Abstract
Nephrogenic systemic fibrosis (NSF) is a devastating condition associated with gadolinium (Gd3+)-based contrast agents (GBCAs) in patients with kidney disease. The release of toxic Gd3+ from GBCAs likely plays a major role in NSF pathophysiology. The cause and etiology of Gd3+ release from GBCAs is unknown. Increased Acidic Serine Aspartate Rich MEPE-associated peptides (ASARM peptides) induce bone mineralization abnormalities and contribute to renal phosphate-handling defects in inherited hypophosphatemic rickets and tumor-induced osteomalacia. The proteolytic cleavage of related bone matrix proteins with ASARM motifs results in release of ASARM peptide into bone and circulation. ASARM peptides are acidic, reactive, phosphorylated inhibitors of mineralization that bind Ca2+ and hydroxyapatite. Since the ionic radius of Gd3+ is close to that of Ca2+, we hypothesized that ASARM peptides increase the risk of NSF by inducing release of Gd3+ from GBCAs. Here, we show 1) ASARM peptides bind and induce release of Gd3+ from GBCAs in vitro and in vivo; 2) A bioengineered peptide (SPR4) stabilizes the Gd3+-GBCA complex by specifically binding to ASARM peptide in vitro and in vivo; and 3) SPR4 peptide infusion prevents GBCA-induced NSF-like pathology in a murine model with increased ASARM peptide (Hyp mouse). We conclude ASARM peptides may play a role in NSF and SPR4 peptide is a candidate adjuvant for preventing or reducing risk of disease.
Keywords: nephrogenic system fibrosis, chronic kidney disease, osteopontin, MEPE, DMP1, FGF23, PHEX, sclerostin
magnetic resonance imaging (MRI) is an invaluable imaging tool that can be markedly enhanced in some cases with use of gadolinium-based contrast agents (GBCAs). Nephrogenic systemic fibrosis (NSF) is a devastating disease described in patients with diminished renal function and is associated with GBCA use. As a result, contrast-enhanced MRI has largely been avoided in patients with kidney disease (9, 11). Free gadolinium (Gd3+) is present in tissues from patients with NSF and thought to be directly responsible for pathophysiology of NSF (9). Factors that lead to release of Gd3+ remain unknown, although transmetallation has been proposed (8, 10).
Calcium and hydroxyapatite bind with high affinity to the bone matrix peptide Acidic Serine Aspartate Rich MEPE (ASARM) peptide. A detailed review describes the role of ASARM peptides in bone renal diseases (13). Importantly, ASARM peptides are physiological substrates for Zn metalloendopeptidase (PHEX), potent inhibitors of mineralization, and chiefly responsible along with renal phosphate wasting for the bone and teeth mineralization defects in inherited hypophosphatemic rickets diseases (x-linked and autosomal) (1, 2, 6, 12, 14–18). In summary, proteolytic cleavage releases ASARM peptides from MEPE and SIBLING proteins of the bone extracellular matrix. The ASARM motif when released as a peptide (ASARM peptide; 2.3 kDa), is highly reactive, acidic, phosphorylated, and protease resistant. We designed a small PHEX-related peptide (SPR4 peptide; 4.2 kDa) that binds with high affinity and specificity to ASARM peptides and motifs in vivo and in vitro (13, 16, 18). Ionized calcium (Ca2+) and Gd3+ share many biophysical properties. We predicted that ASARM peptide would destabilize GBCA and cause Gd3+ release. To determine whether ASARM peptide is involved in early events in NSF, we conducted the following experiments using SPR4 peptide as a control.
METHODS
Animals and diets.
Male (C57BL/6) mice (5 wk) were housed at the University of Kansas Medical Center (KUMC) Department of Laboratory Animal Resources. The policies and procedures of the animal laboratory are in accordance with those detailed in the Guide for the Care and Use of Laboratory Animals published by the US Department of Health and Human Services. Male wild-type (WT) or mutant X-linked hypophosphatemic rickets mice (Hyp) were used for the study (n = 6) and maintained on a 1% phosphorus and 2.41 U/g vitamin-D3 diet (Harlan Teklad Rodent Diet 8604, Indianapolis, IN) (18). As reported previously, Hyp mice had major increases in circulating ASARM peptide compared with WT (6, 13, 17, 18).
Osmotic infusion of GBCA and SPR4.
Micro-osmotic pumps (model 1003D; Durect) containing either 1) vehicle (44 mM Tris, pH 7.4/132 mM NaCl/19.6 μM ZnCl2); 2) gadobenate (32 nmol·h−1·kg−1); or 3) SPR4 peptide (32 nmol·h−1·kg−1) were subcutaneously implanted and left for 3 days (total infusion over 3 days 2.2 μmol/kg; n = 6 mice). Serum, urine, femurs, kidney, and skin were collected for analysis as described previously (16, 18). SPR4 peptide was synthesized using standard techniques by Polypeptide Laboratories (San Diego, CA) as reported previously (12, 16, 18). Peptide purity was >80% via HPLC and mass spectrometry. SPR4 peptide was dissolved as follows: 100 μl/1 mg of peptide of 25 mM acetic acid was first added to dissolve the peptide, then 900 μl of 50 mM Tris, pH 7.4/150 mM NaCl was added and after thorough mixing 20 μl of 1 mM ZnCl2. Final buffer composition was 44 mM Tris pH 7.4/132 mM NaCl/19.6 μM ZnCl2. Zn is required for the Zn-binding motif of SPR4 peptide to structurally optimize SPR4 structure for binding to ASARM peptide (12, 16, 18).
Serum analysis, RNA isolation, and real-time PCR analysis.
Blood samples were collected in serum-separator tubes, and serum was prepared as described previously (12, 16, 18). Gene expression was performed with specific primers using RNA extracted from femurs and whole kidneys (n = 6 mice) as previously described (12, 16, 18).
HPLC and inductively coupled plasma-mass spectrometry.
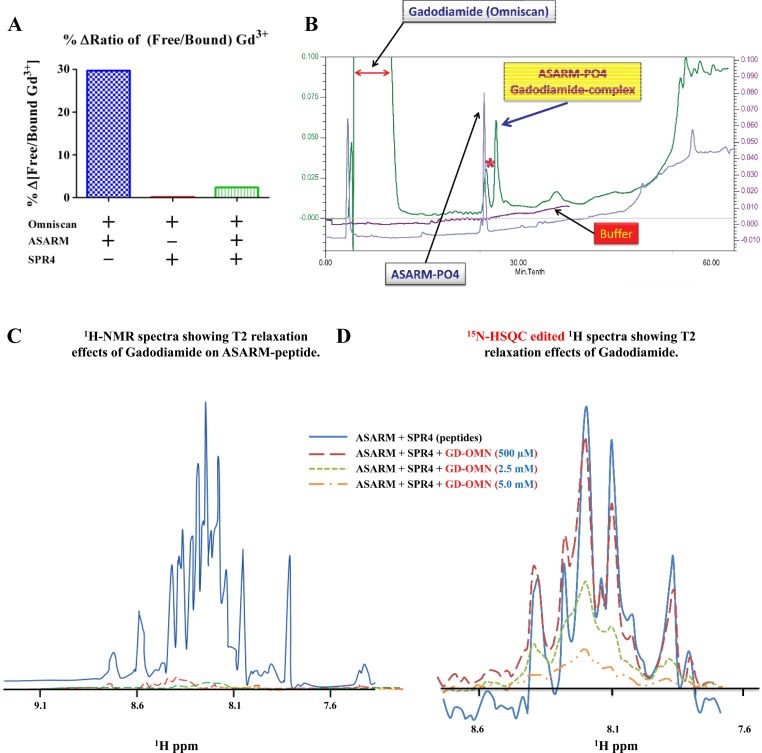
ASARM and SPR4 peptides were synthesized as reported previously (12, 16, 18). A Jupiter-300TM 4μ proteo 90 A C18 reverse-phase HPLC column (150 × 4.6; Phenomenex) with a Bio-Rad HPLC/FPLC system (BioLogic DuoFlow) was used to resolve peptides and GBCAs (Fig. 1B). Measurements of free and bound Gd3+ were undertaken using liquid chromatography-inductively coupled plasma-mass spectrometry (LC-ICP-MS) (4, 8); results are shown graphically in Fig. 1A. The compounds were resolved using an Agilent 7500e ICPMS detector (Collision Cell 2% He) linked to an Agilent HPLC. A Shodex-Ohpak HPLC column (6 μm CB802.5 HQ 80 Å, 8 × 300 mm) was used at 40°C. The percent change in free to bound Gd3+ in Omniscan samples treated with ASARM peptide, SPR4 peptide, or both was measured by LC-ICP-MS and is discussed in the text.
Fig. 1.
A small PHEX-related peptide (SPR4) competitively inhibits binding of Acidic Serine Aspartate Rich MEPE-associated peptides (ASARM peptides) to gadolinium (Gd3+)-based contrast agents (GBCA) in vitro. A: ASARM-induced Gd3+ release from Omniscan is prevented by SPR4 peptide. Percent change in free to bound Gd3+ in Omniscan samples treated with ASARM peptide, SPR4 peptide, or both were measured by HPLC linked to inductively coupled plasma-mass spectrometry (HPLC-ICP-MS) in vitro. Specifically, there was a 3-fold molar excess of SPR4 peptide relative to ASARM peptide (2.9 and 0.96 M, respectively). Omniscan was added at 0.49 M; see figure for combinations. We have shown previously that this ratio of ASARM peptide to SPR4 peptide is optimal for binding in vitro and in vivo (12, 16, 18). The Y-axis represents the percent ratio difference of free to bound gadolinium relative to vehicle. The total free and bound gadolinium for control (vehicle and Omniscan) and experimental conditions were not significantly different [17,400 ppm (μg/g) SD ± 8,508]. For all experimental conditions including vehicle, we detected a significant amount of free Gd3+. The ratios of “free/bound” gadolinium for gadodiamide treated with vehicle, ASARM peptide, SPR4 peptide, and SPR4+ASARM peptide were 0.58, 0.76, 0.59, and 0.60, respectively. B: ASARM peptide binds to gadodiamide: HPLC separation of peptides on a phenomenex C18 5μ jupitor column. Traces A and D show buffer gradient profiles (buffer A; 0.1% TFA/H20, buffer B; 100% acetonitrile/0.1% TFA). ASARM-gadodiamide complex resolves from free ASARM and gadodiamide. C: our previous published studies using 2-dimensional 1H/15N nuclear magnetic resonance (2D-NMR) and surface plasmon resonance (SPR) showed ASARM peptides bind to PHEX and SPR4 in vitro and in vivo (12, 16, 18). The 1H-NMR spectra of the ASARM peptide and SPR4 peptide binding complex and the effects of gadodiamide (Omniscan) on T2 relaxation are shown. A 5.8-fold molar excess of ASARM peptide (2.73 mM) to 15N-labeled SPR4 peptide (0.48 mM) was used as previously described (12). Broadening and quenching of spectral peaks as measured using 1H-NMR even at low concentrations of Gd3+ contrast agent “gadodiamide” occurred. D: in contrast, 15N-HSQC-edited spectra of the same sample run shows vastly reduced 15N-labeled SPR4 peptide line broadening effects. For further discussion and explanation, see the text.
NMR studies: 15N/1H.
All peptides (including 15N isotopically labeled for NMR) were synthesized by Peptide Group (San Diego, CA) as previously described (12, 16, 18). Confirmation of SPR4 binding to peptides was obtained using two-dimensional 1H/15N NMR (12) (Fig. 1, C and D).
Microcomputed tomography for bone and soft tissue.
Microcomputed tomography (μCT) using a Scanco μCT 40 system was carried out as described previously with n = 6 mice (5, 16). Mice bones, kidneys, and skin samples (fixed and ethanol dehydrated) were scanned with high-resolution μCT (μCT40; Scanco Medical, Southeastern, PA) as previously described (5, 16).
MRI: the kidney.
A 9.4 Tesla 31-m horizontal bore Varian system was used for all MRI measurements as described previously (12). A customized RF probe (2-turn solenoid coil, diameter = 7 mm) was used to increase the filling factor, thereby increasing the signal-to-noise ratio. A spin-echo pulse sequence was used to acquire T1-weighted MR images (FOV = 2 cm, resolution = 153 × 153 × 400 μm3, TE/TR = 4/140 ms).
Statistical analyses.
Statistical analyses were performed using PRISM5 (GraphPad Software, La Jolla, CA) as described previously (16, 18).
RESULTS
ASARM peptide induces release of Gd3+ from GBCA, and this is prevented by SPR4 peptide.
HPLC linked to LC-ICP-MS was used to measure free and bound gadolinium in physiologically buffered aqueous solution containing mixtures of GBCA, ASARM peptides, and SPR4 peptides (4, 8). Figure 1A shows ASARM peptide-induced release of Gd3+ from gadodiamide in vitro. Addition of excess SPR4 peptide prevented Gd3+ release. This data confirm ASARM peptides bind gadodiamide, induces desequestration of Gd3+, an effect prevented by SPR4 peptide. We then used HPLC to resolve both molecules and complexes (Fig. 1B) after mixing equimolar concentrations of gadodiamide and ASARM peptide. An ASARM-gadodiamide complex peak appeared that eluted later than the free ASARM peptide and gadodiamide peaks. This finding provided additional evidence that ASARM peptide binds to gadodiamide. We then used NMR to study this further (Fig. 1, C and D). Our previous published studies using two-dimensional 1H/15N NMR (2D-NMR) and surface plasmon resonance (SPR) showed ASARM peptides bind to PHEX and SPR4 in vitro and in vivo (12, 13, 16, 18). Figure 1, C and D, shows 1H-NMR spectra and 15N-HSQC-edited 1H-NMR spectra of the ASARM peptide and SPR4 peptide binding complex and the effects of gadodiamide on T2 relaxation. SPR4 and ASARM peptides are of similar molecular size, making Gd3+-induced T2 effects directly comparable. In the presence of gadodiamide, the 1H spectrum with 5.8-fold molar excess ASARM peptide induced major spectral T2 relaxation (Fig. 1C). Specifically, broadening and quenching of spectral peaks occurred even at low concentrations of gadodiamide. The 1H spectrum in Fig. 1C represents the ASARM peptide signal because of the vast excess of ASARM peptide relative to 15N-labeled SPR4 peptide (5.8-fold molar excess). The 15N-HSQC-edited spectra with 15N-labeled SPR4 peptide confirmed this assertion (Fig. 1D). The same interactions analyzed using 15N-HSQC-edited spectra showed vastly reduced SPR4 line-broadening effects (Fig. 1D). Notably, the 15N-HSQC-edited spectra represent the 15N-labeled 15N-SPR4 peptide and not the unlabeled ASARM peptide. From this we infer the spectral T2 relaxation for the GBCA markedly affects ASARM peptide but has no effect on the 15N-labeled SPR4 peptide. Therefore, the GBCA is closer to the ASARM peptide, providing compelling evidence that ASARM peptide binds with gadodiamide but not 15N-SPR4 peptide.
ASARM peptide-associated NSF-like pathology is prevented by SPR4 peptide.
We next used a murine model of X-linked hypophosphatemic rickets (Hyp) that has elevated ASARM peptides levels in the circulation and bone (12, 16, 18). Since ASARM peptides are small (4.2 kDa), acidic, reactive, highly phosphorylated with a low pI, we hypothesized the excess levels in Hyp mice should contribute to reduced GBCA stability and increase the risk of developing NSF-like pathology. To test this theory, we infused gadobenate with and without SPR4 peptide at concentrations known to neutralize excess circulating ASARM peptides (12, 13, 16, 18). SPR4 peptide prevented gadobenate-induced hyperphosphatemia and hypercalcemia (Table 1). The hyperphosphatemia and its prevention by SPR4 was confirmed by increased renal Na-dependent phosphate cotransporter (NPT2A and NPT2C) mRNA expression (Table 1). SPR4 peptide treatment also prevented a gadobenate-induced drop in bone sclerostin expression (Table 1). Of note, a recent study showed a link with serum phosphorus in renal patients with NSF (3). This case control study shows NSF patients with chronic kidney disease have significantly lower phosphorus levels compared with controls. Also, other investigators have shown serum phosphate is responsible for sensitizing rats to the profibrotic effects of gadodiamide (7). This is consistent with the known phosphaturic effect of ASARM peptides shown by us and others and supports the ASARM model (13). Thus exposure to GBCA in renal patients with high ASARM peptide levels (relatively hypophosphatemic) is proposed to induce release of Gd3+. A transient hyperphosphatemia due to released Gd3+ likely occurs, but as the disease progresses high ASARM peptide levels reestablish a relative hypophosphatemia. Fibrosis with organ failure then follows with classic NSF pathologies. The data in Table 1 therefore show compelling evidence for a link with ASARM peptide bioactivity, GBCA effects, and SPR4 in vivo. Further studies are required to confirm a direct link with NSF pathology.
Table 1.
SPR4 prevents GBCA-induced hyperphosphatemia and hypercalcemia in Hyp mice
| Hyp Mice Serum Chemistry (mg/dl; n = 6) |
|||
|---|---|---|---|
| Vehicle | Gadobenate | Gadobenate+SPR4 | |
| Serum PO4+ | 4.36 ± 0.50b | 5.95 ± 0.48a c | 4.12 ± 0.57b |
| Serum Ca2+ | 9.73 ± 0.33b | 11.64 ± 1.13a c | 9.70 ± 0.35b |
| Hyp Mice (mRNA Fold-Expression; n = 6) |
|||
| Tissue | Gene | Vehicle vs. gadobenate | Vehicle vs. gadobenate+SPR4 |
| Kidney | NPT2A | 1.84 ± 0.51* | NS |
| NPT2C | 1.92 ± 0.43* | NS | |
| 1-α-Hydroxylase | NS | NS | |
| Bone | SOST (Sclerostin) | −2.86 ± 0.19* | NS |
Values are means ± SD. GBCA, gadolinium-based contrast agents. Serum chemistry measurements are for male mice with increased ASARM peptide (Hyp; n = 6; 5 wk of age) infused with vehicle, gadobenate, or gadobenate+SPR4 peptide for 3 days. a, b, and c Significant difference (P < 0.05) for vehicle (a), gadobenate (b), and gadobenate+SPR4 peptide (c), respectively. “Fold” mRNA expression levels (quantitative RT/PCR) for vehicle vs. gadobenate and vehicle vs. gadobenate+SPR4 peptide” are also shown for both bone and kidney. Expression analyses were carried out as described previously, PCR efficiencies were calculated for each primer set, and transferrin was used as a housekeeping gene (16, 18). Significant difference was calculated using a Wilcoxon signed rank test (theoretical median = 1). NPT2A and C are renal Na-dependent phosphate cotransporters. The pharmaceutical name for gadobenate is MultiHance. NS, not significant.
Significant difference (P < 0.05) vehicle.
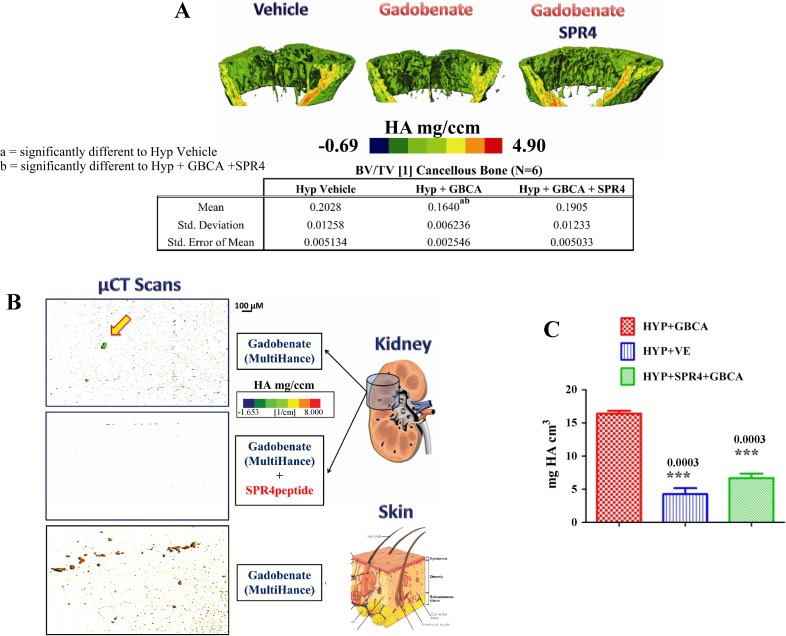
We then tested the effects on bone by scanning resected femurs removed from Hyp mice using μCT (Fig. 2A). We found significant decreased bone volume/tissue volume (BV/TV) after 3 days of infusion. Figure 2A illustrates these changes and the drop in volumetric mineral density in gadobenate-treated mice. We conclude that acute infusion of GBCA and high ASARM peptide levels in Hyp mice induced release of Gd3+ that then displaced Ca2+ and PO4+ from bone even after 3 days. SPR4 peptide co-treatment prevented the gadobenate-induced changes (Fig. 2A). SPR4 peptide likely indirectly prevented the gadobenate-mediated altered mineral content by binding to ASARM peptides. Also, μCT scans of kidneys and dermal sections demonstrate metastatic calcification in gadobenate-treated mice (Fig. 2, B and C). The microcalcified nodules were absent in mice cotreated with SPR4 peptide. This suggests that cotreatment with SPR4 peptide prevents gadobenate-induced NSF-like pathology in Hyp mice.
Fig. 2.
SPR4 infusion reverses GBCA-induced pathology in X-linked hypophosphatemic rickets (Hyp) mice in vivo (n = 6). A: high-resolution microcomputed tomography (μCT; 6 μM) scans of femurs from Hyp mice infused with vehicle, gadobenate, or gadobenate+SPR4 peptide. Note significant reduction in mineral density (mg/cm3) in GBCA-treated mice compared with vehicle- and GBCA+SPR4 peptide-treated mice. Tabulated bone volume/tissue volume (BV/TV) shown below the scheme: a = P < 0.05 significant difference from Hyp+vehicle. b = P < 0.05 significant difference from Hyp+GBCA+SPR4. GBCA used was gadobenate (MultiHance). HA, hydroxyapatite. B: high-resolution (6 μm) scans of kidney and dermal sections (middorsal region) removed from gadobenate- and gadobenate+SPR4 peptide-infused Hyp mice (osmotic pumps). For a more detailed description, see the text. Note metastatic calcifications in gadobenate-infused mice that are absent in mice infused with gadobenate+SPR4 peptide. C: mineral content (mg HA/cm3) of whole kidneys measured using μCT at 6-μm resolution (n = 6). Note that with mice treated with the GBCA MultiHance, an increased mineral density occurs relative to Hyp vehicle mice and mice coinfused with SPR4 peptide and GBCA (see also B). This indicates renal ectopic deposition of mineral that is likely bone derived (see also A).
ASARM peptide destabilizes GBCA in vivo, and this is blocked by SPR4 peptide.
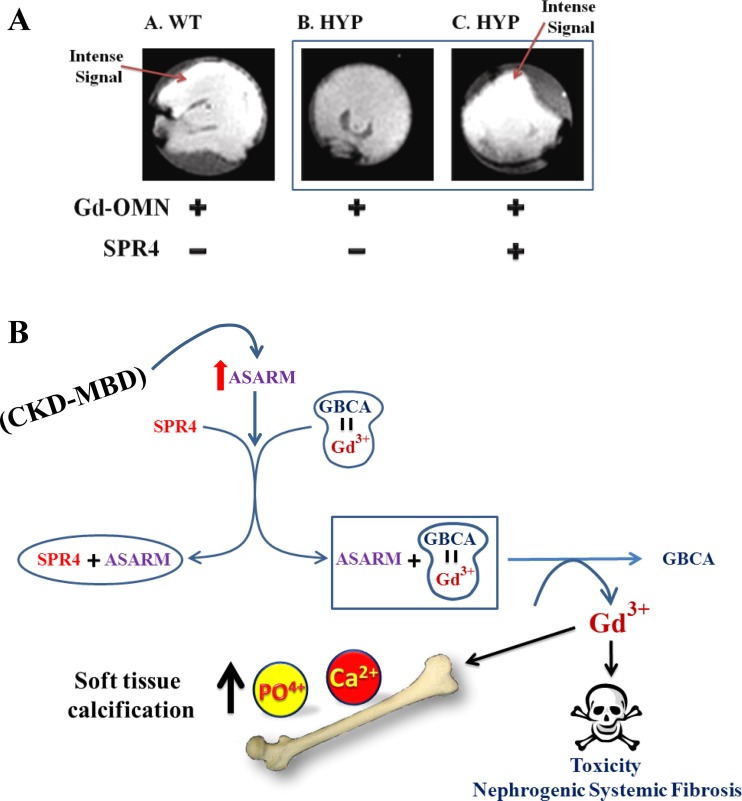
To determine whether ASARM peptides destabilize GBCA in vivo, we carried out the following experiment using Hyp mice: mice were 1) injected (intraperitoneally) with gadodiamide; or 2) pretreated with a bolus of SPR4 peptide (129 nmol) and then treated 30 min later with gadodiamide. After 2 h, mice were euthanized and their kidneys were removed and scanned by T1-weighted MRI. The high-intensity MRI signal reflects an intact Gd3+-gadodiamide complex, and a lower MRI signal reflects a breakdown of the complex (Fig. 3A). We saw a marked quenching of the gadodiamide signal in Hyp mice compared with WT. Hyp mice pretreated with SPR4 peptide showed a striking restoration of signal intensity (Fig. 3A). This is consistent with binding of SPR4 peptide with the excess ASARM peptides with resulting increased stability of the Gd3+-gadodiamide complex. Although the precise biophysical mechanism requires validation, this experiment suggests SPR4 peptide binds to excess ASARM peptide, thereby indirectly increasing the stability of the Gd3+-gadodiamide complex.
Fig. 3.
ASARM-induced Gd3+ release from Omniscan is prevented by SPR4 peptide and increases GBCA stability in renal MRI scans of Hyp mice ex vivo (n = 6). A: MRI (ex vivo) of representative kidneys resected 2 h after intraperitoneal injection with Omniscan (Gd-OMN) or Gd-OMN+SPR4 peptide as indicated in the scheme. Photo A: wild-type mouse (WT). Photos B and C: Hyp mice (Hyp mice overexpress ASARM peptides). Note in Hyp mice treated only with gadodiamide (photo B), the MRI signal is quenched compared with WT mice (photo A) and Hyp mice treated with Gd-OMN+SPR4 peptide (photo C). This indicates preferential desequestration and release of Gd3+ ion from the gadodiamide vehicle in Hyp mice due to excess ASARM peptides (photo B). This is prevented in Hyp mice pretreated with SPR4 peptide (compare photos B and C). Thus SPR4 peptide indirectly stabilizes Omniscan (gadodiamide) by binding to ASARM peptide. Note the contrast images for WT mice, Hyp mice, and Hyp+SPR4-treated mice were identical to Hyp mice treated with Gd-OMN. Thus the quenching of the contrast signal by Gd-OMN is quite marked. See the text for a more detailed description. B: model illustrating the proposed ASARM peptide-induced release of toxic Gd3+ from GBCA resulting in nephrogenic systemic fibrosis (NSF). SPR4 peptide may prevent this by binding to and neutralizing ASARM peptide. Note that free Gd3+ as well as inducing organ/tissue toxicity is also reported to displace Ca2+ and PO4+ from bone and alter expressions of FGF23 and parathyroid hormone. This results in hyperphosphatemia, hypercalcemia, and soft tissue calcification.
DISCUSSION
Our study provides compelling evidence that ASARM peptides bind to GBCAs, induce release of Gd3+ and SPR4 peptide, prevent this in vitro and in vivo. Figure 3B provides a scheme that explains this model. Importantly, our study has limitations since the associated in vivo biological changes we described are insufficient to confirm NSF pathology and could be interesting phenomena unelated to NSF. Also, our study did not investigate a representative range of GBCAs with differing thermodynamic stabilities (Ktherm). Consequently, the implications of these new findings for NSF on pathology, etiology, prevention, and treatment need further investigation. Specifically, further studies should address the following questions: 1) Is there a subset of patients with CKD-MBD who have increased circulating or tissue levels of ASARM peptides and are they at increased risk of developing NSF; 2) Are patients with inherited or tumor-acquired bone-mineral disorders with increased ASARM peptide levels such as autosomal and X-linked hypophosphatemic rickets and tumor-induced osteomalacia (13) at increased risk for NSF; 3) Since SPR4 peptide prevents release of free Gd3+ and indirectly increases GBCA stability, is this peptide an ideal adjuvant for reducing or preventing NSF in susceptible patients; 4) Is the current ELISA test for ASARM peptide measurement in urine and sera a useful prescreen for patients at risk for NSF; and 5) Can we reduce the risk of NSF in MRI-scanned patients by neutralizing ASARM peptides with SPR4 peptide followed by dialysis immediately after the scan?
GRANTS
Research reported in this publication was supported by the National Institute of Arthritis and Musculoskeletal and Skin Diseases (NIAMS) of the National Institutes of Health (NIH) under award number R01AR051598-10 (P. S. Rowe). The Hoglund Brain Imaging Center is supported by grants from the NIH (C76 HF00201, P30 HD002528, S10 RR29577, UL1 TR000001, P30 AG035982) and the Hoglund Family Foundation. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
Author contributions: P.S.N.R. provided conception and design of research; P.S.N.R., L.V.Z., J.S.L., and P.L. performed experiments; P.S.N.R., L.V.Z., J.S.L., P.L., and W.M.B. analyzed data; P.S.N.R., J.S.L., P.L., W.M.B., and E.T.M. interpreted results of experiments; P.S.N.R. prepared figures; P.S.N.R. drafted manuscript; P.S.N.R. and E.T.M. edited and revised manuscript; P.S.N.R., L.V.Z., J.S.L., P.L., W.M.B., and E.T.M. approved final version of manuscript.
REFERENCES
- 1.Addison W, Nakano Y, Loisel T, Crine P, McKee M. MEPE-ASARM peptides control extracellular matrix mineralization by binding to hydroxyapatite—an inhibition regulated by PHEX cleavage of ASARM. J Bone Miner Res 23: 1638–1649, 2008. [DOI] [PubMed] [Google Scholar]
- 2.Barros NM, Hoac B, Neves RL, Addison WN, Assis DM, Murshed M, Carmona AK, McKee MD. Proteolytic processing of osteopontin by PHEX and accumulation of osteopontin fragments in Hyp mouse bone, the murine model of X-linked hypophosphatemia. J Bone Miner Res 28: 688–699, 2013. [DOI] [PubMed] [Google Scholar]
- 3.Bernstein EJ, Isakova T, Sullivan ME, Chibnik LB, Wolf M, Kay J. Nephrogenic systemic fibrosis is associated with hypophosphataemia: a case-control study. Rheumatology (Oxford) 53: 1613–1617, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cleveland D, Long SE, Sander LC, Davis WC, Murphy KE, Case RJ, Rimmer CA, Francini L, Patri AK. Chromatographic methods for the quantification of free and chelated gadolinium species in MRI contrast agent formulations. Anal Bioanal Chem 398: 2987–2995, 2010. [DOI] [PubMed] [Google Scholar]
- 5.David V, Martin A, Hedge AM, Rowe PS. Matrix extracellular phosphoglycoprotein (MEPE) is a new bone renal hormone and vascularization modulator. Endocrinology 150: 4012–4023, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.David V, Martin AC, Hedge AM, Drezner MK, Rowe PS. ASARM peptides: PHEX-dependent and -independent regulation of serum phosphate. Am J Physiol Renal Physiol 300: F783–F791, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fretellier N, Idee J, Bruneval P, Guerret S, Daubine F, Jestin G, Factor C, Poveda N, Dencausse A, Massicot F, Laprevote O, Mandet C, Bouzian N, Port M, Corot C. Hyperphosphataemia sensitizes renally impaired rats to the profibrotic effects of gadodiamide. Br J Pharmacol 165: 1151–1162, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fretellier N, Idee JM, Dencausse A, Karroum O, Guerret S, Poveda N, Jestin G, Factor C, Raynal I, Zamia P, Port M, Corot C. Comparative in vivo dissociation of gadolinium chelates in renally impaired rats: a relaxometry study. Invest Radiol 46: 292–300, 2011. [DOI] [PubMed] [Google Scholar]
- 9.Galan A, Cowper SE, Bucala R. Nephrogenic systemic fibrosis (nephrogenic fibrosing dermopathy). Curr Opin Rheumatol 18: 614–617, 2006. [DOI] [PubMed] [Google Scholar]
- 10.Idee JM, Fretellier N, Robic C, Corot C. The role of gadolinium chelates in the mechanism of nephrogenic systemic fibrosis: a critical update. Crit Rev Toxicol 44: 895–913, 2014. [DOI] [PubMed] [Google Scholar]
- 11.Kanal E, Broome DR, Martin DR, Thomsen HS. Response to the FDA's May 23, 2007, nephrogenic systemic fibrosis update. Radiology 246: 11–14, 2008. [DOI] [PubMed] [Google Scholar]
- 12.Martin A, David V, Laurence JS, Schwarz PM, Lafer EM, Hedge AM, Rowe PS. Degradation of MEPE, DMP1, and release of SIBLING ASARM-peptides (minhibins): ASARM-peptide(s) are directly responsible for defective mineralization in HYP. Endocrinology 149: 1757–1772, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rowe PS. Regulation of bone-renal mineral and energy metabolism: the PHEX, FGF23, DMP1, MEPE ASARM pathway. Crit Rev Eukaryot Gene Expr 22: 61–86, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rowe PSN, Garrett IR, Schwarz PM, Carnes DL, Lafer EM, Mundy GR, Gutierrez GE. Surface plasmon resonance (SPR) confirms MEPE binds to PHEX via the MEPE-ASARM-motif: a model for impaired mineralization in X-linked rickets (HYP). Bone 36: 33–46, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Salmon B, Bardet C, Khaddam M, Naji J, Coyac BR, Baroukh B, Letourneur F, Lesieur J, Decup F, Le Denmat D, Nicoletti A, Poliard A, Rowe PS, Huet E, Vital SO, Linglart A, McKee MD, Chaussain C. MEPE-derived ASARM peptide inhibits odontogenic differentiation of dental pulp stem cells and impairs mineralization in tooth models of X-linked hypophosphatemia. PLoS One 8: e56749, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zelenchuk L, Hedge A, Rowe PS. SPR4-peptide alters bone metabolism of normal and HYP mice. Bone 72: 23–33, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zelenchuk LV, Hedge AM, Rowe PS. Age dependent regulation of bone-mass and renal function by the MEPE ASARM-motif. Bone 79: 131–142, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zelenchuk LV, Hedge AM, Rowe PS. PHEX mimetic (SPR4-peptide) corrects and improves HYP and wild type mice energy-metabolism. PLoS One 9: e97326, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]