those isolated from WT NCD mice. However, there were no differences in responses to Ach or SNP. Data suggest that HFD specifically impairs insulin-stimulated vasodilation through a TLR4-dependent mechanism. ###P < 0.001, #P < 0.05 (WT+NCD vs. WT+HFD), ***P < 0.001 (WT+HFD vs. TLR4KO+HFD).
Impairment of Vasodilator Action of Insulin by Palmitate Is Reversed by Treatment with TUDCA
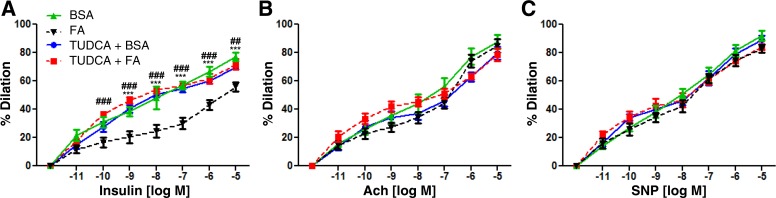
TUDCA is a well-known chemical chaperone that ameliorates ER stress (45). Because SFA-induced ER stress is mediated by TLR4, we examined whether suppressed ER stress by TUDCA improves vasodilator action of insulin or not. Infusion of palmitate impaired the vasodilator action of insulin, and pretreatment with TUDCA prevented the impairment of vasodilation (Fig. 6A). By contrast, neither palmitate nor TUDCA affected Ach- or SNP-stimulated vasodilation. Thus, palmitate-induced ER stress may affect insulin signaling pathways in vascular endothelial cells.
Fig. 6.
Impairment of vasodilatory action of insulin by palmitate is reversed by treatment with tauroursodeoxycholic acid (TUDCA; an ER stress inhibitor). Mesentery arterioles were isolated from 6-wk-old mice. Vascular activities were measured by treatment with various stimulators, including insulin (A), Ach (B), and SNP (C). Intraluminal infusion of palmitate (200 μM) impaired vasodilator action of insulin vs. BSA (control) infusion. Pretreatment with TUDCA (500 μM, 30 min before palmitate infusion) prevented the effect of palmitate. By contrast, there were no differences between groups when vessels were treated with Ach or SNP. Rresults suggest that palmitate-induced ER stress may be the mechanism to cause impairment of vasodilation in response to insulin, and attenuation of ER stress by TUDCA restores the vasodilator action of insulin. ###P < 0.001, ##P < 0.01 (TUDCA+FA vs. FA), ***P < 0.001 (FA vs. BSA).
DISCUSSION
In the present study, we demonstrate that SFA impairs vasodilator actions of insulin through TLR4-induced ER stress. This is the first report suggesting a link between TLR4-induced ER stress and insulin-stimulated vasomotor activities. Moreover, TUDCA may be a potential treatment not only for fatty liver disease but also for the diabetic vascular complications in which ER stress plays important roles in the pathogenesis of these cardiometabolic disorders.
SFA Stimulates Proinflammatory Responses Through a TLR4-Dependent Mechanism
SFAs, including palmitate, stimulate TLRs, which contributes to endothelial dysfunction and insulin resistance (22, 24, 34, 53, 60). Both TLR2 and TLR4 are known to be stimulated by SFAs (33, 34). One study suggests that the activation of TLRs by SFA could be due to contamination of endotoxins in BSA (13). However, the level of endotoxin in the reagents used in this study was negligible (described in materials and methods), and a more recent study strongly confirms that activation of TLR2 and TLR4 are fatty acid specific, because SFA still activates TLRs in the presence of polymixin B, a lipopolysaccharide sequester (21). We (herein, Figs. 1 and 2) and others (5, 22, 24) reported that deficiency of TLR2 or TLR4 prevents the SFA-induced proinflammatory response in vascular endothelial cells. Interestingly, there was no additive effect when either TLR2 or TLR4 was deleted. This suggests that SFA-stimulated proinflammatory responses require both TLR2 and TLR4.
Receptor dimerization is the most proximal step in TLR activation, and palmitate induces dimerization of TLR2 with TLR1 and recruitment of TLR1/2 into lipid rafts, which serve as a platform for the interaction of the receptor with the other signaling molecules to activate the downstream signaling pathways (54). Thus, recruitment of TLRs to lipid raft is a crucial mechanism for proinflammatory responses, and treatment with polyunsaturated fatty acids or apoprotein A-I attenuated the recruitment of TLRs to lipid raft (4, 64). These suggest that SFA may contribute to the microenvironment of plasma membrane promoting the activation of TLRs. Further studies warrant the detailed mechanisms by which SFA specifically activates TLRs.
SFA Stimulates ER Stress Through a TLR4-Dependent Mechanism
There is a close association between ER stress and inflammation, which contributes to obesity-induced metabolic disorders as well as atherosclerosis (20, 31, 51, 59, 68). Imbalanced ER homeostasis causes apoptosis and activation of macrophages. Deficiency of TLR2 or TLR4 attenuated ER stress-induced cell death and atherosclerosis in LDLR KO mice (51). Failure of an adaptive response to ER stress leads to unfolding protein response (UPR), which activates JNK and NF-κB (46, 68). However, it has been unknown whether similar mechanisms exist in vascular endothelial cells. We observed that SFA stimulates ER stress through a TLR4-dependent mechanism (Fig. 2), which is consistent with previous reports in macrophages, hepatic tissues, and hypothalamic neurons (36, 46, 51, 68). Knockdown of TLR4 reduced not only ER stress but also proinflammatory responses. These suggest that activation of TLR4 may perturb cellular homeostasis, leading to ER stress and proinflammatory responses, and these two stress responses are closely associated. One potential mechanism is the activation of double-stranded RNA-dependent protein kinase (PKR), which is a nutrient sensor that transmits signaling to energy metabolism and inflammation (43). Further investigations are needed to clarify whether PKR contributes to SFA-induced inflammation and endothelial dysfunction. Future studies may elucidate the mechanistic link between ER stress and inflammation.
TLR4-KO Mice Are Protected from HFD-Induced Vasodilator Actions of Insulin
TLR4-KO mice are protected from atherosclerosis and inflammatory responses in aorta and macrophages (10, 39, 51). Moreover, TLR4 is involved in impairment of insulin signaling in endothelial cells through activation of NADPH oxidase (24, 35). In the present study, we have demonstrated that lack of TLR4 protects impairment of vasodilator actions of insulin (Figs. 4 and 5). Insulin-stimulated vasomotor activity is important for capillary recruitment in skeletal muscle, which contributes to glucose uptake and insulin sensitivity (3, 42, 48, 67). Since insulin-stimulated capillary recruitment in skeletal muscle facilitates delivery of nutrients and hormones, NO-mediated vascular function plays an important role in energy metabolism (30). Thus, TLR4-mediated impairment of endothelial function may contribute to HFD-induced insulin resistance, which is consistent with a study demonstrating that TLR4-KO mice are protected from HFD-induced insulin resistance (53). Thus, our results demonstrate the potential role of TLR4 linking vascular inflammation and impaired glucose homeostasis. Taken together, the results presented here support the concept that SFA-induced impairment of vascular actions of insulin is one of the mechanisms for the obesity-induced insulin resistance (30).
We observed that HFD caused impairment of insulin-stimulated vasorelaxation (Fig. 5A), which was abolished by deletion of TLR4. Interestingly, HFD affected neither Ach- nor SNP-stimulated vasorelaxation (Fig. 5, B and C). This result suggests that the impaired vasodilator actions of insulin may precede endothelial dysfunction (determined by the response to Ach) and vascular wall damage. The duration of HFD may not be severe enough to exhibit the impaired response to Ach but may be functionally impaired in responsiveness to insulin. This may be clinically important to diagnose insulin resistance/endothelial dysfunction prior to developing serious diseases including diabetes and atherosclerosis.
Impairment of Vasodilator Action of Insulin by Palmitate Is Reversed by Treatment with TUDCA
ER stress causes accumulation of misfolded proteins due to insufficient chaperones, which triggers cellular stress. TUDCA is a chemical chaperone that can reduce ER stress (46). Treatment with chemical chaperone may promote proper folding of proteins that leads to reduction of ER stress and inflammatory responses. TUDCA can restore insulin sensitivity and promote glucose uptake in both human subjects and ob/ob mice (23, 45, 46). We observed that pretreatment with TUDCA restored vasodilator action of insulin (Fig. 6A). It is possible that the restored vascular action of insulin may contribute to enhanced glucose uptake and insulin sensitivity. The specific molecular target of TUDCA and the detailed mechanisms by which ER stress affects insulin signaling are unknown. Future studies warrant the link between ER stress and insulin signaling pathways that affects both cardiovascular and metabolic functions.
In summary, we have demonstrated that SFA induces ER stress through a TLR4-mediated mechanism in vascular endothelium, and that reduction of ER stress restores vasodilator actions of insulin. These results support a notion that TLR4 and ER stress are important therapeutic targets to treat/prevent metabolic and cardiovascular disorders.
GRANTS
This study was supported by the American Diabetes Association (1-09-JF-33, 1-12-BS-99 to J.-a. Kim American Heart Association (13GRNT17220057 to J. Kim), and UAB Diabetes Research Center sponsored pilot and feasibility program supported by National Institute of Diabetes and Digestive and Kidney Diseases (P60 DK-079626), and UAB Comprehensive Diabetes Center, and USDA-ARS Program Project (5306-51530-017-00D) and USDA/NIFA competitive grant (2013-03477 to D. H. Hwang).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: J.-a.K. conception and design of research; J.-a.K. and H.-J.J. analyzed data; J.-a.K. and D.H.H. interpreted results of experiments; J.-a.K. prepared figures; J.-a.K. drafted manuscript; J.-a.K. and D.H.H. edited and revised manuscript; J.-a.K., H.-J.J., and D.H.H. approved final version of manuscript; H.-J.J. performed experiments.
REFERENCES
- 1.Baron AD. Insulin resistance and vascular function. J Diabetes Complications 16: 92–102, 2002. [DOI] [PubMed] [Google Scholar]
- 2.Baron AD. Vascular reactivity. Am J Cardiol 84: 25J–27J, 1999. [DOI] [PubMed] [Google Scholar]
- 3.Baron AD, Tarshoby M, Hook G, Lazaridis EN, Cronin J, Johnson A, Steinberg HO. Interaction between insulin sensitivity and muscle perfusion on glucose uptake in human skeletal muscle: evidence for capillary recruitment. Diabetes 49: 768–774, 2000. [DOI] [PubMed] [Google Scholar]
- 4.Cheng AM, Handa P, Tateya S, Schwartz J, Tang C, Mitra P, Oram JF, Chait A, Kim F. Apolipoprotein A-I attenuates palmitate-mediated NF-kappaB activation by reducing Toll-like receptor-4 recruitment into lipid rafts. PLos One 7: e33917, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Civelek M, Manduchi E, Riley RJ, Stoeckert CJ Jr, Davies PF. Chronic endoplasmic reticulum stress activates unfolded protein response in arterial endothelium in regions of susceptibility to atherosclerosis. Circ Res 105: 453–461, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Coats P, Johnston F, MacDonald J, McMurray JJ, Hillier C. Endothelium- derived hyperpolarizing factor: identification and mechanisms of action in human subcutaneous resistance arteries. Circulation 103: 1702–1708, 2001. [DOI] [PubMed] [Google Scholar]
- 7.Corpeleijn E, Feskens EJ, Jansen EH, Mensink M, Saris WH, de Bruin TW, Blaak EE. Improvements in glucose tolerance and insulin sensitivity after lifestyle intervention are related to changes in serum fatty acid profile and desaturase activities: the SLIM study. Diabetologia 49: 2392–2401, 2006. [DOI] [PubMed] [Google Scholar]
- 8.Dawson D, Vincent MA, Barrett EJ, Kaul S, Clark A, Leong-Poi H, Lindner JR. Vascular recruitment in skeletal muscle during exercise and hyperinsulinemia assessed by contrast ultrasound. Am J Physiol Endocrinol Metab 282: E714–E720, 2002. [DOI] [PubMed] [Google Scholar]
- 9.Dimmeler S, Fleming I, Fisslthaler B, Hermann C, Busse R, Zeiher AM. Activation of nitric oxide synthase in endothelial cells by Akt-dependent phosphorylation. Nature 399: 601–605, 1999. [DOI] [PubMed] [Google Scholar]
- 10.Ding Y, Subramanian S, Montes VN, Goodspeed L, Wang S, Han CY, Teresa AS 3rd, Kim J, O'Brien KD, Chait A. Toll-Like Receptor 4 deficiency decreases atherosclerosis but does not protect against inflammation in obese low-density lipoprotein receptor-deficient mice. Arterioscler Thromb Vasc Biol 2012. [DOI] [PMC free article] [PubMed]
- 11.Edfeldt K, Swedenborg J, Hansson GK, Yan ZQ. Expression of toll-like receptors in human atherosclerotic lesions: a possible pathway for plaque activation. Circulation 105: 1158–1161, 2002. [PubMed] [Google Scholar]
- 12.Ehses JA, Meier DT, Wueest S, Rytka J, Boller S, Wielinga PY, Schraenen A, Lemaire K, Debray S, Van Lommel L, Pospisilik JA, Tschopp O, Schultze SM, Malipiero U, Esterbauer H, Ellingsgaard H, Rutti S, Schuit FC, Lutz TA, Boni-Schnetzler M, Konrad D, Donath MY. Toll-like receptor 2-deficient mice are protected from insulin resistance and beta cell dysfunction induced by a high-fat diet. Diabetologia 53: 1795–1806, 2011. [DOI] [PubMed] [Google Scholar]
- 13.Erridge C, Samani NJ. Saturated fatty acids do not directly stimulate Toll-like receptor signaling. Arterioscler Thromb Vasc Biol 29: 1944–1949, 2009. [DOI] [PubMed] [Google Scholar]
- 14.Fan W, Morinaga H, Kim JJ, Bae E, Spann NJ, Heinz S, Glass CK, Olefsky JM. FoxO1 regulates Tlr4 inflammatory pathway signalling in macrophages. EMBO J 29: 4223–4236, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Frantz S, Ertl G, Bauersachs J. Mechanisms of disease: Toll-like receptors in cardiovascular disease. Nat Clin Pract Cardiovasc Med 4: 444–454, 2007. [DOI] [PubMed] [Google Scholar]
- 16.Fulton D, Gratton JP, McCabe TJ, Fontana J, Fujio Y, Walsh K, Franke TF, Papapetropoulos A, Sessa WC. Regulation of endothelium-derived nitric oxide production by the protein kinase Akt. Nature 399: 597–601, 1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gregor MF, Hotamisligil GS. Inflammatory mechanisms in obesity. Annu Rev Immunol 29: 415–445, 2011. [DOI] [PubMed] [Google Scholar]
- 18.Hadi HA, Suwaidi JA. Endothelial dysfunction in diabetes mellitus. Vasc Health Risk Manag 3: 853–876, 2007. [PMC free article] [PubMed] [Google Scholar]
- 19.Hoshino K, Takeuchi O, Kawai T, Sanjo H, Ogawa T, Takeda Y, Takeda K, Akira S. Cutting edge: Toll-like receptor 4 (TLR4)-deficient mice are hyporesponsive to lipopolysaccharide: evidence for TLR4 as the Lps gene product. J Immunol 162: 3749–3752, 1999. [PubMed] [Google Scholar]
- 20.Hotamisligil GS. Inflammation and endoplasmic reticulum stress in obesity and diabetes. Int J Obes 32, Suppl 7: S52–S54, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Huang S, Rutkowsky JM, Snodgrass RG, Ono-Moore KD, Schneider DA, Newman JW, Adams SH, Hwang DH. Saturated fatty acids activate TLR-mediated proinflammatory signaling pathways. J Lipid Res 53: 2002–2013, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jang HJ, Kim HS, Hwang DH, Quon MJ, Kim JA. Toll-like receptor 2 mediates high-fat diet-induced impairment of vasodilator actions of insulin. Am J Physiol Endocrinol Metab 304: E1077–E1088, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kars M, Yang L, Gregor MF, Mohammed BS, Pietka TA, Finck BN, Patterson BW, Horton JD, Mittendorfer B, Hotamisligil GS, Klein S. Tauroursodeoxycholic Acid may improve liver and muscle but not adipose tissue insulin sensitivity in obese men and women. Diabetes 59: 1899–1905, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kim F, Pham M, Luttrell I, Bannerman DD, Tupper J, Thaler J, Hawn TR, Raines EW, Schwartz MW. Toll-like receptor-4 mediates vascular inflammation and insulin resistance in diet-induced obesity. Circ Res 100: 1589–1596, 2007. [DOI] [PubMed] [Google Scholar]
- 25.Kim F, Pham M, Maloney E, Rizzo NO, Morton GJ, Wisse BE, Kirk EA, Chait A, Schwartz MW. Vascular inflammation, insulin resistance, and reduced nitric oxide production precede the onset of peripheral insulin resistance. Arterioscler Thromb Vasc Biol 28: 1982–1988, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kim JA, Formoso G, Li Y, Potenza MA, Marasciulo FL, Montagnani M, Quon MJ. Epigallocatechin gallate, a green tea polyphenol, mediates NO-dependent vasodilation using signaling pathways in vascular endothelium requiring reactive oxygen species and Fyn. J Biol Chem 282: 13736–13745, 2007. [DOI] [PubMed] [Google Scholar]
- 27.Kim JA, Montagnani M, Koh KK, Quon MJ. Reciprocal relationships between insulin resistance and endothelial dysfunction: molecular and pathophysiological mechanisms. Circulation 113: 1888–1904, 2006. [DOI] [PubMed] [Google Scholar]
- 28.Kolattukudy PE, Niu J. Inflammation, endoplasmic reticulum stress, autophagy, and the monocyte chemoattractant protein-1/CCR2 pathway. Circ Res 110: 174–189, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Konner AC, Bruning JC. Toll-like receptors: linking inflammation to metabolism. Trends Endocrinol Metab 22: 16–23, 2011. [DOI] [PubMed] [Google Scholar]
- 30.Kubota T, Kubota N, Kumagai H, Yamaguchi S, Kozono H, Takahashi T, Inoue M, Itoh S, Takamoto I, Sasako T, Kumagai K, Kawai T, Hashimoto S, Kobayashi T, Sato M, Tokuyama K, Nishimura S, Tsunoda M, Ide T, Murakami K, Yamazaki T, Ezaki O, Kawamura K, Masuda H, Moroi M, Sugi K, Oike Y, Shimokawa H, Yanagihara N, Tsutsui M, Terauchi Y, Tobe K, Nagai R, Kamata K, Inoue K, Kodama T, Ueki K, Kadowaki T. Impaired insulin signaling in endothelial cells reduces insulin-induced glucose uptake by skeletal muscle. Cell Metab 13: 294–307, 2011. [DOI] [PubMed] [Google Scholar]
- 31.Kumashiro N, Erion DM, Zhang D, Kahn M, Beddow SA, Chu X, Still CD, Gerhard GS, Han X, Dziura J, Petersen KF, Samuel VT, Shulman GI. Cellular mechanism of insulin resistance in nonalcoholic fatty liver disease. Proc Natl Acad Sci USA 108: 16381–16385, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kuo LH, Tsai PJ, Jiang MJ, Chuang YL, Yu L, Lai KT, Tsai YS. Toll-like receptor 2 deficiency improves insulin sensitivity and hepatic insulin signalling in the mouse. Diabetologia 54: 168–179, 2011. [DOI] [PubMed] [Google Scholar]
- 33.Lee JY, Plakidas A, Lee WH, Heikkinen A, Chanmugam P, Bray G, Hwang DH. Differential modulation of Toll-like receptors by fatty acids: preferential inhibition by n-3 polyunsaturated fatty acids. J Lipid Res 44: 479–486, 2003. [DOI] [PubMed] [Google Scholar]
- 34.Lee JY, Sohn KH, Rhee SH, Hwang D. Saturated fatty acids, but not unsaturated fatty acids, induce the expression of cyclooxygenase-2 mediated through Toll-like receptor 4. J Biol Chem 276: 16683–16689, 2001. [DOI] [PubMed] [Google Scholar]
- 35.Maloney E, Sweet IR, Hockenbery DM, Pham M, Rizzo NO, Tateya S, Handa P, Schwartz MW, Kim F. Activation of NF-kappaB by palmitate in endothelial cells: a key role for NADPH oxidase-derived superoxide in response to TLR4 activation. Arterioscler Thromb Vasc Biol 29: 1370–1375, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Martinon F, Chen X, Lee AH, Glimcher LH. TLR activation of the transcription factor XBP1 regulates innate immune responses in macrophages. Nat Immunol 11: 411–418, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mather KJ, Steinberg HO, Baron AD. Insulin resistance in the vasculature. J Clin Invest 123: 1003–1004, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Michelsen KS, Doherty TM, Shah PK, Arditi M. TLR signaling: an emerging bridge from innate immunity to atherogenesis. J Immunol 173: 5901–5907, 2004. [DOI] [PubMed] [Google Scholar]
- 39.Michelsen KS, Wong MH, Shah PK, Zhang W, Yano J, Doherty TM, Akira S, Rajavashisth TB, Arditi M. Lack of Toll-like receptor 4 or myeloid differentiation factor 88 reduces atherosclerosis and alters plaque phenotype in mice deficient in apolipoprotein E. Proc Natl Acad Sci USA 101: 10679–10684, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Montagnani M, Chen H, Barr VA, Quon MJ. Insulin-stimulated activation of eNOS is independent of Ca2+ but requires phosphorylation by Akt at Ser(1179). J Biol Chem 276: 30392–30398, 2001. [DOI] [PubMed] [Google Scholar]
- 41.Montagnani M, Ravichandran LV, Chen H, Esposito DL, Quon MJ. Insulin receptor substrate-1 and phosphoinositide-dependent kinase-1 are required for insulin-stimulated production of nitric oxide in endothelial cells. Mol Endocrinol 16: 1931–1942, 2002. [DOI] [PubMed] [Google Scholar]
- 42.Muniyappa R, Montagnani M, Koh KK, Quon MJ. Cardiovascular actions of insulin. Endocr Rev 28: 463–491, 2007. [DOI] [PubMed] [Google Scholar]
- 43.Nakamura T, Furuhashi M, Li P, Cao H, Tuncman G, Sonenberg N, Gorgun CZ, Hotamisligil GS. Double-stranded RNA-dependent protein kinase links pathogen sensing with stress and metabolic homeostasis. Cell 140: 338–348, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Nguyen MT, Favelyukis S, Nguyen AK, Reichart D, Scott PA, Jenn A, Liu-Bryan R, Glass CK, Neels JG, Olefsky JM. A subpopulation of macrophages infiltrates hypertrophic adipose tissue and is activated by free fatty acids via Toll-like receptors 2 and 4 and JNK- dependent pathways. J Biol Chem 282: 35279–35292, 2007. [DOI] [PubMed] [Google Scholar]
- 45.Ozcan L, Ergin AS, Lu A, Chung J, Sarkar S, Nie D, Myers MG Jr, Ozcan U. Endoplasmic reticulum stress plays a central role in development of leptin resistance. Cell Metab 9: 35–51, 2009. [DOI] [PubMed] [Google Scholar]
- 46.Ozcan U, Yilmaz E, Ozcan L, Furuhashi M, Vaillancourt E, Smith RO, Gorgun CZ, Hotamisligil GS. Chemical chaperones reduce ER stress and restore glucose homeostasis in a mouse model of type 2 diabetes. Science 313: 1137–1140, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Rastaldo R, Pagliaro P, Cappello S, Penna C, Mancardi D, Westerhof N, Losano G. Nitric oxide and cardiac function. Life Sci 81: 779–793, 2007. [DOI] [PubMed] [Google Scholar]
- 48.Rattigan S, Clark MG, Barrett EJ. Hemodynamic actions of insulin in rat skeletal muscle: evidence for capillary recruitment. Diabetes 46: 1381–1388, 1997. [DOI] [PubMed] [Google Scholar]
- 49.Reyna SM, Ghosh S, Tantiwong P, Meka CS, Eagan P, Jenkinson CP, Cersosimo E, Defronzo RA, Coletta DK, Sriwijitkamol A, Musi N. Elevated toll-like receptor 4 expression and signaling in muscle from insulin-resistant subjects. Diabetes 57: 2595–2602, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Saberi M, Woods NB, de Luca C, Schenk S, Lu JC, Bandyopadhyay G, Verma IM, Olefsky JM. Hematopoietic cell-specific deletion of toll-like receptor 4 ameliorates hepatic and adipose tissue insulin resistance in high-fat-fed mice. Cell Metab 10: 419–429, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Seimon TA, Nadolski MJ, Liao X, Magallon J, Nguyen M, Feric NT, Koschinsky ML, Harkewicz R, Witztum JL, Tsimikas S, Golenbock D, Moore KJ, Tabas I. Atherogenic lipids and lipoproteins trigger CD36-TLR2-dependent apoptosis in macrophages undergoing endoplasmic reticulum stress. Cell Metab 12: 467–482, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Senn JJ. Toll-like receptor-2 is essential for the development of palmitate-induced insulin resistance in myotubes. J Biol Chem 281: 26865–26875, 2006. [DOI] [PubMed] [Google Scholar]
- 53.Shi H, Kokoeva MV, Inouye K, Tzameli I, Yin H, Flier JS. TLR4 links innate immunity and fatty acid-induced insulin resistance. J Clin Invest 116: 3015–3025, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Snodgrass RG, Huang S, Choi IW, Rutledge JC, Hwang DH. Inflammasome-mediated secretion of IL-1beta in human monocytes through TLR2 activation; modulation by dietary fatty acids. J Immunol 191: 4337–4347, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Steinberg HO, Baron AD. Insulin-dependent diabetes mellitus and nitrovasodilation. Important and complex interactions. Circulation 95: 560–561, 1997. [DOI] [PubMed] [Google Scholar]
- 56.Steinberg HO, Brechtel G, Johnson A, Fineberg N, Baron AD. Insulin-mediated skeletal muscle vasodilation is nitric oxide dependent. A novel action of insulin to increase nitric oxide release. J Clin Invest 94: 1172–1179, 1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Steinberg HO, Chaker H, Leaming R, Johnson A, Brechtel G, Baron AD. Obesity/insulin resistance is associated with endothelial dysfunction. Implications for the syndrome of insulin resistance. J Clin Invest 97: 2601–2610, 1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Sundstrom J, Lind L, Vessby B, Andren B, Aro A, Lithell H. Dyslipidemia and an unfavorable fatty acid profile predict left ventricular hypertrophy 20 years later. Circulation 103: 836–841, 2001. [DOI] [PubMed] [Google Scholar]
- 59.Tabas I. The role of endoplasmic reticulum stress in the progression of atherosclerosis. Circ Res 107: 839–850, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Tsukumo DM, Carvalho-Filho MA, Carvalheira JB, Prada PO, Hirabara SM, Schenka AA, Araujo EP, Vassallo J, Curi R, Velloso LA, Saad MJ. Loss-of-function mutation in Toll-like receptor 4 prevents diet-induced obesity and insulin resistance. Diabetes 56: 1986–1998, 2007. [DOI] [PubMed] [Google Scholar]
- 61.Vincent MA, Clerk LH, Lindner JR, Klibanov AL, Clark MG, Rattigan S, Barrett EJ. Microvascular recruitment is an early insulin effect that regulates skeletal muscle glucose uptake in vivo. Diabetes 53: 1418–1423, 2004. [DOI] [PubMed] [Google Scholar]
- 62.Vincent MA, Montagnani M, Quon MJ. Molecular and physiologic actions of insulin related to production of nitric oxide in vascular endothelium. Curr Diabet Reports 3: 279–288, 2003. [DOI] [PubMed] [Google Scholar]
- 63.Wang N, Verna L, Chen NG, Chen J, Li H, Forman BM, Stemerman MB. Constitutive activation of peroxisome proliferator-activated receptor-gamma suppresses pro- inflammatory adhesion molecules in human vascular endothelial cells. J Biol Chem 277: 34176–34181, 2002. [DOI] [PubMed] [Google Scholar]
- 64.Wong SW, Kwon MJ, Choi AM, Kim HP, Nakahira K, Hwang DH. Fatty acids modulate Toll-like receptor 4 activation through regulation of receptor dimerization and recruitment into lipid rafts in a reactive oxygen species-dependent manner. J Biol Chem 284: 27384–27392, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Zeng G, Nystrom FH, Ravichandran LV, Cong LN, Kirby M, Mostowski H, Quon MJ. Roles for insulin receptor, PI3-kinase, and Akt in insulin-signaling pathways related to production of nitric oxide in human vascular endothelial cells. Circulation 101: 1539–1545, 2000. [DOI] [PubMed] [Google Scholar]
- 66.Zeng G, Quon MJ. Insulin-stimulated production of nitric oxide is inhibited by wortmannin. Direct measurement in vascular endothelial cells. J Clin Invest 98: 894–898, 1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Zhang L, Vincent MA, Richards SM, Clerk LH, Rattigan S, Clark MG, Barrett EJ. Insulin sensitivity of muscle capillary recruitment in vivo. Diabetes 53: 447–453, 2004. [DOI] [PubMed] [Google Scholar]
- 68.Zhang X, Zhang G, Zhang H, Karin M, Bai H, Cai D. Hypothalamic IKKbeta/NF-kappaB and ER stress link overnutrition to energy imbalance and obesity. Cell 135: 61–73, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]